Investigation of the Interface Effects and Frosting Mechanism of Nanoporous Alumina Sheets
Abstract
1. Introduction
2. Sample Preparation and Characterization
2.1. Preparation of the Nanoporous Alumina Sheets
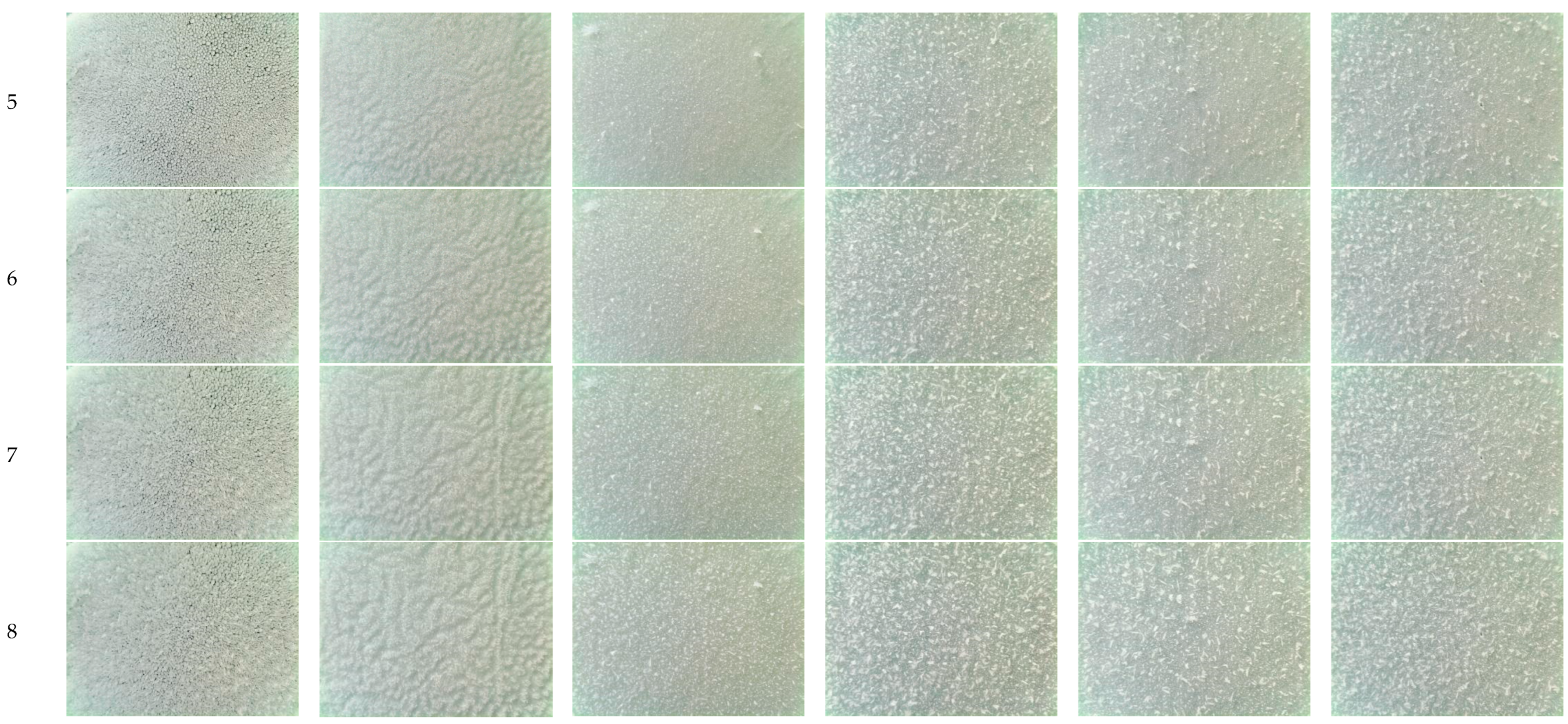
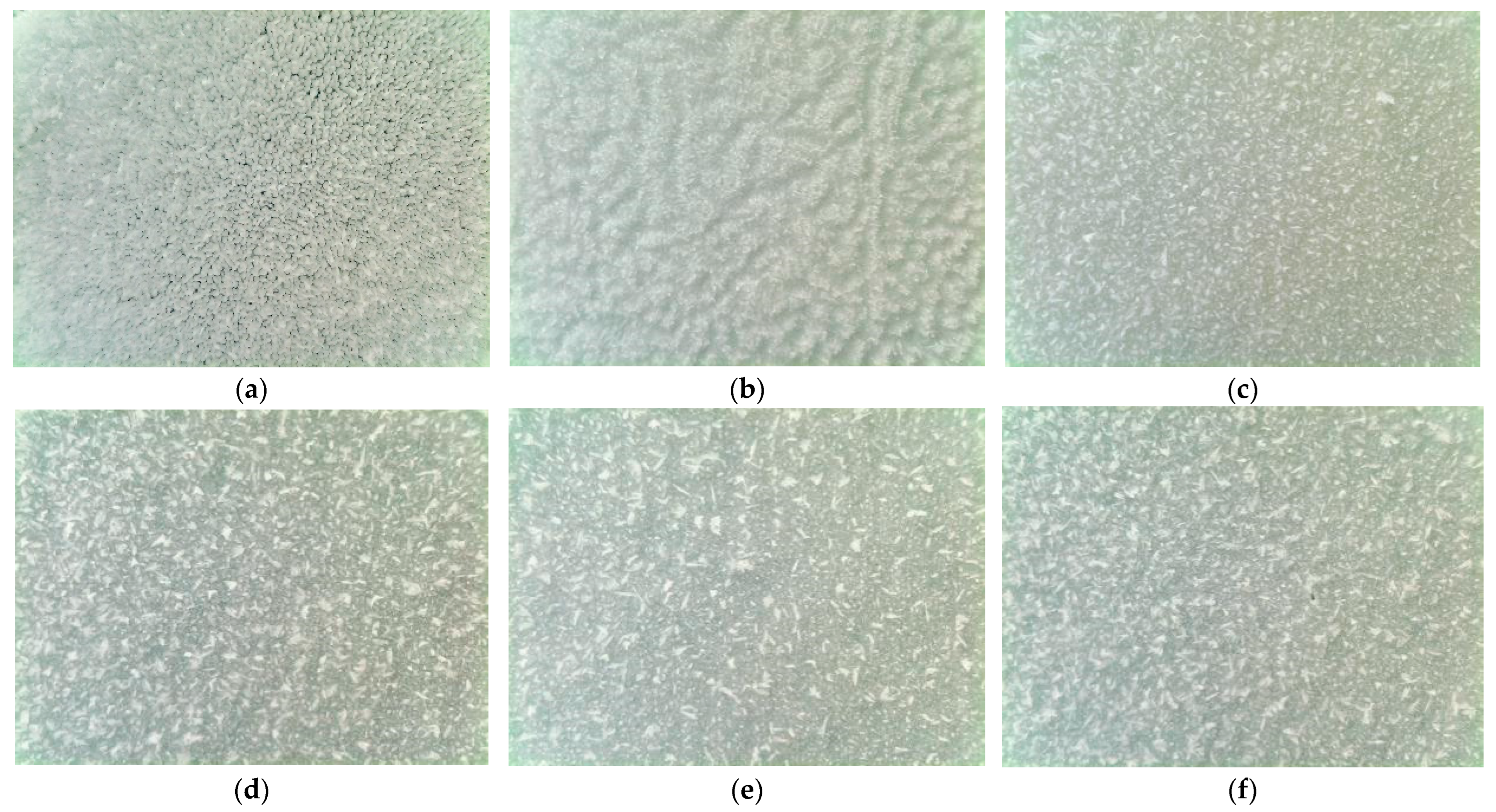
2.2. Surface Topography
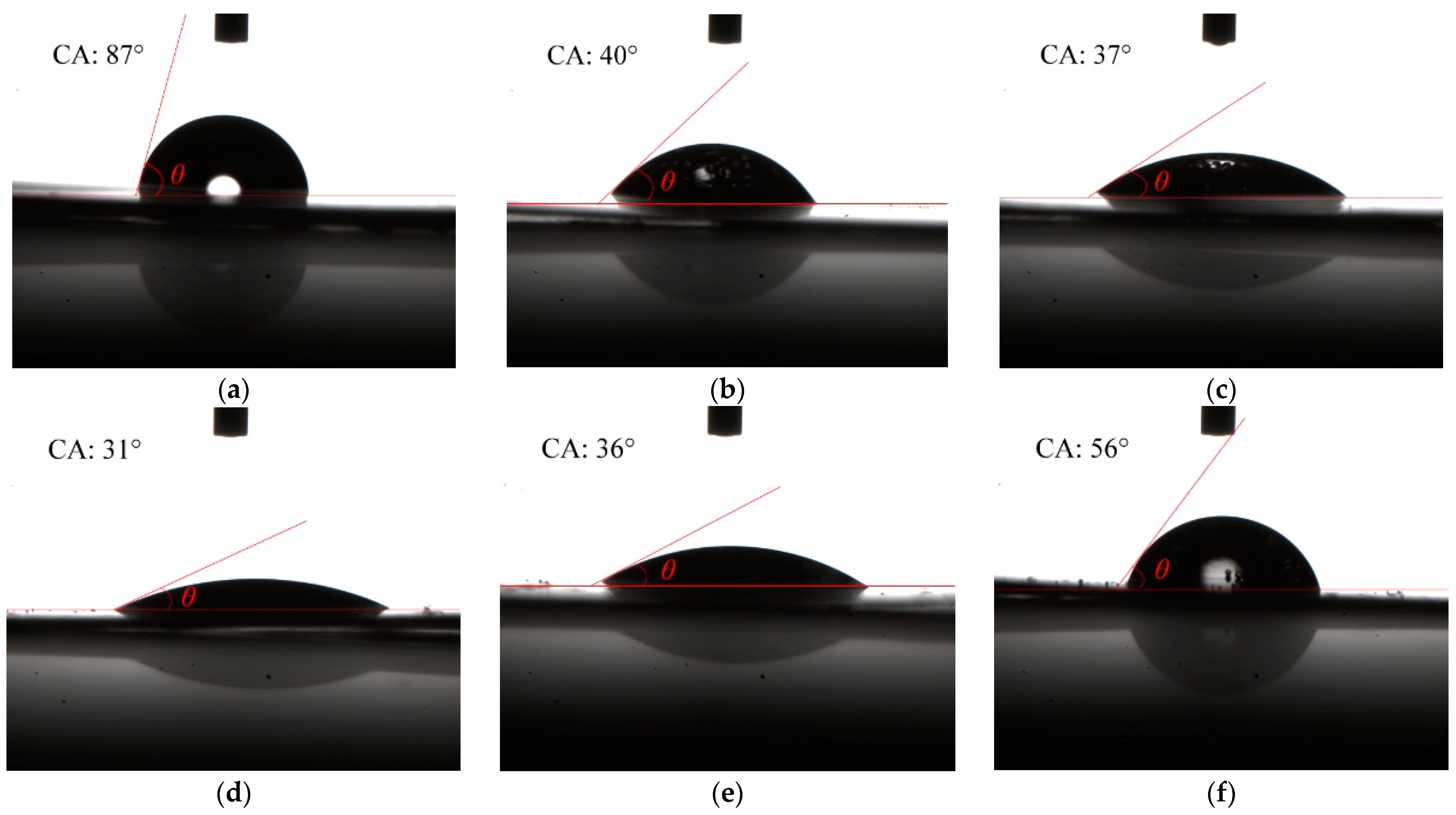
2.3. Contact Angle and Surface Energy
2.4. Surface Fractal Dimension
3. Theoretical Analysis
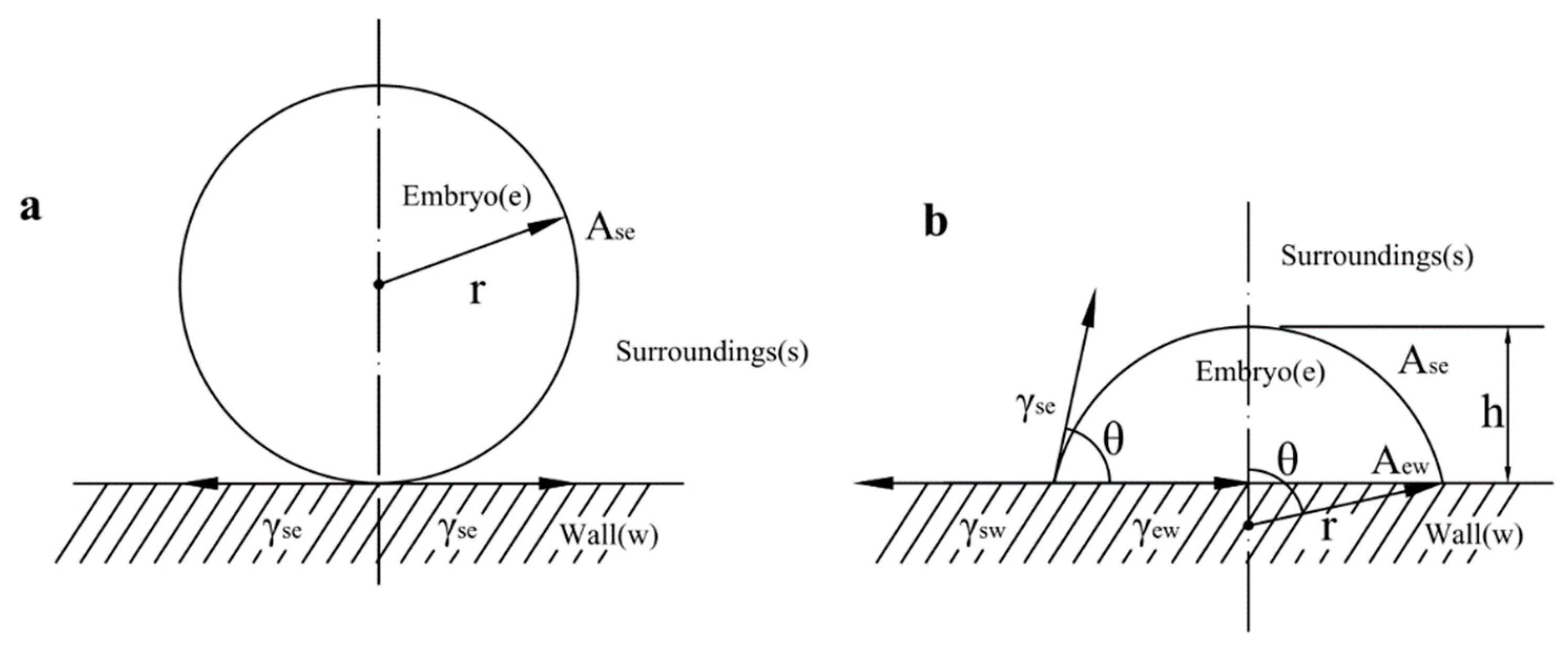
3.1. Thermodynamitical Analysis
3.2. Fractal Dimension Analysis
3.3. Surface Adsorption
4. Experiment and Discussion
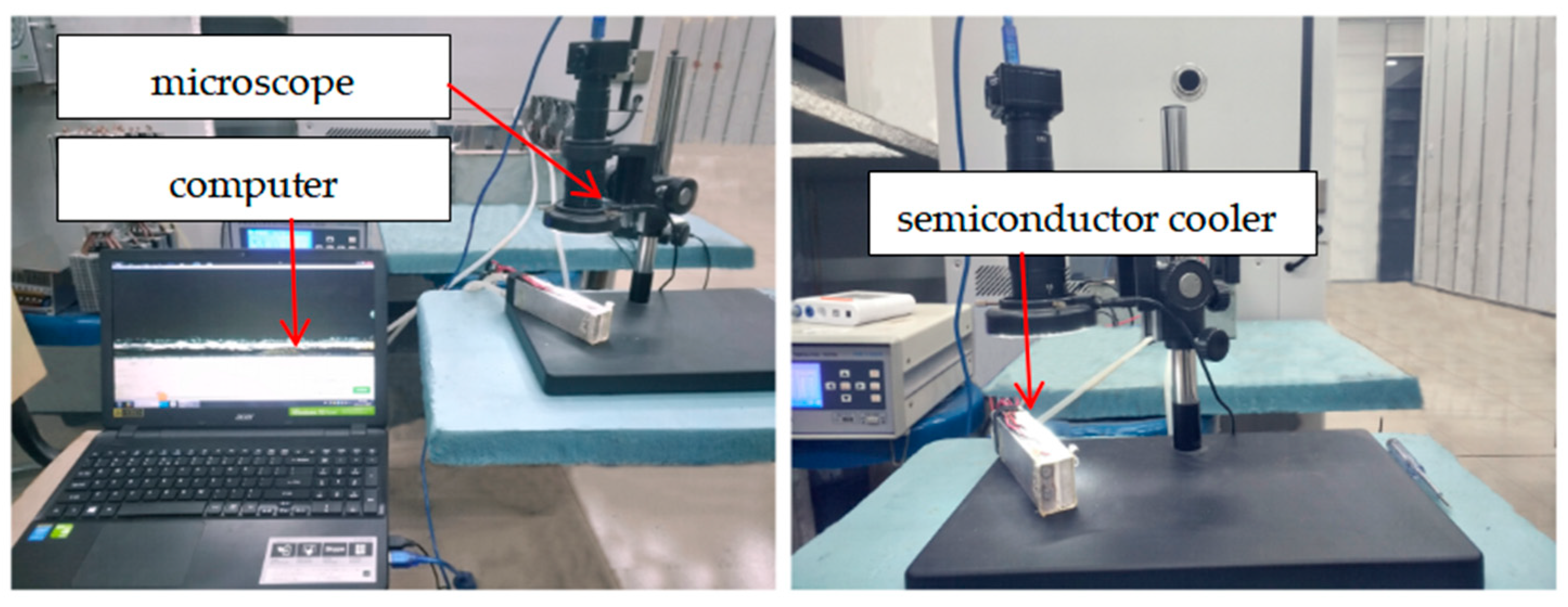
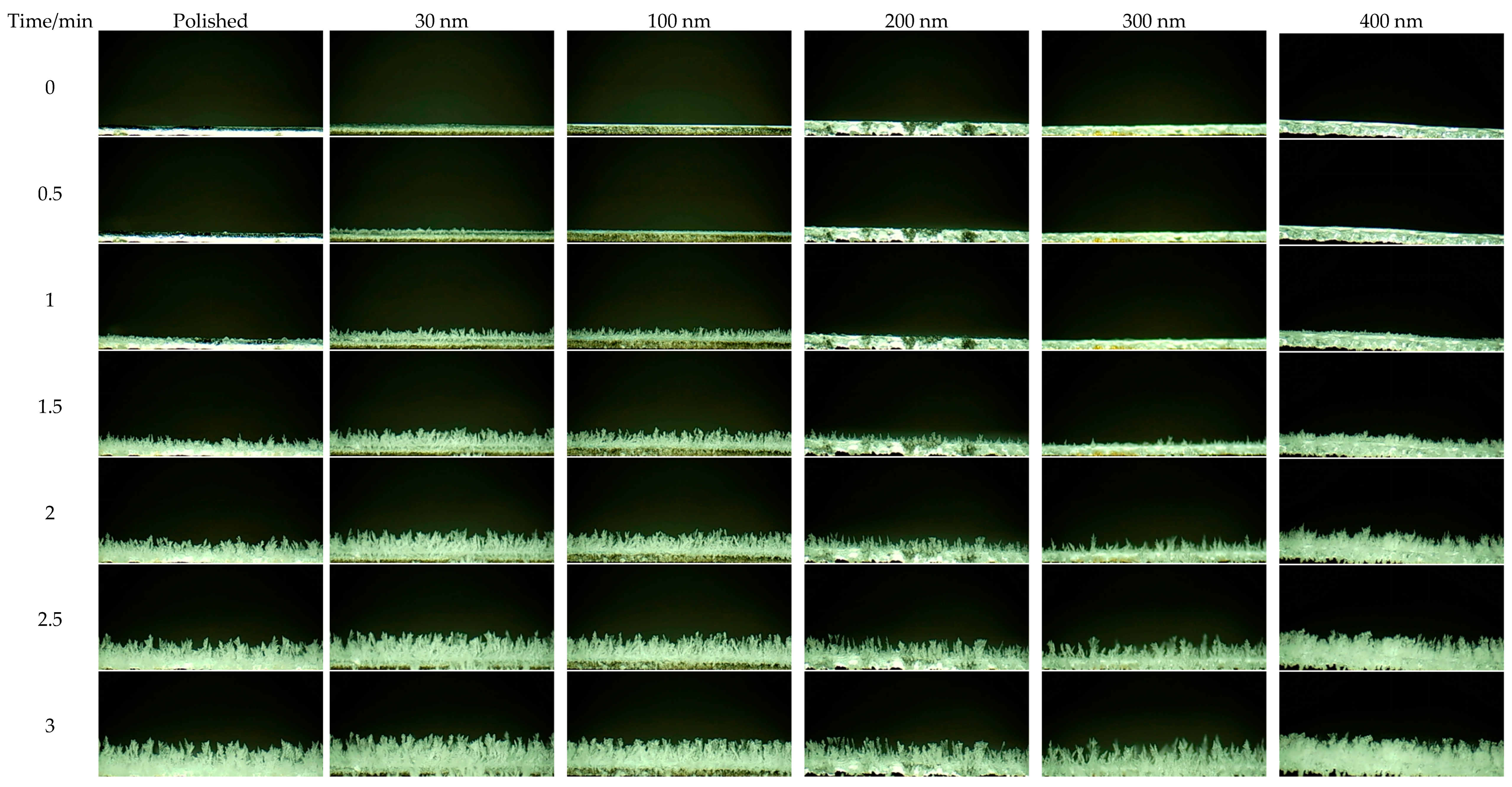
4.1. Experimental Test
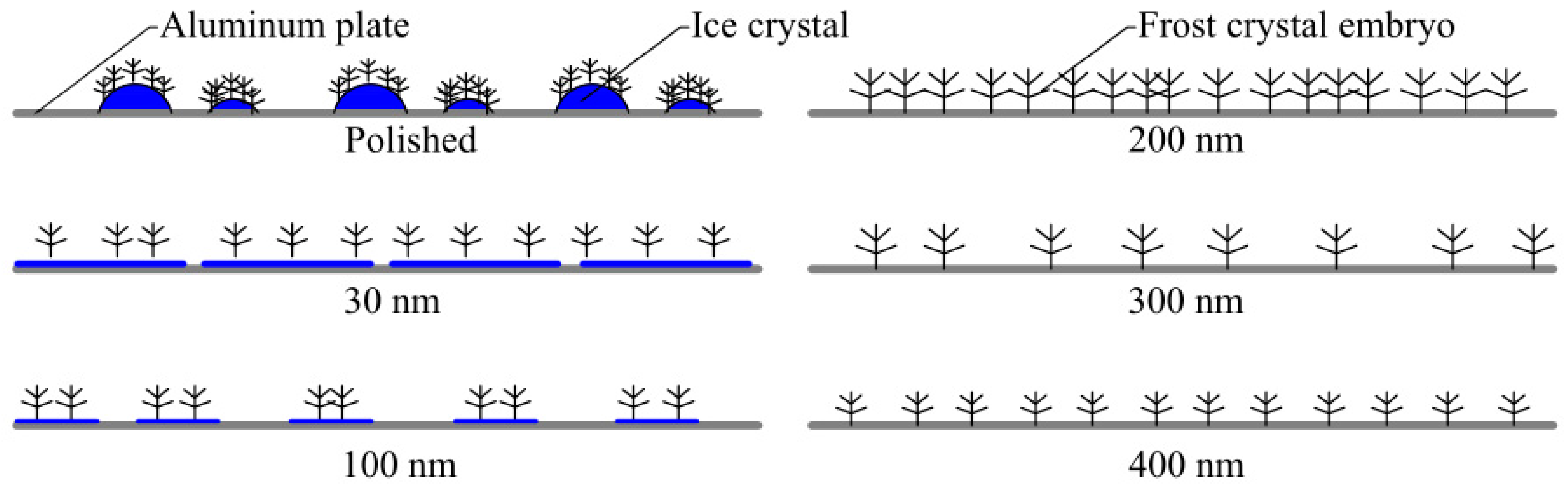
4.2. Interface Effect of Nanoporous Alumina Sheets
- (1)
- The polished aluminum sheet has the best thermal conductivity. The contact area of the surface ice crystal with air is large. Therefore, the polished aluminum sheet has the highest average frosting rate when compared to the other aluminum sheets.
- (2)
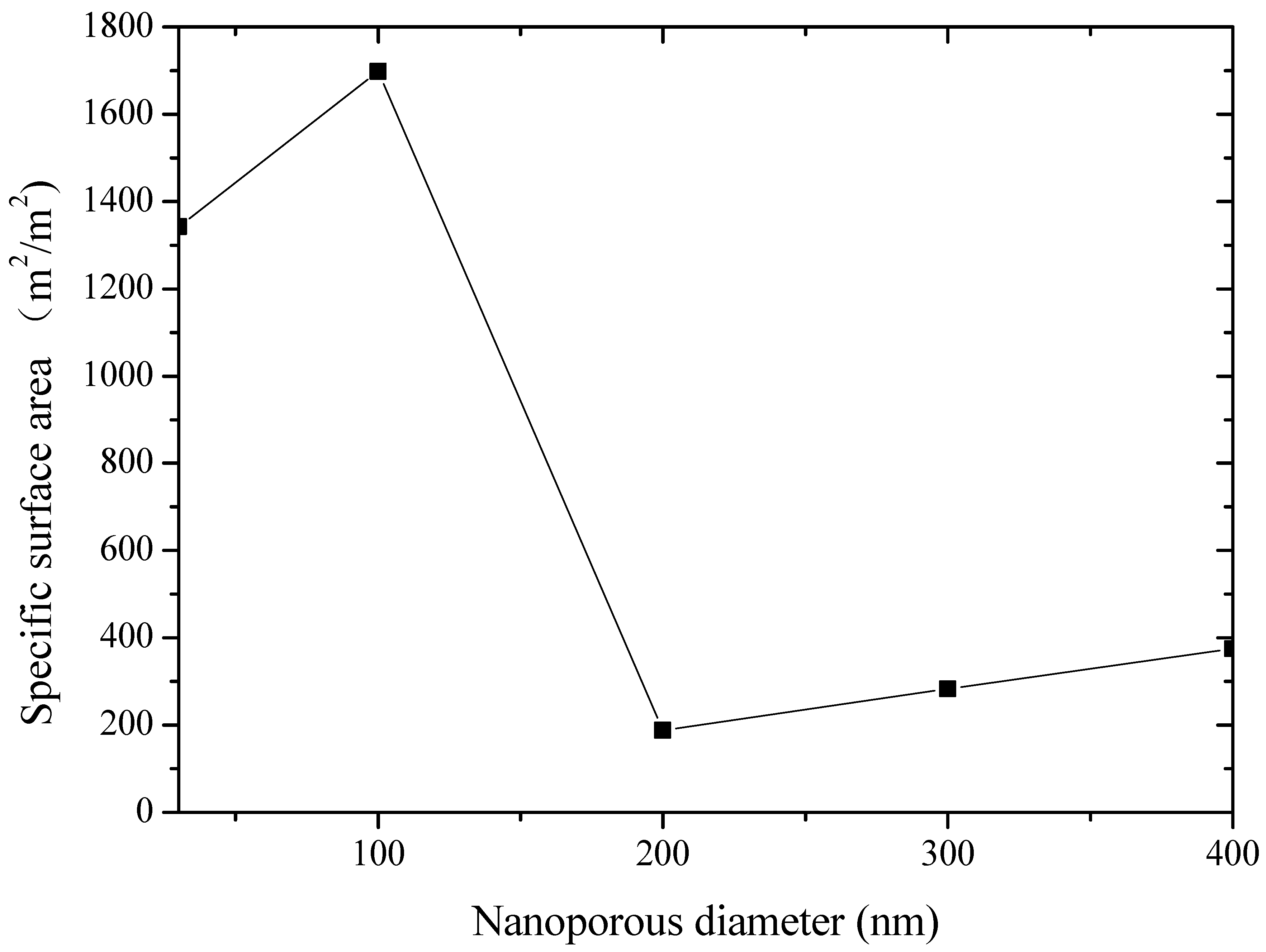
- The formed frost crystals on the 100 nm pore diameter alumina sheet are distributed relatively and sparsely due to the sparsely distributed surface-active points. During the initial stage of frosting, the ice crystals play the role of overhead insulation on the cold surface to prevent frost formation. Hence, the 100 nm pore diameter alumina sheet offers good anti-frosting performance even in high humidity environments.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | area (m2) |
