Sweet Potatoes Puree Mixed with Herbal Aqueous Extracts: A Novel Ready-to-Eat Product for Lactating Mothers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Preparation
2.2.1. Aqueous Extracts of Galactogogue Herbs
2.2.2. Preparation of Sweet Potato Puree Mixed with Herbal Aqueous Extract
2.3. Heat Induced Changes
2.3.1. Cooking Loss Measurements and Calculation
2.3.2. Differential Scanning Calorimetry (DSC)
2.4. Phytochemical Characterization
2.5. Determination of the In Vitro Release of Phenols from Sweet Potato Purees
2.6. Color Evaluation of Sweet Potato Purees
2.7. Rheological Analysis
2.8. Instrumental Determination of Texture
2.9. FT-IR Spectroscopy
2.10. Sensory Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Heat-Induced Changes
3.1.1. Cooking Loss Measurements and Calculation
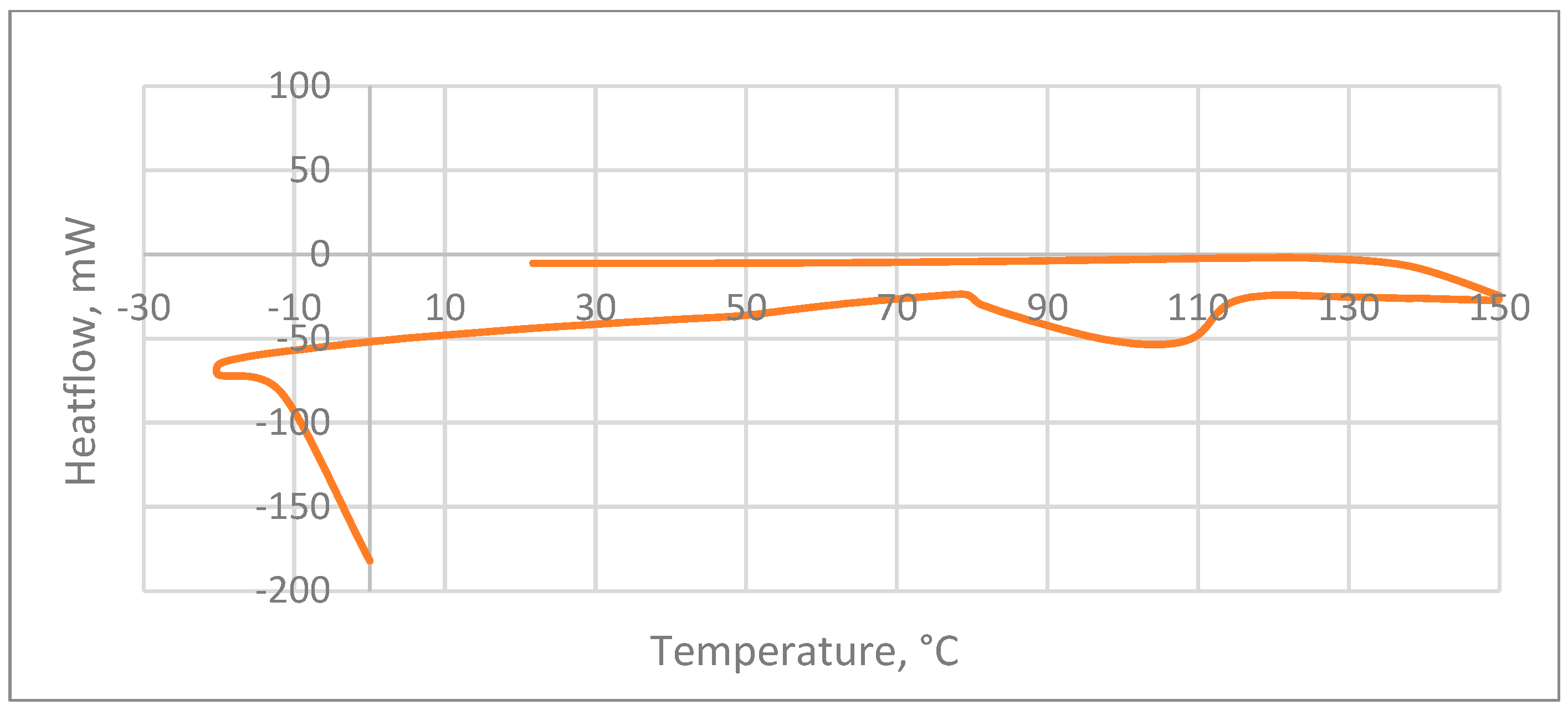
3.1.2. Differential Scanning Calorimetry (DSC)
3.2. Phytochemical Content
3.3. Determination of the In Vitro Release of Remanent Phenolic Content from Sweet Potatoes Purees
3.4. Influence of Heat Treatments on Color Parameters of Sweet Potatoes Purees
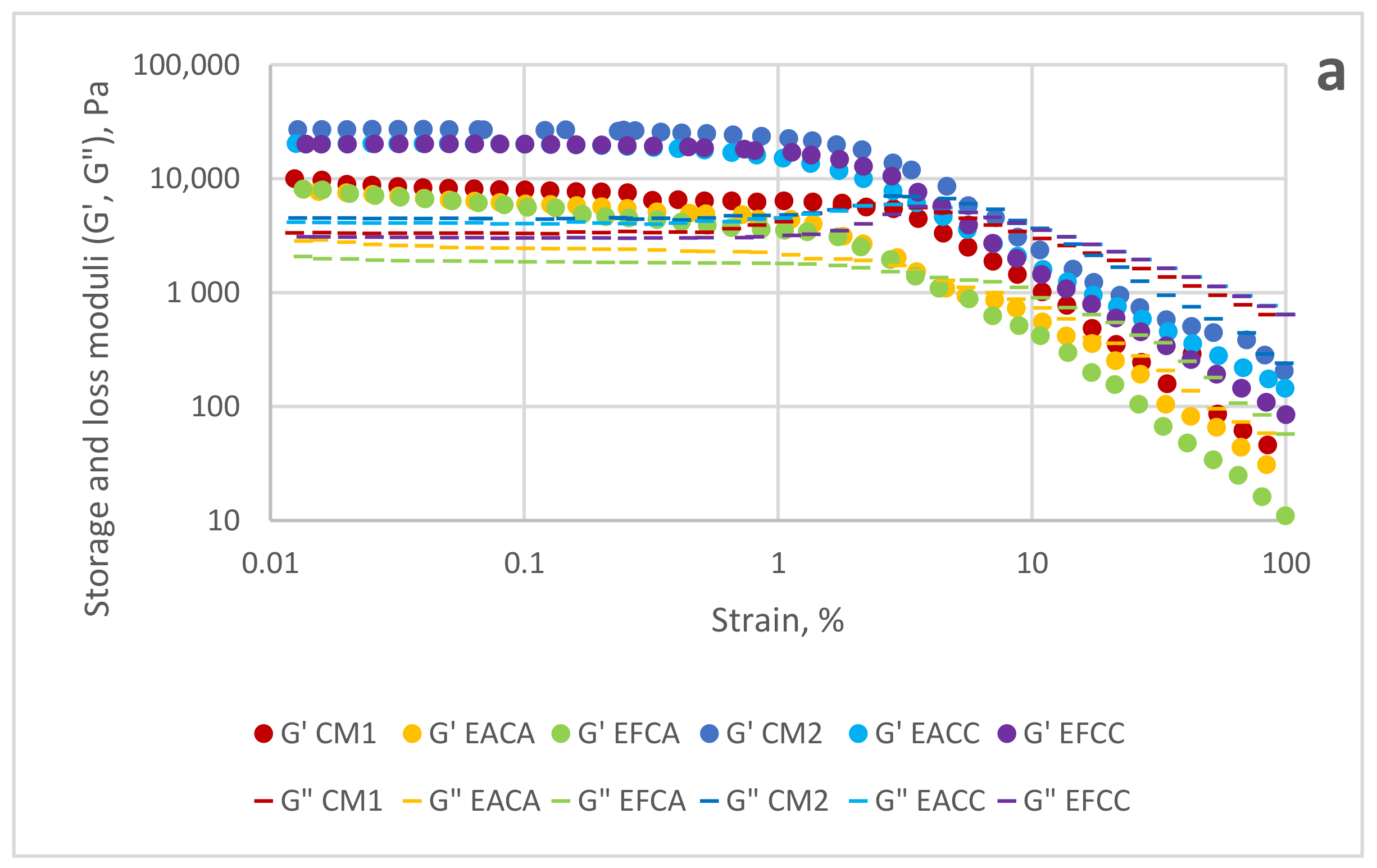
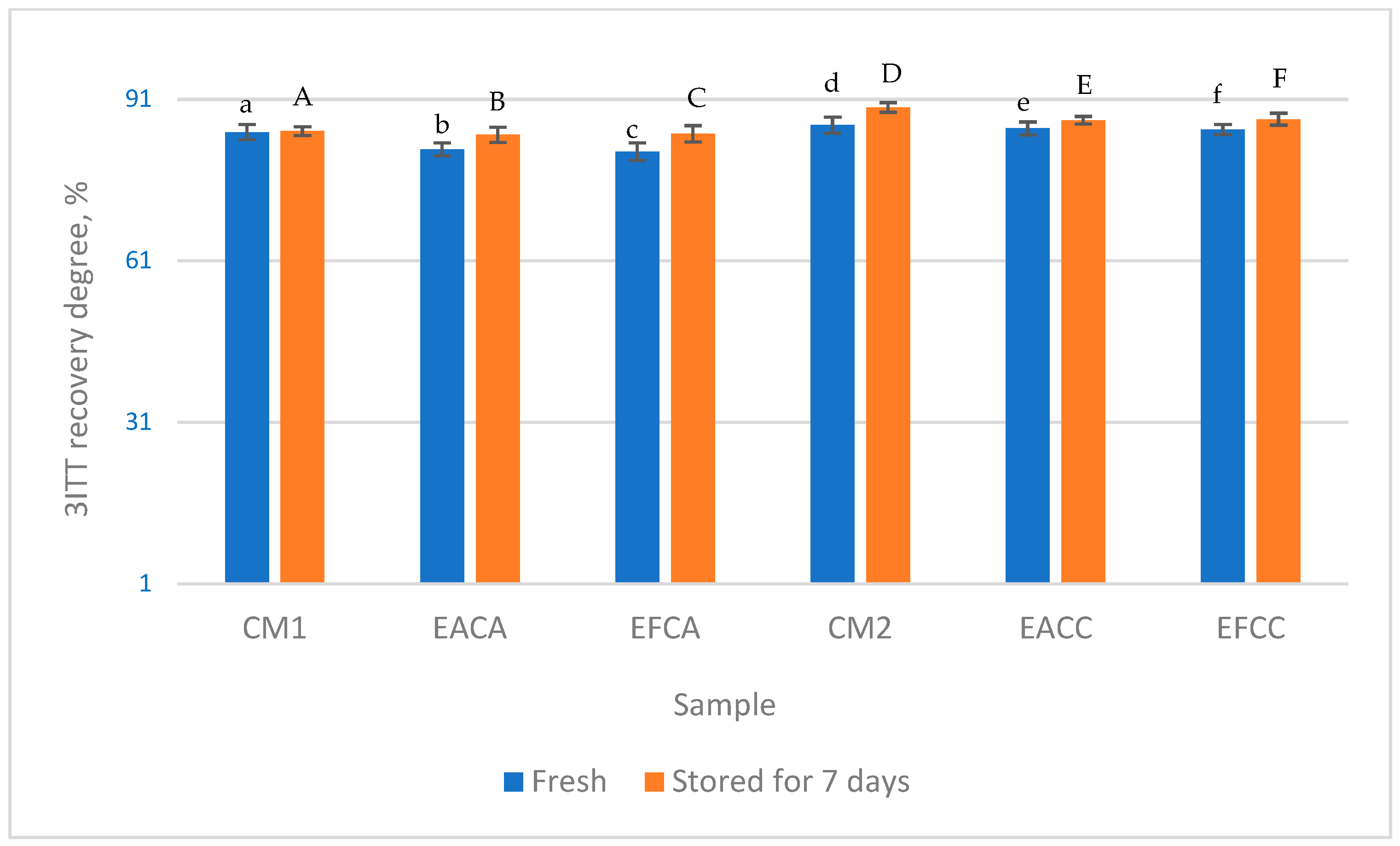
3.5. Rheological Analysis
3.6. Instrumental Determination of Texture
3.7. FT-IR Spectroscopy
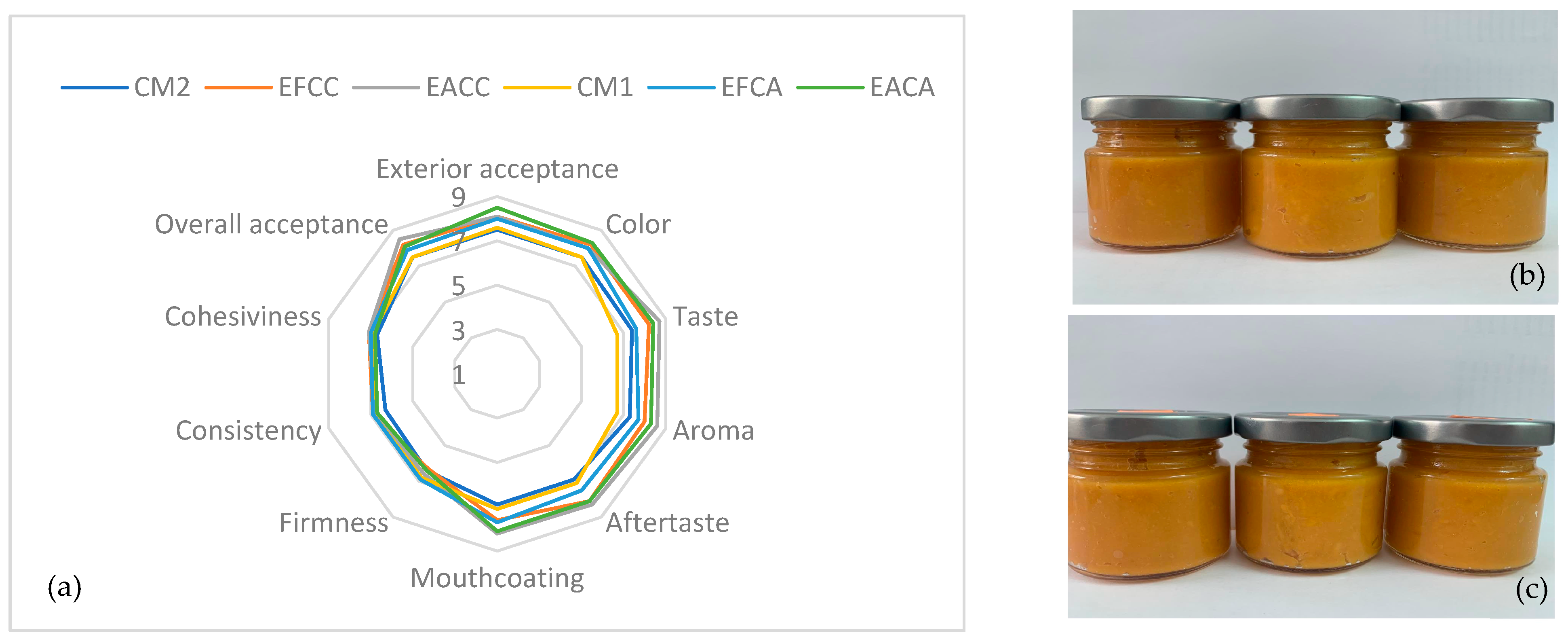
3.8. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chamorro, S.; Cueva-Mestanza, R.; de Pascual-Teresa, S. Effect of Spray Drying on the Polyphenolic Compounds Present in Purple Sweet Potato Roots: Identification of New Cinnamoylquinic Acids. Food Chem. 2021, 345, 128679. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Y.; Cao, Y.; Xia, W.; Jiang, Q. Application of Simultaneous Combination of Microwave and Steam Cooking to Improve Nutritional Quality of Cooked Purple Sweet Potatoes and Saving Time. Innov. Food Sci. Emerg. Technol. 2016, 36, 303–310. [Google Scholar] [CrossRef]
- Jiang, Q.; Liang, S.; Zeng, Y.; Lin, W.; Ding, F.; Li, Z.; Cao, M.; Li, Y.; Ma, M.; Wu, Z. Morphology, Structure and In Vitro Digestibility of Starches Isolated from Ipomoea Batatas (L.) Lam. by Alkali and Ethanol Methods. Int. J. Biol. Macromol. 2019, 125, 1147–1155. [Google Scholar] [CrossRef]
