Fig Leaves (Ficus carica L.): Source of Bioactive Ingredients for Industrial Valorization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Samples
2.2. Samples Preparation
2.3. Color Measurement
2.4. Preparation of Hydroethanolic Extracts
2.5. Chromatographic Analysis of Chemical Constituents
2.5.1. Organic Acids
2.5.2. Free Sugars
2.5.3. Tocopherols
2.5.4. Fatty Acids
2.5.5. Phenolic Compounds
2.6. Evaluation of Bioactive Properties
2.6.1. Antioxidant Activity
2.6.2. Cytotoxic and Hepatotoxic Activities
2.6.3. Inhibition of Nitric Oxide Production Evaluation
2.6.4. Antimicrobial Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Color Measurement
3.2. Chemical Composition
3.2.1. Organic Acids
3.2.2. Free Sugars
3.2.3. Tocopherols
3.2.4. Fatty Acids
3.2.5. Phenolic Compounds
3.3. Bioactivity Evaluation
3.3.1. Antioxidant Activity
3.3.2. Cytotoxic Activities
3.3.3. Inhibition of Nitric Oxide Production Evaluation
4. Antimicrobial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stover, E.; Aradhya, M.; Ferguson, L.; Crisosto, C.H. The Fig: Overview of an Ancient Fruit. HortScience 2007, 42, 1083–1087. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H.; Mahajan, R.T. Traditional Uses, Phytochemistry and Pharmacology of Ficus carica: A Review. Pharm. Biol. 2014, 52, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.R.; Romano, A. Chemical and Biological Characteristics of Ficus carica L. Fruits, Leaves, and Derivatives (Wine, Spirit, and Liqueur). In Modern Fruit Industry; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Valentão, P.; Pereira, J.A.; Silva, B.M.; Tavares, F.; Andrade, P.B. Ficus carica L.: Metabolic and Biological Screening. Food Chem. Toxicol. 2009, 47, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Raskovic, B.; Lazic, J.; Polovic, N. Characterisation of General Proteolytic, Milk Clotting and Antifungal Activity of Ficus carica Latex during Fruit Ripening. J. Sci. Food Agric. 2016, 96, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Okiura, A.; Saito, K.; Kohno, M. Identification of Phenylpropanoids in Fig (Ficus carica L.) Leaves. J. Agric. Food Chem. 2014, 62, 10076–10083. [Google Scholar] [CrossRef]
- Dueñas, M.; Pérez-Alonso, J.J.; Santos-Buelga, C.; Escribano-Bailón, T. Anthocyanin Composition in Fig (Ficus carica L.). J. Food Compos. Anal. 2008, 21, 107–115. [Google Scholar] [CrossRef]
- Shahinuzzaman, M.; Yaakob, Z.; Anuar, F.H.; Akhtar, P.; Kadir, N.H.A.; Hasan, A.K.M.; Sobayel, K.; Nour, M.; Sindi, H.; Amin, N.; et al. In Vitro Antioxidant Activity of Ficus carica L. Latex from 18 Different Cultivars. Sci. Rep. 2020, 10, 10852. [Google Scholar] [CrossRef]
- Ayuso, M.; Carpena, M.; Taofiq, O.; Albuquerque, T.G.; Simal-Gandara, J.; Oliveira, M.B.P.P.; Prieto, M.A.; Ferreira, I.C.F.R.; Barros, L. Fig “Ficus carica L.” and Its by-Products: A Decade Evidence of Their Health-Promoting Benefits towards the Development of Novel Food Formulations. Trends Food Sci. Technol. 2022, 127, 1–13. [Google Scholar] [CrossRef]
