Study on the Remediation of Pyrene-Contaminated Soil with Surfactants and their Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Samples and Reagents
Reagents
2.2. Preparation of Pyrene-Contaminated Samples
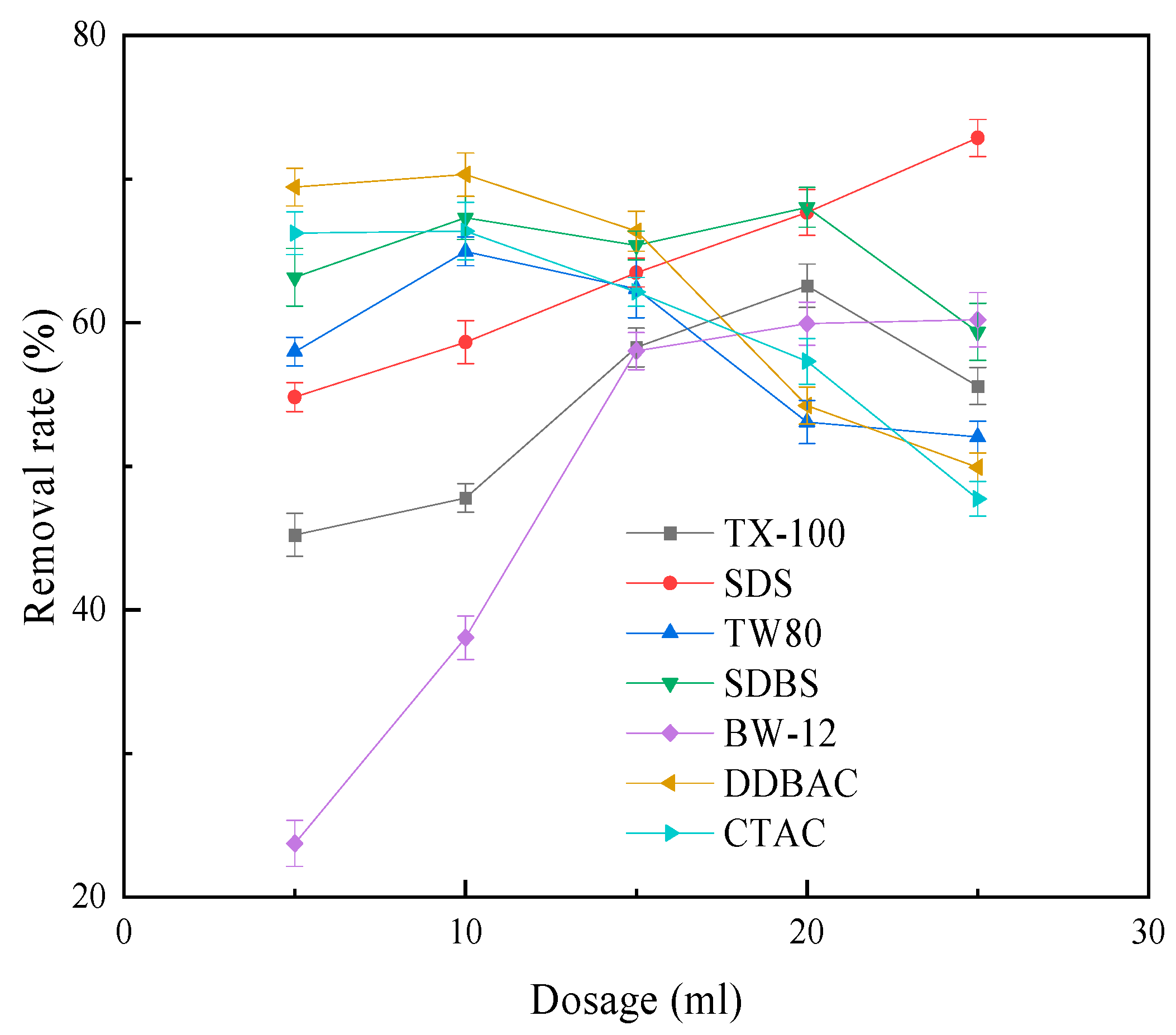
2.3. Desorption Test
2.4. Molecular Dynamics Simulation
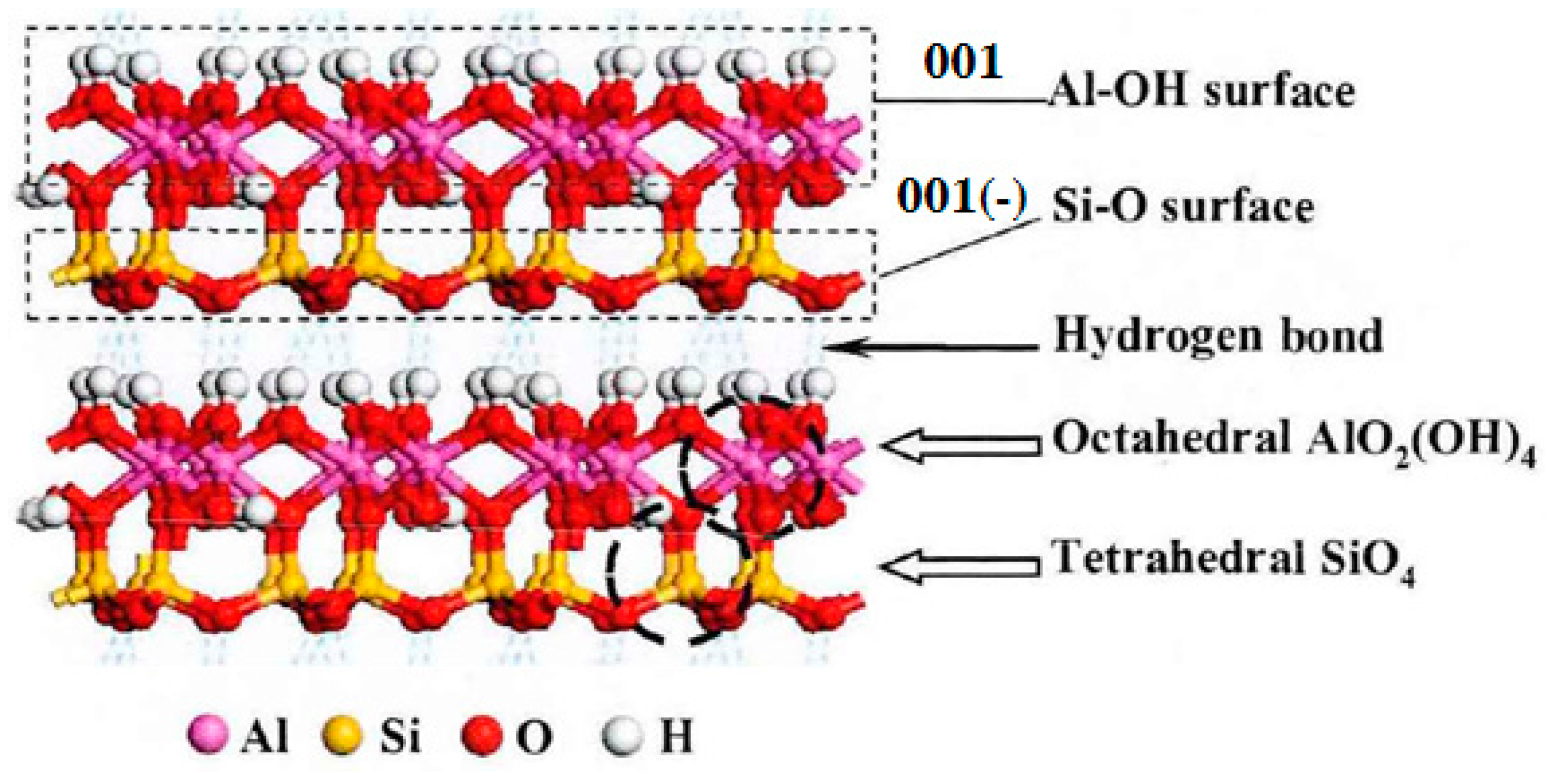
2.4.1. Adsorption Model
2.4.2. Simulation Method
3. Results and Discussion
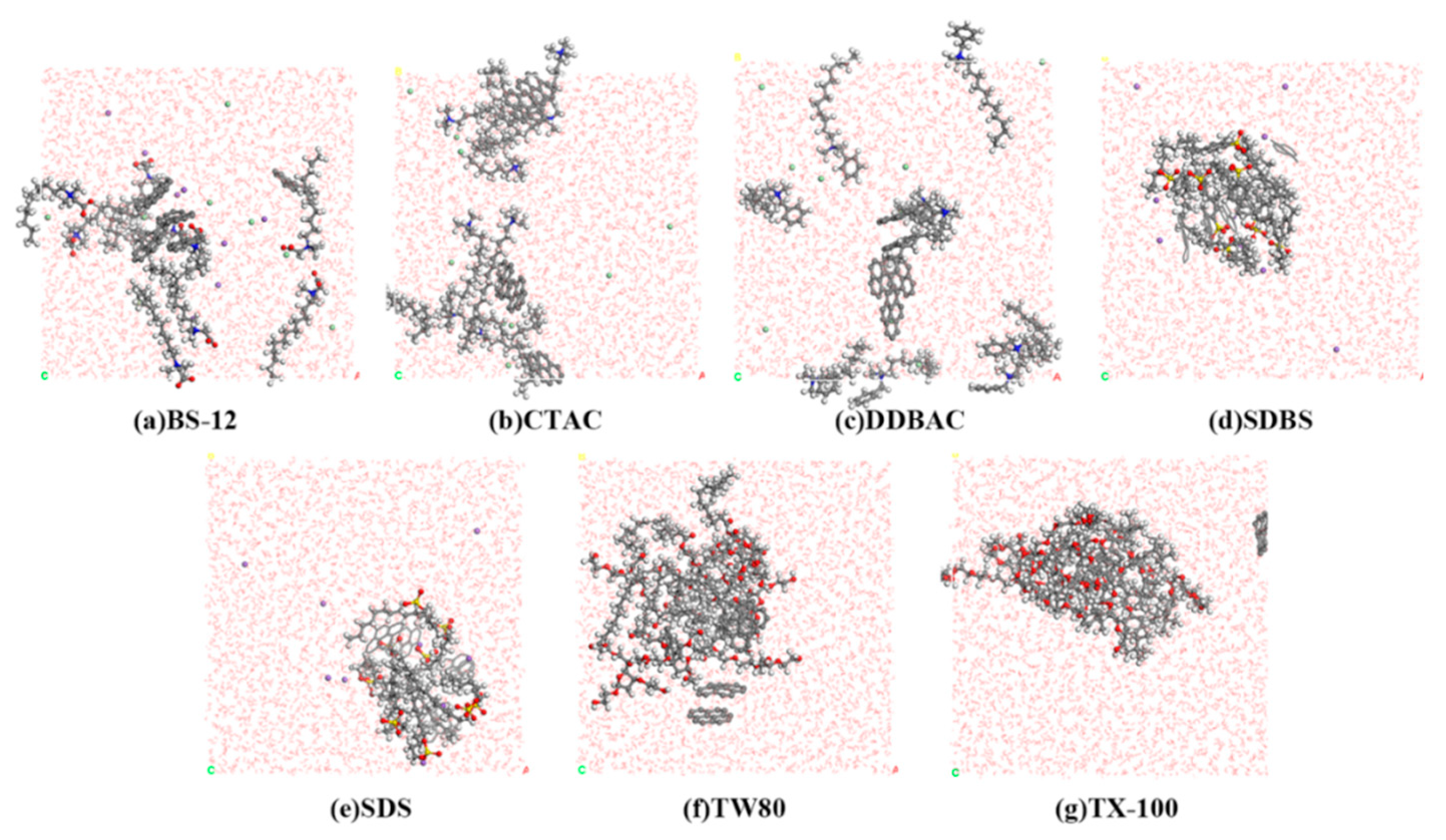
3.1. Molecular Dynamics Simulation Results and Molecular Structure
3.1.1. Adsorption Equilibrium Configuration
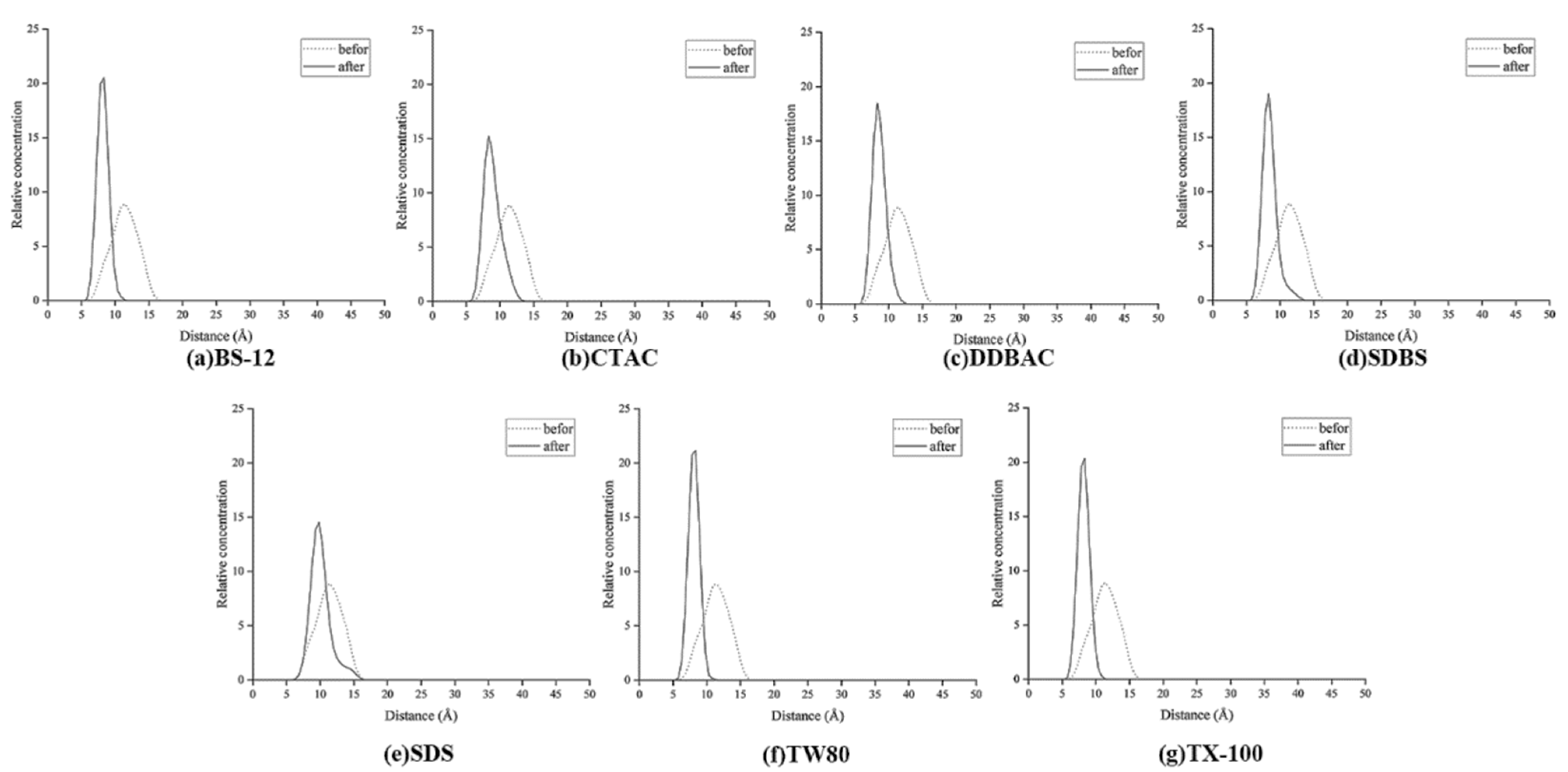
3.1.2. Relative Concentration Distribution
3.2. Desorption Test Results’ Interaction between Collector and Kaolinite Surface
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef]
- Motorykin, O.; Matzke, M.M.; Waters, K.M.; Massey Simonich, S.L. Association of Carcinogenic Polycyclic Aromatic Hydrocarbon Emissions and Smoking with Lung Cancer Mortality Rates on a Global Scale. Environ. Sci. Technol. 2013, 47, 3410–3416. [Google Scholar] [CrossRef]