- Qin, Y.; Naumovski, N.; Ranadheera, C.S.; D’Cunha, N.M. Nutrition-Related Health Outcomes of Sweet Potato (Ipomoea batatas) Consumption: A Systematic Review. Food Biosci. 2022, 50, 102208. [Google Scholar] [CrossRef]
- Sibeko, L.; Johns, T. Global Survey of Medicinal Plants during Lactation and Postpartum Recovery: Evolutionary Perspectives and Contemporary Health Implications. J. Ethnopharmacol. 2021, 270, 113812. [Google Scholar] [CrossRef]
- Barnes, L.A.J.; Rolfe, M.I.; Barclay, L.; McCaffery, K.; Aslani, P. Women’s Reasons for Taking Complementary Medicine Products in Pregnancy and Lactation: Results from a National Australian Survey. Complement. Ther. Clin. Pract. 2022, 49, 101673. [Google Scholar] [CrossRef]
- Fryer, P.J.; Robbins, P.T. Heat Transfer in Food Processing: Ensuring Product Quality and Safety. Appl. Therm. Eng. 2005, 25, 2499–2510. [Google Scholar] [CrossRef]
- Richardson, P. (Ed.) Improving the Thermal Processing of Foods; Woodhead Publishing Limited: Cambridge, UK, 2004. [Google Scholar] [CrossRef]
- Tanase (Butnariu), L.A.; Nistor, O.-V.; Andronoiu, D.-G.; Mocanu, G.-D.; Botez, E. Effects of Heat Treatments on Various Characteristics of Ready-to-Eat Zucchini Purees Enriched with Anise or Fennel. Molecules 2022, 27, 7964. [Google Scholar] [CrossRef]
- Butnariu, L.T.; Nistor, O.; Andronoiu, D.; Mocanu, G.-D.; Botezatu Dediu, A.V.; Botez, E. Different Types of Meatballs Enriched with Wild Thyme/Lemon. Molecules 2022, 27, 3920. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Yildiz Turp, G.; Icier, F.; Kendirci, P.; Kor, G. Effects of Ohmic Heating for Pre-Cooking of Meatballs on Some Quality and Safety Attributes. LWT—Food Sci. Technol. 2014, 55, 232–239. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic Profile and Antioxidant Activity in Selected Seeds and Sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Turturicə, M.; Stənciuc, N.; Bahrim, G.; Râpeanu, G. Effect of Thermal Treatment on Phenolic Compounds from Plum (Prunus Domestica) Extracts—A Kinetic Study. J. Food Eng. 2015, 171, 200–207. [Google Scholar] [CrossRef]
