A Review on the Microwave-Assisted Pyrolysis of Waste Plastics
Abstract
1. Introduction
2. Raw Materials and MAP Technology
2.1. Normal Properties of Plastics
2.2. MAP Technology
3. Influencing Factors, Pyrolysis Residue, and Energy Consumption of MAP of Plastics
3.1. Plastic Types
3.2. Microwave Power
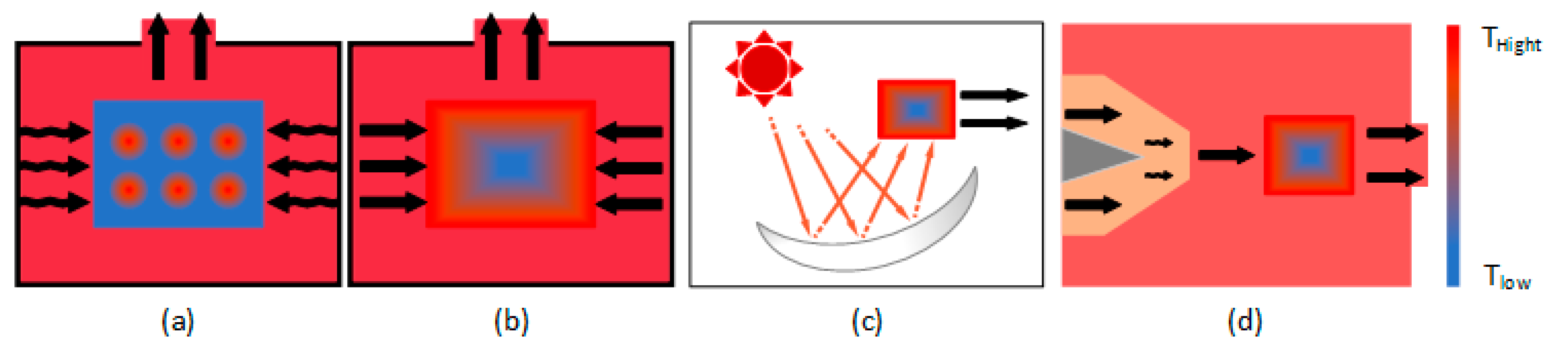
3.3. Microwave Absorbers
3.4. Biomass Co-Pyrolysis
3.5. Catalyst
3.5.1. Molecular Sieve Catalyst
3.5.2. Metal-Based Catalyst
3.6. Pyrolysis Temperature
3.7. The Device Used for MAP
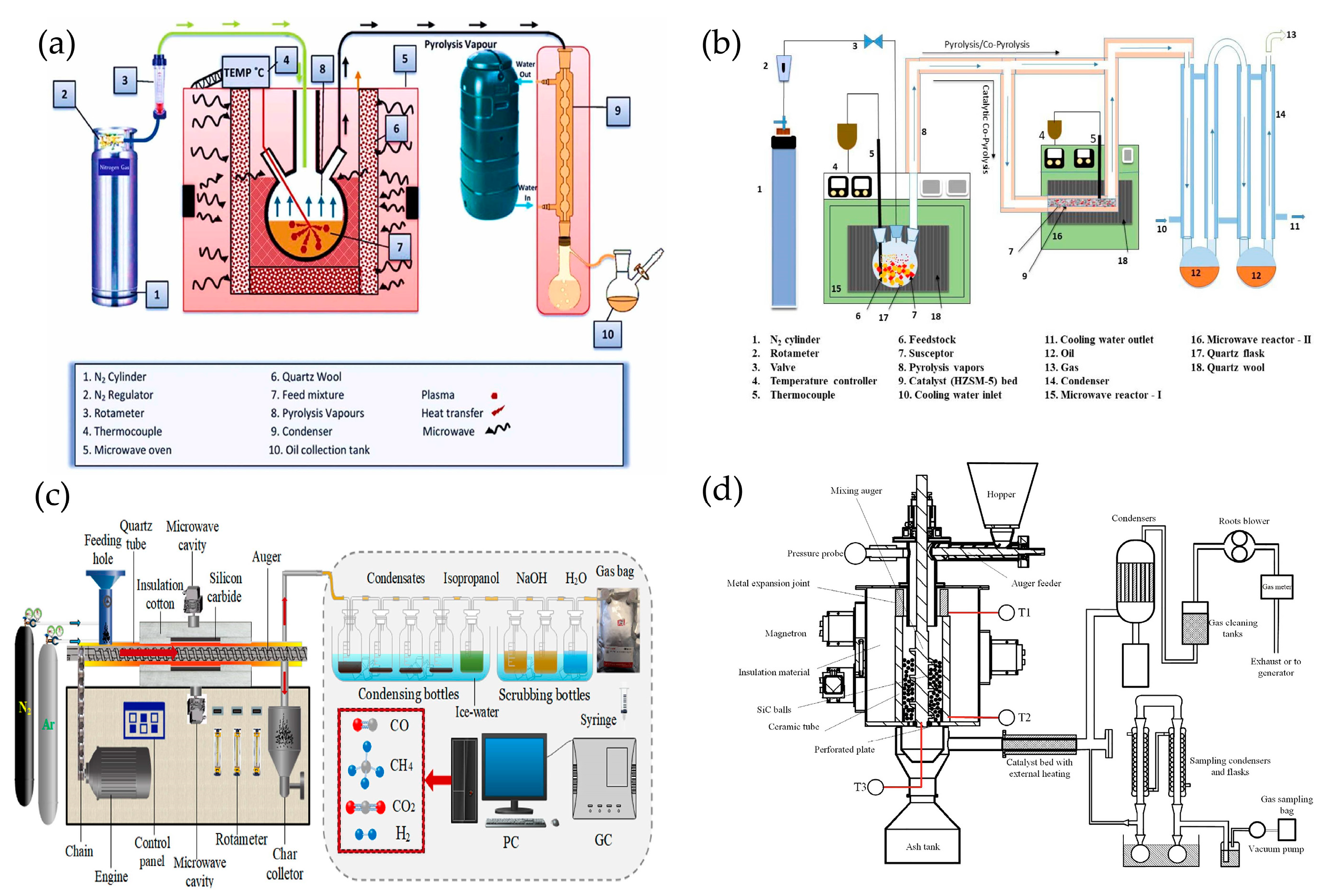
3.8. Residues from MAP of Plastics
3.9. Energy Consumption of MAP of Plastics
4. Modeling and Simulation Research
5. Future Development and Challenges
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, S.H. Plastic waste as pyrolysis feedstock for plastic oil production: A review. Sci. Total Environ. 2023, 877, 162719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Huserbraten, M.B.O.; Hattermann, T.; Broms, C.; Albretsen, J. Trans-polar drift-pathways of riverine European microplastic. Sci. Rep. 2022, 12, 3016. [Google Scholar] [CrossRef]
- Peng, G.; Bellerby, R.; Zhang, F.; Sun, X.; Li, D. The ocean’s ultimate trashcan: Hadal trenches as major depositories for plastic pollution. Water Res. 2020, 168, 115121. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Gao, L.; Xie, Y.; Su, Y.; Mehmood, T.; Bao, R.; Fan, H.; Peng, L. Elucidating the negatively influential and potentially toxic mechanism of single and combined micro-sized polyethylene and petroleum to Chlorella vulgaris at the cellular and molecular levels. Ecotoxicol. Environ. Saf. 2022, 245, 114102. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-X.; Wu, S.-L.; Chuang, K.-H.; Wey, M.-Y. Co-production of carbon nanotubes and hydrogen from waste plastic gasification in a two-stage fluidized catalytic bed. Renew. Energy 2020, 159, 10–22. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Kusenberg, M.; Zayoud, A.; Roosen, M.; Vermeire, F.; Madanikashani, S.; Kuzmanovic, M.; Parvizi, B.; Kresovic, U.; De Meester, S.; et al. Thermal pyrolysis of waste versus virgin polyolefin feedstocks: The role of pressure, temperature and waste composition. Waste Manag. 2023, 165, 108–118. [Google Scholar] [CrossRef]
- Hussain, I.; Ganiyu, S.A.; Alasiri, H.; Alhooshani, K. A state-of-the-art review on waste plastics-derived aviation fuel: Unveiling the heterogeneous catalytic systems and techno-economy feasibility of catalytic pyrolysis. Energy Convers. Manag. 2022, 274, 116433. [Google Scholar] [CrossRef]
- Soni, V.K.; Singh, G.; Vijayan, B.K.; Chopra, A.; Kapur, G.S.; Ramakumar, S.S.V. Thermochemical Recycling of Waste Plastics by Pyrolysis: A Review. Energy Fuels 2021, 35, 12763–12808. [Google Scholar] [CrossRef]
- Lam, S.S.; Wan Mahari, W.A.; Ma, N.L.; Azwar, E.; Kwon, E.E.; Peng, W.; Chong, C.T.; Liu, Z.; Park, Y.K. Microwave pyrolysis valorization of used baby diaper. Chemosphere 2019, 230, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Wan Mahari, W.A.; Azwar, E.; Foong, S.Y.; Ahmed, A.; Peng, W.; Tabatabaei, M.; Aghbashlo, M.; Park, Y.-K.; Sonne, C.; Lam, S.S. Valorization of municipal wastes using co-pyrolysis for green energy production, energy security, and environmental sustainability: A review. Chem. Eng. J. 2021, 421, 129749. [Google Scholar] [CrossRef]
- Conk, R.J.; Hanna, S.; Shi, J.X.; Yang, J.; Ciccia, N.R.; Qi, L.; Bloomer, B.J.; Heuvel, S.; Wills, T.; Su, J.; et al. Catalytic deconstruction of waste polyethylene with ethylene to form propylene. Science 2022, 377, 1561–1566. [Google Scholar] [CrossRef]
- Galaly, A.R. Sustainable Development Solutions for the Medical Waste Problem Using Thermal Plasmas. Sustainability 2022, 14, 11045. [Google Scholar] [CrossRef]
- Salema, A.A.; Ani, F.N.; Mouris, J.; Hutcheon, R. Microwave dielectric properties of Malaysian palm oil and agricultural industrial biomass and biochar during pyrolysis process. Fuel Process. Technol. 2017, 166, 164–173. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Basak, T. A review on the susceptor assisted microwave processing of materials. Energy 2016, 97, 306–338. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, B.; Jing, H.; Wei, Y.; Wang, J.; Qu, Z.; Duan, J. A transparent ultra-broadband microwave absorber based on flexible multilayer structure. Opt. Mater. 2022, 128, 112173. [Google Scholar] [CrossRef]
