Green Separation and Extraction of Isofraxidin from Acanthopanax senticosus Using Deep Eutectic Solvent Synthesized from Choline Chloride and Citric Acid
Abstract
1. Introduction
2. Material and Methods
2.1. Plants Materials
2.2. Chemicals
2.3. Synthesis of DES
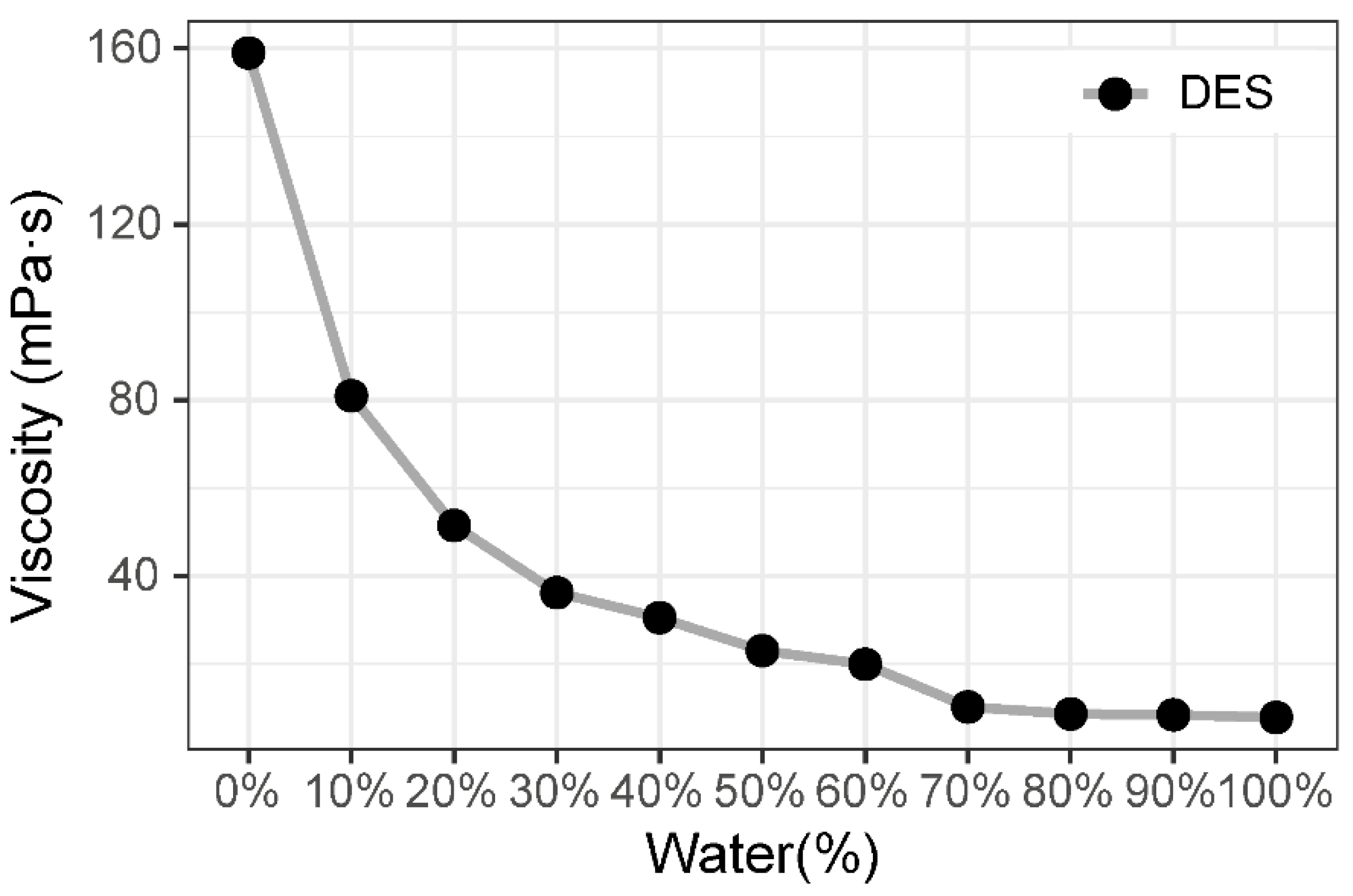
2.4. Viscosity Test
2.5. UHPLC-MS/MS
2.6. Extraction of Specific Components from AS
2.7. Analysis Methodology Validation
2.7.1. Specific Test
2.7.2. Linear Regression Test
2.7.3. Quantitative Limit
2.7.4. Precision Test
2.7.5. Accuracy Test
2.7.6. Statistical Analysis
3. Results
3.1. Chemical and Physical Characterization of DES
3.1.1. The Stability of DES
3.1.2. Viscosity Test
3.2. DES Separation and Extraction of Pharmacologically Active Compounds from AS
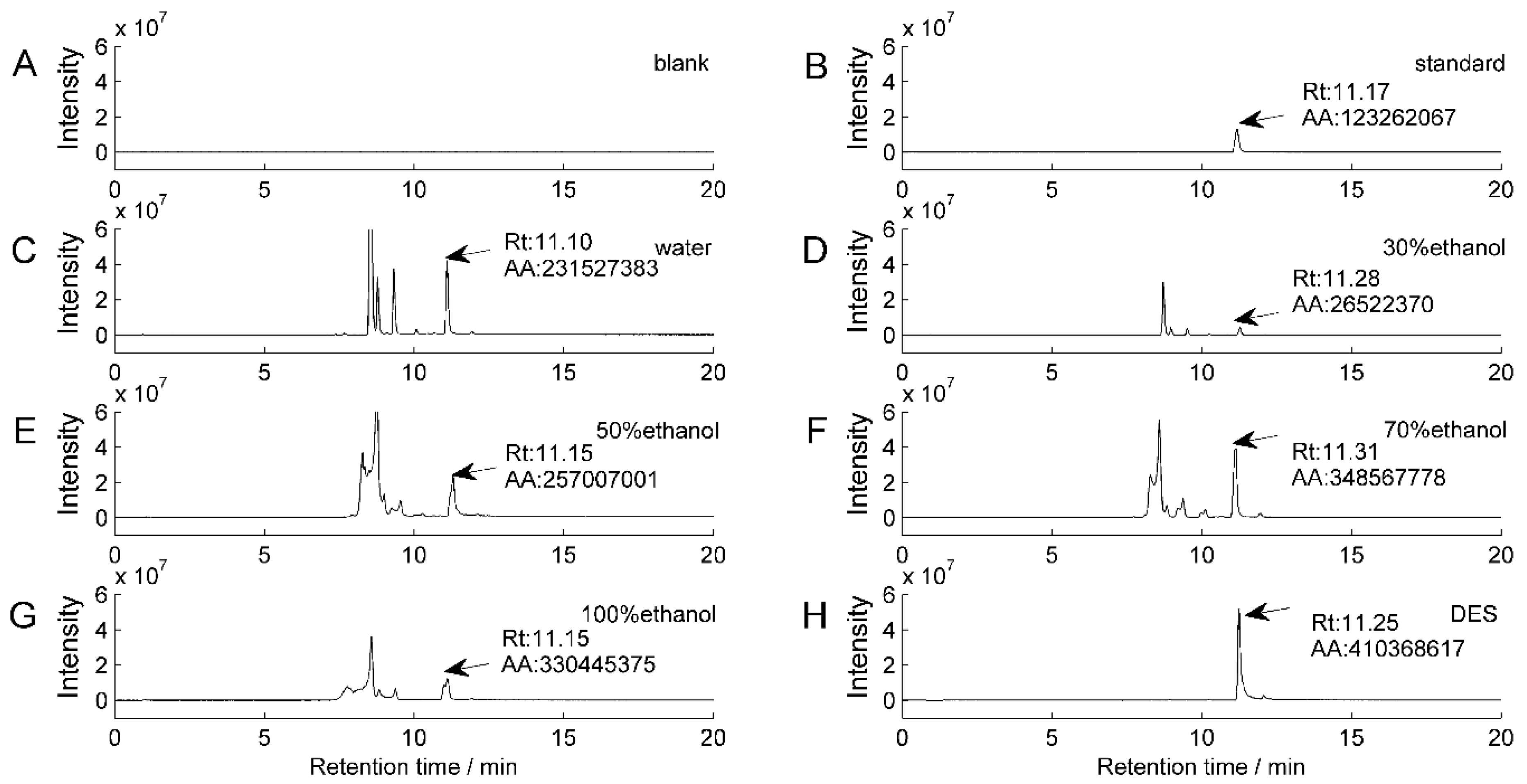
3.2.1. Identification of Isofraxidin from AS
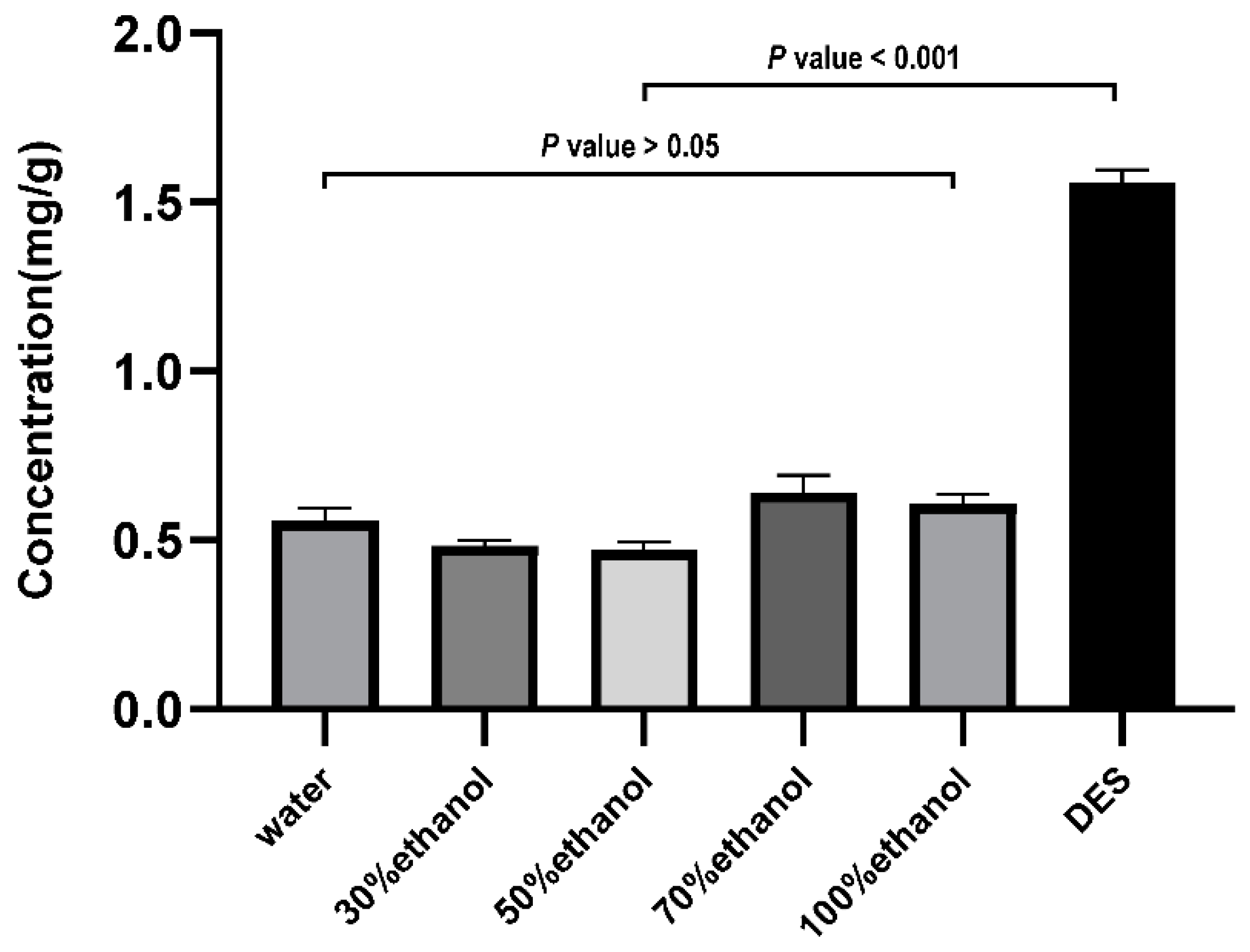
3.2.2. The Capacity to Extract Isofraxidin from Different Solvents
3.3. Methodology Validation
3.3.1. Specific Test
3.3.2. Linear Regression Test
3.3.3. Quantitative Limit
3.3.4. Precision Test
3.3.5. Accuracy Test
3.3.6. The Highest Content of DES Solvent Extraction Isofraxidin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nisibe, S.; Kinoshita, H.; Takeda, H.; Okano, G. Phenolic Compounds from Stem Bark of Acanthopanax senticosus and Their Pharmacological Effect in Chronic Swimming Stressed Rats. Chem. Pharm. Bull. 1990, 38, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.; Chen, K.W.; Cheng, I.S.; Tsai, P.H.; Lu, Y.J.; Lee, N.Y. The effect of eight weeks of supplementation with Eleutherococcus senticosus on endurance capacity and metabolism in human. Chin. J. Physiol. 