- Dewanto, V.; Xianzhong, W.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Escoto, D.F.; Ramborger, B.P.; Gayer, M.C.; Rodrigues, D.T.; Gasparoto Denardin, E.L.; Roehrs, R.; Roehrs, M. Lycopene: Food Sources, Potential Role in Human Health and Antioxidant Effects; Bailey, J.R., Ed.; Nova Science Publishers: New York, NY, USA, 2015. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food-an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- He, C.; Zhang, M.; Devahastin, S. Investigation on Spontaneous Shape Change of 4D Printed Starch-Based Purees from Purple Sweet Potatoes as Induced by Microwave Dehydration; ACS Publications: Washington, DC, USA, 2020; Volume 12. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid and Semisolid Foods: Principles and Applications (Google EBook); Springer Science & Business: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Nwosisi, S.; Nandwani, D.; Ravi, R. Texture profile analysis (tpa) of organic sweetpotato (Ipomoea batatas) cultivars as affected by different processing methods Sochinwechi Nwosisi, Dilip Nandwani and Ramasamy Ravi. J. Microbiol. Biotech. Food Sci. 2019, 8, 1254–1259. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, Y.; Zhang, F.; Guo, X.; Liao, Z. Effect of Thermal Processing on Carotenoids and Folate Changes in Six Varieties of Sweet Potato (Ipomoes Batata L.). Foods 2019, 8, 215. [Google Scholar] [CrossRef]
- Phan, K.T.L.; Chittrakorn, S.; Tai, H.P.; Ruttarattanamongkol, K. Effects of Cooking Methods on the Changes of Total Anthocyanins, Phenolics Content and Physical Characteristics of Purple-Fleshed Sweet Potato (Ipomoea Batatas L.) Grown in Vietnam. Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 227–233. [Google Scholar] [CrossRef]
- Roos, Y.H. Food Processing and Storage. In Phase Transitions in Foods; Elsevier: Amsterdam, The Netherlands, 1995; pp. 313–347. [Google Scholar] [CrossRef]