- Nienow, A.A.; Chaves, A.; Lajús, C.R.; Calvete, E.O. Produção Da Figueira Em Ambiente Protegido Submetida a Diferentes Épocas de Poda e Número de Ramos. Rev. Bras. Frutic. 2006, 28, 421–424. [Google Scholar] [CrossRef]
- Ballardini, R.M.; Kaisto, J.; Similä, J. Developing Novel Property Concepts in Private Law to Foster the Circular Economy. J. Clean. Prod. 2021, 279, 123747. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; León-González, M.E.; Ferreira, I.C.F.R.; Barros, L. Valorisation of Black Mulberry and Grape Seeds: Chemical Characterization and Bioactive Potential. Food Chem. 2021, 337, 127998. [Google Scholar] [CrossRef] [PubMed]
- Soria-Lopez, A.; Garcia-Perez, P.; Carpena, M.; Garcia-Oliveira, P.; Otero, P.; Fraga-Corral, M.; Cao, H.; Prieto, M.A.; Simal-Gandara, J. Challenges for Future Food Systems: From the Green Revolution to Food Supply Chains with a Special Focus on Sustainability. Food Front. 2022, 4, 9–20. [Google Scholar] [CrossRef]
- Othman, S.; Añibarro-Ortega, M.; Dias, M.I.; Ćirić, A.; Mandim, F.; Soković, M.; Ferreira, I.C.F.R.; Pinela, J.; Barros, L. Valorization of Quince Peel into Functional Food Ingredients: A Path towards “Zero Waste” and Sustainable Food Systems. Heliyon 2022, 8, e11042. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of Kiwi Agricultural Waste and Industry By-Products by Recovering Bioactive Compounds and Applications as Food Additives: A Circular Economy Model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Liu, M.; Zhang, C.; Shao, J.; Hou, X.; Tian, J.; Cui, Q. A Comprehensive Review on Phytochemistry, Bioactivities, Toxicity Studies, and Clinical Studies on Ficus carica Linn. Leaves. Biomed. Pharmacother. 2021, 137, 111393. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Mandrone, M.; Chiocchio, I.; Sweeney, E.M.; Tirelli, E.; Uberti, D.; Memo, M.; Poli, F.; Mastinu, A.; Abate, G. Different Seasonal Collections of Ficus carica L. Leaves Diversely Modulate Lipid Metabolism and Adipogenesis in 3T3-L1 Adipocytes. Nutrients 2022, 14, 2833. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Â.; Antonio, A.L.; Barreira, J.C.M.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effects of Gamma Irradiation on Physical Parameters of Lactarius Deliciosus Wild Edible Mushrooms. Postharvest Biol. Technol. 2012, 74, 79–84. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and Chemical Characterization in Hydrophilic and Lipophilic Compounds of Chenopodium Ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic Profile and Antioxidant Activity of Coleostephus Myconis (L.) Rchb.f.: An Underexploited and Highly Disseminated Species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, Â.; Finimundy, T.C.; Pereira, C.; Alves, M.J.; Calhelha, R.C.