Production of Value-Added Products as Food Ingredients via Microbial Fermentation
Abstract
1. Introduction
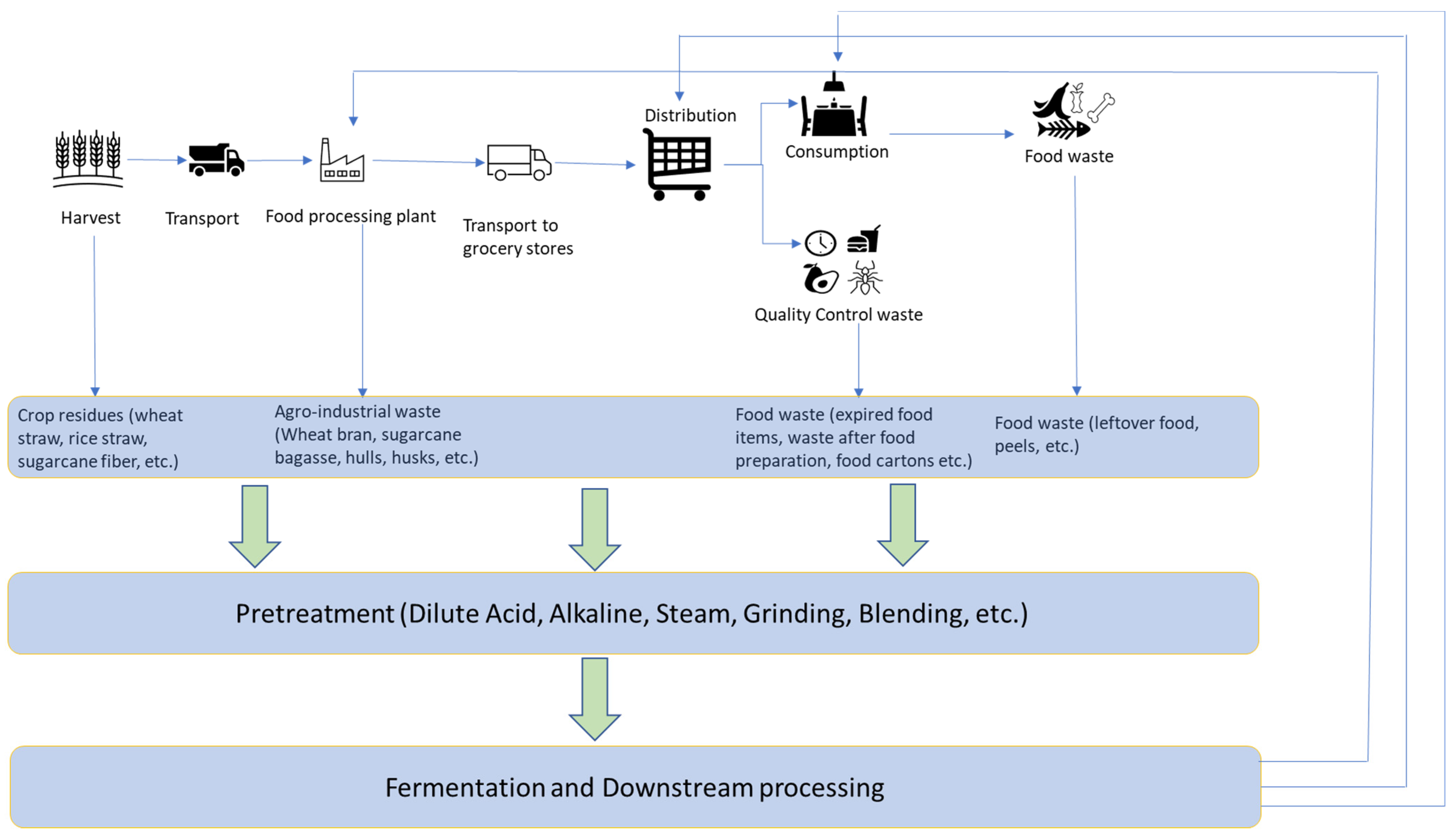
2. Food Ingredients as the Fermentation Products
2.1. Enzymes
| Inexpensive Feedstock | Products | Examples | Pretreatment Conditions | Refs. |
|---|---|---|---|---|
| Agricultural waste | Enzymes | Crop straw, Poplar wood, sawdust | Grinding | [65,66] |
| Other value-added products | Cattle dung, rice straw, wheat straw | Hydrothermal treatment, Mild chemical treatment | [67,68] | |
| Food waste | Enzymes | Banana skin, bagasse | Sulfuric acid hydrolysis | [65,69] |
| Monosaccharides | Wheat bran, coffee waste | Mild chemical treatment, Hydrothermal treatment | [70] | |
| Other value-added products | Cucumber, tomato, lettuce, lemon peel | ultrasonic and ozone pretreatment | [71] | |
| Oceanic seaweed | Lactic acid | Brown, red or green alga | Acid and/or enzymatic hydrolysis | [72] |
| Other value-added products | Brown seaweed | Ethanol extraction | [73] |
| Category | Value-Added Ingredient | Application in Food Industry | Refs. |
|---|---|---|---|
| Enzymes | Protease | Coagulation of milk, Bread quality enhancement, Meat tenderization, Brewing | [74] |
| Amylase | Baking, Brewing, Clarification of fruit juices | [74] | |
| Cellulase | Clarification of fruit juices, Animal feed | [74] | |
| Hemicellulase | Beer improvement | [75] | |
| Antimicrobials | Nisin | Shelf-life extension | [76] |
| Lysozyme | Decreasing the microbial population in food | [76] | |
| Natamycin | Inhibiting the growth of harmful mold | [77] | |
| Vitamins | B2, B12, K | Improve food quality | [3] |
| Sweeteners | Sugar Alcohols | Improve the flavor, health concerns, diabetic food industry | [78] |
| Cultured meat | Non-animal-based meat | Vegetarian/vegan industry | [79] |
| Stabilizers | Xanthan gum | Shelf-life extension | [80] |
| Gellan | [80] | ||
| Curdlan | [80] |
2.2. Antimicrobials
2.3. Vitamins
2.4. Organic Acids
2.5. Sweeteners
2.6. Flavonoids
2.7. Cultured Meat Products
2.8. Oligosaccharides and Polysaccharides
2.9. Amino Acids
2.10. Food Colorants
2.11. Antioxidants
2.12. Lipids and Fatty Acids
2.13. Alcohols
3. Inexpensive Substrates for Such Fermentations
3.1. Agricultural Wastes
3.2. Food Wastes
4. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martí-Quijal, F.J.; Khubber, S.; Remize, F.; Tomasevic, I.; Roselló-Soto, E.; Barba, F.J. Obtaining antioxidants and natural preservatives from food by-products through fermentation: A review. Fermentation 2021, 7, 106. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Simpson, B.K. Enzymes in food bioprocessing—Novel food enzymes, applications, and related techniques. Curr. Opin. Food Sci. 2018, 19, 30–35. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Jin, Z.; Zhang, D. Microbial Cell Factories for Green Production of Vitamins. Front. Bioeng. Biotechnol. 2021, 9, 473. [Google Scholar] [CrossRef]
- Gürler, H.N.; Erkan, S.B.; Ozcan, A.; Yılmazer, C.; Karahalil, E.; Germec, M.; Yatmaz, E.; Ogel, Z.B.; Turhan, I. Scale-up processing with different microparticle agent for β-mannanase production in a large-scale stirred tank bioreactor. J. Food Process. Preserv. 2021, 45, e14915. [Google Scholar] [CrossRef]
- Yilmazer, C.; Germec, M.; Turhan, I. Solid-state fermentation for the production of a recombinant β-mannanase from Aspergillus fumigatus expressed in Aspergillus sojae grown on renewable resources. J. Food Process. Preserv. 2021, 45, e14584. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; Zhang, T.; Wang, Z.; Xu, J.; Xia, J.; He, A.; Yan, Y.; Xu, J. Novel two-stage solid-state fermentation for erythritol production on okara–buckwheat husk medium. Bioresour. Technol. 2018, 266, 439–446. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wu, W.J.; Xu, Y.Y.; Zhou, B.; Niu, K.; Liu, Z.Q.; Zheng, Y.G. Calcium Carbonate Addition Improves L-Methionine Biosynthesis by Metabolically Engineered Escherichia coli W3110-BL. Front. Bioeng. Biotechnol. 2020, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.; Gonçalves, D.A.; Teixeira, J.A.; Rodrigues, L.R. One-step co-culture fermentation strategy to produce high-content fructo-oligosaccharides. Carbohydr. Polym. 2018, 201, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zabed, H.; Zhang, H.; Wang, X.; Yun, J.; Zhang, G.; Yang, M.; Sun, W.; Qi, X. Optimization of fermentation medium for a newly isolated yeast strain (Zygosaccharomyces rouxii JM-C46) and evaluation of factors affecting biosynthesis of D-arabitol. LWT 2019, 99, 319–327. [Google Scholar] [CrossRef]
- Elumalai, P.; Lim, J.-M.; Park, Y.-J.; Cho, M.; Shea, P.J.; Oh, B.-T. Agricultural waste materials enhance protease production by Bacillus subtilis B22 in submerged fermentation under blue light-emitting diodes. Bioprocess Biosyst. Eng. 2020, 43, 821–830. [Google Scholar] [CrossRef]
- Lario, L.D.; Pillaca-Pullo, O.S.; Sette, L.D.; Converti, A.; Casati, P.; Spampinato, C.; Pessoa, A. Optimization of protease production and sequence analysis of the purified enzyme from the cold adapted yeast Rhodotorula mucilaginosa CBMAI 1528. Biotechnol. Reports 2020, 28, e00546. [Google Scholar] [CrossRef]
- Suleiman, A.D.; Rahman, A.; Mohd Yusof, H.; Mohd Shariff, F.; Yasid, N.A. Effect of cultural conditions on protease production by a thermophilic Geobacillus thermoglucosidasius SKF4 isolated from Sungai Klah hot Spring Park, Malaysia. Molecules 2020, 25, 2609. [Google Scholar] [CrossRef]
- Rocha, F.T.B.; Brandão-Costa, R.M.P.; Neves, A.G.D.; Cardoso, K.B.B.; Nascimento, T.P.; Albuquerque, W.W.C.; Porto, A.L.F. Purification and characterization of a protease from Aspergillus sydowii URM5774: Coffee ground residue for protease production by solid state fermentation. An. Acad. Bras. Cienc. 2021, 93. [Google Scholar] [CrossRef] [PubMed]
