1. Introduction
Cyanobacteria, or Cyanophyta, are aquatic Gram-negative bacteria [
1]. It is well known that cyanobacteria were the first organisms to have created oxygen via photosynthesis. In addition, by creating and evolving molecular oxygen as a byproduct of photosynthesis, the living and multiplying of cyanobacteria changed the early oxygen insufficiency of the Earth’s atmosphere and allowed for the emergence of diverse life forms on Earth [
2]. Unlike purple bacteria or other organisms that likely perform anoxygenic photosynthesis, cyanobacterial thylakoid membranes are not continuous with the plasma membrane but have separate compartments [
3]. These thylakoid membranes have an important function in photosynthesis, as they host the PBSs, which function as the primary light-harvesting antennae for their photosystems [
4]. These PBSs are composed of chromophorylated protein stacks, which are referred to as phycobiliproteins (PBPs) and linker proteins. PBSs present a classical structure, wherein allophycocyanin (APC) functions as the core of the model and is surrounded by several outwardly oriented rods made up of stacked disks of phycocyanin (PC) and phycoerythrin (PE). In an antenna-like assembly, the geometrical arrangement of PBPs is exquisite. This intricate arrangement facilitates a high efficacy in terms of the transmission of the excitation of PBSs [
5].
To provide a precise understanding of the energy transfer process in PBSs, scientists have explored models of the protein’s energy distribution and energy transfer through steady-state and transient spectra concerning the unique spectral properties of different PBPs [
6,
7]. The spectral properties of PBPs are primarily determined by their prosthetic groups, which are linear tetrapyrroles known as phycobilin, including phycocyanobilin (PCB), phycoerythrobilin (PEB), and phycourobilin (PUB) [
8]. Each PBP has a distinct absorption and fluorescence emission in the visible range of light. As a result of their presence and specific arrangement within the PBSs, light energy can be absorbed and transferred uni-directionally to the chlorophyll of photosystem II. In this way, PBPs take advantage of the available light wavelengths (in the 500–650 nm range) that chlorophyll cannot access and use their energy for photosynthesis [
9,
10]. This is especially beneficial deeper in the water column, where longer-wavelength light is less transmitted and thus less directly available to the chlorophyll. The light-trapping function of phycobilisome and phycobilin arises from variations in the protein arrangement and microenvironment of the prosthetic groups. The sole reliance on the spectral results of phycobilin falls short in explaining the intricate energy transfer process, which must be understood through a synergistic approach, combining spectral properties, protein structures, and compositions to derive functional insights from structural features. It is much to be regretted that only five detailed architectures of PBSs have been described by cryo-electron microscopy (cryo-EM) with great resolution at the near-atom level: two red alga species
Griffithsia pacifica [
11],
Porphyridium purpureum [
12], and three cyanobacterial species,
Anabaena 7120,
Synechococcus 7002 [
13], and
Thermosynechococcus vulcanus 2134 [
14]. Therefore, it is challenging to interpret the complex energy transfer processes and mechanisms in phycobilisomes.