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Lovage (Levisticum Officinale W.D.J. Koch) Roots: A Source of Bioactive Compounds towards a Circular Economy. Resources 2020, 9, 81. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; de Souza, A.H.P.; Calhelha, R.C.; Barros, L.; Glamoclija, J.; Sokovic, M.; Peralta, R.M.; Bracht, A.; Ferreira, I.C.F.R. Bioactive Formulations Prepared from Fruiting Bodies and Submerged Culture Mycelia of the Brazilian Edible Mushroom Pleurotus Ostreatoroseus Singer. Food Funct. 2015, 6, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Mocan, A.; García, P.A.; Barros, L.; Ferreira, I.C.F.R. Exploring the Phytochemical Profile of Cytinus Hypocistis (L.) L. as a Source of Health-Promoting Biomolecules behind Its in Vitro Bioactive and Enzyme Inhibitory Properties. Food Chem. Toxicol. 2020, 136, 111071. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Rui, H.L. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R.P. Anti-Hepatocellular Carcinoma Activity Using Human HepG2 Cells and Hepatotoxicity of 6-Substituted Methyl 3-Aminothieno[3,2-b]Pyridine-2-Carboxylate Derivatives: In Vitro Evaluation, Cell Cycle Analysis and QSAR Studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef]
- Mandim, F.; Graça, V.C.; Calhelha, R.C.; Machado, I.L.F.; Ferreira, L.F.V.; Ferreira, I.C.F.R.; Santos, P.F. Synthesis, Photochemical and In Vitro Cytotoxic Evaluation of New Iodinated Aminosquaraines as Potential Sensitizers for Photodynamic Therapy. Molecules 2019, 24, 863. [Google Scholar] [CrossRef]
- Mandim, F.; Barros, L.; Calhelha, R.C.; Abreu, R.M.V.; Pinela, J.; Alves, M.J.; Heleno, S.; Santos, P.F.; Ferreira, I.C.F.R. Calluna Vulgaris (L.) Hull: Chemical Characterization, Evaluation of Its Bioactive Properties and Effect on the Vaginal Microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, B.; Barros, L.; Sopik, T.; Calhelha, R.C.; Heleno, S.; Alves, M.J.; Walcott, S.; Kuban, V.; Ferreira, I.C.F.R. Non-Edible Parts of Solanum Stramoniifolium Jacq.—A New Potent Source of Bioactive Extracts Rich in Phenolic Compounds for Functional Foods. Food Funct. 2017, 8, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and Antimicrobial Properties of Dried Portuguese Apple Variety (Malus Domestica Borkh. Cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C.F.R. Melissa Officinalis L. Decoctions as Functional Beverages: A Bioactive Approach and Chemical Characterization. Food Funct. 2015, 6, 2240–2248. [Google Scholar] [CrossRef]
- Pande, G.; Akoh, C.C. Organic Acids, Antioxidant Capacity, Phenolic Content and Lipid Characterisation of Georgia-Grown Underutilized Fruit Crops. Food Chem. 2010, 120, 1067–1075. [Google Scholar] [CrossRef]
- Nuñez-Estevez, B.; Finimundy, T.C.; Carpena, M.; Barral-Martinez, M.; Calhelha, R.; Pires, T.C.S.P.; Otero, P.; Garcia-Perez, P.; Simal-Gandara, J.; Ferreira, I.C.F.R.; et al. Bioactive Compound Profiling and Nutritional Composition of Three Species from the Amaranthaceae Family. Chem. Proc. 2021, 5, 20. [Google Scholar] [CrossRef]