- Salema, A.A.; Yeow, Y.K.; Ishaque, K.; Ani, F.N.; Afzal, M.T.; Hassan, A. Dielectric properties and microwave heating of oil palm biomass and biochar. Ind. Crops Prod. 2013, 50, 366–374. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Peng, P.; Min, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Fan, L.; Liu, C.; et al. Effects of feedstock characteristics on microwave-assisted pyrolysis—A review. Bioresour. Technol. 2017, 230, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bagci, F.; Gulsu, M.S.; Akaoglu, B. Dual-band measurement of complex permittivity in a microwave waveguide with a flexible, thin and sensitive metamaterial-based sensor. Sens. Actuator A Phys. 2022, 338, 113480. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Ureel, Y.; Eschenbacher, A.; Vermeire, F.H.; Varghese, R.J.; Oenema, J.; Stefanidis, G.D.; Van Geem, K.M. Challenges and opportunities of light olefin production via thermal and catalytic pyrolysis of end-of-life polyolefins: Towards full recyclability. Prog. Energy Combust. Sci. 2023, 96, 101046. [Google Scholar] [CrossRef]
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Efficient disposal of waste polyolefins through microwave assisted pyrolysis. Fuel 2014, 116, 662–671. [Google Scholar] [CrossRef]
- Terapalli, A.; Kamireddi, D.; Sridevi, V.; Tukarambai, M.; Suriapparao, D.V.; Rao, C.S.; Gautam, R.; Modi, P.R. Microwave-assisted in-situ catalytic pyrolysis of polystyrene: Analysis of product formation and energy consumption using machine learning approach. Process Saf. Environ. Prot. 2022, 166, 57–67. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Ke, L.; Dai, L.; Wu, Q.; Cobb, K.; Zeng, Y.; Zou, R.; Liu, Y.; Ruan, R. A review on catalytic pyrolysis of plastic wastes to high-value products. Energy Convers. Manag. 2022, 254, 115243. [Google Scholar] [CrossRef]
- Rex, P.; Masilamani, I.P.; Miranda, L.R. Microwave pyrolysis of polystyrene and polypropylene mixtures using different activated carbon from biomass. J. Energy Inst. 2020, 93, 1819–1832. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Nagababu, G.; Yerrayya, A.; Sridevi, V. Optimization of microwave power and graphite susceptor quantity for waste polypropylene microwave pyrolysis. Process Saf. Environ. Prot. 2021, 149, 234–243. [Google Scholar] [CrossRef]
- Bing, W.; Hongbin, Z.; Zeng, D.; Yuefeng, F.; Yu, Q.; Rui, X. Microwave fast pyrolysis of waste tires: Effect of microwave power on product composition and quality. J. Anal. Appl. Pyrolysis 2021, 155, 104979. [Google Scholar] [CrossRef]
- Jing, X.; Wen, H.; Gong, X.; Xu, Z. Heating strategies for the system of PP and Spherical Activated Carbon during microwave cracking for obtaining value-added products. Fuel Process. Technol. 2020, 199, 106265. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.; Li, T.; Xue, Z.; Wang, X.; Ruan, R. Biofuel production from distillers dried grains with solubles (DDGS) co-fed with waste agricultural plastic mulching films via microwave-assisted catalytic fast pyrolysis using microwave absorbent and hierarchical ZSM-5/MCM-41 catalyst. J. Anal. Appl. Pyrolysis 2018, 130, 1–7. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Basak, T. Susceptor-Assisted Enhanced Microwave Processing of Ceramics—A Review. Crit. Rev. Solid State Mater. 2016, 42, 433–469. [Google Scholar] [CrossRef]
- Amini, A.; Latifi, M.; Chaouki, J. Electrification of materials processing via microwave irradiation: A review of mechanism and applications. Appl. Therm. Eng. 2021, 193, 117003. [Google Scholar] [CrossRef]
- Rajasekhar Reddy, B.; Malhotra, A.; Najmi, S.; Baker-Fales, M.; Coasey, K.; Mackay, M.; Vlachos, D.G. Microwave assisted heating of plastic waste: Effect of plastic/susceptor (SiC) contacting patterns. Chem. Eng. Process. 2022, 182, 109202. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Liu, S.; Fan, L.; Zhou, N.; Peng, P.; Wang, Y.; Guo, F.; Min, M.; Cheng, Y.; et al. Fast microwave-assisted pyrolysis of wastes for biofuels production—A review. Bioresour. Technol. 2020, 297, 122480. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Wang, C.; Qian, M.; Huo, E.; Kong, X.; Wang, Y.; Dai, L.; Wang, L.; Zhang, X.; Mateo, W.C.; et al. Catalytic co-pyrolysis of solid wastes (low-density polyethylene and lignocellulosic biomass) over microwave assisted biochar for bio-oil upgrading and hydrogen production. J. Clean. Prod. 2022, 374, 133971. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R. Resource recovery from synthetic polymers via microwave pyrolysis using different susceptors. J. Anal. Appl. Pyrolysis 2015, 113, 701–712. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, W.; Liu, T.; Zhang, Y.; Li, B. Microwave pyrolysis of polyethylene terephthalate (PET) plastic bottle sheets for energy recovery. J. Anal. Appl. Pyrolysis 2022, 161, 105414. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Frediani, M.; Undri, A.; Frediani, P. Depolymerization of polystyrene at reduced pressure through a microwave assisted pyrolysis. J. Anal. Appl. Pyrolysis 2015, 113, 281–287. [Google Scholar] [CrossRef]
