1. Introduction
Achievement of the precision health continuum that bridges basic, clinical, and population research, and enables transformative technologies that extend and improve lives, depends on overcoming a few obstacles inherent in the 1945 report “Science the Endless Frontier” prepared for President Roosevelt. This includes resource availability to mitigate the risk of bold development, prototype impactful technologies, and advanced, robust manufacturing. Today, at least three obstacles impede industry-driven innovations that create applications of scientific discoveries in precision medicine (PM): (i) inability to successfully translate and apply new knowledge; (ii) insufficient near-term market; (iii) uncertainty of long-term profit.
Early on in the health informatics field, it was well-recognized that the enormous volume of data generated by large-scale ‘omics’ technologies presents a substantial challenge yet an opportunity for information management; in particular, as they move towards clinical implementation and better healthcare. This is evidenced nowadays with widespread artificial intelligence (AI) and machine learning (ML) platforms tools and/or utility. It is even more conspicuous when additional clinical, drug, and patient data are superimposed upon electronic health records (EHR).
Healthcare data are growing exponentially along three trajectories: volume, variety, and velocity. However, our capacity to acquire insightful information to improve health outcomes is limited and falling behind compared to technological advancements. Challenges or barriers are numerous and include but are not limited to data that is siloed, of poor structure/quality, or has inadequate ethnic representation.
Despite the aspirational promises of precision medicine at the turn of the century, the health sector has made very little progress in achieving truly individualized care. An immense amount of data are available from innumerable sources (i.e., data and information are distributed); however, a lack of cooperation between stakeholders results in the underutilization of much of these data. Today, patient care remains reactive and therapeutic rather than predictive and preventive. Patients continue to receive inefficient, expensive, and delayed care from convoluted healthcare systems that are nearly impossible for laypersons to understand. Hence, an urgent need exists for a reimagined healthcare macrocosm that harnesses AI and other modern computing technologies to put the world’s enormously valuable data to good use and provide truly patient-centered care.
The intent of this paper is to discuss a novel framework with case studies that bring together divergent stakeholders including patients to the improved healthcare system which will accelerate the process of data-driven healthcare. This effort builds on an earlier described healthcare fusion innovative framework and process [
1].
2. Towards AI Dependent Healthcare
Humans learn from experience; machines learn from data. Much like human experience, more and more data lead to more precise models and better decisions. AI systems need to be ‘trained’ through data, structured and un-structured, that are generated from patient interviews, and routine clinical activities, such as screening, diagnosis, treatment assignment, etc. The clinical data (demographics, medical notes, electronic recordings from medical devices, physical examinations, and clinical laboratory and images) can be used by AI applications for machine learning (structured data). Deep learning and natural language processing tools can use unstructured data (patient interviews and narrations).
Therefore, AI feeds on big data. Well, how big is big data? What types of analytics are enablers and important?
Due to the fast development of digital technology, there is a massive amount of digital data generated every second. Almost every human activity nowadays leaves permanent trails of individuals’ digital traces.
In celebrating its 25th anniversary, the journal Nature Medicine recently dedicated a special issue, focusing on Digital Medicine. The issue discusses the new technologies that are transforming healthcare, and also highlights the limitations and the regulatory aspects.
Eric Topol, Professor of Molecular Medicine at The Scripps Institute, California reviewed the potential applications and limitations of AI in medicine [
2]. He describes the powerful impacts of AI in medicine at three different levels:
clinicians, the
health system, and
patients. For
clinicians, he argued that AI so far has its most significant impact on the accurate interpretation of digitized images. Not only AI allows for very fast and cost-effective screening of millions of digitized images (
$1000, for 250 M images), but it also exhibits diagnostic power that, in many cases, exceeds that of experienced clinicians. For
health systems, he explained how AI via EHR helps improve overall processes: facilitates administrative tasks, enhances workflow efficiency, and minimizes medical errors. He also emphasized the long-term potential of big data including individual patients’ medical, socioeconomic, and behavioral attributes. AI can process these enormous data to inform decisions related to the best methods, treatments, and outcomes of myriads of medical conditions. Finally, Topol discussed the impact of AI at the
patient’s level. He showed several AI applications that enable patients to process their data, monitor their medical adherence, and obtain virtual health coaching. In other words, AI allows patients to take their health and well-being into their own hands. Several articles on the same issue also highlighted some concerns and limitations such as potential data bias, privacy and security issues, and the legal and ethical consequences of using personal data. Notably, there are some overlooked humanitarian aspects to the increasing use of technology in medicine. For instance, it is not immediately obvious how AI and its associated technology would affect the personal relationship between patients and physicians.