- Mumecha, T.K.; Zewde, F.T. Alkaline protease production using eggshells and membrane-based substrates: Process modeling, optimization, and evaluation of detergent potency. Eng. Appl. Sci. Res. 2021, 48, 171–180. [Google Scholar]
- Ma, X.; Kexin, Z.; Yonggang, W.; Ebadi, A.G.; Toughani, M. Optimization of Low-Temperature Lipase Production Conditions and Study on Enzymatic Properties of Aspergillus Niger. Iran. J. Chem. Chem. Eng. 2021, 40, 1364–1374. [Google Scholar]
- Amin, M.; Bhatti, H.N.; Sadaf, S.; Bilal, M. Optimization of lipase production by response surface methodology and its application for efficient biodegradation of polyester vylon-200. Catal. Letters 2021, 151, 3603–3616. [Google Scholar] [CrossRef]
- Izmirlioglu, G.; Demirci, A. Strain selection and medium optimization for glucoamylase production from industrial potato waste by Aspergillus niger. J. Sci. Food Agric. 2016, 96, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Jeevarathinam, G.; Kumar, S.K.S.; Muniraj, I.; Uthandi, S. Optimization and scale-up of α-amylase production by Aspergillus oryzae using solid-state fermentation of edible oil cakes. BMC Biotechnol. 2021, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Salt and nitrogen amendment and optimization for cellulase and xylanase production using dilute acid hydrolysate of distillers’ dried grains with solubles (DDGS) as the feedstock. Bioprocess Biosyst. Eng. 2022, 45, 527–540. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Britt, D.W.; Demirci, A. Development of Bioactive Solid Support for Immobilized Lactococcus lactis Biofilms in Bioreactors for the Production of Nisin. Food Bioprocess Technol. 2022, 15, 132–143. [Google Scholar] [CrossRef]
- Ercan, D.; Demirci, A. Production of human lysozyme in biofilm reactor and optimization of growth parameters of Kluyveromyces lactis K7. Appl. Microbiol. Biotechnol. 2013, 97, 6211–6221. [Google Scholar] [CrossRef] [PubMed]
- Ercan, D.; Demirci, A. Enhanced human lysozyme production in biofilm reactor by Kluyveromyces lactis K7. Biochem. Eng. J. 2014, 92, 2–8. [Google Scholar] [CrossRef]
- He, H.; Wu, S.; Mei, M.; Ning, J.; Li, C.; Ma, L.; Zhang, G.; Yi, L. A combinational strategy for effective heterologous production of functional human lysozyme in Pichia pastoris. Front. Bioeng. Biotechnol. 2020, 8, 118. [Google Scholar] [CrossRef]
- Xie, C.; Coda, R.; Chamlagain, B.; Edelmann, M.; Varmanen, P.; Piironen, V.; Katina, K. Fermentation of cereal, pseudo-cereal and legume materials with Propionibacterium freudenreichii and Levilactobacillus brevis for vitamin B12 fortification. LWT 2021, 137, 110431. [Google Scholar] [CrossRef]
- Mahdinia, E.; Demirci, A.; Berenjian, A. Optimization of Bacillus subtilis natto growth parameters in glycerol-based medium for vitamin K (menaquinone-7) production in biofilm reactors. Bioprocess Biosyst. Eng. 2018, 41, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Mahdinia, E.; Demirci, A.; Berenjian, A. Implementation of fed-batch strategies for vitamin K (menaquinone-7) production by Bacillus subtilis natto in biofilm reactors. Appl. Microbiol. Biotechnol. 2018, 102, 9147–9157. [Google Scholar] [CrossRef]
- Turhan, I.; Bialka, K.L.; Demirci, A.; Karhan, M. Enhanced lactic acid production from carob extract by Lactobacillus casei using invertase pretreatment. Food Biotechnol. 2010, 24, 364–374. [Google Scholar] [CrossRef]
- Chen, P.-T.; Hong, Z.-S.; Cheng, C.-L.; Ng, I.-S.; Lo, Y.-C.; Nagarajan, D.; Chang, J.-S. Exploring fermentation strategies for enhanced lactic acid production with polyvinyl alcohol-immobilized Lactobacillus plantarum 23 using microalgae as feedstock. Bioresour. Technol. 2020, 308, 123266. [Google Scholar] [CrossRef]
- Ali, R.; Saravia, F.; Hille-Reichel, A.; Gescher, J.; Horn, H. Propionic acid production from food waste in batch reactors: Effect of pH, types of inoculum, and thermal pre-treatment. Bioresour. Technol. 2021, 319, 124166. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, B.; Zhang, T.; Chen, J. Combined mutagenesis and metabolic regulation to enhance d-arabitol production from Candida parapsilosis. J. Ind. Microbiol. Biotechnol. 2020, 47, 425–435. [Google Scholar] [CrossRef]
- Yang, L.B.; Kong, W.; Yang, W.; Li, D.; Zhao, S.; Wu, Y.; Zheng, S. High D-arabitol production with osmotic pressure control fed-batch fermentation by Yarrowia lipolytica and proteomic analysis under nitrogen source perturbation. Enzyme Microb. Technol. 2022, 152, 109936. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, X.; Chen, S.; Yan, Y.; He, J.; Xu, J.; Xu, J. Enhancing erythritol production by wheat straw biochar-incorporated solid-state fermentation of agricultural wastes using defatted Schizochytrium sp. biomass as supplementary feedstock. Ind. Crops Prod. 2021, 170, 113703. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. By-products of sugar factories and wineries as feedstocks for erythritol generation. Food Bioprod. Process. 2021, 126, 345–355. [Google Scholar] [CrossRef]
- Deshpande, M.S.; Kulkarni, P.P.; Kumbhar, P.S.; Ghosalkar, A.R. Erythritol production from sugar based feedstocks by Moniliella pollinis using lysate of recycled cells as nutrients source. Process Biochem. 2022, 112, 45–52. [Google Scholar] [CrossRef]
- Rice, T.; Sahin, A.W.; Lynch, K.M.; Arendt, E.K.; Coffey, A. Isolation, characterisation and exploitation of lactic acid bacteria capable of efficient conversion of sugars to mannitol. Int. J. Food Microbiol. 2020, 321, 108546. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. Mannitol bioproduction from surplus grape musts and wine lees. LWT 2021, 151, 112083. [Google Scholar] [CrossRef]
- de la Rosa, O.; Múñiz-Marquez, D.B.; Contreras-Esquivel, J.C.; Wong-Paz, J.E.; Rodríguez-Herrera, R.; Aguilar, C.N. Improving the fructooligosaccharides production by solid-state fermentation. Biocatal. Agric. Biotechnol. 2020, 27, 101704. [Google Scholar] [CrossRef]