- Teruel-Andreu, C.; Sendra, E.; Hernández, F.; Cano-Lamadrid, M. How Does Cultivar Affect Sugar Profile, Crude Fiber, Macro-and Micronutrients, Total Phenolic Content, and Antioxidant Activity on Ficus carica Leaves? Agronomy 2023, 13, 30. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Aktumsek, A.; Karatas, S. Fatty Acid Composition, Total Sugar Content and Anti-Diabetic Activity of Methanol and Water Extracts of Nine Different Fruit Tree Leaves Collected from Mediterranean Region of Turkey. Int. J. Food Prop. 2015, 18, 2268–2276. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Konyalιoğlu, S.; Sağlam, H.; Kιvçak, B.; Konyaloglu, S.; Sniye Saglam, H.; Kvçak, B. α-Tocopherol, Flavonoid, and Phenol Contents and Antioxidant Activity of Ficus carica Leaves. Pharm. Biol. 2008, 43, 683–686. [Google Scholar] [CrossRef]
- Palmeira, L.; Pereira, C.; Dias, M.I.; Abreu, R.M.V.; Corrêa, R.C.G.; Pires, T.C.S.P.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Nutritional, Chemical and Bioactive Profiles of Different Parts of a Portuguese Common Fig (Ficus carica L.) Variety. Food Res. Int. 2019, 126, 108572. [Google Scholar] [CrossRef]
- Clifford, M.N.; Zheng, W.; Kuhnert, N. Profiling the Chlorogenic Acids of Aster by HPLC-MSn. Phytochem. Anal. 2006, 17, 384–393. [Google Scholar] [CrossRef]
- Pereira, O.R.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Identification of Phenolic Constituents of Cytisus multiflorus. Food Chem. 2012, 131, 652–659. [Google Scholar] [CrossRef]
- Essid, A.; Aljane, F.; Ferchichi, A. Mineral and Phenolic Content of Leaves of Tunisian Caprifig (Ficus carica L.) Accessions. J. New Sci. 2015, 21, 977–984. [Google Scholar]
- Nadeem, M.; Zeb, A. Impact of Maturity on Phenolic Composition and Antioxidant Activity of Medicinally Important Leaves of Ficus carica L. Physiol. Mol. Biol. Plants 2018, 24, 881. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Khali, M.; Benkhaled, A.; Benamirouche, K.; Baiti, I. Phenolic and Flavonoid Contents, Antioxidant and Antimicrobial Activities of Leaf Extracts from Ten Algerian Ficus carica L. Varieties. Asian Pac. J. Trop. Biomed. 2016, 6, 239–245. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic Acids: Chemistry, Biosynthesis, Occurrence, Analytical Challenges, and Bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef] [PubMed]
- Ergül, M.; Ergül, M.; Eruygur, N.; Ataş, M.; Uçar, E. In Vitro Evaluation of the Chemical Composition and Various Biological Activities of Ficus carica Leaf Extracts. Turk. J. Pharm. Sci. 2019, 16, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Nirwana, I.; Rianti, D.; Helal Soekartono, R.; Listyorini, R.D.; Basuki, D.P. Antibacterial Activity of Fig Leaf (Ficus carica Linn.) Extract against Enterococcus Faecalis and Its Cytotoxicity Effects on Fibroblast Cells. Vet. World 2018, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, Y.; Huo, B.; Li, B.; Jin, Y.; Hu, X. Extracts and Components of Ficus carica Leaves Suppress Survival, Cell Cycle, and Migration of Triple-Negative Breast Cancer MDA-MB-231 Cells. Onco Targets Ther. 2018, 11, 4377. [Google Scholar] [CrossRef] [PubMed]