- Zhao, Z.; Abdo, S.M.A.; Wang, X.; Li, H.; Li, X.; Gao, X. Process intensification on co-pyrolysis of polyethylene terephthalate wastes and biomass via microwave energy: Synergetic effect and roles of microwave susceptor. J. Anal. Appl. Pyrolysis 2021, 158, 105239. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, K.M.; Hussain, K. Microwave–metal interaction pyrolysis of polystyrene. J. Anal. Appl. Pyrolysis 2010, 89, 39–43. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, K.; Mao, C.; Huang, J.; Xu, Q.; Liao, L.; Wang, R.; Chen, S.; Li, P.; Zhang, C. Microwave assisted catalytic pyrolysis of bagasse to produce hydrogen. Int. J. Hydrogen 2022, 47, 35626–35634. [Google Scholar] [CrossRef]
- Li, M.; Yu, Z.; Bin, Y.; Huang, Z.; He, H.; Liao, Y.; Zheng, A.; Ma, X. Microwave-assisted pyrolysis of eucalyptus wood with MoO3 and different nitrogen sources for coproducing nitrogen-rich bio-oil and char. J. Anal. Appl. Pyrolysis 2022, 167, 105666. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Z.; Lu, X.; Ma, X. Catalytic co-pyrolysis of microwave pretreated chili straw and polypropylene to produce hydrocarbons-rich bio-oil. Bioresour. Technol. 2021, 319, 124191. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xu, X.; Yu, Z.; Chen, L.; Liao, Y.; Ma, X. Effects of microwave pretreatment on catalytic fast pyrolysis of pine sawdust. Bioresour. Technol. 2019, 293, 122080. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wei, D.; Zhao, J.; Huang, X.; Fan, D.; Qi, Q.; Bi, Y.; Liao, L. Study on co-pyrolysis and products of Chlorella vulgaris and rice straw catalyzed by activated carbon/HZSM-5 additives. Bioresour. Technol. 2022, 360, 127594. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Luo, J.; Lin, J.; Ma, R.; Sun, S.; Fang, L.; Li, H. Study of co-pyrolysis endpoint and product conversion of plastic and biomass using microwave thermogravimetric technology. Energy 2022, 247, 123547. [Google Scholar] [CrossRef]
- Zhang, X.; Ke, L.; Wu, Q.; Zhang, Q.; Cui, X.; Zou, R.; Tian, X.; Zeng, Y.; Liu, Y.; Ruan, R.; et al. Microwave catalytic co-pyrolysis of low-density polyethylene and spent bleaching clay for monocyclic aromatic hydrocarbons. J. Anal. Appl. Pyrolysis 2022, 168, 105709. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Sheikh, H.M.A.; El-Naggar, A.H.; Wang, Q. Microwave vacuum co-pyrolysis of waste plastic and seaweeds for enhanced crude bio-oil recovery: Experimental and feasibility study towards industrialization. Renew. Sustain. Energy Rev. 2021, 149, 111335. [Google Scholar] [CrossRef]
- Sun, J.; Luo, J.; Ma, R.; Lin, J.; Fang, L. Effects of microwave and plastic content on the sulfur migration during co-pyrolysis of biomass and plastic. Chemosphere 2022, 314, 137680. [Google Scholar] [CrossRef]
- Sridevi, V.; Suriapparao, D.V.; Tukarambai, M.; Terapalli, A.; Ramesh, P.; Sankar Rao, C.; Gautam, R.; Moorthy, J.V.; Suresh Kumar, C. Understanding of synergy in non-isothermal microwave-assisted in-situ catalytic co-pyrolysis of rice husk and polystyrene waste mixtures. Bioresour. Technol. 2022, 360, 127589. [Google Scholar] [CrossRef]
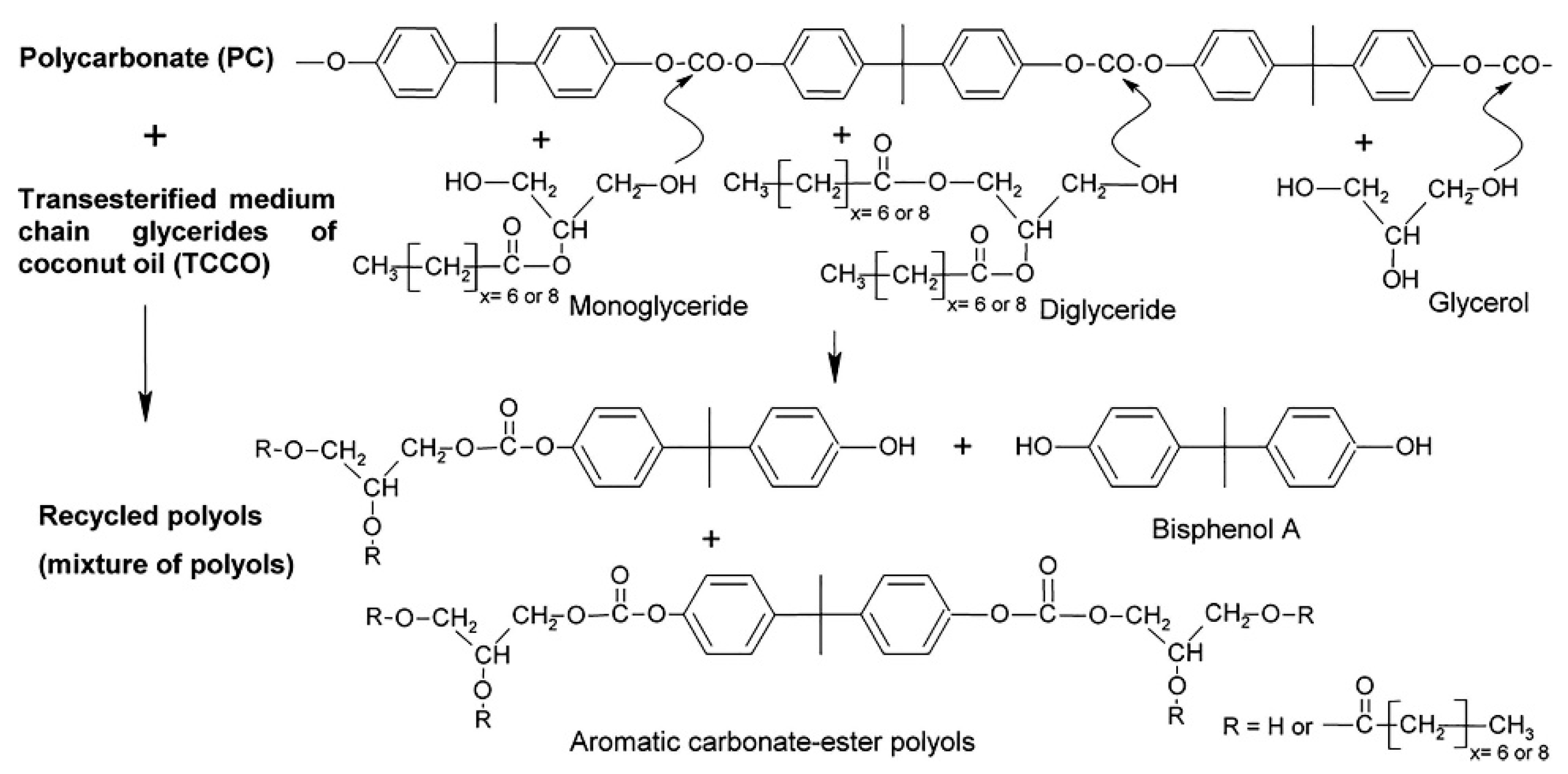
- Beneš, H.; Paruzel, A.; Trhlíková, O.; Paruzel, B. Medium chain glycerides of coconut oil for microwave-enhanced conversion of polycarbonate into polyols. Eur. Polym. J. 2017, 86, 173–187. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Hemanth Kumar, T.; Reddy, B.R.; Yerrayya, A.; Srinivas, B.A.; Sivakumar, P.; Prakash, S.R.; Sankar Rao, C.; Sridevi, V.; Desinghu, J. Role of ZSM5 catalyst and char susceptor on the synthesis of chemicals and hydrocarbons from microwave-assisted in-situ catalytic co-pyrolysis of algae and plastic wastes. Renew. Energy 2022, 181, 990–999. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Awang, S.; Zahariman, N.A.Z.; Peng, W.; Man, M.; Park, Y.K.; Lee, J.; Sonne, C.; Lam, S.S. Microwave co-pyrolysis for simultaneous disposal of environmentally hazardous hospital plastic waste, lignocellulosic, and triglyceride biowaste. J. Hazard. Mater. 2022, 423, 127096. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Duan, D.; Ruan, R.; Fan, L.; Zhou, Y.; Dai, L.; Lv, J.; Liu, Y. Fast microwave-assisted ex-catalytic co-pyrolysis of bamboo and polypropylene for bio-oil production. Bioresour. Technol. 2018, 249, 69–75. [Google Scholar] [CrossRef]