- Ahmed, J.; Ramaswamy, H.S. Viscoelastic Properties of Sweet Potato Puree Infant Food. J. Food Eng. 2006, 74, 376–382. [Google Scholar] [CrossRef]
- Varadaraju, R. Health Benefits of Vegetables. Int. J. Chem. Stud. 2019, 7, 82–87. [Google Scholar]
- Oloniyo, R.O.; Omoba, O.S.; Awolu, O.O. Biochemical and Antioxidant Properties of Cream and Orange-Fleshed Sweet Potato. Heliyon 2021, 7, e06533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Patel, J.; Bhunia, K.; Al-Ghamdi, S.; Sonar, C.R.; Ross, C.F.; Tang, J.; Sablani, S.S. Color, Vitamin C, β-Carotene and Sensory Quality Retention in Microwave-Assisted Thermally Sterilized Sweet Potato Puree: Effects of Polymeric Package Gas Barrier during Storage. Food Packag. Shelf Life 2019, 21, 100324. [Google Scholar] [CrossRef]
- Fadavi, A.; Yousefi, S.; Darvishi, H.; Mirsaeedghazi, H. Comparative Study of Ohmic Vacuum, Ohmic, and Conventional-Vacuum Heating Methods on the Quality of Tomato Concentrate. Innov. Food Sci. Emerg. Technol. 2018, 47, 225–230. [Google Scholar] [CrossRef]
- Dutta, D.; Dutta, A.; Raychaudhuri, U.; Chakraborty, R. Rheological Characteristics and Thermal Degradation Kinetics of Beta-Carotene in Pumpkin Puree. J. Food Eng. 2006, 76, 538–546. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, R.; Jia, R.; Deng, Y.; Wang, Z. Impact of Different Cooking Methods on the Chemical Profile of Orange-Fleshed Sweet Potato (Ipomoea Batatas L.). Lwt 2023, 173, 114288. [Google Scholar] [CrossRef]
- Liu, D.; Dhital, S.; Wu, P.; Chen, X.D.; Gidley, M.J. In Vitro Digestion of Apple Tissue Using a Dynamic Stomach Model: Grinding and Crushing Effects on Polyphenol Bioaccessibility. J. Agric. Food Chem. 2020, 68, 574–583. [Google Scholar] [CrossRef]
- Ziółkiewicz, A.; Kasprzak-Drozd, K.; Wójtowicz, A.; Oniszczuk, T.; Gancarz, M.; Kowalska, I.; Mołdoch, J.; Kondracka, A.; Oniszczuk, A. The Effect of In Vitro Digestion on Polyphenolic Compounds and Antioxidant Properties of Sorghum (Sorghum Bicolor (L.) Moench) and Sorghum-Enriched Pasta. Molecules 2023, 28, 1706. [Google Scholar] [CrossRef]