| G | Gibbs free energy (J) |
| G′ | critical Gibbs free energy (J) |
| G | unit volume Gibbs free energy (J) |
| H | enthalpy (J) |
| I | embryo formation rate (embryo cm−2 s−1) |
| K | Boltzmann constant |
| M | molar mass (kg mol−1) |
| R | ideal gas constant (J mol−1 K−1) |
| R | radius (m) |
| r´ | critical radius (m) |
| S | entropy (J) |
| T | temperature (K) |
| V | volume (m3) |
| Γ | surface energy (J m−2) |
| Ρ | embryo density (kg m3) |
| Θ | contact angle (degrees) |
| Ω | humidity ratio (kgv kga−1) |
| Δ | deviation |
| Subscripts | |
| E | embryo |
| Ew | interface embryo–wall |
| Lat | latent |
| S | surroundings |
| Sat | saturation |
| Se | interface embryo–surroundings |
| Sw | interface surroundings–wall |
| Tot | total |
| W | wall |
References
- Song, M.J.; Mao, N.; Xu, Y.J.; Deng, S.M. Challenges in, and the development of building energy saving techniques, illustrated with the example of an air source heat pump. Therm. Sci. Eng. Prog. 2019, 10, 337–356. [Google Scholar] [CrossRef]
- Ma, Z.J.; Song, J.L.; Zhang, J.L. Energy consumption prediction of air conditioning systems in buildings by selecting similar days based on combined weights. Energy Build. 2017, 151, 157–166. [Google Scholar] [CrossRef]