- Vaštyl, M.; Jankovská, Z.; Cruz, G.J.F.; Matějová, L. A case study on microwave pyrolysis of waste tyres and cocoa pod husk; effect on quantity and quality of utilizable products. J. Environ. Chem. Eng. 2022, 10, 106917. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bhoi, P.R.; Saha, A.; Patil, V.; Adhikari, S. Thermo-catalytic co-pyrolysis of biomass and high-density polyethylene for improving the yield and quality of pyrolysis liquid. Energy 2021, 225, 120231. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Van Geem, K.M.; Fathi, M.; Bazgir, H.; Ghadiri, M. The pyrolysis of oak with polyethylene, polypropylene and polystyrene using fixed bed and stirred reactors and TGA instrument. Energy 2021, 232, 121085. [Google Scholar] [CrossRef]
- Ahmed, N.; Zeeshan, M.; Iqbal, N.; Farooq, M.Z.; Shah, S.A. Investigation on bio-oil yield and quality with scrap tire addition in sugarcane bagasse pyrolysis. J. Clean. Prod. 2018, 196, 927–934. [Google Scholar] [CrossRef]
- Sanahuja-Parejo, O.; Veses, A.; Navarro, M.V.; Lopez, J.M.; Murillo, R.; Callen, M.S.; Garcia, T. Drop-in biofuels from the co-pyrolysis of grape seeds and polystyrene. Chem. Eng. J. 2019, 377, 120246. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.M.A.W.; Ramalingam, S.; Azemi, M.N.B.; Sahu, J.N. Co-pyrolysis of palm shell and polystyrene waste mixtures to synthesis liquid fuel. Fuel 2013, 108, 311–318. [Google Scholar] [CrossRef]
- Vo, T.A.; Tran, Q.K.; Ly, H.V.; Kwon, B.; Hwang, H.T.; Kim, J.; Kim, S.S. Co-pyrolysis of lignocellulosic biomass and plastics: A comprehensive study on pyrolysis kinetics and characteristics. J. Anal. Appl. Pyrolysis 2022, 163, 105464. [Google Scholar] [CrossRef]
- Li, H.Y.; Jiang, X.; Cui, H.R.; Wang, F.Y.; Zhang, X.L.; Yang, L.; Wang, C.P. Investigation on the co-pyrolysis of waste rubber/plastics blended with a stalk additive. J. Anal. Appl. Pyrolysis 2015, 115, 37–42. [Google Scholar] [CrossRef]
- Abnisa, F.; Daud, W.M.A.W.; Sahu, J.N. Pyrolysis of mixtures of palm shell and polystyrene: An optional method to produce a high-grade of pyrolysis oil. Environ. Prog. Sustain. 2014, 33, 1026–1033. [Google Scholar] [CrossRef]
- Dai, M.Q.; Xu, H.; Yu, Z.S.; Fang, S.W.; Chen, L.; Gu, W.L.; Ma, X.Q. Microwave-assisted fast co-pyrolysis behaviors and products between microalgae and polyvinyl chloride. Appl. Therm. Eng. 2018, 136, 9–15. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Boruah, B.; Raja, D.; Vinu, R. Microwave assisted co-pyrolysis of biomasses with polypropylene and polystyrene for high quality bio-oil production. Fuel Process. Technol. 2018, 175, 64–75. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Gautam, R.; Rao Jeeru, L. Analysis of pyrolysis index and reaction mechanism in microwave-assisted ex-situ catalytic co-pyrolysis of agro-residual and plastic wastes. Bioresour. Technol. 2022, 357, 127357. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Sridevi, V.; Ramesh, P.; Sankar Rao, C.; Tukarambai, M.; Kamireddi, D.; Gautam, R.; Dharaskar, S.A.; Pritam, K. Synthesis of sustainable chemicals from waste tea powder and Polystyrene via Microwave-Assisted in-situ catalytic Co-Pyrolysis: Analysis of pyrolysis using experimental and modeling approaches. Bioresour. Technol. 2022, 362, 127813. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Chong, C.T.; Cheng, C.K.; Lee, C.L.; Hendrata, K.; Yuh Yek, P.N.; Ma, N.L.; Lam, S.S. Production of value-added liquid fuel via microwave co-pyrolysis of used frying oil and plastic waste. Energy 2018, 162, 309–317. [Google Scholar] [CrossRef]
- Neha, S.; Remya, N. Co-production of biooil and biochar from microwave co-pyrolysis of food-waste and plastic using recycled biochar as microwave susceptor. Sustain. Energy Technol. Assess. 2022, 54, 102892. [Google Scholar] [CrossRef]
- Huo, E.; Lei, H.; Liu, C.; Zhang, Y.; Xin, L.; Zhao, Y.; Qian, M.; Zhang, Q.; Lin, X.; Wang, C.; et al. Jet fuel and hydrogen produced from waste plastics catalytic pyrolysis with activated carbon and MgO. Sci. Total Environ. 2020, 727, 138411. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, K.M.; Hussain, K.; Perveen, S. Microwave-metal Interaction Pyrolysis of Waste Polystyrene in a Copper Coil Reactor. Energy Sources Part A 2014, 36, 1982–1989. [Google Scholar] [CrossRef]