As mentioned above, the energy transfer dynamics of thermophilic cyanobacterium
T. 2134 are controversial in the absence of a high-resolution protein structure. In 2003, Adir et al. discovered a minor fraction of PC that absorbed maximally at 612 nm during the isolation of APC from
T. 2134 [
15]. This unique PC
612 trimer was thought to cover the space between the lower-base core cylinders and the higher cylinders in the APC core at the ends of two of the rods. Based on this structural model, they discovered a quick energy transfer pathway that took 888 fs to go from the rod to the core via linker proteins, and then 17 ps to get from the core to the terminal emitters (L
CM) [
16]. Then, using ps fluorescence and fs pump-probe spectroscopies, Kawakami et al. discovered an energy transfer of 7.3 ps between the PC trimers in the rods, 53 ps from the PC rod to the APC core, and 180 ps in the APC core complex [
17]. They also confirmed that the APC trimer, not the PC
612 trimer, is located between the lower-base core A cylinders and the higher B cylinder [
18]. Although these works have provided the energy transfer pathways and relative time constants in the PBSs of
T. 2134, the precise energy transfer pathways between chromophores are still uncertain due to the lack of more precise structural information. Until recently, the APC core and PC rod of
T. 2134 were resolved at 3.7 and 4.2 resolutions, respectively, yielding the results that the PBS molecule of
T. 2134 consists of five APC core cylinders (A, A′, B, C, and C′) surrounded by eight PC rods (Rb, Rb′, Rt, Rt′, Rs1, Rs1′, Rs2, and Rs2′), as shown in
Figure 1, thus providing the precise structural basis for energy transfer research. In this paper, with a focus on the PBS rod–core assembly [
19], we aim to re-identify the precise energy transfer dynamics between the chromophores of PBSs of
T. 2134 via fs time-resolved fluorescence spectra on the basis of a near-atomic resolution structure.
3. Results
The steady-state absorption spectra of the isolated PC trimer and APC core from
T. 2134, as well as the entire PBSs, were resolved at room temperature [
19]. The absorption band of the intact PBSs peaked at 620 nm. The absorption band of the isolated PC rod also peaked at 620 nm but exhibited narrower results than those of the intact PBSs, whereas the absorption band of the APC core performed as a broadening on the red side of the PC trimer, peaking at 650 nm with a shoulder at 600 nm. The PC rod then exhibited a fluorescence peak at 650 nm, while the peak of the APC fluorescence was around 675 nm. We further measured the spectral properties of the C-cylinder of the core, where the absorption band peaked at 612 nm and the emission band peaked at 645 nm [
