Study on the Combustion Mechanism of Diesel/Hydrogen Dual Fuel and the Influence of Pilot Injection and Main Injection
Abstract
1. Introduction
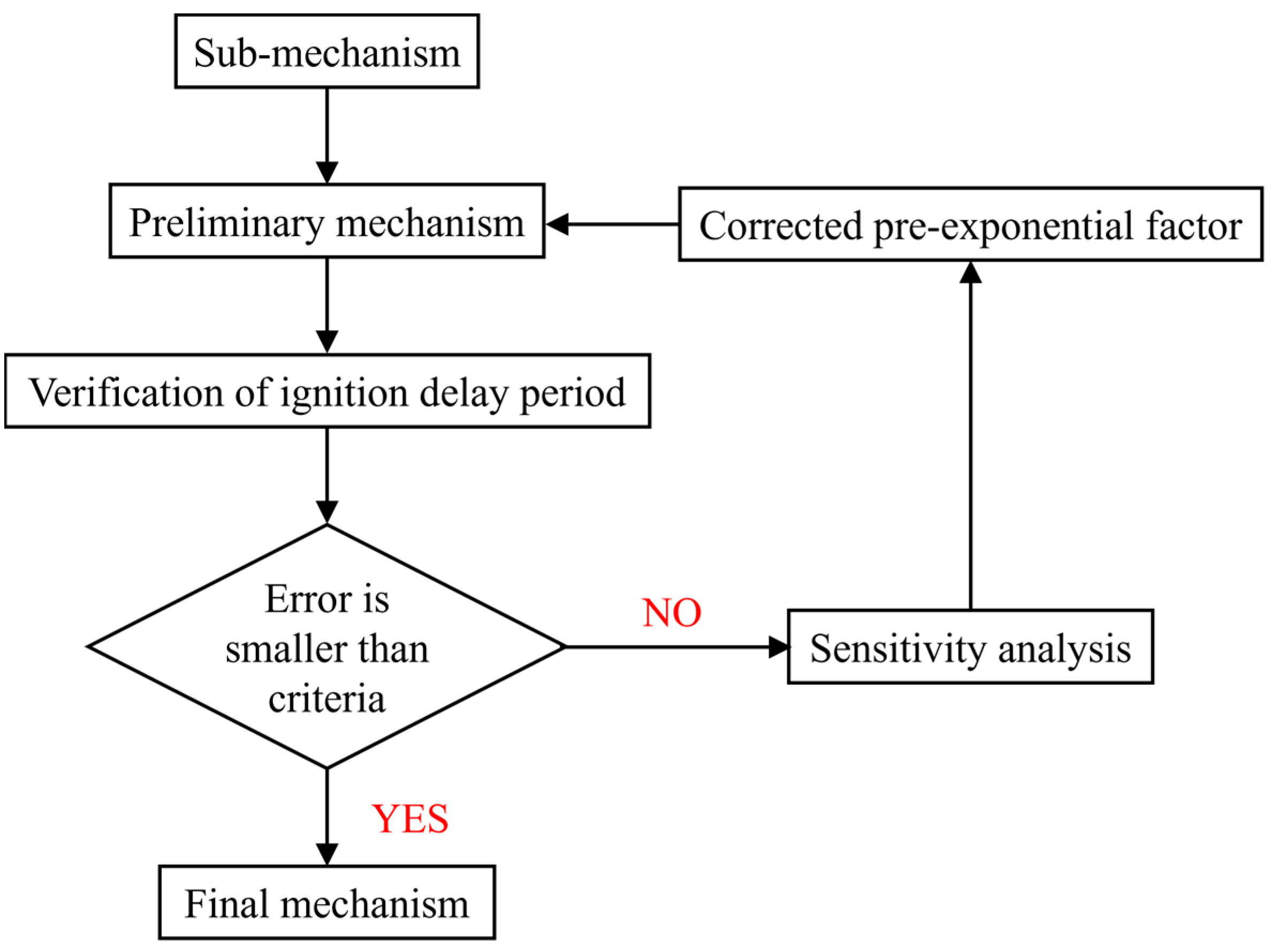
2. Mechanism Development
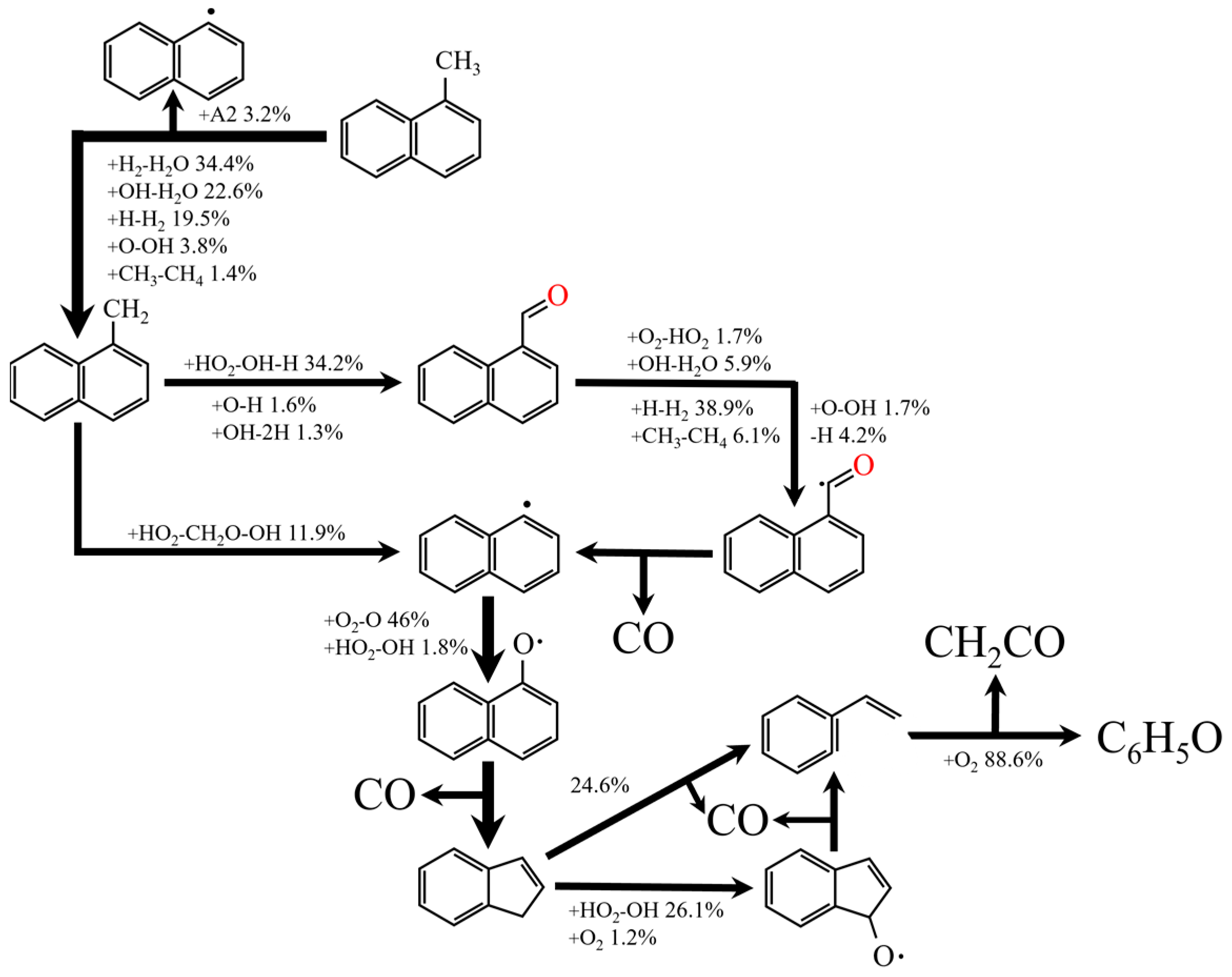
2.1. The Reduce Mechanism of N-Decane and A-Methylnaphthalene
2.2. Construction of Diesel/Hydrogen Dual Fuel Mechanism