- Fekadu, G.; Subudhi, S. Renewable energy for liquid desiccants air conditioning system: A review. Renew. Sustain. Energy Rev. 2018, 93, 364–379. [Google Scholar] [CrossRef]
- Wang, F.; Liang, C.H.; Zhang, X.S. Research of anti-frosting technology in refrigeration and air conditioning fields: A review. Renew. Sustain. Energy Rev. 2018, 81, 707–722. [Google Scholar] [CrossRef]
- Badri, D.; Toublanc, C.; Rouaud, O.; Havet, M. Review on frosting, defrosting and frost management techniques in industrial food freezers. Renew. Sustain. Energy Rev. 2021, 151, 111545. [Google Scholar] [CrossRef]
- Zhang, L.; Song, M.J.; Shen, J.; Zhang, X.; Xu, Y.J.; Hu, Y.X. Impacts of initial cooling rate on local frosting characteristics of horizontal cold plate surface with edge effect considered. Int. Commun. Heat Mass 2023, 143, 106654. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wu, C.Y.; Zhao, G.L.; Sun, J.; Yao, X.; Ma, X.H.; Wang, Z.K. Condensation frosting and passive anti-frosting. Cell Rep. Phys. Sci. 2021, 2, 100474. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wang, H.Y.; Zhang, X.H.; Meng, S.; Ma, C.F. An experimental study on minimizing frost deposition on a cold surface under natural convection conditions by use of a novel anti-frosting paint. Part II. Long-term performance, frost layer observation and mechanism analysis. Int. J. Refrig. 2006, 29, 236–242. [Google Scholar] [CrossRef]
