Failure Analysis of the Crack and Leakage of a Crude Oil Pipeline under CO2-Steam Flooding
Abstract
1. Introduction
2. Materials and Methods
2.1. Nondestructive Test
2.2. Physical and Chemical Performance Test
2.3. Microscopic Characterisation Analysis
2.4. Corrosion Test
3. Results
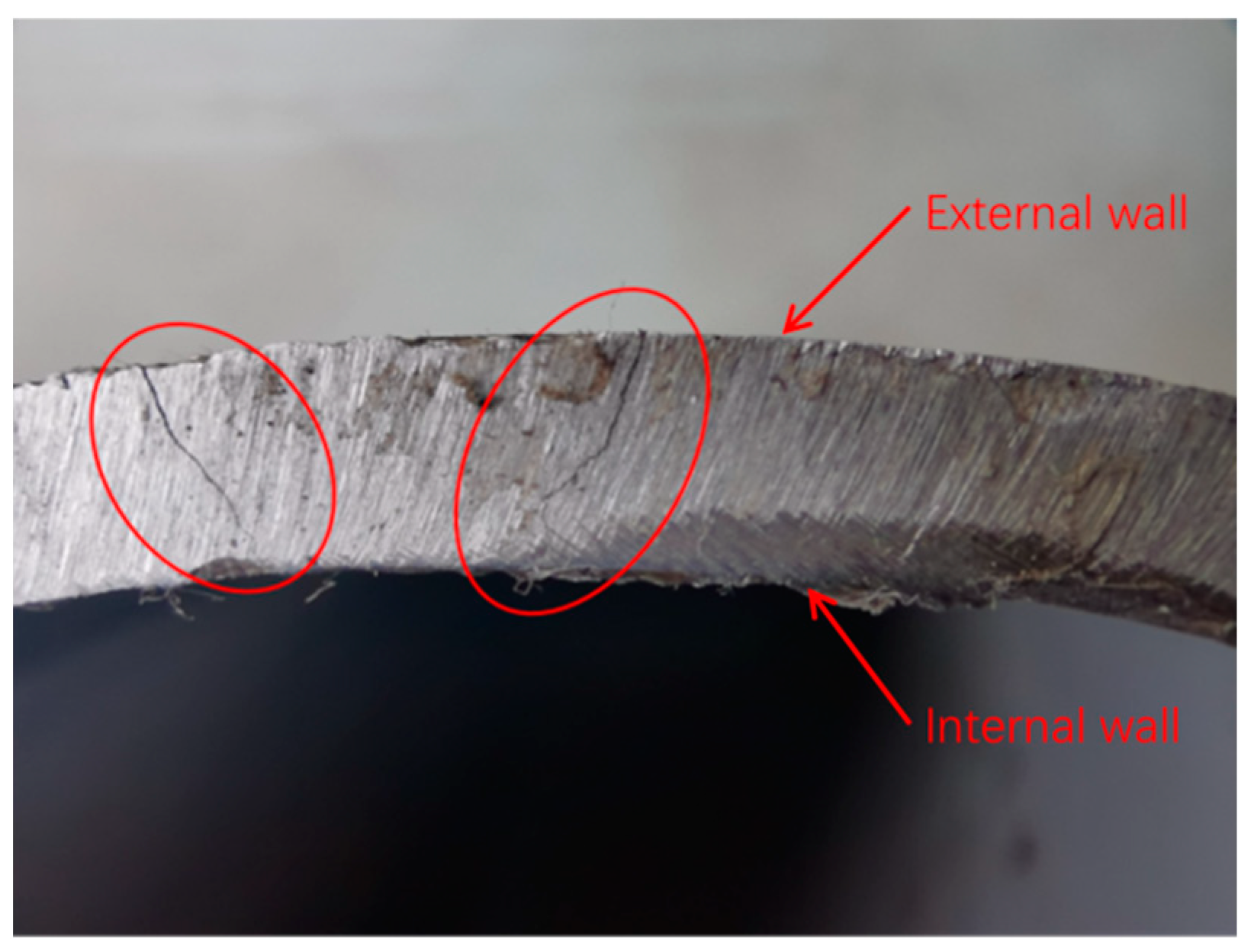
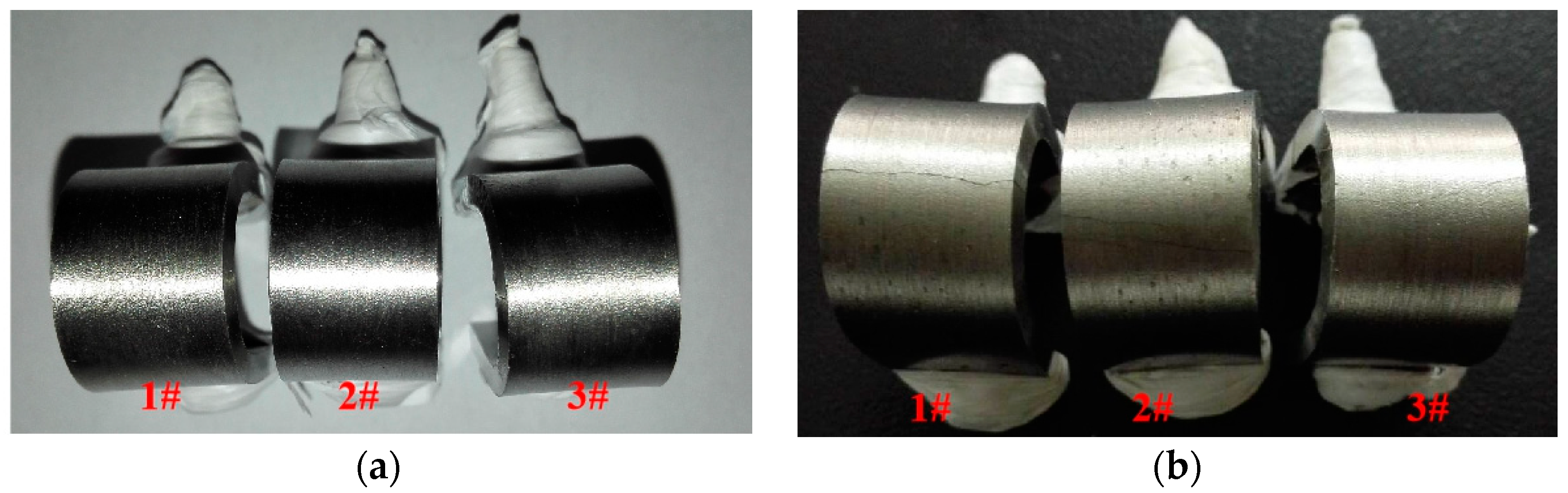
3.1. Visual Inspection
3.2. Nondestructive Test
3.3. Chemical Composition
3.4. Tensile Property
