1. Introduction
Hydrometallurgy is a technology of separating, enriching, and extracting metals in which the valuable metal components of ores, calcine, and other materials are dissolved or precipitated in the solution by a leaching agent. As the concentration of mineable materials declines and the guidelines for environmental protection increase, hydrometallurgy technology at atmospheric pressure struggles to satisfy the demand for the extraction of non-ferrous metals from complex minerals and the comprehensive utilization of rare metals. Therefore, pressure hydrometallurgy has been rapidly developed and has become the most important modern hydrometallurgy technology.
As the name suggests, pressure hydrometallurgy is carried out in higher-pressure conditions. In essence, the reaction temperature of an aqueous solution can be much higher than its boiling point in the atmosphere if the operational pressure increases, e.g., 200~300 °C. This will greatly enhance the reaction driving force and increase the chemical reaction rate in the metallurgical process. Compared with traditional hydrometallurgy, pressure hydrometallurgy technology has the following characteristics [
1]:
- (1)
A good adaptability of raw materials and a high comprehensive utilization of resources. In the leaching kinetic conditions of high temperature and pressure, the pressure hydrometallurgy process has a fast leaching rate and is able to treat a variety of metal sulfide and oxide ores (see
Figure 1) as well as complex low-grade materials, including low-grade minerals [
2], arsenic-containing materials [
3], multi-metal-accompanying minerals, intermediate metallurgical materials, and secondary renewable resources. While extracting the main metal, it can also selectively leach and separate the accompanying rare metals or precious metals [
4]. Consequently, it helps to improve the comprehensive recovery of the accompanying elements whose economic benefit even exceeds that of the main metal. Therefore, pressure hydrometallurgy technology has been widely used in the fields of extraction metallurgy and materials preparation for aluminum, uranium, copper, zinc, nickel, cobalt, tungsten, and a variety of rare and precious metals.
- (2)
A short technological process and environmental friendliness. Pressure hydrometallurgy can directly leach sulfide ores and convert the sulfur element in the ore into sulfur monomers. In this way, the processes of oxidation roasting and sulfuric acid production are not needed. Compared with traditional hydrometallurgy, pressure hydrometallurgy significantly shortens the production process and avoids releasing SO2 pollution into the air. In addition, the sulfur monomers from pressure hydrometallurgy are easier to store and transport than the sulfuric acid from traditional hydrometallurgy.
Figure 1.
Some minerals that are suitable for pressure hydrometallurgy [
1].
Figure 1.
Some minerals that are suitable for pressure hydrometallurgy [
1].
High cost and difficult operation. Compared to traditional hydrometallurgy, a larger cost is necessary to build the production line for pressure hydrometallurgy because the high-temperature and high-pressure reactors and their ancillary equipment are always very expensive. Meanwhile, it is difficult to operate the high-temperature and high-pressure equipment smoothly, which results in technicians with a high level of operation and management being needed.
Despite the advantages of pressure hydrometallurgy, it does come with significant challenges. Among these is the management of the leaching process, which is a key aspect of pressure hydrometallurgy and has become a major topic in the field, such as catalysts for the leaching process [
5], leaching medium [
6], dispersants [
7], etc. Additionally, the phenomenon of flash boiling presents potential safety risks that must be carefully managed.
When the slurry or solution with a high temperature and pressure enters the procedure equipment in the atmosphere (such as solid–liquid separation, electrowinning, etc.), the liquid is in a superheat state, and then the flash boiling phenomenon occurs. If the pressure drop between the two pieces of equipment is too large, a burst boil will take place in the solution, and a large amount of steam will be produced. Then, a steam flow with high speed will be formed and will violently impact the vessel and pipeline. The burst boil greatly affects the steady running of the production and brings safety risks to the production process. In order to avoid the burst boiling phenomenon, high-temperature and high-pressure reactors are generally equipped with multi-stage flash vessels, so that the high-temperature and high-pressure solution can be depressurized step by step to meet the pressure and temperature requirements of the subsequent processes.
By controlling the pressure of multi-stage flash vessels, different grades of steam can be obtained, which is used to heat the solution or slurry through a heat exchanger, and then condensed into liquid water in a condenser. In this way, the step utilization of the steam waste heat is realized to improve the thermal efficiency of the system, and the water is recycled in the system to reduce the discharge of industrial wastewater.
Obviously, in the process of pressure hydrometallurgy, a befitting pressure for each flash stage is an important factor to ensure smooth and safe production and to improve the thermal efficiency of the system and reduce energy consumption. However, the basic scientific research on flash evaporation phenomena in pressure hydrometallurgy is scarce. Zinc and aluminum are two kinds of bulk non-ferrous metal, and the oxygen pressure leaching of zinc sulfide concentrate and alumina Bayer production process are two typical pressure hydrometallurgy technologies. Thus, taking the two processes as a representative sample, this paper introduces the flashing process as well as its function in pressure hydrometallurgy, and reviews the status of related studies. The remainder of this paper is structured as follows: After a detailed introduction of the flashing phenomena in the zinc leaching and Bayer aluminum production processes in
Section 2 and
Section 3, respectively, their features and methods of classifications are discussed in
Section 4. It helps to connect them with the flashing phenomena in other fields. As follows, an overview of the status of research is given in
Section 5, while the relevant progresses made on flashing flows in other fields are summarized in
Section 6. Finally, the status of research, recommendable numerical methods as well as aspects needing further efforts are concluded in
Section 7.
2. Flashing Phenomena in the Oxygen Pressure Leaching Process of Zinc Sulfide Concentrate
2.1. The Oxygen Pressure Leaching Technology of Zinc Sulfide Concentrate
In the oxygen pressure direct leaching process of zinc sulfide concentrate, the finely ground concentrate with a particle size of 40~60 μm is mixed with waste electrolyte and a sulfuric acid solution. The mixture, called a slurry, is pumped into an autoclave by diaphragm pumps, where acid leaching is conducted. The oxygen is injected into the autoclave to promote the conversion of zinc sulfide into zinc sulfate and sulfur monomers. The main chemical reaction is as follows:
The higher the slurry temperature, the faster the leaching reaction rate [
8]. But, when the temperature exceeds the melting point of sulfur, the product sulfur will be melted and cover the unreacted sulfide. This phenomenon will hinder the leaching reaction, which is called a passivation reaction. The following measures can be applied to avoid the passivation reaction:
- (1)
The leaching slurry temperature is maintained below the melting point of sulfur monomers (about 120 °C) by adjusting the steam flux of the autoclave.
- (2)
When the leaching slurry temperature is demanded to be over the melting point of sulfur monomers, some additives, such as lignosulfonates [
9], are used to disperse the sulfur covering the unreacted sulfide.
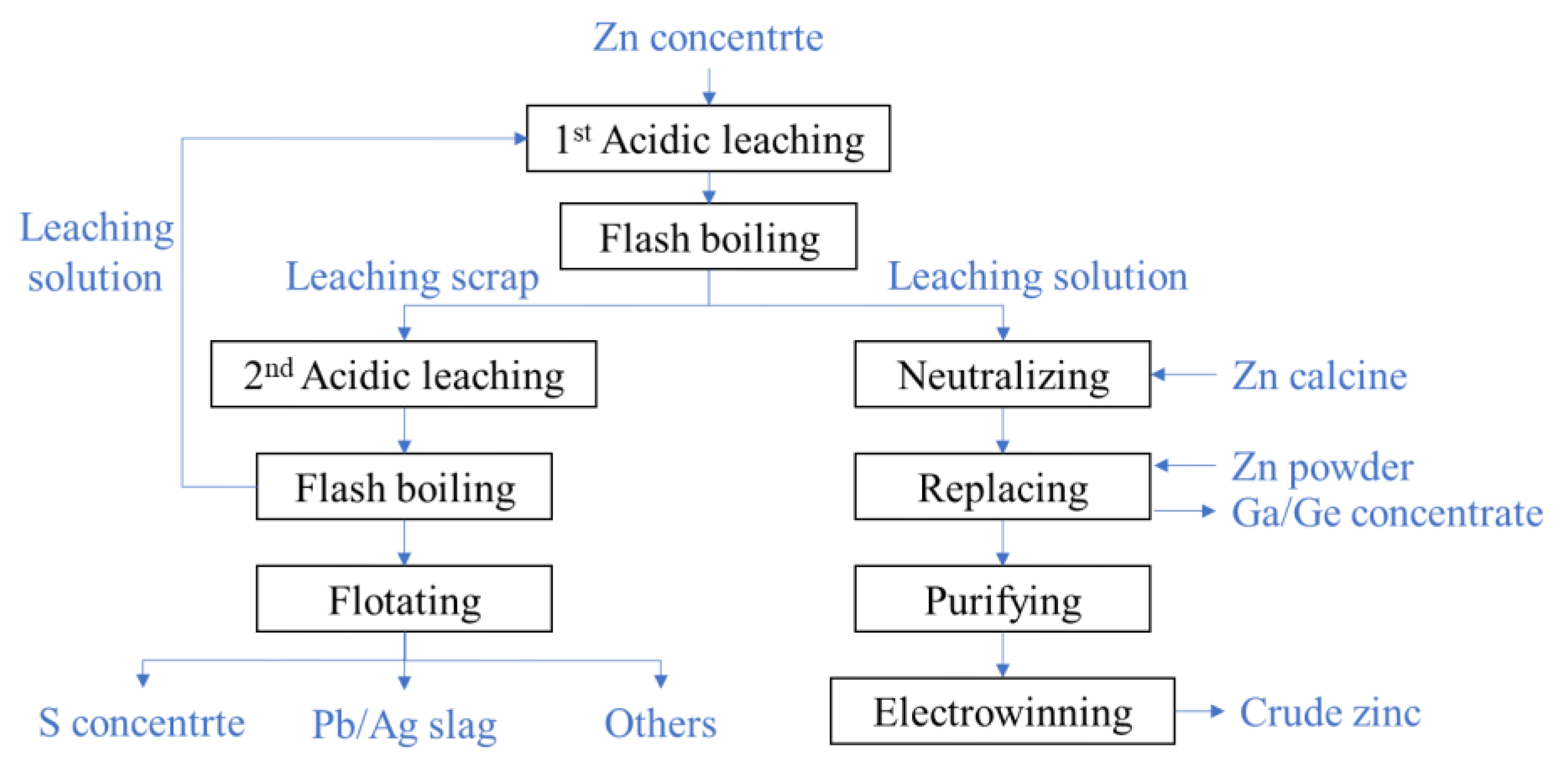
The two-stage oxygen pressure leaching technology of zinc sulfide concentrates is widely used in China. Its flowchart is shown in
Figure 2, and the typical technological parameters are listed in
Table 1.
