In Vitro Hypoglycemic Potential, Antioxidant and Prebiotic Activity after Simulated Digestion of Combined Blueberry Pomace and Chia Seed Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bioactive Extracts and Their Mixture
2.3. Determination of Total Phenolic Content (TPC), Total Flavonoids Content (TFC) and Total Carbohydrate Content
2.4. HPLC Analysis
2.5. Determination of α-Amylase Activity Inhibition
2.6. Determination of Free Radical Scavenging Capacity
2.7. In Vitro Simulated Gastrointestinal Digestion
2.8. In Vitro Cytocompatibility Testing by Neutral Red Assay
2.9. Determination of Intracellular Reactive Oxygen Species (ROS) Production by Flow Cytometry
2.10. Determination of the Prebiotic Effect
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Bioactive Extracts
3.2. Inhibition of α-Amylase Activity
3.3. Free Radical Scavenging Activity
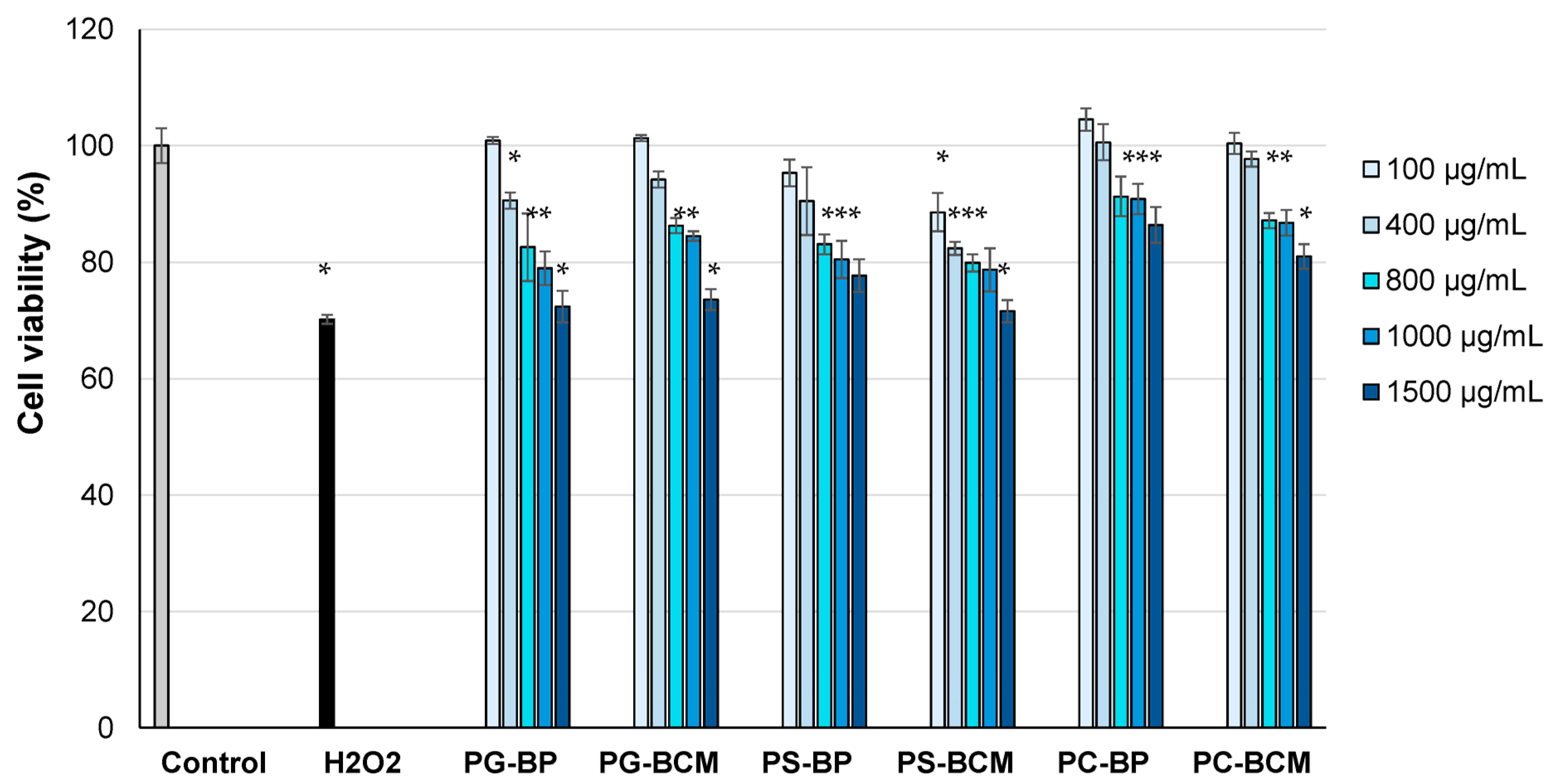
3.4. In Vitro Cell Cytocompatibility
3.5. Effect on Intracellular ROS Production
3.6. Prebiotic Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Nagarajaiah, S.B.; Prakash, J. Chemical Composition and Bioactivity of Pomace from Selected Fruits. Int. J. Fruit Sci. 2016, 16, 423–443. [Google Scholar] [CrossRef]
- Artem, V.; Negreanu–Pirjol, T.; Ranca, A.; Ciobanu, C.; Bratu, M.M.; Popoviciu, D.R.; Moldovan, L.; Vasile, M.; Negreanu-Pirjol, B.S. Total Phenolic Content Correlated with Antioxidant Activity of Some Grape Pomace Biomass Hydroalcoholic Extracts, White and Red Varieties. UPB. Sci. Bull. Ser. B Chem. Mat. Sci. 2021, 83, 61–72. [Google Scholar]
- Negreanu-Pirjol, B.S.; Negreanu–Pirjol, T.; Bratu, M.M.; Popescu, A.; Roncea, F.; Miresan, H.; Jurja, S.; Paraschiv, G.M. Antioxidative Activity of Indigen Bitter Cherry Fruits Extract Corellated with Polyfenols and Minerals Content. Geo. Conf. SGEM Adv. Biotechnol. 2014, 1, 239–244. [Google Scholar]
- Xiong, J.; Chan, Y.H.; Rathinasabapathy, T.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Enhanced Stability of Berry Pomace Polyphenols Delivered in Protein-Polyphenol Aggregate Particles to an In Vitro Gastrointestinal Digestion Model. Food Chem. 2020, 331, 127279. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic Acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kropat, C.; Betz, M.; Kulozik, U.; Leick, S.; Rehage, H.; Boettler, U.; Teller, N.; Marko, D. Effect of Microformulation on the Bioactivity of an Anthocyanin-rich Bilberry Pomace Extract (Vaccinium myrtillus L.) In Vitro. J. Agric. Food Chem. 2013, 61, 4873–4881. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, T.; Negreanu-Pirjol, B.; Popescu, A.; Bratu, M.M.; Udrea, M.; Busuricu, F. Comparative Antioxidant Properties of some Romanian Foods Fruits Extracts. J. Environ. Prot. Ecol. 2014, 15, 1139–1148. [Google Scholar]
