Abstract
UV-C irradiation and high hydrostatic pressure (HHP) successfully reduce the number of bacteria and their growth but can also affect phenolic and sugar content, as well as other physicochemical properties. Therefore, in this work, the effect of UV-C irradiation, HHP, and their combination, UV-C/HHP, on total aerobic mesophilic bacteria count (TAMBC), chlorogenic acid and sugar content, and other physicochemical properties of raw FCP were examined. Acrylamide and polycyclic aromatic hydrocarbons (PAH) were also monitored in treated FCP after frying. Vacuum-packed potato slices pretreated with an antibrowning agent were irradiated with UV-C (2.70 kJ m−2), treated with HHP (400 MPa/3 min) and combined UV-C/HHP, and stored for 15 days. The greatest reduction in TAMBC was achieved in the UV-C/HHP-treated samples, followed by the HHP treatment, and they both resulted in the slowest bacterial growth during storage. All treatments decreased the contents of chlorogenic acid, but the greatest reduction was observed in the HHP-treated samples. All treatments increased the content of reducing sugars, and UV-C/HHP did so significantly, which also led to an increase in acrylamide content in the fried FCP. PAH levels were below the established limits. Acceptable sensory attributes of all samples (raw, boiled, and fried) remained relatively stable during storage.
1. Introduction
Damage of the tissue during minimal processing of fruits and vegetables creates favorable conditions for the growth of microorganisms and increased enzyme activity, which, together with other undesirable changes, leads to a deterioration in the quality and safety of the product. Consequently, the mentioned changes shorten the shelf life of the products. The application of UV-C irradiation and high hydrostatic pressure (HHP) to fruits and vegetables has been shown to have a germicidal effect [1,2,3]. In potatoes, UV-C can significantly slow the growth of total aerobic mesophilic bacteria during storage when applied to potato tubers and slices [1,4], while application of HHP can delay microbial growth during storage or completely inactivate Enterobacteriaceae [3,5]. UV light affects the structure of DNA, which prevents cell replication [6,7,8], and its effectiveness depends on the applied intensity and irradiation time, among other factors. HHP has a complex effect; inter alia, it affects the denaturation of proteins and the destruction of ribosomes, which affects the structural organization of the cell and metabolic processes [9,10,11]. Its effectiveness depends on the pressure applied, the duration and temperature of the treatment, the properties of the suspension media used, and intrinsic factors such as pH, water activity, microorganism-related factors or the type of enzymes [3,12,13]. In addition, other factors may affect the efficacy of these methods, such as the characteristics of the plant material, packaging, or the antibrowning agent used.
Among the undesirable changes caused by minimal processing of potatoes is enzymatic browning. Polyphenol oxidase (PPO) enzymes catalyze the hydroxylation of monophenols to o-diphenols and oxidation to o-quinone that polymerize to dark melanin pigment [14,15]. Reducing enzyme activity can prevent browning of fresh-cut potatoes (FCP). Furthermore, the wound-induced phenylalanine ammonia-lyase (PAL) enzyme catalyzes the synthesis of phenolics, which serve as substrates for PPO and peroxidase (POD). Phenolics are not only involved in browning processes but also form an important group of compounds in potatoes due to their antioxidant properties. The most abundant phenolic component in potatoes is chlorogenic acid [16]. It has numerous beneficial effects on health, such as an anti-inflammatory effect or the prevention of cancer, cardiovascular disease, and diabetes [17,18,19]. In addition, the chlorogenic acid from potatoes can lead to an increase in insulin sensitivity and a decrease in glucose uptake in the intestine [17,20]. According to previous studies, the application of UV-C light and HHP treatment to peeled potato tubers and slices can reduce the content of chlorogenic acid [3,21].
Research on the effects of UV-C light are relatively scarce, and most of it is based on studying the effects on weight loss, germination, rot resistance, or changes in sugar content of potato tubers during storage. Reducing sugars (fructose and glucose), along with asparagine, are precursors of the probably carcinogenic acrylamide. Therefore, increasing their content in raw potatoes can potentially increase the acrylamide content in fried potatoes. Acrylamide is formed by Maillard reactions during frying at temperatures above 120 °C, and the maximum permitted level for potato products set by EU Commission Regulation 2017/2158 is 750 µg kg−1 [22]. UV-C treatment of potato tubers may increase acrylamide content in fried potatoes [23] or increase reducing sugars and acrylamide content in irradiated potato slices, depending on the irradiation dose applied [21]. As for the effect of HHP on potato sticks, no significant effect on acrylamide content was found [24].
In addition to acrylamide, polycyclic aromatic hydrocarbons (PAH) are another important group of toxic chemicals to monitor in fried potatoes. PAH are known for their genotoxicity and mutagenicity, which is particularly attributed to PAH heavy fraction with more than four aromatic rings [25]. Balbino et al. (2020) [26] determined the PAH content in fried fresh-cut potato samples and found levels of benzo(a)pyrene and ΣPAH4 of 0.62 and 1.36 µg kg−1, respectively, both below the limits established in EU Regulation 835/2011 [27]. Environmental contamination of potatoes was identified as the most likely source of PAH in fried potatoes.
By monitoring the changes in the color parameters L*, a*, and b*, possible enzymatic browning of fresh-cut potatoes (FCP) is evaluated as an indicator of the effectiveness of the applied UV-C and HHP treatment. According to previous studies, the application of UV-C light can increase the brightness (L*) of raw FCP and preserve the sensory properties of subsequently thermally treated FCP [1]. HHP can have a negative effect on color in terms of browning, leading to lower L* but also to increased PPO activity [28], or on the contrary, to a complete inactivation of PPO [29]. It is important to point out that there are numerous factors that affect the final result, and it is necessary to adapt the overall treatment conditions to a particular product.
The available scientific data on the influence of UV-C and HHP on the physicochemical and sensory properties of FCP are relatively modest. To our knowledge, the synergistic effect of UV-C and HHP on fresh-cut potatoes has not been studied so far. Considering the promising results of our previous studies [1,5,21], the aim of this study was to investigate which treatment, UV-C light, HHP, or the combined UV-C/HHP, could achieve the desirable quality and safety of FCP in terms of microbiological stability, physicochemical parameters, chlorogenic acid and sugar content, and sensory attributes in raw samples, as well as sensory attributes in subsequently boiled and fried FCP and also acrylamide and PAH in fried samples.
2. Materials and Methods
2.1. Chemicals and Standards
Standards of acrylamide (>99%), chlorogenic acid, fructose (≥99% GC), D-(+)-glucose (≥99.5% GC), and D-(+)-sucrose (≥99.5% GC) where purchased from Sigma-Aldrich (Steinheim, Germany), as well as solvents: formic acid, n-hexane, acetonitrile (HPLC-grade), and methanol (HPLC-grade). The QueChERS salt packet (4 g MgSO4 and 0.5 g NaCl) and QueChERS d-SPE salts (150 mg MgSO4 and 50 mg PSA) were purchased from Agilent Technologies (Santa Clara, CA, USA). The water was of Milli-Q quality (Millipore Corp., Bedford, MA, USA). Isopropyl alcohol, acetonitrile, and ethyl acetate were provided by T.J. Parker (Deventer, The Netherlands) and were of HPLC grade. Mixture of PAH at various concentrations in methylene chloride, methanol (1:1), were purchased from Supelco (Bellefonte, PA, USA). This standard mixture contains the 16 EPA PAH; however, not all 16 EPA PAH can be detected with this method.
2.2. Plant Material, Fresh-Cut Sample Preparation and Further Handling
Potato (Solanum tuberosum L.) tubers of cv. Birgit grown in 2019 in the Slavonia region (Croatia) were used for the experiment. Tubers were treated with Gro Stop Basis and Gro Stop Fog sprout inhibitors (Certis Europe, Great Abington, UK) and stored in wooden pallet boxes in the dark for 4 months (8 °C/RH approx. 100%) in a warehouse specialized for potatoes, where, as regular procedure, they were kept at 16 °C for 5 days before processing.
Undamaged and uniform potato tubers were selected for fresh-cut processing. Following the procedure described by Dite Hunjek et al. (2020) [30], tubers were washed with tap water, drained, peeled by hand, and sliced (0.4 cm) using a commercial slicer. After cutting, potatoes were dipped for 3 min at room temperature in a sodium ascorbate solution (2%, m/V). The drained potato samples (4–6 slices) were vacuum-packed(SmartVac SV 750; Status, Metlika, Slovenia) in a single layer in a polyamide/polyethylene (PA/PE) vacuum bag (Status, Metlika, Slovenia).