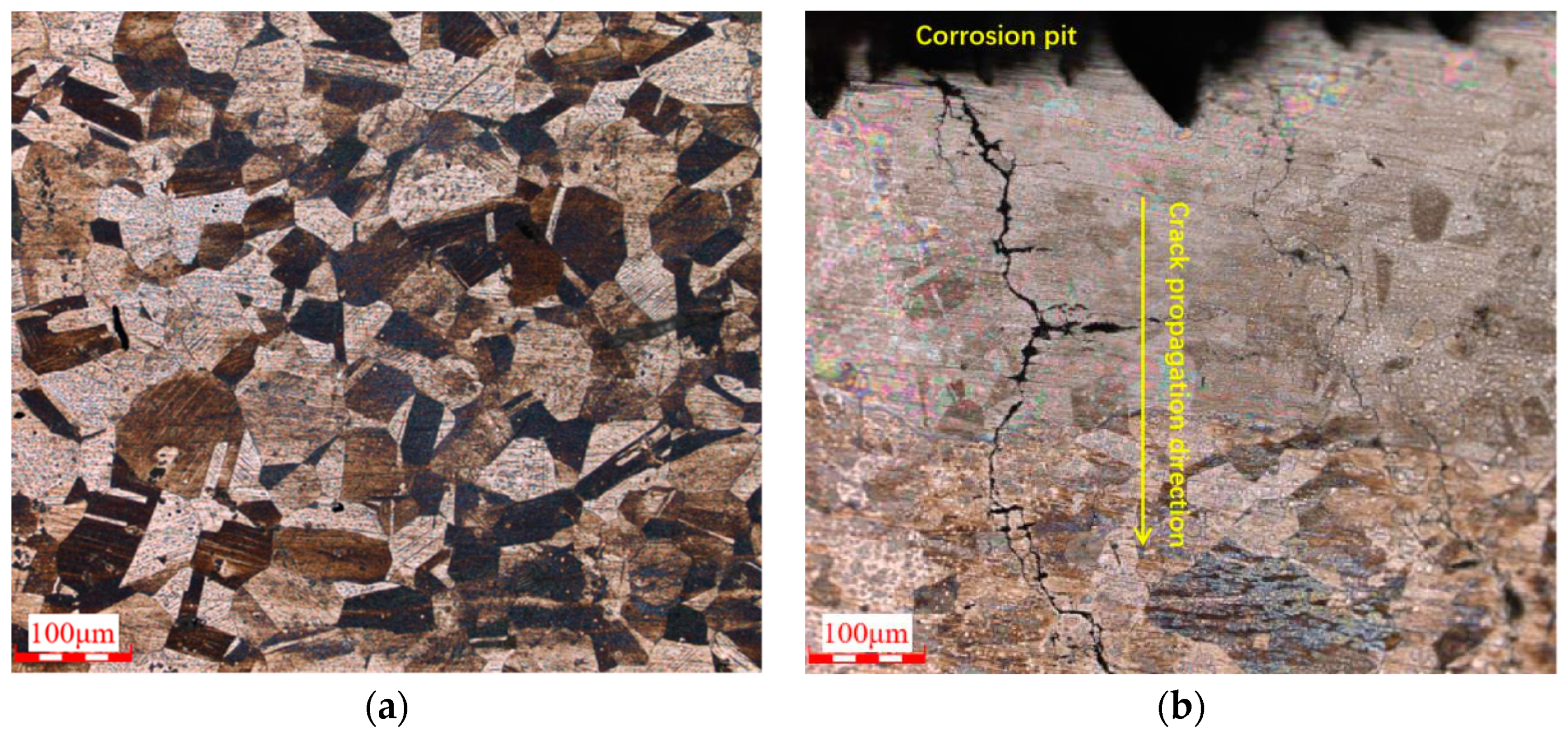
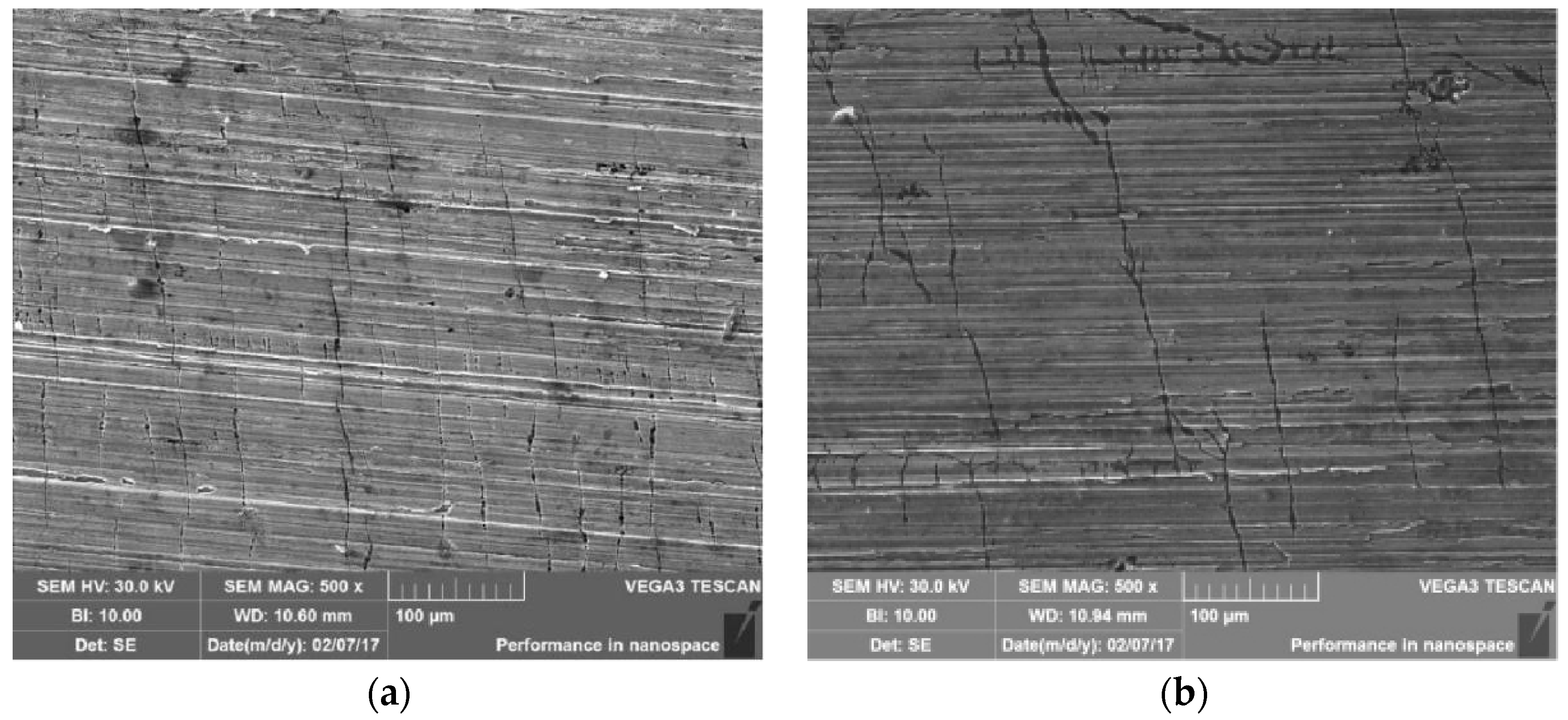
3.5. Metallographic Analysis
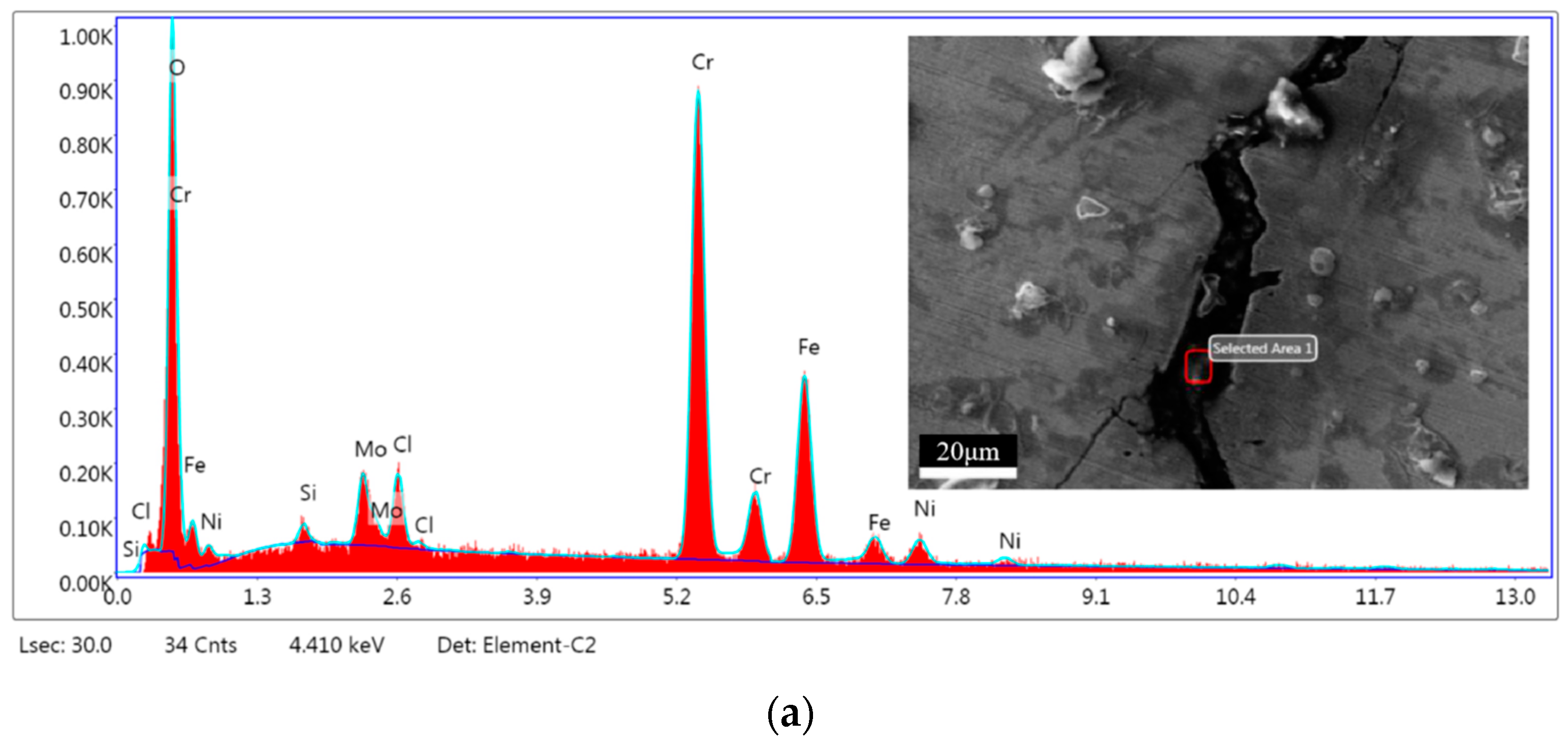
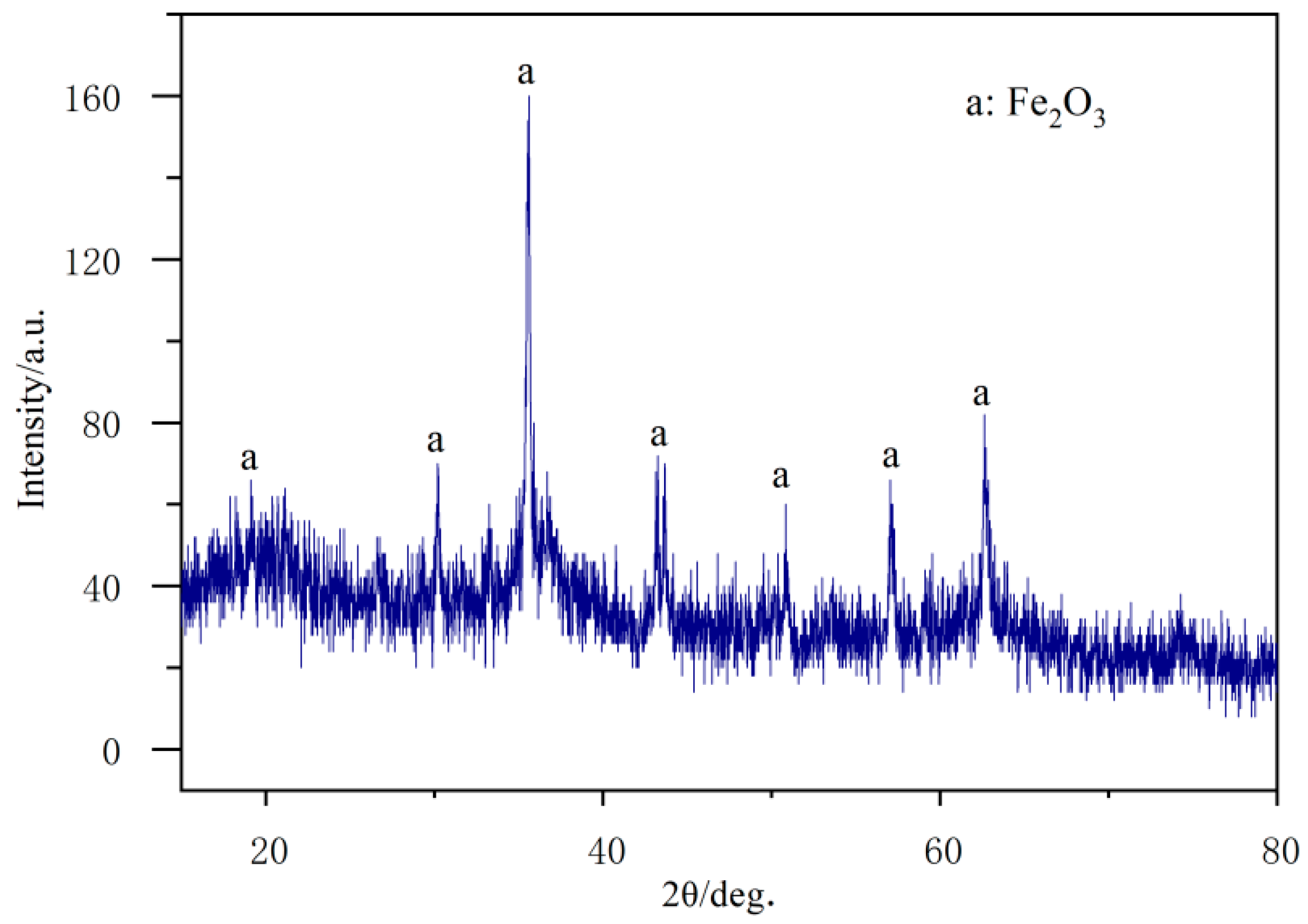
3.6. Microanalysis of Fracture
3.7. Chloride SCC Test