3. Mechanism Validation
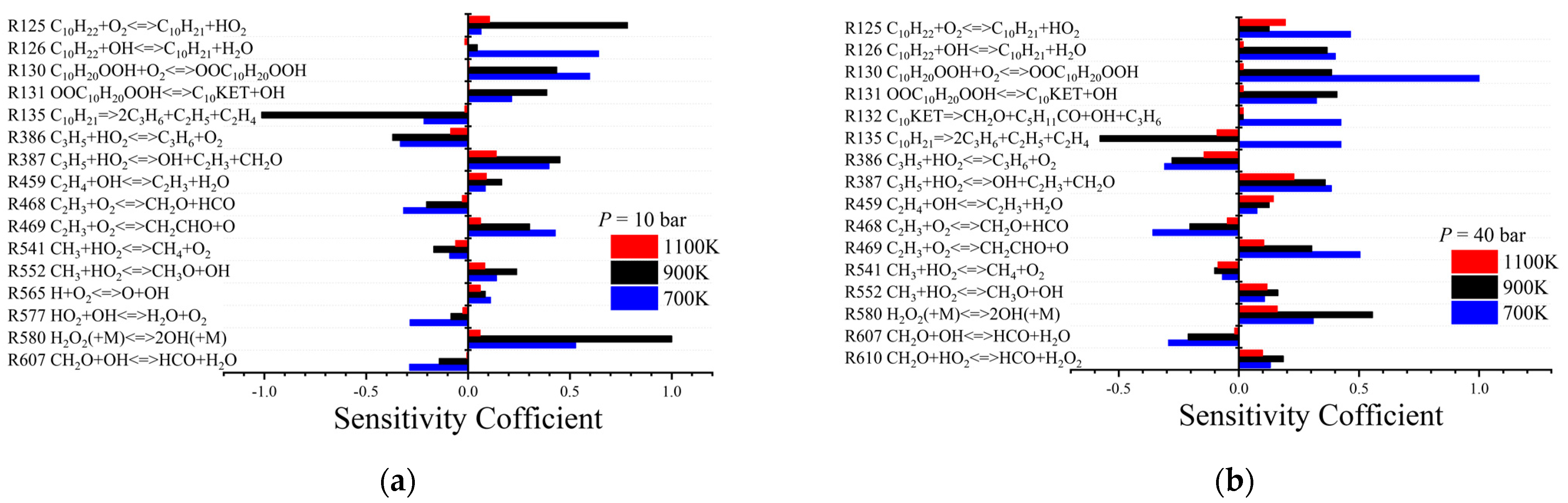
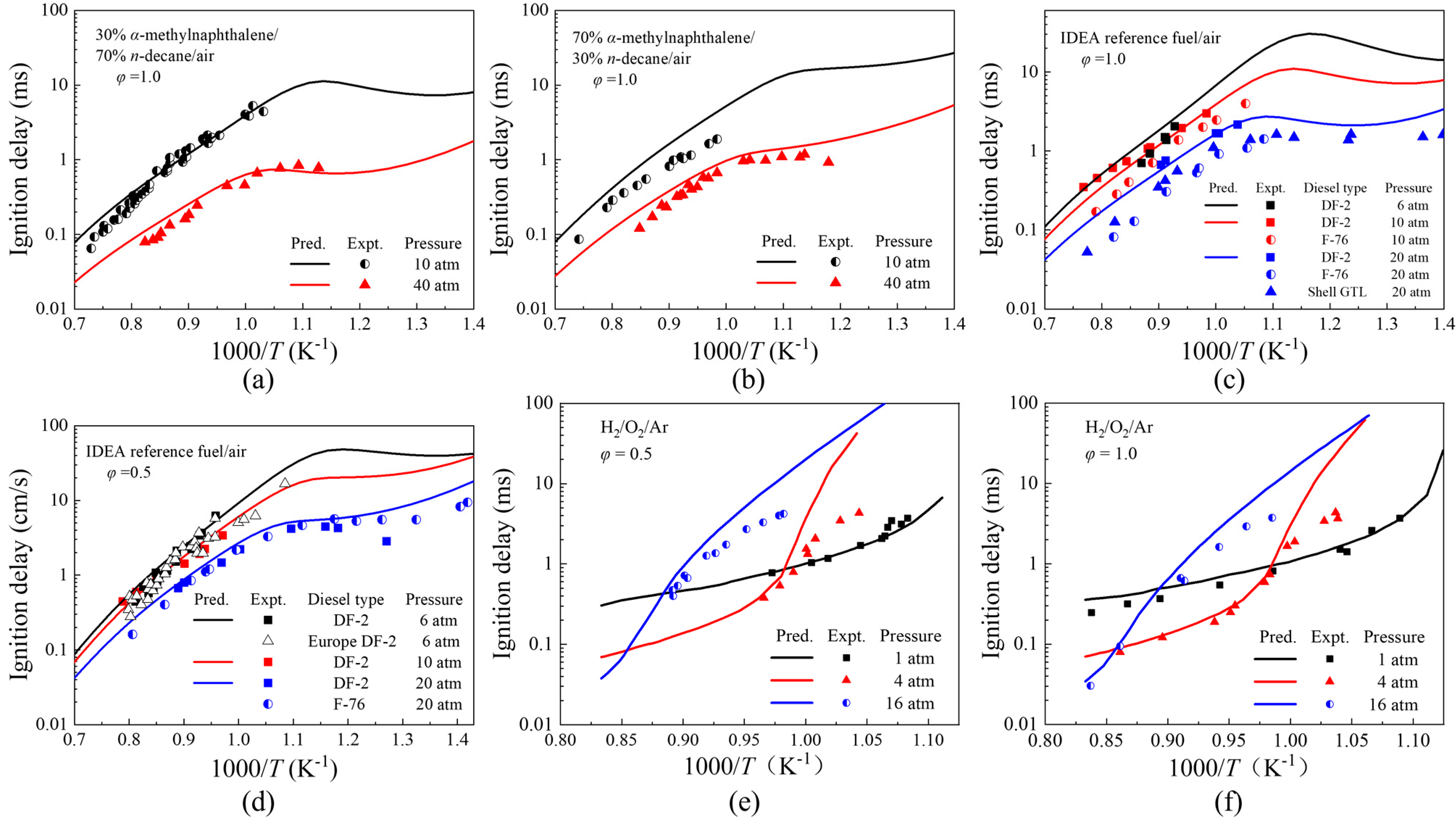
3.1. Validation of Ignition Delays
3.2. Species Concentration Profile
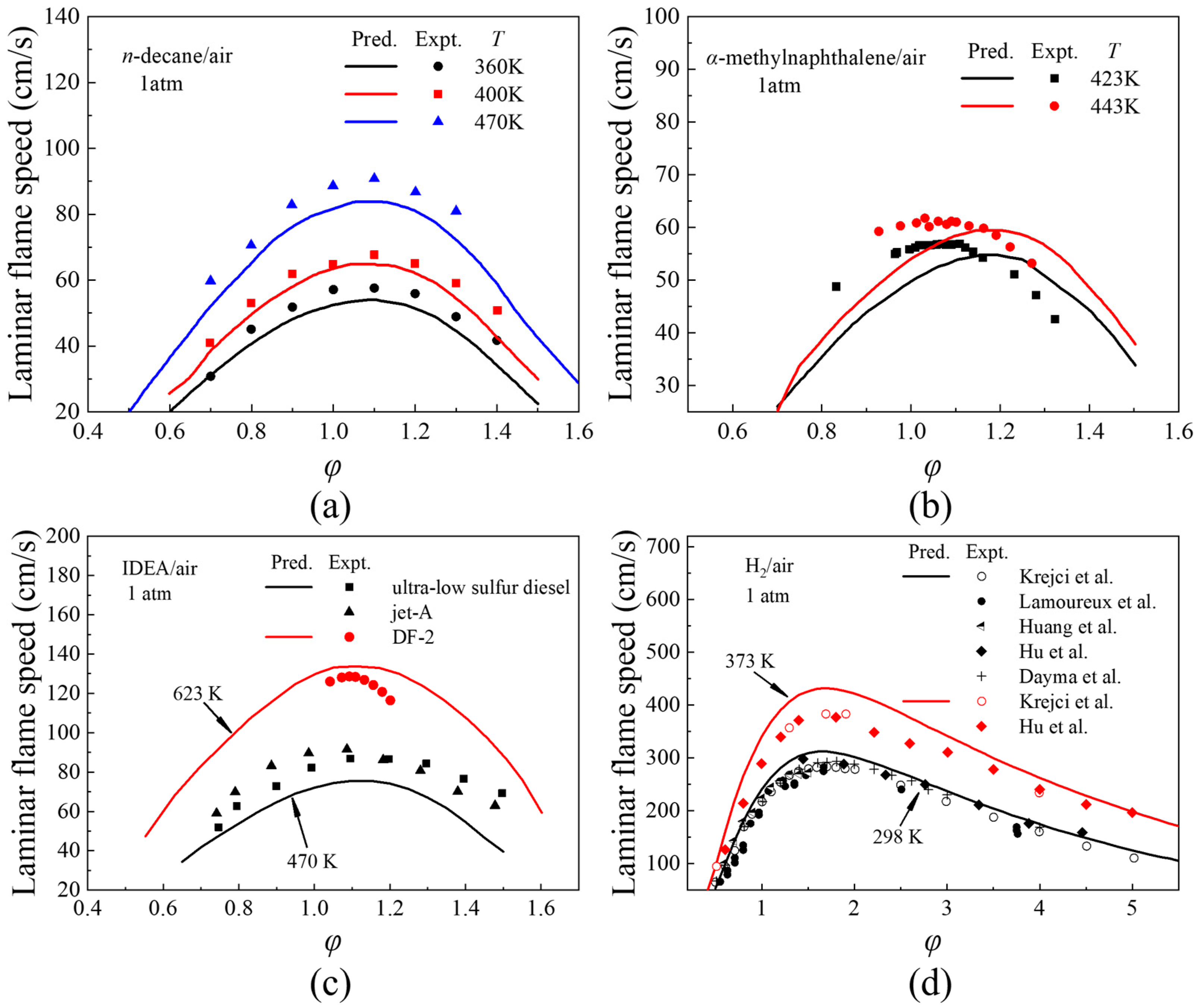
3.3. Laminar Flame Speeds
4. CFD Model Construction and Validation
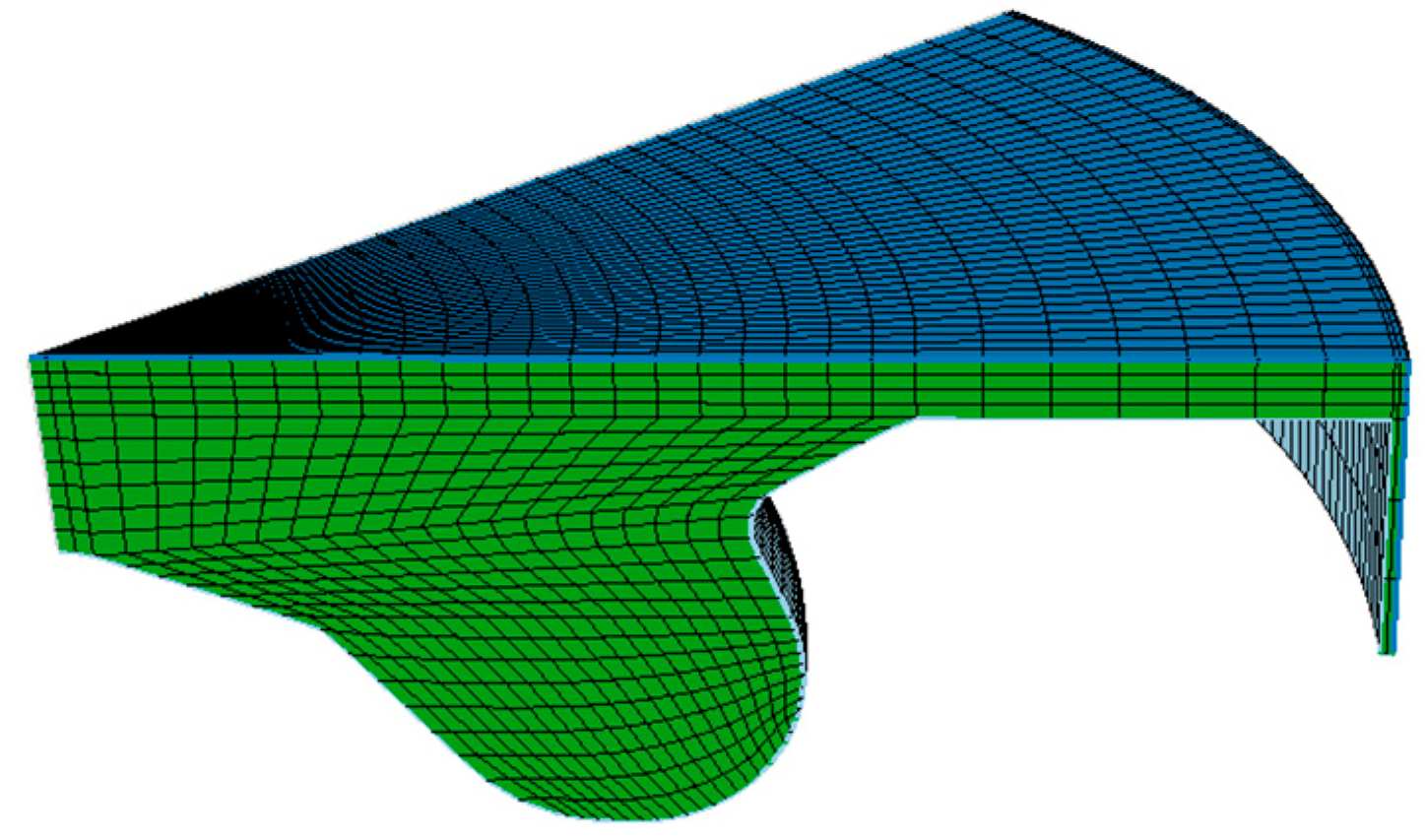
4.1. CFD Model Construction