- Chu, W.K.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2011; pp. 55–73. [Google Scholar]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Mikulic-Petkovsek, M. White Versus Blue: Does the Wild ‘albino’ Bilberry (Vaccinium myrtillus L.) Differ in Fruit Quality Compared to the Blue One? Food Chem. 2016, 211, 876–882. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-Derived Non-Phenolic α-Amylase and α-Glucosidase Inhibitors for Controlling Starch Digestion Rate and Guiding Diabetes-Friendly Recipes. LWT Food Sci. Technol. 2022, 153, 112455. [Google Scholar] [CrossRef]
- Kanner, J. Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants 2020, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Karcheva-Bahchevanska, D.; Lukova, P.; Nikolova, M.M.; Mladenov, R.D.; Iliev, I.N. Effect of Extracts of Bilberries (Vaccinium myrtillus L.) on Amyloglucosidase and -Glucosidase Activity. Folia Med. 2017, 59, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Može, S.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and Blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhang, W.; Tian, H.; Li, R.; Huang, S.; Li, X.; Qi, G.; Liu, X. EGCG Evokes Nrf2 Nuclear Translocation and Dampens PTP1B Expression to Ameliorate Metabolic Misalignment under Insulin Resistance Condition. Food Funct. 2018, 9, 1510–1523. [Google Scholar] [CrossRef]
- Prince, P.D.; Rodriguez Lanzi, C.; Fraga, C.G.; Galleano, M. Dietary (-)-Epicatechin Affects NF-kB Activation and NADPH Oxidases in the Kidney Cortex of High-Fructose-Fed Rats. Food Funct. 2019, 10, 26–32. [Google Scholar] [CrossRef]
- Karppinen, K.; Hirvelä, E.; Nevala, T.; Sipari, N.; Suokas, M.; Jaakola, L. Changes in the Abscisic Acid Levels and Related Gene Expression during Fruit Development and Ripening in Bilberry (Vaccinium myrtillus L.). Phytochemistry 2013, 95, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Qa’dan, F.; Verspohl, E.J.; Nahrstedt, A.; Petereit, F.; Matalka, K.Z. Cinchonain Ib Isolated from Eriobotrya japonica Induces Insulin Secretion In Vitro and In Vivo. J. Ethnopharmacol. 2009, 124, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a Source of Bioactive Compounds for the Treatment of Obesity, Type 2 Diabetes and Chronic Inflammation. J. Funct. Foods. 2017, 30, 16–29. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Long, J.; Guan, P.; Hu, X.; Yang, L.; He, L.; Lin, Q.; Luo, F.; Li, J.; He, X.; Du, Z.; et al. Natural Polyphenols as Targeted Modulators in Colon Cancer: Molecular Mechanisms and Applications. Front. Immunol. 2021, 12, 635484. [Google Scholar] [CrossRef]