4. Discussion
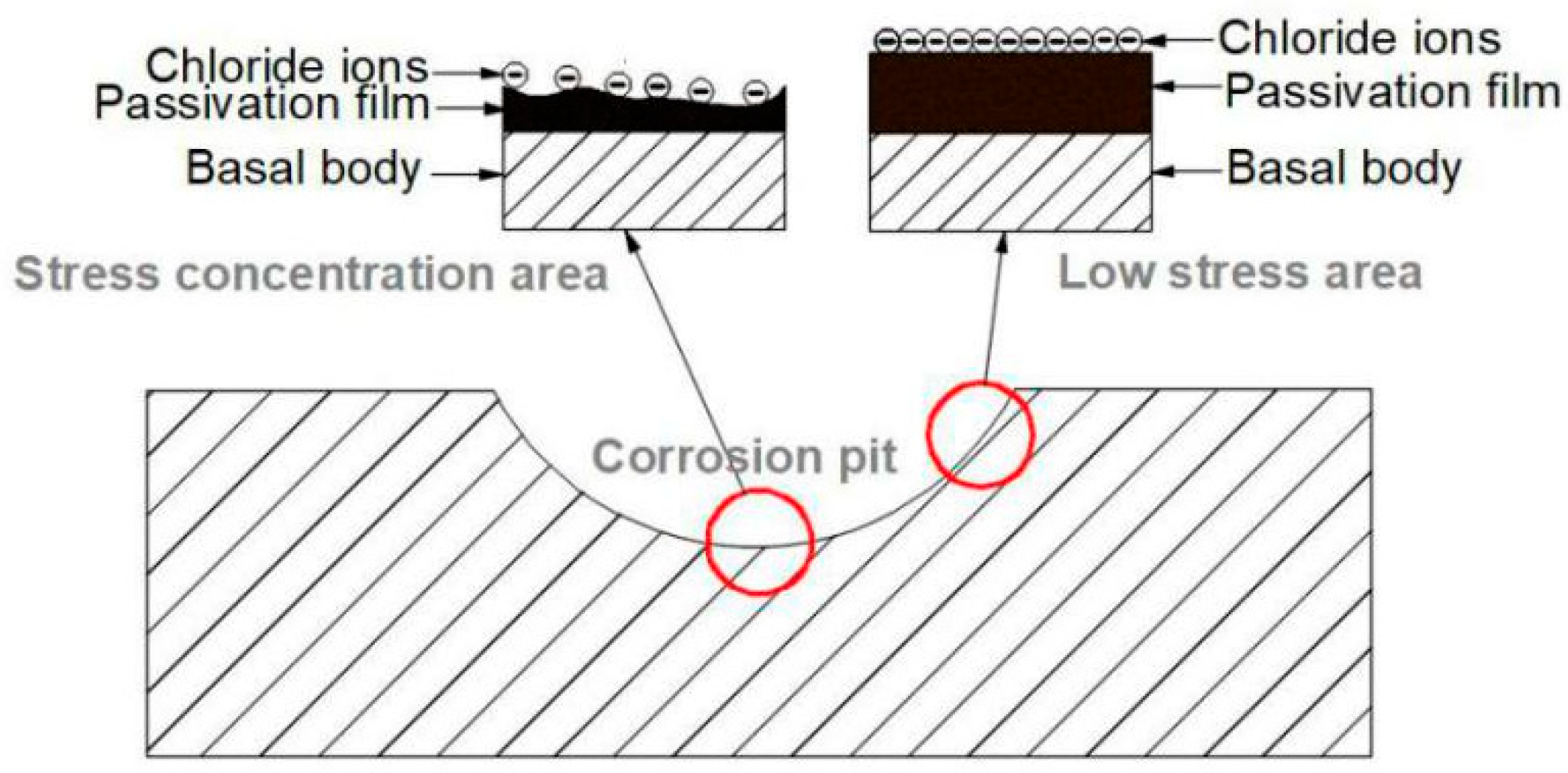
4.1. Corrosion Environment Analysis
4.2. Stress Analysis
4.3. Material Analysis
5. Methods for Chloride SCC Control of Stainless Steel
6. Conclusions and Recommendations
- (1)
- The pipeline experienced localized external corrosion in groundwater containing dissolved oxygen and Cl ions, and leakage failure occurred due to chloride SCC in the stress concentration area at the bottom of the corrosion pits.