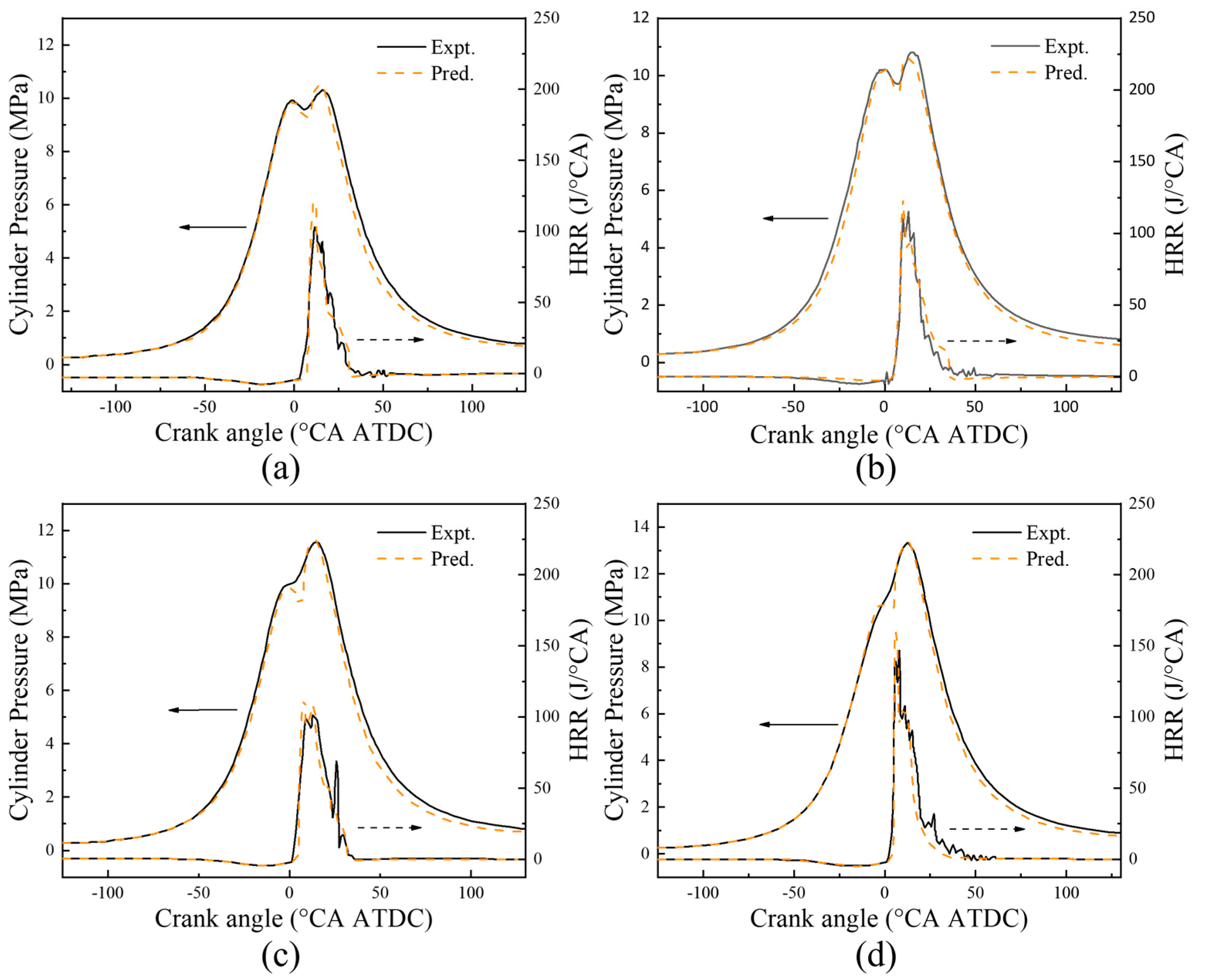
4.2. CFD Model Validation
5. Results and Discussions
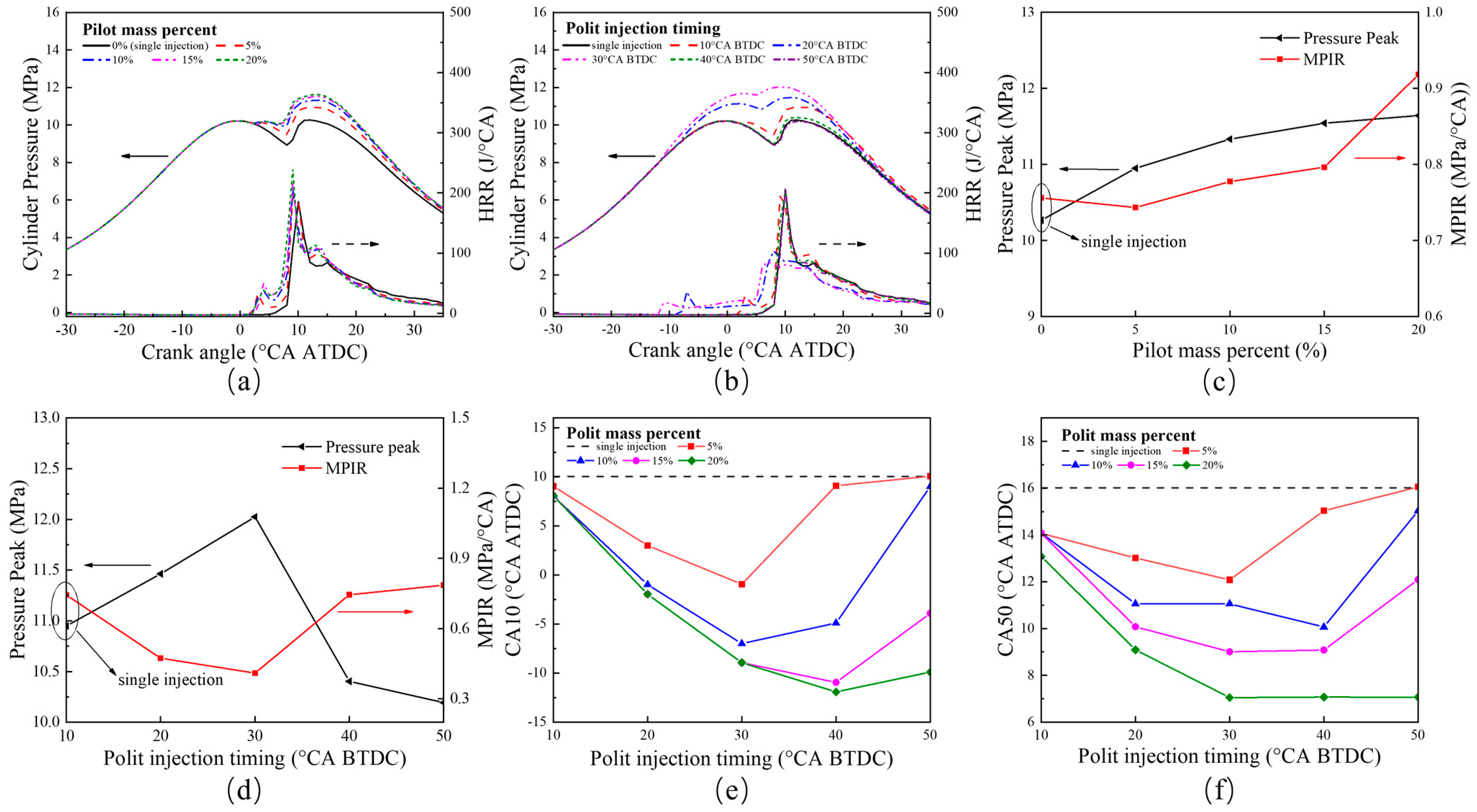
5.1. Effect of Pilot Injection Strategies on Combustion and Emissions Characteristics of Diesel/Hydrogen Dual Fuel Engine
5.1.1. Combustion Characteristics
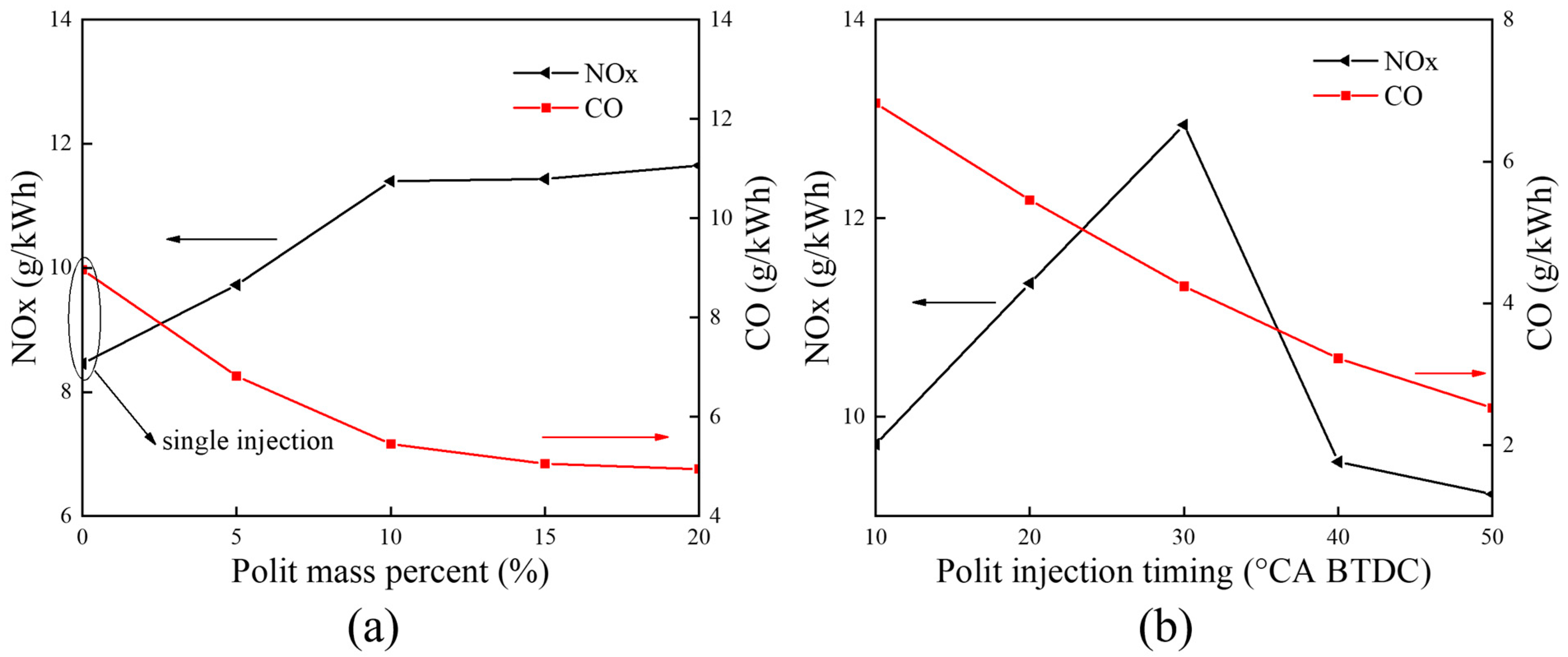
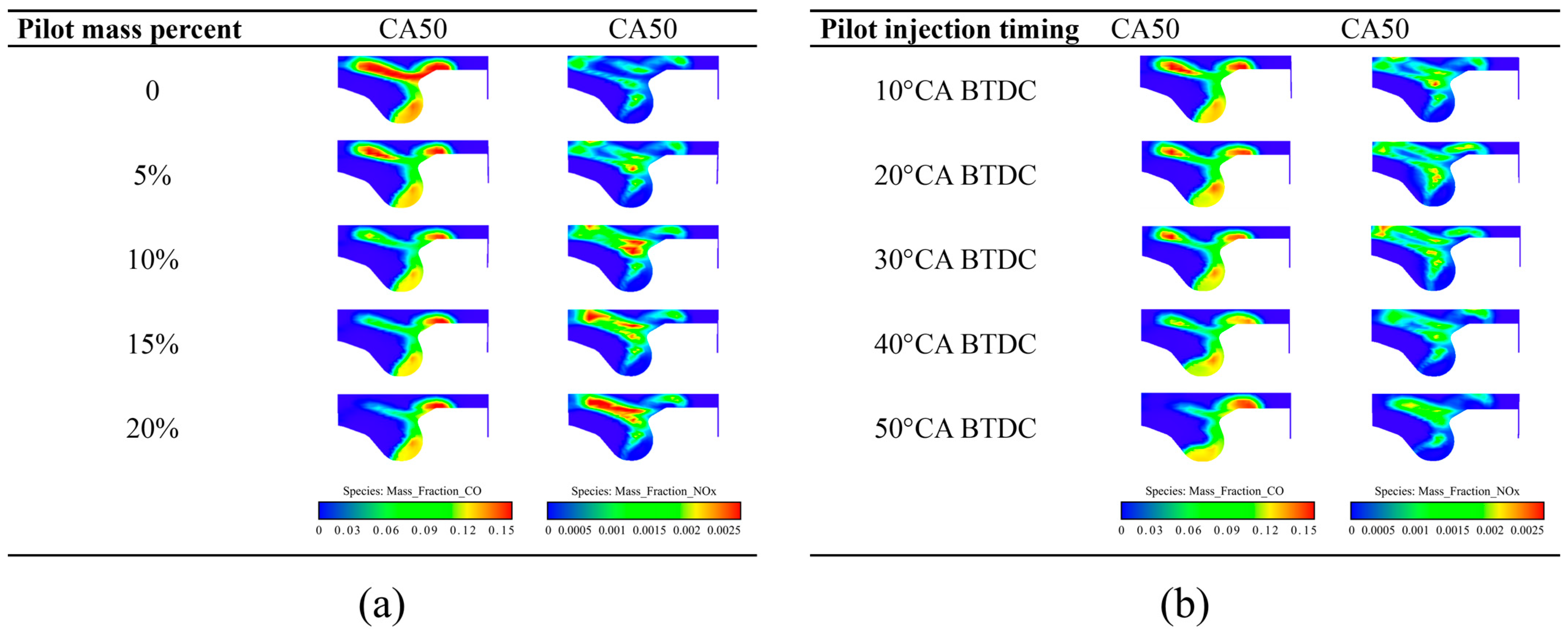
5.1.2. Emission Characteristics