- Purnamasari, R.; Winarni, D.; Permanasari, A.A.; Agustina, E.; Hayaza, S.; Darmanto, W. Anticancer Activity of Methanol Extract of Ficus carica Leaves and Fruits Against Proliferation, Apoptosis, and Necrosis in Huh7it Cells. Cancer Inform. 2019, 18, 1176935119842576. [Google Scholar] [CrossRef]
- Ali, B.; Mujeeb, M.; Aeri, V.; Mir, S.R.; Faiyazuddin, M.; Shakeel, F. Anti-Inflammatory and Antioxidant Activity of Ficus carica Linn. Leaves. Nat. Prod. Res. 2012, 26, 460–465. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
| PA | LA | DA | BN | MA | |
|---|---|---|---|---|---|
 |  |  |  |  | |
| CIELAB | |||||
| L* | 35.0 ± 0.3 c | 38.2 ± 0.8 b | 36.8 ± 0.5 b | 41.1 ± 0.6 a | 37.1 ± 0.3 b |
| a* | −7.1 ± 0.7 b | −11.0 ± 0.4 a | −7.7 ± 0.7 b | −7.7 ± 0.8 b | −7.3 ± 0.3 b |
| b* | 11.1 ± 0.4 b | 16.1 ± 0.9 a | 12.0 ± 0.6 b | 17.7 ± 0.4 a | 12.6 ± 0.4 b |
| Color |  |  |  |  |  |
| Organic Acids (g/100 g dw) | |||||
|---|---|---|---|---|---|
| PA | LA | DA | BN | MA | |
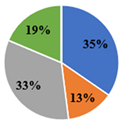
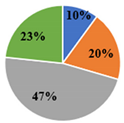
| ▊ Oxalic | 41.6 ± 0.6 c | 60.0 ± 0.6 a | 11.90 ± 0.08 d | 48.8 ± 0.2 b | 41.0 ± 0.7 c |
| ▊ Quinic | 16.1 ± 0.2 c | 19.03 ± 0.08 b | 23.0 ± 0.2 a | 23.3 ± 0.2 a | 19.5 ± 0.6 b |
| ▊ Malic | 40.1 ± 0.4 c | 2.54 ± 0.03 d | 55.8 ± 0.2 a | 52.5 ± 0.6 b | 55.49 ± 0.6 a |
| ▊ Citric | 22.4 ± 0.3 d | 23.1 ± 0.2 c | 27.6 ± 0.4 a | 21.8 ± 0.4 e | 24.1 ± 0.1 b |
| ▊ Shikimic | tr | nd | nd | 0.11 ± 0.06 | nd |
| Ascorbic | nd | tr | nd | tr | tr |
| Fumaric | tr | tr | tr | tr | tr |
| Total | 120.2 ± 0.7 c | 104.7 ± 0.8 d | 118.3 ± 0.4 c | 146.5 ± 1.1 a | 140.1 ± 0.3 b |
 |  |  |  |  | |
| Free Sugars (g/100 g dw) | |||||
|---|---|---|---|---|---|
| PA | LA | DA | BN | MA | |
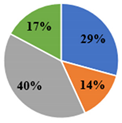
| ▊ Fructose | 1.36 ± 0.02 e | 1.94 ± 0.02 c | 7.34 ± 0.06 a | 3.58 ± 0.02 b | 1.75 ± 0.01 d |
| ▊ Glucose | 1.48 ± 0.02 e | 1.77 ± 0.02 d | 7.72 ± 0.04 a | 6.32 ± 0.007 b | 3.59 ± 0.02 c |
| ▊ Sucrose | 1.62 ± 0.01 c | 0.133 ± 0.007 e | 0.276 ± 0.008 d | 4.27 ± 0.03 a | 3.59 ± 0.01 b |
| ▊ Trehalose | 0.62 ± 0.02 c | 0.63 ± 0.01 c | 1.130 ± 0.009 a | 0.61 ± 0.01 c | 0.72 ± 0.01 b |
| ▊ Raffinose | 0.26 ± 0.01 e | 0.47 ± 0.02 d | 0.87 ± 0.01 a | 0.74 ± 0.01 b | 0.687 ± 0.008 c |
| Total | 5.34 ± 0.02 d | 4.95 ± 0.02 e | 17.36 ± 0.08 a | 15.57 ± 0.03 b | 10.08 ± 0.06 c |
 | 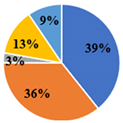 | 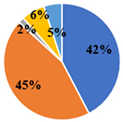 | 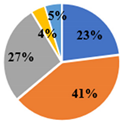 | 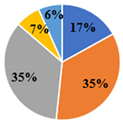 | |
| Tocopherols (mg/100 g dw) | |||||
|---|---|---|---|---|---|
| PA | LA | DA | BN | MA | |
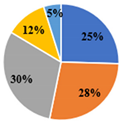