- Bordiga, M.; Montella, R.; Travaglia, F.; Arlorio, M.; Coisson, J.D. Characterization of Polyphenolic and Oligosaccharidic Fractions Extracted from Grape Seeds Followed by the Evaluation of Prebiotic Activity Related to Oligosaccharides. Int. J. Food Sci. Technol. 2019, 54, 1283–1291. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of Polysaccharides in Food, Digestion, and Health. Crit. Rev. Food Sci. Nutr. 2015, 57, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-Amylase and α-Glucosidase: Potential Linkage for Whole Cereal Foods on Prevention of Hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Benalla, W.; Bellahcen, S. Antidiabetic Medicinal Plants as a Source of Alpha Glucosidase Inhibitors. Curr. Diab. Rep. 2010, 6, 247–254. [Google Scholar] [CrossRef]
- Reyes-Caudillo, E.; Tecante, A.; Valdivia-López, M.A. Dietary Fibre Content and Antioxidant Activity of Phenolic Compounds Present in Mexican Chia (Salvia hispanica L.) Seeds. Food Chem. 2008, 107, 656–663. [Google Scholar] [CrossRef]
- Kulczynski, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michalowska, A. The Chemical Composition and Nutritional Value of Chia Seeds—Current State of Knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef]
- Motyka, S.; Koc, K.; Ekiert, H.; Blicharska, E.; Czarnek, K.; Szopa, A. The Current State of Knowledge on Salvia hispanica and Salviae hispanicae semen (Chia Seeds). Molecules 2022, 27, 1207. [Google Scholar] [CrossRef]
- Knez Hrnčič, M.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia hispanica L.): An Overview-Phytochemical Profile, Isolation Methods, and Application. Molecules 2019, 25, 11. [Google Scholar] [CrossRef]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of Synergistic Polyphenol Combinations Using Biopolymer-Based Systems: Advances in Physicochemical Properties, Stability and Bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar] [CrossRef]
- Lavelli, V.; Sri Harsha, P.S.C.; Ferranti, P.; Scarafoni, A.; Lametti, S. Grape Skin Phenolics as Inhibitors of Mammalian α-Glucosidase and α-Amylase—Effect of Food Matrix and Processing on Efficacy. Food Funct. 2016, 7, 1655. [Google Scholar] [CrossRef] [PubMed]
- Saponjac, V.T.; Cetkovic, G.; Canadanovic-Brunet, J.; Pajin, B.; Djilas, S.; Petrovic, J.; Loncarevic, I.; Stajcic, S.; Vulic, J. Sour cherry Pomace Extract Encapsulated in Whey and Soy Proteins: Incorporation in Cookies. Food Chem. 2016, 207, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, L.; Craciunescu, O.; Balan, M.; Gaspar, A.; Gherghina, E. The Purification, Physico-Chemical Characterization and Bioactivity of Polysaccharides from Viscum album. Rev. Chim. 2008, 59, 1022–1025. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Prelipcean, A.M.; Mihai, E.; Ciucan, T.; Anton, R.E.; Popescu, A.F.; Tomescu, J.; Craciunescu, O. Free Radicals Scavenging, Acetylhydrolase Inhibition and Antimicrobial Activity of Aromatic Plant Hydrolate Mixtures. J. Food Measur. Charact. 2022, 16, 3589–3596. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of Antioxidant and Cytoprotective Activities of Arnica Montana L. and Artemisia Absinthium L. Ethanolic Extracts. Chem. Cent. J. 2012, 6, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory Potential of Herb, Fruit, and Fungal-Enriched Cheese Against Key Enzymes Linked to Type 2 Diabetes and Hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Celep, E.; Charehsaz, M.; Akyuz, S.; Acar, E.T.; Yesilada, E. Effect of In Vitro Gastrointestinal Digestion on the Bioavailability of Phenolic Components and the Antioxidant Potentials of Some Turkish Fruit Wines. Food Res. Int. 2015, 78, 209–215. [Google Scholar] [CrossRef]
- Voicescu, M.; Craciunescu, O.; Angelescu, D.G.; Tatia, R.; Moldovan, L. Spectroscopic, Molecular Dynamics Simulation and Biological Studies of Flavin Mononucleotide and Flavin Adenine Dinucleotide in Biomimetic Systems. Spectrochim Acta A Molec. Biomolec. Spectrosc. 2021, 246, 118997. [Google Scholar] [CrossRef]
- Iosageanu, A.; Ilie, D.; Craciunescu, O.; Seciu-Grama, A.M.; Oancea, A.; Zarnescu, O.; Moraru, I.; Oancea, F. Effect of Fish Bone Bioactive Peptides on Oxidative, Inflammatory and Pigmentation Processes Triggered by UVB Irradiation in Skin Cells. Molecules 2021, 26, 2691. [Google Scholar] [CrossRef]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol Content and In Vitro Evaluation of Antioxidant, Antimicrobial and Prebiotic Properties of Red Fruit Extracts. Eur. Food Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Tagliani, C.; Perez, C.; Curutchet, A.; Arcia, P.; Cozzano, S. Blueberry Pomace, Valorization of an Industry by-Product Source of Fibre with Antioxidant Capacity. Food Sci. Technol. 2019, 39, 644–651. [Google Scholar] [CrossRef]
- Loncaric, A.; Celeiro, M.; Jozinovic, A.; Jelinic, J.; Kovac, T.; Jokic, S.; Babic, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Jenkins, A.L.; Brissette, C.; Choleva, L.; Jovanovski, E.; Gibbs, A.L.; Bazinet, R.P.; Au-Yeung, F.; Zurbau, A.; Ho, H.V.T.; et al. Salba-Chia (Salvia hispanica L.) in the Treatment of Overweight and Obese Patients with Type 2 Diabetes: A Double-Blind Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ogunsuyi, O.B.; Ogunbadejo, M.D.; Adefegha, S.A. Influence of Gallic Acid on α-Amylase and α-Glucosidase Inhibitory Properties of Acarbose. J. Food Drug Anal. 2016, 24, 627–634. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of Porcine Pancreatic α-Amylase Activity by Chlorogenic Acid. J. Funct. Food. 2020, 64, 103587. [Google Scholar] [CrossRef]
- Riyaphan, J.; Pham, D.C.; Leong, M.K.; Weng, C.F. In Silico Approaches to Identify Polyphenol Compounds as α-Glucosidase and α-Amylase Inhibitors Against Type-II Diabetes. Biomolecules 2021, 11, 1877. [Google Scholar] [CrossRef]
