Abstract
Core–shell nanoparticles are functional materials with tailored properties, able to improve the requirements of various applications. Both core and shell components can be inorganic or organic, and there are numerous studies in this field regarding their synthesis methods, properties, and applications. This review aims to study core–shell nanostructures with Fe3O4 cores and different shell types, observing their antibacterial and anticancer properties. By the type of coating, Fe3O4 core–shell nanoparticles (NPs) are classified into four categories: metal-coated NPs, metal-organic framework (MOF) coated NPs, metal oxide coated NPs, and polymer-coated NPs. Each category is briefly presented, emphasizing anticancer or antibacterial properties and specific applications (cancer diagnosis or therapy, drug carrier). Moreover, synthesis methods and particle size for both core and shell nanostructures, as well as the magnetic properties of the final core–shell material, are summarized in this review. Most of the consulted papers discussed sphere-like core–shell nanoparticles obtained by chemical methods such as coprecipitation, hydrothermal, and green synthesis methods using plant extract. These types of core–shell nanoparticles could be used as drug nanocarriers for tumor-targeted drug delivery, hyperthermia treatment, or contrast agents. Further work needs to be conducted to understand nanoparticles’ interaction with living cells and their traceability in the human body.
1. Introduction
Fe3O4 nanoparticles, due to their physical–chemical properties, low toxicity, and high saturation magnetization values, have received great attention in the biomedical field. Their pharmaceutical applications, such as anticancer agents against various cancer cells and antiviral (e.g., influenza virus, HBV, HIV) or antibacterial agents, have been recently considered. They are studied in several areas of interest from a medical point of view, such as magnetic hyperthermia, drug delivery, magnetic resonance imaging, photothermal therapy of tumors, magnetic bioseparation, magnetofection agents, DNA molecule detection, infectious diseases, and cancer therapy [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30].
In chemotherapy, several drug delivery systems can be used, but among them, one has shown great potential in nanomedicine: superparamagnetic iron oxide nanoparticles, also known as SPION, have become a priority choice for the delivery of cancer drugs because they effectively target cancer cells, through a magnetic field, improving the accumulation of magnetic nanoparticles at the target site. Moreover, these nanoparticles (NPs) also make simultaneous drug delivery and magnetic resonance imaging possible [31,32]. SPIONs have been extensively studied for cancer treatment by magnetic hyperthermia. The principle of this technique is as follows: when iron oxide NPs are exposed to an external magnetic field, magnetic losses are dissipated as heat appears. If SPIONs are placed near tumors, they raise the temperature of the tumor to a therapeutic level (42–45 °C) and induce weakness or death of cancer cells without damaging the surrounding healthy tissue or cells [1,18,33,34,35]. Several studies have provided evidence that the overuse of antibiotics has led to the development of bacterial resistance to numerous drugs, as in the case of nosocomial infections in hospitals [36,37]. Recently, researchers found that iron oxide nanoparticles could be used as magnetic drug delivery systems for antibiotics (e.g., amoxicillin). It means that Fe3O4 is loaded with various antibiotics as agents for killing bacteria in the respective damaged tissue, reducing the dose of medication needed in the classical method. When the external magnetic field is applied, the affected tissue is heated, and antibiotics act as bacteria-killing heat agents. A new approach is represented by the green synthesis method of Fe3O4 in the presence of plant extracts [36]. For example, Fe3O4 prepared using garlic extract has potential antibacterial action and is tested in the antimicrobial therapy of numerous multidrug-resistant bacterial strains and fungi. However, bare SPION oxidizes easily in the air, losing its magnetic properties and dispersibility due to its high chemical activity [38]. Therefore, surface coating is recommended to maintain the stability of magnetic Fe3O4 and to improve iron oxide antimicrobial applications. Moreover, the surface coating prevents agglomeration of nanoparticles, protects nanoparticles against reticuloendothelial system (RES) uptake and elimination, and improves internalization efficiency [4,34]. One of the most popular surface coating techniques is the obtaining of core–shell nanostructures. Core–shell particles serve as storage and carrier platforms for many applications [39,40,41]. Several types of core–shell structures based on Fe3O4 (core) have been investigated and have been used in the field of chemotherapy due to both their antibacterial properties and their superparamagnetic properties [32,33,42,43,44,45,46]. This structure is frequently used as a platform for targeted drug delivery in cancer treatment [46]. Bacterial infections and cancer are connected diseases that have become a global health threat. Bacteria accelerate the development of cancer, prompting researchers to find an antibacterial and anticancer drug delivery agent. A lot of articles have studied organic coatings of iron oxide nanoparticles, the most popular being natural and synthetic polymers such as chitosan, dextran, starch, polyethylene glycol (PEG), polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP), polyethyleneimine (PEI), poloxamers, polysorbate 20 and 80, thermo-responsive poly (N-isopropyl acrylamide) (PNIPAAm), or small organic molecules with functional groups, such as thiols, amines, or carboxyls [2,4,32,34,47]. In this mini-review, core–shell nanoparticles having Fe3O4 as a core will be discussed, describing briefly their properties, synthesis methods, and biomedical applications and focusing on the inorganic shell types. Each synthesis method can produce different sizes, shapes, or magnetic properties for a specific application. The main characteristics of the inorganic core–shell materials discussed in this paper are presented synthetically in the form of tables.
2. Different Types of Core–Shell Structures with Fe3O4 Core for Biomedical Applications
Core–shell nanostructures are defined as heterogeneous nanoparticles composed of two or more nanomaterials that can be identified and are separated by distinct boundaries. Both core and shell components can be inorganic (metals, metal oxides) or organic (polymers, biomolecules) [48,49,50]. Core/shell composite nanostructures (NSs) have attracted much attention in recent years due to their diverse and unique material properties not shown by the core or shell materials alone, such as good mechanical, thermal, and optical properties [48,51]. These properties are significantly enhanced compared to pure compounds [51]. The interaction between the core and the shell of a nanostructure can lead to new properties and functions [45].
There are numerous core–shell materials with various applications and much literature about their classification and detailed descriptions of the preparation method. This paper presents only inorganic core–shell materials with Fe3O4 as the core and their medical applications.
Fe3O4 can be coated with different types of shells, such as metals (Ag, Au) [52,53,54,55,56], metal–organic frameworks (Cu–MOF), metal oxides (SiO2, TiO2, ZnO), and organic polymers (polyethyleneimine: PEI, polyacrylic acid: PAA, etc.), to obtain core–shell nanostructures with desired properties [3].
Core–shell nanostructures with Fe3O4 as a core have been a popular research topic over the last decade, with more than 700 articles published in the field, as shown in Figure 1a. As can be seen from Figure 1b, most of the papers published on this topic were research articles (>700 papers) and short communications (>40 papers). The data presented in Figure 1 were obtained using the ScienceDirect database (https://www.sciencedirect.com/) and searching for “Fe3O4 core–shell nanoparticles for biomedical applications”. The results were refined by year (selecting from 2012 to 2023) in Figure 1a and by article type in Figure 1b. These data were collected in May 2023.