In the first acidic leaching, the slurry is made from the ground zinc concentrate mixed with the waste acid and the solution of the second leaching and then pumped into the autoclave. About 50% zinc is leached from the minerals after the slurry stay 1.5~2 h in the autoclave at a temperature of 105~115 °C. The leached slurry with a high temperature and high pressure is depressurized into the atmosphere state in the flash boiling devices. By filtrating the slurry, the leaching solution and scrap are separated. The solution is rich in gallium, germanium, zinc, and other metal ions as well as residual sulfuric acid. So, zinc oxide is firstly added to neutralize the residual sulfuric acid, then zinc powder is added to replace gallium, germanium, and other metal ions. By precipitating and filtrating the zinc sulfate solution, the gallium–germanium enrichments [
11] are extracted. The zinc sulfate solution is purified by iron removal and then the crude zinc is produced by electrowinning procedure.
About 50% zinc element is left in the leaching scrap of the first acidic leaching, and the scrap is needed to be further leached in the second acidic leaching. The low grade of zinc and high content of sulfur monomers make the leaching dynamic condition poorer than that of the first stage. To improve the leaching rate, the second acidic leaching is conducted in the case of a high acid concentration (80~85 g/L) and high temperature (150~155 °C), and the leaching time extends to 2~4 h. The slurry is also depressurized through the flash boiling devices. The sulfur and Pb/Ag slag are orderly separated from the scrap of the second leaching by flotation. The solution of the second leaching contains a high concentration of acid and is returned to the first acidic leaching.
2.2. The Flashing of Zinc Sulfide Leaching Slurry
As shown in
Figure 2, each leaching procedure connects to a flash boiling procedure in the two-stage oxygen pressure leaching process of zinc sulfide concentrate. The flash boiling occurs when the leaching slurry flows into the flash tank whose pressure is lower than the saturation pressure of the slurry. The boiling rate is controlled by adjusting the pressure of the discharge steam pipeline to regulate the superheat degree of the slurry. In the flash boiling process, a large amount of steam is generated and takes away heat energy from the slurry, which makes the slurry temperature and pressure decrease rapidly until saturation is achieved.
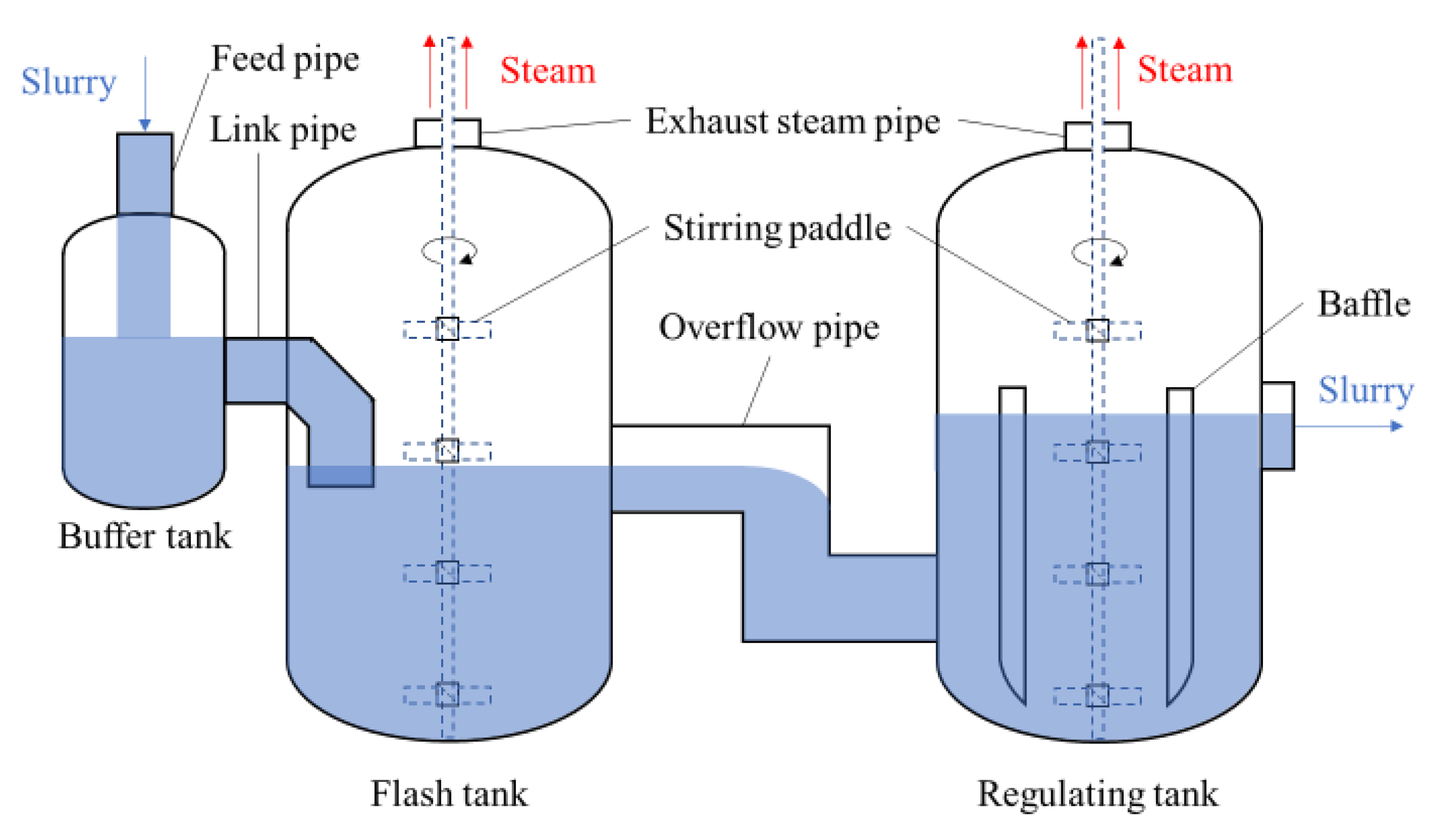
The same flashing devices are configured for the first and second leaching stage, including the flash tank (FT), buffer tank, and regulating tank (RT) (seeing
Figure 3). Their different operation parameters are shown in
Table 2. It is known that the temperature and pressure of the slurry in the second leaching are higher than those in the first leaching. Correspondingly, the temperature of FT for the second leaching is higher than that of FT for the first leaching.
The slurry passes through the buffer tank, flash tank, and regulating tank in turn. The flash tank is the main vessel for the depressurization of the slurry. The buffer tank is first used to pre-reduce the pressure partially, which can avoid the shock wave caused by rapid depressurizing at the outlet of the pipe. The primary function of the regulating tank lies in further depressurization of the slurry and controlling the crystallization process of sulfur.
There is only one inlet and one outlet in the buffer tank. The leached slurry flows into the buffer tank via the feed pipe at the top. The mixture of flash steam and the slurry flows into the flash tank. To achieve moderate depressurization, the link pipe of the flash tank is immersed in the liquid. The slurry outflows from the flash tank into the regulating tank through the overflow pipe, which connects the middle of the flash tank and the bottom of the regulating tank. Both the flash tank and the regulating tank are equipped with stirring paddles for continuous stirring, which can prevent the slurry from settling and make the temperature of the slurry even. This measure is good for improving the flashing efficiency. Four baffles are installed on the inner wall of the regulating tank to enhance the convection of the slurry in the vertical direction. The flash steam is discharged through the exhaust steam pipe at the top of both the flash tank and the regulating tank.
In the production of oxygen pressure leaching process of zinc sulfide concentrate, if the pressure in the flash tank is too large, the slurry in the regulating tank will boil violently and lead to a false liquid level and even over-swelling. Meanwhile, when the convection of the slurry in a regulating tank is choked, a large amount of thermal energy is gathered at the bottom and high-pressure bubbles are generated. As the pressure increases and exceeds a critical value, the bubbles will break, resulting in huge instantaneous steam flux and severe pressure fluctuations [
11]. On the contrary, if the pressure in the flash tank is too small, the burst boil will happen at the outlet of the link pipe, which has a serious impact on the valve of the pipeline and obviously shortens the life span of the vessels. Therefore, a comprehensive understanding of the flash boiling behavior in the flash tank and regulating tank is of great significance in guiding the structure design and operation of the equipment.
3. Flashing Phenomena in BAYER Method for Alumina Production
3.1. The Alumina Production Technology in Bayer Method
The Bayer method is most widely used in the alumina production process, and the major reactions are as follows:
where
x = 1 for monohydrate hard alumina bauxite or monohydrate soft alumina bauxite;
x = 3 for the trihydrate alumina bauxite. Among them, trihydrate alumina bauxite is the easiest to leach, with a typical leaching temperature of 140~145 °C and Na
2O mass concentration of 120~140 g/L. Monohydrate soft alumina bauxite is more difficult to leach, requiring a leaching temperature over 200 °C and a Na
2O mass concentration of 180~240 g/L. Monohydrate hard bauxite is the most difficult to leach, with a leaching temperature of 240~270 °C and a Na
2O mass concentration of 240~300 g/L.
The leaching of alumina from bauxite and the crystallization of aluminum hydroxide are alternatively conducted by controlling the reaction condition. In the case of high temperatures and the concentration of the caustic soda solution, the reaction proceeds in the forward direction, and then the alumina is leached into the solution from the bauxite, and the sodium aluminate solution is produced. In the case of lower temperatures and the concentration of caustic solution, the reaction proceeds in the reverse direction, and then the sodium aluminate in the solution decomposes into aluminum hydroxide and a caustic solution. After filtration, the caustic solution is recovered and recycled, and therefore the liquor in the Bayer process is also called circulating mother liquor.
The main process flow of alumina in the Bayer method is shown in
Figure 4. The bauxite is ground and mixed with caustic soda liquor. Their mixture is called a slurry, which is pumped into the leaching device (a leaching tube is generally used) by a diaphragm pump. The slurry is first heated to a high temperature where the alumina is subsequently leached into the solution. Then, the high temperature and pressure mixture is cooled down and depressurized by a multi-stage flash boiling device. Through filtration, the flash-boiled slurry is separated into the insoluble material (namely red mud) and the liquid (namely aluminate liquor). After cooling down, the aluminate liquor becomes supersaturated and starts decomposing. When some crystalline seeds are added to the aluminate liquor, the crystallization of aluminum hydroxide is converted from primary nucleation to secondary nucleation [
12], which helps raise the crystallizing rate. The anions and shaping additives are often used to regulate the morphology of aluminum hydroxide crystals [
13]. The aluminum hydroxide product and spent liquor are separated by filtering. The spent liquor is evaporated through multi-effect evaporation and flash evaporation, and then causticized to obtain a caustic soda solution, which goes back to the raw material preparation procedure.
