Lignocellulose-Derived Arabinose for Energy and Chemicals Synthesis through Microbial Cell Factories: A Review
Abstract
1. Introduction
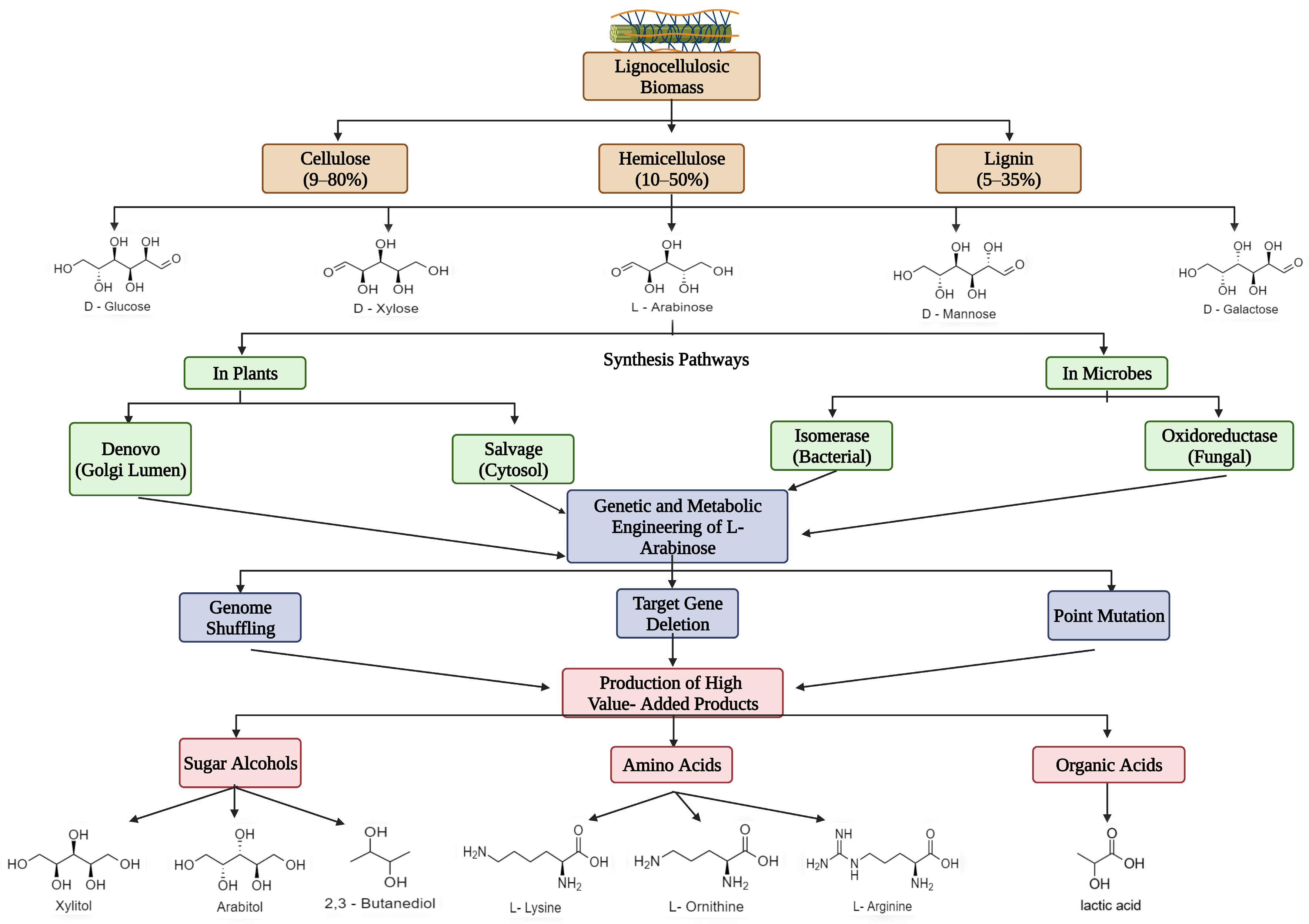
2. Abundance and Significance of L-Ara as a Bioresource
2.1. 2,3-Butanediol
2.2. Other Value-Added Products
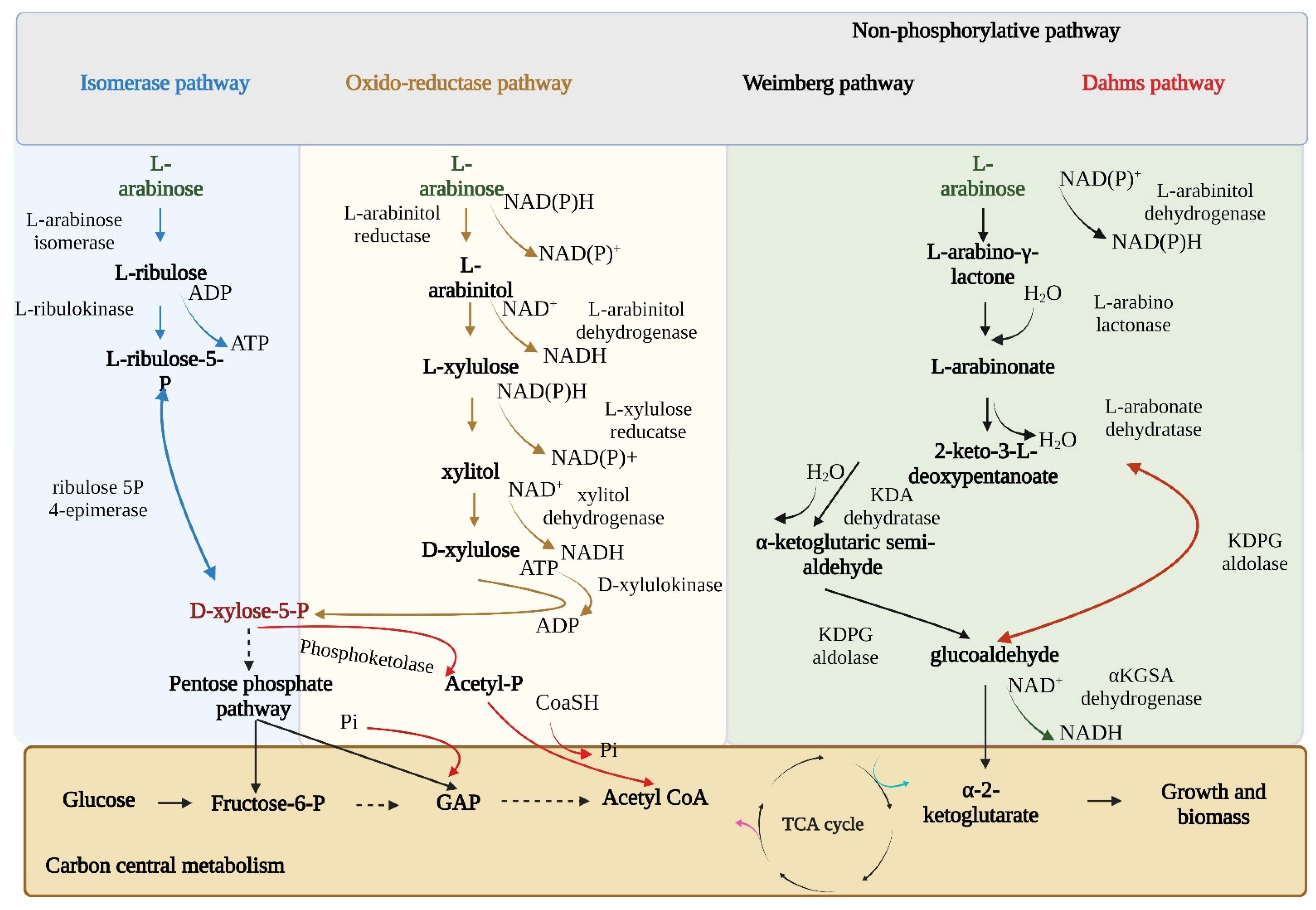
3. Overview of Distinct Natural Metabolic Pathways of L-Ara Assimilation by Microbes
Kinetics of L-Ara Uptake by Different Microbes
4. Native L-Ara Fermenting Strains and Its Metabolic Pathway
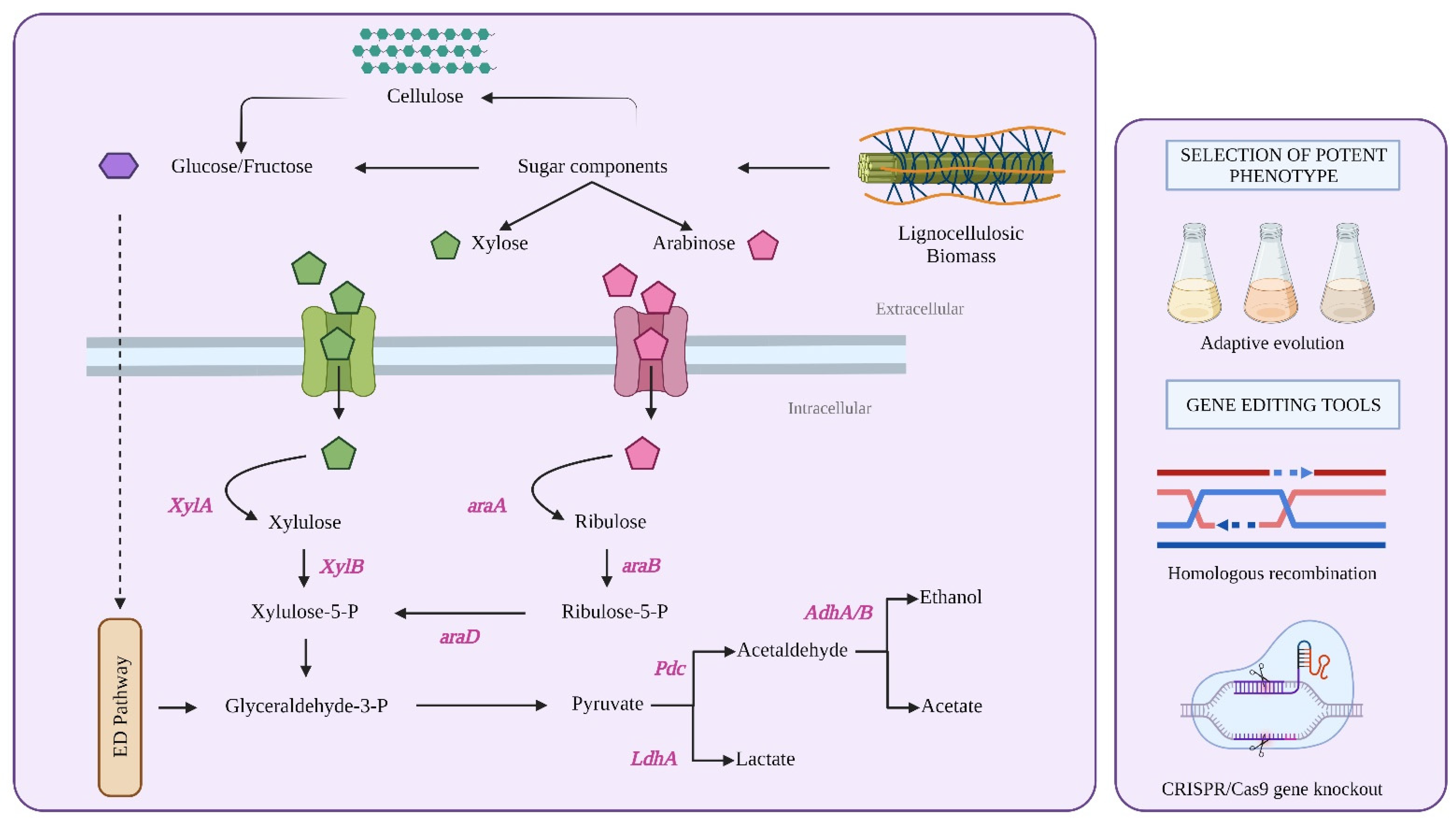
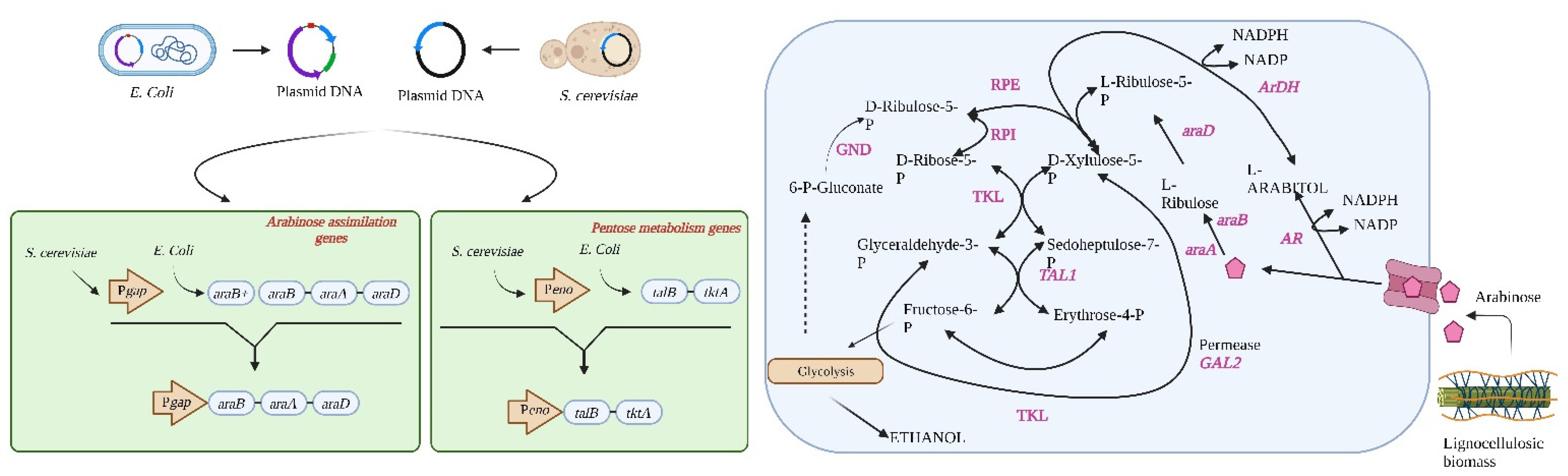
5. Metabolic Engineering of Microbial Cell Factories for Improved L-Ara Fermentation
5.1. Engineering Zymomonas Mobilis for L-Ara Fermentation
5.2. Engineering Saccharomyces Cerevisiae for L-Ara Fermentation
5.3. Fusants-A Distinct Hybrid Yeast
5.4. Engineering Bacteria for L-Ara Fermentation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francois, J.; Alkim, C.; Morin, N. Engineering microbial pathways for production of bio-based chemicals from lignocellulosi/.c sugars: Current status and perspectives. Biotechnol. Biofuels 2020, 13, 118. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Holtzapple, M.T. Cellulose, hemicelluloses, and lignin. In Encyclopedia of Food Science, Food Technology, and Nutrition; Macrae, R., Robinson, R.K., Sadler, M.J., Eds.; Academic Press: London, UK, 1993; pp. 2731–2738. [Google Scholar]
- Sekeri, S.H.; Ibrahim, M.N.M.; Umar, K.; Yaqoob, A.A.; Azmi, M.N.; Hussin, M.H.; Othman, M.B.H.; Malik, M.F.I.A. Preparation and characterization of nanosized lignin from oil palm (Elaeis guineensis) biomass as a novel emulsifying agent. Int. J. Biol. Macromol. 2020, 164, 3114–3124. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Sekeri, S.H.; Othman, M.B.H.; Ibrahim, M.N.M.; Feizi, Z.H. Thermal degradation and kinetics stability studies of oil palm (Elaeis Guineensis) biomass-derived lignin nanoparticle and its application as an emulsifying agent. Arab. J. Chem. 2021, 14, 103182. [Google Scholar] [CrossRef]
- Schneider, J.; Niermann, K.; Wendisch, V. Production of the amino acids L-glutamate, L-lysine, L-ornithine and L-arginine from L-Ara by recombinant Corynebacterium glutamicum. J. Biotechnol. 2011, 154, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Meiswinkel, T.; Gopinath, V.; Lindner, S.; Nampoothiri, K.; Wendisch, V. Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine. Microb. Biotechnol. 2012, 6, 131–140. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; de Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [Google Scholar] [CrossRef]
- Banu, J.R.; Kavitha, P.S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic biomass based biorefinery: A successful platform towards circular bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- Market Watch. Food Grade L-Arabinose Market Demand by 2030. Available online: https://www.marketwatch.com/press-release/food-grade-l-arabinose-market-demand-by-2030-2023-04-06 (accessed on 10 April 2023).
- Lane, S.; Xu, H.; Oh, E.J.; Kim, H.; Lesmana, A.; Jeong, D.; Zhang, G.; Tsai, C.S.; Jin, Y.S.; Kim, S.R. Glucose repression can be alleviated by reducing glucose phosphorylation rate in Saccharomyces cerevisiae. Sci. Rep. 2018, 8, 2613. [Google Scholar] [CrossRef]
- Mohamed, E.T.; Mundhada, H.; Landberg, J.; Cann, I.; Mackie, R.I.; Nielsen, A.T.; Herrgard, M.J.; Feist, A.M. Generation of an E. coli platform strain for improved sucrose utilization using adaptive laboratory evolution. Microb. Cell Fact. 2019, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, F.; Carbonell, P.; François, J.M.; Haynes, K.A. Editorial–Synthetic biology: Engineering complexity and refactoring cell capabilities. Front. Bioeng. Biotechnol. 2015, 3, 120. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Park, J.H.; Choi, I.S.; Wi, S.G.; Ha, S.; Chun, H.H.; Hwang, I.M.; Chang, J.Y.; Choi, H.-J.; Kim, J.-C.; et al. Effective approach to organic acid production from agricultural kimchi cabbage waste and its potential application. PLoS ONE 2018, 13, e0207801. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Eberhardt, D.; Wendisch, V.F. Improving putrescine production by Corynebacterium glutamicum by fine-tuning ornithine transcarbamoylase activity using a plasmid addiction system. Appl. Microbiol. Biotechnol. 2012, 95, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Venkateswar Rao, L.; Goli, J.; Gentela, J.; Koti, S. Bioconversion of lignocellulosic biomass to xylitol: An overview. Bioresour. Technol. 2016, 213, 299–310. [Google Scholar] [CrossRef]