- Nasir, A.; Sattar, F.; Ashfaq, I.; Lindemann, S.R.; Chen, M.H.; Van den Ende, W.; Öner, E.T.; Kirtel, O.; Khaliq, S.; Ghauri, M.A.; et al. Production and characterization of a high molecular weight levan and fructooligosaccharides from a rhizospheric isolate of Bacillus aryabhattai. LWT 2020, 123, 109093. [Google Scholar] [CrossRef]
- Han, S.; Pan, L.; Zeng, W.; Yang, L.; Yang, D.; Chen, G.; Liang, Z. Improved production of fructooligosaccharides (FOS) using a mutant strain of Aspergillus oryzae S719 overexpressing β-fructofuranosidase (FTase) genes. LWT 2021, 146, 111346. [Google Scholar] [CrossRef]
- Abdeshahian, P.; Ascencio, J.J.; Philippini, R.R.; Antunes, F.A.F.; dos Santos, J.C.; da Silva, S.S. Utilization of sugarcane straw for production of β-glucan biopolymer by Lasiodiplodia theobromae CCT 3966 in batch fermentation process. Bioresour. Technol. 2020, 314, 123716. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Koczoń, P.; Błażejak, S.; Kozera, J.; Kieliszek, M. Valorization of Deproteinated Potato Juice Water into β-Glucan Preparation of C. utilis Origin: Comparative Study of Preparations Obtained by Two Isolation Methods. Waste Biomass Valorization 2020, 11, 3257–3271. [Google Scholar] [CrossRef]
- Acosta, S.B.P.; Marchioro, M.L.K.; Santos, V.A.Q.; Calegari, G.C.; Lafay, C.B.B.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; da Cunha, M.A.A. Valorization of Soybean Molasses as Fermentation Substrate for the Production of Microbial Exocellular β-Glucan. J. Polym. Environ. 2020, 28, 2149–2160. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; He, C.Y.; Wang, G.L.; Zhang, G.C.; Wang, C.L.; Wang, D.H.; Zou, X.; Wei, G.Y. Improved production of β-glucan by a T-DNA–based mutant of Aureobasidium pullulans. Appl. Microbiol. Biotechnol. 2021, 105, 6887–6898. [Google Scholar] [CrossRef] [PubMed]
- Rishi, V.; Sandhu, A.K.; Kaur, A.; Kaur, J.; Sharma, S.; Soni, S.K. Utilization of kitchen waste for production of pullulan to develop biodegradable plastic. Appl. Microbiol. Biotechnol. 2020, 104, 1307–1317. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Zhang, Y.; Wang, G.; Wang, C.; Wang, D.; Wei, G. Efficient pullulan production by Aureobasidium pullulans using cost-effective substrates. Int. J. Biol. Macromol. 2021, 186, 544–553. [Google Scholar] [CrossRef]
- Haghighatpanah, N.; Khodaiyan, F.; Kennedy, J.F.; Hosseini, S.S. Optimization and characterization of pullulan obtained from corn bran hydrolysates by Aerobasidiom pullulan KY767024. Biocatal. Agric. Biotechnol. 2021, 33, 101959. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan production in stirred tank reactor by a colour-variant strain of Aureobasidium pullulans FB-1. Carbohydr. Polym. Technol. Appl. 2021, 2, 100086. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Innasimuthu, G.M.; Rajoo, B.; Alanzi, K.F.; Rajaram, S.K. Optimization of glutamic acid production by Corynebacterium glutamicum using response surface methodology. J. King Saud Univ.-Sci. 2020, 32, 1403–1408. [Google Scholar] [CrossRef]
- Fahimitabar, A.; Razavian, S.M.H.; Rezaei, S.A. Application of RSM for optimization of glutamic acid production by Corynebacterium glutamicum in bath culture. Heliyon 2021, 7, e07359. [Google Scholar] [CrossRef]
- Tang, X.L.; Du, X.Y.; Chen, L.J.; Liu, Z.Q.; Zheng, Y.G. Enhanced production of l-methionine in engineered Escherichia coli with efficient supply of one carbon unit. Biotechnol. Lett. 2020, 42, 429–436. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Wang, H.; Liu, L.; Fang, H.; Chen, T.; Zhang, D. Engineering Escherichia coli to improve tryptophan production via genetic manipulation of precursor and cofactor pathways. Synth. Syst. Biotechnol. 2020, 5, 200–205. [Google Scholar] [CrossRef]
- Lim, Y.H.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Rahim, R.A. Rapid evaluation and optimization of medium components governing tryptophan production by pediococcus acidilactici tp-6 isolated from Malaysian food via statistical approaches. Molecules 2020, 25, 779. [Google Scholar] [CrossRef]
- Guo, L.; Ding, S.; Liu, Y.; Gao, C.; Hu, G.; Song, W.; Liu, J.; Chen, X.; Liu, L. Enhancing tryptophan production by balancing precursors in Escherichia coli. Biotechnol. Bioeng. 2022, 119, 983–993. [Google Scholar] [CrossRef]
- Xu, J.Z.; Ruan, H.Z.; Yu, H.B.; Liu, L.M.; Zhang, W. Metabolic engineering of carbohydrate metabolism systems in Corynebacterium glutamicum for improving the efficiency of l-lysine production from mixed sugar. Microb. Cell Fact. 2020, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Singh, R.; Jahan, S.; Alreshidi, M.; Hamadou, W.S.; Khan, A.; Ahmad, A.; Patel, M.; Abdelmuhsin, A.A.; Sulieman, A.M.E.; et al. Enzymes in Food Fermentations. In African Fermented Food Products- New Trends; Elhadi Sulieman, A.M., Adam Mariod, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 101–133. ISBN 978-3-030-82902-5. [Google Scholar]
- Hyseni, B.; Aytekin, A.Ö.; Nikerel, E. Solid state fermentation for enzyme production for food industry. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 615–622. [Google Scholar] [CrossRef]
- Shah, M.A.; Mir, S.A.; Paray, M.A. Plant proteases as milk-clotting enzymes in cheesemaking: A review. Dairy Sci. Technol. 2014, 94, 5–16. [Google Scholar] [CrossRef]
- Visser, S. Proteolytic enzymes and their relation to cheese ripening and flavor: An overview. J. Dairy Sci. 1993, 76, 329–350. [Google Scholar] [CrossRef]
- Fatima, S.; Faryad, A.; Ataa, A.; Joyia, F.A.; Parvaiz, A. Microbial lipase production: A deep insight into the recent advances of lipase production and purification techniques. Biotechnol. Appl. Biochem. 2021, 68, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Mala, J.G.S.; Takeuchi, S. Understanding Structural Features of Microbial Lipases–-An Overview. Anal. Chem. Insights 2008, 3, ACI-S551. [Google Scholar] [CrossRef]
