Study on the Optimal Double-Layer Electrode for a Non-Aqueous Vanadium-Iron Redox Flow Battery Using a Machine Learning Model Coupled with Genetic Algorithm
Abstract
1. Introduction
2. Methods
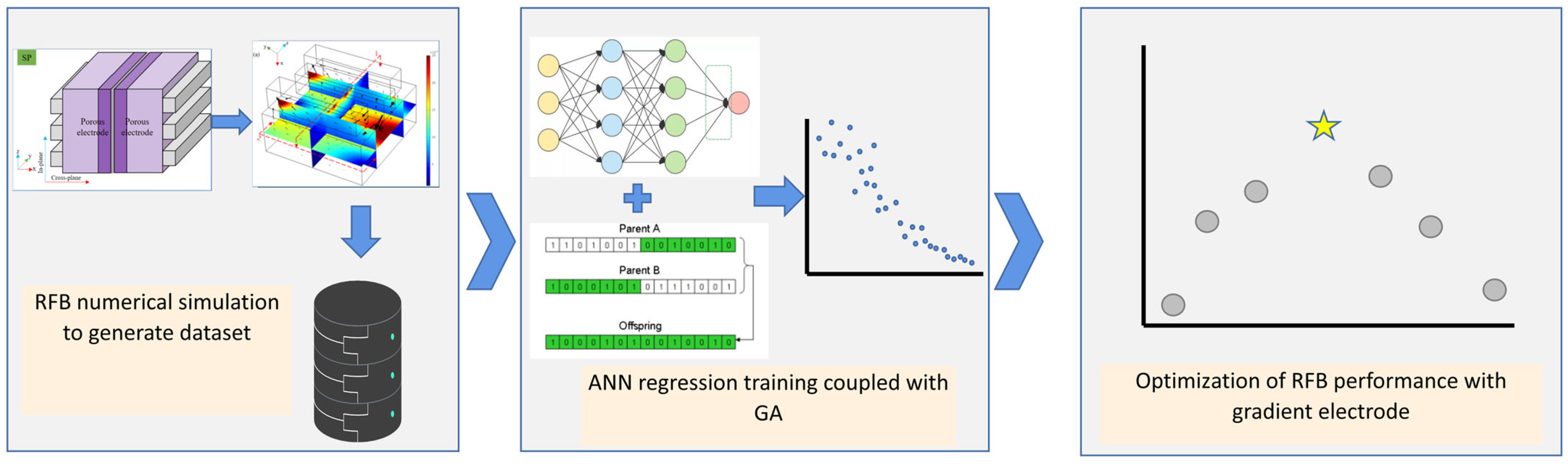
2.1. Scheme of Machine Learning Model Coupled with Genetic Algorithm
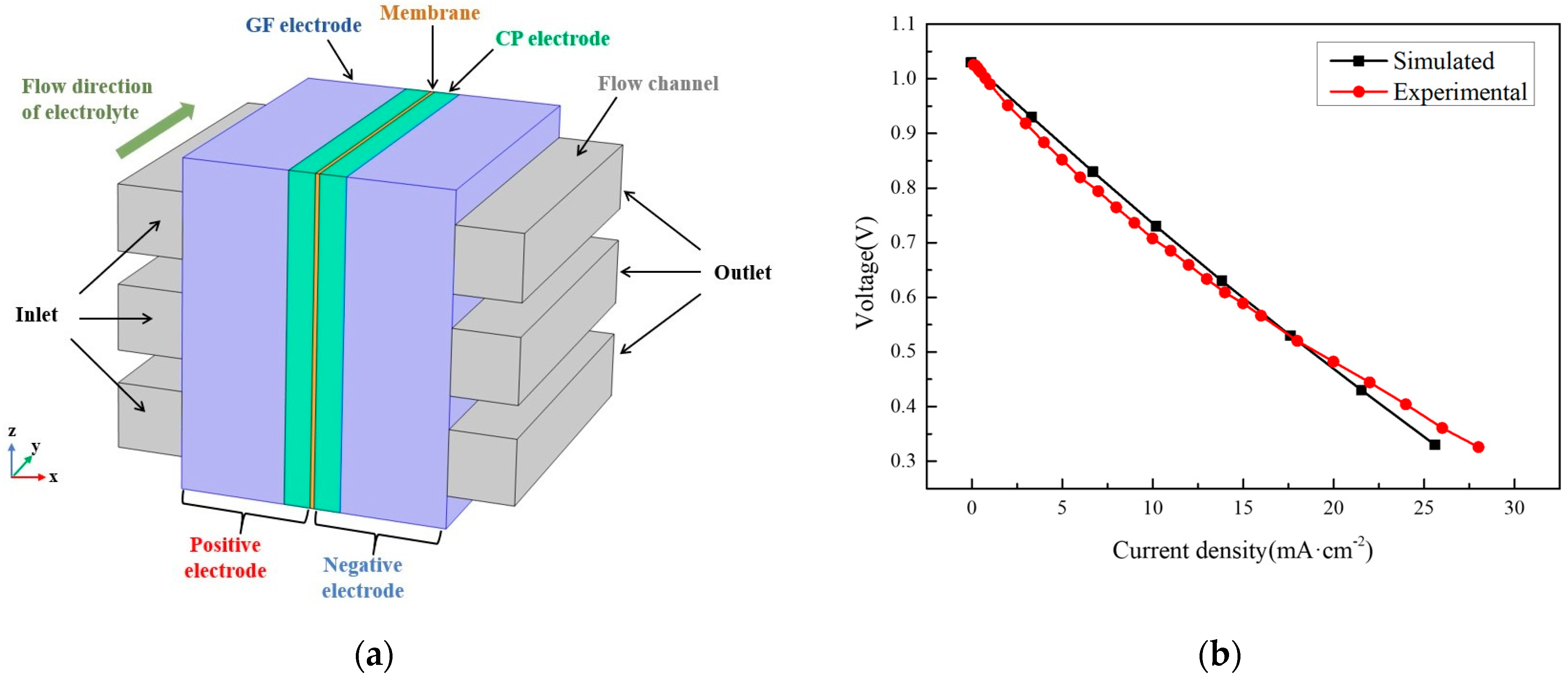