- Peng, J.; Tang, J.; Barrett, D.M.; Sablani, S.S.; Anderson, N.; Powers, J.R. Thermal Pasteurization of Ready-to-Eat Foods and Vegetables: Critical Factors for Process Design and Effects on Quality. Crit. Rev. Food Sci. Nutr. 2017, 57, 2970–2995. [Google Scholar] [CrossRef]
- Gallego-Castillo, S.; Ayala-Aponte, A.A. Changes in Physical Properties of Sweet Potato Due to Effects of Thermal Pre-Treatments for Puree Production. DYNA 2018, 85, 135–142. [Google Scholar] [CrossRef]
- Maskan, M. Drying, Shrinkage and Rehydration Characteristics of Kiwifruits during Hot Air and Microwave Drying. J. Food Eng. 2001, 48, 177–182. [Google Scholar] [CrossRef]
- Grace, M.H.; Yousef, G.G.; Gustafson, S.J.; Den Truong, V.; Yencho, G.C.; Lila, M.A. Phytochemical Changes in Phenolics, Anthocyanins, Ascorbic Acid, and Carotenoids Associated with Sweetpotato Storage and Impacts on Bioactive Properties. Food Chem. 2014, 145, 717–724. [Google Scholar] [CrossRef]
- Espinosa-Muñoz, L.; Renard, C.M.G.C.; Symoneaux, R.; Biau, N.; Cuvelier, G. Structural Parameters That Determine the Rheological Properties of Apple Puree. J. Food Eng. 2013, 119, 619–626. [Google Scholar] [CrossRef]
- Fasina, O.O.; Walter, W.M.; Fleming, H.P.; Simunovic, N. Viscoelastic Properties of Restructured Sweetpotato Puree. Int. J. Food Sci. Technol. 2003, 38, 421–425. [Google Scholar] [CrossRef]
- He, C.; Zhang, M.; Guo, C. 4D Printing of Mashed Potato/Purple Sweet Potato Puree with Spontaneous Color Change. Innov. Food Sci. Emerg. Technol. 2020, 59, 102250. [Google Scholar] [CrossRef]
- Mei, X.; Mu, T.H.; Han, J.J. Composition and Physicochemical Properties of Dietary Fiber Extracted from Residues of 10 Varieties of Sweet Potato by a Sieving Method. J. Agric. Food Chem. 2010, 58, 7305–7310. [Google Scholar] [CrossRef]