- Okoroafor, E.U.; Newborough, M. Minimising frost growth on cold surfaces exposed to humid air by means of crosslinked hydrophilic polymeric coatings. Appl. Therm. Eng. 2000, 20, 736–758. [Google Scholar] [CrossRef]
- Lee, H.; Shin, J.; Ha, S.; Choi, B.; Lee, J. Frost formation on a plate with different surface hydrophilicity. Int. J. Heat Mass Transf. 2004, 47, 4881–4893. [Google Scholar] [CrossRef]
- Yang, W.; Zeng, B.; Zhang, Y.; He, S.; Zhao, X. Frosting performance of a nanoporous hydrophilic aluminum surface. Energies 2018, 11, 3483. [Google Scholar] [CrossRef]
- Wang, F.; Tang, R.; Wang, Z.; Yang, W. Experimental study on anti-frosting performance of superhydrophobic surface under high humidity conditions. Appl. Therm. Eng. 2022, 217, 119193. [Google Scholar] [CrossRef]
- Wang, G.Y.; Shen, Y.Z.; Tao, J.; Luo, X.Y.; Jin, M.M.; Xie, Y.H.; Li, Z.Z.; Guo, S.M. Facilely constructing micro-nanostructure superhydrophobic aluminum surface with robust ice-phobicity and corrosion resistance. Surf. Coat. Technol. 2017, 329, 224–231. [Google Scholar] [CrossRef]
- Lei, S.; Wang, F.; Fang, X.; Ou, J.; Li, W. Icing behavior of water droplets impinging on cold superhydrophobic surface. Surf. Coat. Technol. 2019, 363, 362–368. [Google Scholar] [CrossRef]
- Fan, P.Y.; Li, Y.X.; Liu, Z.L.; Yu, F.J.; Chen, Y.L.; Li, Y. Preparation and performance of nanoparticles-based anti-frosting transparent hydrophobic surfaces. Int. J. Refrig. 2021, 130, 404–412. [Google Scholar] [CrossRef]