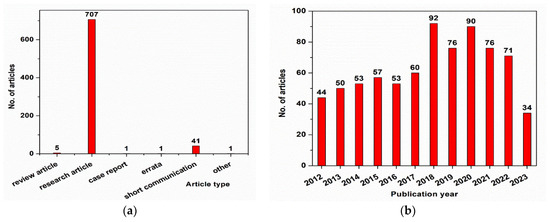
Figure 1.
(a) Evolution of the published articles in the field of Fe3O4 core–shell nanoparticles; (b) types of papers published in the field of Fe3O4 core–shell nanoparticles.
2.1. Metal-Coated Fe3O4
Silver-coated Fe3O4 nanohybrids have been used in a broad range of applications, including chemical and biological sensors [48,57], drug delivery—as successful drug carriers with focused antimicrobial, anticancer properties [48,58], diagnosis, and cancer therapy [48,59,60].
Different methods were used to synthesize Ag-coated Fe3O4 nanoparticles. Generally, a two-step synthesis procedure is applied: magnetite is prepared by a solvothermal, co-precipitation, or microemulsion route [57,61,62], obtaining spherical-shaped particles, and then Fe3O4 nanoparticles are dispersed in AgNO3 solution in the presence of an organic solvent (ethanol, di-chlorobenzene), a surfactant (oleylamine, cetyltrimethylammonium bromide—CTAB), and a reduction agent for Ag (butylamine, sodium borohydride). Another approach uses combined phyto- and hydrothermal synthesis, preparing the magnetite core in the presence of a plant extract (neem leaf extract, leaf extract of Eryngium planum, Vitis vinifera (grape) stem extract, Euphorbia peplus Linn leaf extract), followed by hydrothermal synthesis of Fe3O4–Ag (silver nitrate was added in the magnetite suspension). Plant extract acts as a reducing agent for silver shells [44,59,63,64]. Spherical core–shell structures with 7–80 nm are obtained in these cases [44,57,59,61,62,63,64]. Moreover, brick-like Ag-coated Fe3O4 nanoparticles with ~13 nm in width and ~15 nm in length were prepared by single-step thermal decomposition of the magnetite precursors in the presence of AgNO3 salt and 1,2-hexadecane-diol reduction agent [58].
It has been discovered that Fe3O4–Ag nanocomposites present a self-sterilizing property that avoids the formation of biofilms, which are the most dangerous source capable of spreading toxic bacteria into the environment [61], improving the contrast of magnetic resonance imaging (MRI) in cancer detection [48].
Similar synthesis methods as in the case of silver-doped magnetite core–shell structures (coprecipitation, thermal decomposition of Fe3O4), followed by reduction of HAuCl4 or gold acetate with various agents (NaBH4, sodium citrate, 1,2-hexadecane-diol), as well as combined phyto-hydrothermal synthesis (with Juglans regia green husk as reducing and stabilizing agent for HAuCl4), were reported in [65,66,67,68,69,70,71,72] for gold-coated magnetite nanostructures. In 2023, Danafar et al. [65] prepared Fe3O4–Au hybrid nanoparticles coated with bovine serum albumin (BSA) by co-precipitation of magnetite at 60 °C followed by the reduction of HAuCl4 with sodium citrate and NaBH4, resulting in Fe3O4–Au hybrids that were further coated with BSA under magnetic stirring at room temperature. They studied their potential application as a contrast agent in magnetic resonance imaging (cancer diagnosis). Gold nanoparticles represent a good option for Fe3O4 coating due to their good biocompatibility, large specific surface area, “surface plasmon” property, and well-known attraction for thiol groups from organic molecules [66]. Fe3O4–Au core–shell nanoparticles can be used in biomedical applications such as magnetic resonance imaging, hyperthermia, biosensors, immunosensors, photothermal therapy, controlled drug delivery, targeted gene delivery, protein separation, DNA detection, and DNA/RNA interaction [67,68,69,70,71].
2.2. Metal–Organic Framework (MOF) Coated Fe3O4
Fe3O4 nanoparticle was used as a core for improving the physicochemical properties and the thermal stability of the Cu–MOF compound. Metal–organic frameworks (MOFs) are a class of crystalline, porous materials composed of metal ions surrounded by multi-dented organic molecules. The metal ions form nodes that bind the arms of the organic ligands which act as linkers in the cage-like network structure. MOFs have a high surface area, significant porosity, tunable pore size, and high thermal stability in comparison to other nanostructures. Azizabadi et al. [51] prepared Fe3O4–Cu–MOFs by an ultrasonic-assisted reverse micelle synthesis (ultrasonic irradiation time of 10 min, temperature of 25 °C, power of 80 W) and found that this core–shell composite has good antibacterial activities against both Gram-positive and Gram-negative bacteria, which recommends it for advanced biomedical applications.
2.3. Metal Oxide-Coated Fe3O4
One of the most studied metal oxides as a shell for the Fe3O4 core was SiO2, due to the powerful attraction of magnetic nanoparticles to silica [73]. SiO2 particles are non-toxic, highly biocompatible, and abundant in surface hydroxyl groups, which makes them an ideal surface functional coating for magnetic nanoparticles in the medical field [3,74,75,76,77,78,79]. Fe3O4 nanoparticles coated with SiO2 shells obtained by Ta et al. through hydrolysis and condensation [75] showed increased biocompatible properties and provided new ideas for future bioconjugation studies [3]. Moreover, the Fe3O4–SiO2 core–shell structure prepared by Lu et al. using an ultrasound-assisted method [80] has good opportunities in the field of biomedicine [3].
TiO2 is another metal oxide with interesting properties such as biocompatibility, chemical inertness, high stability, and resistance to body fluids that lead to its use in cosmetics, pharmaceutics, and malignant tumor therapy [43,81,82]. The coating of magnetite nanoparticles with a TiO2 shell protects the core from environmental damage and improves biocompatible properties [43]. Fe3O4–TiO2 core–shell structures with various Fe3O4:TiO2 molar ratios were synthesized by a modified sol–gel method [83] or hydrothermal process [84]. The obtained Fe3O4–TiO2 core–shell nanorods are superparamagnetic and could be further used for magnetic hyperthermia applications [43].