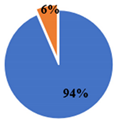
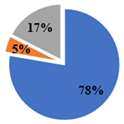
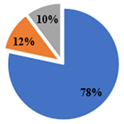
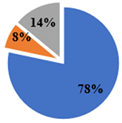
| ▊ α-Tocoferol | 3.36 ± 0.05 a | 3.32 ± 0.05 a | 2.26 ± 0.02 b | 1.438 ± 0.004 c | 1.45 ± 0.01 c |
| ▊ β-Tocoferol | 0.29 ± 0.01 a | 0.23 ± 0.02 b | 0.14 ± 0.01 c | 0.21 ± 0.01 b | 0.142 ± 0.008 c |
| ▊ γ-Tocoferol | 0.49 ± 0.01 a | nd | 0.51 ± 0.02 a | 0.188 ± 0.007 c | 0.26 ± 0.01 b |
| ▊ δ-Tocoferol | nd | nd | nd | nd | nd |
| Total | 4.14 ± 0.04 a | 3.56 ± 0.06 b | 2.91 ± 0.03 c | 1.84 ± 0.01 d | 1.85 ± 0.01 d |
 |  |  |  |  | |
| Fatty Acids (% of Total) | |||||
|---|---|---|---|---|---|
| PA | LA | DA | BN | MA | |
| C12:0 | nd | 0.911 ± 0.004 | nd | nd | nd |
| C13:0 | nd | 2.171 ± 0.008 b | 1.65 ± 0.02 d | 2.87 ± 0.15 a | 1.77 ± 0.06 c |
| C14:0 | nd | 3.11 ± 0.02 a | 1.39 ± 0.04 d | 2.30 ± 0.04 b | 1.87 ± 0.01 c |
| C14:1 | nd | nd | 0.316 ± 0.004 | nd | nd |
| C15:1 | nd | nd | 0.346 ± 0.003 | nd | nd |
| C16:0 | 18.9 ± 0.4 b | 23.19 ± 0.07 a | 19.05 ± 0.05 b | 18.1 ± 0.3 c | 16.5 ± 0.1 d |
| C16:1 | nd | nd | 1.457 ± 0.002 | nd | 1.66 ± 0.03 |
| C17:0 | nd | nd | 0.756 ± 0.001 | nd | nd |
| C18:0 | 7.8 ± 0.2 a | nd | 4.77 ± 0.02 d | 6.5 ± 0.2 b | 5.26 ± 0.02 c |
| C18:1n9c | nd | nd | 4.62 ± 0.04 a | nd | 3.941 ± 0.006 b |
| C18:2n6c | 13.5 ± 0.5 c | 19.68 ± 0.05 a | 12.25 ± 0.09 d | 15.27 ± 0.02 b | 10.318 ± 0.004 e |
| C18:3n3 | 52.9 ± 0.2 a | 44.23 ± 0.02 b | 40.334 ± 0.001 c | 36.8 ± 0.3 e | 38.99 ± 0.02 d |
| C20:0 | nd | nd | 3.41 ± 0.02 b | nd | 4.63 ± 0.05 a |
| C20:3n3 | nd | 6.71 ± 0.07 b | 1.420 ± 0.001 d | 18.4 ± 0.2 a | 3.6 ± 0.1 c |
| C22:0 | 6.7 ± 0.2 a | nd | 4.045 ± 0.006 c | nd | 6.26 ± 0.02 b |
| C24:0 | nd | nd | 4.19 ± 0.04 | nd | 5.3 ± 0.1 |
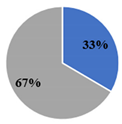
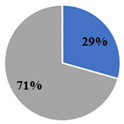
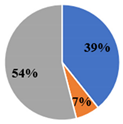
| ▊ SFA | 33.4 ± 0.3 c | 29.4 ± 0.1 d | 39.3 ± 0.1 b | 29.7 ± 0.2 d | 41.5 ± 0.09 a |
| ▊ MUFA | nd | nd | 6.73 ± 0.03 | nd | 5.60 ± 0.03 |
| ▊ PUFA | 66.4 ± 0.2 b | 70.6 ± 0.1 a | 54.00 ± 0.09 c | 70.5 ± 0.1 a | 52.9 ± 0.1 d |
 |  |  |  |  | |
| Quantification (mg/g dw Extract) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak | Rt (min) | λmax (nm) | [M−H]− (m/z) | MS2 (m/z) | TentativeIdentification | PA | LA | DA | BN | MA |
| 1 | 5.99 | 320 | 341 | 179 (100), 161 (18), 135 (5) | Caffeic acid hexoside | 0.747 ± 0.007 b | 0.56 ± 0.02 c | 1.08 ± 0.02 a | 0.427 ± 0.006 d | 0.54 ± 0.01 c |
| 2 | 7.08 | 324 | 353 | 191 (100), 179 (12), 161 (7), 135 (5) | cis 5-O-caffeoylquinic acid | 1.52 ± 0.04 a | 1.22 ± 0.02 b | 1.27 ± 0.05 b | 0.548 ± 0.007 d | 0.67 ± 0.02 c |
| 3 | 7.26 | 324 | 353 | 191 (100), 179 (9), 161 (8), 135 (5) | trans 5-O-Caffeoylquinic acid | nd | nd | 2.361 ± 0.003 | nd | nd |
| 4 | 10.12 | 328 | 579 | 459 (22), 429 (83), 357 (63), 327 (100), 309 (54) | Luteolin O-pentosyl-C-hexoside | 3.5 ± 0.1 c | 7.7 ± 0.1 a | 3.99 ± 0.07 b | 0.092 ± 0.001 e | 3.1 ± 0.1 d |