The samples prepared in this way were divided into (1) samples that were not to be subjected to further treatment (control); (2) samples for UV-C treatment (UV-C); (3) samples for high hydrostatic pressure (HHP) treatment; and (4) samples for combined UV-C and HHP (UV-C/HHP) treatment. The control samples were immediately stored at 6 °C in the refrigerator until further processing or analysis, while UV-C, HHP, and UV-C/HHP samples were subjected to irradiation and/or pressure treatments. After the treatments, these samples were stored in the refrigerator at 6 °C, like the control samples. On day 0, 8, 11, and 15 of storage, the treated and untreated samples were boiled or fried and analyzed together with the raw treated and untreated samples. On the mentioned storage days, the raw samples were analyzed for pH, total solids, total soluble solids, firmness, and color (instrumentally), while sensory analysis was performed on raw, boiled, and fried samples. In addition, fried and raw samples were frozen at −60 °C/24 h, freeze-dried (CoolSafe PRO, Labogene, Denmark), homogenized by grinding, and stored at −20 °C until further analysis of phenolics and sugars in raw and acrylamide and polycyclic aromatic hydrocarbons (PAH) in fried FCP.
2.3. UV-C, HHP, and Combined UV-C/HHP Treatment
UV-C irradiation of FCP was performed in a UV-C chamber (UVpro EKB 100; Orca GmbH, Kürten, Germany) equipped with 4 UV-C lamps (4 × HNSL 24W, maximum emission at 253.7 nm, UVpro), according to the procedure described by Pelaić et al. (2021) [31]. The vacuum-packed FCP samples were placed on the perforated shelf and irradiated for 5 min to obtain a dose of 2.70 kJ m−2. This dose was chosen based on our previous research [1,21], primarily for its germicidal effect but also for the tested properties of FCP treated in this way.
HHP treatment of the vacuum-packed FCP was performed in a device from Stansted Fluid Power LTD (Stanford, UK) at a pressure of 400 MPa, with a pressure holding time of 3 min and a pressure fluid temperature of 25 °C. The compression rate was 10 MPa/s, and the decompression rate was 50 MPa/s. Propylene glycol with water (1:1) was used as a pressure fluid. These conditions were chosen after experiments in our laboratory [5], with the specific difference being the packaging of the treated samples. Instead of dipping the potato slices in a sodium ascorbate solution and pressing them, the slices were previously vacuum-packed without liquid and then pressed.
The combined UV-C/HHP treatment was performed such that the samples were first irradiated with UV-C for 5 min and then subjected to the HHP treatment at a pressure of 400 MPa, with a pressure holding time of 3 min and a pressure fluid temperature of 25 °C.
2.4. Microbiological Analysis
Total aerobic mesophilic bacteria count (TAMBC) was determined by the Horizontal method—Colony count technique at 30 °C (HRN EN ISO 4833-1:2013) [32]. Dilutions (in peptone water, 0.1%, w/v) were applied as surface smears (1 mL) on plate count agar (Biolife, Milan, Italy) and incubated at 30 ± 1 °C for 72 ± 3 h in a drying oven (FN -500, Nüve, Ankara, Turkey). Analyses were performed on raw samples in duplicate (n = 2), and results are expressed in log CFU g−1 as mean ± standard error (SE).
2.5. Determination of Total Solids, Total Soluble Solids, and pH
Homogenized raw potato slices (Bosch MSM89160 blender, Robert Bosch GmbH, Gerlingen-Schillerhöhe, Germany) were used for determination of total solids (TS), total soluble solids (TSS), and pH. Potato samples were dried at 103 ± 2 °C (FN -500, Nüve) to a constant mass (AOAC, 1990), and TS was calculated as a percentage of the mass ratio before and after drying. The TSS were determined in homogenized FCP at 20 °C using a digital refractometer (DR201-95, A. Krüss Optronic GmbH, Hamburg, Germany) and expressed as °Brix. The pH was determined using a pH meter (SevenEasy pH Meter S20, Mettler Toledo, Greifensee, Switzerland). All measurements were performed in duplicate (n = 2), and results are expressed as mean ± (SE).
2.6. Firmness Analysis
The firmness analysis was performed on raw FCP samples using the TA.HD.plus Texture Analyser (StableMicro Systems, Godalming, UK) with a 5 kg load cell and a 2 mm stainless steel cylinder penetration probe. The pretest speed was 1 mm/s, and the test speed was 0.5 mm/s. Measurements were performed using Exponent Stable Micro System software v 6.1.18. in triplicate (n = 3), and results are expressed as mean (N) ± SE.
2.7. Color Analysis
The color of the raw samples was determined using a colorimeter (Spectrophotometer CM -3500d, Konica Minolta, Tokyo, Japan) equipped with a D65 light source with a 2° angular observer and a measuring plate with a 30 mm diameter hole. Measurements were performed on six slices (n = 6), and the CIELAB color parameters L* (brightness), a* (red/green), and b* (yellow/blue) were determined. Results are expressed as mean ± SE.
2.8. Cooking Treatments
Boiling of FCP was performed in water (m(water):m(sample) = 5:1) at 100 °C/15 min and frying in sunflower oil (V(oil):m(sample)) = 1.0 L:120 g) at an initial temperature of 180 °C/5 min. Excess water or oil from the potatoes prepared in this way was removed with a paper towel. Samples prepared in this way were used for sensory evaluation and additionally fried for acrylamide and PAH analyses.
2.9. Analysis of Phenolics
2.9.1. Extraction of Phenolics
The extraction method previously described by Dite Hunjek et al. (2020) [31] was used for phenolics extraction. Briefly, 0.5 g of homogenized freeze-dried samples was extracted with 5 mL of 80% methanol with 1% formic acid (v/v) in an ultrasonic bath (Elmasonic 40H, Elma, Germany) at 50 °C/30 min and centrifuged at 3000 rpm/10 min (Hettich® Rotofix 32a, Tuttlingen, Germany). Extraction solvent (5 mL) was added to precipitate, and the procedure was repeated. Such obtained supernatants were combined in a 10 mL flask and made up with extraction solvent, filtered into vials (0.45 µm membrane filter, Macherey-Nagel GmbH & Co. KG, Düren, Germany), and stored at −20 °C until UPLC MS2 analysis. Extractions were performed in duplicate (n = 2).
2.9.2. UPLC MS2 Analysis of Phenolics
An Agilent 1290 series RRLC instrument with a triple quadrupole mass spectrometer (6430) (Agilent Technologies, Santa Clara, CA, USA) was used for UPLC MS2 analysis. Zorbax Eclipse Plus C18 column (100 × 2.1 mm, 1.8 µm) (Agilent Technologies) was used for separation. The analysis was performed according to the previously described conditions and instrument settings by Elez Garofulić et al. (2018) [33]. Column temperature was 35 °C, the injection volume was 2.5 µL, and the flow rate was 0.3 mL min−1. Eluent A was 0.1% formic acid (v/v) and eluent B 0.1% formic acid in acetonitrile (v/v). Ionization was performed by electrospray (ESI) in negative and positive mode (m/z 100–1000), and the ionization source parameters were capillary voltage of +4000/−3500 V, nitrogen temperature of 300 °C/flow rate 11 L h−1, and nebulizer pressure of 40 psi. Data acquisition was performed in dynamic multiple reaction monitoring (dMRM) mode. Since in our previous study the other phenolic components were below the LOQ or not detected, in this study, we continued to only observe the changes in chlorogenic acid as the most abundant phenolic component in potatoes. Chlorogenic acid was identified using the retention time and mass spectra of the chlorogenic acid standard and quantified using a calibration curve obtained from the standard. Analytics parameters were as described in our previous study [21]. Results are expressed in mg 100 g−1 of dry weight (DW) as mean ± SE.
2.10. Sugar Analyses
2.10.1. Extraction of Sugars
Sugars were extracted according to the method described by Dite Hunjek et al. (2020) [31]. Homogenized freeze-dried samples (0.4 g) were mixed with 4 mL of 80% methanol (v/v), vortex homogenized, thermostated in a water bath at 60 °C/60 min, and centrifuged at 6000 rpm/15 min. The supernatant obtained was filtered into a 5 mL flask and made up with extraction solvent. The extracts were filtered through a 0.45 µm membrane filter into vials and stored at +4 °C until the analysis. Extractions were performed in duplicate (n = 2).
2.10.2. HPLC Analysis of Sugars
The determination of sugars (fructose, glucose, and sucrose) was performed as previously described by Dite Hunjek et al. (2020) [31] using an Agilent 1260 Infinity quaternary LC system (Agilent Technologies) equipped with a refractive index detector (RID). Cosmosil Sugar-D, 5 µm, 250 × 4.6 mm I.D. column (Nacalai Tesque, Inc., Kyoto, Japan) was used to separate compounds. Briefly, 80% acetonitrile (v/v) was used as the mobile phase in isocratic elution mode, and the chromatographic conditions were flow rate of 1.3 mL min−1, injection volume of 10 µL, and column temperature of 45 °C. Sugars were identify by comparing retention times with those of standard solutions, while a fixed concentration of each sugar standard was used for quantification. Results are expressed in g 100 g−1 DW as mean ± SE.