3.2. The Flashing of Alumina Leaching Slurry
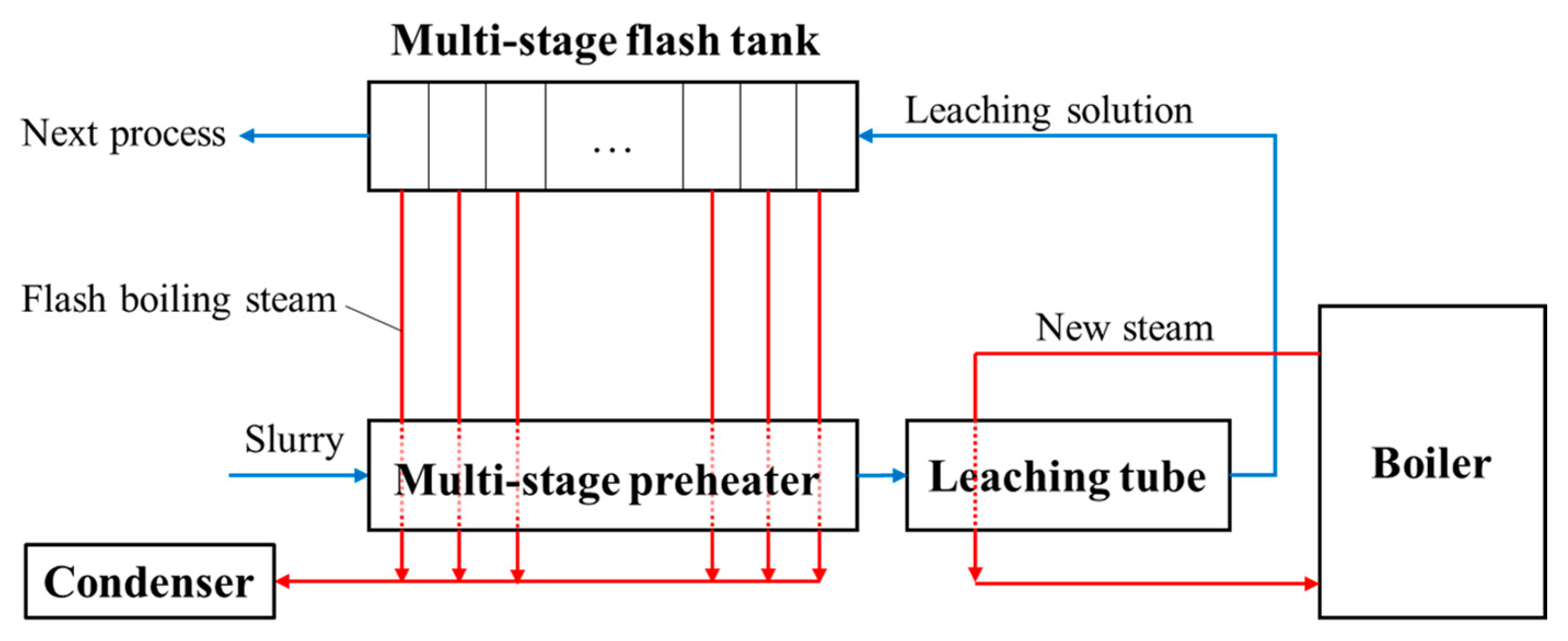
The multi-stage preheating process, leaching process, and multi-stage flashing process are shown in
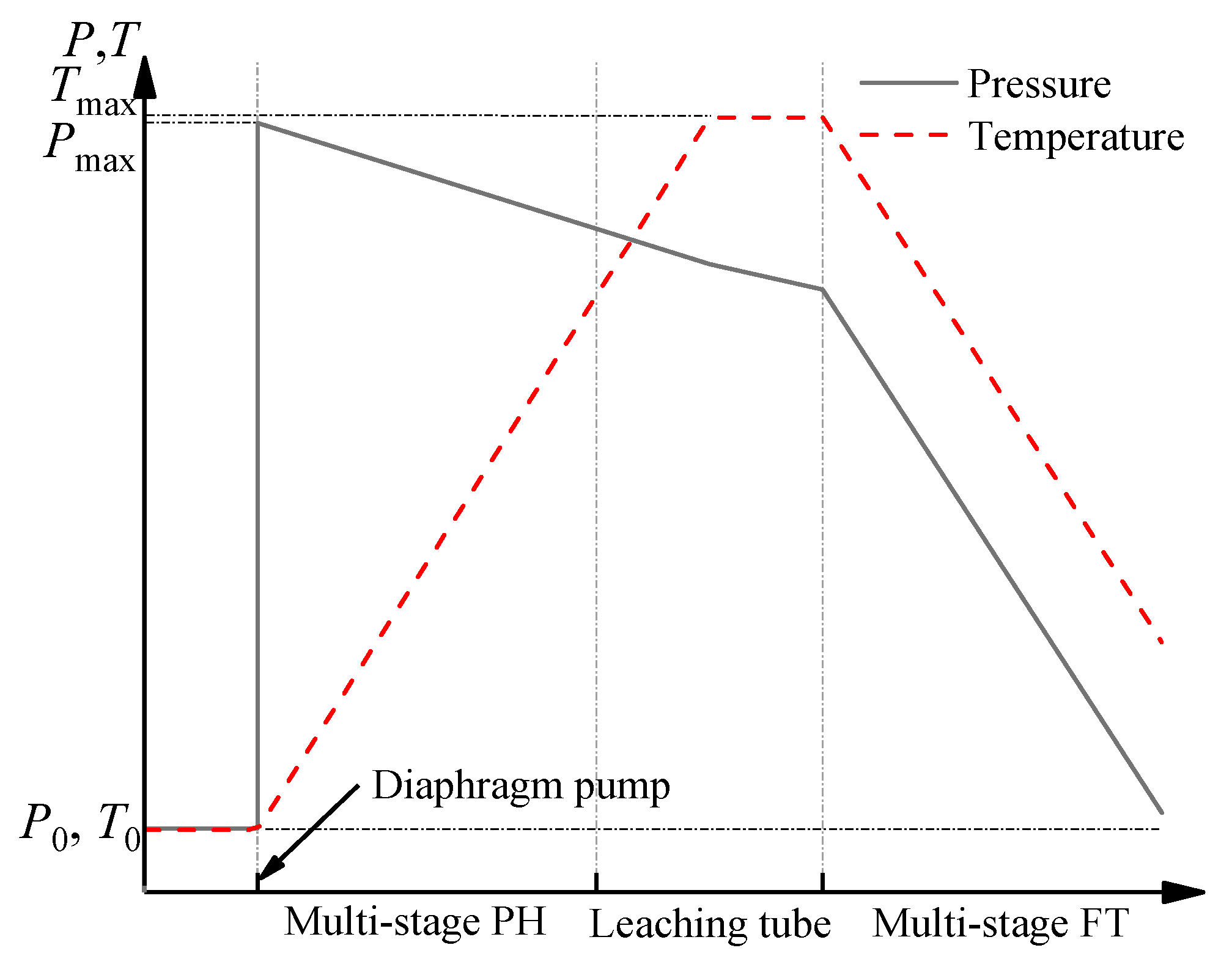
Figure 5. The slurry at atmospheric pressure is boosted to a high-pressure state by a diaphragm pump and pumped into a multi-stage preheater (PH), where it is heated in turn by the flash steam at different temperatures. The slurry flows into the leaching tube and is heated by the new steam at a higher temperature from the boiler. In this way, the temperature of the slurry increases step by step and reaches the required temperature of leaching, and the alumina is leached. The leached slurry enters the multi-stage flash tank, and its temperature and pressure gradually decrease as flash boiling occurs. The flash steam with a different temperature is correspondingly used as the heat sources for the multi-stage preheater. The trend of temperature and pressure of the slurry in the preheating, leaching, and flash boiling process is shown in
Figure 6. The combination of multi-stage flash boiling and multi-stage preheater can realize the graded utilization of thermal energy. A typical case is the combination of a 10-stage preheater and 10-stage flash tank, and the pressure in the 10-stage flash tank decreases from 3.0 MPa to 0.1 MPa (referring to
Table 3).
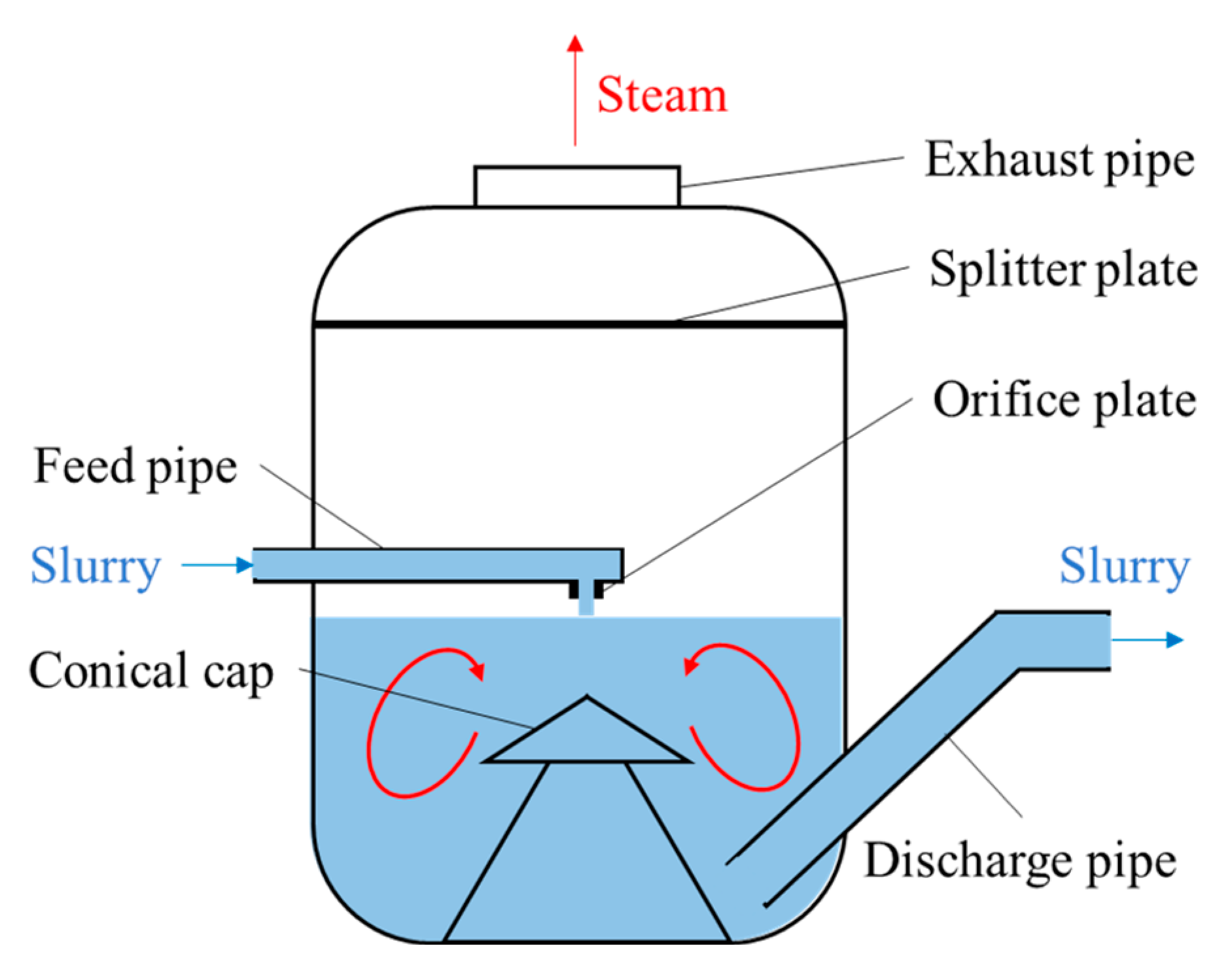
The typical structure of an alumina slurry flash tank is shown in
Figure 7. The slurry is injected downward into a flash tank from the feed pipe exit, which is located at the center of the flash tank and above the liquid level. An orifice plate is installed at the feed pipe exit to reduce the pressure of the outflowing slurry. Otherwise, burst boiling and a shock wave will occur, which will lead to a severe impact on the flash tank. Therefore, the superheat degree of the slurry in the flash tank is controlled by adjusting the pressure in the exhaust pipe. The steam is discharged through the exhaust pipe and entrains some solution droplets, which are blocked by the splitter plate. A conical cap is set under the feed pipe exit to protect the bottom lining of the flash tank from the direct impact of the slurry. The flash tank is one of the key pieces of equipment in alumina production due to its harsh working environment (high temperature, high pressure, high speed, multi-phase, scarring, etc.). Despite the efforts made in this area, failures still occur from time to time, resulting in expensive production downtime and maintenance costs [
14].