- Hilton, S.; Buckley, J.T. Studies on the reaction mechanism of a microbial lipase/acyltransferase using chemical modification and site-directed mutagenesis. J. Biol. Chem. 1991, 266, 997–1000. [Google Scholar] [CrossRef]
- Pandey, A.; Benjamin, S.; Soccol, C.R.; Nigam, P.; Krieger, N.; Soccol, V.T. The realm of microbial lipases in biotechnology. Biotechnol. Appl. Biochem. 1999, 29, 119–131. [Google Scholar] [PubMed]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Screening of bacterial and fungal strains for cellulase and xylanase production using distillers’ dried grains with solubles (DDGS) as the main feedstock. Biomass Convers. Biorefinery 2020, 11, 1955–1964. [Google Scholar] [CrossRef]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Ideal Feedstock and Fermentation Process Improvements for the Production of Lignocellulolytic Enzymes. Processes 2021, 9, 38. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal laccase production from lignocellulosic agricultural wastes by solid-state fermentation: A review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef]
- Raina, N.; Slathia, P.S.; Sharma, P. Experimental optimization of thermochemical pretreatment of sal (Shorea robusta) sawdust by Central Composite Design study for bioethanol production by co-fermentation using Saccharomyces cerevisiae (MTCC-36) and Pichia stipitis (NCIM-3498). Biomass Bioenergy 2020, 143, 105819. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Raza, W.; Luo, J.; Zhang, F.; Shen, Q. Solid-state fermentation of agro-industrial wastes to produce bioorganic fertilizer for the biocontrol of Fusarium wilt of cucumber in continuously cropped soil. Bioresour. Technol. 2011, 102, 3900–3910. [Google Scholar] [CrossRef]
- Kumar, V.; Bahuguna, A.; Ramalingam, S.; Kim, M. Developing a sustainable bioprocess for the cleaner production of xylooligosaccharides: An approach towards lignocellulosic waste management. J. Clean. Prod. 2021, 316, 128332. [Google Scholar] [CrossRef]
- Panakkal, E.J.; Sriariyanun, M.; Ratanapoompinyo, J.; Yasurin, P.; Cheenkachorn, K.; Rodiahwati, W.; Tantayotai, P. Influence of sulfuric acid pretreatment and inhibitor of sugarcane bagasse on the production of fermentable sugar and ethanol. Appl. Sci. Eng. Prog. 2022, 15. [Google Scholar] [CrossRef]
- Javed, U.; Ansari, A.; Aman, A.; Qader, S.A.U. Fermentation and saccharification of agro-industrial wastes: A cost-effective approach for dual use of plant biomass wastes for xylose production. Biocatal. Agric. Biotechnol. 2019, 21, 101341. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; Gonzalez-Fernandez, C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef]
- Lin, H.-T.V.; Huang, M.-Y.; Kao, T.-Y.; Lu, W.-J.; Lin, H.-J.; Pan, C.-L. Production of lactic acid from seaweed hydrolysates via lactic acid bacteria fermentation. Fermentation 2020, 6, 37. [Google Scholar] [CrossRef]
- Hoffmann, S.L.; Kohlstedt, M.; Jungmann, L.; Hutter, M.; Poblete-Castro, I.; Becker, J.; Wittmann, C. Cascaded valorization of brown seaweed to produce l-lysine and value-added products using Corynebacterium glutamicum streamlined by systems metabolic engineering. Metab. Eng. 2021, 67, 293–307. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Beevi Ummalyma, S.; Abraham, A.; Kuruvilla Mathew, A.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef]
- Bhat, M.K. Cellulases and related enzymes in biotechnology. Biotechnol. Adv. 2000, 18, 355–383. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A natural preservative for food applications—A review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Sweeteners as food additives in the XXI century: A review of what is known, and what is to come. Food Chem. Toxicol. 2017, 107, 302–317. [Google Scholar] [CrossRef]
- Hong, T.K.; Shin, D.-M.; Choi, J.; Do, J.T.; Han, S.G. Current issues and technical advances in cultured meat production: A review. Food Sci. Anim. Resour. 2021, 41, 355. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Brum, L.F.W.; dos Santos, C.; Zimnoch Santos, J.H.; Brandelli, A. Structured silica materials as innovative delivery systems for the bacteriocin nisin. Food Chem. 2022, 366, 130599. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.; Sandhu, P.; Ror, P.; Dash, E.; Sharma, S.; Arakha, M.; Jha, S.; Akhter, Y.; Saleem, M. Lipid-II independent antimicrobial mechanism of nisin depends on its crowding and degree of oligomerization. Sci. Rep. 2016, 6, 37908. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, D.; Guo, K.; Dong, K.; Xu, D.; Wu, Z. Online recovery of nisin during fermentation coupling with foam fractionation. J. Food Eng. 2015, 162, 25–30. [Google Scholar] [CrossRef]
- Kördikanlıoğlu, B.; Şimşek, Ö.; Saris, P.E.J. Nisin production of Lactococcus lactis N 8 with hemin-stimulated cell respiration in fed-batch fermentation system. Biotechnol. Prog. 2015, 31, 678–685. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Yan, G.; Cui, Y.; Cheng, Q.; Zhang, Z.; Meng, Q.; Teng, L.; Ren, X. Aeration and fermentation strategies on nisin production. Biotechnol. Lett. 2015, 37, 2039–2045. [Google Scholar] [CrossRef]
- Ariana, M.; Hamedi, J. Enhanced production of nisin by co-culture of Lactococcus lactis sub sp. lactis and Yarrowia lipolytica in molasses based medium. J. Biotechnol. 2017, 256, 21–26. [Google Scholar] [CrossRef]
- Davidson, P.M.; Doan, C. Natamycin. In Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 339–356. ISBN 0429058195. [Google Scholar]
- Masschalck, B.; Michiels, C.W. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 2003, 29, 191–214. [Google Scholar] [CrossRef]