In another review published in
BMJ Leader, Erwin Loh, Clinical Professor at Monash University discussed the implications of the increasing use of AI for medical leaders [
3]. He hinted at the lack of awareness among health professionals regarding the potential impacts of technology on the future of medicine. He suggested that medical leaders should engage in collaborative efforts to ensure the appropriate oversight of the new technologies as they come, in terms of safety, cost, staff training, the legality of data handling, etc. Medical leaders should also be aware and prepared for the impact of AI and automation on the health workforce in the near future. AI systems will not only assist and perhaps replace some health professions, but they could also support, and potentially replace, the role of managers. In his concluding remark, Loh said: “
Regardless of whether the AI singularity comes to pass or not, AI in health will continue to improve, and these improvements appear to be accelerating. There are clear challenges for the adoption of AI in health for health services, organizations and governments, and a need to develop a policy framework around this issue. As doctors and health leaders, we need to start preparing the profession to be supported by, partnered with, and, in future, potentially be replaced by, AI and advanced robotics systems. We have an opportunity now to literally shape the development of humanity’s future autonomous health providers, and we should be leaders in this space rather than passive observers”.
With its transformative power, AI can truly be life-changing if appropriate data sets are fused together for these machine brains to learn about the patient’s values and health conditions. AI has the potential to enable clinicians and patients to work together to make decisions and select tests, treatments, and care plans based on clinical evidence that balances risks and expected outcomes with patient preferences and values (shared decision-making). Though it is expected for these benefits to accrue incrementally over time, AI applications have the potential to address the issues of “access, affordability and effectiveness” by improving them.
3. Challenges to the Adoption and Implementation of AI
Widespread adoption of AI tolls will need stronger end-user and stakeholder involvement. Healthcare providers will need to see value in the applications and trust the machine-suggested options generated by AI applications. Clinical validation of these suggestions will go a long way in addressing the skepticism about adopting AI tools. Similarly, patients will have to gain the trust of such tools too as they may not understand enough about how AI works. Scalability and cost of implementation may be another challenge in the adoption of AI tools, however, with time and more advanced technology some of these issues can be overcome. The tardiness of governmental organizations in developing regulatory policies and laws will pose a challenge of a different type. Given the diverse stakeholders involved in the provision of healthcare services, AI adoption and implementation will see numerous challenges in foreseeable future.
Apart from AI tools and stakeholders/inter–intrasectoral engagements, data remains the foremost central matter in any process improvement. This ranges from resolving issues of data being distributed, to being of meager quality or that lack underprivileged populations representation in order to drive the healthcare processes.
4. Healthcare Fusion
4.1. Healthcare Data Fusion and Management Framework
The healthcare fusion concept and framework were elaborated on in a recent publication [
1]. We aimed to create an organized, regulated framework for healthcare data management. The intent is to accelerate patient-centered care and improve interoperability within healthcare systems.
Briefly, we envisioned and proposed the healthcare fusion framework, which fuses healthcare data and stakeholders involved in healthcare delivery to realize culturally and demographically relevant outcomes in precision and population health.
The foundation of this is a national repository healthcare data system healthcare information center, HIC, that is a centralized, cloud-based information management system (IMS). A HIC umbrella utilizes patient- and population-level health data. Healthcare stakeholders would contribute a variety of information to the IMS. AI and ML platforms are leveraged to analyze the data with high precision and throughput. Participants would have varying levels of access to these data.
To ensure processing capability and scalability to achieve healthcare fusion objectives, the IMS must maintain the quality and integrity of data in the fused system. Moreover, it is necessary to meet certain standards for accessibility, efficacy, and efficiency. Five such critical criteria are specified within the acronym ROBIN [Responsive to demand, Open to stakeholders, Bridges data, Information shared, Networks capable].