In practice, due to the inappropriate operating pressure of the flash tank, part of the slurry is entrained by the flash steam into the piping of the preheater and leaves scars on the wall [
15]. The scarring will damage the heat transfer efficiency of the preheater, which will cause the slurry preheating temperature to decrease sharply. In this case, the energy consumption of the system would increase and the production capacity would decrease. Serious abrasions of the steam pipes and internal components of the preheater would occur. In addition, flash boiling and the turbulence of the fluid in the feed pipe can cause vibrations in the equipment [
16], which will disrupt normal production and reduce equipment life.
3.3. The Flashing of the Spent Liquor
The evaporation process of spent liquor plays the key role of liquid balance in the whole production system and is one of the important parts of Bayer technology. The evaporation methods mainly include multi-effect evaporation, multi-stage flash evaporation, and the combination of the two methods. Compared with the first two, the combination of multi-effect evaporation and multi-stage flash evaporation can provide higher concentration efficiency of the spent liquor [
17], and thus is widely used in Bayer technology.
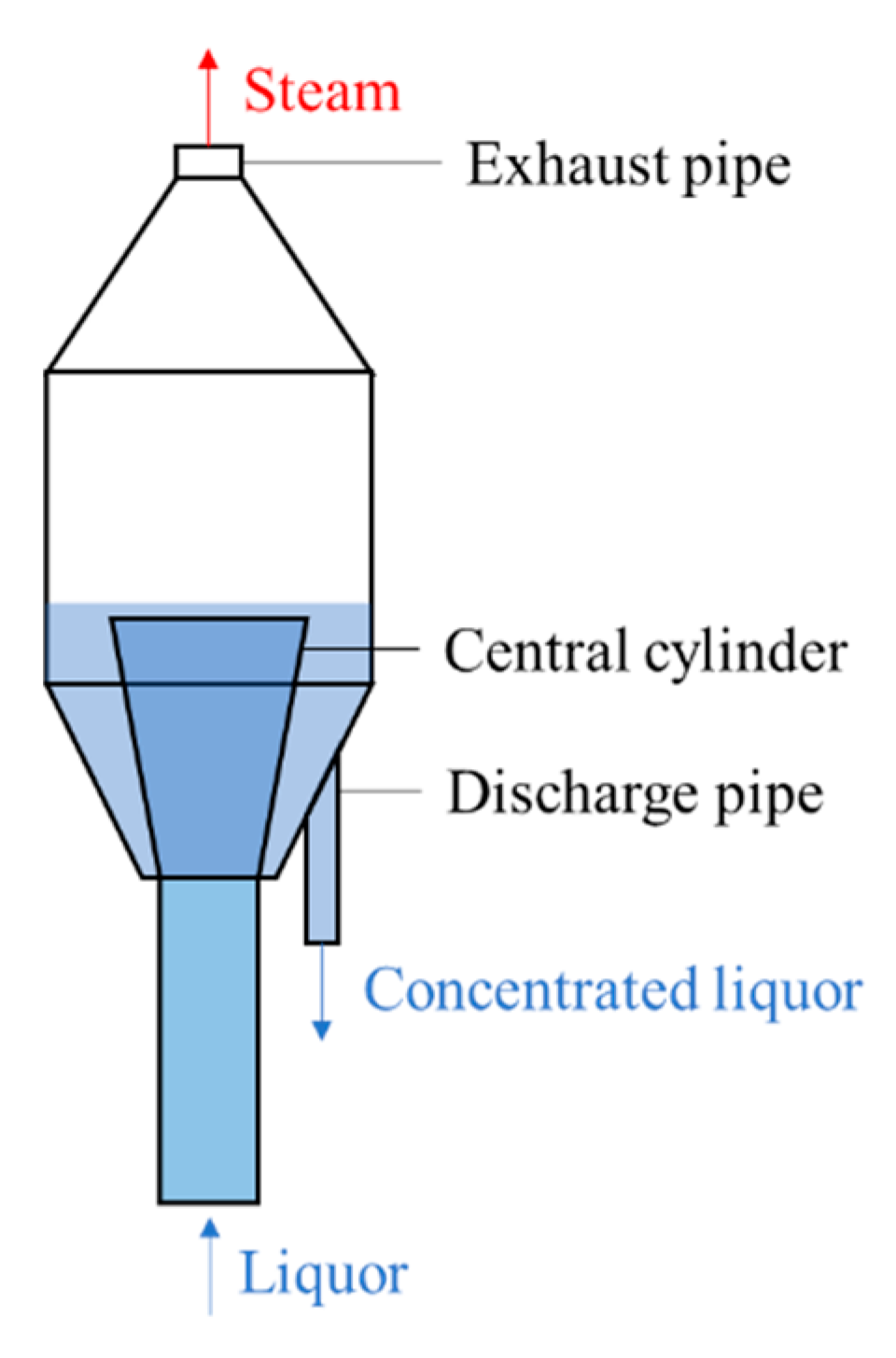
Firstly, multi-effect evaporation is used to quickly concentrate the spent liquor until close to its saturation state. Then, 3~4 stage flashes are applied. The structure diagram of the spent liquor flash tank is shown in
Figure 8. The spent liquor is fed from the bottom of the evaporator and flows upward. As the spent liquor moves upward, the pressure gradually decreases to below the saturation pressure, followed by flash boiling. The flash steam is discharged through the exhaust pipe at the top, and the concentrated liquor flows out from the edge of the cylinder and enters the next stage. To evaporate the water of the spent liquor rapidly, the flash tank in the latter stage operates between slightly positive and slightly negative pressure (compared to atmosphere), so the finial temperature will be lowered below 100 °C (about 80 °C). The design of flash stages and pressure control still relies on experience and lacks theoretical guidance, because the kinetic characteristics of the spent liquor flashing process have not been well understood.
4. Characteristics of Flashing in Pressure Hydrometallurgy
The flashing processes encountered in pressure hydrometallurgy can be seen as a combination of several characteristic processes, which will be analyzed separately in this section to provide a reference for the related research. First, the flashing process in pressure hydrometallurgy has the same basic characteristics as the general one-component flashing process: the liquid is at a superheated state during the depressurization process, and after reaching a certain threshold, phenomena such as nucleation, growth, coalescence, and breakup of vapor bubbles take place.
The flashing process in pressure hydrometallurgy differs from similar processes in other fields such as seawater desalination, nuclear safety analysis, and engine fuel atomization, mainly in the presence of solid. According to the solid content, it can be classified into two categories, namely, slurry flashing and solution flashing. For the former, it is reasonable to speculate that the presence of solids would have an effect on the flashing process. Unfortunately, the particle effect during the evaporation process has not been studied yet to our knowledge. Furthermore, in slurry flashing, active or passive stirring devices are usually installed in the flash tank to prevent solids from settling and accelerating the evaporation of water. In this way, the solid particles are approximately uniformly distributed in the slurry, but the stirring devices make the structure of the flash tank complex and may generate intense turbulence. Nevertheless, in some flashing procedures of hydrometallurgy, the solid content of liquor after multiple filtrations and precipitation is sufficiently low, and the effect of the solid on flow could be ignored. For example, the solid content of spent liquor is lower than 3 g/L in the evaporation procedure of Bayer technology. This flashing process is referred to as solution flashing. Besides the absence of solid effects, solution flashing usually has a simple flash tank structure and a lower solution velocity compared with slurry flashing. Furthermore, in both slurry and solution flashing, the liquid is a multi-component mixture, whose flashing is more complex than that of single-component liquid due to the thermodynamic property differences among the components.
The flashing process in pressure hydrometallurgy can also be classified according to the flashing location. There are types of flashing, i.e., pool flashing, jet flashing, and tube flashing. The evaporation of an alumina slurry in a flash tank, and the evaporation of a zinc slurry in a buffer tank, flash tank, and regulating tank belong to pool flashing, while the flashing of the slurry in a feed pipe and the flashing of spent liquor in a vertical pipe can be classified as tube flashing. As shown in
Figure 3 and
Figure 7, a jet flow is formed at the outlet of the feed pipe and leads to slurry flashing in the upper space of the buffer tank and flash tank. The three types of flashing can also be found in other engineering fields, e.g., pool flashing in the flash chamber of multi-stage flash desalination technology [
18,
19], jet flashing in the fuel atomization of engines [
20,
21,
22], and tube flashing in the cooling devices of nuclear reactor. More research has been reported about the flashing phenomena in these fields than in pressure hydrometallurgy, and this work aims at bridging the related activities and state-of-the-art knowledge from different fields.