- Xue, W.; Warshawsky, D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, X.; Lin, W.; Guo, C.; Dang, Z. Clay mineral dependent desorption of pyrene from soils by single and mixed anionic–nonionic surfactants. Chem. Eng. J. 2015, 264, 807–814. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, Z.; Cai, G.; Wang, C.; Cheng, G.; Wang, X. Adsorption behaviour and mechanism of benzene, toluene and m-xylene (BTX) solution onto kaolinite: Experimental and molecular dynamics simulation studies. Sep. Purif. Technol. 2022, 291, 120940. [Google Scholar] [CrossRef]
- Yang, L.; Jin, M.; Tong, C.; Xie, S. Study of dynamic sorption and desorption of polycyclic aromatic hydrocarbons in silty-clay soil. J. Hazard. Mater. 2013, 244–245, 77–85. [Google Scholar] [CrossRef]
- Zhang, G.; He, L.; Guo, X.; Han, Z.; Ji, L.; He, Q.; Han, L.; Sun, K. Mechanism of biochar as a biostimulation strategy to remove polycyclic aromatic hydrocarbons from heavily contaminated soil in a coking plant. Geoderma 2020, 375, 114497. [Google Scholar] [CrossRef]
- Dai, C.; Han, Y.; Duan, Y.; Lai, X.; Fu, R.; Liu, S.; Leong, K.H.; Tu, Y.; Zhou, L. Review on the contamination and remediation of polycyclic aromatic hydrocarbons (PAHs) in coastal soil and sediments. Environ. Res. 2022, 205, 112423. [Google Scholar] [CrossRef]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Xing, B. Enhanced Soil Washing of Phenanthrene by Mixed Solutions of TX100 and SDBS. Environ. Sci. Technol. 2006, 40, 4274–4280. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem. Eng. J. 2017, 314, 98–113. [Google Scholar] [CrossRef]
- Chong, Z.-Y.; Liao, X.-Y.; Yan, X.-L.; Sun, L.; Zhao, D.; Liang, T. Enhanced Desorption of PAHs from Manufactured Gas Plant Soils Using Different Types of Surfactants. Pedosphere 2014, 24, 209–219. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, M.; Guo, C.; Abel, S.; Yi, X.; Lu, G.; Yang, C.; Dang, Z. Competitive solubilization of low-molecular-weight polycyclic aromatic hydrocarbons mixtures in single and binary surfactant micelles. Chem. Eng. J. 2014, 244, 522–530. [Google Scholar] [CrossRef]