Fe3O4–ZnO core–shell nanoparticles represent some of the most studied materials for magnetic hyperthermia and bio-imaging applications [33,85,86,87,88,89]. ZnO is well known for its anti-bacterial and biocompatible properties and possesses unique physical and chemical characteristics due to its wide bandgap and elevated exciton binding energy (piezoelectricity, photoluminescence, chemical stability) [90,91,92]. It has been demonstrated that ZnO–Fe3O4 composites combine the magnetic properties of Fe3O4 with the antibacterial activity of ZnO, resulting in a material with improved biocompatibility and enhanced antibacterial activity. ZnO–Fe3O4 composites inhibit microorganisms’ biofilm formation due to their synergetic activity of ion lixiviation (Fe3+, Zn2+) and oxidative activity. The material’s magnetic properties play a major role in reducing the ability of microorganisms to attach to different surfaces, inhibiting biofilm formation [85]. It is very important to hinder the formation of biofilm because its existence makes microorganisms more resistant to antibiotics. ZnO/Fe3O4 composites have shown enhanced antibacterial ability under visible light irradiation compared to single ZnO [93]. In 2021, Gupta et al. [33] reported the hydrothermal synthesis of Fe3O4–ZnO core–shell nanoparticles. The obtained material preserved the photoluminescence capacity of ZnO and the superparamagnetic properties of Fe3O4, demonstrating its potential use for hyperthermia therapy and fluorescent-based cellular imaging. Fe3O4–ZnO nanoparticles significantly reduced the viability of human cervical cancer cells (HeLa) under the applied AC magnetic field. However, in 2018, Madhubala et al. [87] found that only the lowest concentrations of Fe3O4–ZnO core–shell nanoparticles are non-toxic for cells and could be used for cancer treatment using magnetic hyperthermia therapy (MHT). Moreover, the authors concluded that Fe3O4–ZnO with a molar ratio of 1:20 has a small particle size and high crystallinity, and Fe3O4 is completely encapsulated in the ZnO nanoparticles [87].
2.4. Polymer-Coated Fe3O4
Magnetite surface coating with natural or synthetic polymers has been widely investigated [3,32,94,95,96,97,98,99,100] due to their good biocompatibility, biodegradability, non-toxicity, stability, and ability to modify physical-chemical surface properties. Covering magnetite with polymers improves the antibacterial and anticancer properties of core–shell nanoparticles. Different polymers such as polyethylene glycol (PEG), chitosan, poly-N-vinylpyrrolidone (PVP), hydroxyl ethylene cellulose (HEC), nanocrystalline cellulose (NCC), heparin-poloxamer (HP), poly(N-isopropyl acrylamide) (PNIPAAm), polyethyleneimine (PEI), and polyacrylic acid (PAA) have been coated on the Fe3O4 surface for tumor-targeted drug delivery. In 2021, Mohammadi et al. [95] synthesized magnetic nanoparticles with cross-linked PEG coatings using plasma treatment. The plasma-induced graft polymerization creates a cross-linked network of PEG chains, resulting in a rigid surface that hinders the burst release of the drug. The classical coprecipitation method of magnetite core followed by direct addition of chitosan or PEG shell and heating at 80 °C for 30 min [96] leads to an irregular and dendrimer-like surface morphology with small and large grain sizes. Fe3O4 surface functionalized with PEG has significant results at 20 mg/mL against antimicrobial activities. The anticancer activity was tested against HepG2 liver cancer cell lines, and magnetite-polymer nanoparticles are suitable for hyperthermia therapy to treat carcinoma.
When superparamagnetic iron oxide nanoparticles (SPIONs) were coated with heparin-poloxamer (HP) and the core–shell system was tested for anticancer drug delivery, doxorubicin (DOX) was entrapped in the polymer shell, showing a controlled release up to 120 h without any initial burst effect [98]. Moradi et al. [32] prepared Fe3O4 core–shell nanoparticles as drug nanocarriers, having PNIPAAm grafted with chitosan as a polymer shell. PNIPAAm is a thermo-responsive polymer, while chitosan is a pH-responsive moiety. Therefore, the highest release percentage of methotrexate (MTX) as a negatively charged anticancer drug has been observed at T = 40 °C and pH = 5.5.
A schematic representation of Fe3O4-based core–shell nanoparticles with various types of shells for biomedical applications is shown in Figure 2.
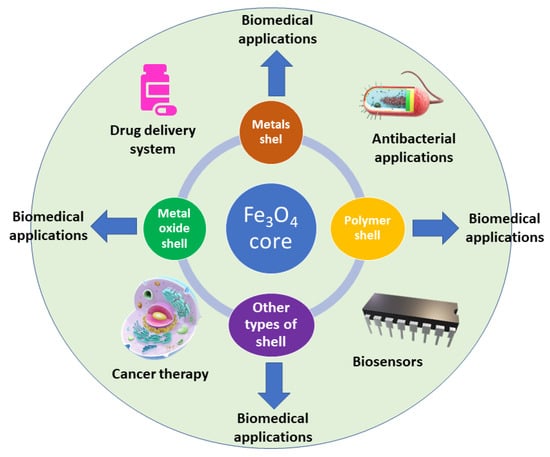
Figure 2.
Various types of Fe3O4 core–shell nanoparticles with biomedical applications.
Table 1 shows the main synthesis methods and applications of Fe3O4 core–shell nanoparticles. Table 2 presents the sizes and properties of core, shell, and core–shell nanoparticles in correlation with the synthesis conditions of the core–shell nanostructure.

Table 1.
Synthesis methods for Fe3O4 core–shell nanoparticles and their applications.

Table 2.
Size and properties of Fe3O4-based core–shell nanostructures.
3. Conclusions and Perspectives
Core–shell nanoparticles are an important class of materials for biomedical applications. This mini-review has been focused on nanoparticles having Fe3O4 as a core and various types of shells, especially inorganic ones. It has been shown that medical applications of these nanostructures depend on their size, shape, and properties. The most common morphology is spherically shaped nanoparticles. The most efficient particle sizes seem to be in the range of 6–50 nm.
In some cases, the shell thickness was higher than the core diameter, leading to a decrease in magnetic saturation value, which affects its use as a contrast agent (MRI contrast ability is lower).
Generally, the most suitable nanoparticles for cancer therapy are those with diameters between 10 and 100 nm. Particles with around 10 nm diameter have a higher surface area-to-volume ratio and are more effective for drug delivery and imaging, while particles with approximately 50 nm diameter may be more effective in hyperthermia treatment.
Too small particles (less than 2 nm) can easily leak from the normal vasculature, and particles below 10 nm can be filtered by the kidneys. Particles larger than 100 nm can be cleared from circulation by phagocytes.
Fe3O4 core–shell nanoparticles are still under development regarding their use as magnetic contrast agents, hyperthermia agents, or drug delivery systems in clinical applications on human patients. It is necessary to investigate and understand the interaction between core –shell nanoparticles and human tissues before clinical trials.
In hyperthermia, the damaged body tissue is exposed to high temperatures in order to damage and kill cancer cells or make them more sensitive to radiation and anticancer drugs. In magnetic hyperthermia, an external magnetic field is used to control magnetic nanoparticles, which are introduced into the human body through intravenous injection, intratumoral injection, or targeted delivery to damaged organs or tissues. Fe3O4 nanoparticles absorb electromagnetic energy and convert it into heat (>41.5 °C). The inductive heating effect of iron oxide nanoparticles appears when an alternating magnetic field suddenly changes the magnetic orientation of the superparamagnetic magnetite particles. Rapid alternation of the magnetic orientation produces particle vibration and further generates heat after internalization, causing cell death when temperatures reach approximately 42 °C. The heat generated by the magnetic nanoparticles can kill cancer cells without damaging healthy tissue.
Another method to treat cancer using Fe3O4 core–shell nanoparticles is to apply mechanical pressure to cancer cells in order to cause magnetic particle vibrations, which will finally cause cell death.