5. Research of Flashing in Pressure Hydrometallurgy
The multi-component three-phase flashing process in pressure hydrometallurgy is very complex, and few studies focus directly on it. On the Web of Science, up until 2023, there have been 3745 search records using the keyword of hydrometallurgy, while there have only been 15 search records using the keywords of hydrometallurgy and flashing, in which almost no literature has directly studied the physical phenomena of the flashing process. The relevant studies fall into the following categories:
- (a)
Process simulation: Hydrometallurgical processes have been embedded in many commercial process simulation software, such as ASPEN [
23] and HSC Sim [
24]. The performance of a flashing system and the impact of the process parameters on its production efficiency can be studied and analyzed using the above software. Kirandoudis et al. [
25] built a modular object-oriented steady-state simulation software named PRISMA and used it to perform a simulation of the Bayer process, including leaching, flashing, washing, and settling processes. In general, the applicability of process simulation software is limited due to the simplicity of the mathematical model, the restricted range of physical parameters, and the inappropriate description of local physical phenomena in the process.
- (b)
Thermal analysis and energy conservation of the spent liquor evaporation process: As mentioned above, the evaporation process of spent liquor is an important process in the alumina production process. However, the flashing of spent liquor in an alumina refinery has the disadvantages of high steam consumption per ton, severe heat exchanger tube scarring, and low evaporation capacity [
26]. In order to improve the energy efficiency of this process, Wu et al. [
26] analyzed the thermal and exergy state of a four-effect, three-stage flash evaporation system and found that the first, third, and fourth evaporators had larger heat losses. To recover the waste heat in the condensate process of aluminum production, Rahimi et al. [
17] proposed a re-concentration process driven by a low-grade heat source. The re-concentration process combines two-stage flash evaporation and three-effect falling film evaporation. In this process, the condensate at approximately 85 °C heats the process liquid of the first-effect evaporator and then heats the feed of the flash tank. A mass, energy, and component balance analysis of the per effect evaporator of each stage showed that the liquid yield of its optimized process was about 36% higher than that of the conventional multi-effect evaporation process in the case of a given inlet heat source temperature of 75 °C and 85 °C. So far, energy conservation measures have mainly relied on the operational experience, whether the evaporation equipment was improved and updated or the operating parameters were adjusted, while there has been less analytical research on the evaporation process itself.
- (c)
The optimized design for the feed orifice plate of an alumina slurry flash tank: As shown in
Figure 7, the alumina slurry flash tank is generally a large cylinder. Its bottom half contains the slurry, while its top half is used for separating the flash steam from the slurry and discharging the steam from the top of the tank. The slurry with a high temperature and high pressure is sprayed into the flash tank through a vertical downward orifice plate located at the end of the feed pipe. As mentioned earlier, many efforts in optimizing the flash tank have been made to prolong its life and reduce production costs. Most of them focused on the design of the feed orifice plate. A conventional feed orifice plate consists of a plug valve and a section of equal cross-section plunger [
27,
28] where most of the pressure drop occurs. This feed orifice plate is prone to generating explosive flashing when the volume expansion rate of the slurry is high (e.g., at low pressure), which will cause severe wear on the flash tank wall and on the components near the orifice plate. Williams [
29] suggested that an expansion cone be added below the plunger, and adjusting the diameter of the plunger and the expansion cone exit can decrease the pressure drop between the upstream of orifice plate and inside of flash tank, which hence weakens the shock wave intensity of the slurry. Smith et al. [
27,
28] called Williams’ design a “rocket nozzle” and pointed out that it does prevent explosive flashing and minimize the wear and tear of the pipe from explosive flashing, but the jetting flow can cause severe wear on the bottom of the groove or on the conical cap (see
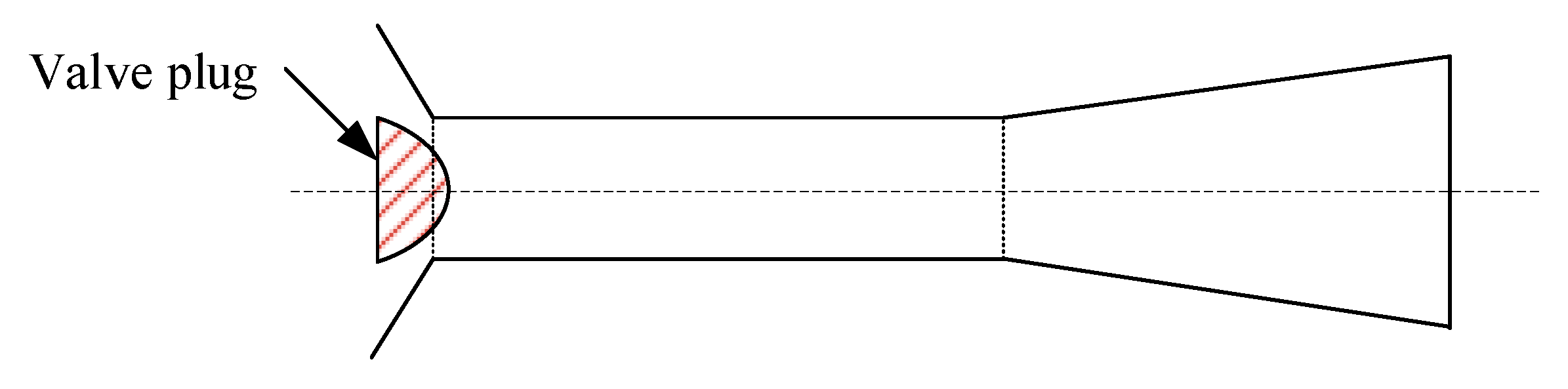
Figure 7). They designed a “flashtube” (
Figure 9), whose opening cross-sectional area is proportional to the linear position of the plug with a parabolic profile. By adjusting the plug position to expand the slurry to a low enough pressure in the flashtube, the shock wave will be formed inside the flashtube rather than in the receiving tank. When shock is formed and the resistance loss increases, the velocity and kinetic energy of the slurry at the outlet of the flashtube drop, thus reducing the impact wear on the bottom and the conical cap of the flash tank.
- (d)
Numerical simulation of multi-phase flow and heat transfer process: Numerical simulations of the flashing process in pressure hydrometallurgy are scarce and still in the early stages of development. Smith et al. [
27] performed a simple one-dimensional numerical analysis for their design of a flashtube feed nozzle. The slurry flow in the flashtube was assumed to be in adiabatic and interphase thermal equilibrium, i.e., no heat exchange between the liquid, vapor, and solid particles. These assumptions are acceptable in the case of a large number of solid particles uniformly dispersed within the slurry. Since these particles could provide enough bubble nucleation sites to allow the liquid to vaporize rapidly after the saturation temperature is exceeded, which shortens the metastable state time of the superheated liquid. In addition, they assumed that there is no relative slip between the vapor, liquid, and solid phases, and that the three phases flow at the same velocity throughout the system, which is difficult to satisfy when there are large solid particles or bubbles in the system. Also, the authors pointed out that it is difficult to obtain reliable data to verify the model due to the harsh environment in which flashtubes actually operate, but such simplified models are generally recognized as useful basic design tools in engineering fields.
Blackmore et al. [
30] reviewed the current numerical methods and their challenges based on the flowing physical processes in the feed nozzle and its outlet jetting in the flash tank. They concluded that the flow characteristics in the flash tank are largely impacted by the following three processes: (1) the interaction of the slurry jet with the liquid surface in the flash tank; (2) the diffusion of the jet flow in the upper space above the liquid surface as well as the trajectory of the solid particles; and (3) the separation of the vapor from the slurry. The first two processes are related to the nozzle exit conditions, which proves that the feed nozzle or orifice plate are key components of the flash tank. The authors simulated a 2D flow in the feed nozzle using the commercial CFD software ANSYS FLUENT v.12.0, and the nozzle is of the geometry of the flashtube designed by Smith et al. [
27]. The results showed that the back pressure in the flash tank determines the location of the shock wave front and the expansion rate of the jet. At a suitable back pressure, the shock wave front is located above the liquid level, and the velocity of the slurry is low when entering the liquid region. However, a too low back pressure makes the jet over-expanded, and serious equipment failure will be caused if the jet reaches the bottom of the flash tank. If the back pressure further decreases, the over-expanded jet with a large radius enters the slurry pool. In this case, the refractory lining of the tank will be severely worn due to the impact of the high-speed particle flow. It should be noted that, in their numerical simulation, Blackmore et al. [
30] did not consider particles and relative slip between phases but used a homogeneous mixture model. In addition, they used the Volume of Fluid (VOF) method to analyze the interaction of the slurry jet with the slurry pool in the flash tank and the penetration depth of the jet. The slurry flow field was simulated in isothermal conditions, i.e., the phase change was not considered, and the mixture of particles and liquid was approximately treated as a single-phase fluid with a mass-weighted density and an apparent viscosity as a function of the local strain rate. The simulation results show that, due to the impact of the high-velocity jet, the upper vapor will be sucked into the slurry pool, and a large number of slurry droplets will splash onto the flash tank walls. Knowledge is still insufficient for a precise description of these complex phenomena.
Lv et al. [
16] found that the last stage flash tank in a 10-stage slurry flash system of an alumina refinery exhibits periodic vibrations with an amplitude of 7–8 mm. To find the cause of the vibration, they simulated the flash tank (
Figure 7) using a mixture multiphase model in ANSYS FLUENT software, and the slurry was treated as a pseudo-single-phase liquid, as in the above study. The results show that, under industrial operating conditions, flashing occurs in the feed pipe, and then turbulent gas–liquid two-phase flow is formed, triggering the pipe vibration. Meanwhile, the pressure fluctuations in the flash tank generate slight vibrations, which in turn induce resonant vibrations in the pipe.
The summary of the main published paper related to flashing phenomena in pressure hydrometallurgy is shown in
Table 4. For the flashing process in pressure hydrometallurgy, flashing of the slurry is the primary one, and the large solid content is the most prominent feature that distinguishes it from other flashing processes, but the related works have not been published yet. Blackmore et al. [
30] pointed out that the design of a slurry flash tank is closely related to feed pipe exit conditions, and future research should focus on how to consider the effect of the solid particles on the vapor nucleation, the interphase thermodynamic non-equilibrium and velocity slip, as well as the acoustic velocity of multiphase flows.
6. Status of Relevant Research from Other Fields
As shown in
Section 5, the direct research on the flashing process in pressure hydrometallurgy is very limited. Therefore, we extend the review on the relevant work in other fields, including thermal analysis, the numerical simulation of gas–liquid two-phase flashing as well as the solid effects in three-phase flows. This can be considered a reference for the future study of the flashing process in pressure hydrometallurgy.