- Safian, M.T.-U.; Sekeri, S.H.; Yaqoob, A.A.; Serra, A.; Jamudin, M.D.; Ibrahim, M.N.M. Utilization of lignocellulosic biomass: A practical journey towards the development of emulsifying agent. Talanta 2022, 239, 123109. [Google Scholar] [CrossRef]
- Gírio, F.; Fonseca, C.; Carvalheiro, F.; Duarte, L.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Fehér, C. Novel approaches for biotechnological production and application of L-arabinose. J. Carbohydr. Chem. 2018, 37, 251–284. [Google Scholar] [CrossRef]
- Kennedy, M.; List, D.; Lu, Y.; Foo, L.Y.; Newman, R.H.; Sims, I.M.; Bain, P.J.S.; Hamilton, B.; Fenton, G. Apple pomace and products derived from apple pomace: Use, composition and analysis. In Modern Methods of Plant Analysis, Analysis of Plant Waste Materials; Linskens, H.F., Jackson, J.F., Eds.; Springer-Verlag: Berlin, Germany, 1999; Volume 20, pp. 75–119. [Google Scholar]
- Doran, J.B.; Cripe, J.; Sutton, M.; Foster, B. Fermentations of pectin rich biomass with recombinant bacteria to produce fuel ethanol. Appl. Biochem. Biotechnol. 2000, 84, 141–152. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Zhou, W.; Widmer, W.; Grohmann, K. Developments in ethanol production from citrus peel waste. Proc. Fla. State Hort. Soc. 2008, 121, 307–310. [Google Scholar]
- Edwards, M.; Doran-Peterson, J. Pectin-rich biomass as feedstock for fuel ethanol production. Appl. Microbiol. Biotechnol. 2012, 95, 565–575. [Google Scholar] [CrossRef]
- Seiboth, B.; Metz, B. Fungal arabinan and L-arabinose metabolism. Appl. Microbiol. Biotechnol. 2011, 89, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, J.; Lindhauer, M. Pilot-scale isolation of glucuronoarabinoxylans from wheat bran. Carbohydr. Polym. 2005, 59, 225–230. [Google Scholar] [CrossRef]
- Fehér, C. Integrated process of arabinose biopurification and xylitol fermentation based on the diverse action of Candida boidinii. Chem. Biochem. Eng. Q. 2016, 29, 587–597. [Google Scholar] [CrossRef]
- Kühnel, S.; Schols, H.; Gruppen, H. Aiming for the complete utilization of sugar-beet pulp: Examination of the effects of mild acid and hydrothermal pretreatment followed by enzymatic digestion. Biotechnol. Biofuels 2011, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Treimo, J.; Westereng, B.; Horn, S.J.; Forssell, P.; Robertson, J.A.; Faulds, C.B.; Waldron, K.W.; Buchert, J.; Eijsink, V.G.H. Enzymatic solubilization of brewers’ spent grain by combined action of carbohydrases and peptidases. J. Agric. Food Chem. 2009, 57, 3316–3324. [Google Scholar] [CrossRef]
- Gottschalk, L.; Oliveira, R.; Bon, E. Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochem. Eng. J. 2010, 51, 72–78. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.; Jonglertjunya, W. Rice straw and sugarcane bagasse degradation mimicking lignocellulose decay in nature: An alternative approach to biorefinery. ScienceAsia 2012, 38, 364. [Google Scholar] [CrossRef]
- Sabiha-Hanim, S.; Siti-Norsafurah, A.M. Physical properties of hemicellulose films from sugarcane bagasse. Procedia Eng. 2012, 42, 1390–1395. [Google Scholar] [CrossRef]
- Tsigie, Y.; Wang, C.; Truong, C.; Ju, Y. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour. Technol. 2011, 102, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Y.; Yue, W.; Wang, W.; Wang, Y.-Y.; Yuan, T.-Q.; Sun, R.-C. Valorization of lignin and cellulose in acid-steam-exploded corn stover by a moderate alkaline ethanol post-treatment based on an integrated biorefinery concept. Biotechnol. Biofuels. 2016, 9, 238. [Google Scholar] [CrossRef]
- Cellulosic Biofuel Process Can also Improve Ruminant Forage Digestibility. MSU Extension. Available online: https://www.canr.msu.edu/news/cellulosic_biofuel_process_can_also_improve_ruminant_forage_digestibility. (accessed on 28 April 2022).