Targeted cancer therapies are developed to interrupt the uncontrolled proliferation of cancer cells. Core–shell nanoparticles can be designed to deliver drugs only after entering the tumor tissues. This could reduce side effects and increase the accuracy of tumor targeting with improved treatment efficacy. Fe3O4 core–shell nanoparticles present various reactive sites for contact with drugs and can be triggered for binding to specific sites, release the drug at a certain time/temperature/pH, in a controlled manner (shell thickness dependent, ROS-mediated cytotoxicity, microwave-triggered), etc. The drugs embedded in core–shell nanostructures accumulate in cancer cells under the influence of the external magnetic field through enhanced permeability and retention effects.
For nanoparticles, as in the case of medicines, in parallel with the effectiveness, the safety of use is also evaluated. Over the past 40 years, the number and variety of controlled-release drug delivery systems have greatly increased, but despite all the successes achieved, the delivery systems have not been fully accepted due to issues with the regulatory process. Many of the nanoformulations of oncology drugs were retracted from the market, although they had already received Food and Drug Administration (FDA) approval. Newly investigated therapeutic nanoparticles with anticancer properties have some limitations and fail to pass clinical trials. The major drawback is the lack of understanding of the mechanism of nanoparticles’ interaction with biomolecules in the human body. Other important limitations are cellular internalization of the drug (heterogeneous accumulation in the tumor cells). Less than 1% of injected nanoparticles reach the tumor because of the complexity of the tumor; drug release rate; nanoparticles should remain in circulation long enough to allow for significant tumor accumulation; drugs should not be dispersed and distributed in the entire body; nanoparticles that serve as drug nanocarriers should be capable of targeting only tumor cells; and the prediction of the response of the immune system to the newly introduced nanoparticles in the human body tissues.
Researchers are investigating the possibility of creating multifunctional nanoparticles that, after detecting the tumor in the body, can also proceed to its treatment, a fact that would revolutionize oncological practice, replacing the classical therapeutic methods such as chemotherapy and radiotherapy that affect not only cancer cells but also those healthy, destroying them. With the help of nanotechnologies, cancer cells could be destroyed in a targeted manner without harming healthy tissue in any way.
It is necessary to enhance the selectivity and accuracy of delivery for core–shell nanoparticles to target cancer cells. The major challenges are to design nanoparticles that are stable in the patient’s bloodstream and possess improved precision and efficacy for the therapeutic treatment of tumors. Consequently, there is a need to improve synthesis methods to obtain new core–shell nanoparticles for local control of tumors and improved targeted delivery of agents for cancer therapy. Among the synthesis methods briefly presented in this review, hydrothermal synthesis of Fe3O4 core–shell nanoparticles combined with phyto-synthesis of one component (either magnetite or shell) using different plant extracts is eco-friendly, cost-effective, and can ensure control of particle size and morphology for tailored applications (drug delivery nanocarriers or magnetic hyperthermia therapy).
In the future, it should be used under mild synthesis conditions of the hydrothermal method: A shorter reaction time (≤3 h) and lower temperature (≤200 °C) coupled with supplementary pressure (from an external source through inert gas bubbling such as Ar or N2) created in addition to the vapor pressure formed above the reaction system could lead to core–shell nanoparticles with improved properties. Inorganic core–shell precursors such as chloride, nitrate, and sulfate are cheaper than organic–metallic ones and should be further used to avoid any environmental problems created by organic solvents or precursors.
Future research should focus on biological, technological, and design aspects regarding the use of Fe3O4 core–shell nanoparticles in cancer treatment. Thus, biological aspects refer to the interactions of core–shell nanostructures in the human body with living cells, organs, and species. An important issue to be solved in the future is what happens with insoluble or very little soluble nondegradable nanoparticles in the human body. Moreover, the linkage between drug-loaded nanoparticles and cells should be studied to understand the mechanism of cellular uptake. Technological aspects refer to scale-up synthesis and performance predictions. It should be possible to find cost-effective synthesis routes capable of yielding large quantities of chemicals that can be produced by pharmaceutical companies. Currently, predicting nanoparticle efficacy and performance in real human tissues is hard because nanoparticle therapy is applied to patients after several classical routes (surgery, chemotherapy, phototherapy, and radiation therapy) have been administered and their immune systems have already been affected by the respective treatment. Computational or theoretical modeling, along with experimental results, can be designed to imitate physiological tissue and the surrounding environment or to study the interaction between drug carriers and cells. The design of drug nanocarriers based on core–shell nanostructures should consider colloidal stability, drug loading capacity, tracking, the release of drug components only at the target sites, biocompatibility, toxicity, and minimal risk (to avoid spreading or accumulation of the drug in other tissues or organs). The particle sizes and size distributions must be reproducible. Fighting cancer is an old dream of physicians and researchers, and it makes them confident that one day the cure for this disease will be discovered.
Author Contributions