- Leśnierowski, G.; Yang, T. Lysozyme and its modified forms: A critical appraisal of selected properties and potential. Trends Food Sci. Technol. 2021, 107, 333–342. [Google Scholar] [CrossRef]
- Mahdinia, E.; Demirci, A.; Berenjian, A. Utilization of glucose-based medium and optimization of Bacillus subtilis natto growth parameters for vitamin K (menaquinone-7) production in biofilm reactors. Biocatal. Agric. Biotechnol. 2018, 13, 219–224. [Google Scholar] [CrossRef]
- Duan, Y.; Mehariya, S.; Kumar, A.; Singh, E.; Yang, J.; Kumar, S.; Li, H.; Kumar Awasthi, M. Apple orchard waste recycling and valorization of valuable product-A review. Bioengineered 2021, 12, 476–495. [Google Scholar] [CrossRef]
- Paulino, B.N.; Molina, G.; Pastore, G.M.; Bicas, J.L. Current perspectives in the biotechnological production of sweetening syrups and polyols. Curr. Opin. Food Sci. 2021, 41, 36–43. [Google Scholar] [CrossRef]
- Rice, T.; Zannini, E.; Arendt, E.K.; Coffey, A. A review of polyols–biotechnological production, food applications, regulation, labeling and health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef]
- Felipe Hernández-Pérez, A.; Jofre, F.M.; De Souza Queiroz, S.; Vaz De Arruda, P.; Chandel, A.K.; Felipe, M.D.G.D.A. Biotechnological production of sweeteners. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 261–292. ISBN 9780444643230. [Google Scholar]
- Liu, X.; Yu, X.; Gao, S.; Dong, X.; Xia, J.; Xu, J.; He, A.; Hu, L.; Yan, Y.; Wang, Z. Enhancing the erythritol production by Yarrowia lipolytica from waste oil using loofah sponge as oil-in-water dispersant. Biochem. Eng. J. 2019, 151, 107302. [Google Scholar] [CrossRef]
- Goris, T.; Pérez-Valero, Á.; Martínez, I.; Yi, D.; Fernández-Calleja, L.; San Leon, D.; Bornscheuer, U.T.; Magadán-Corpas, P.; Lombo, F.; Nogales, J. Repositioning microbial biotechnology against COVID-19: The case of microbial production of flavonoids. Microb. Biotechnol. 2021, 14, 94–110. [Google Scholar] [CrossRef]
- Post, M.J. Cultured meat from stem cells: Challenges and prospects. Meat Sci. 2012, 92, 297–301. [Google Scholar] [CrossRef]
- Yue, P.; Hu, Y.; Tian, R.; Bian, J.; Peng, F. Hydrothermal pretreatment for the production of oligosaccharides: A review. Bioresour. Technol. 2022, 343, 126075. [Google Scholar] [CrossRef]
- Bhatia, L.; Sharma, A.; Bachheti, R.K.; Chandel, A.K. Lignocellulose derived functional oligosaccharides: Production, properties, and health benefits. Prep. Biochem. Biotechnol. 2019, 49, 744–758. [Google Scholar] [CrossRef]
- Su, Z.; Luo, J.; Li, X.; Pinelo, M. Enzyme membrane reactors for production of oligosaccharides: A review on the interdependence between enzyme reaction and membrane separation. Sep. Purif. Technol. 2020, 243, 116840. [Google Scholar] [CrossRef]
- Mano, M.C.R.; Neri-Numa, I.A.; da Silva, J.B.; Paulino, B.N.; Pessoa, M.G.; Pastore, G.M. Oligosaccharide biotechnology: An approach of prebiotic revolution on the industry. Appl. Microbiol. Biotechnol. 2018, 102, 17–37. [Google Scholar] [CrossRef]
- Bych, K.; Mikš, M.H.; Johanson, T.; Hederos, M.J.; Vigsnæs, L.K.; Becker, P. Production of HMOs using microbial hosts—From cell engineering to large scale production. Curr. Opin. Biotechnol. 2019, 56, 130–137. [Google Scholar] [CrossRef]
- de la Rosa, O.; Flores-Gallegos, A.C.; Muñíz-Marquez, D.; Nobre, C.; Contreras-Esquivel, J.C.; Aguilar, C.N. Fructooligosaccharides production from agro-wastes as alternative low-cost source. Trends Food Sci. Technol. 2019, 91, 139–146. [Google Scholar] [CrossRef]
- Santibáñez, L.; Henríquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from lignocellulosic biomass: A comprehensive review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef] [PubMed]
- Himashree, P.; Sengar, A.S.; Sunil, C.K. Food thickening agents: Sources, chemistry, properties and applications—A review. Int. J. Gastron. Food Sci. 2022, 27, 100468. [Google Scholar] [CrossRef]
- Horue, M.; Rivero Berti, I.; Cacicedo, M.L.; Castro, G.R. Microbial production and recovery of hybrid biopolymers from wastes for industrial applications- a review. Bioresour. Technol. 2021, 340, 125671. [Google Scholar] [CrossRef]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2016, 36, 594–606. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 2019, 217, 46–57. [Google Scholar] [CrossRef]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef]
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino acids production focusing on fermentation technologies—A review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef]
- Wendisch, V.F. Metabolic engineering advances and prospects for amino acid production. Metab. Eng. 2020, 58, 17–34. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, X.; Liang, X.; Wang, Y.; Feng, D.; Zhang, Y.; Xian, M.; Zou, H. Improving the Microbial Production of Amino Acids: From Conventional Approaches to Recent Trends. Biotechnol. Bioprocess Eng. 2021, 26, 708–727. [Google Scholar] [CrossRef]
- Han, X.; Li, L.; Bao, J. Microbial extraction of biotin from lignocellulose biomass and its application on glutamic acid production. Bioresour. Technol. 2019, 288, 121523. [Google Scholar] [CrossRef]
- Coban, H.B.; Demirci, A.; Patterson, P.H.; Elias, R.J. Enhanced phenylpyruvic acid production with Proteus vulgaris in fed-batch and continuous fermentation. Prep. Biochem. Biotechnol. 2016, 46, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Rana, B.; Bhattacharyya, M.; Patni, B.; Arya, M.; Joshi, G.K. The Realm of Microbial Pigments in the Food Color Market. Front. Sustain. Food Syst. 2021, 5, 54. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef]