- Zhu, L.; Feng, S. Synergistic solubilization of polycyclic aromatic hydrocarbons by mixed anionic–nonionic surfactants. Chemosphere 2003, 53, 459–467. [Google Scholar] [CrossRef]
- Duran, S.; Anwar, J.; Moin, S.T. Interaction of gentamicin and gentamicin-AOT with poly-(lactide-co-glycolate) in a drug delivery system—Density functional theory calculations and molecular dynamics simulation. J. Hazard. Mater. 2023, 294, 106958. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Y.; Sui, C.; Sang, Y.; Hao, W.; Li, J.; Wu, J.; He, X.; Wang, C. Molecular dynamics simulations of Carbyne/Carbon nanotube gigahertz oscillators. Comput. Mater. Sci. 2023, 222, 112105. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, L. Adsorption of Naphthalene on Clay Minerals: A Molecular Dynamics Simulation Study. Materials 2022, 15, 5120. [Google Scholar] [CrossRef]
- Wu, G.; He, L.; Chen, D. Sorption and distribution of asphaltene, resin, aromatic and saturate fractions of heavy crude oil on quartz surface: Molecular dynamic simulation. Chemosphere 2013, 92, 1465–1471. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, S.; Jing, R.; Shao, Y.; Liu, X.; Lv, F.; Hu, X.; Zhang, Q.; Meng, Z.; Liu, A. Competitive adsorption of antibiotic tetracycline and ciprofloxacin on montmorillonite. Appl. Clay Sci. 2019, 180, 105175. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Hu, L. Thermal desorption mechanism of n-dodecane on unsaturated clay: Experimental study and molecular dynamics simulation. Environ. Pollut. 2023, 323, 121228. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, L.; Xu, L. Sorption characteristics of CTMA–bentonite complexes as controlled by surfactant packing density. Colloids Surf. A 2007, 294, 221–227. [Google Scholar] [CrossRef]
- Tran, H.T.; Lin, C.; Hoang, H.G.; Bui, X.T.; Le, V.G.; Vu, C.T. Soil washing for the remediation of dioxin-contaminated soil: A review. J. Hazard. Mater. 2022, 421, 126767. [Google Scholar] [CrossRef]
- Liu, H.-Q.; Liu, F.; Wei, G.-X.; Zhang, R.; Zhu, Y.-W. Effects of Surfactants on the Removal of Carbonaceous Matter and Dioxins from Weathered Incineration Fly Ash. Aerosol Air Qual. Res. 2017, 17, 2338–2347. [Google Scholar] [CrossRef]