- Diamantino, T.C.; Gonçalves, R.; Nunes, A.; Páscoa, S.; Carvalho, M.J. Durability of different selective solar absorber coatings in environments with different corrosivity. Sol. Energy Mater. Sol. Cells 2017, 166, 26–38. [Google Scholar] [CrossRef]
- Wilson, P.W.; Lu, W.; Xu, H.J.; Kim, P.; Kreder, M.J.; Alvarengad, J.; Aizenbergb, J. Inhibition of ice nucleation by slippery liquid-infused porous surfaces. Appl. Surf. Sci. 2013, 15, 581–584. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6576. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Z.; Liu, Y.; Guo, Y.; Wang, L. Effect of contact angle on water droplet freezing process on a cold flat surface. Exp. Therm. Fluid Sci. 2012, 40, 73–80. [Google Scholar] [CrossRef]
- Piucco, R.O.; Hermes, C.J.L.; Melo, C.; Barbosa, J.R. A study of frost nucleation on flat surfaces. Exp. Therm. Fluid Sci. 2008, 32, 1710–1715. [Google Scholar] [CrossRef]
- Mangini, D.; Antonini, C.; Marengo, M.; Amirfazli, A. Runback ice formation mechanism on hydrophilic and superhydrophobic surfaces. Cold Reg. Sci. Technol. 2015, 109, 53–60. [Google Scholar] [CrossRef]
- Na, B.; Webb, R.L. A fundamental understanding of factors that affecting frost nucleation. Int. J. Heat Mass Transf. 2003, 46, 3797–3808. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Zhang, X.; Meng, S.; Ma, C. An experimental study on minimizing frost deposition on a cold surface under natural convection conditions by use of a novel anti-frosting paint. Part I. Anti-frosting performance and comparison with the uncoated metallic surface. Int. J. Refrig. 2006, 29, 229–236. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, C.Q.; Liu, K.; Jiang, Y.; Song, Y.L.; Francisco, J.S.; Zeng, X.C.; Wang, J.J. Distinct ice patterns on solid surfaces with various wettabilities. Proc. Natl. Acad. Sci. USA 2017, 114, 11285–11290. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.J.; Jeong, H.J.; Son, H.; Kim, D.R.; Lee, K.S. Frost formation from general-low to ultra-low temperatures: A review. Int. J. Heat Mass Transf. 2022, 195, 123164. [Google Scholar] [CrossRef]
- Jing, T.; Kim, Y.; Lee, S.; Kim, D.; Kim, J.; Hwang, W. Frosting and defrosting on rigid superhydrohobic surface. Appl. Surf. Sci. 2013, 276, 37–42. [Google Scholar] [CrossRef]
- Fletcher, N.H. The Chemical Physics of Ice; Cambridge University Press: Cambridge, UK, 1970; Chapters 4–5. [Google Scholar]
- Bai, G.Y.; Gao, D.; Liu, Z.; Zhou, X.; Wang, J.J. Probing the critical nucleus size for ice formation with graphene oxide nanosheets. Nature 2019, 576, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Doring, W. Kinetische behandlung der keimbildung in übersättigten dämpfen. Ann. Phys. Lpz. 1935, 24, 719. [Google Scholar] [CrossRef]