Conceptualization, L.-M.C. and A.-G.Ș.; literature search—M.-A.I.; writing—original draft preparation, M.-A.I.; writing—review and editing, L.-M.C. and A.-M.M.; supervision, R.M.P. and A.-G.Ș.; project administration, I.A.T.; funding acquisition, A.-M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MCID, project no. 23250202, through the Core Program within the National Research Development and Innovation Plan 2022–2027 and INOVADIT project of the Ministry of Research, Innovation, and Digitization through Program 1—Development of the national research-development system, Subprogram 1.2-Institutional performance-Projects for financing excellence in RDI, Contract no. 9PFE/2021.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This work was performed through the Core Program within the National Research Development and Innovation Plan 2022–2027, carried out with the support of MCID, project no. 23250202, and the INOVADIT project of the Ministry of Research, Innovation, and Digitization through Program 1—Development of the national research-development system, Subprogram 1.2—Institutional Performance-Projects for Financing Excellence in RDI, Contract no. 9PFE/2021. M.-A. Ioța and A.-G. Șchiopu gratefully acknowledge the Interdisciplinary Doctoral School, University of Pitești, for administrative and technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Musielak, M.; Piotrowski, I.; Suchorska, W.M. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as a Multifunctional Tool in Various Cancer Therapies. Rep. Pract. Oncol. Radiother. 2019, 24, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, Surface Functionalization and Application of Fe3O4 Magnetic Nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic Iron Oxide Nanoparticles for Delivery of Therapeutic Agents: Opportunities and Challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef]
- Shaw, S.K.; Sharma, A.; Kailashiya, J.; Gupta, S.K.; Meena, S.S.; Dash, D.; Maiti, P.; Prasad, N.K. Mesoporous Fe3O4 Nanoparticle: A Prospective Nano Heat Generator for Thermo-Therapeutic Cancer Treatment Modality. J. Magn. Magn. Mater. 2023, 578, 170817. [Google Scholar] [CrossRef]
- Shubitidze, F.; Kekalo, K.; Stigliano, R.; Baker, I. Magnetic Nanoparticles with High Specific Absorption Rate of Electromagnetic Energy at Low Field Strength for Hyperthermia Therapy. J. Appl. Phys. 2015, 117, 094302. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Ahmad Khairudin, N.B.B.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green Biosynthesis of Superparamagnetic Magnetite Fe3O4 Nanoparticles and Biomedical Applications in Targeted Anticancer Drug Delivery System: A Review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Fu, S.; Wang, S.; Zhang, X.; Qi, A.; Liu, Z.; Yu, X.; Chen, C.; Li, L. Structural Effect of Fe3O4 Nanoparticles on Peroxidase-like Activity for Cancer Therapy. Colloids Surf. B Biointerfaces 2017, 154, 239–245. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-Engineered Magnetic Nanoparticle Platforms for Cancer Imaging and Therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, T.; Zhao, E.; Docter, D.; Yang, W.; Stauber, R.H.; Gao, M. Small Is Smarter: Nano MRI Contrast Agents—Advantages and Recent Achievements. Small 2016, 12, 556–576. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Datta, N.R.; Krishnan, S.; Speiser, D.E.; Neufeld, E.; Kuster, N.; Bodis, S.; Hofmann, H. Magnetic Nanoparticle-Induced Hyperthermia with Appropriate Payloads: Paul Ehrlich’s “Magic (Nano)Bullet” for Cancer Theranostics? Cancer Treat Rev. 2016, 50, 217–227. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in Hyperthermia Cancer Therapy: From Fundamental Principles to Advanced Applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Kaewsaneha, C.; Tangboriboonrat, P.; Polpanich, D.; Elaissari, A. Multifunctional Fluorescent-Magnetic Polymeric Colloidal Particles: Preparations and Bioanalytical Applications. ACS Appl. Mater. Interfaces 2015, 7, 23373–23386. [Google Scholar] [CrossRef]
- Sahadevan, J.; Sojiya, R.; Padmanathan, N.; Kulathuraan, K.; Shalini, M.G.; Sivaprakash, P.; Esakki Muthu, S. Magnetic Property of Fe2O3 and Fe3O4 Nanoparticle Prepared by Solvothermal Process. Mater. Today Proc. 2022, 58, 895–897. [Google Scholar] [CrossRef]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic Nanoparticles and Targeted Drug Delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; González-Carreño, T.; Veintemillas-Verdaguer, S.; Serna, C.J. Advances in Magnetic Nanoparticles for Biotechnology Applications. J. Magn. Magn. Mater. 2005, 290–291 Pt 1, 28–34. [Google Scholar] [CrossRef]
- Majewski, P.; Thierry, B. Functionalized Magnetite Nanoparticles—Synthesis, Properties, and Bio-Applications. Crit. Rev. Solid State Mater. Sci. 2007, 32, 203–215. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; Von Rechenberg, B. Superparamagnetic Nanoparticles for Biomedical Applications: Possibilities and Limitations of a New Drug Delivery System. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Mona, L.P.; Songca, S.P.; Ajibade, P.A. Synthesis and Encapsulation of Iron Oxidenanorods for Application in Magnetichyperthermia and Photothermal Therapy. Nanotechnol. Rev. 2022, 11, 176–190. [Google Scholar] [CrossRef]
- Thomas, R.; Park, I.K.; Jeong, Y.Y. Magnetic Iron Oxide Nanoparticles for Multimodal Imaging and Therapy of Cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in Magnetic Resonance Imaging. Rom Simple to Dual Contrast Agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional Nanostructured Materials for Multimodal Imaging, and Simultaneous Imaging and Therapy. Chem. Soc. Rev. 2009, 38, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Govindan, B.; Sabri, M.A.; Hai, A.; Banat, F.; Haija, M.A. A Review of Advanced Multifunctional Magnetic Nanostructures for Cancer Diagnosis and Therapy Integrated into an Artificial Intelligence Approach. Pharmaceutics 2023, 15, 868. [Google Scholar] [CrossRef] [PubMed]