- Jie, X.; Li, W.; Slocombe, D.; Gao, Y.; Banerjee, I.; Gonzalez-Cortes, S.; Yao, B.; AlMegren, H.; Alshihri, S.; Dilworth, J.; et al. Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons. Nat. Catal. 2020, 3, 902–912. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.; Liang, C.; Luo, D.; Li, B.; Ma, J. In-situ exsolution of Fe-Ni alloy catalysts for H2 and carbon nanotube production from microwave plasma-initiated decomposition of plastic wastes. J. Hazard. Mater. 2023, 445, 130609. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, C.; Wu, M.; Chen, X.; Liu, D.; Ma, J. High-efficient microwave plasma discharging initiated conversion of waste plastics into hydrogen and carbon nanotubes. Energy Convers. Manag. 2022, 268, 116017. [Google Scholar] [CrossRef]
- Marczewski, M.; Kamińska, E.; Marczewska, H.; Godek, M.; Rokicki, G.; Sokołowski, J. Catalytic decomposition of polystyrene. The role of acid and basic active centers. Appl. Catal. B 2013, 129, 236–246. [Google Scholar] [CrossRef]
- Zhang, Q.; Shang, H.; Zhang, W.; Al-harahsheh, M. The influence of microwave electric field on the sulfur vacancy formation over MoS2 clusters and the corresponding properties: A DFT study. Chem. Eng. Sci. 2021, 234, 116441. [Google Scholar] [CrossRef]
- Zhang, Q.; Shang, H.; Xue, Z.; Duan, A. The effect of microwave electric field on sulfur vacancies formation over the edge sites of Co/Ni-promoted and unpromoted MoS2 catalysts through DFT investigations. Fuel 2022, 318, 123553. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Liu, Y.; Dai, L.; Wu, Q.; Xia, M.; Zhang, S.; Ke, L.; Zou, R.; Ruan, R. Microwave catalytic co-pyrolysis of waste cooking oil and low-density polyethylene to produce monocyclic aromatic hydrocarbons: Effect of different catalysts and pyrolysis parameters. Sci. Total Environ. 2022, 809, 152182. [Google Scholar] [CrossRef]
- Ding, K.; Liu, S.; Huang, Y.; Liu, S.; Zhou, N.; Peng, P.; Wang, Y.; Chen, P.; Ruan, R. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production. Energy Convers. Manag. 2019, 196, 1316–1325. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Yadavalli, G.; Zhu, L.; Wei, Y.; Liu, Y. Gasoline-range hydrocarbons produced from microwave-induced pyrolysis of low-density polyethylene over ZSM-5. Fuel 2015, 144, 33–42. [Google Scholar] [CrossRef]
- Bu, Q.; Cao, M.; Wang, M.; Zhang, X.; Mao, H. The effect of torrefaction and ZSM-5 catalyst for hydrocarbon rich bio-oil production from co-pyrolysis of cellulose and low density polyethylene via microwave-assisted heating. Sci. Total Environ. 2021, 754, 142174. [Google Scholar] [CrossRef]
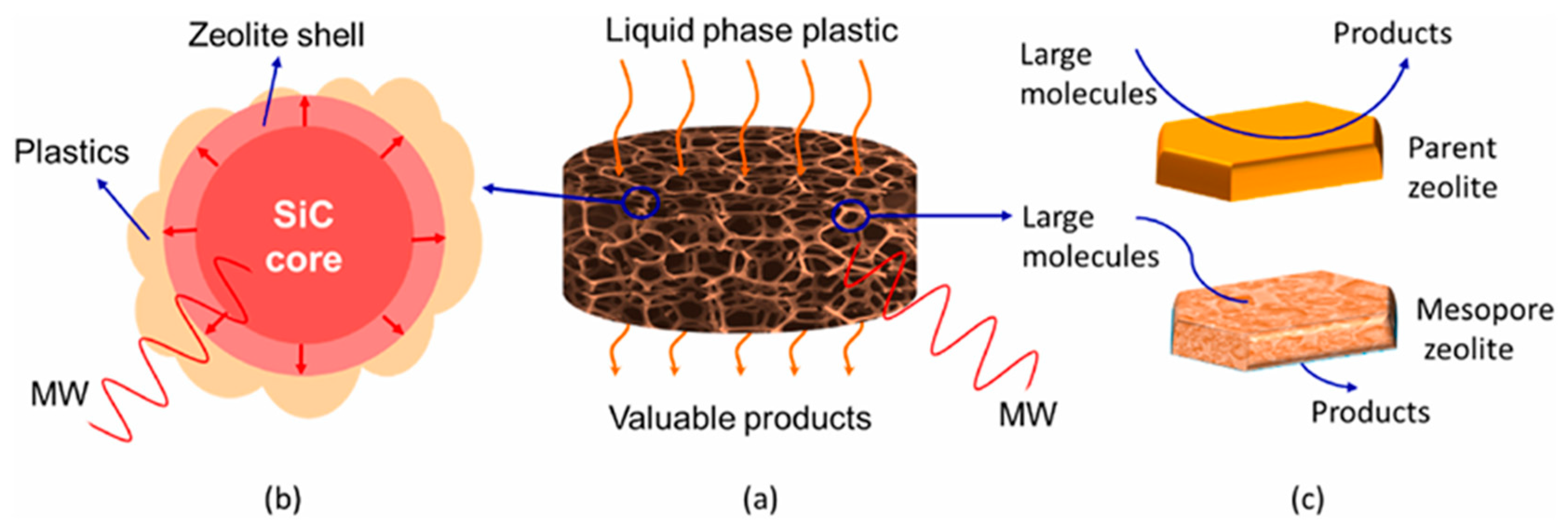
- Chen, Z.; Monzavi, M.; Latifi, M.; Samih, S.; Chaouki, J. Microwave-responsive SiC foam@zeolite core-shell structured catalyst for catalytic pyrolysis of plastics. Environ. Pollut. 2022, 307, 119573. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, Z.; Li, H.; Gao, X.; Fan, X. Microwave-assisted pyrolysis of plastics with iron-based catalysts for hydrogen and carbon nanotubes production. Mater. Today Chem. 2022, 26, 101166. [Google Scholar] [CrossRef]
- Yao, L.; Yi, B.; Zhao, X.; Wang, W.; Mao, Y.; Sun, J.; Song, Z. Microwave-assisted decomposition of waste plastic over Fe/FeAl2O4 to produce hydrogen and carbon nanotubes. J. Anal. Appl. Pyrolysis 2022, 165, 105577. [Google Scholar] [CrossRef]
- Karaismailoğlu, M.; Figen, H.E.; Baykara, S.Z. Methane decomposition over Fe-based catalysts. Int. J. Hydrogen 2020, 45, 34773–34782. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710. [Google Scholar] [CrossRef]
- Genuino, D.A.D.; de Luna, M.D.G.; Capareda, S.C. Improving the surface properties of municipal solid waste-derived pyrolysis biochar by chemical and thermal activation: Optimization of process parameters and environmental application. Waste Manag. 2018, 72, 255–264. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Liu, S.; Zhou, N.; Chen, P.; Liu, Y.; Wang, Y.; Peng, P.; Cheng, Y.; Addy, M.; et al. Ex-situ catalytic upgrading of vapors from microwave-assisted pyrolysis of low-density polyethylene with MgO. Energy Convers. Manag. 2017, 149, 432–441. [Google Scholar] [CrossRef]
- Ramzan, F.; Shoukat, B.; Naz, M.Y.; Shukrullah, S.; Ahmad, F.; Naz, I.; Makhlouf, M.M.; Farooq, M.U.; Kamran, K. Single step microwaves assisted catalytic conversion of plastic waste into valuable fuel and carbon nanotubes. Thermochim. Acta 2022, 715, 179294. [Google Scholar] [CrossRef]