2.11. Acrylamide Analysis
2.11.1. Extraction of Acrylamide
Acrylamide was extracted from homogenized, freeze-dried, and fried FCP according to the method described in our previous study [21]. To 1 g of sample, 5 mL of n-hexane was added, followed by 10 mL of water and 10 mL of acetonitrile, and shaken at vortex for 3 min. The QueChERS salt packet was added, shaken strongly for 1 min, and centrifuged at 5000 rpm/5 min. After centrifugation and discarding the hexane layer, 1 mL of the acetonitrile layer was transferred to a 2 mL vial containing QueChERS d-SPE salts. After homogenization by vortex and centrifugation at 5000 rpm/1 min, 0.5 mL of the supernatant was transferred to vials and analyzed by UPLC MS2. Extractions were performed in duplicate (n = 2).
2.11.2. UPLC MS2 Analysis of Acrylamide
UPLC MS2 analysis of acrylamide was performed using an Agilent UPLC system (Section 2.9.2). A Hypercarb TM column (5 µm, 50 mm × 2.1 mm) with a guard column (5 µm, 10 mm × 2 mm) (Thermo Hypersil-Keystone, Bellefonte, PA, USA) was used for separation. Chromatographic conditions and instrument settings were as previously described by Dite Hunjek et al. (2020) [31]. The temperature of the column was set at 22 °C, the injection volume was 10 µL, and the flow rate was 0.7 mL min−1, and the mobile phase was 10% methanol with 0.1% formic acid. Electrospray (ESI) in positive ion mode was used for ionization. Acrylamide from the sample extracts was identified by comparing the peak ratios of the MRM transitions m/z 72→55.1 with those of the acrylamide standard. The calibration curve obtained from the extracted acrylamide standard solution was used for quantification, and results are expressed in µg kg−1 DW. The analytical parameters were as described in our previous study [21].
2.12. Polycyclic Aromatic Hydrocarbons (PAH) Analysis
Freeze-dried and subsequently fried FCP samples were extracted with acetonitrile and QuEChERS extraction salts, and supernatant was separated by centrifugation. The extract was purified with another type of QuEChERS salt, centrifuged, and transferred to a vial. PAH compounds were determined by the gas chromatography (GC) method coupled by mass spectrometer detection (GCMS/MS Thermo Scientific: Trace 1300 GC and TSQ 8000 Evo MS). GC analysis was carried out according to a temperature program with an initial temperature of 60 °C. Temperature was then increased from 35 °C/min to 160 °C, 3.5 °C/min to 260 °C, and 15 °C/min to 290 °C, where it was kept for 15 min. Separation was made on a TG -5SILMS W/5m Safeguard (Thermo Scientific, Waltham, MA, USA) column (30 m × 0.25 mm × 0.25 µm). The injector, electron source, and detector were set at temperatures of 310 °C, 320 °C, and 250 °C, respectively. Individual PAH compounds were identified based on qualifier ions (m/z): acenaphthylene (152.1; 151.1); acenaphthene (153.1; 152.1); fluorene (165.1; 164.1); phenanthrene (178.1; 152.1); anthracene (178.10; 152.1); fluoranthene (202.1; 200.1); pyrene (202.10; 201.10); benzo[a]anthracene (228.1; 226.1); crisis (228.1; 226.1); benzo(b)fluoranthene (250.1; 252.1); benzo(k)fluoranthene (250.1; 252.1); benzo(a)pyrene (250.1; 252.1); indeno(1,2,3-c, d)pyrene (276.1; 274.0); dibenzo(a, h)anthracene (278.1; 276.0); and benzo(g, h, i)perylene (276.1; 274.1). They were quantified through their calibration curves. Results are expressed in µg kg−1 DW as mean ± SE.
2.13. Sensory Monitoring
Sensory monitoring was performed according to the procedure described by Dite Hunjek et al. (2020) [30]. Quantitative Descriptive Analysis (QDA) of raw, boiled, and fried potato samples was performed at room temperature (20 °C) by a sensory panel consisting of a group of 6 trained people, following the procedure of ISO (1985, 2012) [34,35]. Quality was assessed using a standard scale from 0 (lowest score) to 5 (highest score). Color, i.e., browning intensity of raw and boiled FCP, was scored as follows: 0—no browning and 5—complete browning. The color of the fry is described as characteristic color. Odor and off-odor intensity were rated from 0—absent to 5—very pronounced, moistness from 0—very dry to 5—very wet, and firmness from 0—very soft to 5—very firm. For boiled and fried FCP, characteristic taste was evaluated and rated from 1—absent to 5—very pronounced. Oiliness and crispness (fried potato) were rated from 0—absent to 5—very pronounced, while creaminess (boiled potato) was scored from 0—absence of creamy texture to 5—melting in the mouth. The results (n = 7) are statistically analyzed and displayed graphically.
2.14. Statistical Analysis
The obtained experimental data were analyzed using XLSTAT ver. 2020.5.1 software (Addisoft, Paris, France). The influence of treatment (UV-C, HHP, and UV-C/HHP) and storage time (0, 8, 11, and 15 days) on TAMBC, chlorogenic acid and sugar content, pH, SS, TSS, firmness and color parameters of raw FCP, and acrylamide and PAH content in fried FCP were investigated. Analysis of variance (ANOVA) followed by Tukey’s HSD test was used for dependent variables examination and determination of effect of factors and differences between the applied factor levels. In the case of TAMBC, chlorogenic acid, reducing sugars, and acrylamide, factor interaction was also considered. Standard deviation of the sampling distribution for all analyzed samples taken in statistical processing is expressed as SE. Normality and homoscedasticity of the residuals were tested by Shapiro–Wilk and Levene’s test, respectively. In case the ANOVA assumptions were violated, adequate statistical analysis was applied. In the case of heteroscedastic variance, SE were calculated using the HC3 correction. In the case of normality violation, Kruskal–Wallis nonparametric test was used for dependent variables examination, followed by Dunn’s post hoc test. The mean of all results obtained for a given property is listed at the end of the tables as the grand mean. Principal Component Analysis (PCA) was conducted for the sensory attributes of the raw and thermally treated samples using principal components (PC) with eigenvalue >1, and variables with communalities ≥0.5 were included. The significance level for all tests was p ≤ 0.05.
3. Results and Discussion
3.1. Microbiological Analysis
All applied treatments significantly reduced TAMBC (p < 0.01) (Figure 1). The initial TAMBC of the control samples at the beginning of the storage (day 0) was 2.6 log CFU g−1. The observed reductions on the same day were 1.0, 1.1, and 1.4 log CFU g−1 for UV-C, HHP, and UV-C/HHP, respectively.
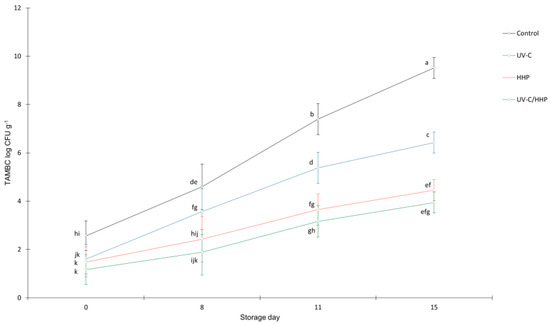
Figure 1.
Total aerobic mesophilic bacteria count (TAMBC) of untreated (control) and treated (UV-C, HHP, and UV-C/HHP) raw, fresh-cut potatoes during storage. Error bars represent standard errors; lowercase letters represent a significant difference within means (p < 0.05).
During the 15-day storage period, TAMBC increased in all samples, especially in the control (from 2.6 to 9.5 log CFU g−1), followed by UV-C. The increase in UV-C samples could be attributed to a strong repair mechanism—photoreactivation—as well as to replication of bacteria that remained functional after treatment [8,11]. Due to the rugged topography of FCP, some microorganisms may remain in the shade, so UV-C does not affect them. However, the slowest bacterial growth during the 15 days was observed in the HHP and UV-C/HHP samples, from 1.5 to 4.5 and from 1.2 to 3.9 log CFU g−1, respectively. The microbial inactivation caused by the treatments with HHP is probably related to the damage it causes to the structural organization of the cells, which can lead to damage of the cells, resulting in leakage of the cell contents and consequently cell death [13]. A reduction of 15% of TAMBC was found by Levaj et al. (2020) [5] by the same pressure conditions (400 MPa/3 min) applied on potato slices dipped in 2% sodium ascorbate solution. The greater efficacy of HHP in this study (reduction of 47%) may be due to the different sample preparation for HHP and further handling. In addition, Tsikrika et al. (2021) [3] also observed a significant reduction in aerobic bacterial counts when HHP (600) MPa was applied to peeled and vacuumed potato tubers. UV-C irradiation has also been shown to have an inhibitory effect on fresh-cut products [2,36]. In our previous study [1], UV-C treatment (2.70 kJ m−2) was as effective in slowing down bacterial growth during storage, as in this study.