- Tănase, L.A.; Nistor, O.-V.; Andronoiu, D.-G.; Mocanu, G.; Ciortan, S.; Ioni, E.; Dediu, A.V.B.; Botez, E. Galactogogue Herbs: Antioxidant Activity and Bioactive Compounds’ Content Determined from Aqueous Extracts. In 10th Central European Congress on Food. CE-Food; Brka, M., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Dawid, C.; Hofmann, T. Identification of Sensory-Active Phytochemicals in Asparagus (Asparagus officinalis L.). J. Agric. Food Chem. 2012, 60, 11877–11888. [Google Scholar] [CrossRef]



| Parameters | Baking | Steaming |
|---|---|---|
| Cooking loss, % | 36.88 ± 0.08 A | 5.72 ± 0.31 B |
| Cooking yield, % | 63.12 ± 0.08 B | 94.28 ± 0.32 A |
| Phytochemicals and Antioxidant Activity of Sweet Potato Samples | T0—Samples in the First Day of Storage | |||||
|---|---|---|---|---|---|---|
| Steaming | Baking | |||||
| CM1 | EFCA | EACA | CM2 | EFCC | EACC | |
| Antioxidant Activity, µM Trolox/g DW | 35.4 ± 0.70 A,B | 40.72 ± 2.24 A | 40.52 ± 0.48 A | 30.96 ± 1.64 B | 36.95 ± 0.85 A | 35.85 ± 3.75 A,B |
| TPC, mg GAE/g DW | 4.59 ± 0.18 B | 5.03 ± 0.09 A | 5.04 ± 0.19 A | 3.38 ± 0.10 D | 3.73 ± 0.04 C,D | 3.85 ± 0.17 C |
| TFC, mg EQ/g DW | 5.98 ± 0.46 A,B,C | 6.56 ± 0.17 A | 6.28 ± 6.28 A,B | 4.73 ± 0.09 D | 5.29 ± 0.18 C,D | 5.63 ± 0.19 B,C |
| BC, mg/g DW | 0.68 ± 0.09 B,C | 1.27 ± 0.11 A | 0.81 ± 0.09 B | 0.47 ± 0.03 D | 0.56 ± 0.01 C,D | 0.6 ± 0.02 C,D |
| LYC, mg/g DW | 0.45 ± 0.02 B | 0.59 ± 0.07 A | 0.5 ± 0.05 A,B | 0.28 ± 0.03 C | 0.33 ± 0.01 C | 0.32 ± 0.02 C |
| TC, mg/g DW | 0.72 ± 0.04 B | 1.38 ± 0.11 A | 0.82 ± 0.10 B | 0.46 ± 0.03 C | 0.5 ± 0.02 C | 0.64 ± 0.01 B,C |
| T7—Samples after 7 Days Refrigeration Storage | ||||||
| Steaming | Baking | |||||
| Antioxidant Activity, µM Trolox/g DW | 28.9 ± 1.35 D | 40.69 ± 0.43 B | 33.68 ± 0.93 C | 32.44 ± 0.55 C | 44.97 ± 1.23 A | 46.5 ± 1.29 A |
| TPC, mg GAE/g DW | 6.68 ± 0.05 A | 6.71 ± 0.11 A | 6.8 ± 0.04 A | 3.55 ± 0.01 C | 5.32 ± 0.18 B | 5.45 ± 0.09 B |
| TFC, mg EQ/g DW | 6.1 ± 0.25 B | 6.8 ± 0.04 A | 7.15 ± 0.18 A | 3.97 ± 0.03 D | 5.52 ± 0.04 C | 5.39 ± 0.07 C |
| BC, mg/g DW | 0.67 ± 0.01 D | 1.14 ± 0.04 A | 1.01 ± 0.04 B | 0.43 ± 0.01 E | 0.78 ± 0.02 C | 0.67 ± 0.00 D |
| LYC, mg/g DW | 0.48 ± 0.01 C | 0.61 ± 0.03 A | 0.54 ± 0.03 B | 0.23 ± 0.01 E | 0.46 ± 0.01 C | 0.4 ± 0.00 D |