- Fehér, A. Combined approaches to xylose production from corn stover by dilute acid hydrolysis. Chem. Biochem. Eng. Q. 2017, 31, 77–87. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, M.; Zhou, Z.; Huang, T.; Chen, X.; Wang, Y. Isolation of cellulose with ionic liquid from steam exploded rice straw. Ind. Crops Prod. 2011, 33, 734–738. [Google Scholar] [CrossRef]
- Pinzi, S.; Dorado, M. Vegetable-based feedstocks for biofuels production. In Handbook of Biofuels Production: Processes and Technologies; Luque, R., Campelo, J., Clark, J., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2011; pp. 61–94. [Google Scholar]
- Roberto, I.; Mussatto, S.; Rodrigues, R. Dilute-acid hydrolysis for optimization of xylose recovery from rice straw in a semi-pilot reactor. Ind. Crops. Prod. 2003, 17, 171–176. [Google Scholar] [CrossRef]
- Nigam, J.N. Bioconversion of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to motor fuel ethanol by xylose-fermenting yeast. J. Biotechnol. 2002, 97, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, J.R.; Daza, L.T.; Lindado, G.; Peláez, H.C.; Córdoba, Á.P. Acid hydrolysis of water hyacinth to obtaining fermentable sugars. Cienc. Tecnol. Futuro. 2013, 5, 101–112. [Google Scholar] [CrossRef][Green Version]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Gírio, F.M. Wheat straw autohydrolysis: Process optimization and products characterization. Appl. Biochem. Biotechnol. 2009, 153, 84–93. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Hubbe, M.; Taha, M.; Becquart, F.; Ayoub, A. A review of water-resistant hemicellulose-based materials: Processing and applications. Chem. Sus. Chem. 2016, 10, 305–323. [Google Scholar] [CrossRef]
- Tozluoğlu, A.; Özyurek, Ö.; Çöpür, Y.; Özdemir, H. Integrated production of biofilm, bioethanol, and papermaking pulp from wheat Straw. Bioresour. 2015, 10, 7834–7854. [Google Scholar] [CrossRef]
- Olmos, J.C.; Hansen, M.Z. Enzymatic depolymerization of sugar beet pulp: Production and characterization of pectin and pectic-oligosaccharides as a potential source for functional carbohydrates. Chem. Eng. J. 2012, 192, 29–36. [Google Scholar] [CrossRef]
- Saric, L.; Filipcev, B.; Simurina, O.; Plavsic, D. Sugar beet molasses: Properties and applications in osmotic dehydration of fruits and vegetables. Food Feed Res. 2016, 43, 135–144. [Google Scholar] [CrossRef]
- Dinand, E.; Chanzy, H.; Vignon, M. Parenchymal cell cellulose from sugar beet pulp: Preparation and properties. Cellulose 1996, 3, 183–188. [Google Scholar] [CrossRef]
- Eveleigh, D.E. Comprehensive biotechnology: The principles, applications and regulations of biotechnology in industry, agriculture and medicine. In The Principles of Biotechnology: Scientific Fundamentals; Moo-Young, M., Bull, A.T., Dalton, H., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 1, p. 688. [Google Scholar]
- Gama, R.; Dyk, J.V.; Pletschke, B. Optimisation of enzymatic hydrolysis of apple pomace for production of biofuel and biorefinery chemicals using commercial enzymes. 3 Biotech 2015, 5, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of orange peel waste and valorization to obtain reducing sugars. Molecules 2021, 26, 1348. [Google Scholar] [CrossRef]
- Torrado, A.M.; Cortés, S.; Salgado, J.M.; Max, B.; Rodríguez, N.; Bibbins, B.P.; Converti, A.; Domínguez, J.M. Citric acid production from orange peel wastes by solid-state fermentation. Braz. J. Microbiol. 2011, 42, 394–409. [Google Scholar] [CrossRef]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Kheiralla, Z.H.; El-Gendy, N.S.; Ahmed, H.A.; Shaltout Th, H.; Hussein, M.M.D. Upgrading of Tomato (Solanum lycopersicum) Agroindustrial Wastes. J. Microb. Biochem. Technol. 2018, 10, 46–48. [Google Scholar] [CrossRef]
- Del Valle, M.; Cámara, M.; Torija, M. Chemical characterization of tomato pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef]
- Ma, C.; Wang, A.; Qin, J.; Li, L.; Ai, X.; Jiang, T.; Tang, H.; Xu, P. Enhanced 2, 3-butanediol production by Klebsiella pneumoniae SDM. Appl. Microbiol. Biotechnol. 2009, 82, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z. Recent advances on production of 2, 3-butanediol using engineered microbes. Biotechnol. Adv. 2019, 37, 569–578. [Google Scholar] [CrossRef]
- Saha, B.C.; Bothast, R.J. Production of 2,3-butanediol by newly isolated Enterobacter cloacae. Appl. Microbiol. Biotechnol. 1999, 52, 321–326. [Google Scholar] [CrossRef]