26]. At steady state, the emissions from the various fluorescence components combined to form the fluorescence spectra; therefore, to investigate the overall energy transfer dynamics in the PBSs, the time-resolved fluorescence spectra were analyzed, and the femtosecond laser at a wavelength of 498 nm and 570 nm was selected to excite the PBS samples, respectively. The steady-state absorption spectra measurements showed that only the PC trimer was excited under a wavelength of 500 nm, as the APC core has no absorption at a wavelength of 498 nm [
19,
27]; however, both the PC trimer and APC core could be excited under a wavelength of 570 nm.
The time-resolved fluorescence spectra of the PBSs of
T. 2134 at 77 K were examined in detail. High signal-to-noise ratios were made possible by its ability to ignore solvent and molecule vibration effects at 77 K, which ensured that the results of the time-resolved fluorescence spectroscopy closely matched the energy transfer assumptions required in the theoretical models [
28].
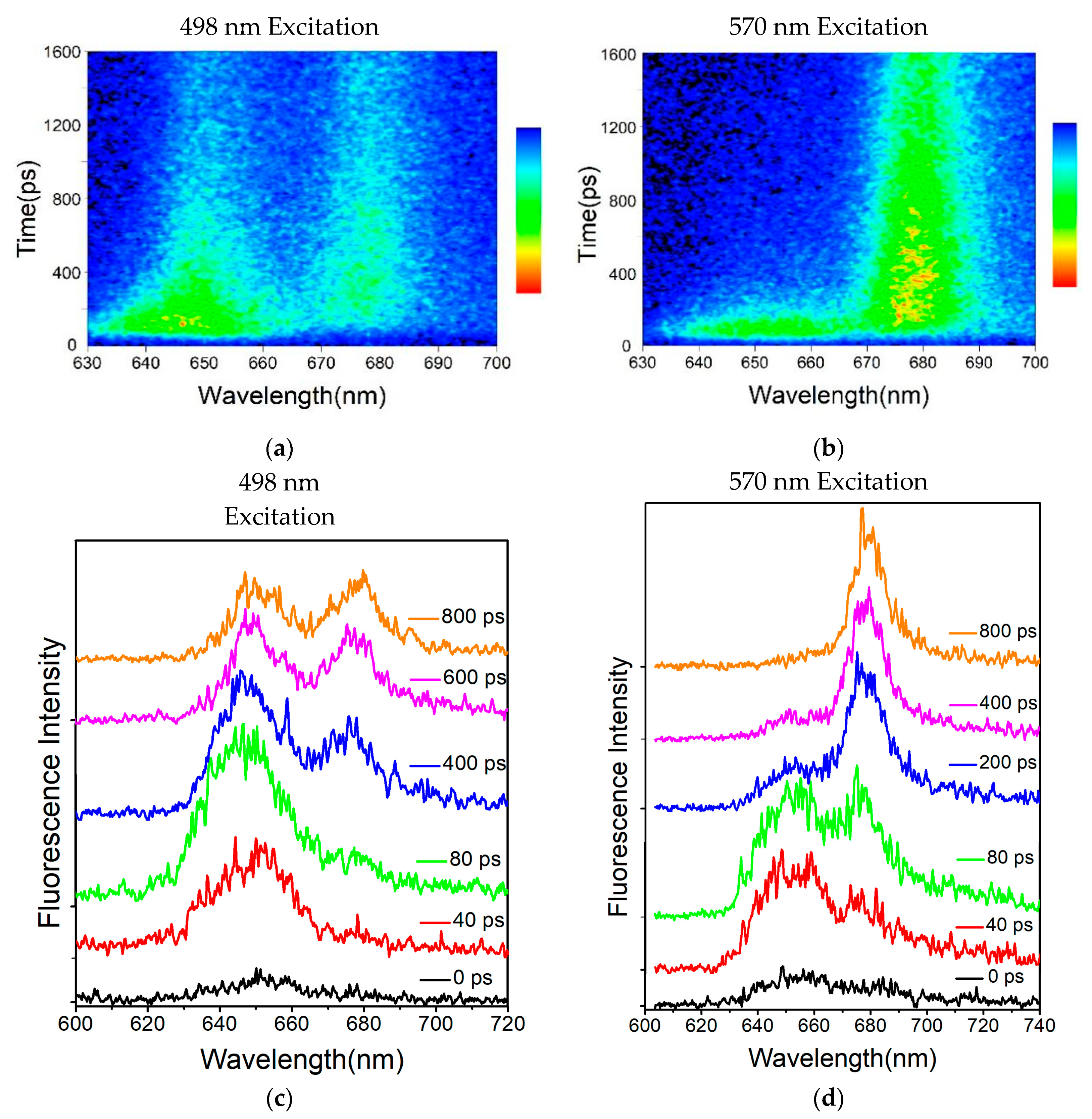
Figure 3a exhibits the reconstructed three-dimensional time-resolved fluorescence spectra of the PBSs from
T. 2134 excited at 498 nm. The expression aided us in conceptualizing the energy flow. Following excitation at 498 nm, which preferentially excited the PCBs of β155 in the PC rods [
27], the time-resolved fluorescence spectra of the PBSs revealed a PC fluorescence band with a central wavelength of around 655 nm, which was followed immediately by the fluorescence band of the C-cylinder at 645 nm, and the fluorescence band of the APC core at around 675 nm rose concurrently with the decay of the C-cylinder. The immediate evidence for the energy transfer from PC → C-cylinder → APC came from the changes in the relative intensities of the fluorescence components. Different from the results of the 498 nm excitation time-resolved fluorescence spectra, the PC rod and APC core were excited simultaneously under an excitation wavelength of 570 nm. The time-resolved fluorescence spectra results showed that the PC and APC emissions were both discovered at the same time, as shown in
Figure 3b.
It is obvious from
Figure 3c that there was a large difference among the fluorescence spectra obtained by the synchronscan technology. It is reasonable to assume that the 650 nm emission could be initially clearly identified. In addition, the APC emission appeared at 40 ps after excitation, indicating that the terminal emitter in the core accepted the energy transferred from the PC rod within 40 ps. However, the PC emission quickly decayed and nearly disappeared at 400 ps after the excitation (
Figure 3d), indicating that the energy transfer process between PC and APC was completed within 400 ps.