- (2)
- The Ni content of the pipeline material was lower than the standard product requirements and within the sensitive content range of chloride SCC, which accelerated the cracking of the pipeline. As well as the high temperature of the recovered medium under CO2 and steam combined flooding promoted the progress of chloride SCC.
- (3)
- Several Specific and practical recommendations are then proposed from both manufacturing and maintenance points of view. First, replacing the pipeline with 2205 duplex stainless steel pipe is feasible. Second, by reducing the operating temperature of the pipeline, the development of SCC will be slowed. Third, the buried pipelines should adopt an anticorrosion layer + cathodic protection to slow the occurrence of external corrosion damage under the new process of CO2-steam combined flooding. Fourth, similar pipelines need to be excavated for defect inspection and safety evaluation, and the severely corroded external pipeline section needs to repair by B-type sleeve, carbon fiber (or glass fiber) reinforcement, etc.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A review of recent progress of carbon capture, utilization, and storage (CCUS) in China. Appl. Sci. 2023, 13, 1169. [Google Scholar] [CrossRef]
- Zhou, X.; Yuan, Q.; Peng, X.; Zeng, F.; Zhang, L. A critical review of the CO2 huff ‘n’ puff process for enhanced heavy oil recovery. Fuel 2018, 215, 813–824. [Google Scholar] [CrossRef]
- JLuo, S.; Hou, Z.M.; Feng, G.Q.; Liao, J.X.; Haris, M.; Xiong, Y. Effect of reservoir heterogeneity on CO2 flooding in tight oil reservoirs. Energies 2022, 15, 3015–3022. [Google Scholar]
- Jiang, K.; Ashworth, P.; Zhang, S.; Liang, X.; Angus, D. China’s carbon capture, utilization and storage (CCUS) policy: A critical review. Renew. Sustain. Energy Rev. 2019, 119, 109601. [Google Scholar] [CrossRef]
- Makarova, A.A.; Mantorova, I.V.; Kovalev, D.A.; Kutovoy, I.N. The modeling of mineral water fields data structure. In Proceedings of the 2021 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (ElConRus), Moscow, Russia, 26–29 January 2021; pp. 517–521. [Google Scholar]
- Makarova, A.A.; Kaliberda, I.V.; Kovalev, D.A.; Pershin, I.M. Modeling a production well flow control system using the example of the Verkhneberezovskaya area. In Proceedings of the 2022 Conference of Russian Young Researchers in Electrical and Electronic Engineering (ElConRus), Saint Petersburg, Russia, 25–28 January 2022; pp. 760–764. [Google Scholar]
- Wang, T.; Wang, L.; Meng, X.; Chen, Y.; Song, W.; Yuan, C. Key parameters and dominant EOR mechanism of CO2 miscible flooding applied in low-permeability oil reservoirs. Geoenergy Sci. Eng. 2023, 225, 211724. [Google Scholar] [CrossRef]
- Morgan, A.; Ampomah, W.; Grigg, R.; Dai, Z.; You, J.; Wang, S. Techno-economic life cycle assessment of CO2-EOR operations towards net negative emissions at Farnsworth field unit. Fuel 2023, 342, 127897. [Google Scholar] [CrossRef]
- Heidari, P.; Kordestany, A.; Sepahvand, A. An experimental evaluation of oil recovery by steam alternative CO2 injection in naturally fractured reservoirs. Energy Sources 2013, 35, 1498–1507. [Google Scholar] [CrossRef]
- Yuan, R.; Yang, Z.D.; Guo, B.; Wang, X.W.; Zhang, L.Q.; Lin, R.Y. Potential analysis of enhanced oil recovery by superheated steam during steam-assisted gravity drainage. Energy Technol. Gener. Conversion. Storage Distrib. 2021, 9, 2100135. [Google Scholar] [CrossRef]
- Liu, W.; Du, L.; Zou, X.; Liu, T.; Wu, X.; Wang, Y.; Dong, J. Experimental study on the enhanced ultra-heavy oil recovery using an oil-soluble viscosity reducer and CO2 assisted steam flooding. Geoenergy Sci. Eng. 2023, 222, 211409. [Google Scholar] [CrossRef]
- ASTM-A312-2018; Standard specification for seamless, welded, and heavily cold-worked austenitic stainless steel pipes. ASTM: West Conshohocken, PA, USA, 2018.
- Yu, H.; Luo, Z.; Zhang, X.; Feng, Y.; Xie, G. A comparative study of the microstructure and corrosion resistance of Fe-based/B4C composite coatings with Ni-added or Cu-added by vacuum cladding. Mater. Lett. 2023, 335, 133730. [Google Scholar] [CrossRef]
- Zhang, S.; Bian, T.; Mou, L.; Yan, X.; Zhang, J.; Zhang, Y.; Liu, B. Alloy design employing Ni and Mo low alloying for 3Cr steel with enhanced corrosion resistance in CO2 environments. J. Mater. Res. Technol. 2023, 24, 1304–1321. [Google Scholar] [CrossRef]
- API RP 571-2020; Damage Mechanisms Affecting Fixed Equipment in the Refining Industry. API: Minnesota City, MN, USA, 2020.