- Białkowska, A.M. Strategies for efficient and economical 2, 3-butanediol production: New trends in this field. World J. Microbiol. Biotechnol. 2016, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hagerdal, B.; Karhumaa, K.; Fonseca, C.; Spencer-Martins, I.; Gorwa-Grauslund, M.F. Towards industrial pentose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 2007, 74, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Meiswinkel, T.M.; Wendisch, V.F.; Nampoothiri, K.M. Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2011, 92, 985–996. [Google Scholar] [CrossRef]
- Sasaki, M.; Jojima, T.; Kawaguchi, H.; Inui, M.; Yukawa, H. Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars. Appl. Microbiol. Biotechnol. 2009, 85, 105–115. [Google Scholar] [CrossRef]
- Jojima, T.; Omumasaba, C.; Inui, M.; Yukawa, H. Sugar transporters in efficient utilization of mixed sugar substrates: Current knowledge and outlook. Appl. Microbiol. Biotechnol. 2009, 85, 471–480. [Google Scholar] [CrossRef]
- Chiang, C.; Knight, S.G. L-Arabinose metabolism by cell-free extracts of Penicillium chrysogenum. Biochim. Biophys. Acta 1961, 46, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, C.F.B.; Busink, R.; van de Vondervoort, P.; Dijkema, C.; Swart, K.; Visser, J. L-Arabinose and D-xylose catabolism in Aspergillus niger. J. Gen. Microbiol. 1989, 135, 2163–2171. [Google Scholar] [CrossRef][Green Version]
- Umai, D.; Kayalvizhi, R.; Kumar, V.; Jacob, S. Xylitol: Bioproduction and applications-A Review. Front. Sustain. 2022, 3, 826190. [Google Scholar] [CrossRef]
- Jin, Y.; Cruz, J.; Jeffries, T. Xylitol production by a Pichia stipitis D-xylulokinase mutant. Appl. Microbiol. Biotechnol. 2005, 68, 42–45. [Google Scholar] [CrossRef]
- Dahms, A.S. 3-Deoxy-D-pentulosonic acid aldolase and its role in a new pathway of D-xylose degradation. Biochem. Biophys. Res. Commun. 1974, 60, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Weimberg, R. Pentose oxidation by Pseudomonas fragi. J. Biol. Chem. 1961, 236, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Atomi, H. Novel metabolic pathways in Archaea. Curr. Opin. Microbiol. 2011, 14, 307–314. [Google Scholar] [CrossRef] [PubMed]
- McClintock, M.K.; Wang, J.; Zhang, K. Application of nonphosphorylative metabolism as an alternative for utilization of Lignocellulosic Biomass. Front Microbiol. 2017, 8, 2310. [Google Scholar] [CrossRef]
- Burley, S.; Bonanno, J. Structural genomics of proteins from conserved biochemical pathways and processes. Curr. Opin. Struct. Biol. 2002, 12, 383–391. [Google Scholar] [CrossRef]
- Liu, J.; Rost, B. Comparing function and structure between entire proteomes. Protein Sci. 2001, 10, 1970–1979. [Google Scholar] [CrossRef]
- Vermersch, P.S.; Tesmer, J.J.; Lemon, D.D.; Quiocho, F.A. A Pro to Gly mutation in the hinge of the arabinose-binding protein enhances binding and alters specificity. Sugar-binding and crystallographic studies. J. Biol. Chem. 1990, 265, 16592–16603. [Google Scholar] [CrossRef] [PubMed]
- deGroot, M.; Prathumpai, W.; Visser, J.; Ruijter, G. Metabolic control analysis of Aspergillus niger L-arabinose catabolism. Biotechnol. Prog. 2005, 21, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.P.; Flipphi, M.J.; Witteveen, C.F.; Visser, J. Characterization of an Aspergillus nidulans L-arabitol dehydrogenase mutant. FEMS Microbial. Lett. 1994, 123, 83–90. [Google Scholar] [CrossRef]
- Fonseca, C.; Spencer-Martins, I.; Hahn-Hägerdal, B. L-arabinose metabolism in Candida arabinofermentans PYCC 5603T and Pichia guilliermondii PYCC 3012: Influence of sugar and oxygen on product formation. Appl. Microbiol. Biotechnol. 2007, 75, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Lee, S.-J.; Kim, S.-B.; Lee, S.J.; Lee, S.H.; Lee, D.-W. Structural insights into conserved L-arabinose metabolic enzymes reveal the substrate binding site of a thermophilic L-arabinose isomerase. FEBS. Lett. 2014, 588, 1064–1070. [Google Scholar] [CrossRef]
- Ye, S.; Kim, J.; Kim, S. Metabolic engineering for improved fermentation of L-arabinose. J. Microbiol. Biotechnol. 2019, 29, 339–346. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Karhumaa, K.; Jeppsson, M.; Gorwa-Grauslund, M. Metabolic engineering for pentose utilization in Saccharomyces cerevisiae. Biofuels 2007, 108, 147–177. [Google Scholar] [CrossRef]