5.2. Effect of Main Injection Timing on Combustion and Emissions Characteristics of Diesel/Hydrogen Dual Fuel Engine
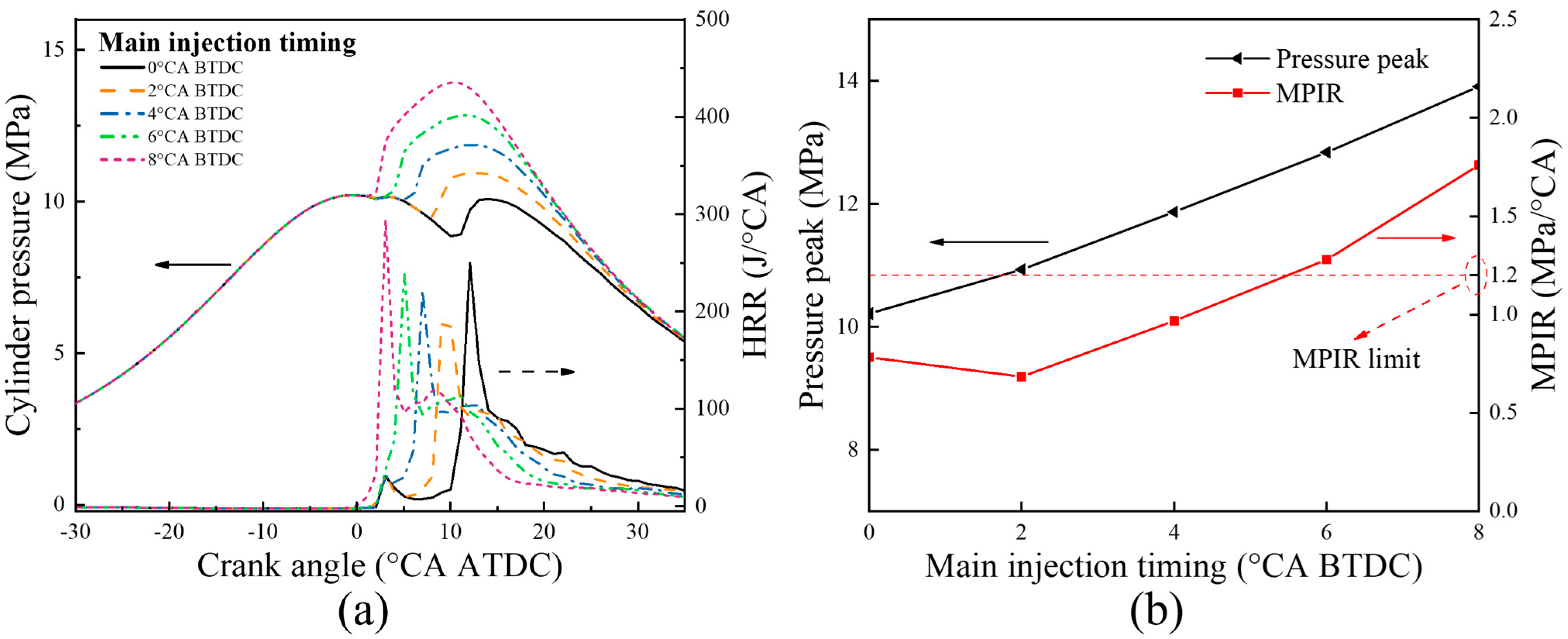
5.2.1. Combustion Characteristics
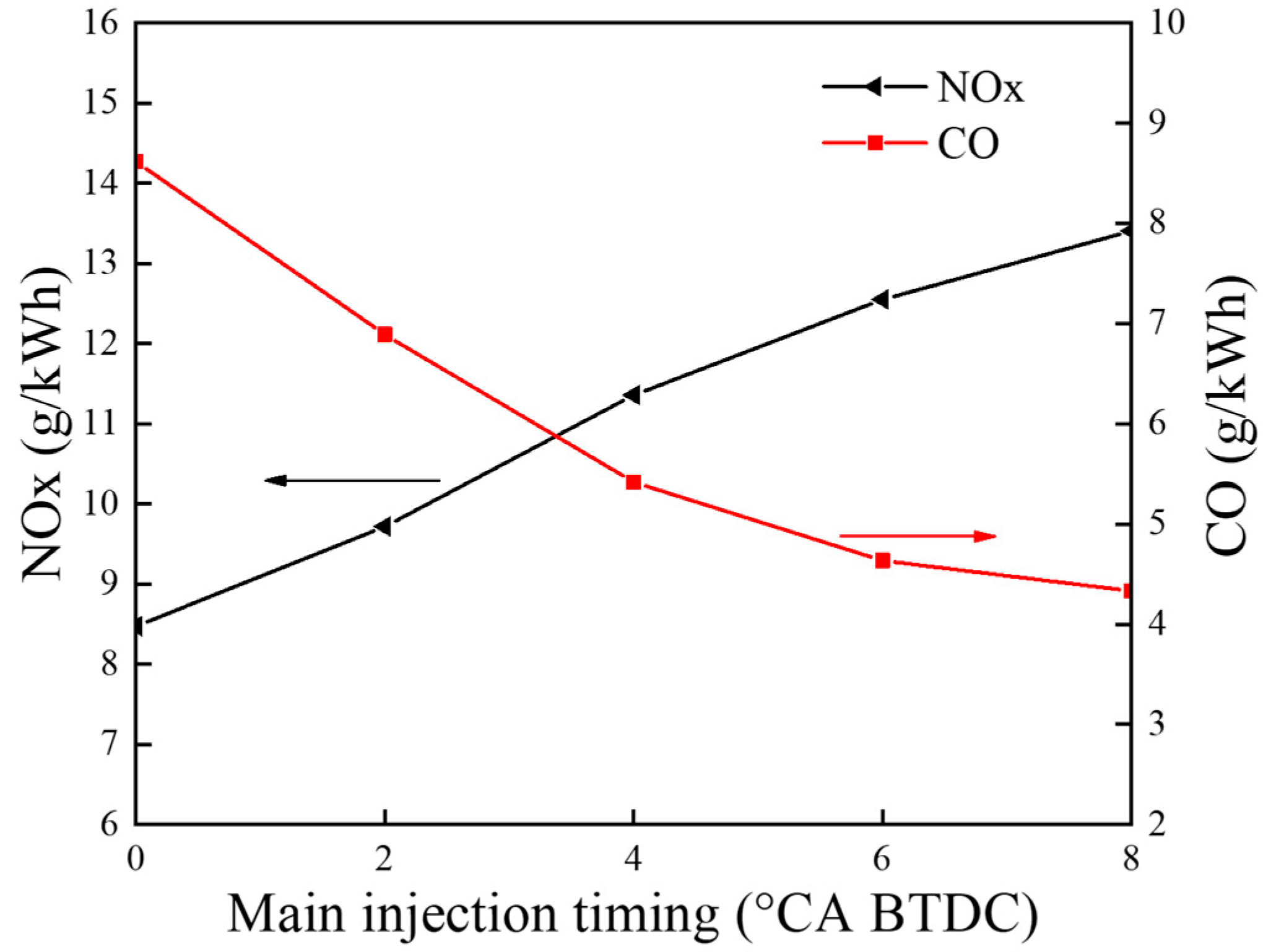
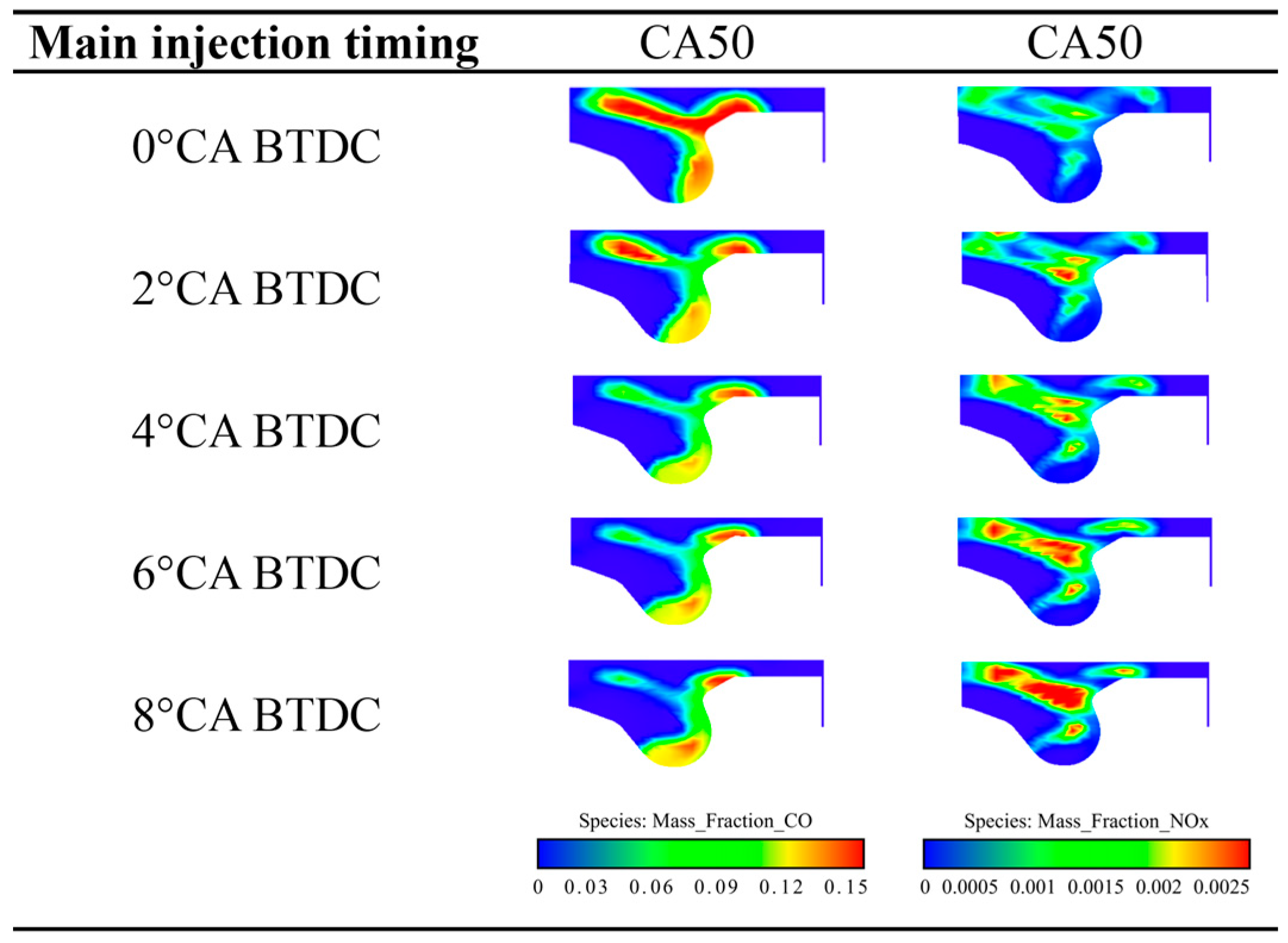
5.2.2. Emission Characteristics
6. Conclusions
- (1)
- 70% n-decane and 30% α-methylnaphthalene (IDEA reference fuel) were selected as diesel substitutes. The sub-mechanisms of n-decane, α-methylnaphthalene, NOX, PAH, soot and H2/C1-C3 were combined to obtain the diesel/hydrogen dual fuel combustion mechanism. A reduced diesel/hydrogen dual fuel combustion mechanism with 191 components and 847 elementary reactions was finally obtained by optimization.