- Olusegun, S.J.; Osial, M.; Majkowska-Pilip, A.; Żelechowska-Matysiak, K.; Nieciecka, D.; Krajewski, M.; Pękała, M.; Krysinski, P. Synthesis and Characterization of Sr2+ and Gd3+ Doped Magnetite Nanoparticles for Magnetic Hyperthermia and Drug Delivery Application. Ceram. Int. 2023, 49, 19851–19860. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite Nanoparticles for Cancer Diagnosis, Treatment, and Treatment Monitoring: Recent Advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Nuzhina, J.V.; Shtil, A.A.; Prilepskii, A.Y.; Vinogradov, V.V. Preclinical Evaluation and Clinical Translation of Magnetite-Based Nanomedicines. J. Drug Deliv. Sci. Technol. 2019, 54, 101282. [Google Scholar] [CrossRef]
- Attia, N.F.; El-Monaem, E.M.A.; El-Aqapa, H.G.; Elashery, S.E.A.; Eltaweil, A.S.; El Kady, M.; Khalifa, S.A.M.; Hawash, H.B.; El-Seedi, H.R. Iron Oxide Nanoparticles and Their Pharmaceutical Applications. Appl. Surf. Sci. Adv. 2022, 11, 100284. [Google Scholar] [CrossRef]
- Alamdari, S.G.; Amini, M.; Jalilzadeh, N.; Baradaran, B.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Oroojalian, F. Recent Advances in Nanoparticle-Based Photothermal Therapy for Breast Cancer. J. Control. Release 2022, 349, 269–303. [Google Scholar] [CrossRef]
- Rodrigues, G.R.; López-Abarrategui, C.; de la Serna Gómez, I.; Dias, S.C.; Otero-González, A.J.; Franco, O.L. Antimicrobial Magnetic Nanoparticles Based-Therapies for Controlling Infectious Diseases. Int. J. Pharm. 2019, 555, 356–367. [Google Scholar] [CrossRef]
- Nieciecka, D.; Celej, J.; Żuk, M.; Majkowska-pilip, A.; Żelechowska-Matysiak, K.; Lis, A.; Osial, M. Hybrid System for Local Drug Delivery and Magnetic Hyperthermia Based on Spions Loaded with Doxorubicin and Epirubicin. Pharmaceutics 2021, 13, 480. [Google Scholar] [CrossRef]
- Moradi, S.; Najjar, R.; Hamishehkar, H.; Lotfi, A. Triple-Responsive Drug Nanocarrier: Magnetic Core-Shell Nanoparticles of Fe3O4@poly(N-Isopropylacrylamide)-Grafted-Chitosan, Synthesis and In Vitro Cytotoxicity Evaluation against Human Lung and Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 72, 103426. [Google Scholar] [CrossRef]
- Gupta, J.; Hassan, P.A.; Barick, K.C. Core-Shell Fe3O4@ZnO Nanoparticles for Magnetic Hyperthermia and Bio-Imaging Applications. AIP Adv. 2021, 11, 025207. [Google Scholar] [CrossRef]
- Rybka, J.D. Radiosensitizing Properties of Magnetic Hyperthermia Mediated by Superparamagnetic Iron Oxide Nanoparticles (SPIONs) on Human Cutaneous Melanoma Cell Lines. Rep. Pract. Oncol. Radiother. 2019, 24, 152–157. [Google Scholar] [CrossRef]
- Katifelis, H.; Mukha, I.; Lyberopoulou, A.; Vityuk, N.; Grammatikaki, M.; Pylypchuk, I.; Lazaris, F.; Storozhuk, L.; Kouloulias, V.; Gazouli, M. In Vitro Effect of Hyperthermic Ag and Au Fe3O4 Nanoparticles in Cancer Cells. Beilstein Arch. 2019, 2019101. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Abdulkreem AL-Huqail, A.; Ali, E.; Alkhalifah, T.; Alturise, F.; Ali, H.E. Green Synthesis of Fe3O4 Nanoparticles Using Alliaceae Waste (Allium sativum) for a Sustainable Landscape Enhancement Using Support Vector Regression. Chemosphere 2023, 334, 138638. [Google Scholar] [CrossRef]
- Roy, S.; Hasan, I.; Guo, B. Recent Advances in Nanoparticle-Mediated Antibacterial Applications. Coord. Chem. Rev. 2023, 482, 215075. [Google Scholar] [CrossRef]
- Singh, M.; Savchenko, A.; Shetinin, I.; Majouga, A. An Original Route to Target Delivery via Core-Shell Modification. Mater. Today Proc. 2016, 3, 2652–2661. [Google Scholar] [CrossRef]
- Mélinon, P.; Begin-Colin, S.; Duvail, J.L.; Gauffre, F.; Boime, N.H.; Ledoux, G.; Plain, J.; Reiss, P.; Silly, F.; Warot-Fonrose, B. Engineered Inorganic Core/Shell Nanoparticles. Phys. Rep. 2014, 543, 163–197. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, X.; Luo, W.; Yue, Q.; Zhang, Y.; Cheng, X.; Li, W.; Kong, B.; Deng, Y.; Zhao, D. Interfacial Engineering of Magnetic Particles with Porous Shells: Towards Magnetic Core–Porous Shell Microparticles. Nano Today 2016, 11, 464–482. [Google Scholar] [CrossRef]
- Su, H.; Tian, Q.; Hurd Price, C.A.; Xu, L.; Qian, K.; Liu, J. Nanoporous Core@shell Particles: Design, Preparation, Applications in Bioadsorption and Biocatalysis. Nano Today 2020, 31, 100834. [Google Scholar] [CrossRef]
- Ren, Q.; Yi, C.; Pan, J.; Sun, X.; Huang, X. Smart Fe3O4@ZnO Core-Shell Nanophotosensitizers Potential for Combined Chemo and Photodynamic Skin Cancer Therapy Controlled by UVA Radiation. Int. J. Nanomed. 2022, 17, 3385–3400. [Google Scholar] [CrossRef] [PubMed]
- Madhubala, V.; Nagarajan, C.; Baskaran, P.; Raguraman, V.; Kalaivani, T. Formulation of Magnetic Core-Shell Nanostructured Fe3O4@TiO2 for Cytotoxic Activity against Huh-7 Cells. Inorg. Chem. Commun. 2023, 149, 110430. [Google Scholar] [CrossRef]
- Madhubala, V.; Nagarajan, C.; Baskaran, P.; Raguraman, V.; Kalaivani, T. Influences of Superparamagnetic Fe3O4@Ag Core-Shell Nanoparticles on the Growth Inhibition of Huh-7 Cells. Mater. Today Commun. 2023, 35, 106139. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Shameli, K.; Teow, S.Y.; Yusefi, M.; Kia, P.; Rasouli, E.; Tareq, M.A. Anticancer Activity of 5-Fluorouracil-Loaded Nanoemulsions Containing Fe3O4/Au Core-Shell Nanoparticles. J. Mol. Struct. 2021, 1245, 131075. [Google Scholar] [CrossRef]
- Sánchez-Orozco, J.L.; García-Cerda, L.A.; Puente-Urbina, B.; Meléndez-Ortiz, H.I. Poly(N-Vinylcaprolactam-Co-2-(Diethylamino)Ethylmethacrylate) Coated Fe3O4@SiO2 Core-Shell Magnetic Nanoparticles for Controlled Doxorubicin Delivery. J. Drug Deliv. Sci. Technol. 2023, 81, 104253. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Yeneayehu, K.; Senbeta, T.; Mesfin, B. Enhancement of the Optical Response of Fe3O4@Ag Core-Shell Nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2021, 134, 114822. [Google Scholar] [CrossRef]
- Díez, A.G.; Rincón-Iglesias, M.; Lanceros-Méndez, S.; Reguera, J.; Lizundia, E. Multicomponent Magnetic Nanoparticle Engineering: The Role of Structure-Property Relationship in Advanced Applications. Mater. Today Chem. 2022, 26, 101220. [Google Scholar] [CrossRef]
- Khatami, M.; Alijani, H.Q.; Nejad, M.S.; Varma, R.S. Core@shell Nanoparticles: Greener Synthesis Using Natural Plant Products. Appl. Sci. 2018, 8, 411. [Google Scholar] [CrossRef]