- Wisselink, H.W.; Toirkens, M.J.; del Rosario Franco Berriel, M.; Winkler, A.A.; van Dijken, J.P.; Pronk, J.T.; van Maris, A.J.A. Engineering of Saccharomyces cerevisiae for efficient anaerobic alcoholic fermentation of l-arabinose. Appl. Environ. Microbiol. 2007, 73, 4881–4891. [Google Scholar] [CrossRef]
- Servinsky, M.D.; Germane, K.L.; Liu, S.; Kiel, J.T.; Clark, A.M.; Shankar, J.; Sund, C.J. Arabinose is metabolized via a phosphoketolase pathway in Clostridium acetobutylicum ATCC 824. J. Ind. Microbiol. Biotechnol. 2012, 39, 1859–1867. [Google Scholar] [CrossRef]
- Dien, B.; Kurtzman, C.; Saha, B.; Bothast, R. Screening for L-arabinose fermenting yeasts. Appl. Biochem. Biotechnol. 1996, 57-58, 233–242. [Google Scholar] [CrossRef]
- McMillan, J.D.; Boynton, B.L. Arabinose utilization by xylose-fermenting yeasts and fungi. Appl. Biochem. Biotechnol. 1994, 45-46, 569–584. [Google Scholar] [CrossRef]
- Lucas, C.; van Uden, N. Transport of hemicellulose monomers in the xylose-fermenting yeast Candida shehatae. Appl. Microbiol. Biotechnol. 1986, 23, 491–495. [Google Scholar] [CrossRef]
- Kordowska-Wiater, M. Production of arabitol by yeasts: Current status and future prospects. J. Appl. Microbiol. 2015, 119, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-K.; Nielsen, J. Metabolic engineering of Saccharomyces cerevisiae: A key cell factory platform for future biorefineries. Cell. Mol. Life Sci. 2012, 69, 2671–2690. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Tian, K.; Kumar, A.; Singh, S.; Prior, B.A.; Wang, Z. Metabolic engineering of Escherichia coli: A sustainable industrial platform for bio-based chemical production. Biotechnol. Adv. 2013, 31, 1200–1223. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Larsson, C.; Maris, A.V.; Pronk, J. Metabolic engineering of yeast for production of fuels and chemicals. Curr. Opin. Biotechnol. 2013, 24, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.L.; Jeon, Y.J.; Lee, K.J.; Lawford, H.G. Zymomonas mobilis for fuel ethanol and higher value products. Biofuels 2007, 108, 263–288. [Google Scholar] [CrossRef]
- Wallace-Salinas, V.; Gorwa-Grauslund, M. Adaptive evolution of an industrial strain of Saccharomyces cerevisiae for combined tolerance to inhibitors and temperature. Biotechnol. Biofuels 2013, 6, 151. [Google Scholar] [CrossRef]
- Çakar, Z.; Turanlı-Yıldız, B.; Alkım, C.; Yılmaz, Ü. Evolutionary engineering of Saccharomyces cerevisiae for improved industrially important properties. FEMS Yeast Res. 2011, 12, 171–182. [Google Scholar] [CrossRef]
- Demeke, M.M.; Dietz, H.; Li, Y.; Foulquié-Moreno, M.R.; Mutturi, S.; Deprez, S.; Abt, T.D.; Bonini, B.M.; Liden, G.; Dumortier, F.; et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol. Biofuels 2013, 6, 89. [Google Scholar] [CrossRef]
- Dhar, R.; Sägesser, R.; Weikert, C.; Yuan, J.; Wagner, A. Adaptation of Saccharomyces cerevisiae to saline stress through laboratory evolution. J. Evol. Biol. 2011, 24, 1135–1153. [Google Scholar] [CrossRef]
- Hua, Q.; Joyce, A.R.; Palsson, B.Ø.; Fong, S.S. Metabolic characterization of Escherichia coli strains adapted to growth on lactate. Appl. Environ. Microbiol. 2007, 73, 4639–4647. [Google Scholar] [CrossRef]
- Lee, D.-H.; Palsson, B.Ø. Adaptive evolution of Escherichia coli K-12 mg1655 during growth on a non-native carbon source, l-1,2-propanediol. Appl. Environ. Microbiol. 2010, 76, 4158–4168. [Google Scholar] [CrossRef]
- Agrawal, M.; Wang, Y.; Chen, R. Engineering efficient xylose metabolism into an acetic acid-tolerant Zymomonas mobilis strain by introducing adaptation-induced mutations. Biotechnol. Lett. 2012, 34, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Sootsuwan, K.; Thanonkeo, P.; Keeratirakha, N.; Thanonkeo, S.; Jaisil, P.; Yamada, M. Sorbitol required for cell growth and ethanol production by Zymomonas mobilis under heat, ethanol, and osmotic stresses. Biotechnol. Biofuels 2013, 6, 180. [Google Scholar] [CrossRef]
- Zhang, M.; Eddy, C.; Deanda, K.; Finkelstein, M.; Picataggio, S. Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis. Science 1995, 267, 240–243. [Google Scholar] [CrossRef]