4.2. The Healthcare Fusion Frontier: AI-Dependent Healthcare Value vs. Chaos
As advancements continue to be made in health-related technologies, the healthcare field will become increasingly dependent on AI and ML. The frontier currently lies at the intersection between data and medicine, and advancement from the current status quo depends on strong end-user and stakeholder involvement. At this point, patients and healthcare providers remain skeptical about the diagnostic accuracy of AI platforms, whereas governments and legislative bodies are tardy in developing relevant regulations. Furthermore, patients often continue to lack ownership of their health data, and are rightfully concerned about data security and privacy. These factors constitute significant hindrances to innovative parties, and delay the full adoption of AI in healthcare.
Through its linking of all parties involved in health and medicine, the healthcare fusion framework facilitates the advancement of the frontier through collaboration. Clinical validation of AI decision-making is critical to address existing skepticism surrounding AI; this objective can be achieved through the fusion of hospitals, providers, and technology corporations, such that physicians and AI systems can share diagnostic data and workflows in the interest of quality control. Under these conditions, AI platforms and healthcare providers at a hospital should be able to access and utilize data from all other hospitals within the fused system to enhance their diagnostic capabilities. Additionally, based on accuracy and throughput data from existing AI tools, computing system manufacturers can optimize platforms to meet the specific needs of hospitals and health systems.
Healthcare fusion also entails significant involvement of governmental and regulatory sectors, which should address emerging technologies in a timely manner. Legislative bodies must keep pace with private sector innovation, and ensure that regulations do not pose undue burdens to the stakeholders in health. Data privacy and security must be given high priority; for the protection of patients, universal best practices must be developed and implemented to prevent the stealing and selling of personal medical data [
4].
With proper implementation and oversight, AI platforms can increase efficiency, accuracy, and profitability in medicine. Clinicians and hospitals stand to benefit from the rapid screening of medical images, increases in workflow efficiency, and reductions in medical errors, whereas patients will be able to process their own medical data, monitor their treatment adherence, and obtain virtual health coaching [
2]. Further, AI systems can assist both health professionals and managers, and enable patient-centered care under the shared decision-making model [
3]. Under the proposed healthcare fusion framework, these benefits will accrue incrementally, as all stakeholders stand to benefit and will therefore invest in the modernization of healthcare.
5. Population Health
The components of healthcare fusion that drive precision health can also deliver population-level impact (
Figure 1). Aggregated qualitative data, collected from patient interviews and social media posts and subjected to qualitative analysis, are a tool to elucidate sentiments and attitudes towards diseases, treatments, and health; these data may also be harnessed to analyze patient demographics and cultures [
5]. Pharmaceutical companies, healthcare corporations, and insurance providers stand to profit from detailed knowledge of patient populations, whereas individual patients will benefit from improved, culturally conscious healthcare delivery mechanisms.
From a public health standpoint, the key benefits of quantitative big data analysis utilizing the ROBIN approach are surveillance and predictive power. Under the healthcare fusion model, epidemiological and social media data facilitate the detection and mapping of disease outbreaks; analysis of this data can reveal patterns and trends in the occurrence and spread of diseases [
5,
6]. Predictive power, which is derived from pattern analysis, can constitute part of an early warning system for infectious diseases. In this regard, population-level metadata on risk factors, which include smoking, poverty, and social isolation, may prove instrumental in identifying areas where COVID-19 and similar diseases will have the greatest impact; this is further discussed within the case study below.
Beyond preventative medicine and disease mapping, healthcare fusion also offers solutions for treatment development. Disease mechanisms must be understood prior to drug discovery; as such, the combination of omics data with clinical data can provide insights into the genetic and molecular mechanisms of disease [
7]. Finally, quantitative omics data can support the design and implementation of cloud computing and AI-driven molecular modeling workflows for drug discovery and development [
8].
The value of such an approach (full integration and analysis of big data) will become evident per the case 1 study discussed below.
6. Real Data and Evidence
According to data published by the Precision Medicine World Conference—PMWC (pmwcintl.com), real-world evidence (RWE)-based approaches will certainly impact clinical decision-making as real-world data (RWD) analysis methodologies not only improve but also the utilization of RWD from non-traditional and novel sources. However, this advancement will necessitate that the precision medicine stakeholders safeguard against RWD bias and increase population diversity. Undeniably, achieving precision health will rest with sanctioning RWD to undertake health disparity issues. At present, regulatory authorities accept RWE clinical evidence of therapeutics or medical devices derived from RWD analyses.