| TC, mg/g DW | 0.97 ± 0.01 C | 1.18 ± 0.02 A | 1.06 ± 0.04 B | 0.43 ± 0.01 F | 0.78 ± 0.02 D | 0.69 ± 0.00 E |
| T0 | |||||
|---|---|---|---|---|---|
| Raw | EFCA | EACA | EFCC | EACC | |
| L* | 54.59 ± 0.00 A | 49.59 ± 0.02 B | 49.07 ± 0.00 C | 47.51 ± 0.06 E | 47.81 ± 0.11 D |
| a* | 25.37 ± 0.00 A | 21.13 ± 0.01 C | 20.15 ± 0.00 D | 21.32 ± 0.03 B | 19.84 ± 0.15 E |
| b* | 40.2 ± 0.00 D | 41.59 ± 0.20 B | 41.11 ± 0.06 C | 41.76 ± 0.02 B | 42.22 ± 0.13 A |
| ΔE | - | 6.70 ± 0.05 D | 7.65 ± 0.01 C | 8.30 ± 0.06 B | 8.97 ± 0.14 A |
| C* | 47.54 ± 0.00 A | 46.65 ± 0.18 B | 45.78 ± 0.05 C | 46.88 ± 0.03 B | 46.65 ± 0.18 B |
| h* | 32.26 ± 0.00 D | 63.06 ± 0.10 C | 63.89 ± 0.03 B | 62.95 ± 0.02 C | 64.82 ± 0.09 A |
| WI | 34.26 ± 0.00 A | 31.31 ± 0.13 C | 31.51 ± 0.04 B | 29.61 ± 0.02 E | 30.0 ± 0.04 D |
| BI | 152.41 ± 0.00 C | 178.78 ± 1.33 B | 177.46 ± 0.38 B | 192.94 ± 0.22 A | 192.15 ± 0.39 A |
| YI | 105.2 ± 0.00 C | 119.83 ± 0.61 B | 119.68 ± 0.17 B | 125.57 ± 0.10 A | 126.13 ± 0.11 A |
| T7 | |||||
| L* | 54.59 ± 0.00 A | 44.64 ± 0.20 B, C | 44.95 ± 0.51 B | 44.79 ± 0.43 B, C | 44.03 ± 0.30 C |
| a* | 25.37 ± 0.00 A | 16.74 ± 0.23 C | 17.0 ± 0.02 C | 17.94 ± 0.16 B | 17.00 ± 0.03 C |
| b* | 40.2 ± 0.00 A | 33 ± 0.06 C | 34.19 ± 0.84 C | 37.93 ± 0.70 B | 36.66 ± 0.18 B |
| ΔE | - | 15.01 ± 0.24 A | 14.12 ± 0.71 A | 12.52 ± 0.55 B | 13.94 ± 0.25 A |
| C* | 47.54 ± 0.00 A | 37.0 ± 0.05 D | 38.18 ± 0.76 D | 41.95 ± 0.69 B | 40.41 ± 0.15 C |
| h* | 32.26 ± 0.00 C | 63.1 ± 0.36 B | 63.55 ± 0.53 B | 64.69 ± 0.21 A | 65.13 ± 0.14 A |
| WI | 34.26 ± 0.00 A | 33.41 ± 0.14 B | 33.0 ± 0.02 C | 30.65 ± 0.08 E | 30.96 ± 0.15 D |
| BI | 152.41 ± 0.00 C | 147.17 ± 0.98 D | 153.07 ± 2.46 C | 179.58 ± 2.28 A | 174.30 ± 0.66 B |
| YI | 105.2 ± 0.00 C | 105.61 ± 0.67 C | 108.65 ± 1.43 B | 120.96 ± 1.06 A | 118.96 ± 0.21 A |
| Textural Parameters | T0 | |||||
|---|---|---|---|---|---|---|
| CM1 | EFCA | EACA | CM2 | EFCC | EACC | |
| Firmness, N | 5.71 ± 0.11 B,C | 3.59 ± 0.10 C | 4.27 ± 0.69 C | 13.24 ± 1.21 A | 9.87 ± 0.95 A | 9.52 ± 3.26 A,B |