- Deanda, K.; Zhang, M.; Eddy, C.; Picataggio, S. Development of an arabinose-fermenting Zymomonas mobilis strain by metabolic pathway engineering. Appl. Environ. Microbiol. 1996, 62, 4465–4470. [Google Scholar] [CrossRef]
- Zhang, M.; Chou, Y.-C.; Picataggio, S.K.; Finkelstein, M. Single Zymomonas mobilis Strain for Xylose and Arabinose Fermentation. US Patent 5843760, 1 December 1998. [Google Scholar]
- Mohagheghi, A.; Evans, K.; Chou, Y.; Zhang, M. Cofermentation of glucose, xylose, and arabinose by genomic DNA-integrated xylose/arabinose fermenting strain of Zymomonas mobilis AX101. Appl. Biochem. Biotechnol. 2002, 98–100, 885–898. [Google Scholar] [CrossRef] [PubMed]
- He, M.X.; Wu, B.; Qin, H.; Ruan, Z.Y.; Tan, F.R.; Wang, J.L.; Shui, Z.X.; Dai, L.C.; Zhu, Q.L.; Pan, K.; et al. Zymomonas mobilis: A novel platform for future biorefineries. Biotechnol. Biofuels 2014, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Isaacs, F.J.; Carr, P.A.; Sun, Z.Z.; Xu, G.; Forest, C.R.; Church, G.M. Programming cells by multiplex genome engineering and accelerated evolution. Nature 2009, 460, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A. Genome engineering using site-specific recombinases. Cloning Stem Cells 2002, 4, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Wirth, D.; Gama-Norton, L.; Riemer, P.; Sandhu, U.; Schucht, R.; Hauser, H. Road to precision: Recombinase-based targeting technologies for genome engineering. Curr. Opin. Biotechnol. 2007, 18, 411–419. [Google Scholar] [CrossRef]
- Zhang, Y.; Perry, K.; Vinci, V.; Powell, K.; Stemmer, W.; del Cardayré, S. Genome shuffling leads to rapid phenotypic improvement in bacteria. Nature 2002, 415, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D. Genome engineering with zinc-finger nucleases. Genetics 2011, 188, 773–782. [Google Scholar] [CrossRef]
- Becker, J.; Boles, E. A modified Saccharomyces cerevisiae strain that consumes L-arabinose and produces ethanol. Appl. Environ. Microbiol. 2003, 69, 4144–4150. [Google Scholar] [CrossRef]
- Sedlak, M.; Ho, N. Expression of E. coli araBAD operon encoding enzymes for metabolizing L-arabinose in Saccharomyces cerevisiae. Enzyme Microb. Technol. 2001, 28, 16–24. [Google Scholar] [CrossRef]
- Wiedemann, B.; Boles, E. Codon-optimized bacterial genes improve L-arabinose fermentation in recombinant Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2008, 74, 2043–2050. [Google Scholar] [CrossRef]
- Wang, C.; Shen, Y.; Zhang, Y.; Suo, F.; Hou, J.; Bao, X. Improvement of L-arabinose fermentation by modifying the metabolic pathway and transport in Saccharomyces cerevisiae. Biomed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Richard, P.; Verho, R.; Putkonen, M.; Londesborough, J.; Penttila, M. Production of ethanol from L-arabinose by containing a fungal L-arabinose pathway. FEMS Yeast Res. 2003, 3, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Sedlak, M.; Khan, A.; Ho, N. Establishment of L-arabinose fermentation in glucose/xylose co-fermenting recombinant Saccharomyces cerevisiae 424A(LNH-ST) by genetic engineering. Appl. Microbiol. Biotechnol. 2010, 87, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Qiu, C.; Wang, S.; Shen, Y.; Du, B.; Ding, Y.; Bao, X. Co-utilization of D-glucose, D-xylose, and L-arabinose in Saccharomyces cerevisiae by co expressing the metabolic pathways and evolutionary engineering. BioMed. Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Verho, R.; Putkonen, M.; Londesborough, J.; Penttilä, M.; Richard, P. A novel NADH-linked L-xylulose reductase in the L-arabinose catabolic pathway of yeast. J. Biol. Chem. 2004, 279, 14746–14751. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, Y.; Jin, Y.; Seo, J. Strain engineering of Saccharomyces cerevisiae for enhanced xylose metabolism. Biotechnol. Adv. 2013, 31, 851–861. [Google Scholar] [CrossRef]
- Jansen, M.L.A.; Bracher, J.M.; Papapetridis, I.; Verhoeven, M.D.; de Bruijn, H.; de Waal, P.P.; van Maris, A.J.A.; Klaassen, P.; Pronk, J.T. Saccharomyces cerevisiae strains for second-generation ethanol production: From academic exploration to industrial implementation. FEMS Yeast Res. 2017, 17, fox044. [Google Scholar] [CrossRef]
- Leandro, M.; Fonseca, C.; Gonasalves, P. Hexose and pentose transport in ascomycetous yeasts: An overview. FEMS Yeast Res. 2009, 9, 511–525. [Google Scholar] [CrossRef]