2.2. 3D Numerical Model of DES Electrolyte-Based Vanadium-Iron RFB
2.3. Dataset Generation
2.4. Artificial Neural Network Model Coupled with Genetic Algorithm
3. Results and Discussions
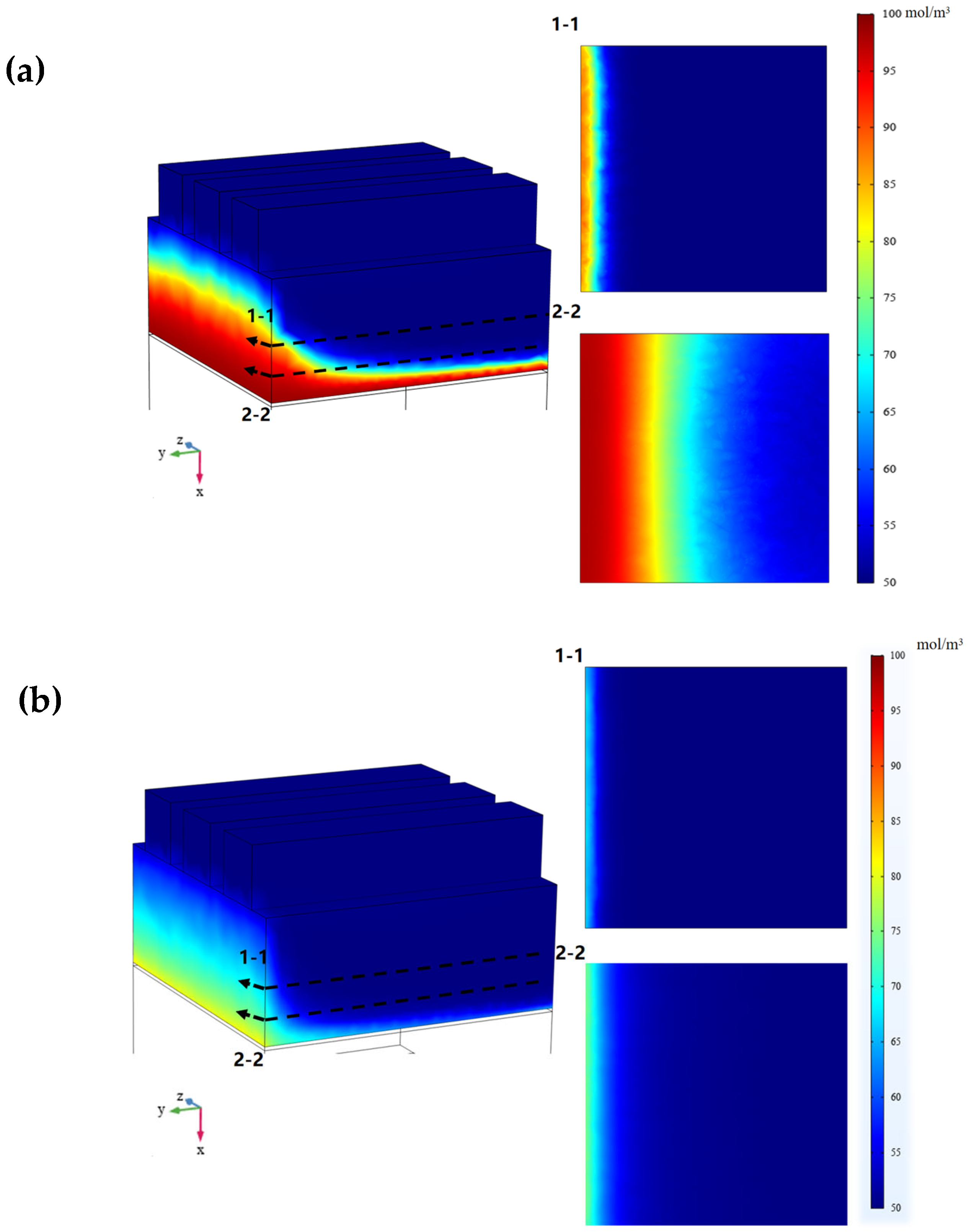
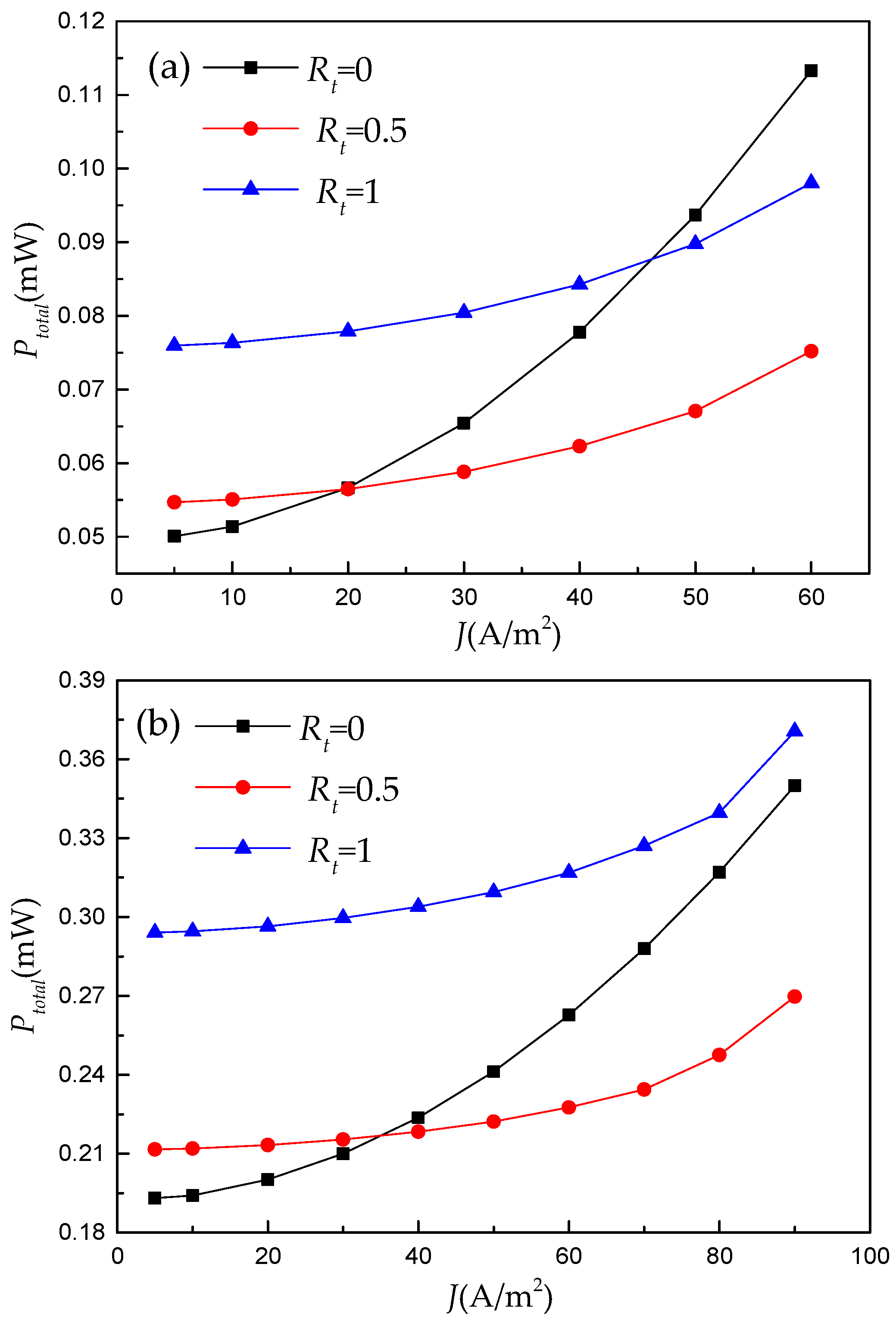
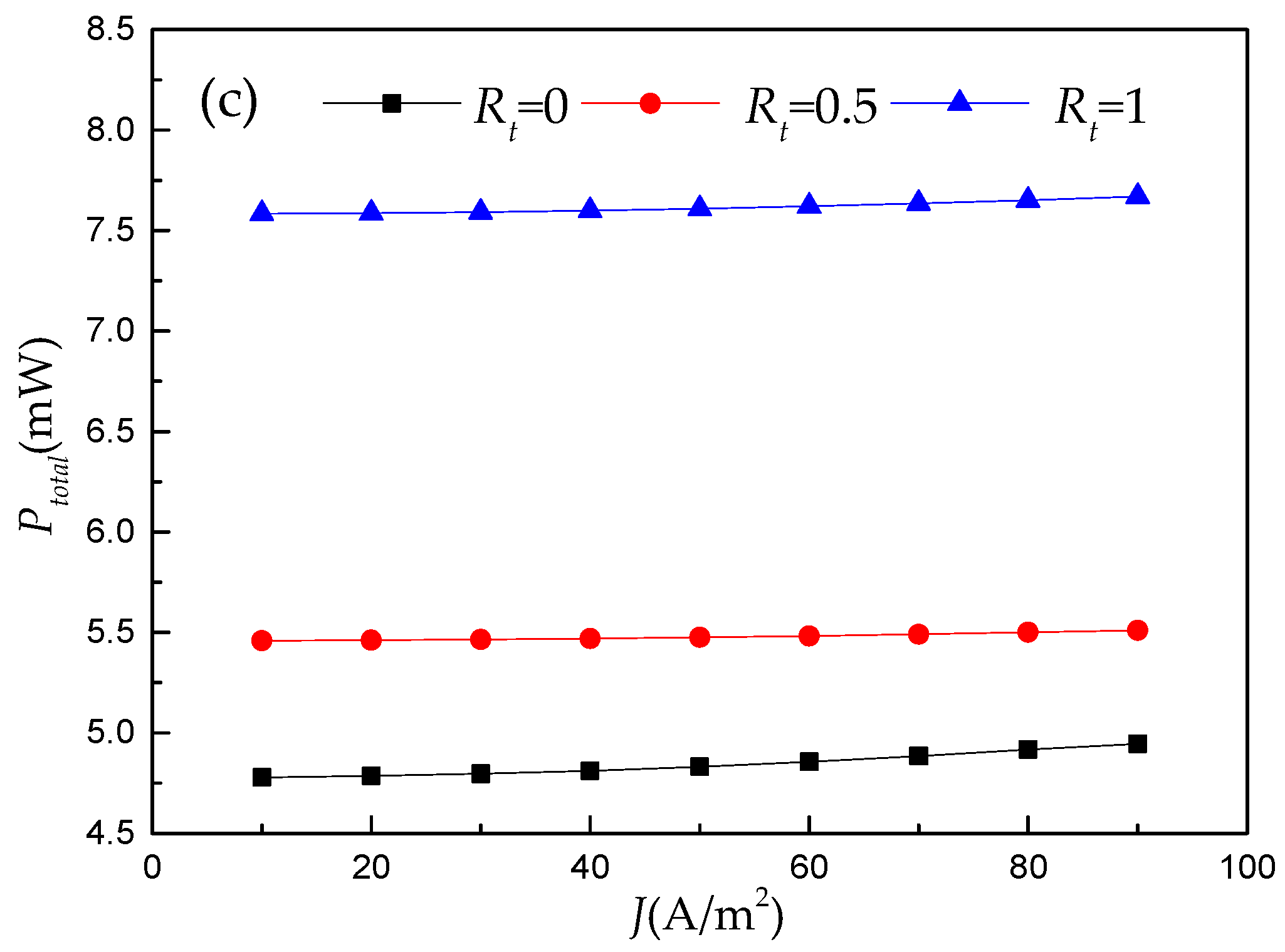