2010, 53, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Ren, F.; Huang, W.; Ding, R.T.; Zhou, Q.S.; Liu, X.W. Anti-fatigue activity of extracts of stem bark from Acanthopanax senticosus. Molecules 2010, 16, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, J.; Yoon, J.; Kim, M.Y.; Choi, H.Y.; Kim, H. Neuroprotective effects of Eleutherococcus senticosus bark on transient global cerebral ischemia in rats. J. Ethnopharmacol. 2012, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, H.; Zhao, L.; Li, X.; You, J.; Jiang, Q.; Li, S.; Jin, L.; Xu, Y. Protective effects of aqueous extract from Acanthopanax senticosus against corticosterone-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2013, 148, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Bohn, B.; Nebe, C.T.; Birr, C. Flow-cytometric studies with eleutherococcus senticosus extract as an immunomodulatory agent. Arzneimittelforschung 1987, 37, 1193–1196. [Google Scholar]
- Chen, G.; Song, X.; Lin, D.; Xu, P. Isofraxidin Alleviates Myocardial Infarction Through NLRP3 Inflammasome Inhibition. Inflammation 2020, 43, 712–721. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, X. Syringaresinol-di-O-β-D-glucoside, a phenolic compound from Polygonatum sibiricum, exhibits an antidiabetic and antioxidative effect on a streptozotocin-induced mouse model of diabetes. Mol. Med. Rep. 2018, 18, 5511–5519. [Google Scholar] [CrossRef]
- Yamazaki, T.; Tokiwa, T. Isofraxidin, a coumarin component from Acanthopanax senticosus, inhibits matrix metalloproteinase-7 expression and cell invasion of human hepatoma cells. Biol. Pharm. Bull. 2010, 33, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yu, X.; Hu, Z.; Tang, S.; Zhong, X.; Xu, J.; Shang, P.; Huang, Y.; Liu, H. Isofraxidin targets the TLR4/MD-2 axis to prevent osteoarthritis development. Food Funct. 2018, 9, 5641–5652. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.L.; Zhai, S.K.; Chen, H.L.; Luo, Y.D.; Tu, G.R.; Ou, D.W. Immunomopharmacological effects of polysaccharides from Acanthopanax senticosus on experimental animals. Int. J. Immunopharmacol. 