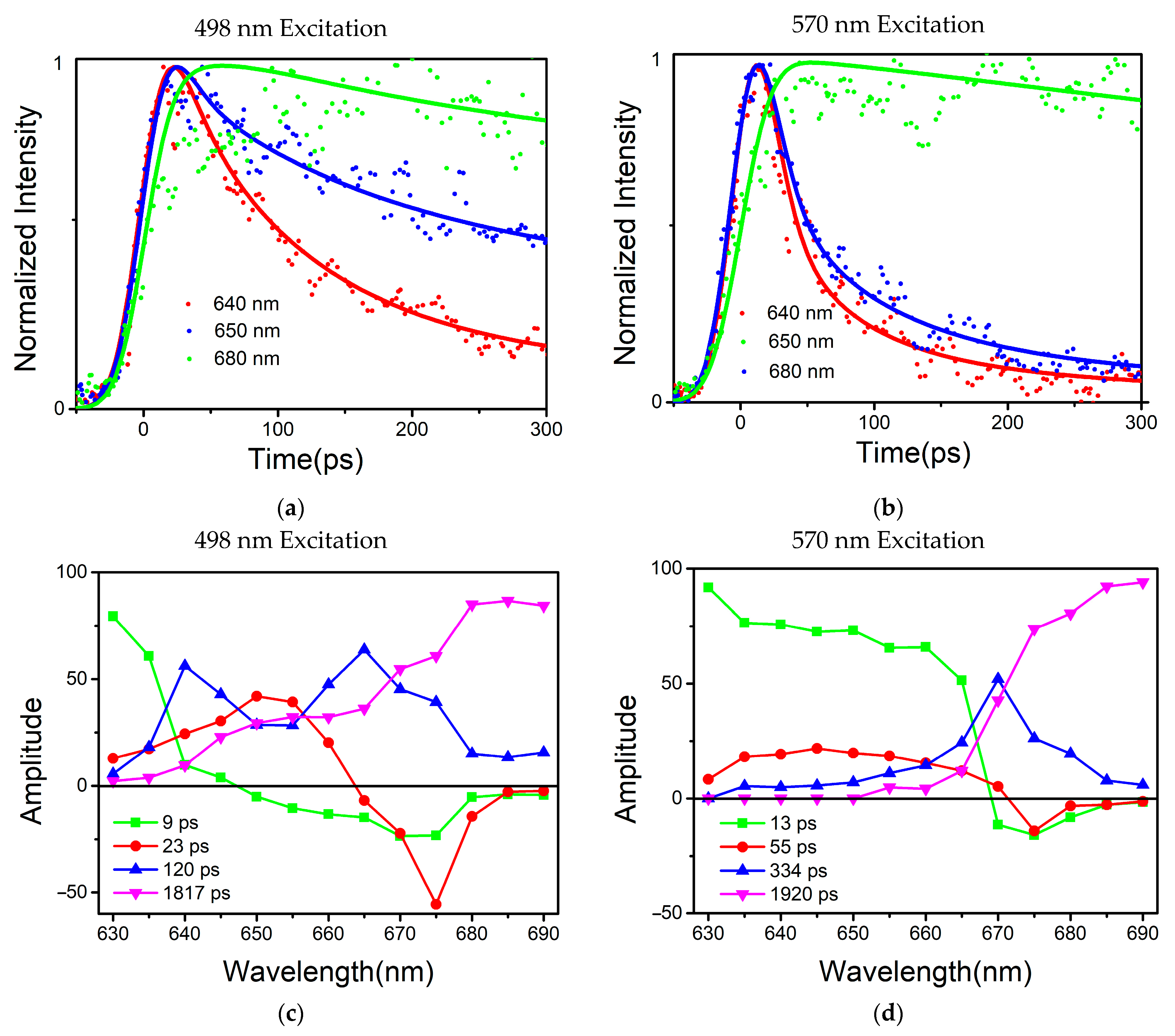
Global optimization deconvolution was used to find out the fluorescence decay constants in the PBSs during energy transferring. Multi-exponential deconvolution was used to resolve the fluorescence decay curves at various measured wavelengths, and the Monte-Carlo approach was used to analyze the experimental data. An energy acceptor is represented by the component (A%) with a negative amplitude of the fluorescence’s rising phase, while an energy donor is represented by the component (A%) with a positive pre-exponential of the fluorescence’s falling phase.