- Volmer, M.; Flood, H. Tröpfchenbildung in dämpfen. Z. Phys. Chem. A 1934, 170, 273. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. How long is the coast of britain? statistical self-similarity and fractional dimension. Science 1967, 156, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Liang, C.; Yu, W.-P. Experimental study and fractal analysis of ice crystal structure at initial period of frost formation. J. Appl. Sci. 2007, 25, 193–197. [Google Scholar]
- Mei, M.; Yu, B.; Cai, J.; Liang, L. A fractal analysis of dropwise condensation heat transfer. Int. J. Heat Mass Transf. 2009, 52, 4823–4828. [Google Scholar] [CrossRef]
- Ding, Y.F.; Yin, S.; Liao, Y.D.; Wu, H.J. Frosting mechanism and suppression on nano/micro-structured hydrophobic surfaces. CIESC J. 2012, 63, 3213–3219. [Google Scholar]
- Carrales, D.H.; Rodríguez, I.; Leyva, R.; Mendoza, E.; Villela, D.E. Effect of surface area and physical-chemical properties of graphite and graphene-based materials on their adsorption capacity towards metronidazole and trimethoprim antibiotics in aqueous solution. Chem. Eng. J. 2020, 402, 126154. [Google Scholar]
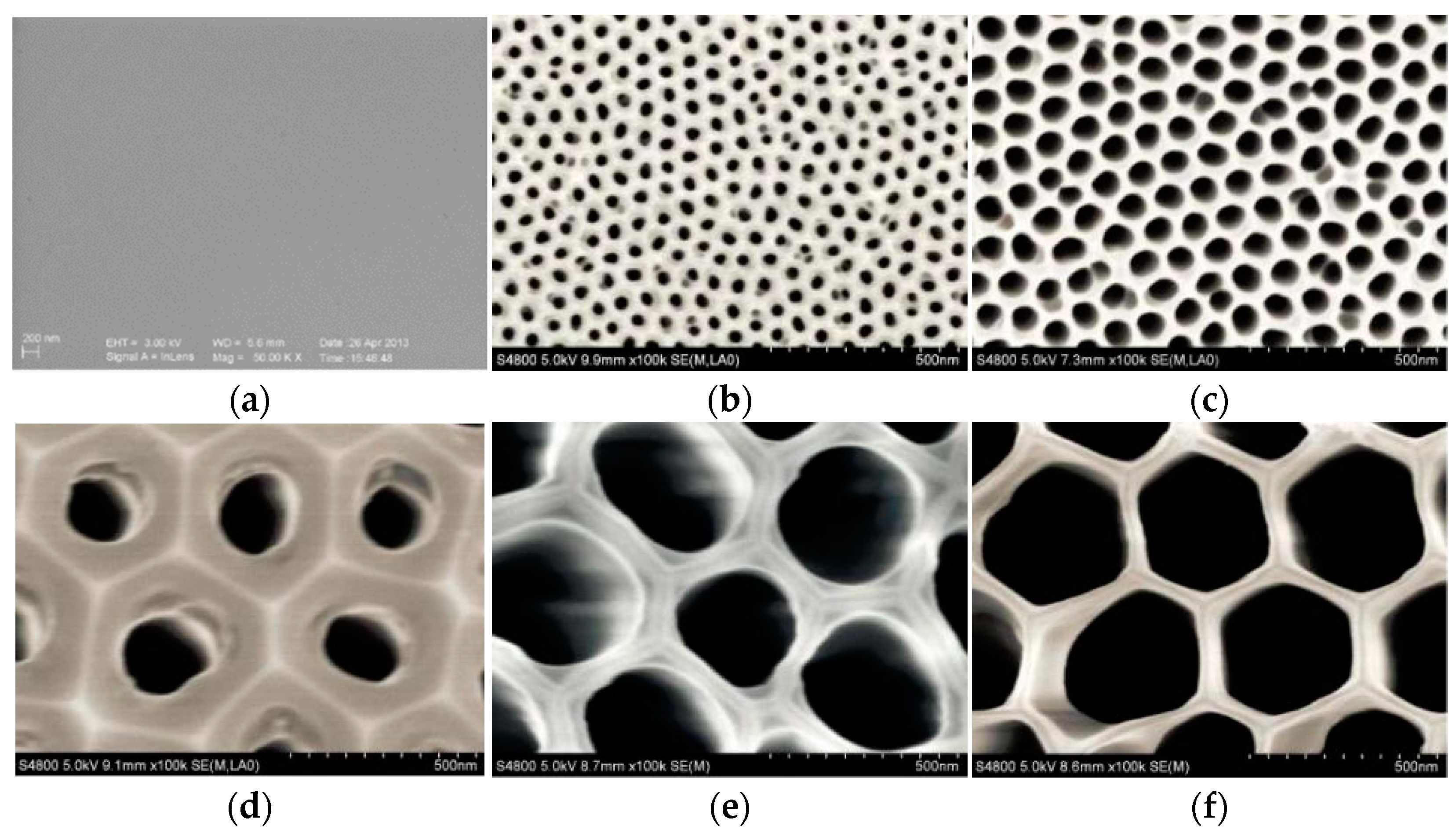



| Simple | Diameter | Spacing | Depth | Current | Size |
|---|---|---|---|---|---|
| 1 | 0 nm | 0 nm | 0 μm | 0 A | 20 × 20 × 0.2 mm |
| 2 | 30 nm | 65 nm | 60 ± 5 μm | 0.2 A | |
| 3 | 100 nm | 100 nm | 0.5 A | ||
| 4 | 200 nm | 450 nm | 1.2 A | ||
| 5 | 300 nm | 450 nm | 1.2 A | ||
| 6 | 400 nm | 450 nm | 1.2 A |