- (2)
- The combustion mechanism of diesel/hydrogen dual fuel was verified by ignition delay, JSR and laminar flame speed. In the verification of the ignition delay time of n-decane/α-methylnaphthalene blends and IDEA reference fuel for diesel and hydrogen, the average error is within an order of magnitude; in the JSR oxidation verification of diesel and hydrogen by IDEA reference fuel, the average error is less than 6%. In the laminar flame verification of diesel and hydrogen with n-decane, α-methylnaphthalene and IDEA reference fuel, the average error is less than 16%.
- (3)
- The reduced diesel/hydrogen dual fuel mechanism is coupled with the three-dimensional CFD model to verify the combustion and emission characteristics of the dual fuel engine under four operation conditions. The results show that the variation trend of in-cylinder pressure and combustion heat release rate is consistent with the simulated value, and the average error is less than 5%. The variation trend of NOX, CO and HC emissions is consistent with the simulated values, and the average error is less than 17%.
- (4)
- With the increase in the pilot injection quantity, the peak heat release rate, the peak cylinder pressure and MPIR show an upward trend, the corresponding phase of CA10 and CA50 is advanced, the CO emissions is reduced, and the NOX emission is increased. With the advance of pilot injection timing, the peak value of cylinder pressure increases first and then decreases, the peak value of MPIR and heat release rate decreases first and then increases, the corresponding phases of CA10 and CA50 advance first and then delay, CO emissions decrease gradually, and NOX emissions increase first and then decrease. With the advance of the main injection timing, the peak heat release rate and MPIR first decreased slightly and then increased, the peak cylinder pressure increased, the corresponding phase of the peak heat release rate and the peak cylinder pressure moved forward as a whole, the CO emission decreased, and the NOX emission increased.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Zhang, Z.; Hou, X.; Lv, J.; Lan, G.; Yang, G.; Hu, J. The environmental potential of hydrogen addition as complementation for diesel and biodiesel: A comprehensive review and perspectives. Fuel 2023, 342, 127794. [Google Scholar] [CrossRef]
- Geng, P.; Cao, E.; Tan, Q.; Wei, L. Effects of alternative fuels on the combustion characteristics and emission products from diesel engines: A review. Renew. Sustain. Energy Rev. 2017, 71, 523–534. [Google Scholar] [CrossRef]
- Wei, L.; Geng, P. A review on natural gas/diesel dual fuel combustion, emissions and performance. Fuel Process. Technol. 2016, 142, 264–278. [Google Scholar] [CrossRef]
- Wei, L.; Yao, C.; Wang, Q.; Wang, P.; Han, G. Combustion and emission characteristics of a turbocharged diesel engine using high premixed ratio of methanol and diesel fuel. Fuel 2015, 140, 156–163. [Google Scholar] [CrossRef]
- Asongu, S.A.; Agboola, M.O.; Alola, A.A.; Bekun, F.V. The criticality of growth, urbanization, electricity and fossil fuel consumption to environment sustainability in Africa. Sci. Total Environ. 2020, 712, 136376. [Google Scholar] [CrossRef]
- Ike, G.N.; Usman, O.; Sarkodie, S.A. Fiscal policy and CO2 emissions from heterogeneous fuel sources in Thailand: Evidence from multiple structural breaks cointegration test. Sci. Total Environ. 2020, 702, 134711. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Jones, M.W.; O‘sullivan, M.; Andrew, R.M.; Bakker, D.C.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. J. Earth Syst. Sci. 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Chen, J.; Jiaqiang, E.; Kang, S.; Zhao, X.; Zhu, H.; Deng, Y.; Peng, Q.; Zhang, Z. Modeling and characterization of the mass transfer and thermal mechanics of the power lithium manganate battery under charging process. Energy 2019, 187, 115924. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, J.; Lv, J.; Xu, W.; Tan, D.; Jiang, F.; Huang, H. Investigation on the effects of non-uniform porosity catalyst on SCR characteristic based on the field synergy analysis. J. Environ. Chem. Eng. 2022, 10, 107056. [Google Scholar] [CrossRef]
- Song, X.; Zhu, T.; Pan, D.; Wang, Z.; Ji, C.; Zhao, D. Numerical investigations on the beating behavior of self-excited combustion instability in a hydrogen-fueled Rijke type combustor. Aerosp. Sci. Technol. 2022, 126, 107624. [Google Scholar] [CrossRef]
- Zhao, D.; Li, X. Minimizing transient energy growth of nonlinear thermoacoustic oscillations. Int. J. Heat Mass Transf. 2015, 81, 188–197. [Google Scholar] [CrossRef]
- Choi, W.; Yoo, E.; Seol, E.; Kim, M.; Song, H. Greenhouse gas emissions of conventional and alternative vehicles: Predictions based on energy policy analysis in South Korea. Appl. Energy 2020, 265, 114754. [Google Scholar] [CrossRef]
- Falcone, P.M.; Hiete, M.; Sapio, A. Hydrogen economy and sustainable development goals: Review and policy insights. Curr. Opin. Green Sustain. Chem. 2021, 31, 100506. [Google Scholar] [CrossRef]
- Hafner, M.; Raimondi, P.P. Priorities and challenges of the EU energy transition: From the European Green Package to the new Green Deal. Russ. J. Econ. 2020, 6, 374–389. [Google Scholar] [CrossRef]
- Chapman, A.; Itaoka, K.; Farabi-Asl, H.; Fujii, Y.; Nakahara, M. Societal penetration of hydrogen into the future energy system: Impacts of policy, technology and carbon targets. Int. J. Hydrogen Energy 2020, 45, 3883–3898. [Google Scholar] [CrossRef]
- Tang, O.; Rehme, J.; Cerin, P.; Huisingh, D. Hydrogen production in the Swedish power sector: Considering operational volatilities and long-term uncertainties. Energy Policy 2021, 148, 111990. [Google Scholar] [CrossRef]
- Edwards, R.L.; Font-Palma, C.; Howe, J. The status of hydrogen technologies in the UK: A multi-disciplinary review. Sustain. Energy Technol. Assess. 2021, 43, 100901. [Google Scholar] [CrossRef]
- Ciniviz, M.; Köse, H. Hydrogen use in internal combustion engine: A review. Int. J. Automot. Eng. Technol. 2012, 1, 1–15. [Google Scholar]
- Kumar, V.; Gupta, D.; Kumar, N. Hydrogen use in internal combustion engine: A review. Int. J. Adv. Cult. Technol. 2015, 3, 87–99. [Google Scholar] [CrossRef]
- Saravanan, N.; Nagarajan, G.; Dhanasekaran, C.; Kalaiselvan, K.M. Experimental investigation of hydrogen port fuel injection in DI diesel engine. Int. J. Hydrogen Energy 2007, 32, 4071–4080. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, Z.; Jia, C.; Duan, J.; Wang, L. Experimental study on combustion characteristics of diesel-hydrogen dual-fuel engine. J. Therm. Anal. Calorim. 2020, 142, 1483–1491. [Google Scholar] [CrossRef]