- Xu, W.; Shao, R.; Xiao, J.B. Is There Consistency between the Binding Affinity and Inhibitory Potential of Natural Polyphenols as Alpha-Amylase Inhibitors? Crit. Rev. Food Sci. Nutr. 2016, 56, 1630–1639. [Google Scholar] [CrossRef]
- Xiao, J.; Hogger, P. Dietary Polyphenols and Type 2 Diabetes: Current Insights and Future Perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Sieminska-Kuczer, A.; Szymanska-Chargot, M.; Zdunek, A. Recent Advances in Interactions between Polyphenols and Plant Cell Wall Polysaccharides as Studied using an Adsorption Technique. Food Chem. 2022, 373, 131487. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; de la Rosa, L.A.; Alvarez-Parrilla, E. Effect of Pectin on the Interactions Among Phenolic Compounds Determined by Antioxidant Capacity. J. Mol. Struct. 2020, 1199, 126967. [Google Scholar] [CrossRef]
- Piechowiak, T.; Skora, B.; Grzelak-Blaszczyk, K.; Sojka, M. Extraction of Antioxidant Compounds from Blueberry Fruit Waste and Evaluation of Their In Vitro Biological Activity in Human Keratinocytes (HaCaT). Food Anal. Methods. 2021, 14, 2317–2327. [Google Scholar] [CrossRef]
- Vuong, T.; Matar, C.; Ramassamy, C.; Haddad, P.S. Biotransformed Blueberry Juice Protects Neurons from hydrogen Peroxide-Induced Oxidative Stress and Mitogen-Activated Protein Kinase Pathway Alterations. Br. J. Nutr. 2010, 104, 656–663. [Google Scholar] [CrossRef]
- Sanchez-Velazquez, O.A.; Mulero, M.; Cuevas-Rodriguez, E.O.; Mondor, M.; Arcand, Y.; Hernandez-Alvarez, A.J. In Vitro Gastrointestinal Digestion Impact on Stability, Bioaccessibility and Antioxidant Activity of Polyphenols from Wild and Commercial Blackberries (Rubus spp.). Food Funct. 2021, 12, 7358–7378. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Kalariya, H.M.; Poulev, A.; Ribnicky, D.M.; Jaja-Chimedza, A.; Roopchand, D.E.; Raskin, I. Grape polyphenols Reduce Gut-Localized Reactive Oxygen Species Associated with the Development of Metabolic Syndrome in Mice. PLoS ONE 2018, 13, e0198716. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry Polyphenols and Human Health: Evidence of Antioxidant, Anti-Inflammatory, Microbiota Modulation, and Cell-Protecting Effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant Activity of High Purity Blueberry Anthocyanins and the Effects on Human Intestinal Microbiota. LWT 2020, 117, 108621. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry Polyphenols Extract as a Potential Prebiotic with Anti-Obesity Effects on C57BL/6 J Mice by Modulating the Gut Microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Westfall, S.; Lomis, N.; Prakash, S. A Novel Polyphenolic Prebiotic and Probiotic Formulation Have Synergistic Effects on the Gut Microbiota Influencing Drosophila melanogaster Physiology. Artif. Cells Nanomed. Biotechnol. 2018, 46, 441–455. [Google Scholar] [CrossRef]