Based on the results of the global multiexponential analysis,
Figure 4c,d show the fluorescence-decay-associated spectra (FDAS) obtained by the phycobilisome of
T. 2134 under excitation at 498 nm and 570 nm, respectively. In combination with the high-resolution model, the FDAS could further help to reveal the energy transfer in the PBSs. Typically, to explore the decay kinetics of the same decay constant in different wavelength regions, it is necessary to consider the kinetic increase and reduction in the excited component, that is, the change in the amplitude of the kinetic phase. Moreover, if the sign of the FDAS factor with the same decay constant is inverted, this indicates that there was a coupling that occurred between the positive and negative bands, i.e., that there was energy transferred from the complex of the former band that gave energy to the latter band.
From the results, the two excitation wavelengths produced two different sets of decay constants, 10 ps, 20 ps, 120 ps, and 1817 ps for the 498 nm excitation, and 13 ps, 55 ps, 344 ps and 1920 ps for the 570 nm excitation. In the
Supplementary Material, the fluorescence decay curves and the correspondent fitting results of the PBSs at different wavelengths are depicted in
Figures S1 and S2, and Tables S1 and S2 show the amplitudes of each time component. Among these results, the four rapidly decaying DAS components, 9 ps, 13 ps, 23 ps, and 55 ps, exhibited sign changes from positive to negative, which are indicative of the decay of one fluorescence band in parallel with the rise of another, showing the characteristics of EET. The other four components had positive peaks without sign changes, indicating that their decay only occurred during the trapping or relaxation. In addition, considering the position of the sign shift and the peak in the horizontal direction, the four decay constants were clearly distinguished. The sign change in the 9 ps component appeared between 645 nm and 650 nm, while the sign change in the 13 ps component occurred between 665 nm and 670 nm; similarly, the sign change in# the 23 ps component occurred between 660 nm and 665 nm, and that of the 55 ps component occurred between 665 nm and 670 nm. The positive peaks occurred at decay constants of 120 ps and 334 ps, while the decay constants of 1817 ps and 1920 ps gradually increased with increasing the wavelength. Therefore, combined with the steady-state spectra and structural information, the FDAS results allowed us to assign the following decay constants:
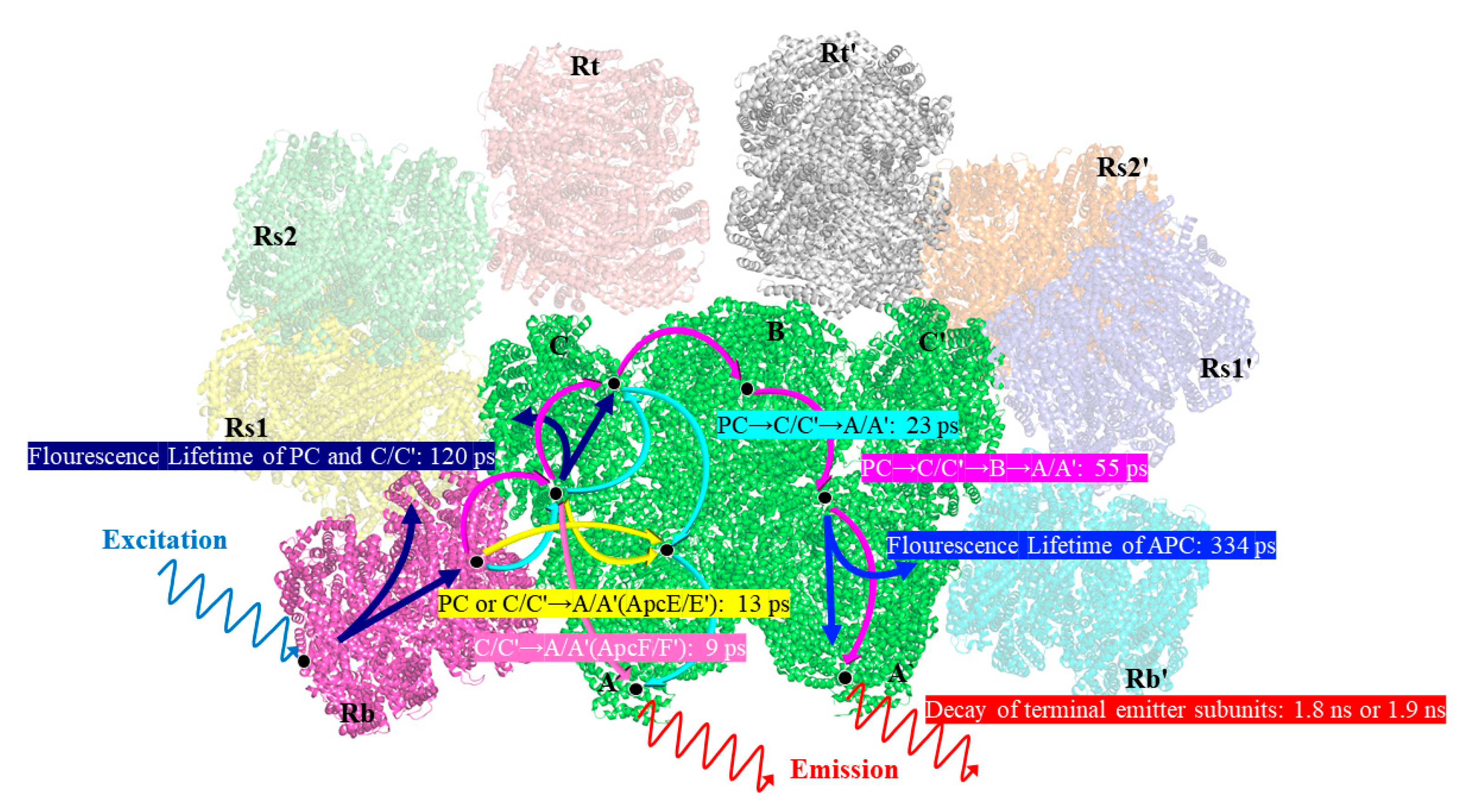
The 9 ps and 13 ps components corresponded to the fastest decay constants, as shown in
Figure 4c,d, respectively. The 9 ps component was constant in terms of fluorescence decay in the PBSs of
T. 2134 and could be assigned to the time of the energy transfer from the C/C′ cylinders directly to the terminal emitter ApcF/F′ in the A/A′ cylinders of the APC core, owing to the fact that its amplitude began positively on the blue side before swiftly turning negative and having a minimum at 670 nm (as shown in
Figure 4c). A negative amplitude corresponds to a fluorescence increase and energy transfer from a donor to an acceptor. Moreover, the latest APC cryo-EM structure showed that there were strongly coupled pigment pairs between the C/C′ cylinders and the A/A′ cylinders, i.e.,
Cα
84–
Aα
84. The transferring of energy from the C/C′ cylinders to the A/A′ cylinders occurring via
Cα
84–
Aα
84 (35 Å) resulted in a fast energy transfer time of 9 ps [
14]. Therefore, the quick transfer of energy between the C/C′-cylinders and the terminal emitter ApcF/F′ was assigned a decay time of 9 ps. The 13 ps constant of the fluorescence decay curve of the PBSs of
T. 2134 could be attributed to the process of energy transfer from the rod Rb/Rb′ and the C/C′ cylinders to the terminal emitter ApcE/E′ in the A/A′ cylinders with the same transfer constant, as shown in
Figure 4d, from 630 to 660 nm, and its amplitude was positive. It then started to decline at 665 nm, turned negative at 670 nm, and finally reached a minimum at 675 nm. The following is a description of this amplitude variation: An acceptor with an emission peak of 675 nm received energy from a donor with an emission peak between 630 and 660 nm. The light absorbed by the PCBs in the rod Rb/Rb′ was transferred directly to the ApcE/E′ in A/A′ cylinders via a rod–core linker, L
RC. At the same time, the light absorbed by the PCBs in the C/C′ cylinders was transferred to the terminal emitter ApcE/E′ in the A/A′ cylinders via a core linker, L
C. Thus, combining the fluorescence spectra and the cryo-EM structure of the APC core, it was reasonable to assign a decay time of 13 ps to the energy transfer time from the rod or the C/C′ cylinders or the B cylinder to the terminal emitter ApcE/E′ in the A/A′ cylinders with the same transfer constant.