The EC regulations [37,38] on microbiological criteria for food safety related to TAMBC do not provide any information for fresh-cut food intended for further preparation. According to the Croatian Agency for Agriculture and Food [39], the borderline level of TAMBC for ready-to-eat vacuum-packed and refrigerated vegetables is given as 6 ≤ 8 log CFU g−1. In the present study, TAMBC was below 6.5 log CFU g−1 in all samples, except the control sample in the 15th day with 9.52 log CFU g−1.
3.2. TS, TSS, pH, and Firmness Analysis
According to the statistical results, treatment had a significant effect on TS, TSS, and pH and storage on TSS and pH (Table 1) of raw FCP. Firmness was not affected by treatment or storage.

Table 1.
The influence of UV-C, HHP, and UV-C/HHP treatment and storage time on total solids content (TS), total soluble solids content (TSS), pH, and firmness of raw, fresh-cut potatoes.
The content of TS in the control FCP was 25.2%, which was slightly higher than in the previously studied potatoes of the same Birgit cultivar [1,30]. This difference is probably due to different conditions during growth but also to the different age of the potatoes. The application of HHP and HHP/UV-C treatments resulted in TS increase. HHP treatment increases cell membrane permeability, improves diffusion, and increases the mass transfer, resulting in water loss [40,41]. According to Douardo et al. [24], an equal or lower percentage of moisture was observed in HHP-treated potato samples (0.1, 100, 200, and 400 MPa/5 min) than in untreated samples. The same effect was reported by Tsikrika et al. [42] when FCP was subjected to HHP treatment (600 MPa/3 min), but it differed according to cultivar used. As mentioned earlier, in this study, TS content did not change significantly during storage.
The obtained mean value for TSS of the control sample was 3.90 °Bx, which is lower than the TSS obtained in our previous study [1]. Samples subjected to HHP and combined UV-C/HHP treatment showed a significantly increased content of TSS. This increase could be due to the cell damage caused by treatments, which subsequently leads to the release of sugars and other soluble solids from the cells [43,44]. Similar to our results, Douardo et al. (2020) [24] also found up to a five-fold increase in TSS in external potato water (400 MPa) when compared with the control. According to our results, the content of TSS increased during storage, which may be related to the starch breakdown into SS or cell wall hydrolysis [45].
The pH of the control samples was 5.60, similar to that found in our previous study (5.64) [1]. UV-C treatment slightly decreased pH, while HHP significantly increased it, as did UV-C/HHP. A decrease in pH in potato slices treated with UV-C (2.70 kJ m−2) was previously found by Pelaić et al. (2022) [1], where the value was similar to that in this work (5.59). A lower pH can have an effect on the inhibition of enzymes or create more unfavorable conditions for the growth of microorganisms, but in this way the browning processes can also be controlled [13]. Lu et al. (1991) [46] also observed a decrease in pH in UV-C-treated peaches and apples. As in this study, an increase in the pH of HHP-treated (200, 400, and 600 MPa/5, 15, and 25 min) spinach puree was also observed by Wang et al. (2012) [47]. During storage, the pH decreased until the 11th day, after which it was stable. A decrease in pH during storage has also been observed in vacuum-packed potatoes or potato slices [30,48]. It could be related to the increased respiration rate and CO2 production [49] which was found for vacuum-packed potatoes stored in refrigerators [50].
The grand mean value of raw FCP firmness was 7.14 N (Table 1). Although the applied treatments resulted in changes in TS and TSS and could damage tuber structure and cell walls, these changes did not significantly affect the measured potato firmness. According to our previous study [1], UV-C decreased the firmness of FCP, while similar observations as in the present study were reported by Levaj et al. (2020) [5] when potato slices were immersed in sodium ascorbate solution and treated with HHP (400 MPa). Douardo et al. (2020) [24] observed a decrease in firmness when potato sticks, immersed in either water or asparaginase solution, were treated with HHP (200 and 400 MPa).
3.3. Color Analysis
The mean values of L*, a*, and b* for the control samples were 62.3, 0.37, and 31.6, respectively (Table 2). All values were lower when compared with the same potato variety previously investigated [1,30], probably due to various effects such as the growing conditions of potatoes or age.

Table 2.
The influence of UV-C, HHP, and UV-C/HHP treatment and storage time on the color parameters of raw, fresh-cut potatoes.
All applied treatments significantly increased the L* value, which expresses brightness, and decreased the a* value, which as a positive value means redness and as negative value means greenness. The b*, as yellowness, was not affected by the treatment. The UV-C/HHP-treated samples were the brightest, whereas a* was more pronounced in the control samples. Our previous study [1] also found that UV-C treatment could increase the brightness of raw potato slices. Although HHP may create favorable conditions for tissue browning due to possible cellular damage, allowing better contact between PPO and phenolic substrate, it can be concluded that this was not the case in the present study, as no browning was observed (L* increased). In addition, HHP could affect the distribution of surface reflectance due to texture changes, which may be the cause of brighter slices [51,52]. According to previously published studies, Sánchez-Moreno et al. (2006) [53] reported an increase in L* in tomato puree treated with HHP (400 MPa/15 min), and Zhou et al. (2014) [54] in pumpkin slices treated with HHP. In contrast to our results, Tsikrika et al. (2021) [3] found a decrease in L* and b* in peeled potato tubers treated with higher pressure (600 MPa/3 min).
Storage significantly affected the values of L*, a*, and b*. L* increased significantly on the 8th day and remained stable thereafter until the end of storage. Although the changes in a* values during storage were significant, no trend of changes was observed, and they remained at values below 1 throughout the storage period. These values of L* and a* indicate that no browning occurred during storage. Although the values of b* (yellow coloration) were statistically the highest on the 8th day of storage, no trend of changes was observed. The yellow color is characteristic of the potato tissue of the Birgit variety (European Cultivated Potato Database [55]).
It can be concluded that UV-C, HHP, and the combined UV-C/HHP treatment can maintain the natural color of the potato tissue during the 15-day storage period.
3.4. Chlorogenic Acid Analysis
Chlorogenic acid is the most abundant phenolic constituent of potato, found primarily in the skin and outer tissue but also in significant amounts in the inner tissue [16]. Because of its important role in the browning processes and its antioxidant properties, the influence of UV-C light, HHP, and the combination of UV-C/HHP treatments on its content, as well as the changes during 15 days of storage, were examined.
The grand mean value of chlorogenic acid was 8.95 mg 100 g−1 DW (Table 3). The value obtained in the control samples (15.0 mg 100 g−1 DW) was somewhat lower than in our previous study (17.28 mg 100 g−1 DW), probably due to the age or growth conditions of the potatoes. In comparison with the control samples, the treatments significantly reduced the content of chlorogenic acid in the raw FCP, which was the most pronounced with the HHP and UV-C/HHP treatments. UV-C reduced the chlorogenic acid content by 30.7%, UV-C/HHP by 58.0%, and HHP by 72.7%. In our previous study [21], a nonsignificant decrease in chlorogenic acid content with increasing UV-C dose was found. However, according to the study by Teoh et al. (2016) [56], the content of total phenolics in fresh-cut potatoes (FCP) increased under the influence of UV-C light. Given the different experimental conditions and the fact that total phenolics were monitored by the aforementioned authors, these results are difficult to compare. Regarding the HHP treatment, Tsikrika et al. (2021) [3] reported a significant decrease in chlorogenic acid (about 65% or 84% depending on cultivar) and an increase in caffeic acid content in treated (600 MPa/3 min), vacuum-packed whole potato tubers, which the authors suggested might be due to the degradation of freeform chlorogenic acid to its constituent, i.e., caffeic acid. It can be assumed that the mentioned degradation could be the cause of such a high decrease in chlorogenic acid content in the examined samples in this study. The decrease in chlorogenic acid content due to the effect of HHP (450, 550, and 650 MPa/5, 10, and 15 min) was also observed in other vegetables [57].

Table 3.
The influence of UV-C, HHP, and UV-C/HHP treatment and storage time on the chlorogenic acid and sugar content of raw, fresh-cut potatoes.