- Subtil, T.; Boles, E. Improving L-arabinose utilization of pentose fermenting Saccharomyces cerevisiae cells by heterologous expression of L-arabinose transporting sugar transporters. Biotechnol. Biofuels 2011, 4, 38. [Google Scholar] [CrossRef]
- Verhoeven, M.D.; Bracher, J.M.; Nijland, J.G.; Bouwknegt, J.; Daran, J.-M.G.; Driessen, A.J.M.; van Maris, A.J.A.; Pronk, J.T. Laboratory evolution of a glucose-phosphorylation-deficient, arabinose-fermenting S. cerevisiae strain reveals mutations in GAL2 that enable glucose-insensitive L-arabinose uptake. FEMS Yeast Res. 2018, 18, foy062. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Cai, P.; Wang, B.; Ma, J.Y.; Benz, P.; Tian, C. Functional analysis of two L-Arabinose transporters from filamentous fungi reveals promising characteristics for improved pentose utilization in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2015, 81, 4062–4070. [Google Scholar] [CrossRef]
- Fonseca, C.; Romão, R.; de Sousa, H.R.; Hahn-Hägerdal, B.; Spencer-Martins, I. L-Arabinose transport and catabolism in yeast. FEBS J. 2007, 274, 3589–3600. [Google Scholar] [CrossRef]
- Bettiga, M.; Bengtsson, O.; Hahn-Hägerdal, B.; Gorwa-Grauslund, M.F. Arabinose and xylose fermentation by recombinant Saccharomyces cerevisiae expressing a fungal pentose utilization pathway. Microb. Cell Factories 2009, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, A.; Tamalampudi, S.; Srivastava, A.; Fukuda, H.; Bisaria, V.S.; Kondo, A. Alcoholic fermentation of xylose and mixed sugars using recombinant Saccharomyces cerevisiae engineered for xylose utilization. Appl. Microbiol. Biotechnol. 2009, 82, 1037–1047. [Google Scholar] [CrossRef]
- Hamacher, T.; Becker, J.; Gárdonyi, M.; Hahn-Hägerdal, B.; Boles, E. Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiology 2002, 148, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Subtil, T.; Boles, E. Competition between pentoses and glucose during uptake and catabolism in recombinant Saccharomyces cerevisiae. Biotechnol. Biofuels 2012, 5, 14. [Google Scholar] [CrossRef]
- Ye, S.; Jeong, D.; Shon, J.C.; Liu, K.-H.; Kim, K.H.; Shin, M.; Kim, S.R. Deletion of PHO13 improves aerobic l-arabinose fermentation in engineered Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2019, 46, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Hsieh, P.; Mau, J.; Teng, D. Construction of an intergeneric fusion from Schizosaccharomyces pombe and Lentinula edodes for xylan degradation and polyol production. Enzyme Microb. Technol. 2005, 36, 107–117. [Google Scholar] [CrossRef]
- Kordowska-Wiater, M.; Kubik-Komar, A.; Targoński, Z. Optimization of arabitol production by karyoductant SP-K 7 of S. cerevisiae V30 and P. stipitis CCY 39501 using response surface methodology. Pol J Microbiol. 2012, 61, 291–297. [Google Scholar] [CrossRef]
- Akinterinwa, O.; Khankal, R.; Cirino, P. Metabolic engineering for bioproduction of sugar alcohols. Curr. Opin. Biotechnol. 2008, 19, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Dien, B.S.; Hespell, R.B.; Wyckoff, H.A.; Bothast, R.J. Fermentation of hexose and pentose sugars using a novel ethanologenic Escherichia coli strain. Enzyme Microb. Technol. 1998, 23, 366–371. [Google Scholar] [CrossRef]
- Bothast, R.; Saha, B.; Flosenzier, A.; Ingram, L. Fermentation of L-arabinose, D-xylose and D-glucose by ethanologenic recombinant Klebsiella oxytoca strain P2. Biotechnol. Lett. 1994, 16, 401–406. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, X.; Chen, S. Engineering of an L-arabinose metabolic pathway in Rhodococcus jostii RHA1 for biofuel production. J. Ind. Microbiol. Biotechnol. 2016, 43, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Sasaki, M.; Vertès, A.; Inui, M.; Yukawa, H. Identification and functional analysis of the gene cluster for L-arabinose utilization in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2009, 75, 3419–3429. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Sakakibara, Y.; Cotta, M. Production of D-arabitol by a newly isolated Zygosaccharomyces rouxii. J. Ind. Microbiol. Biotechnol. 2007, 34, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Bothast, R. Production of L -arabitol from L -arabinose by Candida entomaea and Pichia guilliermondii. Appl. Microbiol. Biotechnol. 1996, 45, 299–306. [Google Scholar] [CrossRef]