- Azizabadi, O.; Akbarzadeh, F.; Danshina, S.; Chauhan, N.P.S.; Sargazi, G. An Efficient Ultrasonic Assisted Reverse Micelle Synthesis Route for Fe3O4@Cu-MOF/Core-Shell Nanostructures and Its Antibacterial Activities. J. Solid State Chem. 2021, 294, 121897. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, Y.; He, M.; Zhao, Q.; Chen, C.; Hu, C.; Liu, Y. Controlled Synthesis of Fe3O4/Ag Core-Shell Composite Nanoparticles with High Electrical Conductivity. J. Electron. Mater. 2012, 41, 519–523. [Google Scholar] [CrossRef]
- Amarjargal, A.; Tijing, L.D.; Im, I.T.; Kim, C.S. Simultaneous Preparation of Ag/Fe3O4 Core-Shell Nanocomposites with Enhanced Magnetic Moment and Strong Antibacterial and Catalytic Properties. Chem. Eng. J. 2013, 226, 243–254. [Google Scholar] [CrossRef]
- Iglesias-Silva, E.; Rivas, J.; León Isidro, L.M.; López-Quintela, M.A. Synthesis of Silver-Coated Magnetite Nanoparticles. J. Non-Cryst. Solids 2007, 353, 829–831. [Google Scholar] [CrossRef]
- Mandal, M.; Kundu, S.; Ghosh, S.K.; Panigrahi, S.; Sau, T.K.; Yusuf, S.M.; Pal, T. Magnetite Nanoparticles with Tunable Gold or Silver Shell. J. Colloid Interface Sci. 2005, 286, 187–194. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Wykowska, U.; Satuła, D. Magnetic Nanoparticles of Core-Shell Structure. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 527–536. [Google Scholar] [CrossRef]
- Ghazanfari, M.; Johar, F.; Yazdani, A. Synthesis and Characterization of Fe3O4 @Ag Core-Shell: Structural, Morphological, and Magnetic Properties. J. Ultrafine Grained Nanostruct. Mater. 2014, 47, 97–103. [Google Scholar]
- Brollo, M.E.F.; López-Ruiz, R.; Muraca, D.; Figueroa, S.J.A.; Pirota, K.R.; Knobel, M. Compact Ag@Fe3O4 Core-Shell Nanoparticles by Means of Single-Step Thermal Decomposition Reaction. Sci. Rep. 2014, 4, 6839. [Google Scholar] [CrossRef]
- Dehghan, Z.; Ranjbar, M.; Govahi, M.; Khakdan, F. Green Synthesis of Ag/Fe3O4 Nanocomposite Utilizing Eryngium Planum L. Leaf Extract and Its Potential Applications in Medicine. J. Drug Deliv. Sci. Technol. 2022, 67, 102941. [Google Scholar] [CrossRef]
- Ding, Q.; Liu, D.; Guo, D.; Yang, F.; Pang, X.; Che, R.; Zhou, N.; Xie, J.; Sun, J.; Huang, Z.; et al. Shape-Controlled Fabrication of Magnetite Silver Hybrid Nanoparticles with High Performance Magnetic Hyperthermia. Biomaterials 2017, 124, 35–46. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Nguyen, V.T.; Nguyen, T.A. Biological Activity and Nanostructuration of Fe3O4-Ag/High Density Polyethylene Nanocomposites. J. Compos. Sci. 2019, 3, 34. [Google Scholar] [CrossRef]
- Singh, P.; Upadhyay, C. Role of Silver Nanoshells on Structural and Magnetic Behavior of Fe3O4 Nanoparticles. J. Magn. Magn. Mater. 2018, 458, 39–47. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Natesh Kumar, B.; Prathima, B.; Anitha, K.; Jyothi, N.V.V. A Novel Green Synthesis of Fe3O4-Ag Core Shell Recyclable Nanoparticles Using Vitis Vinifera Stem Extract and Its Enhanced Antibacterial Performance. Phys. B Condens. Matter 2015, 457, 30–35. [Google Scholar] [CrossRef]
- Sajjadi, M.; Nasrollahzadeh, M.; Mohammad Sajadi, S. Green Synthesis of Ag/Fe3O4 Nanocomposite Using Euphorbia Peplus Linn Leaf Extract and Evaluation of Its Catalytic Activity. J. Colloid Interface Sci. 2017, 497, 1–13. [Google Scholar] [CrossRef]
- Danafar, H.; Baghdadchi, Y.; Barsbay, M.; Ghaffarlou, M.; Mousazadeh, N.; Mohammadi, A. Synthesis of Fe3O4-Gold Hybrid Nanoparticles Coated by Bovine Serum Albumin as a Contrast Agent in MR Imaging. Heliyon 2023, 9, e13874. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Determination of Calreticulin Using Fe3O4@AuNPs Core-Shell Functionalized with PT(COOH)2 Polymer Modified Electrode: A New Platform for the Impedimetric Biosensing of Cancer Biomarkers. Sens. Actuators B Chem. 2022, 367, 132099. [Google Scholar] [CrossRef]
- Tarhan, T.; Ulu, A.; Sariçam, M.; Çulha, M.; Ates, B. Maltose Functionalized Magnetic Core/Shell Fe3O4@Au Nanoparticles for an Efficient L-Asparaginase Immobilization. Int. J. Biol. Macromol. 2020, 142, 443–451. [Google Scholar] [CrossRef]
- Wang, W.; Luo, J.; Fan, Q.; Suzuki, M.; Suzuki, I.S.; Engelhard, M.H.; Lin, Y.; Kim, N.; Wang, J.Q.; Zhong, C.J. Monodispersed Core-Shell Fe3O4@Au Nanoparticles. J. Phys. Chem. B 2005, 109, 21593–21601. [Google Scholar] [CrossRef]
- Chatterjee, K.; Sarkar, S.; Jagajjanani Rao, K.; Paria, S. Core/Shell Nanoparticles in Biomedical Applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef]
- Salihov, S.V.; Ivanenkov, Y.A.; Krechetov, S.P.; Veselov, M.S.; Sviridenkova, N.V.; Savchenko, A.G.; Klyachko, N.L.; Golovin, Y.I.; Chufarova, N.V.; Beloglazkina, E.K.; et al. Recent Advances in the Synthesis of Fe3O4@AU Core/Shell Nanoparticles. J. Magn. Magn. Mater. 2015, 394, 173–178. [Google Scholar] [CrossRef]
- Rajkumar, S.; Prabaharan, M. Multi-Functional Core-Shell Fe3O4@Au Nanoparticles for Cancer Diagnosis and Therapy. Colloids Surf. B Biointerfaces 2019, 174, 252–259. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Shameli, K.; Miyake, M.; Teow, S.Y.; Peh, S.C.; Mohamad, S.E.; Mohd Taib, S.H. Green Fabrication of Biologically Active Magnetic Core-Shell Fe3O4/Au Nanoparticles and Their Potential Anticancer Effect. Mater. Sci. Eng. C 2019, 96, 51–57. [Google Scholar] [CrossRef]
- Mostafaei, M.; Hosseini, S.N.; Khatami, M.; Javidanbardan, A.; Sepahy, A.A.; Asadi, E. Isolation of Recombinant Hepatitis B Surface Antigen with Antibody-Conjugated Superparamagnetic Fe3O4/SiO2 Core-Shell Nanoparticles. Protein Expr. Purif. 2018, 145, 1–6. [Google Scholar] [CrossRef]
- Shao, H.; Qi, J.; Lin, T.; Zhou, Y. Preparation and Characterization of Fe3O4@SiO2@NMDP Core-Shell Structure Composite Magnetic Nanoparticles. Ceram. Int. 2018, 44, 2255–2260. [Google Scholar] [CrossRef]
- Ta, T.K.H.; Trinh, M.T.; Long, N.V.; Nguyen, T.T.M.; Nguyen, T.L.T.; Thuoc, T.L.; Phan, B.T.; Mott, D.; Maenosono, S.; Tran-Van, H.; et al. Synthesis and Surface Functionalization of Fe3O4-SiO2 Core-Shell Nanoparticles with 3-Glycidoxypropyltrimethoxysilane and 1,1′-Carbonyldiimidazole for Bio-Applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 376–383. [Google Scholar] [CrossRef]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W. Synthesis and Characterization of Silica-Coated Iron Oxide Nanoparticles in Microemulsion: The Effect of Nonionic Surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar] [CrossRef]