3.1. Numerical Results of RFB’s Galvanostatic Discharge Process
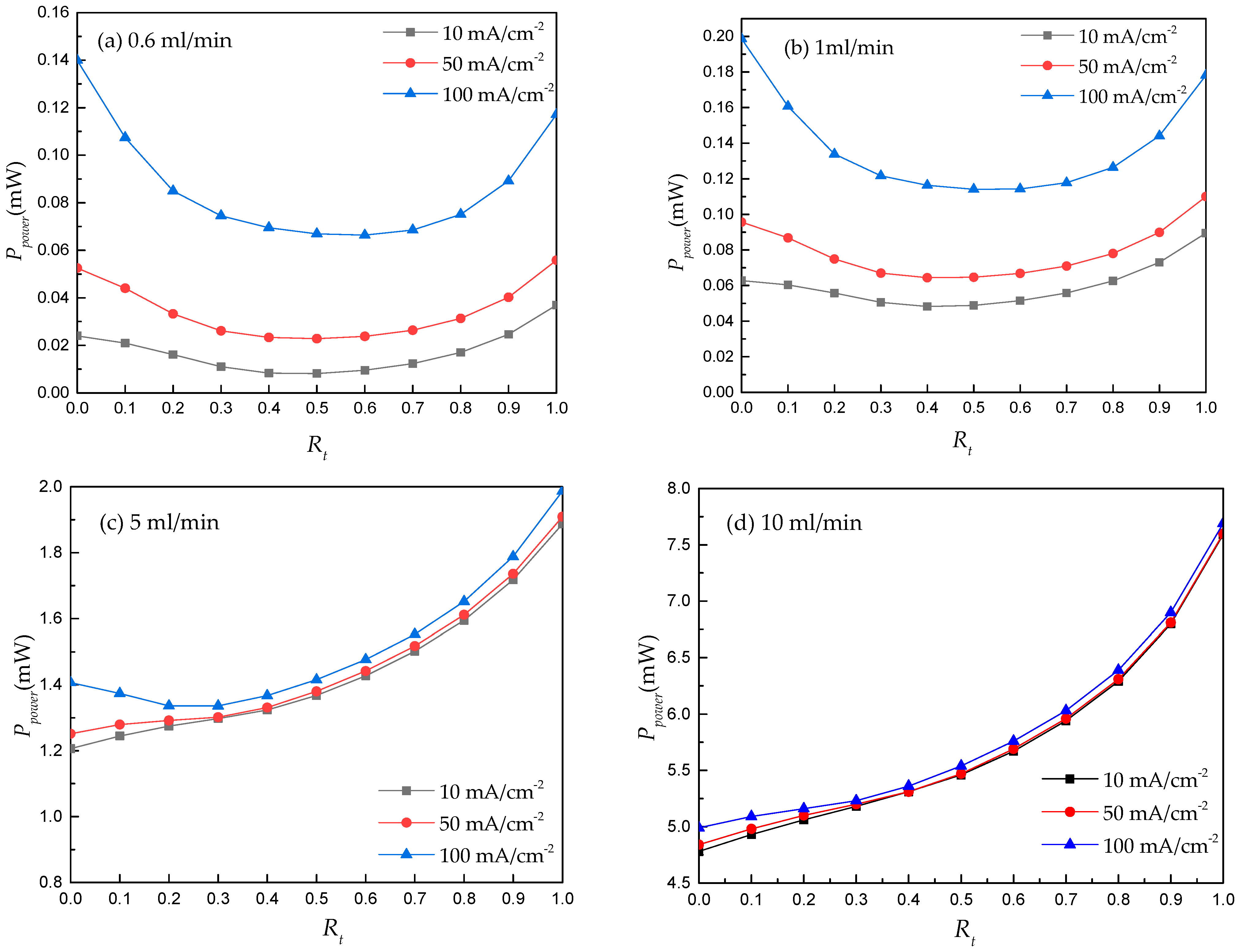
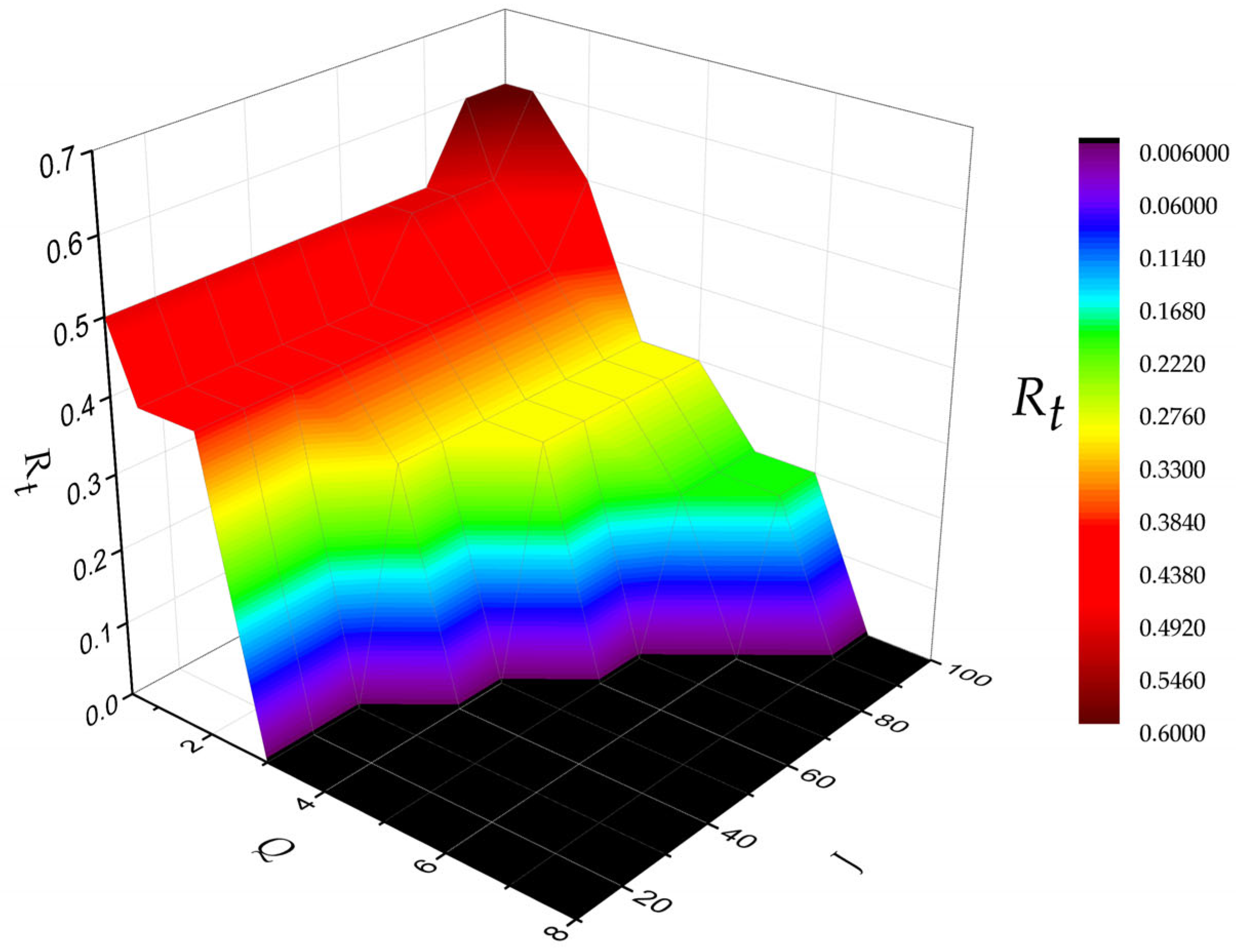
3.2. RFB’s Performance Prediction Using ANN Regression Model Coupled with GA
4. Conclusions
- (1)
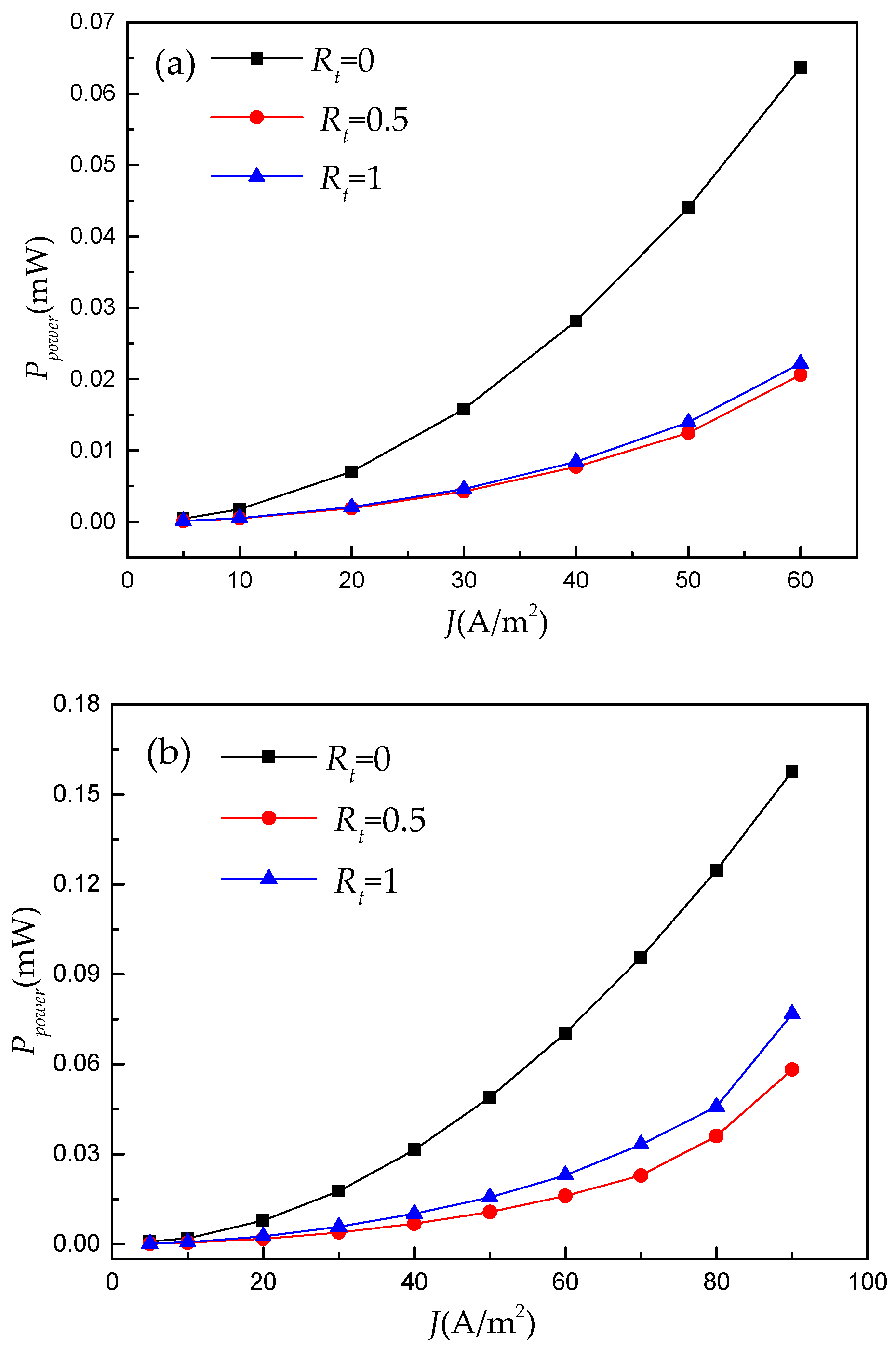
- The numerical results reveal that this double-layer electrode only plays a positive role under limited operating conditions. Hence, using an ML model to illustrate the regression relationship between RFB’s performance and electrode architectures under different operating conditions is necessary.
- (2)
- The accuracy of this well-trained ML model is tested and compared with other popular regression models. The comparative result shows that the MSE of this multi-layer ANN coupled with GA can reach 1.54 × 10−8. This predictive error is significantly lower than other ANNs without GA parameter optimization and is also lower than other popular ML regression models. The results show that the GA approach is one of the promising tools for training this multi-layer ANN. At the same time, intensive research should be conducted by comparing this optimization approach of GA with the commonly used optimizer, thus determining the applicable scope of this approach that has distinct advantages over other optimizers.