6.1. Thermal Analysis of Pool Flash Evaporation
Pool flash evaporation has been extensively paid attention to because of its application in multi-stage flash desalination technology. The existing experimental and theoretical studies have mainly focused on thermodynamic performance analysis. Besides the evaporation rate, the boiling point elevation (BPE) and non-equilibrium allowance (NEA) are important design parameters.
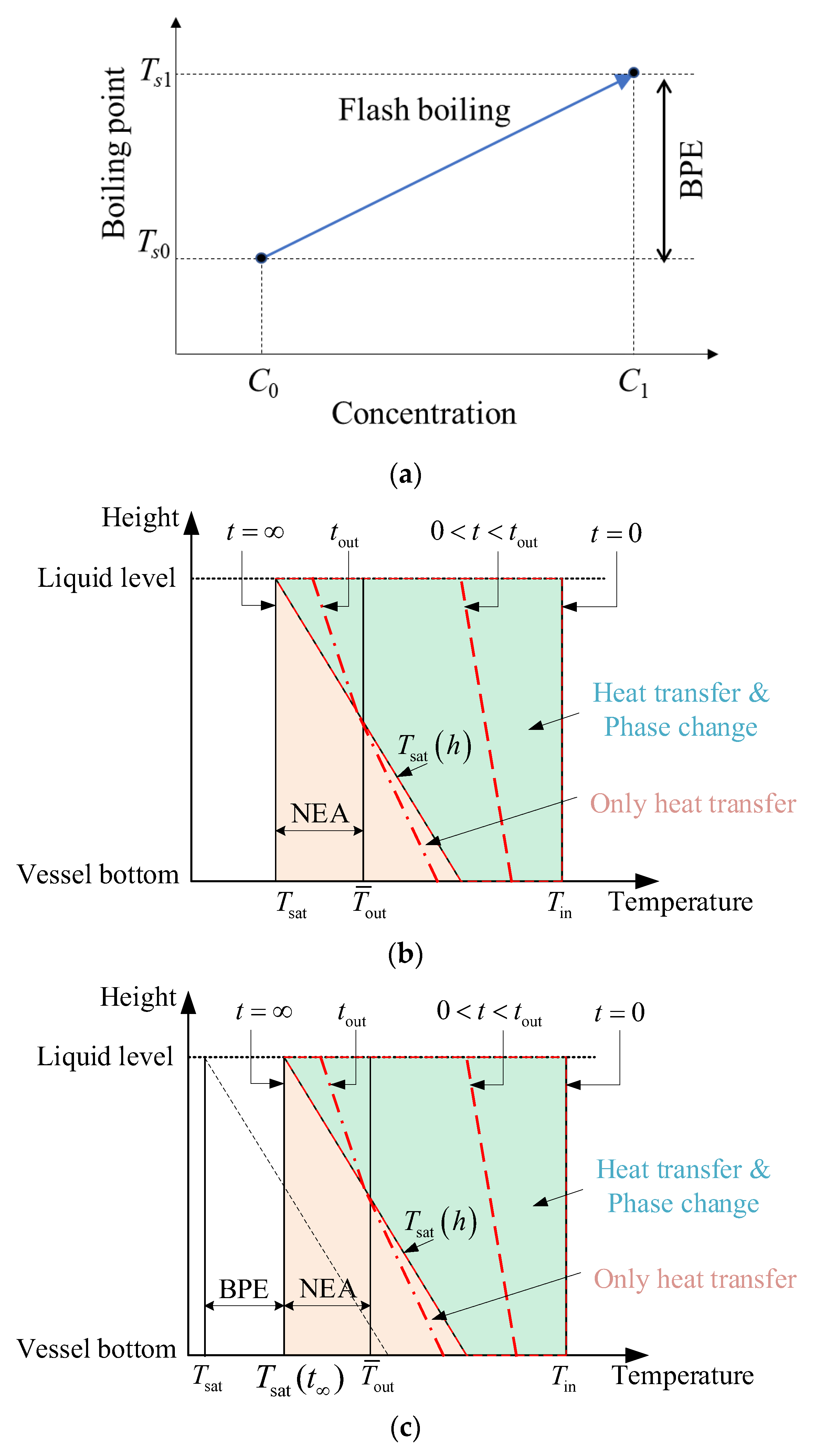
Figure 10a shows that in flash evaporation, the boiling point of the liquid rises as the solute concentration increases [
31], and the value of boiling point elevation is defined as boiling point elevation. The BPE is related to the solute concentration before and after flash evaporation. At present, BPE is mainly determined on practical experiences, and the BPE of the NaOH solution can be found in the literature [
32]. In addition, further studies [
33] found that the BPE slows down the growth of vapor bubbles by increasing the bubble delay time or reducing the liquid superheat.
Figure 10b shows that at the beginning of flashing, the liquid at different depths is of the same initial temperature
Tin, but the saturation temperature increases with the depth because of the increase in static pressure, so the liquid superheat decreases with depth. With the flashing of the liquid, its temperature gradually drops and is close to the corresponding saturation temperature because of phase change and heat transfer. In addition, the natural convection driven by the temperature difference between the top and bottom liquid can homogenize the temperature. The average temperature
Tout of the final exit liquid is higher than its saturation temperature corresponding to its steam pressure in the flash tank, and the difference between the average exit temperature and the saturation temperature of the liquid is called as non-equilibrium allowance (NEA). Obviously, the non-equilibrium allowance is related to the flash rate and heat transfer process at different depths. In fact, the saturation temperature of the solution or slurry will also increase due to BPE; the simultaneous effect of BPE and NEA is plotted in
Figure 10c.
Through the experiments, Saury et al. [
34] studied the effect of the initial water depth and depressurization rate on the flash rate of pool liquid and proposed the corresponding experimental correlation equation. Wang et al. [
35] derived the relationship between the superheat degree and depressurization rate based on the ideal gas assumption and predicted the flash evaporation temperature and depressurization rate close to the experimental results. Wu et al. [
36] found that the propagation depth changed with the initial temperature and the superheat by the flashing experiment of water and fitted the relationship of propagation depth with superheat at the initial temperatures of 50 °C and 90 °C, respectively. Zhang et al. [
37] proposed the non-equilibrium fraction (NEF) considering the saturation temperature corresponding to the BPE of the solution:
The NEF equal to 1 represents the initial of the flashing process, while NEF close to 0 means a sufficient flashing process. It was found in the experiment that the NEF in cyclic flash evaporation was smaller than that in static flash evaporation.
All BPE, NEA, and NEF depend on the distribution of the state parameters of the solution, such as concentration, pressure, temperature, etc. Their distribution further depends on the flow, heat transfer, and mass transfer of the solution during the flashing process.
6.2. Numerical Simulation of Gas–Liquid Two-Phase Flow in Flashing Process
With the development of high-performance computing, more and more scholars researched the two-phase flow and thermodynamic features in the flashing process through the CFD method. But so far, they have mainly focused on single-component flow systems and less on multi-component pool flash evaporation. Liao and Lucas [
38] provided a detailed review of various typical models, including the homogeneous equilibrium model, the homogeneous relaxation model, and the two-fluid model.
The key to numerical simulation lies in a correct understanding of the sub-physical phenomena governing the flashing process and an accurate description of these phenomena with suitable mathematical models. Nucleation is the initiation or inception of a flashing process and in practice it is usually shown as a heterogeneous nucleation on the vessel wall and particle surface. The mechanism of heterogeneous nucleation on the wall has been reviewed in detail by Liao and Lucas [
38] and Yin et al. [
39], and various types of nucleation models have been summarized in the literature [
40].
Bubble growth is the most important heat and mass transfer in the flashing process, and the growth rate depends on the temperature and pressure gradient of liquid around the bubbles. There are two main approaches to simulate the growth process of a vapor bubble in a superheated liquid:
- (a)
Front-tracking methods, such as the VOF method [
41] or the level set method. In the VOF method, the gas–liquid interface is captured by solving the volume fraction equation and sharpened by considering the Continuum Surface Force (CSF) in the momentum equation. In the level set method, a signed distance function is used to track the interface. With a sufficiently fine grid, these methods can directly resolve the heat and mass transfer processes at the vapor–liquid interface [
42] or solve the vapor bubble growth process [
43] by combining the direct identification of the interface position with the heat/mass transfer models of the interface. Front-tracking methods are suitable for mechanistic studies or small-scale process simulations, but it is difficult to balance accuracy and computational cost at industrial scales.
- (b)
The two-fluid model uses two sets of governing equations for the gas phase and liquid phase, respectively, considers their interaction through source terms, and distinguishes them by solving volume fraction equations. The two-fluid method is available for numerical studies of industrial processes, but the accuracy largely depends on the closure models, e.g., a suitable heat and mass transfer model [
44]. In the flashing process with high temperature and high pressure, the bubble growth mainly depends on the heat transfer process and is dominated by the heat transfer between the superheated liquid and the interface. The rapid bubble growth, high flow velocity, and strong turbulence in the flashing flow bring a great challenge to the determination of the interfacial heat transfer coefficient. So far, two methods have been commonly used. One is to use the experimental correlation equation obtained from the flash flow experiment [
45]. The other is to independently analyze heat transfer mechanisms, such as conduction and convection, to derive theoretical analytical solutions or semi-empirical correlations, and then to linearly accumulate the Nusselt (
Nu) numbers for different heat transfer mechanisms [
40]:
where the subscripts
cond,
conv, and
turb mean the heat transfer mechanism of conduction, convection, and turbulence, respectively.
In the two-fluid model, an additional model is needed to describe the variation of the bubble diameter. The monodisperse approach [
46,
47] (i.e., prescribing bubble size) is simple but not accurate enough when applied to the simulation of industrial flashing flows [
48,
49]. A large number of experiments have shown that after nucleation, bubbles rapidly grow during flash evaporation and are accompanied by intense breakup and coalescence behavior, resulting in a wide distribution of bubble size.
Liao and Lucas extended the coupled computational fluid dynamics with population balance model (CFD-PBM) method [
50]. This method divides the size of bubbles into several groups, solves the volume fraction equation for each group, tracks the growth, breakup, and coalescence of the bubbles with source terms, and then redistributes the bubbles among different groups. They applied this method to simulate the flashing flow in a vertical pipe and compared the numerical results with the experimental data. The results verified that the method could effectively capture the processes of bubble nucleation and growth. The above works provide a reliable numerical method for exploring the solution flashing process in pressure hydrometallurgy. However, the various closure models, such as nucleation, heat transfer, breakup, and coalescence, need to be further developed and improved. For example, the effects of solute concentration on boiling point and of mass transfer on breakup and coalescence have not been considered yet.