According to the statistical results, the storage had a significant effect on the chlorogenic acid content. The content increased during storage from 7.8 mg 100 g−1 DW to 10.3 mg 100 g−1 DW on the 15th day. The increase in chlorogenic acid content during storage is consistent with previously published studies [58,59]. The influence of the HHP and HHP/UV-C treatments (Figure 2), which resulted in the greatest reduction in chlorogenic acid, is also evidenced by the lowest increase in its content during 15-day storage (0.16 and 1.06 mg 100 g−1 DW, respectively). Such minimal increase in chlorogenic acid content in the aforementioned samples during storage could probably be related to the decreased activity of enzymes involved in chlorogenic acid biosynthetic pathways.
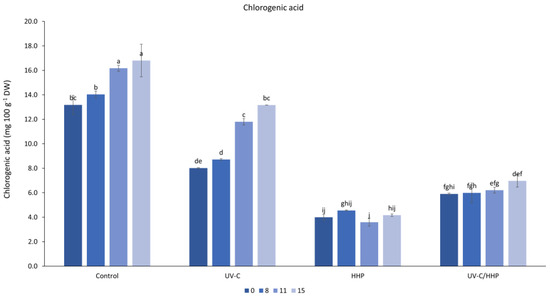
Figure 2.
Interaction of treatment (control, UV-C, HHP, and UV-C/HHP) and storage time on chlorogenic acid content. Error bars represent standard errors; lowercase letters represent a significant difference within means (p < 0.05).
Observed reduction due to the treatments is useful in terms of preventing the browning process, which, according to the color analysis (Table 2), did not occur in the treated samples.
3.5. Sugars Analysis
The grand mean contents of reducing sugars and sucrose were 1.13 g 100 g−1 DW and 0.36 g 100 g−1 DW, respectively (Table 3). The mean value of reducing sugars content for the raw control (0.79 g 100 g−1 DW) was slightly higher when compared with the raw control samples of the same cultivar in a previous study [21].
Statistical analysis revealed that the UV-C, HHP, and UV-C/HHP treatments significantly increased the content of reducing sugars (glucose and fructose) and sucrose. Such an effect of UV-C irradiation on the content of reducing sugars content in potatoes was found in our previous study [21]. This change in sugar content could be related to the effect of UV-C irradiation on the activity of various enzymes involved in sugar metabolism [60,61]. Similar to our results for the increased content of reducing sugars by HHP, Ghafoor et al. (2012) [62] observed a significant increase in HHP-treated (400–600 MPa/1 min) red ginseng, whereas Shigematsu et al. (2017) [63] found no change in sugar content in HHP-treated (100–600 MPa/25 °C), vacuum-packed fresh-cut sweet potatoes. The highest increase in reducing sugar (Table 3) was in the UV-C/HHP-treated samples, in which the sugar content approximately doubled when compared with the control samples. The increase in sugar content caused by treatments involving HHP is likely due to the effect of HHP on cell structure [62], which may subsequently lead to higher sugar release and availability. Additional studies are needed to explain this observed effect of combined treatment.
Statistical analysis showed that storage, expressed in days, had a significant effect on the content of reducing sugars. The content increased during storage and reached its maximum on the 15th day (1.29 g 100 g−1 DW). According to the results (Figure 3), the content of reducing sugars increased in all samples regardless of treatment, but it was more pronounced in samples treated with UV-C on the 8th and 15th day. Such an increase during the refrigerated storage of fruits and vegetables has been reported previously [21,31]. Sweetening at low temperatures [61,64] during storage may cause such a change.
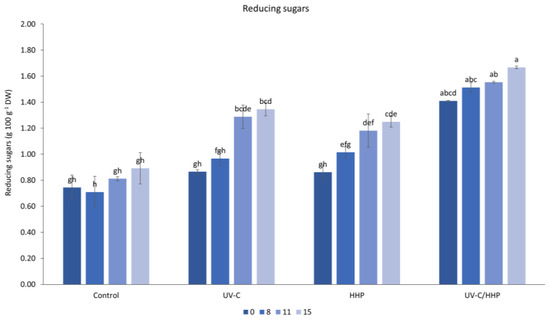
Figure 3.
Interaction of treatment (control, UV-C, HHP, and UV-C/HHP) and storage time on reducing sugars. Error bars represent standard errors; lowercase letters represent a significant difference within means (p < 0.05).
3.6. Acrylamide Analysis
Acrylamide is a neurotoxic organic compound, classified by the International Agency for Research on Cancer (IARC) [65] as a probable carcinogenic to humans in Group 2A. Its metabolization into glycinamide, after being distributed throughout the organs, is considered to be the basis for its genotoxicity and carcinogenicity (EFSA 2015) [66]. Therefore, the effect of UV-C, HHP and UV-C/HHP treatment on acrylamide content in subsequently fried FCP was investigated.
The acrylamide content obtained in fried FCP ranged from 598 to 2611 µg kg−1 DW (Table 3). Although all applied treatments increased acrylamide content, it was about three-fold higher when the combined UV-C/HHP treatment was applied. The increase in reducing sugars is significant with regard to the possible increase in the content of carcinogenic acrylamide in fried potatoes, as they are one of the precursors of acrylamide. The observed increase in reducing sugars (Table 1, Figure 3) in raw UVC- and HHP-treated samples is followed by an increase in acrylamide content (Figure 4) in fried FCP. However, a disproportionately large increase in acrylamide can be observed for the UV-C/HHP treatment. Since reducing sugars and the free amino acid asparagine are precursors in Maillard reactions in which acrylamide is formed, it could be suggested that this combined treatment, among others, affected some of the precursors or the reaction process itself. Ghafoor et al. (2012) [62] observed an increase in the content of reducing sugars as well as amino acids, including asparagine, under the influence of HHP (400–600 MPa) on red ginseng roots. Additional studies are needed to better elucidate the effects of the combined treatment. However, acrylamide levels in the control, UV-C, and HHP were always below the adopted limit for acrylamide in potato products, which is 750 µg kg−1 according to ECR 2017/2158 [22]. It is important to emphasize that the obtained values are expressed as DW and are about three-fold higher than the values expressed as the mass of the fried samples. For UV-C/HHP, acrylamide content exceeded the limit, making them unsafe for consumption. As for previous studies, Sobol et al. (2020) [23] reported an increase in acrylamide content when potato tubers were irradiated with UV-C. In our previous study [21], it was found that the irradiation of raw FCP with UV-C light can slightly increase acrylamide content in fried FCP, depending on the applied radiation dose. A recent study by Douardo et al. (2020) [24] showed that there was no significant change in acrylamide content when HHPs of 100, 200, or 400 MPa/5 min were applied on raw potato sticks immersed in water or asparaginase solution. During storage, a slight increase in acrylamide content was observed on day 8, which remained stable thereafter.
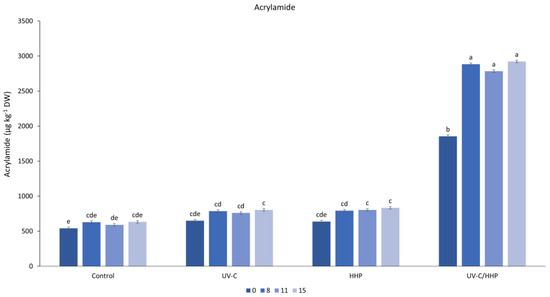
Figure 4.
Interaction of treatment (control, UV-C, HHP, and UV-C/HHP) and storage time on acrylamide. Error bars represent standard errors; lowercase letters represent a significant difference within means (p < 0.05).
Under the conditions of our experiment, the samples treated with UV-C and HHP were safe in terms of acrylamide content.
3.7. PAH Content
For additional evaluation of the safety of UV-C- and HHP-treated fried FCP, the content of PAH as environmental and processing pollutants was assessed in addition to the acrylamide content, following the recommendations of EFSA (2007) (Figure 5). The analyses of PAH were performed only for the single influence of UV-C and HHP treatment, since the determination was made after the analysis of acrylamide, which showed the inadequacy of the combined treatment in terms of a large increase acrylamide content in fried potatoes.
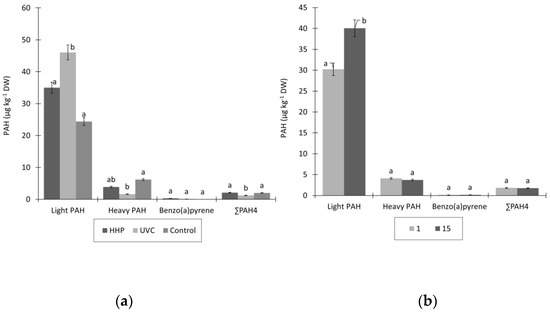
Figure 5.