- Luo, J.; Liu, Z.; Wang, J.; Xu, H.; Tie, Y.; Yang, D.; Zhang, Z.; Zhang, C.; Wang, H. Investigation of hydrogen addition on the combustion, performance, and emission characteristics of a heavy-duty engine fueled with diesel/natural gas. Energy 2022, 260, 125082. [Google Scholar] [CrossRef]
- Juknelevičius, R.; Rimkus, A.; Pukalskas, S.; Matijošius, J. Research of performance and emission indicators of the compression-ignition engine powered by hydrogen-Diesel mixtures. Int. J. Hydrogen Energy 2019, 44, 10129–10138. [Google Scholar] [CrossRef]
- Szwaja, S.; Grab-Rogalinski, K. Hydrogen combustion in a compression ignition diesel engine. Int. J. Hydrogen Energy 2009, 34, 4413–4421. [Google Scholar] [CrossRef]
- Rorimpandey, P.; Yip, H.L.; Srna, A.; Zhai, G.; Wehrfritz, A.; Kook, S.; Hawkes, E.R.; Chan, Q.N. Hydrogen-diesel dual-fuel direct-injection (H2DDI) combustion under compression-ignition engine conditions. Int. J. Hydrogen Energy 2023, 48, 766–783. [Google Scholar] [CrossRef]
- Suzuki, Y.; Tsujimura, T.; Mita, T. The Performance of Multi-Cylinder Hydrogen / Diesel Dual Fuel Engine. SAE Int. J. Engines 2015, 8, 2240–2252. [Google Scholar] [CrossRef]
- Jabbr, A.I.; Gaja, H.; Koylu, U.O. Multi-objective optimization of operating parameters for a H2/Diesel dual-fuel compression-ignition engine. Int. J. Hydrogen Energy 2020, 45, 19965–19975. [Google Scholar] [CrossRef]
- Xu, L.; Chang, Y.; Treacy, M.; Zhou, Y.; Jia, M.; Bai, X. A skeletal chemical kinetic mechanism for ammonia/n-heptane combustion. Fuel 2023, 331, 125830. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Y.; Huang, H.; Chen, Y.; Liu, M.; Lei, H.; Deng, B.; Chen, C. Development of a Diesel/Natural Gas Mechanism Model for the CFD Simulation of Dual-Fuel Engine. ACS Omega 2021, 6, 21543–21555. [Google Scholar] [CrossRef]
- Schuh, S.; Frühhaber, J.; Lauer, T.; Winter, F. A novel dual fuel reaction mechanism for ignition in natural gas–diesel combustion. Energies 2019, 12, 4396. [Google Scholar] [CrossRef]
- Wang, H.; Reitz, R.D.; Yao, M.; Yang, B.; Jiao, Q.; Qiu, L. Development of an n-heptane-n-butanol-PAH mechanism and its application for combustion and soot prediction. Combust. Flame 2013, 160, 504–519. [Google Scholar] [CrossRef]
- Hentschel, W.; Schindler, K.; Haahtele, O. European diesel research idea-experimental results from DI diesel engine investigations. SAE Trans. 1994, 103, 1168–1187. [Google Scholar]
- Barths, H.; Hasse, C.; Peters, N. Computational fluid dynamics modelling of non-premixed combustion in direct injection diesel engines. Int. J. Engine Res. 2000, 1, 249–267. [Google Scholar] [CrossRef]
- Barths, H.; Pitsch, H.; Peters, N. 3d Simulation of Di Diesel Combustion and Pollutant Formation Using a Two-Component Reference Fuel. Oil Gas Sci. Technol. 1999, 54, 233–244. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Liu, Y.; Li, Y.; Xie, M.; Yin, H. Application of a decoupling methodology for development of skeletal oxidation mechanisms for heavy n-alkanes from n-octane to n-hexadecane. Energy Fuels 2013, 27, 3467–3479. [Google Scholar] [CrossRef]
- Mati, K.; Ristori, A.; Pengloan, G.; Dagaut, P. Oxidation of 1-methylnaphthalene at 1–13 atm: Experimental study in a JSR and detailed chemical kinetic modeling. Combust. Sci. Technol. 2007, 179, 1261–1285. [Google Scholar] [CrossRef]
- Wang, H.; Yao, M.; Yue, Z.; Jia, M.; Reitz, R.D. A reduced toluene reference fuel chemical kinetic mechanism for combustion and polycyclic-aromatic hydrocarbon predictions. Combust. Flame 2015, 162, 2390–2404. [Google Scholar] [CrossRef]
- Wang, H.; Dempsey, A.B.; Yao, M.; Jia, M.; Reitz, R.D. Kinetic and Numerical Study on the Effects of Di-tert-butyl Peroxide Additive on the Reactivity of Methanol and Ethanol. Energy Fuels 2014, 28, 5480–5488. [Google Scholar] [CrossRef]
- Slavinskaya, N.A.; Riedel, U.; Dworkin, S.B.; Thomson, M. Detailed numerical modeling of PAH formation and growth in non-premixed ethylene and ethane flames. Combust. Flame 2012, 159, 979–995. [Google Scholar] [CrossRef]
- Frenklach, M.; Wang, H. Detailed modeling of soot particle nucleation and growth. Sympos on Combustion. Soot Form. Combust. 1994, 59, 165–192. [Google Scholar]
- Wang, H.; Warner, S.J.; Oehlschlaeger, M.A.; Bounaceur, R.; Biet, J.; Glaude, P.A.; Battin-Leclerc, F. An experimental and kinetic modeling study of the autoignition of a-methylnaphthalene/air and a methylnaphthalene/n-decane/air mixtures at elevated pressures. Combust. Flame 2010, 157, 1976–1988. [Google Scholar] [CrossRef]
- Alturaifi, S.A.; Rebagay, R.L.; Mathieu, O.; Guo, B.; Petersen, E.L. A shock-tube autoignition study of jet, rocket, and diesel fuels. Energy Fuels 2019, 33, 2516–2525. [Google Scholar] [CrossRef]
- Gowdagiri, S.; Wang, W.; Oehlschlaeger, M.A. A shock tube ignition delay study of conventional diesel fuel and hydroprocessed renewable diesel fuel from algal oil. Fuel 2014, 128, 21–29. [Google Scholar] [CrossRef]
- Haylett, D.R.; Lappas, P.P.; Davidson, D.F.; Hanson, R.K. Application of an aerosol shock tube to the measurement of diesel ignition delay times. Proc. Combust. Inst. 2009, 32, 477–484. [Google Scholar] [CrossRef]
- Herzler, J.; Naumann, C. Shock-tube study of the ignition of methane /ethane /hydrogen mixtures with hydrogen contents from 0% to 100% at different pressures. Proc. Combust. Inst. 2009, 32, 213–220. [Google Scholar] [CrossRef]
- Battin-Leclerc, F. Cleaner Combustion: Developing Detailed Chemical Kinetic Models (Green Energy and Technology); Springer: London, UK, 2013; pp. 183–210. [Google Scholar]
- Ramirez, H.P.; Hadj-Ali, K.; Diévart, P.; Dayma, G.; Togbé, C.; Moréac, G.; Dagaut, P. Kinetics of oxidation of commercial and surrogate diesel fuels in a jet-stirred reactor: Experimental and modeling studies. Energy Fuels 2010, 24, 1668–1676. [Google Scholar] [CrossRef]
- Cong, T.L.; Dagaut, P. Oxidation of H2/CO2 mixtures and effect of hydrogen initial concentration on the combustion of CH4 and CH4/CO2 mixtures: Experiments and modeling. Proc. Combust. Inst 2009, 32, 427–435. [Google Scholar] [CrossRef]
- Zou, C. Basic Combustion Science; Huazhong University of Science and Technology Press: Wuhan, China, 2021; p. 93. [Google Scholar]
- Gu, F.; Huang, Y.; Liu, D. Fundamentals of Combustion; Southeast University Press: Nanjing, China, 2019; pp. 128–133. [Google Scholar]
- Kumar, K.; Sung, C.J. Laminar flame speeds and extinction limits of preheated n-decane/o-2/n-2 and n-dodecane/o-2/n-2 mixtures. Combust. Flame 2007, 51, 209–224. [Google Scholar] [CrossRef]
- Goulier, J.; Ghaumeix, N.; Gourmel, F.; Abid, S.; Dubois, T. Experimental Study of a-methylnaphthalene Oxidation under Engine Relevant Conditions. In Proceedings of the Western States Section of the Combustion Institute (Spring Meeting), Tempe, AZ, USA, 19–20 March 2012; pp. 645–655. [Google Scholar]