6.3. The Effect of Solid Particles on Gas–Liquid Flow and Heat Transfer
In bubbling fluidized beds, which are broadly used in engineering, the influence of particles on gas–liquid flow and heat transfer has attracted scholar’s attentions, although solid–liquid mixtures are often treated as a pseudo-single-phase fluid. Ahmadi et al. [
51] found in their experiments that the presence of particles can affect the turbulence intensity of the liquid phase. Zhou et al. [
52] found through numerical simulations that the presence of particles increases the viscosity and density of the slurry and makes its flow regime change.
The solid particles in the slurry also affect the nucleation of vapor bubbles. A large number of solid particles can trigger the heterogeneous nucleation whose rate depends on the number density, shape, and contact parameters of the particles. The nucleation process on the particle surface is more complex compared to the wall nucleation. By molecular dynamics simulations, Chen et al. [
53] studied the effect of different foreign atoms on the nucleation process of liquid argon and found that it is related to the magnitude of its energy parameter. Wu et al. [
54] put a carbon particle in the sulfuric acid vapor system and analyzed the effect of the interaction between vapor and particles as well as the shape of the particle on the nucleation behavior. It was found that heterogeneous nucleation on the particle surface became gradually dominant as the interaction between the particle and vapor increased. Moreover, it was pointed out that the larger the specific surface area of particles, the faster the growth of bubble nucleus embryos, while heterogeneous nucleation tended to occur on flat surfaces for non-spherical particles.
The high concentration of solid particles will inevitably affect the motion of the vapor bubble. Xia et al. [
55] established a particle–bubble–liquid multiphase flow model including the interaction between the rising bubble and the particles and found that hydrophobic particles would hinder the bubble rise, but hydrophilic particles would promote the bubble rise. Recently, Liao et al. [
56] combined the front-tracking method with the Lagrangian particle tracking method and deeply studied the effect of particles on the bubble coalescence process. Mokhtari et al. [
57] took into account the effect of a solid on bubble breakup and coalescence, revised the bubble coalescence probability, and improved the CFD-PBM model for simulating slurry bubble flow.
In general, the simulation of the slurry flashing process is more complicated than that of the solution flashing process because of the solid particles. The numerical simulation methods are able to be referred to for the solid–liquid–vapor three-phase flow process, and some qualitative conclusions were reported concerning the effect of solid particle properties on the vapor bubble nucleation process. However, the quantitative models that can calculate the flash nucleation rate at different solid holdups are still not available. Moreover, the effects of particles on liquid phase turbulence, interphase heat transfer, bubble breakup, and coalescence still need further studies.
7. Conclusions and Outlook
7.1. Conclusions
In pressure hydrometallurgy, the flashing process is mainly applied to connect the high-pressure leaching equipment with atmospheric pressure equipment, as well as for evaporation and concentration of the solution. It plays an important role in the smooth running of the metallurgical process, lowering the steam consumption and balancing the liquid of the metallurgical system. A better understanding of the flashing process may contribute to optimizing the design, enhancing the device’s lifetime, and reducing operational costs. This review takes the flashing process in the alumina industry and zinc metallurgy as an example and presents the flashing process in pressure hydrometallurgy. A special focus was put on reviewing the direct research on the flashing process in pressure hydrometallurgy and related research and progress made in other fields.
The flashing process in pressure hydrometallurgy can be divided into slurry and solution flashing according to the solid holdup in the fluid. After the leaching process of concentrate at high pressure and high temperatures, slurry flashing is used to reduce its pressure and recover its waste heat, while solution flashing is used for the evaporation and concentration of the solution. The combination of multi-effect evaporation and multi-stage flashing can enhance the concentration efficiency. BPE and NEA exist in the flashing process of both slurry and solution, and accurate BPE and NEA are helpful for guiding reasonable multi-stage flashing design to achieve energy conservation and emission reduction. There are hardly any studies on flash evaporation in pressure hydrometallurgy with a few exceptions based on extremely simplified models, such as the one-dimensional homogeneous phase equilibrium model. CFD is a promising tool for the analysis of complex multiphase problems, and considerable progress has been made in other fields.
7.2. Outlook
Future research areas and problems that need to be solved in the study of the flashing process in pressure hydrometallurgy are summarized as follows:
- (1)
The flashing of solutions takes place in vapor–liquid two-phase systems. In this case, the two-fluid model with the CFD-PBM coupled method is experimentally validated and suitable for a wide range of bubble size distributions caused by nucleation, growth, breakup, and coalescence processes. However, the complex morphological changes in the gas–liquid interface and interphase heat transfer mechanism in the flashing process pose great challenges to the reliability of the existing closure models. Furthermore, phenomenological models are needed to describe the effects of solute concentration on the boiling point of the solution, of interphase mass transfer and phase change on bubble breakup and coalescence, and of turbulence on interphase heat transfer.
- (2)
Slurry flashing is one of the major procedures in pressure hydrometallurgy, and the high solid holdup is the most prominent feature that distinguishes it from other flashing processes. Slurry flashing is also more complicated because solid particles affect the liquid flow, two-phase transfer, vapor bubble dynamic behavior, and even the flow regime. So far, in three-phase flow, the slurry has often been treated as a pseudo-single-phase fluid, and the influence of solids has been taken into account by modifying the physical property parameters, whereas studies have shown that solid particles have an effect on liquid flow, vapor bubble rise, coalescence, and breakup behavior. Some mathematical models are available as a reference for the slurry flashing simulation, but further improvement and development are still needed. For example, the effect of solid particles on vapor bubble nucleation has also been studied qualitatively, but quantitative analyses for modifying the nucleation rate are not yet available. In addition, experimental data such as bubble size and volume fraction distribution required for model development and validation are still lacking.
Author Contributions
Conceptualization, P.Z. and B.L.; investigation, P.Z. and J.L.; resources, C.W.; data curation, D.W.; writing—original draft preparation, P.Z. and J.L.; writing—review and editing, P.Z. and J.L.; visualization, D.W. and B.L.; supervision, P.Z.; project administration, P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 52206128).
Data Availability Statement
In this study, the technical data of flash evaporation processes in pressure hydrometallurgy were provided by Chalco Guangxi Branch and Dan Xia smelter, and are available with the permission of Chalco Guangxi Branch and Dan Xia smelter. Other data are available in open literature.
Acknowledgments
The authors acknowledge Liao Yixiang, research fellow of Helmholtz-Zentrum Dresden, Rossendorf, for her help with the writing. The authors acknowledge Zou Qiang (Engineer of Dan Xia smelter) and Liao Mingchao (Engineer of Dan Xia smelter) for the data support. The authors acknowledge Chalco Guangxi Branch for the data support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, K. Pressure Hydrometallurgy; Metallurgical Industry Press: Beijing, China, 2016; ISBN 978-7-5024-7098-2. [Google Scholar]
- Karimov, K.A.; Rogozhnikov, D.A.; Naboichenko, S.S.; Karimova, L.M.; Zakhar’yan, S.V. Autoclave Ammonia Leaching of Silver from Low-Grade Copper Concentrates. Metallurgist 2018, 62, 783–789. [Google Scholar] [CrossRef]
- Neustroev, V.I.; Karimov, K.A.; Naboichenko, S.S.; Kovyazin, A.A. Autoclave Leaching of Arsenic from Copper Concentrate and Matte. Metallurgist 2015, 59, 177–179. [Google Scholar] [CrossRef]
- Nesterov, K.N.; Smirnov, K.M. Autoclave Leaching of Rare-Earth Metals from Hydroxide Precipitate. Metallurgist 2018, 62, 163–168. [Google Scholar] [CrossRef]
- Liang, D.; Wang, J.; Wang, Y.; Zhang, H. Catalytic Role of Manganese in Autoclave Oxidation of Germanium-Rich Sphalerite Concentrates. Can. J. Chem. Eng. 2009, 87, 106–109. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Rosales, G.D.; Pinna, E.G.; Suarez, D.S. Effect of Na + Ion on the Dissolution of Ferrocolumbite in Autoclave. Hydrometallurgy 2016, 159, 60–64. [Google Scholar] [CrossRef]
- Khazieva, E.B.; Naboichenko, S.S.; Bolatbaev, K.N. Influence of Lignosulfonates on the Cementation Velocity of Copper with Zinc. Russ. J. Non-Ferr. Met. 2015, 56, 142–145. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, T.; Liu, Y.; Mu, W.; Zhang, W.; Dou, Z.; Jiang, X. Pressure Acid Leaching of Zinc Sulfide Concentrate. Trans. Nonferrous Met. Soc. China 2010, 20, s136–s140. [Google Scholar] [CrossRef]
- Yang, Z. Effect of the Amount of Dispersant Addition on Zinc Concentrate Oxygen Pressure Leaching Process. China Nonferrous Metall. 2019, 48, 5–7+39. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, X.; Zhou, K.; Wu, C.; Zhang, D.; Chen, L.; Deng, M. Production Practice of Zinc Powder Replacement Ga and Ge Slag by Pressure Oxidation Leaching. Nonferrous Met. Sci. Eng. 2020, 11, 142–147. [Google Scholar] [CrossRef]