- Yuwen, C.; Liu, B.; Rong, Q.; Hou, K.; Zhang, L.; Guo, S. Enhanced microwave absorption capacity of iron-based catalysts by introducing Co and Ni for microwave pyrolysis of waste COVID-19 Masks. Fuel 2023, 340, 127551. [Google Scholar] [CrossRef]
- Yuwen, C.; Liu, B.; Rong, Q.; Hou, K.; Zhang, L.; Guo, S. Mechanism of microwave-assisted iron-based catalyst pyrolysis of discarded COVID-19 masks. Waste Manag. 2023, 155, 77–86. [Google Scholar] [CrossRef] [PubMed]
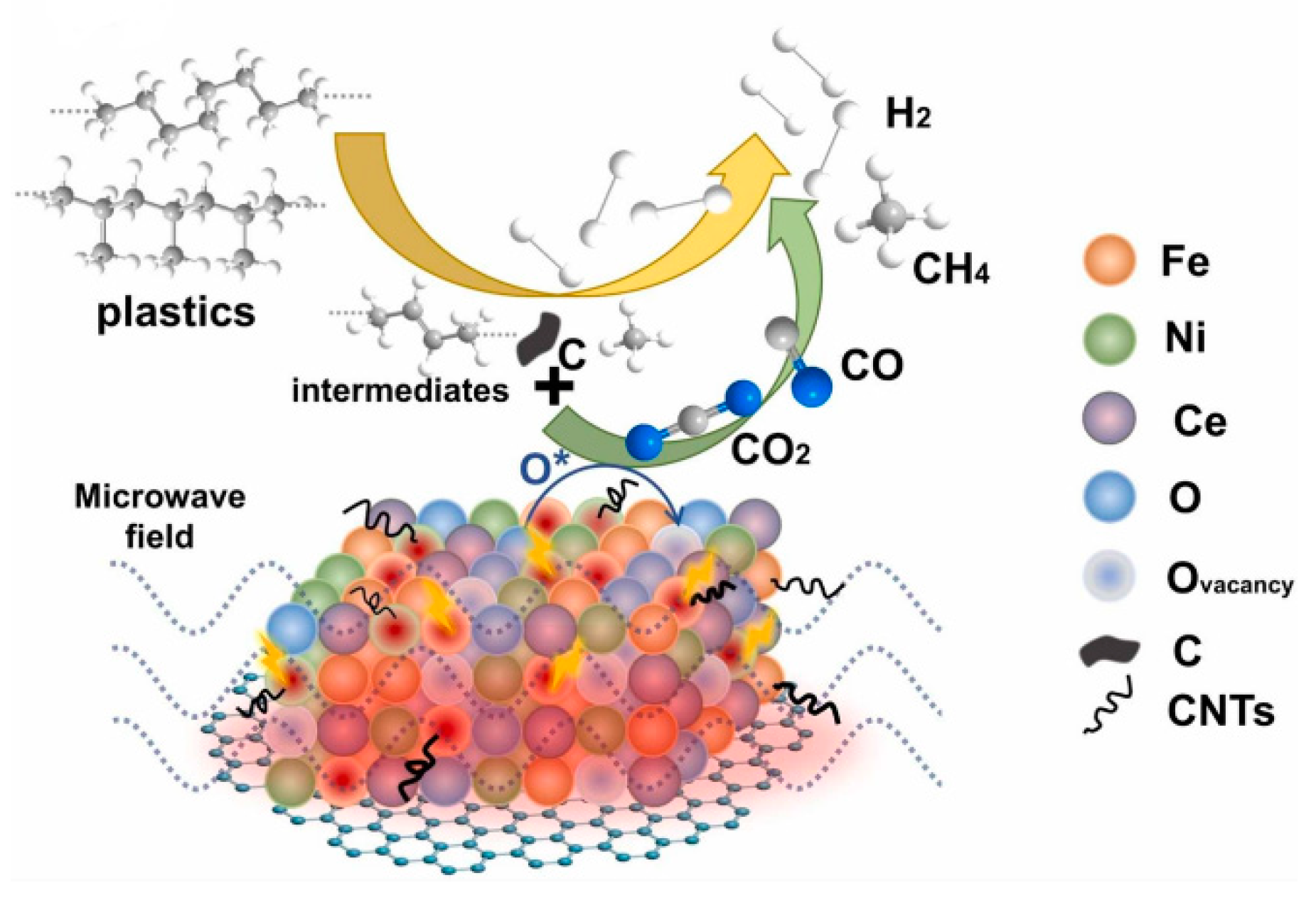
- Wang, J.; Pan, Y.; Song, J.; Huang, Q. A high-quality hydrogen production strategy from waste plastics through microwave-assisted reactions with heterogeneous bimetallic iron/nickel/cerium catalysts. J. Anal. Appl. Pyrolysis 2022, 166, 105612. [Google Scholar] [CrossRef]
- Cao, Q.; Dai, H.-C.; He, J.-H.; Wang, C.-L.; Zhou, C.; Cheng, X.-F.; Lu, J.-M. Microwave-initiated MAX Ti3AlC2-catalyzed upcycling of polyolefin plastic wastes: Selective conversion to hydrogen and carbon nanofibers for sodium-ion battery. Appl. Catal. B 2022, 318, 121828. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Y.; Liu, T.; Fu, W.; Li, B. Microwave-assisted pyrolysis of polystyrene for aviation oil production. J. Anal. Appl. Pyrolysis 2022, 162, 105425. [Google Scholar] [CrossRef]
- Fu, Z.; Hua, F.; Yang, S.Q.; Wang, H.Z.; Cheng, Y. Evolution of light olefins during the pyrolysis of polyethylene in a two-stage process. J. Anal. Appl. Pyrolysis 2023, 169, 105877. [Google Scholar] [CrossRef]
- Bai, M.Q.; Liu, Y.; Liu, L.; Yin, J.; Zhang, Y.G.; Zhao, D.F.; Roy, N.T. Kinetics of polyethylene pyrolysis in the atmosphere of ethylene. J. Therm. Anal. Calorim. 2021, 144, 383–391. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Lam, S.S.; Chase, H.A. A Review on Waste to Energy Processes Using Microwave Pyrolysis. Energies 2012, 5, 4209–4232. [Google Scholar] [CrossRef]
- Zhou, N.; Dai, L.; Lv, Y.; Li, H.; Deng, W.; Guo, F.; Chen, P.; Lei, H.; Ruan, R. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem. Eng. J. 2021, 418, 129412. [Google Scholar] [CrossRef]
- Fan, L.; Liu, L.; Xiao, Z.; Su, Z.; Huang, P.; Peng, H.; Lv, S.; Jiang, H.; Ruan, R.; Chen, P.; et al. Comparative study of continuous-stirred and batch microwave pyrolysis of linear low-density polyethylene in the presence/absence of HZSM-5. Energy 2021, 228, 120612. [Google Scholar] [CrossRef]
- Jing, X.; Dong, J.; Huang, H.; Deng, Y.; Wen, H.; Xu, Z.; Ceylan, S. Interaction between feedstocks, absorbers and catalysts in the microwave pyrolysis process of waste plastics. J. Clean. Prod. 2021, 291, 125857. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.; Liu, Y.; Zhang, T.; Li, X.; Wang, L.; Dai, J.; Qu, J.; Zhang, C.; Yu, M.; et al. Product characteristics and potential energy recovery for microwave assisted pyrolysis of waste printed circuit boards in a continuously operated auger pyrolyser. Energy 2022, 239, 122383. [Google Scholar] [CrossRef]
- Pan, Y.; Du, X.; Zhu, C.; Wang, J.; Xu, J.; Zhou, Y.; Huang, Q. Degradation of rubber waste into hydrogen enriched syngas via microwave-induced catalytic pyrolysis. Int. J. Hydrogen 2022, 47, 33966–33978. [Google Scholar] [CrossRef]
- Prathiba, R.; Shruthi, M.; Miranda, L.R. Pyrolysis of polystyrene waste in the presence of activated carbon in conventional and microwave heating using modified thermocouple. Waste Manag. 2018, 76, 528–536. [Google Scholar] [CrossRef]
- Talib Hamzah, H.; Sridevi, V.; Seereddi, M.; Suriapparao, D.V.; Ramesh, P.; Sankar Rao, C.; Gautam, R.; Kaka, F.; Pritam, K. The role of solvent soaking and pretreatment temperature in microwave-assisted pyrolysis of waste tea powder: Analysis of products, synergy, pyrolysis index, and reaction mechanism. Bioresour. Technol. 2022, 363, 127913. [Google Scholar] [CrossRef]
- Kappe, C.O. How to measure reaction temperature in microwave-heated transformations. Chem. Soc. Rev. 2013, 42, 4977–4990. [Google Scholar] [CrossRef]
- Durka, T.; Stefanidis, G.D.; Gerven, T.V.; Stankiewicz, A. On the accuracy and reproducibility of fiber optic (FO) and infrared (IR) temperature measurements of solid materials in microwave applications. Meas. Sci. Technol. 2010, 21, 045108. [Google Scholar] [CrossRef]
- Bartoli, M.; Frediani, M.; Briens, C.; Berruti, F.; Rosi, L. An Overview of Temperature Issues in Microwave-Assisted Pyrolysis. Processes 2019, 7, 658. [Google Scholar] [CrossRef]