Cost/benefit analysis of RWD resulting from AI/ML platforms will increase. Its impact is already felt in the drug discovery/development sector. The development of the COVID vaccine including its mutation is an excellent vivid example of AI contribution.
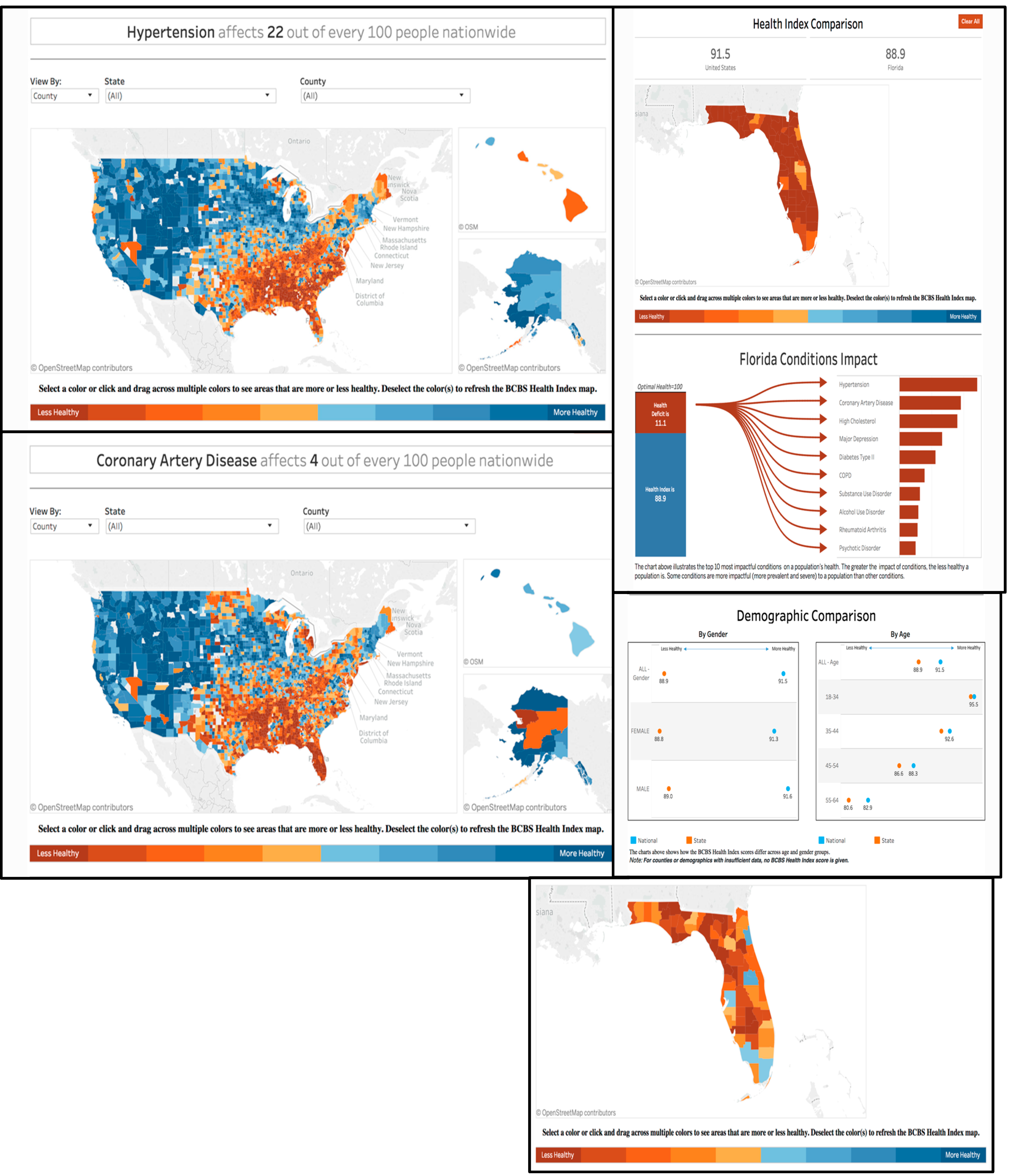
As healthcare becomes increasingly big data-driven, standards for evidence-based medicine will naturally shift. Clinical decision-making will be guided by real-world evidence derived from real-time data analysis; diagnostic and therapeutic guidelines could be updated on a regular basis as new data become available around the world. As such, clinical decision support systems will become powerful allies to healthcare providers as they navigate the new data-filled macrocosm. An example of real-world data analysis is the Blue Cross Blue Shield Health of America Index
SM described below [
9].