- Masrat, R.; Majid, K. Solubilization of pyrene by mixed polymer-cationic/nonionic surfactant systems: Effect of polymer concentration. Colloids Surf. A 2022, 653, 129974. [Google Scholar] [CrossRef]
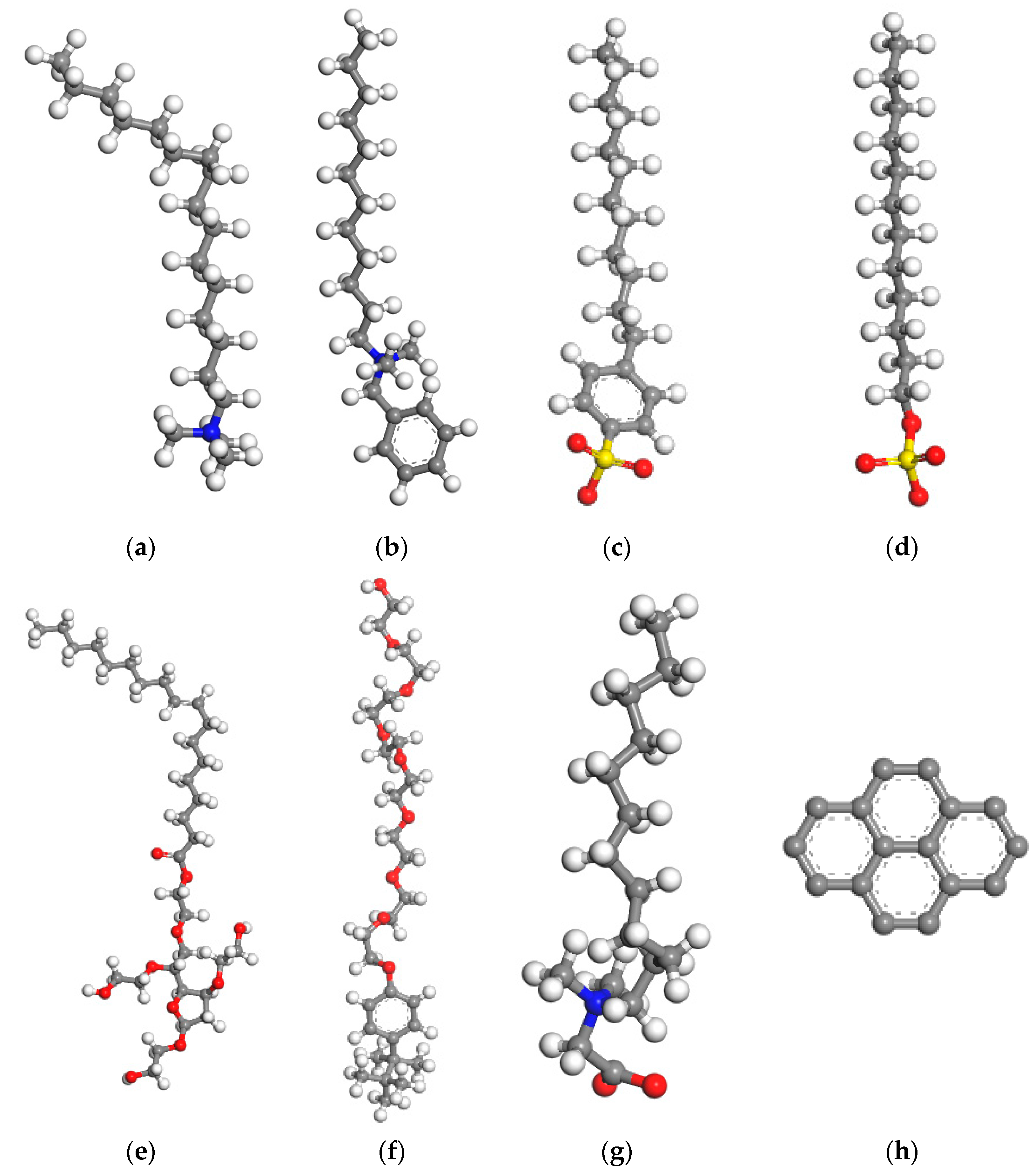




| Collector | Chemical Formulas | Abbreviations |
|---|---|---|
| Pyrene | C16H10 | - |
| Tramadol X-100 | C14H22O(C2H4O)n | TX-100 |
| Sodium dodecyl sulfate | C12H25SO3Na | SDS |
| Hexadecyltrimethylammonium chloride | C19H42ClN | CTAC |
| Dodecyldimethylbenzylammonium chloride | C21H38ClN | DDBAC |
| Lauryl betaine | C16H33NO2 | BS-12 |
| Sodium dodecylbenzene sulfonate | C18H29NaO3S | SDBS |
| Tween 80 | C24H44O6(C2H4O)n | TW 80 |
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | MnO | Loss |
|---|---|---|---|---|---|---|---|---|---|
| 49.625 | 34.221 | 1.135 | 0.036 | 0.131 | 0.101 | 0.081 | 0.625 | 0.015 | 14.03 |
| Systems | Components | Size/(Å × Å × Å) |
|---|---|---|
| 1 | 1000 water + kaolinite + 5 pyrene | 26 × 26 × 45 |