- API RP 581-2016; Risk-based inspection methodology. API: Minnesota City, MN, USA, 2016.
- Luo, B.W.; Zhou, J.; Bai, P.P.; Zheng, S.Q.; An, T.; Wen, X.L. Comparative study on the corrosion behavior of X52, 3Cr, and 13Cr steel in an O2–H2O–CO2 system: Products, reaction kinetics, and pitting sensitivity. Int. J. Miner. Metall. Mater. 2017, 24, 646–656. [Google Scholar] [CrossRef]
- Song, C.L.; Liu, X.B.; Pan, X. Failure analysis of the leakage and ignition of an oil–gas mixture transportation pipeline. J. Fail. Anal. Preven. 2022, 22, 259–266. [Google Scholar] [CrossRef]
- Zhang, S.H.; Hou, L.F.; Wei, Y.H.; Du, H.Y.; Wei, H.; Liu, B.S.; Chen, X.B. Dual functions of chloride ions on corrosion behavior of mild steel in CO2 saturated aqueous solutions. Mater. Corros. 2019, 70, 888–896. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.-X.; Jin, J.; Tang, D.-Z.; Zhang, L. Selective corrosion mechanism of CoCrFeMoNi high-entropy alloy in the transpassive region based on the passive film characterization by ToF-SIMS. Corros. Sci. 2023, 218, 111206. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Chen, L.-Y.; Chu, Y.-H.; Zhang, L.; Li, R.; Lu, S.; Wang, L.; Zhang, L.-C. Metastable pitting corrosion behavior and characteristics of passive film of laser powder bed fusion produced Ti–6Al–4V in NaCl solutions with different concentrations. Corros. Sci. 2023, 215, 111017. [Google Scholar] [CrossRef]
- Cong, S.; Tong, K.; Li, D.F.; Chen, Z.X.; Cai, K. Leakage failure analysis of the ERW steel pipeline. Mater. Sci. Forum 2020, 993, 1224–1229. [Google Scholar] [CrossRef]
- Hua, Y.; Shamsa, A.; Barker, R.; Neville, A. Protectiveness, morphology and composition of corrosion products formed on carbon steel in the presence of Cl−, Ca2+ and Mg2+ in high-pressure CO2 environments. Appl. Surf. Sci. 2018, 455, 667–682. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, X.L. A review—Pitting corrosion initiation investigated by TEM. J. Mater. Sci. Technol. 2019, 35, 1455–1465. [Google Scholar] [CrossRef]
- Congleton, J.; Shih, H.C.; Shoji, T.; Parkins, R.N. The stress corrosion cracking of type 316 stainless steel in oxygenated and chlorinated high-temperature water. Corros. Sci. 1985, 25, 769–788. [Google Scholar] [CrossRef]
- Andresen, P.L.; Morra, M.M. IGSCC of non-sensitized stainless steels in high-temperature water. J. Nucl. Mater. 2008, 383, 97–111. [Google Scholar] [CrossRef]
- Du, D.H.; Chen, K.; Lu, H.; Zhang, L.F.; Shi, X.Q.; Xu, X.L.; Andresen, P.L. Effects of chloride and oxygen on stress corrosion cracking of cold worked 316/316L austenitic stainless steel in high-temperature water. Corros. Sci. 2016, 110, 134–142. [Google Scholar] [CrossRef]
- Andresen, P.L.; Young, L.M. Characterization of the Roles of Electrochemistry, Convection and Crack Chemistry in Stress Corrosion Cracking; No. CONF-950816-; NACE International: Houston, TX, USA, 1995; pp. 579–596. [Google Scholar]
- Congleton, J.; Shoji, T.; Parkins, R.N. The stress corrosion cracking of reactor pressure vessel steel in high-temperature water. Corros. Sci. 1985, 25, 633–650. [Google Scholar] [CrossRef]
- Xu, L.; Wu, P.; Zhu, X.; Zhao, G.; Ren, X.; Wei, Q.; Xie, L. Structural characteristics and chloride intrusion mechanism of the passive film. Corros. Sci. 2022, 207, 110563. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M.; Rehim, S.S.A.E.; Hamza, M.M. Corrosion behavior of some austenitic stainless steels in chloride environments. Mater. Chem. Phys. 2009, 115, 80–85. [Google Scholar] [CrossRef]
- Chu, T.; Shao, C.; Wang, Y.; Ma, N.; Lu, F. Crack branching behavior and amorphous film formation mechanism during SCC expanding test for multi-layers weld metal of NiCrMoV steels. Mater. Des. 2022, 216, 110520. [Google Scholar] [CrossRef]