PAH content (µg kg−1 DW) in (a) control, UV-C-, and HHP-treated fried fresh-cut potatoes at (b) 1st and 15th day of storage. Results are expressed as least square (LS) means. Different letters within columns mean statistically different values at p ≤ 0.05.
The GC–MS method was able to identify a total of 15 PAH, which for clarification purposes were divided into major light fraction (naphthalene, acenaphtylene, acenaphtene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo(a)anthracene, and chrysene), containing up to four aromatic rings, and heavy fraction (benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, dibenzo(a,h)anthracene, and indeno(1,2,3,-c,d)pyrene), with five or six aromatic rings, while the levels of benzo(g,h,i)perilene were below the detection limit of the method in all samples tested. Phenantrene (4.90–28.63 µg kg−1) and benzo(k)fluoranthene (0.70–6.21 µg kg−1) were the dominant PAH molecules in the light and heavy fractions, respectively. These results differ somewhat from those obtained by Balbino et al. (2020) [26], who considered naphthalene to be dominant in the light fraction and benzo(g,h,i)perylene in the heavy fraction. Moreover, the levels of benzo(a)pyrene and PAH4 were below the limits of EU Regulation 835/2011 [27] in all the samples studied. However, the composition of PAH was influenced by the treatments applied. The contents of the heavy PAH fraction and PAH4 were significantly lower in UV-C-treated samples, while the contents of the light fraction were higher compared with the control. Several authors have studied the effect of UV-C lights of different wavelengths on the degradation and decomposition of PAH in soil, water, and wastewater [67,68]. Their results show that UV-C irradiation causes photocatalytic degradation of PAH at different levels, which depend on the chemical composition of the matrix [69]. The UV-C treatment of fried, fresh-cut potatoes applied in this study could cause the decomposition of heavy PAH compounds to PAH with lower molecular weights. On the other hand, the increase in light PAH fraction after 15 days of storage could be due to partial cell destruction and loss of water content by the applied treatments.
3.8. Results of PCA Analysis of Sensory Data in Relation to UV-C, HHP, and UV-C/HHP Treatment and Storage Time
PCA analysis of sensory data was performed for raw, boiled, and fried samples in order to visualize the relations between the analyzed parameters and to define a possible grouping of samples in relations to the applied treatments (UV-C, HHP, and combined UV-C/HHP) and storage time (days).
Sensory attributes of color (browning), odor, off-odor, firmness, and moistness were included in the PCA evaluation of the raw FCP (Figure 6). PC1 and PC2 described 84.24% of the total data variance. PC1 showed a strong correlation (>0.73) with all sensory attributes, while PC2 correlated strongly with color (browning) (r = −0.619) and off-odor (r = −0.604) and moderately with odor (r = −0.524) and firmness (r = −0.407). With regard to treatment, all UV-C-treated samples were placed on the positive PC1 due to their higher firmness and odor scores and lower off-odor scores. Almost all HHP- and UV-C/HHP-treated samples were located in the positive PC2 range with the highest scored moistness. In terms of storage, clustering was observed at day 0 and day 8, with almost all samples distributed at positive PC1, as they were characterized by higher scores of firmness and odor. Samples evaluated on the 11th and 15th day were situated mostly on negative PC1 due to higher moistness and color (browning) scores.
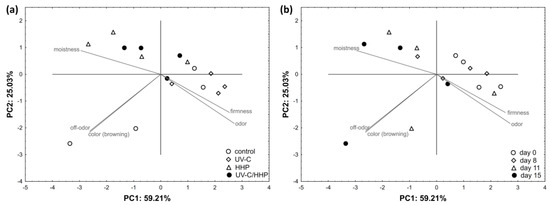
Figure 6.
Distribution of raw, fresh-cut potatoes in two-dimensional coordinate system defined by the first two principal components (PC1 and PC2) in relation to the (a) treatment and (b) storage time.
The boiled FCP sensory attributes of color (browning), odor, off-odor, moistness, firmness, creaminess, and characteristic taste were put in relations with the treatment applied and storage time (Figure 7). PC1 and PC2 explained 83.86% of the total data variance, with PC1 showing a very strong correlation with odor (r = −0.978) and characteristic taste (r = −0.958), a strong correlation with color (browning) (r = 0.808), off-odor (r = 0.885), and moistness (r = 0.669), and a moderate correlation with creaminess (r = −0.539). It was noticeable that the samples treated with UV-C were grouped in the upper left quadrant, which is characterized by more pronounced creaminess and odor. Control samples were grouped on positive PC2, defined by increased creaminess and color (as browning). Pronounced firmness was the most evident in the HHP- and UV-C/HHP-treated samples. Separation of samples stored for 0 and 8 days was noticeable at negative PC1 and correlated with higher odor, firmness, creaminess, and characteristic taste scores. Most samples on day 15 were associated with increased color (browning) values.
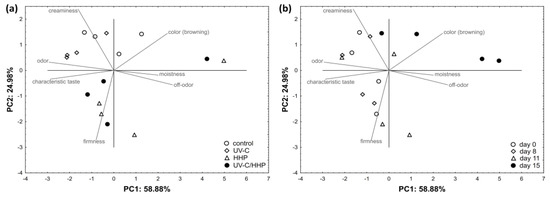
Figure 7.
Distribution of boiled, fresh-cut potatoes in two-dimensional coordinate system defined by the first two principal components (PC1 and PC2) in relation to the (a) treatment and (b) storage time.
PCA results for fried FCP are shown in Figure 8. PC1 and PC2 described 79.86% of the total data variance. The sensory attributes of color, odor, oiliness, firmness, crispiness, and taste were evaluated. A strong correlation was found between odor (r = −0.890), oiliness (r = 0.714), crispiness (r = 0.850), and taste (r = −0.863) and PC1, as well as between color (r = −0.773), and firmness (r = 0.763), and PC2. Color and firmness correlated moderately with PC1 and oiliness with PC2. Clear separation from other samples by PC2 was visible for the control and UV-C samples. UV-C samples were grouped on the negative PC2 based on higher color and odor scores, while the control samples showed high correlation with color and oiliness. The HHP- and UV-C/HHP-treated samples were situated on the positive side of PC2, mainly due to the lower color and oiliness scores and higher firmness scores. Regarding storage, all samples scored at day 0 and day 8 were distributed on the negative PC1 side due to the more pronounced positive attributes of color, odor, crispness, and taste. In contrast, all samples on the 15th day were placed on the positive PC1 due to the lower scored attributes previously mentioned but also due to the more pronounced oiliness.
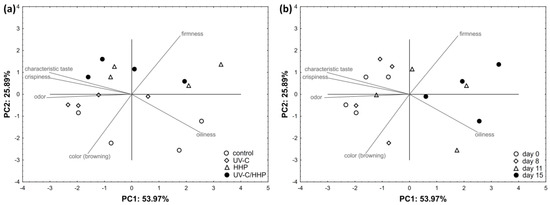
Figure 8.
Distribution of fried, fresh-cut potatoes in two-dimensional coordinate system defined by the first two principal components (PC1 and PC2) in relation to the (a) treatment and (b) storage time.
Overall, the occurrence of some negative changes in sensory attributes was mainly influenced by the storage time, which on the 15th day resulted in a slightly pronounced browning and off-odor of the raw and boiled control samples and oiliness in the control fried samples. From all of the above, it appears that the sensory attributes of the UV-C, HHP-, and UV-C/HHP-treated samples were satisfactorily maintained, regardless of the treatment itself or the storage time.
4. Conclusions
All applied treatments reduced the TAMBC of raw FCP, but the combined treatment of UV-C/HHP was the most effective, followed by HHP alone. Both treatments slowed bacterial growth during storage when compared with the UV-C treatment and even more to the control. After 15 days of storage, only the control had unsatisfactory TAMBC. In addition, all treatments, but especially HHP, reduced the chlorogenic acid content in the raw FCP. The reducing sugar content was the most increased when UV-C/HHP was applied, and this treatment significantly increased the acrylamide content in the subsequently fried FCP, even above the benchmark level (EU Commission Regulation 2017/2158). The UV-C and HHP treatments kept the acrylamide content below the specified limit. Additional studies are needed to clarify the effect of the combined treatment. PAH levels were below the limits set by the EU Regulation 835/2011 in all analyzed samples. Photocatalytic degradation of heavy PAH to PAH with lower molecular weights might have occurred with UV-C treatment. Despite some changes in sensory attributes, the raw and thermally treated samples were sensory acceptable during the 15-day storage period. The combined UV-C/HHP treatment gives very desirable results in terms of suppressing the growth of microorganisms under the conditions tested but is not applicable due to the high acrylamide content in fried samples; so, an adjustment of the conditions is recommended. However, a relatively short UV-C treatment, despite lower antimicrobial effect, and HHP treatment when an antibrowning agent and vacuum packaging are used, have the potential to ensure safety and satisfactory quality and to somewhat extend the shelf life of FCP. Nevertheless, at the level of the fresh-cut potato industry, it is necessary to examine their effect on a real scale.