1991, 13, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Yoon, Y.D.; Ahn, H.J.; Lee, H.S.; Lee, C.W.; Yoon, W.K.; Park, S.K.; Kim, H.M. Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int. Immunopharmacol. 2003, 3, 1301–1312. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Liu, Y.; Wu, K.; Zhu, Y.; Lu, H.; Liang, B. Insights into the relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents. Environ. Sci. Pollut. Res. Int. 2021, 28, 35537–35563. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Feng, C.; Hu, L.; Zhao, X.; Tang, X.; Huang, Y.; Luo, J.; Xu, M.; Xie, W. Exploration of a ternary deep eutectic solvent for the efficient extraction of plantamajoside, acteoside, quercetin and kaempferol from Plantago asiatica L. Phytochem. Anal. 2022, 33, 94–104. [Google Scholar] [CrossRef]
- Osamede Airouyuwa, J.; Mostafa, H.; Riaz, A.; Maqsood, S. Utilization of natural deep eutectic solvents and ultrasound-assisted extraction as green extraction technique for the recovery of bioactive compounds from date palm (Phoenix dactylifera L.) seeds: An investigation into optimization of process parameters. Ultrason. Sonochem. 2022, 91, 106233. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, F.; Cheng, J.; Wu, X.; Xu, J.; Li, C.; Li, W.; Xie, N.; Wang, Y.; He, L. Natural Deep Eutectic Solvent-Assisted Extraction, Structural Characterization, and Immunomodulatory Activity of Polysaccharides from Paecilomyces hepiali. Molecules 2022, 27, 20. [Google Scholar] [CrossRef]
- Song, C.; Li, S.; Duan, F.; Liu, M.; Shan, S.; Ju, T.; Zhang, Y.; Lu, W. The Therapeutic Effect of Acanthopanax senticosus Components on Radiation-Induced Brain Injury Based on the Pharmacokinetics and Neurotransmitters. Molecules 2022, 27, 1106. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, W. Image Effect Observation of Acanthopanax senticosus on Antifatigue Activity after Exercise. Scanning 2022, 2022, 7588680. [Google Scholar] [CrossRef]