| 2 | 3000 water + 9 surfactants + 5 pyrene | 45 × 45 × 45 |
| 3 | 1000 water + kaolinite + 5 pyrene + 9 surfactants | 26 × 26 × 45 |
| 4 | 1000 water + kaolinite + 5 pyrene + 5 surfactants | 26 × 26 × 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Liu, Y.; Gong, J.; Qiao, E. Study on the Remediation of Pyrene-Contaminated Soil with Surfactants and their Mechanisms. Processes 2023, 11, 2199. https://doi.org/10.3390/pr11072199
Shen L, Liu Y, Gong J, Qiao E. Study on the Remediation of Pyrene-Contaminated Soil with Surfactants and their Mechanisms. Processes. 2023; 11(7):2199. https://doi.org/10.3390/pr11072199
Chicago/Turabian StyleShen, Liang, Yifang Liu, Jiabao Gong, and Erle Qiao. 2023. "Study on the Remediation of Pyrene-Contaminated Soil with Surfactants and their Mechanisms" Processes 11, no. 7: 2199. https://doi.org/10.3390/pr11072199
APA StyleShen, L., Liu, Y., Gong, J., & Qiao, E. (2023). Study on the Remediation of Pyrene-Contaminated Soil with Surfactants and their Mechanisms. Processes, 11(7), 2199. https://doi.org/10.3390/pr11072199