For the decay constants of the 23 ps and 55 ps components, the experimental results at different excitation wavelengths clearly demonstrated that the terminal received an energy flow that was transferred from the PC rod or C/C′ cylinders after 40 ps. Therefore, the 23 ps constant of fluorescence decay in the PBSs of
T. 2134 could be assigned to the energy transfer time from the PC rod Rs/Rs′ → C/C′ cylinders → terminal emitter ApcE/E′ in the A/A′ cylinders because at the blue side, the amplitude of the FDAS was positive with a maximum wavelength of around 650 nm, while at the red side, the amplitude shifted to negative with a minimum at about 675 nm (
Figure 4c). Compared with the constants of the 9 ps component, the higher amplitude of the FDAS curve of the constant of the 23 ps component showed that the energy transfer pathway with an energy transfer time of 23 ps began to dominate in the energy transfer from the rods to the core. This agrees with the cryo-EM findings, which showed that the C/C′ cylinder served as a conduit for energy transfer from the PC rods to the terminal emitters. Therefore, combining the fluorescence spectra and the cryo-EM structure of the PC rods and APC core, it was appropriate to provide the energy transfer between the Rs/Rs′ rods and terminal emitter ApcE/E′ a decay time of 23 ps. The 55 ps constant of the fluorescence decay curve in the PBSs of
T. 2134 could be attributed to the process of the energy transfer time from the PC rod Rs/Rs′ → the C/C′ cylinders → the B cylinder → the terminal emitter ApcD/D′ in the A/A′ cylinders, which is a long energy transfer pathway from the rod to the terminal emitter. This is because on the blue side, the amplitude of the FDAS was positive with a maximum of 645 nm, while on the red side, the amplitude shifted to negative with a minimum of about 670 nm. Moreover, the amplitude of the 55 ps component was low compared with that of the 13 ps component, indicating this energy transfer pathway was not the main pathway of the energy transfer from the rod to the core. Therefore, combining the fluorescence spectra and the cryo-EM structure of the PC rods and APC core, it was appropriate to give a decay time of 55 ps to the energy transfer from the PC rod Rs/Rs′ → the C/C′ cylinder → the B cylinder → the terminal emitter ApcD/D′ in the A/A′ cylinders.
For the four decay constants without EET, the possible energy transfer paths were assigned according to their peak and the trend of the FDAS factor. The fluorescence isotropic decay constants of 1920 ps and 1817 ps reflected the emission of the terminal emitter ApcE (ApcE′). The 120 ps constant of fluorescence decay possibly reflected the emission of the rods and the C/C′ cylinders because a positive band with two peaks at 640 nm and 665 nm was resolved in the FDAS. Due to the fact that only a positive band with a maximum at 670 nm was determined, the fluorescence decay constants of the 334 ps component may represent the emission of the APC core.