- Tadyszak, K.; Kertmen, A.; Coy, E.; Andruszkiewicz, R.; Milewski, S.; Kardava, I.; Scheibe, B.; Jurga, S.; Chybczyńska, K. Spectroscopic and Magnetic Studies of Highly Dispersible Superparamagnetic Silica Coated Magnetite Nanoparticles. J. Magn. Magn. Mater. 2017, 433, 254–261. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmed, R.M.; Taha, M.; Soliman, T.S. Fe3O4 Nanoparticles and Fe3O4 @SiO2 Core-Shell: Synthesize, Structural, Morphological, Linear, and Nonlinear Optical Properties. J. Alloys Compd. 2023, 947, 169639. [Google Scholar] [CrossRef]
- Asgari, M.; Miri, T.; Soleymani, M.; Barati, A. A Novel Method for in Situ Encapsulation of Curcumin in Magnetite-Silica Core-Shell Nanocomposites: A Multifunctional Platform for Controlled Drug Delivery and Magnetic Hyperthermia Therapy. J. Mol. Liq. 2021, 324, 114731. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, G.H.; Yu, B.; Cong, H.L.; Kong, L.M.; Guo, L. Design and Synthesis of Fe3O4@SiO2 Core-Shell Nanomaterials. Integr. Ferroelectr. 2017, 182, 46–52. [Google Scholar] [CrossRef]
- Chen, X.; Selloni, A. Introduction: Titanium Dioxide (TiO2) Nanomaterials. Chem. Rev. 2014, 114, 9281–9282. [Google Scholar] [CrossRef] [PubMed]
- Madhubala, V.; Pugazhendhi, A.; Thirunavukarasu, K. Cytotoxic and Immunomodulatory Effects of the Low Concentration of Titanium Dioxide Nanoparticles (TiO2 NPs) on Human Cell Lines—An In Vitro Study. Process Biochem. 2019, 86, 186–195. [Google Scholar] [CrossRef]
- Khashan, S.; Dagher, S.; Tit, N.; Alazzam, A.; Obaidat, I. Novel Method for Synthesis of Fe3O4@TiO2 Core/Shell Nanoparticles. Surf. Coat. Technol. 2017, 322, 92–98. [Google Scholar] [CrossRef]
- Rani, N.; Dehiya, B.S. Influence of Anionic and Non-Ionic Surfactants on the Synthesis of Core-Shell Fe3O4@TiO2 Nanocomposite Synthesized by Hydrothermal Method. Ceram. Int. 2020, 46, 23516–23525. [Google Scholar] [CrossRef]
- Medina-Ramírez, I.E.; Díaz de León-Macias, C.E.; Pedroza-Herrera, G.; Gonzáles-Segovia, R.; Zapien, J.A.; Rodríguez-López, J.L. Evaluation of the Biocompatibility and Growth Inhibition of Bacterial Biofilms by ZnO, Fe3O4 and ZnO@Fe3O4 Photocatalytic Magnetic Materials. Ceram. Int. 2020, 46, 8979–8994. [Google Scholar] [CrossRef]
- Liu, H.; Wu, J.; Min, J.H.; Zhang, X.; Kim, Y.K. Tunable Synthesis and Multifunctionalities of Fe3O4-ZnO Hybrid Core-Shell Nanocrystals. Mater. Res. Bull. 2013, 48, 551–558. [Google Scholar] [CrossRef]
- Madhubala, V.; Kalaivani, T. Phyto and Hydrothermal Synthesis of Fe3O4@ZnO Core-Shell Nanoparticles Using Azadirachta Indica and Its Cytotoxicity Studies. Appl. Surf. Sci. 2018, 449, 584–590. [Google Scholar] [CrossRef]
- Manikandan, A.; Yogasundari, M.; Thanrasu, K.; Dinesh, A.; Raja, K.K.; Slimani, Y.; Jaganathan, S.K.; Srinivasan, R.; Baykal, A. Structural, Morphological and Optical Properties of Multifunctional Magnetic-Luminescent ZnO@Fe3O4 Nanocomposite. Phys. E Low Dimens. Syst. Nanostruct. 2020, 124, 114291. [Google Scholar] [CrossRef]
- Ahadpour Shal, A.; Jafari, A. Study of Structural and Magnetic Properties of Superparamagnetic Fe3O4-ZnO Core-Shell Nanoparticles. J. Supercond. Nov. Magn. 2014, 27, 1531–1538. [Google Scholar] [CrossRef]
- Aljohar, A.Y.; Muteeb, G.; Zia, Q.; Siddiqui, S.; Aatif, M.; Farhan, M.; Khan, M.F.; Alsultan, A.; Jamal, A.; Alshoaibi, A.; et al. Anticancer Effect of Zinc Oxide Nanoparticles Prepared by Varying Entry Time of Ion Carriers against A431 Skin Cancer Cells In Vitro. Front. Chem. 2022, 10, 1069450. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Li, X.; Wang, D.; Wei, B.; Song, H.; Li, X.; Fu, S. Preparation and Photocatalytic Properties of Magnetically Reusable Fe3O4@ZnO Core/Shell Nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2016, 75, 66–71. [Google Scholar] [CrossRef]
- Sin, J.C.; Tan, S.Q.; Quek, J.A.; Lam, S.M.; Mohamed, A.R. Facile Fabrication of Hierarchical Porous ZnO/Fe3O4 Composites with Enhanced Magnetic, Photocatalytic and Antibacterial Properties. Mater. Lett. 2018, 228, 207–211. [Google Scholar] [CrossRef]
- Tang, S.; Lan, Q.; Liang, J.; Chen, S.; Liu, C.; Zhao, J.; Cheng, Q.; Cao, Y.C.; Liu, J. Facile Synthesis of Fe3O4@PPy Core-Shell Magnetic Nanoparticles and Their Enhanced Dispersity and Acid Stability. Mater. Des. 2017, 121, 47–50. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Asghari, S.; Aslibeiki, B. Surface Modified Fe3O4 Nanoparticles: A Cross-Linked Polyethylene Glycol Coating Using Plasma Treatment. Surf. Interfaces 2021, 25, 101271. [Google Scholar] [CrossRef]
- Munir, T.; Mahmood, A.; Rasul, A.; Imran, M.; Fakhar-e-Alam, M. Biocompatible Polymer Functionalized Magnetic Nanoparticles for Antimicrobial and Anticancer Activities. Mater. Chem. Phys. 2023, 301, 127677. [Google Scholar] [CrossRef]
- Bekaroğlu, M.G.; Alemdar, A.; İşçi, S. Comparison of Ionic Polymers in the Targeted Drug Delivery Applications as the Coating Materials on the Fe3O4 Nanoparticles. Mater. Sci. Eng. C 2019, 103, 109838. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Tran, D.H.N.; Bach, L.G.; Quang, H.V.; Nguyen, D.C.; Park, K.D.; Nguyen, D.H. Functional Magnetic Core-Shell System-Based Iron Oxide Nanoparticle Coated with Biocompatible Copolymer for Anticancer Drug Delivery. Pharmaceutics 2019, 11, 120. [Google Scholar] [CrossRef]
- Ding, Y.; Shen, S.Z.; Sun, H.; Sun, K.; Liu, F.; Qi, Y.; Yan, J. Design and Construction of Polymerized-Chitosan Coated Fe3O4 Magnetic Nanoparticles and Its Application for Hydrophobic Drug Delivery. Mater. Sci. Eng. C 2015, 48, 487–498. [Google Scholar] [CrossRef]
- Yeamsuksawat, T.; Zhao, H.; Liang, J. Characterization and Antimicrobial Performance of Magnetic Fe3O4@Chitosan@Ag Nanoparticles Synthesized via Suspension Technique. Mater. Today Commun. 2021, 28, 102481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).