- Chong, C.T.; Hochgreb, S. Measurements of laminar flame speeds of liquid fuels: Jet-A1, diesel, palm methyl esters and blends using particle imaging velocimetry. Proc. Combust. Inst. 2011, 33, 979–986. [Google Scholar] [CrossRef]
- Gómez-Meyer, J.S.; Gollahalli, S.R.; Parthasarathy, R.N.; Quiroga, J.E. Laminar flame speed of soy and canola biofuels. CTF Cienc. Tecnol. Futuro 2012, 4, 75–83. [Google Scholar] [CrossRef]
- Krejci, M.C.; Mathieu, O.; Vissotski, A.J.; Ravi, S.; Sikes, T.G.; Petersen, E.L.; Kérmonès, A.; Metcalfe, W.; Curran, H.J. Laminar Flame Speed and Ignition Delay Time Data for the Kinetic Modeling of Hydrogen and Syngas Fuel Blends. In Proceedings of the ASME Turbo Expo 2012: Turbine Technical Conference and Exposition, Copenhagen, Denmark, 11–15 June 2012; pp. 931–952. [Google Scholar]
- Lamoureux, N.; Djebaili-Chaumeix, N.; Paillard, C.E. Laminar flame velocity determination for H2-air-He-CO2 mixtures using the spherical bomb method. Exp. Therm. Fluid Sci. 2003, 27, 385–393. [Google Scholar] [CrossRef]
- Scharl, V.; Sattelmayer, T. Ignition and combustion characteristics of diesel piloted ammonia injections. Fuel 2022, 11, 100068. [Google Scholar] [CrossRef]
- Førby, N.; Thomsen, T.B.; Cordtz, R.F.; Bræstrup, F.; Schramm, J. Ignition and combustion study of premixed ammonia using GDI pilot injection in CI engine. Fuel 2023, 331, 125728. [Google Scholar] [CrossRef]
- Kessharwani, A.; Gupta, R. Evaluation of performance and emission characteristics of a diesel engine using split injection. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 1–14. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jung, D.W.; Lim, O.T.; Pyo, Y.D.; Lee, Y.J. Influence of pilot injection on combustion characteristics and emissions in a DI diesel engine fueled with diesel and DME. Int. J. Automot. Technol. 2014, 15, 861–869. [Google Scholar] [CrossRef]
- Sudarmanta, B.; Yuvenda, D.; Ary Bachtiar, K.P.; Wahjudi, A.; Trihatmojo, A.A. Effect of pilot injection timing using crude palm oil biodiesel on combustion process on dual fuel engines with compressed natural gas as the main fuel. Int. J. Sustain. Eng. 2021, 14, 2097–2113. [Google Scholar] [CrossRef]
- Wu, B.; Yang, P.; Luo, Y.; Jia, Z. Effects and mechanism of pilot diesel injection strategies on combustion and emissions of natural gas engine. Proc. Inst. Mech. Eng. Part D 2023, 09544070221145738. [Google Scholar]
- Xu, M.; Cheng, W.; Zhang, H.; An, T.; Zhang, S. Effect of diesel pre-injection timing on combustion and emission characteristics of compression ignited natural gas engine. Energy Convers. Manag. 2016, 117, 86–94. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Ning, L.; Ke, Z. Effects of pilot injection timing on the combustion noise and particle emissions of a diesel/natural gas dual-fuel engine at low load. Appl. Therm. Eng. 2016, 102, 822–828. [Google Scholar] [CrossRef]
- Shang, T.; Fan, C.; Fu, Z.; Wei, M.; Wang, T. Combination of pilot injection and a NH3-SCR system to reduce NOx emissions of a nonroad compression ignition engine. ACS Omega 2021, 6, 28871–28879. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, M.S.; Yoon, S.K.; Choi, N.J. Effects of pilot injection timing and EGR on combustion, performance and exhaust emissions in a common rail diesel engine fueled with a canola oil biodiesel-diesel blend. Energies 2015, 8, 7312–7325. [Google Scholar] [CrossRef]





| Type of Fuel | Cetane Number | Low Heating Value | Density at 20 °C (kg/m³) | H/C |
|---|---|---|---|---|
| IDEA fuel | 56 | 42.4 | 817 | 1.7 |
| Diesel | 53 | 42.8 | 840 | 1.76 |
| Elementary Reaction | A | ||
|---|---|---|---|
| Reaction Number | Reaction Chemical Equation | Before Adjustment | After Adjustment |
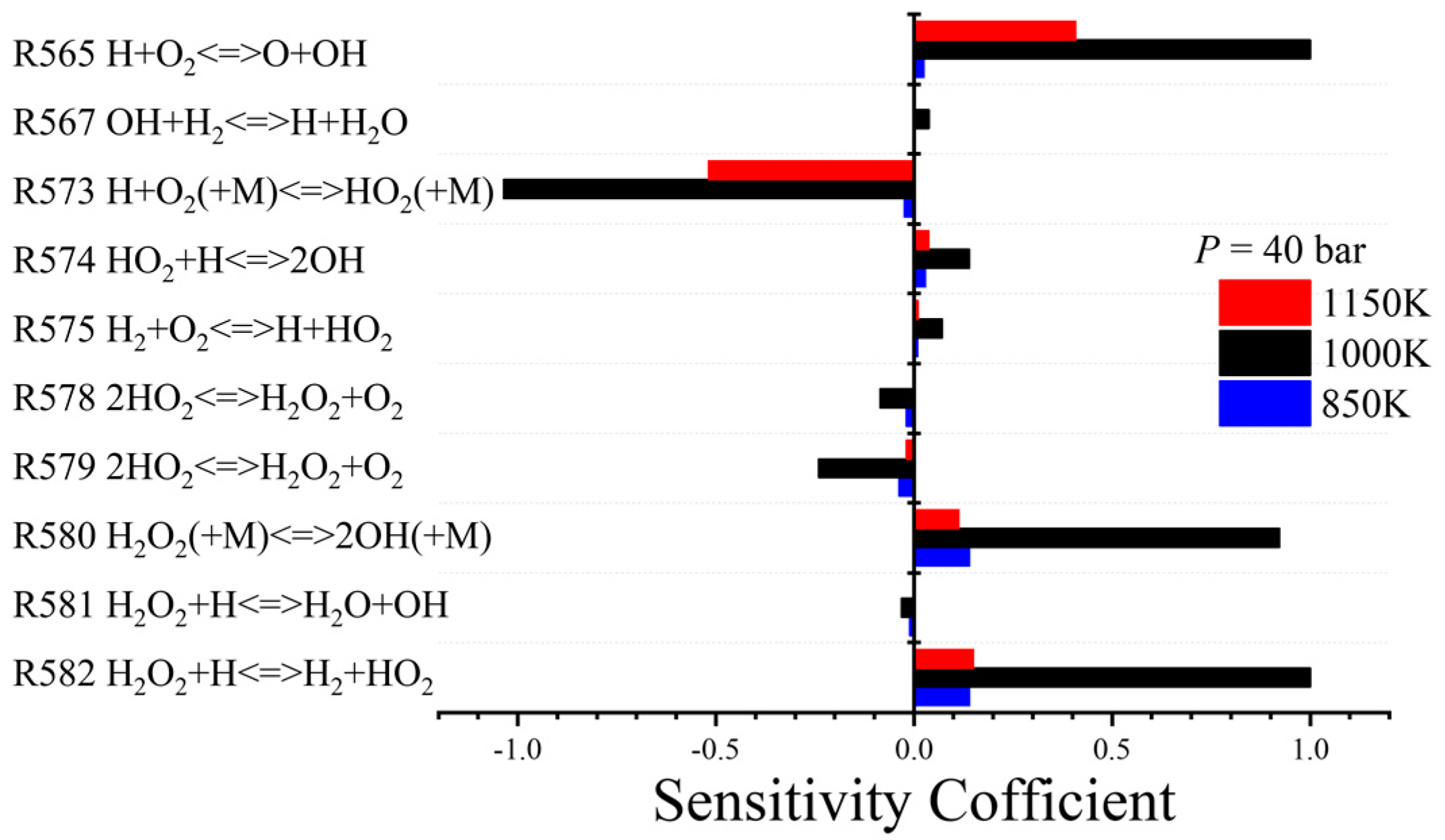
| R565 | H + O2 O + OH | 9.33 × 1013 | 1.90 × 1014 |
| R573 | OH + H2 H + H2O | 1.17 × 109 | 2.20 × 108 |
| R580 | H2O2(+M) 2OH(+M) | 1.30 × 1017 | 1.20 × 1017 |
| R582 | H2O2 + H H2 + HO2 | 1.60 × 1012 | 6.02 × 1013 |
| Model Type | Name |
|---|---|
| Turbulence model | RNG k-ε |
| Droplet collision model | Radius of Influence |
| Spray crushing model | KH-RT |
| Wall oil film model | O’Rourke–Amsden |
| Evaporation model | DMC |
| Wall heat transfer model | Han–Reitz |
| Parameter | Value |
|---|---|
| Engine type | 4 cylinders in line, high-pressure common rail, supercharged medium cold-pressure combustion engine |
| Rated speed (r/min) | 3200 |
| Rated power (kW) | 115 |
| Maximum torque (N·m) | 450 |
| speed at maximum torque (r/min) | 1800 |
| Compression ratio | 16.6:1 |
| Bore×Stroke (mm) | 95 × 105 |
| Displacement (L) | 2.977 |
| Oil atomizer | Bosch, 8-nozzle |
| Parameter | Value |
|---|---|
| Intake pressure (bar) | 2.9 |
| Intake temperature (K) | 390 |
| Turbulent kinetic energy (m2/s2) | 21.6 |
| Turbulent length scale (cm) | 0.483 |
| Swirl ratio | 1.6 |
| Spray angle | 120° |
| Spray cone angle | 12° |
| Nozzle hole number | 8 |
| Nozzle hole diameter (mm) | 0.259 |
| IVC (CA BTDC) | −125° |
| EVO (CA BTDC) | 130° |
| Cylinder head | Wall, 525 K |
| Piston | Mesh movement, 525 K |
| Liner | Wall, 400 K |
| Parameters of Operation | Operation Condition | |||
|---|---|---|---|---|
| a | b | c | d | |
| Engine speed (r/min) | 1800 | 1800 | 1800 | 1800 |
| Torque/(N·m) | 322 | 322 | 365 | 365 |
| Hydrogen replacement rate/% | 0 | 30 | 0 | 27.01 |
| Engine fuel consumption/(kg·h−1) | 12.98 | 8.97 | 14.34 | 10.35 |
| Parameter | Value |
|---|---|
| Rated speed (r/min) | 1800 |
| Torque (N·m) | 322 |
| Hydrogen replacement rate/% | 30% |
| Total injection diesel (mg) | 37.8 |
| Pilot mass percent/% | 0%, 5%, 10%, 15%, 20% |
| Pilot injection timing (CA BTDC) | 10°, 20°, 30°, 40°, 50° |
| Main injection timing (CA BTDC) | 0°, 2°, 4°, 6°, 8° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Dong, H.; Liu, S.; Shen, L.; Bi, Y. Study on the Combustion Mechanism of Diesel/Hydrogen Dual Fuel and the Influence of Pilot Injection and Main Injection. Processes 2023, 11, 2122. https://doi.org/10.3390/pr11072122
Xu L, Dong H, Liu S, Shen L, Bi Y. Study on the Combustion Mechanism of Diesel/Hydrogen Dual Fuel and the Influence of Pilot Injection and Main Injection. Processes. 2023; 11(7):2122. https://doi.org/10.3390/pr11072122
Chicago/Turabian StyleXu, Longlong, Haochuan Dong, Shaohua Liu, Lizhong Shen, and Yuhua Bi. 2023. "Study on the Combustion Mechanism of Diesel/Hydrogen Dual Fuel and the Influence of Pilot Injection and Main Injection" Processes 11, no. 7: 2122. https://doi.org/10.3390/pr11072122
APA StyleXu, L., Dong, H., Liu, S., Shen, L., & Bi, Y. (2023). Study on the Combustion Mechanism of Diesel/Hydrogen Dual Fuel and the Influence of Pilot Injection and Main Injection. Processes, 11(7), 2122. https://doi.org/10.3390/pr11072122