- (3)
- The performance prediction driven by this ML model shows that the pre-determined operating ranges (e.g., Q and J) are critical for designing the electrode architecture of an RFB. For example, based on the known operating ranges of the RFB, the predictive results of the ML algorithm can be used to estimate whether a double-layer electrode should be used in a non-aqueous vanadium-iron RFB and determine an optimal thickness ratio of this double-layer electrode.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Case, J.; Minteer, S.D. Bipolar Redox-Active Molecules in Non-Aqueous Organic Redox Flow Batteries: Status and Challenges. ChemElectroChem 2021, 8, 1215–1232. [Google Scholar] [CrossRef]
- Cao, J.; Tian, J.; Xu, J.; Wang, Y. Organic Flow Batteries: Recent Progress and Perspectives. Energy Fuels 2020, 34, 13384–13411. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Ge, M. Review of the Research Status of Cost-Effective Zinc–Iron Redox Flow Batteries. Batteries 2022, 8, 202. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, T.; Ali, M.; Wu, C.; Chen, W. Recent Progress in Organic Species for Redox Flow Batteries. Energy Storage Mater. 2022, 50, 105–138. [Google Scholar] [CrossRef]
- Chen, H.; Cong, G.; Lu, Y.C. Recent progress in organic redox flow batteries: Active materials, electrolytes and membranes. J. Energy Chem. 2018, 27, 1304–1325. [Google Scholar] [CrossRef]
- Mushtaq, K.; Lagarteira, T.; Zaidi, A.A.; Mendes, A. In-situ crossover diagnostics to assess membrane efficacy for non-aqueous redox flow battery. J. Energy Storage 2021, 40, 102713. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, J.; Zhang, J.; Leung, P.; Ma, Q.; Su, H.; Yang, W.; Xu, Q. Facile segmented graphite felt electrode for iron-vanadium redox flow batteries with deep eutectic solvent (DES) electrolyte. J. Power Sources 2021, 483, 229200. [Google Scholar] [CrossRef]
- Puttaswamy, R.; Mondal, C.; Mondal, D. An account on the deep eutectic solvents-based electrolytes for rechargeable batteries and supercapacitors. Sustain. Mater. Technol. 2022, 33, e00477. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Zhang, H.; Chen, J. Progress on the electrode materials towards vanadium flow batteries (VFBs) with improved power density. J. Energy Chem. 2018, 27, 1292–1303. [Google Scholar] [CrossRef]
- Abbas, A.; Eng, X.E.; Ee, N.; Saleem, F.; Wu, D.; Chen, W.; Handayani, M.; A Tabish, T.; Wai, N.; Lim, T.M. Development of reduced graphene oxide from biowaste as an electrode material for vanadium redox flow battery. J. Energy Storag. 2021, 41, 102848. [Google Scholar] [CrossRef]
- Lv, Y.; Han, C.; Zhu, Y.; Zhang, T.; Yao, S.; He, Z.; Dai, L.; Wang, L. Recent advances in metals and metal oxides as catalysts for vanadium redox flow battery: Properties, structures, and perspectives. J. Mater. Sci. Technol. 2021, 75, 96–109. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, L.; Li, J.; Fu, Q.; Zhu, X.; Liao, Q.; Zhang, Y. 3-D printed gradient porous composite electrodes improve anodic current distribution and performance in thermally regenerative flow battery for low-grade waste heat recovery. J. Power Sources 2020, 473, 228525. [Google Scholar] [CrossRef]
- Jing, M.; Zhang, C.; Qi, X.; Yang, Y.; Liu, J.; Fan, X.; Yan, C.; Fang, D. Gradient-microstructural porous graphene gelatum/flexible graphite plate integrated electrode for vanadium redox flow batteries. Int. J. Hydrogen Energy 2020, 45, 916–923. [Google Scholar] [CrossRef]
- Chen, W.; Kang, J.; Shu, Q.; Zhang, Y. Analysis of storage capacity and energy conversion on the performance of gradient and double-layered porous electrode in all-vanadium redox flow batteries. Energy 2019, 180, 341–355. [Google Scholar] [CrossRef]
- Jiang, H.R.; Zhang, B.W.; Sun, J.; Fan, X.; Shyy, W.; Zhao, T. A gradient porous electrode with balanced transport properties and active surface areas for vanadium redox flow batteries. J. Power Sources 2019, 440, 227159. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.Y.; Yun, N.; Yang, M.; Jeon, Y.; Kim, K.J.; Choi, J.-I. Activity gradient carbon felt electrodes for vanadium redox flow batteries. J. Power Sources 2018, 408, 128–135. [Google Scholar] [CrossRef]
- Wu, Q.; Lv, Y.; Lin, L.; Zhang, X.; Liu, Y.; Zhou, X. An improved thin-film electrode for vanadium redox flow batteries enabled by a dual layered structure. J. Power Sources 2019, 410, 152–161. [Google Scholar] [CrossRef]
- Ma, Q.; Fu, W.; Zhao, L.; Chen, Z.; Su, H.; Xu, Q. A double-layer electrode for the negative side of deep eutectic solvent electrolyte-based vanadium-iron redox flow battery. Energy 2023, 265, 126291. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, N.; Sun, C.; Luo, X. Investigations on physicochemical properties and electrochemical performance of graphite felt and carbon felt for iron-chromium redox flow battery. Int. J. Energy Res. 2020, 44, 3839–3853. [Google Scholar] [CrossRef]
- Takagishi, Y.; Yamanaka, T.; Yamaue, T. Machine Learning Approaches for Designing Mesoscale Structure of Li-Ion Battery Electrodes. Batteries 2019, 5, 54. [Google Scholar] [CrossRef]