- Lin, J.; Sun, S.; Luo, J.; Cui, C.; Ma, R.; Fang, L.; Liu, X. Effects of oxygen vacancy defect on microwave pyrolysis of biomass to produce high-quality syngas and bio-oil: Microwave absorption and in-situ catalytic. Waste Manag. 2021, 128, 200–210. [Google Scholar] [CrossRef]
- Hayden, S.; Damm, M.; Kappe, C.O. On the Importance of Accurate Internal Temperature Measurements in the Microwave Dielectric Heating of Viscous Systems and Polymer Synthesis. Macromol. Chem. Phys. 2013, 214, 423–434. [Google Scholar] [CrossRef]
- Potnuri, R.; Suriapparao, D.V.; Sankar Rao, C.; Sridevi, V.; Kumar, A.; Shah, M. The effect of torrefaction temperature and catalyst loading in Microwave-Assisted in-situ catalytic Co-Pyrolysis of torrefied biomass and plastic wastes. Bioresour. Technol. 2022, 364, 128099. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, N.; Priatharsini, P.; Hakim, S.B. Microwave-Assisted Co-Pyrolysis of Bamboo Biomass with Plastic Waste for Hydrogen-Rich Syngas Production. Am. J. Appl. Sci. 2016, 13, 511–521. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Peng, Y.; Ke, L.; Yang, Q.; Jiang, L.; Dai, L.; Liu, Y.; Ruan, R.; Xia, D.; et al. Microwave-assisted pyrolysis of waste cooking oil for hydrocarbon bio-oil over metal oxides and HZSM-5 catalysts. Energy Convers. Manag. 2020, 220, 113124. [Google Scholar] [CrossRef]
- Kamireddi, D.; Terapalli, A.; Sridevi, V.; Bai, M.T.; Surya, D.V.; Rao, C.S.; Jeeru, L.R. Microwave-Assisted In-situ Catalytic Co-Pyrolysis of Polypropylene and Polystyrene Mixtures: Response Surface Methodology Analysis using Machine Learning. J. Anal. Appl. Pyrolysis 2023, 172, 105984. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Ma, D.; Chen, C.; Wang, Y.; Wang, W.; Li, A. Exergy and energy analysis of pyrolysis of plastic wastes in rotary kiln with heat carrier. Process Saf. Environ. Prot. 2020, 142, 203–211. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Gupta, A.A.; Nagababu, G.; Kumar, T.H.; Sasikumar, J.S.; Choksi, H.H. Production of aromatic hydrocarbons from microwave-assisted pyrolysis of municipal solid waste (MSW). Process Saf. Environ. Prot. 2022, 159, 382–392. [Google Scholar] [CrossRef]
- Potnuri, R.; Suriapparao, D.V.; Sankar Rao, C.; Sridevi, V.; Kumar, A. Effect of dry torrefaction pretreatment of the microwave-assisted catalytic pyrolysis of biomass using the machine learning approach. Renew. Energy 2022, 197, 798–809. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Reddy, B.R.; Rao, C.S.; Jeeru, L.R.; Kumar, T.H. Prosopis juliflora valorization via microwave-assisted pyrolysis: Optimization of reaction parameters using machine learning analysis. J. Anal. Appl. Pyrolysis 2023, 169, 105811. [Google Scholar] [CrossRef]
- Potnuri, R.; Suriapparao, D.V.; Rao, C.S.; Kumar, T.H. Understanding the role of modeling and simulation in pyrolysis of biomass and waste plastics: A review. Bioresour. Technol. Rep. 2022, 20, 101221. [Google Scholar] [CrossRef]
- Neha, S.; Remya, N. Optimization of bio-oil production from microwave co-pyrolysis of food waste and low-density polyethylene with response surface methodology. J. Environ. Manag. 2021, 297, 113345. [Google Scholar] [CrossRef]
- Jing, X.; Wen, H.; Xu, Z. Temperature field simulation of polyolefin-absorber mixture by FDTD-FDM model during microwave heating. Chin. J. Chem. Eng. 2020, 28, 2900–2917. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, F.; Song, Z.; Zhao, X.; Wang, W.; Mao, Y.; Sun, J. ReaxFF MD simulation of microwave catalytic pyrolysis of polypropylene over Fe catalyst for hydrogen. Fuel 2023, 340, 127550. [Google Scholar] [CrossRef]
- Mariappan, M.; Panithasan, M.S.; Venkadesan, G. Pyrolysis plastic oil production and optimisation followed by maximum possible replacement of diesel with bio-oil/methanol blends in a CRDI engine. J. Clean. Prod. 2021, 312, 127687. [Google Scholar] [CrossRef]
| Types | Performance | Monomer | Main Application | Main Pyrolysis Products |
|---|---|---|---|---|
| PET | Melting point range: 250–255 °C Softening temperature: 98 °C Transparent, oil-resistant, tough, and resistant to most solvents. | Terephthalic acid, ethylene glycol | Beverage bottles, packaging bags, etc. | Benzoic acid, 4-vinyl benzoic acid, mono vinyl terephthalate, divinyl terephthalate, ethylene glycol, benzene, vinyl benzoate, terephthalic acid |
| HDPE | Melting point range: 250–260 °C Softening temperature: 90 °C Transparent, tough, and corrosion-resistant. | Ethylene | Shopping bags, toys, water pipes, etc. | C1–C4 alkanes, ethylene, propylene, 1-butenes,1-pentene, butadiene, C6–C25 alkanes and alkenes, coke |