- Lin, Y.; Jain, R.; Yan, Y. Microbial production of antioxidant food ingredients via metabolic engineering. Curr. Opin. Biotechnol. 2014, 26, 71–78. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef] [PubMed]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Bhanja Dey, T.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Béligon, V.; Christophe, G.; Fontanille, P.; Larroche, C. Microbial lipids as potential source to food supplements. Curr. Opin. Food Sci. 2016, 7, 35–42. [Google Scholar] [CrossRef]
- Kuttiraja, M.; Dhouha, A.; Tyagi, R.D. Harnessing the Effect of pH on Lipid Production in Batch Cultures of Yarrowia lipolytica SKY7. Appl. Biochem. Biotechnol. 2018, 184, 1332–1346. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Tyagi, R.D.; Drogui, P. Utilization of methanol in crude glycerol to assist lipid production in non-sterilized fermentation from Trichosporon oleaginosus. Bioresour. Technol. 2018, 253, 8–15. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Wu, D.; Li, J.; Tyagi, R.D.; Surampalli, R.Y. Economical lipid production from Trichosporon oleaginosus via dissolved oxygen adjustment and crude glycerol addition. Bioresour. Technol. 2019, 273, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Triantaphyllidou, I.E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Laddha, H.; Pawar, P.R.; Prakash, G. Bioconversion of waste acid oil to docosahexaenoic acid by integration of “ex novo’’ and “de novo’’ fermentation in Aurantiochytrium limacinum. Bioresour. Technol. 2021, 332, 125062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Wu, Y.H.; Wang, J.H.; Wang, X.X.; Deantes-Espinosa, V.M.; Dao, G.H.; Tong, X.; Hu, H.Y. Heterotrophic cultivation of microalgae in straw lignocellulose hydrolysate for production of high-value biomass rich in polyunsaturated fatty acids (PUFA). Chem. Eng. J. 2019, 367, 37–44. [Google Scholar] [CrossRef]
- Estupiñán, M.; Hernández, I.; Saitua, E.; Bilbao, M.E.; Mendibil, I.; Ferrer, J.; Alonso-sáez, L. Novel Vibrio spp. Strains Producing Omega-3 Fatty Acids Isolated from Coastal Seawater. Mar. Drugs 2020, 18, 99. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, H.; Sen, B.; Xie, Y.; He, Y.; Park, S.; Wang, G. Improved production of docosahexaenoic acid in batch fermentation by newly-isolated strains of Schizochytrium sp. and Thraustochytriidae sp. through bioprocess optimization. Synth. Syst. Biotechnol. 2018, 3, 121–129. [Google Scholar] [CrossRef]
- Du, Z.Y.; Alvaro, J.; Hyden, B.; Zienkiewicz, K.; Benning, N.; Zienkiewicz, A.; Bonito, G.; Benning, C. Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata. Biotechnol. Biofuels 2018, 11, 174. [Google Scholar] [CrossRef]
- Cordova, L.T.; Alper, H.S. Production of α-linolenic acid in Yarrowia lipolytica using low-temperature fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 8809–8816. [Google Scholar] [CrossRef]
- Arbter, P.; Sinha, A.; Troesch, J.; Utesch, T.; Zeng, A.P. Redox governed electro-fermentation improves lipid production by the oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2019, 294, 122122. [Google Scholar] [CrossRef]
- Santos-Merino, M.; Garcillán-Barcia, M.P.; De La Cruz, F. Engineering the fatty acid synthesis pathway in Synechococcus elongatus PCC 7942 improves omega-3 fatty acid production. Biotechnol. Biofuels 2018, 11, 239. [Google Scholar] [CrossRef]
- Liu, H.H.; Wang, C.; Lu, X.Y.; Huang, H.; Tian, Y.; Ji, X.J. Improved Production of Arachidonic Acid by Combined Pathway Engineering and Synthetic Enzyme Fusion in Yarrowia lipolytica. J. Agric. Food Chem. 2019, 67, 9851–9857. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Chen, S.; Sun, X.; Hu, X.; Ji, X.; Huang, H.; Ren, L. Fermentation performance and metabolomic analysis of an engineered high-yield PUFA-producing strain of Schizochytrium sp. Bioprocess Biosyst. Eng. 2019, 42, 71–81. [Google Scholar] [CrossRef]
- Walker, G.M.; Walker, R.S.K. Enhancing Yeast Alcoholic Fermentations; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 105, ISBN 9780128151815. [Google Scholar]
- Sahu, L.; Panda, S.K. Innovative Technologies and Implications in Fermented Food and Beverage Industries: An Overview. In Innovations in Technologies for Fermented Food and Beverage Industries; Springer: Cham, Switzerland, 2018; pp. 1–23. ISBN 9783319748207. [Google Scholar]
- Alperstein, L.; Gardner, J.M.; Sundstrom, J.F.; Sumby, K.M.; Jiranek, V. Yeast bioprospecting versus synthetic biology—Which is better for innovative beverage fermentation? Appl. Microbiol. Biotechnol. 2020, 104, 1939–1953. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Adler, L.; Lidén, G. Strategies for enhancing fermentative production of glycerol—A review. Enzyme Microb. Technol. 2002, 31, 53–66. [Google Scholar] [CrossRef]
- Ivit, N.N.; Longo, R.; Kemp, B. The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation 2020, 6, 77. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Celli, I.; Brunori, E.; Eugeni, M.; Cristinariu, C.A.; Zampilli, M.; Massoli, S.; Bartocci, P.; Caldarelli, V.; Saetta, S.; Bidini, G.; et al. Development of a tool to optimize economic and environmental feasibility of food waste chains. Biomass Convers. Biorefinery 2022, 12, 4307–4320. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Somboonpanyakul, P. B vitamins and prebiotic fructooligosaccharides of cashew apple fermented with probiotic strains Lactobacillus spp., Leuconostoc mesenteroides and Bifidobacterium longum. Process Biochem. 2018, 70, 9–19. [Google Scholar] [CrossRef]
- Germec, M.; Turhan, I. Kinetic modeling and sensitivity analysis of inulinase production in large-scale stirred tank bioreactor with sugar beet molasses-based medium. Biochem. Eng. J. 2021, 176, 108201. [Google Scholar] [CrossRef]
- Sindhu, R.; Gnansounou, E.; Rebello, S.; Binod, P.; Varjani, S.; Thakur, I.S.; Nair, R.B.; Pandey, A. Conversion of food and kitchen waste to value-added products. J. Environ. Manage. 2019, 241, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Kover, A.; Kraljić, D.; Marinaro, R.; Rene, E.R. Processes for the valorization of food and agricultural wastes to value-added products: Recent practices and perspectives. Syst. Microbiol. Biomanufacturing 2022, 2, 50–66. [Google Scholar] [CrossRef]