| 5 | 10.54 | 324 | 179 | 163 (100) | Caffeic acid | 2.55 ± 0.03 b | 3.68 ± 0.03 a | 2.16 ± 0.05 c | 0.71 ± 0.01 e | 1.02 ± 0.03 d |
| 6 | 11.7 | 283 | 337 | 191 (100), 163 (12), 119 (10) | 5-O-p-Coumaroylquinic acid | 3.624 ± 0.002 a | 0.95 ± 0.03 b | 0.92 ± 0.02 b | 0.599 ± 0.001 d | 0.68 ± 0.02 c |
| 7 | 12.88 | 337 | 563 | 473 (58), 443 (100), 383 (15), 353 (20), 311 (5), 297 (5) | Apigenin-C-hexoside-C-pentoside | 6.63 ± 0.04 c | 13.62 ± 0.08 a | 8.83 ± 0.09 b | 6.19 ± 0.06 e | 6.40 ± 0.02 d |
| 8 | 14.37 | 338 | 563 | 473 (58), 443 (100), 383 (15), 353 (20), 311 (5), 297 (5) | Apigenin-C-hexoside-C-pentoside | 0.562 ± 0.002 d | 0.87 ± 0.02 b | 1.01 ± 0.01 a | 0.592 ± 0.009 c | 0.60 ± 0.01 c |
| 9 | 15.72 | 287 | 545 | 501 (100), 459 (13), 313 (5), 167 (98) | Vanilic acid -malonyl-rhamnoside-rhamnoside | 0.104 ± 0.001 d | 0.238 ± 0.003 a | 0.181 ± 0.001 b | 0.175 ± 0.001 c | 0.178 ± 0.003 b,c |
| 10 | 16.82 | 355 | 609 | 301 (100) | Quercetin-O-deoxyhexosyl-hexoside | 6.72 ± 0.03 c | 9.33 ± 0.03 b | 9.903 ± 0.005 a | 5.293 ± 0.004 e | 5.97 ± 0.02 d |
| 11 | 18.21 | 351 | 463 | 301 (100) | Quercetin-O-hexoside | 1.426 ± 0.005 b | 1.00 ± 0.02 c | 1.16 ± 0.02 a | 0.586 ± 0.003 e | 0.668 ± 0.002 d |
| 12 | 19.58 | 355 | 549 | 505 (52), 463 (43), 301 (100) | Quercetin-O-malonyl-hexoside | 3.640 ± 0.005 a | 1.90 ± 0.03 b | 1.39 ± 0.02 c | 0.826 ± 0.001 e | 1.28 ± 0.0 d |
| 13 | 20 | 359 | 593 | 285 (100) | Kaempherol-O-deoxyhexosyl-hexoside | 0.895 ± 0.009 c | 1.38 ± 0.02 a | 1.05 ± 0.01 b | 0.718 ± 0.006 d | 0.735 ± 0.009 d |
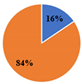
| ▊Total Phenolic Acids | 8.54 ± 0.02 a | 6.6 ± 0.1 c | 7.98 ± 0.05 b | 2.455 ± 0.002 e | 3.08 ± 0.03 d | |||||
| ▊Total Flavonoids | 23.4 ± 0.1 c | 35.80 ± 0.08 a | 27.3 ± 0.1 b | 14.30 ± 0.04 e | 18.7 ± 0.2 d | |||||
 |  |  | 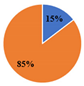 | 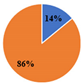 | ||||||
| Total Phenolic Compounds | 31.9 ± 0.1 c | 42.4 ± 0.2 a | 35.3 ± 0.2 b | 16.75 ± 0.04 e | 21.8 ± 0.2 d | |||||
| PA | LA | DA | BN | MA | Positive Control | ||
|---|---|---|---|---|---|---|---|
| Antioxidant Activity | |||||||
| TBARS (EC50, mg/mL) | 0.78 ± 0.02 d | 0.74 ± 0.02 d | 0.35 ± 0.01 b | 0.23 ± 0.01 a | 0.45 ± 0.03 c | 0.0091 ± 0.0003 | |
| CAA (% inhibition by 2 mg/mL) | 65 ± 11 a | 60 ± 4 a | 57 ± 5 a | 40 ± 3 b | 33 ± 3 c | 95 ± 5 | |
| Cytotoxic activity | |||||||
| AGS | (GI50, µg/mL) | >400 | 173 ± 12 b | 158 ± 13 a | 235 ± 23 c | >400 | 1.23 ± 0.03 |
| MCF-7 | 253 ± 12 b,c | 208 ± 7 a | 223 ± 2 a,b | >400 | 279 ± 24 c | 1.02 ± 0.02 | |
| CaCo2 | >400 | >400 | >400 | >400 | >400 | 1.21 ± 0.02 | |
| VERO | >400 | >400 | >400 | >400 | >400 | 1.41 ± 0.06 | |
| PLP2 | >400 | 225 ± 1 a | 248 ± 10 b | >400 | >400 | 1.4 ± 0.1 | |
| Inhibition of nitric oxide production evaluation | |||||||