- Shiokawa, Y.; Miyauchi-Wakuda, S.; Kagota, S.; Maruyama-Fumoto, K.; Yamada, S.; Shinozuka, K. Acanthopanax senticosus Induces Vasorelaxation via Endothelial Nitric Oxide-Dependent and -Independent Pathways. Planta Med. 2019, 85, 1080–1087. [Google Scholar] [CrossRef]
- Liu, S.M.; Li, X.Z.; Zhang, S.N.; Yang, Z.M.; Wang, K.X.; Lu, F.; Wang, C.Z.; Yuan, C.S. Acanthopanax senticosus Protects Structure and Function of Mesencephalic Mitochondria in A Mouse Model of Parkinson’s Disease. Chin. J. Integr. Med. 2018, 24, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Tang, S.Q.; Huang, H.; Luo, J.; Zhang, X.D.; Yook, C.S.; Whang, W.K.; Kim, Y.C.; Liu, X.Q. Acanthopanax henryi: Review of Botany, Phytochemistry and Pharmacology. Molecules 2021, 26, 2215. [Google Scholar] [CrossRef] [PubMed]
- Majnooni, M.B.; Fakhri, S.; Shokoohinia, Y.; Mojarrab, M.; Kazemi-Afrakoti, S.; Farzaei, M.H. Isofraxidin: Synthesis, Biosynthesis, Isolation, Pharmacokinetic and Pharmacological Properties. Molecules 2020, 25, 2040. [Google Scholar] [CrossRef] [PubMed]
- Tsvetov, N.; Pasichnik, E.; Korovkina, A.; Gosteva, A. Extraction of Bioactive Components from Chamaenerion angustifolium (L.) Scop. with Choline Chloride and Organic Acids Natural Deep Eutectic Solvents. Molecules 2022, 27, 4216. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Ren, X.; Shi, X.; Zhong, K.; Zhang, Z.; Wang, Z. Study on enhanced extraction and seasonal variation of secondary metabolites in Eucommia ulmoides leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2022, 209, 114514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, J.; Chu, X.; Wang, X. A Green Method of Extracting and Recovering Flavonoids from Acanthopanax senticosus Using Deep Eutectic Solvents. Molecules 2022, 27, 923. [Google Scholar] [CrossRef]