| PVC | Melting point: 160–180 °C Softening temperature: 80–85 °C | Vinyl chloride | Pipe, packaging film, sealing material, artificial leather, etc. | HCl, H2, C1–C4 hydrocarbon gases, benzene, toluene, PAHs |
| LDPE | Melting point range: about 120 °C. Softening range: about 80–90 °C. Soft and elastic, translucent, and easy to scratch. | Ethylene | Shopping bags, garbage bags, cosmetics and detergent bottles, milk, etc. | C1–C4 hydrocarbon gases, 1-butenes,1-pentene, butadiene, C6–C25 alkanes, and alkenes |
| PP | Melting point range: about 140–160 °C. Softening range: 95–110 °C. Hard, translucent, versatile, and solvent-resistant. | Propylene | Detergent packaging, bottle caps, fasteners, food and steam packaging, food trays in microwave ovens, etc. | Propylene, butadiene, butene methane, propadiene and C7–C9 alkanes, alkenes |
| PS | Melting point range: 140–180 °C Softening range: 80–105 °C Transparent, cheap, rigid, insulating, and printable. | Styrene | Instrument shell, lampshade, disposable plastic tableware, transparent CD box, etc. | Tyrene, toluene, α-methyl styrene, diphenyl propane, benzene, ethylbenzene, cumene, diphenyl butane, and light olefins |
| Others E.g.: PC | Melting point range: 220–230 °C Softening range: 130–140 °C Transparent, heat resistant, flame retardant, and impact resistant. | Bisphenol-A and diphenyl carbonate | CD, packaging, medical equipment, bulletproof glass, helmet, etc. | Phenol, p-methylphenol, p-ethylphenol, p-propylphenol, bisphenol-A, tert-butyl phenol, di (4-tert-butylbenzene) carbonate |
| Materials | Tanδ | References |
|---|---|---|
| Polyethylene glycol terephthalate (PET) | 0.003 | [17] |
| Polyethylene (PE) | 0.001–0.002 | [18] |
| Polypropylene (PP) | 0.003-0.004 | [18] |
| Polyvinyl chloride (PVC) | 0.0056 | [16] |
| Polypropylene (PS) | 0.0002–0.0003 | [19] |
| Polycarbonate (PC) | 0.01 | [20] |
| Natural rubber | 0.002–0.005 | [18] |
| Carborundum (SiC) | 0.25–0.37 | [16] |
| Activated carbon (AC) | 0.31–0.9 | [16] |
| Fe3O4 | 0.199 | [16] |
| Al2O3 | 0.001 | [16] |
| Wood | 0.11 | [18] |
| Sr. No. | Feedstock | Pyrolysis Technology | Temperature/Ratio | Oil Yield (wt.%) | HHV (MJ/kg) | Residual (wt.%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Pine/HDPE | Fixed bed | 500 °C/25:75 | 22.5 | 37.5 | 7.3 | [56] |
| 2 | Red oak/HDPE | Fixed bed | 450 °C/1:1 | 53 | - | 16 | [57] |
| 3 | Sugarcane bagasse/Scrap tire | Fixed bed | 500 °C/1:3 | 49.7 | 41 | 33.8 | [58] |
| 4 | Grape seeds/PS | Fixed bed | 550 °C/80:20 | 51 | 39 | 27 | [59] |
| 5 | Palm shell/PS | Vertical furnace | 600 °C/40:60 | 68.3 | 40.34 | ~12 | [60] |
| 6 | Bamboo/PP | Fixed bed | 500 °C/80:20 | 50.95 | 24.57 | 20.60 | [61] |
| 7 | Rubber/plastic | Fixed bed | 550 °C/4:1 | 33.77 | 39.93 | 39.50 | [62] |
| 8 | Palm shell/PS | Fixed bed | 500 °C/1:1 | 61.63 | 38.01 | 16.24 | [63] |
| 9 | Bamboo/PS | Fixed bed | 500 °C/80:20 | 50.17 | 28.22 | 21.59 | [61] |
| 10 | Microalgae/PVC | MAP | 550 °C/7:3 | 36.68 | 35.87 | 11.72 | [64] |
| 11 | Algae/PS | MAP | 600 °C/1:1 | 65 | 42.2 | 10 | [52] |
| 12 | Rice husk/PP | MAP | 600 °C/(10–11.5):1 | 41.1 | 42.0 | 24.2 | [65] |
| 13 | Rice husk/PS | MAP | 600 °C/(10–11.5):1 | 54.3 | 39.4 | 22.7 | [65] |
| 14 | Wheat Straw/PP | MAP | 600 °C/1:1 | 47.5 | - | 8.8 | [66] |
| 15 | Wheat Straw/PS | MAP | 600 °C/1:1 | 58.4 | - | 7.5 | [66] |
| 16 | Waste tea powder/PS | MAP | 600 °C/3:1 | 80 | - | 10.9 | [67] |
| 17 | Algae/PE | MAP | 600 °C/1:1 | 40 | 42.9 | 10 | [52] |
| 18 | Used frying oil/LDPE | MAP | 550 °C/1:1 | 81 | 42–46 | 1 | [68] |
| 19 | Biochar/PS | MAP | 450–500 °C/1:10 | 86.1 | 46.87 | 3.4 | [25] |
| 20 | Food-waste/plastic | MAP | 550 °C/87:13 | 42 | 20.2 | 42 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Shang, H.; Li, J.; Fan, X.; Sun, J.; Duan, A. A Review on the Microwave-Assisted Pyrolysis of Waste Plastics. Processes 2023, 11, 1487. https://doi.org/10.3390/pr11051487
Yang C, Shang H, Li J, Fan X, Sun J, Duan A. A Review on the Microwave-Assisted Pyrolysis of Waste Plastics. Processes. 2023; 11(5):1487. https://doi.org/10.3390/pr11051487
Chicago/Turabian StyleYang, Changze, Hui Shang, Jun Li, Xiayu Fan, Jianchen Sun, and Aijun Duan. 2023. "A Review on the Microwave-Assisted Pyrolysis of Waste Plastics" Processes 11, no. 5: 1487. https://doi.org/10.3390/pr11051487
APA StyleYang, C., Shang, H., Li, J., Fan, X., Sun, J., & Duan, A. (2023). A Review on the Microwave-Assisted Pyrolysis of Waste Plastics. Processes, 11(5), 1487. https://doi.org/10.3390/pr11051487