- Semwal, A.; Ambatipudi, K.; Navani, N. Development and Characterization of Sodium Caseinate Based Probiotic Edible Film with Chia Mucilage as a Protectant for the Safe Delivery of Probiotics in Functional Bakery. Food Hydrocoll. Health 2022, 2, 100065. [Google Scholar] [CrossRef]




| Sample | Total Phenolic Content (g GAE/100 g Dry Weight) | Total Flavonoids Content (g QE/100 g Dry Weight) | Total Carbohydrates Content (g/100 g Dry Weight) |
|---|---|---|---|
| BP | 30.40 ± 1.28 | 1.89 ± 0.07 | - |
| CS | 3.93 ± 0.15 | 0.72 ± 0.03 | 70.78 ± 4.41 |
| Compound | Quantity (mg/100 g Dry Weight) |
|---|---|
| Gallic acid | 5043.27 ± 181.03 |
| Chlorogenic acid | 1509.41 ± 61.37 |
| Caffeic acid | 200.60 ± 9.45 |
| p-Coumaric acid | 251.85 ± 8.75 |
| Ferulic acid | 118.14 ± 5.18 |
| Rutin | 609.78 ± 23.26 |
| Luteolin 7-glucoside | 262.11 ± 12.91 |
| Kaempferol 3-glucoside | 161.46 ± 6.76 |
| Myricetin | 175.18 ± 7.22 |
| Resveratrol | 103.43 ± 4.69 |
| Quercetin | 316.46 ± 11.84 |
| Histogram | Sample | ROS Production (%) |
|---|---|---|
| B | Oxidative-stressed group | 100.00 ± 4.03 |
| C | PS-BP | 46.70 ± 2.24 *,# |
| D | PS-BCM | 41.43 ± 1.56 *,# |
| E | PC-BP | 36.41 ± 2.13 *,# |
| F | PC-BCM | 34.86 ± 1.06 * |
| G | Ascorbic acid (control) | 30.16 ± 2.12 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, E.; Negreanu-Pirjol, B.-S.; Craciunescu, O.; Ciucan, T.; Iosageanu, A.; Seciu-Grama, A.-M.; Prelipcean, A.-M.; Utoiu, E.; Coroiu, V.; Ghenea, A.-M.; et al. In Vitro Hypoglycemic Potential, Antioxidant and Prebiotic Activity after Simulated Digestion of Combined Blueberry Pomace and Chia Seed Extracts. Processes 2023, 11, 1025. https://doi.org/10.3390/pr11041025
Mihai E, Negreanu-Pirjol B-S, Craciunescu O, Ciucan T, Iosageanu A, Seciu-Grama A-M, Prelipcean A-M, Utoiu E, Coroiu V, Ghenea A-M, et al. In Vitro Hypoglycemic Potential, Antioxidant and Prebiotic Activity after Simulated Digestion of Combined Blueberry Pomace and Chia Seed Extracts. Processes. 2023; 11(4):1025. https://doi.org/10.3390/pr11041025
Chicago/Turabian StyleMihai, Elena, Bogdan-Stefan Negreanu-Pirjol, Oana Craciunescu, Teodora Ciucan, Andreea Iosageanu, Ana-Maria Seciu-Grama, Ana-Maria Prelipcean, Elena Utoiu, Viorica Coroiu, Ana-Maria Ghenea, and et al. 2023. "In Vitro Hypoglycemic Potential, Antioxidant and Prebiotic Activity after Simulated Digestion of Combined Blueberry Pomace and Chia Seed Extracts" Processes 11, no. 4: 1025. https://doi.org/10.3390/pr11041025
APA StyleMihai, E., Negreanu-Pirjol, B.-S., Craciunescu, O., Ciucan, T., Iosageanu, A., Seciu-Grama, A.-M., Prelipcean, A.-M., Utoiu, E., Coroiu, V., Ghenea, A.-M., & Negreanu-Pirjol, T. (2023). In Vitro Hypoglycemic Potential, Antioxidant and Prebiotic Activity after Simulated Digestion of Combined Blueberry Pomace and Chia Seed Extracts. Processes, 11(4), 1025. https://doi.org/10.3390/pr11041025