| Sample | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| Number of nanopores | 9.5 × 1010 | 4 × 1010 | 1.98 × 109 | 1.98 × 109 | 1.98 × 109 |
| Specific surface area (m2/m2) | 1342.5 | 1697.5 | 187.5 | 282.5 | 375 |
| Sample | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Contact angle (degree) | 87 | 40 | 37 | 31 | 36 | 56 |
| Surface energy (mN/m) | 50 | 142 | 147 | 157 | 149 | 111 |
| Sample | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| Fractal dimension | 2.8788 | 2.8084 | 2.8643 | 2.7684 | 2.8247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Liu, H.; Zhang, Y.; Liu, H.; Chen, W. Investigation of the Interface Effects and Frosting Mechanism of Nanoporous Alumina Sheets. Processes 2023, 11, 2019. https://doi.org/10.3390/pr11072019
He S, Liu H, Zhang Y, Liu H, Chen W. Investigation of the Interface Effects and Frosting Mechanism of Nanoporous Alumina Sheets. Processes. 2023; 11(7):2019. https://doi.org/10.3390/pr11072019
Chicago/Turabian StyleHe, Song, Heyun Liu, Yuan Zhang, Haili Liu, and Wang Chen. 2023. "Investigation of the Interface Effects and Frosting Mechanism of Nanoporous Alumina Sheets" Processes 11, no. 7: 2019. https://doi.org/10.3390/pr11072019
APA StyleHe, S., Liu, H., Zhang, Y., Liu, H., & Chen, W. (2023). Investigation of the Interface Effects and Frosting Mechanism of Nanoporous Alumina Sheets. Processes, 11(7), 2019. https://doi.org/10.3390/pr11072019