- Hou, Q.; Liu, Z.Y.; Li, C.T.; Li, X.G.; Shao, J.M. Degradation of the oxide film formed on Alloy 690TT in a high-temperature chloride solution. Appl. Surf. Sci. 2019, 467, 1104–1112. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Liu, L.; Pu, Q.; Jiang, L.; Chu, H.; Luo, Y.; Liu, Q.; Cai, H. Use of XPS for quantitative evaluation of tensile-stress-induced degradation of passive film on carbon steel in simulated concrete pore solution. Constr. Build. Mater. 2021, 274, 121779. [Google Scholar] [CrossRef]
- Wenman, M.R.; Trethewey, K.R.; Jarman, S.E.; Chard-Tuckey, P.R. A finite-element computational model of chloride-induced transgranular stress-corrosion cracking of austenitic stainless steel. Acta Mater. 2008, 56, 4125–4136. [Google Scholar] [CrossRef]
- Beber, V.C.; Schneider, B. Fatigue of structural adhesives under stress concentrations: Notch effect on fatigue strength, crack initiation and damage evolution. Int. J. Fatigue 2020, 140, 105824. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization of corrosion of pre-cracked X70 pipeline steel in a concentrated carbonate/bicarbonate solution. Corros. Sci. 2010, 52, 960–968. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Pezzato, L.; Dabalà, M. Stress corrosion cracking of AISI 304 under chromium variation within the standard limits: Failure analysis implementing microcapillary method. Eng. Fail. Anal. 2022, 142, 106797. [Google Scholar] [CrossRef]
- Das, N.K.; Suzuki, K.; Ogawa, K.; Shoji, T. Early stage SCC initiation analysis of fcc Fe–Cr–Ni ternary alloy at 288 °C: A quantum chemical molecular dynamics approach. Corros. Sci. 2009, 51, 908–913. [Google Scholar] [CrossRef]








| Medium Type | Design Pressure (MPa) | Operating Pressure (MPa) | Design Temperature (°C) | Operating Temperature (°C) | Allowable Flow Rate (m/s) | Operating Flow Rate (m/s) |
|---|---|---|---|---|---|---|
| Crude oil containing water, associated gas | 1.6 | 0.3 | 200 | 98 | 10 | 0.4 |
| Element | C | Si | Mn | P | S | Ni | Cr | Mo | Nb | V | Cu | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pipe body | 0.019 | 0.40 | 0.91 | 0.030 | 0.0017 | 9.92 | 16.12 | 2.03 | 0.008 | 0.082 | 0.25 | 0.008 |
| Pitting area | 0.018 | 0.40 | 0.90 | 0.030 | 0.0017 | 9.89 | 16.14 | 2.01 | 0.007 | 0.083 | 0.25 | 0.009 |
| ASTM-A312 | ≤ 0.035 | ≤ 1.00 | ≤ 2.00 | ≤ 0.045 | ≤ 0.030 | 10.00~ 14.00 | 16.00~ 18.00 | 2.00~ 3.00 | / | / | / | / |
| Sample | Original Gauge Length L1 (mm) | Final Gauge Length L2 (mm) | Yield Force Fm (kN) | Maximal Force FeL (kN) | Original Cross-Sectional Area S (mm2) | Tensile Strength Rm (MPa) | Yield Strength ReL (MPa) | Elongation after Fracture A (%) |
|---|---|---|---|---|---|---|---|---|
| 1# | 50 | 81.92 | 31.92 | 70.19 | 128.9 | 545 | 248 | 64 |
| 2# | 50 | 81.81 | 29.40 | 66.91 | 121.9 | 549 | 241 | 64 |
| 3# | 50 | 81.89 | 32.07 | 70.25 | 128.9 | 545 | 249 | 64 |
| ASTM-A312 | / | / | / | / | / | ≥485 | ≥170 | ≥35 |
| Elements | Area 1 | Area 2 | ||
|---|---|---|---|---|
| In wt.% | In at.% | In wt.% | In at.% | |
| O | 17.14 | 40.99 | 23.26 | 49.37 |
| Si | 0.70 | 0.95 | 1.33 | 1.60 |
| Mo | 5.21 | 2.08 | 5.02 | 1.78 |
| Cl | 3.04 | 3.28 | 9.47 | 9.07 |
| Cr | 43.75 | 32.19 | 28.47 | 18.60 |
| Fe | 25.82 | 17.69 | 27.11 | 16.49 |
| Ni | 4.34 | 2.83 | 5.34 | 3.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Li, Y.; Wu, F.; Luo, J.; Li, L.; Li, G. Failure Analysis of the Crack and Leakage of a Crude Oil Pipeline under CO2-Steam Flooding. Processes 2023, 11, 1567. https://doi.org/10.3390/pr11051567
Song C, Li Y, Wu F, Luo J, Li L, Li G. Failure Analysis of the Crack and Leakage of a Crude Oil Pipeline under CO2-Steam Flooding. Processes. 2023; 11(5):1567. https://doi.org/10.3390/pr11051567
Chicago/Turabian StyleSong, Chengli, Yuanpeng Li, Fan Wu, Jinheng Luo, Lifeng Li, and Guangshan Li. 2023. "Failure Analysis of the Crack and Leakage of a Crude Oil Pipeline under CO2-Steam Flooding" Processes 11, no. 5: 1567. https://doi.org/10.3390/pr11051567
APA StyleSong, C., Li, Y., Wu, F., Luo, J., Li, L., & Li, G. (2023). Failure Analysis of the Crack and Leakage of a Crude Oil Pipeline under CO2-Steam Flooding. Processes, 11(5), 1567. https://doi.org/10.3390/pr11051567