Author Contributions
Conceptualization, B.L.; methodology, B.L. and Z.P.; formal analysis investigation, Z.P., Z.Č., M.R., F.D. and S.B.; writing—original draft preparation, Z.P.; writing—review and editing, M.R. and B.L.; supervision, B.L.; funding acquisition, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Croatian Science Foundation [grant number IP-2016-06-5343].
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pelaic, Z.; Cosic, Z.; Repajic, M.; Pedisic, S.; Zoric, Z.; Scetar, M.; Galic, K.; Levaj, B. Effect of UV-C Irradiation on the Shelf Life of Fresh-Cut Potato and Its Sensory Properties after Cooking. Food Technol. Biotechnol. 2022, 60, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Da Pieve, S.; Maifreni, M. Impact of UV-C light on safety and quality of fresh-cut melon. Innov. Food Sci. Emerg. 2011, 12, 13–17. [Google Scholar] [CrossRef]
- Tsikrika, K.; Walsh, D.; Joseph, A.; Burgess, C.M.; Rai, D.K. High-Pressure Processing and Ultrasonication of Minimally Processed Potatoes: Effect on the Colour, Microbial Counts, and Bioactive Compounds. Molecules 2021, 26, 2614. [Google Scholar] [CrossRef] [PubMed]
- Čošić, Z.; Pelaić, Z.; Repajić, M.; Pedisić, S.; Zorić, Z.; Levaj, B. Effect of uv-c irradiation on microbial load and phenolic content of potato tubers and slices. Carpathian J. Food Sci. Technol. 2021, 13, 25–32. [Google Scholar] [CrossRef]
- Levaj, B.; Ljubas, A.; Čošić, Z.; Pelaić, Z.; Dujmić, F.; Repajić, M. Effect of the high hydrostatic pressure on the quality and shelf-life of fresh-cut potato. In Proceedings of the 18th Ružička Days “Today Science—Tomorrow Industry”, Vukovar, Croatia, 16–18 September 2020. [Google Scholar]
- Livneh, Z.; Cohenfix, O.; Skaliter, R.; Elizur, T. Replication of Damaged DNA and the Molecular Mechanism of Ultraviolet-Light Mutagenesis. Crit. Rev. Biochem. Mol. 1993, 28, 465–513. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry—A critical review. J. Sci. Food Agr. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Allende, A.; Artés, F. Combined ultraviolet-C and modified atmosphere packaging treatments for reducing microbial growth of fresh processed lettuce. LWT Food Sci. Technol. 2003, 36, 779–786. [Google Scholar] [CrossRef]
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schluter, O.; Schwarzenbolz, U.; Jager, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of high hydrostatic pressure food processing: Perspectives on technology and food safety. Compr. Rev. Food Sci. F 2021, 20, 3225–3266. [Google Scholar] [CrossRef]
- Niven, G.W.; Miles, C.A.; Mackey, B.M. The effects of hydrostatic pressure on ribosome conformation in Escherichia coli: An in vivo study using differential scanning calorimetry. Microbiol-Sgm 1999, 145, 419–425. [Google Scholar] [CrossRef]
- Gardner, D.W.M.; Shama, G. The kinetics of Bacillus subtilis spore inactivation on filter paper by uv light and uv light in combination with hydrogen peroxide. J. Appl. Microbiol. 1998, 84, 633–641. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; Nauta, M.; et al. The efficacy and safety of high-pressure processing of food. EFSA J. 2022, 20, e07128. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, J.A.; Barbosa-Cánovas, G.V.; Swanson, B.G. High Hydrostatic Pressure Processing of Fruit and Vegetable Products. Food Rev. Int. 2005, 21, 411–425. [Google Scholar] [CrossRef]
- Nicolaus, R.A.; Piattelli, M.; Fattorusso, E. Structure of Melanins + Melanogenesis. 4. On Some Natural Melanins. Tetrahedron 1964, 20, 1163–1172. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin Pigment in Plants: Current Knowledge and Future Perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Deußer, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and glycoalkaloid contents in potato cultivars grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Plazas, M.; Prohens, J.; Cunat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Andujar, I. Reducing Capacity, Chlorogenic Acid Content and Biological Activity in a Collection of Scarlet (Solanum aethiopicum) and Gboma (S. macrocarpon) Eggplants. Int. J. Mol. Sci. 2014, 15, 17221–17241. [Google Scholar] [CrossRef]
- Andre, C.M.; Schafleitner, R.; Guignard, C.; Oufir, M.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.F.; Evers, D.; Larondelle, Y. Modification of the Health-Promoting Value of Potato Tubers Field Grown under Drought Stress: Emphasis on Dietary Antioxidant and Glycoalkaloid Contents in Five Native Andean Cultivars (Solanum tuberosum L.). J. Agr. Food Chem. 2009, 57, 599–609. [Google Scholar] [CrossRef]
- Pelaic, Z.; Cosic, Z.; Pedisic, S.; Repajic, M.; Zoric, Z.; Levaj, B. Effect of UV-C Irradiation, Storage and Subsequent Cooking on Chemical Constituents of Fresh-Cut Potatoes. Foods 2021, 10, 1698. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU), 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Off. J. Eur. Union 2017, 304, 24–44. [Google Scholar]
- Sobol, Z.; Jakubowski, T.; Surma, M. Effect of Potato Tuber Exposure to UV-C Radiation and Semi-Product Soaking in Water on Acrylamide Content in French Fries Dry Matter. Sustainability 2020, 12, 3426. [Google Scholar] [CrossRef]
- Dourado, C.; Pinto, C.A.; Cunha, S.C.; Casal, S.; Saraiva, J.A. A novel strategy of acrylamide mitigation in fried potatoes using asparaginase and high pressure technology. Innov. Food Sci. Emerg. Technol. 2020, 60, 102310. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Balbino, S.; Repajic, M.; Solaric, T.; Hunjek, D.D.; Skevin, D.; Kraljic, K.; Obranovic, M.; Levaj, B. Oil Uptake and Polycyclic Aromatic Hydrocarbons (PAH) in Fried Fresh-Cut Potato: Effect of Cultivar, Anti-Browning Treatment and Storage Conditions. Agronomy-Basel 2020, 10, 1773. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU), 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, 54, L215/4-8. [Google Scholar]
- Procaccini, L.M.G.; Mu, T.H.; Sun, H.N. Effect of innovative food processing technologies on microbiological quality, colour and texture of fresh-cut potato during storage. Int. J. Food Sci. Technol. 2022, 57, 898–907. [Google Scholar] [CrossRef]
- Eshtiaghi, M.N.; Knorr, D. Potato Cubes Response to Water Blanching and High Hydrostatic-Pressure. J. Food Sci. 1993, 58, 1371–1374. [Google Scholar] [CrossRef]
- Dite Hunjek, D.; Pranjic, T.; Repajic, M.; Levaj, B. Fresh-cut potato quality and sensory: Effect of cultivar, age, processing, and cooking during storage. J. Food Sci. 2020, 85, 2296–2309. [Google Scholar] [CrossRef]
- Dite Hunjek, D.; Pelaic, Z.; Cosic, Z.; Pedisic, S.; Repajic, M.; Levaj, B. Chemical constituents of fresh-cut potato as affected by cultivar, age, storage, and cooking. J. Food Sci. 2021, 86, 1656–1671. [Google Scholar] [CrossRef]
- HRN EN ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorgan Isms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. Croatian Standards Institute: Zagreb, Croatia, 2013. (In Croatian)
- Elez Garofulic, I.; Zoric, Z.; Pedisic, S.; Brncic, M.; Dragovic-Uzelac, V. UPLC-MS(2) Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- ISO 6564:1985; Sensory Analysis-Methodology—Flavour Profile Methods. International Organization for Standardization (ISO): Geneva, Switzerland, 1985.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization (ISO): Geneva, Switzerland, 2007.
- Fonseca, J.M.; Rushing, J.W. Effect of ultraviolet-C light on quality and microbial population of fresh-cut watermelon. Postharvest Biol. Technol. 2006, 40, 256–261. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs (Text with EEA relevance). Off. J. EU 2005, 338, 1–32. Available online: https://eur-lex.europa.eu/legal-content/EN/TX-T/?uri=CELEX%3A02005R2073-20200308 (accessed on 16 January 2023).