| Category | Product | Microorganisms | Fermentation Mode | Productivity | Fermentation Conditions | Refs. |
|---|---|---|---|---|---|---|
| Enzymes | Proteases | Bacillus subtilis B22 | Submerged | 334 ± 1.8 U/mL | 40 °C with pH: 8 and Agricultural waste materials | [10] |
| Proteases | Rhodotorula mucilaginosa CBMAI 1528 | Submerged | 280 ± 1.7 U/mL | 20 °C and a culture medium containing both glucose and casein peptone (20 and 10 g/L, respectively) | [11] | |
| Proteases | Geobacillus thermoglucosidasius SKF4 | Submerged | 175 U/mL | 60 to 65 °C, pH 7 to 8, >1% NaCl with casein and yeast extract | [12] | |
| Proteases | Aspergillus sydowii URM5774 | Submerged | 352.0 U/mL | pH 8.0 at 45 °C with coffee ground residues | [13] | |
| Proteases | Bacillus mojavensis | Submerged | 78.7% | pH 9.08, temperature 39.74 °C with eggshells and membrane-based substrates | [14] | |
| Lipase | A. niger | Submerged | 1.55 U/mL | soluble starch 4%, (NH4)2SO4 0.1%, K2HPO4 0.1%, MgSO4·7H2O 0.05%, peptone 3%, olive oil 1.05%. pH 7. Temperature 30 °C, agitation 213 rpm | [15] | |
| Lipase | Penicillium fellutanum | Submerged | 1038.86 U/gds | pH 5.0, incubation time 24 h, temperature 35 °C | [16] | |
| Glucoamylase | Aspergillus niger van Tieghem | Submerged | 274.4 U/mL | 51.82 g L−1 malt extract, 9.27 g L−1 CaCl2·2H2O and 0.50 g L−1 FeSO4.7H2O 30 °C and 150 rpm | [17] | |
| α-amylase | Aspergillus oryzae | Solid-State | 10,994.74 U/gds | edible oil cakes, temperature of 32.5 °C, pH of 4.5, moisture content of 64% | [18] | |
| Cellulase | A. niger (NRRL 330) | Submerged | 0.54 ± 0.02 IU/mL | pH: 5, Temperature: 30 °C, Peptone: 5 g/L, Yeast extract: 16.5 g/L and Ammonium sulfate: 1.9 g/L | [19] | |
| Hemicellulase | A. niger (NRRL 330) | Submerged | 48.71 ± 2.05 IU/mL | pH: 5, Temperature: 30 °C, Peptone: 5 g/L, Yeast extract: 16.5 g/L and Ammonium sulfate: 1.9 g/L | [19] | |
| Antimicrobials | Nisin | Lactococcus lactis | Submerged | 523.5 ± 256.7 IU/mL | D-glucose (80 g/L), peptone (10 g/L), YE (10 g/L), KH2PO4 (10 g/L), NaCl (2 g/L), and MgSO4∙7H2O (0.2 g/L), at 32 °C | [20] |
| lysozyme | Kluyveromyces lactis K7 | Submerged | 141 U/mL | 25 °C, pH 4, no aeration | [21] | |
| lysozyme | Kluyveromyces lactis K7 | Submerged | 173 U/mL | 16.3% lactose, 1.2% casamino acid, 0.8% yeast nitrogen, no pH control, 25 °C, 150 rpm, and no aeration | [22] | |
| lysozyme | Pichia pastoris GS115 | Submerged | 14,680 ± 300 U/mL | 28 °C Temperature, 250 rpm agitation | [23] | |
| Vitamins | Vitamin B12 | Propionibacterium freudenreichii DSM 20271 and Levilactobacillus brevis | Submerged | 742 ng/g dw | 200 rpm at 25 °C | [24] |
| Vitamin K | Bacillus subtilis natto | Submerged | 12.09 mg/L | temperature (35 °C), agitation (200 rpm) and pH (6.58) | [25] | |
| Vitamin K | Bacillus subtilis natto (NF1) | Submerged | 28.7 ± 0.3 mg/L | aeration (1 vvm), agitation (200 rpm for glycerol and 234 rpm for glucose), pH (6.48 for glucose and 6.6 for glycerol), and temperatures (30 °C for glucose and 35 °C for glycerol) | [26] | |
| Organic acids | Lactic acid | Lactobacillus casei | Submerged | 59.27 g/L | yeast extract was 31.35 (g/L) | [27] |
| Lactic acid | Lactobacillus plantarum 23 | Submerged | 14.2 g/L/h | pH 5.0 and 200 rpm agitation | [28] | |
| Propionic acid | Mixed bacterial culture | Submerged | 26.5 g/L | pH 6, 30 °C | [29] | |
| Sweeteners | Arabitol | Candida parapsilosis SK26.002 Mutant A6 | Submerged | 32.92 g/L | 30 °C, pH: 4.0, %4 initial inoculum and 200 rpm in shake flask with medium containing 200 g/L glucose and 30 g/L yeast extract | [30] |
| Arabitol | Yarrowia lipolytica ARA9 | Submerged | 118.5 g/L | 30 °C, pH: 5.0, 600 rpm agitation speed and 1.0 vvm aeration rate. Medium containing 200 g/L crude glycerol, 3.7 g/L (NH4)2SO4 and 2 g/L yeast extract | [31] | |
| Erythritol | Yarrowia lipolytica M53-S | solid state fermentation | 190.5 mg/gds | 30 °C, 70% initial moisture content, pH: 4.0, 7.5 × 104 cells/gds inoculum size and supplemented with 0.02 g/gds NaCl. Medium containing 60% peanut press cake and 40% sesame meal supplemented with 4% biochar and 20% concentrated enzymatic hydrolysate of the defatted Schizochytrium residue | [32] | |
| Erythritol | Moniliella pollinis MUCL 40570 | Submerged | 106.40 ± 0.42 g/L | 30 °C, pH: 5.5, 3% (v/v) initial inoculum and 200 rpm in shake flask. Sugarcane molasses media: 300 g/L total sugar conc. and 5 g/L yeast extract. Beet molasses media: 200 g/L total sugar and 0.67 g/L yeast extract. Grape musts media: 200 g/L total sugar and 6.7 g/L yeast extract. | [33] | |
| Erythritol | Moniliella pollinis CBS 461.67 | Submerged Fed-Batch | 94 g/L | 30 °C, inital pH: 6.5–6.8 (not controlled during fermentation), 150 rpm agitation speed and 1.0 vvm aeration rate. Sugarcane juice medium: 175 g/L total sugar and 1.63 g/L Moniliella culture lysate. Molasses medium: 219.8 g/L total sugar and 1.63 g/L Moniliella culture lysate | [34] | |
| Mannitol | Leuconostoc citreum TR116 | Submerged | 61.6 g/L | 30 °C, inital pH: 6.5, 1.0% (v/v) initial inoculum and 120 rpm agitation speed. MRS5 medium containing 100.0 g/L fructose and 50.0 g/L glucose. Apple juice medium supplemented with 2.0 g/L yeast extract | [35] | |
| Mannitol | Lactobacillus intermedius NRRL B-3693 | Submerged | 80 g/L | 37 °C, inital pH: 6.0 and 100 rpm agitation speed. Red must medium containing 155.3 g/L sugar, 7.48 g/L yeast extract and 0.047 g/L MnSO4·H2O and white must medium containing 175.7 g/L sugar, 7.54 g/L yeast extract and 0.088 g/L MnSO4·H2O | [36] | |