| NO production inhibition (IC50, µg/mL) | 82 ± 8 b | >400 | 20 ± 5 a | >400 | 89 ± 3 b | 6.3 ± 0.4 | |
| Fig Leaves Varieties | Positive Control | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA | LA | DA | BN | MA | Streptomycin (1 mg/mL) | Methicillin (1 mg/mL) | Ampicillin (20 mg/mL) | |||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBS | MIC | MBC | |
| Gram-negative bacteria | ||||||||||||||||
| E. cloacae | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t | 0.15 | 0.15 |
| E. coli | 10 | >10 | 10 | >10 | 2.5 | >10 | 5 | >10 | 5 | >10 | 0.01 | 0.01 | n.t. | n.t. | 0.15 | 0.15 |
| P. aeruginosa | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.06 | 0.06 | n.t. | n.t. | 0.63 | 0.63 |
| S. enterica | 10 | >10 | 10 | >10 | 5 | >10 | 10 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 |
| Y. enterocolitica | 10 | >10 | 5 | >10 | 1.25 | >10 | 0.6 | >10 | 0.6 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 |
| Gram-positive bacteria | ||||||||||||||||
| B. cereus | 10 | >10 | 10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.007 | 0.007 | n.t. | n.t. | n.t. | n.t. |
| L. monocytogenes | 5 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 |
| S. aureus | 10 | >10 | 10 | >10 | 2.5 | >10 | 10 | >10 | 10 | >10 | 0.007 | 0.007 | 0.007 | 0.007 | 0.15 | 0.15 |
| Fungal strains | Ketaconazole (1 mg/mL) | |||||||||||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |||||
| A. brasiliensis | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 10 | >10 | 0.06 | 0.125 | ||||
| A. fumigatus | 5 | >10 | 10 | >10 | >10 | >10 | 10 | >10 | 10 | >10 | 0.5 | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiraishi, C.S.H.; Zbiss, Y.; Roriz, C.L.; Dias, M.I.; Prieto, M.A.; Calhelha, R.C.; Alves, M.J.; Heleno, S.A.; V., d.C.M.; Carocho, M.; et al. Fig Leaves (Ficus carica L.): Source of Bioactive Ingredients for Industrial Valorization. Processes 2023, 11, 1179. https://doi.org/10.3390/pr11041179
Shiraishi CSH, Zbiss Y, Roriz CL, Dias MI, Prieto MA, Calhelha RC, Alves MJ, Heleno SA, V. dCM, Carocho M, et al. Fig Leaves (Ficus carica L.): Source of Bioactive Ingredients for Industrial Valorization. Processes. 2023; 11(4):1179. https://doi.org/10.3390/pr11041179
Chicago/Turabian StyleShiraishi, Carlos S. H., Yosra Zbiss, Custódio Lobo Roriz, Maria Inês Dias, Miguel A. Prieto, Ricardo C. Calhelha, Maria José Alves, Sandrina A. Heleno, da Cunha Mendes V., Márcio Carocho, and et al. 2023. "Fig Leaves (Ficus carica L.): Source of Bioactive Ingredients for Industrial Valorization" Processes 11, no. 4: 1179. https://doi.org/10.3390/pr11041179
APA StyleShiraishi, C. S. H., Zbiss, Y., Roriz, C. L., Dias, M. I., Prieto, M. A., Calhelha, R. C., Alves, M. J., Heleno, S. A., V., d. C. M., Carocho, M., Abreu, R. M. V., & Barros, L. (2023). Fig Leaves (Ficus carica L.): Source of Bioactive Ingredients for Industrial Valorization. Processes, 11(4), 1179. https://doi.org/10.3390/pr11041179