- Chen, L. Innovative Research and Industrial Application of Oxygen Pressure Leaching Technology for Zinc Sulfide Concentrate in High Altitude Area. World Nonferrous Met. 2021, 15, 1–2. [Google Scholar]
- Sonthalia, R.; Behara, P.; Kumaresan, T.; Thakre, S. Review on Alumina Trihydrate Precipitation Mechanisms and Effect of Bayer Impurities on Hydrate Particle Growth Rate. Int. J. Miner. Process. 2013, 125, 137–148. [Google Scholar] [CrossRef]
- Fan, W.; Li, W. Morphology-Control Techniques for Preparing Aluminum Hydroxide via Wet Chemical Synthesis. Hydrometallurgy 2020, 192, 105256. [Google Scholar] [CrossRef]
- Li, Y.-D.; Xu, N.; Wu, X.-F.; Guo, W.-M.; Zang, Q.-S.; Shi, J.-B. Failure Analysis of the Flash Evaporator in an Alumina Production Plant. Case Stud. Eng. Fail. Anal. 2013, 1, 95–102. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Zhang, W. Analysis of Causes and Hazards of Secondary Steam Strip in Alumina Flash Evaporation System Produced by Bayer Process. World Nonferrous Met. 2018, 10, 25+28. [Google Scholar]
- Lv, C.; Zhang, Z.M.; Zhao, Q.Y.; Zhang, T.A.; Guo, X.H.; Wang, J. Numerical Simulation of Flash Vaporisation in Alumina Production. Can. Metall. Q. 2016, 55, 463–469. [Google Scholar] [CrossRef]
- Rahimi, B.; Regenauer-Lieb, K.; Chua, H.T.; Boom, E.; Nicoli, S.; Rosenberg, S. A Novel Low Grade Heat Driven Process to Re-Concentrate Process Liquor in Alumina Refineries. Hydrometallurgy 2017, 170, 34–42. [Google Scholar] [CrossRef]
- Fiorini, P.; Sciubba, E.; Sommariva, C. A New Formulation for the Non-Equilibrium Allowance in MSF Processes. Desalination 2001, 136, 177–188. [Google Scholar] [CrossRef]
- Mussati, S.; Marcovecchio, M.; Aguirre, P.; Scenna, N. A New Global Optimization Algorithm for Process Design: Its Application to Thermal Desalination Processes. Desalination 2004, 166, 129–140. [Google Scholar] [CrossRef]
- Ju, D.; Huang, Z.; Jia, X.; Qiao, X.; Xiao, J.; Huang, Z. Macroscopic Characteristics and Internal Flow Pattern of Dimethyl Ether Flash-Boiling Spray Discharged through a Vertical Twin-Orifice Injector. Energy 2016, 114, 1240–1250. [Google Scholar] [CrossRef]
- Khan, M.M.; Sheikh, N.A.; Khalid, A.; Lughmani, W.A. Experimental Characterization of Gasoline Sprays under Highly Evaporating Conditions. Heat Mass Transf. 2018, 54, 1531–1543. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Qiu, S.; Li, X.; Hung, D.L.S.; Xu, M. Mechanism of Flash Boiling Bubble Breakup Based on Rim-like Structure. Fuel 2022, 329, 125345. [Google Scholar] [CrossRef]
- Langa, J.M.; Russell, T.G.; O’Neill, G.A.; Gacka, P.; Shah, V.B.; Stephenson, J.L.; Snyder, J.G. Aspen Modeling of the Bayer Process. In Essential Readings in Light Metals: Volume 1 Alumina and Bauxite; Donaldson, D., Raahauge, B.E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 860–869. ISBN 978-3-319-48176-0. [Google Scholar]
- Elomaa, H.; Sinisalo, P.; Rintala, L.; Aromaa, J.; Lundström, M. Process Simulation and Gate-to-Gate Life Cycle Assessment of Hydrometallurgical Refractory Gold Concentrate Processing. Int. J. Life Cycle Assess. 2020, 25, 456–477. [Google Scholar] [CrossRef]
- Kiranoudis, C.T.; Voros, N.G.; Kritikos, T.; Maroulis, Z.B.; Marinoskouris, D.; Papassiopi, N.; Dimitropoulou, O.; Paspaliaris, I.; Kontopoulos, A. Object-Oriented Simulation of Hydrometallurgical Processes: Part II. Application to the Bayer Process. Met. Mater. Trans. B 1997, 28, 785–793. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, X.; Song, Y.; Li, S. Thermal Analysis and Exergy Analysis of Evaporation Process in Alumina Refinery. In Proceedings of the 2011 International Conference on Computer Distributed Control and Intelligent Environmental Monitoring, Changsha, China, 19–20 February 2011; IEEE: New York, NY, USA, 2011; pp. 1002–1006. [Google Scholar]
- Smith, C.C.; Dixon, D.G.; Luque, M.R.; Robison, J.C.; Chipman, S.R. Analysis and Design of Flashtubes for Pressure Letdown in Autoclave Leaching Operations. Hydrometallurgy 2006, 81, 86–99. [Google Scholar] [CrossRef]
- Robison, J.C.; Chipman, S.R.; Smith, C.C.; Luque, M.R. Flash Tube Device. U.S. Patent 6,523,573, 25 February 2002. [Google Scholar]
- Williams, R.; Dixon, D.; Enloe, L.; Wilmot, P. Nozzle for Low Pressure Flash Tanks for Ore Slurry. U.S. Patent 6,482,250, 19 November 2002. [Google Scholar]
- Blackmore, A.; Shah, U.; Pearson, M.; Plikas, A.; Sim, B. Flashing of Pressurized Slurry in Metallurgical Processing: A Review of the State of the Art, Operational Experiences, and Recommendations for Future Research. In Fluids Engineering Division Summer Meeting; American Society of Mechanical Engineers: Houston, TX, USA, 2014. [Google Scholar]
- Sharqawy, M.H.; Lienhard, J.H.; Zubair, S.M. Thermophysical Properties of Seawater: A Review of Existing Correlations and Data. Desalin. Water Treat. 2010, 16, 354–380. [Google Scholar] [CrossRef]
- Rahimi, B.; Chua, H.T. (Eds.) Appendix B—Boiling Point Elevation and Nonequilibrium Allowance. In Low Grade Heat Driven Multi-Effect Distillation and Desalination; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–167. ISBN 978-0-12-805124-5. [Google Scholar]
- Hao, L. A Mathematical Study of Bubble Dynamics in Superheated Sodium Chloride Solution. Int. J. Heat Mass Transf. 2019, 145, 118728. [Google Scholar] [CrossRef]
- Saury, D.; Harmand, S.; Siroux, M. Flash Evaporation from a Water Pool: Influence of the Liquid Height and of the Depressurization Rate. Int. J. Therm. Sci. 2005, 44, 953–965. [Google Scholar] [CrossRef]
- Wang, C.; Xu, R.; Chen, X.; Jiang, P.; Liu, B. Study on Water Flash Evaporation under Reduced Pressure. Int. J. Heat Mass Transf. 2019, 131, 31–40. [Google Scholar] [CrossRef]
- Wu, H.; Liu, W.; Li, X.; Chen, F.; Yang, L. Experimental Study on Flash Evaporation under Low-Pressure Conditions. J. Appl. Sci. Eng. 2019, 22, 213–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Yan, J.; Chong, D.; Liu, J.; Zhang, W.; Wang, C. Experimental Study on Non-Equilibrium Fraction of NaCl Solution Circulatory Flash Evaporation. Desalination 2014, 335, 9–16. [Google Scholar] [CrossRef]
- Liao, Y.; Lucas, D. Computational Modelling of Flash Boiling Flows: A Literature Survey. Int. J. Heat Mass Transf. 2017, 111, 246–265. [Google Scholar] [CrossRef]
- Yin, S.; Wang, N.; Wang, H. Nucleation and Flashing Inception in Flashing Flows: A Review and Model Comparison. Int. J. Heat Mass Transf. 2020, 146, 118898. [Google Scholar] [CrossRef]
- Liao, Y.; Lucas, D. A Review on Numerical Modelling of Flashing Flow with Application to Nuclear Safety Analysis. Appl. Therm. Eng. 2021, 182, 116002. [Google Scholar] [CrossRef]
- Liao, Y.; Li, J.; Lucas, D. Investigation on Pool-Scrubbing Hydrodynamics with VOF Interface-Capturing Method. Nucl. Eng. Des. 2022, 390, 111713. [Google Scholar] [CrossRef]
- Lakehal, D.; Fulgosi, M.; Yadigaroglu, G.; Banerjee, S. Direct Numerical Simulation of Turbulent Heat Transfer Across a Mobile, Sheared Gas-Liquid Interface. J. Heat Transf. 2003, 125, 1129–1139. [Google Scholar] [CrossRef]
- Iyer, S.; Kumar, A.; Coventry, J.; Lipiński, W. Mechanistic Modelling of Bubble Growth in Sodium Pool Boiling. Appl. Math. Model. 2022, 117, 336–358. [Google Scholar] [CrossRef]
- Liao, Y.; Lucas, D. Evaluation of Interfacial Heat Transfer Models for Flashing Flow with Two-Fluid CFD. Fluids 2018, 3, 38. [Google Scholar] [CrossRef]
- Wang, Y.; He, Q.; Li, G.; Yang, Q.; Yan, J. Experimental Investigation on Heat Transfer Characteristics in Circulatory Flash Vaporization of Aqueous NaCl Solution. Desalination 2018, 446, 12–20. [Google Scholar] [CrossRef]
- Li, C.; Cui, Y.; Shi, X.; Gao, J.; Lan, X. CFD Simulation of Mass Transfer in Bubble Columns: Detailed Study of Mass Transfer Models. Chem. Eng. Sci. 2022, 264, 118173. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, P.; Liu, L.; Chen, S.; Song, Y.; Yan, H. CFD Modeling of Reactive Absorption of CO2 in Aqueous NaOH in a Rectangular Bubble Column: Comparison of Mass Transfer and Enhancement Factor Model. Chem. Eng. Sci. 2021, 230, 116218. [Google Scholar] [CrossRef]
- Marsh, C.A.; O’Mahony, A.P. Three-Dimensional Modelling of Industrial Flashing Flows. Prog. Comput. Fluid Dyn. 2009, 9, 393–398. [Google Scholar] [CrossRef]
- Janet, J.P.; Liao, Y.; Lucas, D. Heterogeneous Nucleation in CFD Simulation of Flashing Flows in Converging–Diverging Nozzles. Int. J. Multiph. Flow 2015, 74, 106–117. [Google Scholar] [CrossRef]
- Liao, Y.; Lucas, D. Numerical Analysis of Flashing Pipe Flow Using a Population Balance Approach. Int. J. Heat Fluid Flow 2019, 77, 299–313. [Google Scholar] [CrossRef]
- Ahmadi, F.; Ebrahimian, M.; Sanders, R.S.; Ghaemi, S. Particle Image and Tracking Velocimetry of Solid-Liquid Turbulence in a Horizontal Channel Flow. Int. J. Multiph. Flow 2019, 112, 83–99. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, N.; Li, J. A Conceptual Model for Analyzing Particle Effects on Gas-Liquid Flows in Slurry Bubble Columns. Powder Technol. 2020, 365, 28–38. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Yu, B.; Zou, Y.; Chen, B.-N.; Tao, W.-Q. Study on the Effect of Foreign Particle on Bubble Nucleation by Using Molecular Dynamics Simulation. J. Mol. Liq. 2020, 305, 112876. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Z.; Lyu, S.; Song, Q.; Duan, Y.; Yang, Z. Numerical Investigation of Effects of Particle Properties on Nucleation Process in a Sulfuric Acid–Water Vapor Mixture: Homogeneous versus Heterogeneous. Chem. Phys. Lett. 2022, 797, 139582. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Z.; Liu, J.; Ao, X.; Lin, S.; Yang, Y. Modeling and Numerical Study of Particle-Bubble-Liquid Flows Using a Front-Tracking and Discrete-Element Method. Appl. Math. Model. 2023, 114, 525–543. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, Q.; Caliskan, U.; Miskovic, S. Investigation of Particle Effects on Bubble Coalescence in Slurry with a Chimera MP-PIC and VOF Coupled Method. Chem. Eng. Sci. 2023, 265, 118174. [Google Scholar] [CrossRef]
- Mokhtari, M.; Shabanian, J.; Chaouki, J. Effects of Solid Particles on Bubble Breakup and Coalescence in Slurry Bubble Columns. Chem. Eng. Sci. 2022, 264, 118148. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).