| ValueAdded Products | Yield | Microbe | ‘C’ Source | Reference |
|---|---|---|---|---|
| Organic acids | Lactic Acid: 12.1 g/L Fumaric Acid: 7.4 g/L Acetic Acid: 4.5 g/L | Lactobacillus sakei WiKim31 | Kimchi cabbage waste | [15] |
| Putrescine | 19 g/L | Clostridium glutamicum PUT21 | Glucose | [16] |
| Amino acids | L-Lysine: 9.9 g/L L-Ornithine: 25.8 g/L L-Arginine: 8.4 g/L | C. glutamicum ARG1 | Glucose and L-Ara | [6] |
| Feedstock | Lignin (%) | Hemicellulose (%) | Cellulose (%) | Pre-Treatment Method Used to Obtain L-Ara | Applications | References |
|---|---|---|---|---|---|---|
| Sugarcane bagasse | 25–32 | 19–24 | 32–43 | Acid hydrolysis resulted in 2.78 g/L of L-Ara | Food coatings, hydrogels, packaging films, cationic biopolymers, and other biomedical uses | [32,33,34] |
| Corn stover | 19 | 22 | 36 | Acid hydrolysis resulted in 38.2% L-Ara yield in 8 h reaction time | Advanced biofuels and livestock feed | [35,36,37] |
| Rice straw | 15 | 18 | 35 | Combined pre-treatment methods resulted in 2.7–4.5% of L-Arayield | Biofuel and ethanol production | [38,39,40] |
| Water hyacinth | 10 | 35 | 25 | Sulphuric acid treatment resulted in 33.3 g/L yield of L-Ara | Biothanol production using Pichia stipitis | [41,42] |
| Wheat straw | 16–25 | 23–24 | 28–39 | Hot water and NaOH treatment resulted in 2.37 ± 0.09% of L-Ara | Adsorbents, packing materials, bioplastic industry, and several other industries | [43,44,45] |
| Sugar beet molasses | 6 | 30 | 22–24 | Acid alkali pretreatment along with ultrafiltration resulted in 92% recovery of L-Ara | Food industry, as a bakery or confectionery product, apart from being utilized as a ruminant feed | [46,47] |
| Apple pomace | 19 | 10 | 12 | Sulphuric acid treatment resulted in 90% yield of L-Ara | Bioethanol, animal feed, citric acid, and several other applications | [48,49,50] |
| Orange peels | 20 | 9 | 69 | Acid alkali treatment to extract L-Ara | Bioethanol, essential oils, and biogas | [51,52] |
| Carrot pomace | 17 | 7 | 28 | Acid treatment to extract L-Ara | Fertilizer, feed for livestock, dietary fiber, and production of biofuels | [53,54] |
| Tomato pomace | 7 | 31 | 38 | Acid treatment to extract L-Ara | Fertilizer, feed for livestock, dietary fiber, and production of biofuels | [54,55,56] |
| Type of Organism | Name of Organism/Strain | Product Produced | Product Yield (g g−1) | References |
|---|---|---|---|---|
| Yeast | Debaryomyces nepalensis NCYC 3413 | L-Arabitol | 0.48 | [140] |
| P. guilliermondii | 0.54 | [80] | ||
| C. entomeae | 0.77 | [141] | ||
| Intergeneric fusant | S. pombe and L. edodes hybrid | L-Arabitol | 0.80 | [133] |
| Recombinant | S. cerevisiae AH22 | L-Arabitol | 0.62 | [114] |
| S. cerevisiae TMB 3664 | 0.48 | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, S.; Dilshani, A.; Rishivanthi, S.; Khaitan, P.; Vamsidhar, A.; Rajeswari, G.; Kumar, V.; Rajak, R.C.; Din, M.F.M.; Zambare, V. Lignocellulose-Derived Arabinose for Energy and Chemicals Synthesis through Microbial Cell Factories: A Review. Processes 2023, 11, 1516. https://doi.org/10.3390/pr11051516
Jacob S, Dilshani A, Rishivanthi S, Khaitan P, Vamsidhar A, Rajeswari G, Kumar V, Rajak RC, Din MFM, Zambare V. Lignocellulose-Derived Arabinose for Energy and Chemicals Synthesis through Microbial Cell Factories: A Review. Processes. 2023; 11(5):1516. https://doi.org/10.3390/pr11051516
Chicago/Turabian StyleJacob, Samuel, Aswin Dilshani, Srinivasan Rishivanthi, Pratham Khaitan, Adhinarayan Vamsidhar, Gunasekaran Rajeswari, Vinod Kumar, Rajiv Chandra Rajak, Mohd Fadhil Md. Din, and Vasudeo Zambare. 2023. "Lignocellulose-Derived Arabinose for Energy and Chemicals Synthesis through Microbial Cell Factories: A Review" Processes 11, no. 5: 1516. https://doi.org/10.3390/pr11051516
APA StyleJacob, S., Dilshani, A., Rishivanthi, S., Khaitan, P., Vamsidhar, A., Rajeswari, G., Kumar, V., Rajak, R. C., Din, M. F. M., & Zambare, V. (2023). Lignocellulose-Derived Arabinose for Energy and Chemicals Synthesis through Microbial Cell Factories: A Review. Processes, 11(5), 1516. https://doi.org/10.3390/pr11051516