| Oligosaccharides | Fructooligosagharides | Aspergillus oryzae DIA–MF | Solid state fermentation | 7.64 g/L | 30 °C, pH: 4.5, 70% initial moisture content and 2.0 × 107 spores/g substrat inoculum size. Different fermentation medium including sugarcane bagasse, coffee husk, pineapple peel, prickle pear peel and banana peel waste supplemented with aguamiel | [37] |
| Fructooligosagharides | Bacillus aryabhattai GYC2-3 | Submerged | 26 g/L | 30 °C, pH: 8.0, 5% (v/v) inoculum containing 1 × 106 CFU/mL and 150 rpm in shake flask with medium containing 250 g/L sucrose | [38] | |
| Fructooligosagharides | Mutant strain of Aspergillus oryzae S719 (overexpressed FTase genes) | Submerged | 586 ± 4.7 g/L | 50 °C, pH: 6.0, 160 rpm agitation speed and 1.0 g/L mycelium as inoculum. Medium containing 900 g/L sucrose | [39] | |
| Mannooligosagharide | recombinant Aspergillus sojae AsT3 | solid state fermentation | 983.53 U/mg | 30 °C, pH: 7.0, 1:3 (w/v) solid-to-liquid ratiot and 7.0% inoculum size. Different fermentation medium including 5 g of wheat bran, rye bran, oat husk, barley husk supplemented with 4 g/L yeast extract | [5] | |
| Polysaccharides | Glucan | Lasiodiplodia theobromae CCT 3966 | Submerged | 0.047 g/g | 28 °C, pH: 7.0, 105 CFU/m inoculum and 200 rpm in shake flask. Fermentation medium including Sugarcane straw hydrolysate (40 g/L glucose concentration) | [40] |
| Glucan | Candida utilis ATCC 9950 | Submerged | 82% | 28 °C, 10.0% (v/v) inoculum, 200 rev/min agitation and 2.5 vvm aeration. Medium containing Deproteinated Potato Juice Water (pH 5.0 ± 0.2) supplemented with 10% of glycerol | [41] | |
| Glucan | Lasiodiplodia theobromae MMPI | Submerged | 1.06 g/L | 28 °C, pH: 5.5, 10.0 mL inoculum and 150 rpm in shake flask. Medium including soybean molasses (20 g/L total sugar) | [42] | |
| Glucan | T-DNA − based mutant Aureobasidium pullulans CGMCC 19650 | Submerged | 78.6% | 30 °C, pH: 3.8, 10.0% (v/v) inoculum, 400 rpm agitation speed and 1.0 vvm aeration rate. Medium containing 50 g/L glucose, 3.0 g/L yeast extract | [43] | |
| Pullulan | Aureobasidium pullulans MTCC 2013 | Submerged | 24.77 ± 1.06 g/L | 28 °C, pH: 6.5, 5.0% of 1 × 108 cells inoculum and 150 rpm in shake flask. Medium including hydrolyzed kitchen waste supplemented with 0.25% peptone and yeast extract | [44] | |
| Pullulan | Aureobasidium pullulans CCTCC M 2012259 | Submerged | 50 g/L | 30 °C, pH: 3.8, 10.0% (v/v) inoculum, 400 rpm agitation speed and 1.0 vvm aeration rate. M1 containing 51.59 g/L cassava starch and 4.40 g/L corn steep liquor powder. M2 containing 51.75 g/L cassava starch and 9.47 mL/L soybean meal hydrolysate | [45] | |
| Pullulan | Aerobasidiom pullulans KY 767024 | Submerged | 19.45 ± 0.40 g/L | 28 °C, pH: 5.5, 10.0% inoculum in shake flask. Medium including corn bran hydrolysates 20% (w/v) yeast extract 0.2% (w/v) | [46] | |
| Pullulan | Aureobasidium pullulans FB-1 | Submerged | 4.8%, w/v | 30 °C, pH: 6.5 5.0% (v/v) inoculum, 300 rpm agitation and 0.75 vvm aeration. Medium containing 50 g/L sucrose, 2.0 g/L yeast extract | [47] | |
| Amino Acids | Glutamic acid | Corynebacterium glutamicum NCIM 2168 | Submerged | 16.49 g/L | 30 °C, 5.0% (v/v) inoculum and 200 rpm in shake flask. Medium containing 50 g/L glucose, 10 g/L urea and 19.24% of salt solution | [48] |
| Glutamic acid | Corynebacterium glutamicum PTCC 1532 | Submerged | 19.84 mg/mL | 30 °C, pH: 7.0, 10 mL of the overnight culture inoculum, 180 rpm in shake flask. Medium containing 90 g/L glucose, 9 µg/L biotin and 3 g/L urea | [49] | |
| methionine | Genetically engineered Escherichia coli W3110-BL | Submerged | 1.48 g/L | 37 °C, 5.0% (v/v) inoculum, 1.4 vvm aeration rate and agitation controlled DO 20%. Medium containing 120 g/L glucose, 50 mg/L L-lysine, 100 mg/mL Amp, and 0.1 mmol/L isopropyl b-d-1-thiogalactopyranoside | [7] | |
| methionine | Recombinant Escherichia coli | Submerged (Fed-Batch) | 3.22 g/L | 30 °C, pH: 7.0, 10 mL of the overnight culture inoculum, 180 rpm in shake flask. Medium containing 20 g/L glucose, 2 g/L yeast extract, 0.01 g/L L-lysine and 1.0 mL/L salt solutiın | [50] | |
| tryptophan | Genetically engineered Escherichia coli TS-10 | Submerged | 1.710 g/L | Tryptophan fermentation was carried out in shake flask with lysogeny broth medium for 48 h | [51] | |
| tryptophan | Pediococcus acidilactici TP-6 | Submerged | 68.05 mg/L | 30 °C, 10.0% (v/v) inoculum in shake flask. Medium containing 14.06 g/L molasses, 23.68 g/L meat extract, 5.56 g/L urea 0.024 g/L and FeSO4 | [52] | |
| tryptophan | Genetically modified Escherichia coli CCTCC M20211388 | Submerged | 52.1 g/L | 35 °C, pH: 7.0, 20 mL (OD600: 1.0) inoculum, aeration rate and agitation controlled DO 20–30%. Medium containing 20 g/L glucose, 1 g/L yeast extract and 2 g/L sodium citrate | [53] | |
| Lysine | Metanolic engineered C. glutamicum | Submerged (Fed-Batch) | 221.3 ± 17.6 g/L | fermentation was carried out in bioreactor with 10% (v/v) inoculum. Medium containing 80 g/L glucose, 40 g/L beet | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iram, A.; Ozcan, A.; Turhan, I.; Demirci, A. Production of Value-Added Products as Food Ingredients via Microbial Fermentation. Processes 2023, 11, 1715. https://doi.org/10.3390/pr11061715
Iram A, Ozcan A, Turhan I, Demirci A. Production of Value-Added Products as Food Ingredients via Microbial Fermentation. Processes. 2023; 11(6):1715. https://doi.org/10.3390/pr11061715
Chicago/Turabian StyleIram, Attia, Ali Ozcan, Irfan Turhan, and Ali Demirci. 2023. "Production of Value-Added Products as Food Ingredients via Microbial Fermentation" Processes 11, no. 6: 1715. https://doi.org/10.3390/pr11061715
APA StyleIram, A., Ozcan, A., Turhan, I., & Demirci, A. (2023). Production of Value-Added Products as Food Ingredients via Microbial Fermentation. Processes, 11(6), 1715. https://doi.org/10.3390/pr11061715