- Tsurumaki, A.; Chiarucci, C.; Khaire, S.; Dal Bosco, C.; Gentili, A.; Navarra, M.A. Removal of Copper Corrosion Products by Using Green Deep Eutectic Solvent and Bio-Derivative Cellulose Membrane. Polymers 2022, 14, 2284. [Google Scholar] [CrossRef]
- Maneffa, A.J.; Harrison, A.B.; Radford, S.J.; Whitehouse, A.S.; Clark, J.H.; Matharu, A.S. Deep Eutectic Solvents Based on Natural Ascorbic Acid Analogues and Choline Chloride. ChemistryOpen 2020, 9, 550–558. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]

| Concentration | Isofraxidin | |
|---|---|---|
| (mg/mL) | Mean ± SD | RSD% |
| 0.002 | 0.0019 ± 0.00004 | 2.11% |
| 0.01 | 0.0115 ± 0.00017 | 1.47% |
| 0.1 | 0.1083 ± 0.0021 | 1.93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.-h.; Hou, J.-h.; Sun, S.-y.; Zhang, Y.; Zhang, X.-r.; Mu, L.-t. Green Separation and Extraction of Isofraxidin from Acanthopanax senticosus Using Deep Eutectic Solvent Synthesized from Choline Chloride and Citric Acid. Processes 2023, 11, 943. https://doi.org/10.3390/pr11030943
Sun C-h, Hou J-h, Sun S-y, Zhang Y, Zhang X-r, Mu L-t. Green Separation and Extraction of Isofraxidin from Acanthopanax senticosus Using Deep Eutectic Solvent Synthesized from Choline Chloride and Citric Acid. Processes. 2023; 11(3):943. https://doi.org/10.3390/pr11030943
Chicago/Turabian StyleSun, Chang-hai, Jing-hua Hou, Shi-yuan Sun, Yu Zhang, Xin-ran Zhang, and Li-ting Mu. 2023. "Green Separation and Extraction of Isofraxidin from Acanthopanax senticosus Using Deep Eutectic Solvent Synthesized from Choline Chloride and Citric Acid" Processes 11, no. 3: 943. https://doi.org/10.3390/pr11030943
APA StyleSun, C.-h., Hou, J.-h., Sun, S.-y., Zhang, Y., Zhang, X.-r., & Mu, L.-t. (2023). Green Separation and Extraction of Isofraxidin from Acanthopanax senticosus Using Deep Eutectic Solvent Synthesized from Choline Chloride and Citric Acid. Processes, 11(3), 943. https://doi.org/10.3390/pr11030943