- Shodiev, A.; Duquesnoy, M.; Arcelus, O.; Chouchane, M.; Li, J.; Franco, A.A. Machine learning 3D-resolved prediction of electrolyte infiltration in battery porous electrodes. J. Power Sources 2021, 511, 230384. [Google Scholar] [CrossRef]
- Wan, S.; Liang, X.; Jiang, H.; Sun, J.; Djilali, N.; Zhao, T. A coupled machine learning and genetic algorithm approach to the design of porous electrodes for redox flow batteries. Appl. Energy 2021, 298, 0306–2619. [Google Scholar] [CrossRef]
- van Gorp, R.; van der Heijden, M.; Sadeghi, M.A.; Gostick, J.; Forner-Cuenca, A. Bottom-up design of porous electrodes by combining a genetic algorithm and a pore network model. Chem. Eng. J. 2022, 455, 139947. [Google Scholar] [CrossRef]
- Ding, R.; Ding, Y.; Zhang, H.; Wang, R.; Xu, Z.; Liu, Y.; Yin, W.; Wang, J.; Li, J.; Liu, J. Applying machine learning to boost the development of high-performance membrane electrode assembly for proton exchange membrane fuel cells. J. Mater. Chem. 2021, 9, 6841–6850. [Google Scholar] [CrossRef]
- Moses, I.A.; Joshi, R.P.; Ozdemir, B.; Kumar, N.; Eickholt, J.; Barone, V. Machine Learning Screening of Metal-Ion Battery Electrode Materials. ACS Appl. Mater. Interfaces 2021, 13, 53355–53362. [Google Scholar] [CrossRef]
- Ma, Q.; Mao, C.W.; Zhao, L.J.; Chen, Z.; Su, H.; Xu, Q. A pore-scale study for reactive transport processes in double-layer gradient electrode as negative side of a deep eutectic solvent electrolyte-based vanadium-iron redox flow battery. Electrochim. Acta 2022, 431, 141110. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, L.J.; Xu, J.C.; Su, H.; Zhang, W.; Yang, W.; Xu, Q. Pore-scale investigation of reactive transfer process in a deep eutectic solvent (DES) electrolyte-based vanadium-iron redox flow battery. Electrochim. Acta 2020, 353, 1364863. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, L.; Su, H.N.; Zhang, W.; Yang, W.; Xu, Q. Numerical investigation on the dispersion effect in vanadium redox flow battery. Chem. Eng. J. 2020, 393, 124753. [Google Scholar] [CrossRef]
- Ma, Q.; Mao, C.W.; Fu, W.X.; Li, H.; Su, H.; Xu, Q. Numerical study of deep eutectic solvent electrolyte-based vanadium-iron redox flow battery with three-dimensional multi-layer porous electrode. Int. J. Energy Res. 2022, 46, 12820–12836. [Google Scholar] [CrossRef]
- Mao, C.W.; Ma, Q.; Li, H.H.; Su, H.; Xu, Q. Numerical investigation of coupling effects of gradient porous electrode and flow channel pattern on iron-vanadium redox flow battery. Int. J. Green Energy 2022, 19, 1375–1387. [Google Scholar] [CrossRef]
- Zhao, L.J.; Ma, Q.; Xu, Q.; Su, H.; Zhang, W. Performance improvement of nonaqueous iron-vanadium flow battery using chromium oxide-modified graphite felt electrode. Ionics 2021, 27, 4315–4325. [Google Scholar] [CrossRef]
- Katoch, S.; Chauhan, S.S.; Kumar, V. A review on genetic algorithm: Past, present, and future. Multimed. Tools Appl. 2021, 80, 8091–8126. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]




| Types | Boundaries | Descriptive Equations |
|---|---|---|
| Charge transfer boundary | Galvanostatic boundary on the positive side | |
| Ground boundary on the negative side | ||
| Insulation boundary for other walls | ||
| Momentum transfer boundary | Constant electrolyte flow rate boundary at the inlet | |
| Constant pressure boundary at the outlet | ||
| Non-slip flow condition for other boundaries | ||
| Mass transfer boundary | Constant SOC boundary at the inlet | |
| Full development boundary at the outlet | ||
| Non-permeate boundary for other walls |
| Geometric Parameters | Value | Unit |
|---|---|---|
| Cell height | 10 | mm |
| Cell width | 10 | mm |
| Total electrode thickness | 4 | mm |
| Membrane thickness | 0.12 | mm |
| Channel height | 2 | mm |
| Channel width | 2 | mm |
| Channel length | 10 | mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Fu, W.; Xu, J.; Wang, Z.; Xu, Q. Study on the Optimal Double-Layer Electrode for a Non-Aqueous Vanadium-Iron Redox Flow Battery Using a Machine Learning Model Coupled with Genetic Algorithm. Processes 2023, 11, 1529. https://doi.org/10.3390/pr11051529
Ma Q, Fu W, Xu J, Wang Z, Xu Q. Study on the Optimal Double-Layer Electrode for a Non-Aqueous Vanadium-Iron Redox Flow Battery Using a Machine Learning Model Coupled with Genetic Algorithm. Processes. 2023; 11(5):1529. https://doi.org/10.3390/pr11051529
Chicago/Turabian StyleMa, Qiang, Wenxuan Fu, Jinhua Xu, Zhiqiang Wang, and Qian Xu. 2023. "Study on the Optimal Double-Layer Electrode for a Non-Aqueous Vanadium-Iron Redox Flow Battery Using a Machine Learning Model Coupled with Genetic Algorithm" Processes 11, no. 5: 1529. https://doi.org/10.3390/pr11051529
APA StyleMa, Q., Fu, W., Xu, J., Wang, Z., & Xu, Q. (2023). Study on the Optimal Double-Layer Electrode for a Non-Aqueous Vanadium-Iron Redox Flow Battery Using a Machine Learning Model Coupled with Genetic Algorithm. Processes, 11(5), 1529. https://doi.org/10.3390/pr11051529