| Adhesiveness, mJ | 23.16 ± 3.12 B | 35.68 ± 3.57 A,B | 38.86 ± 3.79 B | 50.24 ± 4.16 A,B | 91.25 ± 3.99 A | 98.87 ± 4.50 A |
| Cohesiveness | 0.53 ± 0.04 A | 0.69 ± 0.02 A | 0.60 ± 0.06 A | 0.51 ± 0.01 A | 0.66 ± 0.09 A | 0.65 ± 0.03 A |
| Springiness, mm | 8.69 ± 0.03 B | 13.13 ± 0.15 A | 13.31 ± 0.66 A | 8.72 ± 0.52 B | 13.92 ± 1.25 A | 14.06 ± 0.91 A |
| T7 | ||||||
| Firmness, N | 5.70 ± 0.25 B | 3.30 ± 0.25 C | 3.96 ± 0.13 C | 13.63 ± 0.88 A | 9.44 ± 0.77 B | 8.98 ± 0.21 B |
| Adhesiveness, mJ | 23.61 ± 2.25 A,B,C | 33.77 ± 5.15 B,C | 20.44 ± 8.90 C | 45.9 ± 15.31 A | 88.01 ± 5.11 A,B | 91.31 ± 4.46 A,B,C |
| Cohesiveness | 0.39 ± 0.02 A | 0.58 ± 0.13 A | 0.54 ± 0.05 A | 0.44 ± 0.07 A | 0.47 ± 0.14 A | 0.60 ± 0.02 A |
| Springiness, mm | 8.05 ± 0.22 A | 12.65 ± 1.30 A | 13.76 ± 0.85 A | 8.71 ± 2.74 A | 12.23 ± 1.26 A | 13.10 ± 0.30 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tănase, L.-A.; Andronoiu, D.-G.; Nistor, O.-V.; Mocanu, G.-D.; Botez, E.; Ștefănescu, B.I. Sweet Potatoes Puree Mixed with Herbal Aqueous Extracts: A Novel Ready-to-Eat Product for Lactating Mothers. Processes 2023, 11, 2219. https://doi.org/10.3390/pr11072219
Tănase L-A, Andronoiu D-G, Nistor O-V, Mocanu G-D, Botez E, Ștefănescu BI. Sweet Potatoes Puree Mixed with Herbal Aqueous Extracts: A Novel Ready-to-Eat Product for Lactating Mothers. Processes. 2023; 11(7):2219. https://doi.org/10.3390/pr11072219
Chicago/Turabian StyleTănase (Butnariu), Luiza-Andreea, Doina-Georgeta Andronoiu, Oana-Viorela Nistor, Gabriel-Dănuț Mocanu, Elisabeta Botez, and Bogdan Ioan Ștefănescu. 2023. "Sweet Potatoes Puree Mixed with Herbal Aqueous Extracts: A Novel Ready-to-Eat Product for Lactating Mothers" Processes 11, no. 7: 2219. https://doi.org/10.3390/pr11072219
APA StyleTănase, L.-A., Andronoiu, D.-G., Nistor, O.-V., Mocanu, G.-D., Botez, E., & Ștefănescu, B. I. (2023). Sweet Potatoes Puree Mixed with Herbal Aqueous Extracts: A Novel Ready-to-Eat Product for Lactating Mothers. Processes, 11(7), 2219. https://doi.org/10.3390/pr11072219