- Commission Regulation (EU). No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbi-ological criteria for foodstuffs. Off. J. EU 2007, 322, 12–29. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32007R1441 (accessed on 16 January 2023).
- Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods; Health Protection Agency: London, UK, 2009; Available online: https://www.hah.hr/wp-content/up¬loads/2015/09/HPA-vodic-za-procjenu-mkb-sigurnosti-RTE-proizvoda.pdf (accessed on 16 January 2023). (In Croatian)
- Janowicz, M.; Lenart, A. The impact of high pressure and drying processing on internal structure and quality of fruit. Eur. Food Res. Technol. 2018, 244, 1329–1340. [Google Scholar] [CrossRef]
- Sopanangkul, A.; Ledward, D.; Niranjan, K. Mass Transfer During Sucrose Infusion into Potatoes under High Pressure. J. Food Sci. 2002, 67, 2217–2220. [Google Scholar] [CrossRef]
- Tsikrika, K.; O’Brien, N.; Rai, D. The Effect of High Pressure Processing on Polyphenol Oxidase Activity, Phytochemicals and Proximate Composition of Irish Potato Cultivars. Foods 2019, 8, 517. [Google Scholar] [CrossRef]
- Oliveira, M.M.d.; Tribst, A.A.L.; Leite Júnior, B.R.d.C.; Oliveira, R.A.d.; Cristianini, M. Effects of high pressure processing on cocoyam, Peruvian carrot, and sweet potato: Changes in microstructure, physical characteristics, starch, and drying rate. Innov. Food Sci. Emerg. Technol. 2015, 31, 45–53. [Google Scholar] [CrossRef]
- Rastogi, N.; Niranjan, K. Enhanced Mass Transfer During Osmotic Dehydration of High Pressure Treated Pineapple. J. Food Sci. 2008, 63, 508–511. [Google Scholar] [CrossRef]
- Iturralde-García, R.D.; Cinco-Moroyoqui, F.J.; Martínez-Cruz, O.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Cornejo-Ramírez, Y.I.; Bernal-Mercado, A.T.; Del-Toro-Sánchez, C.L. Emerging Technologies for Prolonging Fresh-Cut Fruits’ Quality and Safety during Storage. Horticulturae 2022, 8, 731. [Google Scholar] [CrossRef]
- Lu, J.Y.; Stevens, C.; Khan, V.A.; Kabwe, M.; Wilson, C.L. The effect of ultraviolet irradiation on shelf-life and ripening of peaches and apples. J. Food Quality 1991, 14, 299–305. [Google Scholar] [CrossRef]
- Wang, R.; Wang, T.; Zheng, Q.; Hu, X.; Zhang, Y.; Liao, X. Effects of high hydrostatic pressure on color of spinach purée and related properties. J. Sci. Food Agric. 2012, 92, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Coulon, E.; Morais, A.M.M.B. Effects of vacuum packaging on the physical quality of minimally processed potatoes. Food Serv. Technol. 2003, 3, 81–88. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Grigelmo, N.; Hernando, I.; Lluch, M.; Martin-Belloso, O. Effect of minimal processing on the textural and structural properties of fresh-cut pears. J. Sci. Food Agr. 2002, 82, 1682–1688. [Google Scholar] [CrossRef]
- Hunjek, D.; Repajić, M.; Scetar, M.; Karlović, S.; Vahcić, N.; Ježek, D.; Galić, K.; Levaj, B. Effect of anti-browning agents and package atmosphere on the quality and sensory of fresh-cut Birgit and Lady Claire potato during storage at different temperatures. J. Food Process. Preserv. 2020, 44, e14391. [Google Scholar] [CrossRef]
- Oey, I.; Lille, M.; Van Loey, A.; Hendrickx, M. Effect of high-pressure processing on colour, texture and flavour of fruit and vegetable based food products: A review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- MacDougall, D.B. Colour measurement of food: Principles and practice. In Colour in Food, Improving Quality; MacDougall, D.B., Ed.; Woodhead PublishingLimited: Cambridge, UK, 2002; p. 33e63. [Google Scholar]
- Sánchez-Moreno, C.; Plaza, L.; De Ancos, B.; Cano, M.P. Impact of high-pressure and traditional thermal processing of tomato purée on carotenoids, vitamin C and antioxidant activity. J. Sci. Food Agr. 2006, 86, 171–179. [Google Scholar] [CrossRef]
- Zhou, C.-L.; Liu, W.; Zhao, J.; Yuan, C.; Song, Y.; Chen, D.; Ni, Y.-Y.; Li, Q.-H. The effect of high hydrostatic pressure on the microbiological quality and physical–chemical characteristics of Pumpkin (Cucurbita maxima Duch.) during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2014, 21, 24–34. [Google Scholar] [CrossRef]
- The European Cultivated Potato Database; Scottish Agricultural Science Agency: Edinburgh, Scotland, UK; Available online: https://www.europotato.org/varieties/view/Bir¬git-E/ (accessed on 12 December 2022).
- Teoh, L.S.; Lasekan, O.; Adzahan, N.M.; Hashim, N. The effect of ultraviolet treatment on enzymatic activity and total phenolic content of minimally processed potato slices. J. Food Sci. and Technol. 2016, 53, 3035–3042. [Google Scholar] [CrossRef]
- Jeż, M.; Wiczkowski, W.; Zielińska, D.; Białobrzewski, I.; Błaszczak, W. The impact of high pressure processing on the phenolic profile, hydrophilic antioxidant and reducing capacity of purée obtained from commercial tomato varieties. Food Chem. 2018, 261, 201–209. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Jacobo-Velázquez, D.A. Effects of Wounding Stress and Storage Temperature on the Accumulation of Chlorogenic Acid Isomers in Potatoes (Solanum tuberosum). Appl. Sci. 2021, 11, 8891. [Google Scholar] [CrossRef]
- Ramamurthy, M.S.; Maiti, B.; Thomas, P.; Nair, P.M. High-performance liquid chromatographic determination of phenolic acids in potato tubers (Solanum tuberosum) wound healing. J. Agric. Food Chem. 1992, 40, 569–572. [Google Scholar] [CrossRef]
- Zhou, H.J.; Zhang, X.N.; Su, M.S.; Du, J.H.; Li, X.W.; Ye, Z.W. Effects of Ultraviolet-C Pretreatment on Sugar Metabolism in Yellow Peaches during Shelf Life. Hortscience 2020, 55, 416–423. [Google Scholar] [CrossRef]
- Lin, Q.; Xie, Y.J.; Liu, W.; Zhang, J.; Cheng, S.Z.; Xie, X.F.; Guan, W.Q.; Wang, Z.D. UV-C treatment on physiological response of potato (Solanum tuberosum L.) during low temperature storage. J. Food Sci. Technol. Mys. 2017, 54, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Kim, S.O.; Lee, D.U.; Seong, K.; Park, J. Effects of high hydrostatic pressure on structure and colour of red ginseng (Panax ginseng). J. Sci. Food Agric. 2012, 92, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, T.; Furukawa, N.; Takaoka, R.; Hayashi, M.; Sasao, S.; Ueno, S.; Nakajima, K.; Kido, M.; Nomura, K.; Iguchi, A. Effect of high pressure on the saccharification of starch in the tuberous root of sweet potato (Ipomoea batatas). Biophys. Chem. 2017, 231, 105–110. [Google Scholar] [CrossRef]
- De Wilde, T.; De Meulenaer, B.; Mestdagh, F.; Govaert, Y.; Vandeburie, S.; Ooghe, W.; Fraselle, S.; Demeulemeester, K.; Van Peteghem, C.; Calus, A.; et al. Influence of storage practices on acrylamide formation during potato frying. J. Agr. Food Chem. 2005, 53, 6550–6557. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Acrylamide, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Industrial Chemicals. Int. Agency Res. Cancer Lyon 1994, 60, 389–433. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on Acrylamide in Food. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2015.4104 (accessed on 25 May 2021).
- Eldos, H.I.; Ashfaq, M.Y.; Al-Ghouti, M.A. Rapid assessment of the impact of microwave heating coupled with UV-C radiation on the degradation of PAHs from contaminated soil using FTIR and multivariate analysis. Arab. J. Chem. 2020, 13, 7609–7625. [Google Scholar] [CrossRef]
- Salihoglu, K.; Eker Şanli, G.; Salihoglu, G.; Tasdemir, Y. Removal of Polycyclic Aromatic Hydrocarbons from Municipal Sludge Using UV Light. Desalin. Water Treat. 2012, 44, 324–333. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, B.; Jing, L.; Zhang, H.; Lee, K. Photocatalytic Degradation of Polycyclic Aromatic Hydrocarbons in Offshore Produced Water: Effects of Water Matrix. J. Environ. Eng. 2016, 142, 04016054. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).