Case Study 1: Blue Cross Blue Shield (BCBS)
Blue Cross Blue Shield (BCBS) is a US-based health insurance provider which offers a nationwide, personalized approach to healthcare based on the needs of the communities where its members live and work. It boasts over 106 million members and a contract rate greater than 95%—higher than any other US insurer. One of its programs is called the Health of America Initiative, where key healthcare trends are uncovered and shared through advanced analytics and innovative research. The BCBS Health Index
SM quantifies the impact of over 200 medical conditions on the health and well-being of commercially insured Americans [
9]. Its recent data show that hypertension is the number one health condition affecting Americans’ quality of life and longevity.
The data provided on the BCBS website are enormously useful. According to the BCBS Health Index, the top 10 conditions nationally, causing approximately 58% of commercially insured Americans’ overall reduction in health [
9], are the following:
It is worth highlighting that the BCBS framework exemplifies our proposed healthcare fusion framework ROBIN approach [
1], in that its analysis is freely accessible, links individual and population data, and utilizes forefront technology to address immediate and anticipated demands. Stakeholders can view the spread and impact of the top ten adverse health conditions facing commercially insured Americans at-a-glance, and they can also obtain detailed numerical and demographic information at the state and county levels (
Figure 2).
Discussion of risk factors, preventative measures, and lifestyle is outside the scope of this paper. However, it is invaluable to illustrate how these types of data/information may contribute to population health. A case in point, one notable shortcoming of the BCBS interface is its lack of data on the prevalence of major contributing lifestyle risk factors in disease; these include poverty, homeless, lack of access to healthcare, and the Western lifestyle, as well as risky behaviors such as unhealthy food habits, tobacco use, alcohol consumption, substance abuse, eating disorders, and sexual activity to name a few. Recent data on smoking and smoking-related complications within the US almost certainly exist; they are likely inaccessible to the general public due to their scattering among numerous isolated parties. Indeed, lifestyle data in its broadest definition is a critical piece to understanding the overall patient or population health (per
Figure 1, the merge of qualitative and quantitative data). Another instructive example is the COVID-19 pandemic. Although everyday media coverage tends to focus specifically on COVID-19 data, analysis of pre-existing metadata can be instrumental in predicting outcomes at a regional level and elucidating the underlying reasons behind the severe strain posed by COVID-19 on today’s healthcare systems. Italy, for instance, exhibits an exceptionally high COVID-19 death rate—likely a result of shortages of hospital beds, ventilators, and personal protective equipment. In situ research may conclude that Italian medical infrastructure is overwhelmed by the volume of COVID-19 cases. However, a retrospective analysis may reveal foundational weaknesses which remained unknown until the pandemic. In Italy, the prevalence of smoking was 21.7% in 2010 and 21.4% in 2015 and 2016, whereas an estimated 71,445 deaths (12.5% of all deaths) in 2010 were attributed to smoking [
10,
11]. These figures suggest that Italian hospitals may already have been heavily burdened by preventable, smoking-related conditions prior to the COVID-19 outbreak; it is plausible that coronavirus cases overwhelmed a system that already exhibited several red flags.
7. Health Fusion and Clinical Workflow
To achieve precision health, massive integration of personalized health and large-scale epidemiological and molecular data is required. In our drive toward patient-centered care to improve individual and population health outcomes, the journey or odyssey that the patient takes is invaluable to dissect, in particular, the processes that improve a system and are ultimately beneficial to the patient. The clinical workflow is one such process to understand. Furthermore, we articulate and then demonstrate how having the clinical workflow path being an integral part of the healthcare fusion process can be impactful with an impressive outcome.
7.1. Clinical Workflow Assessment
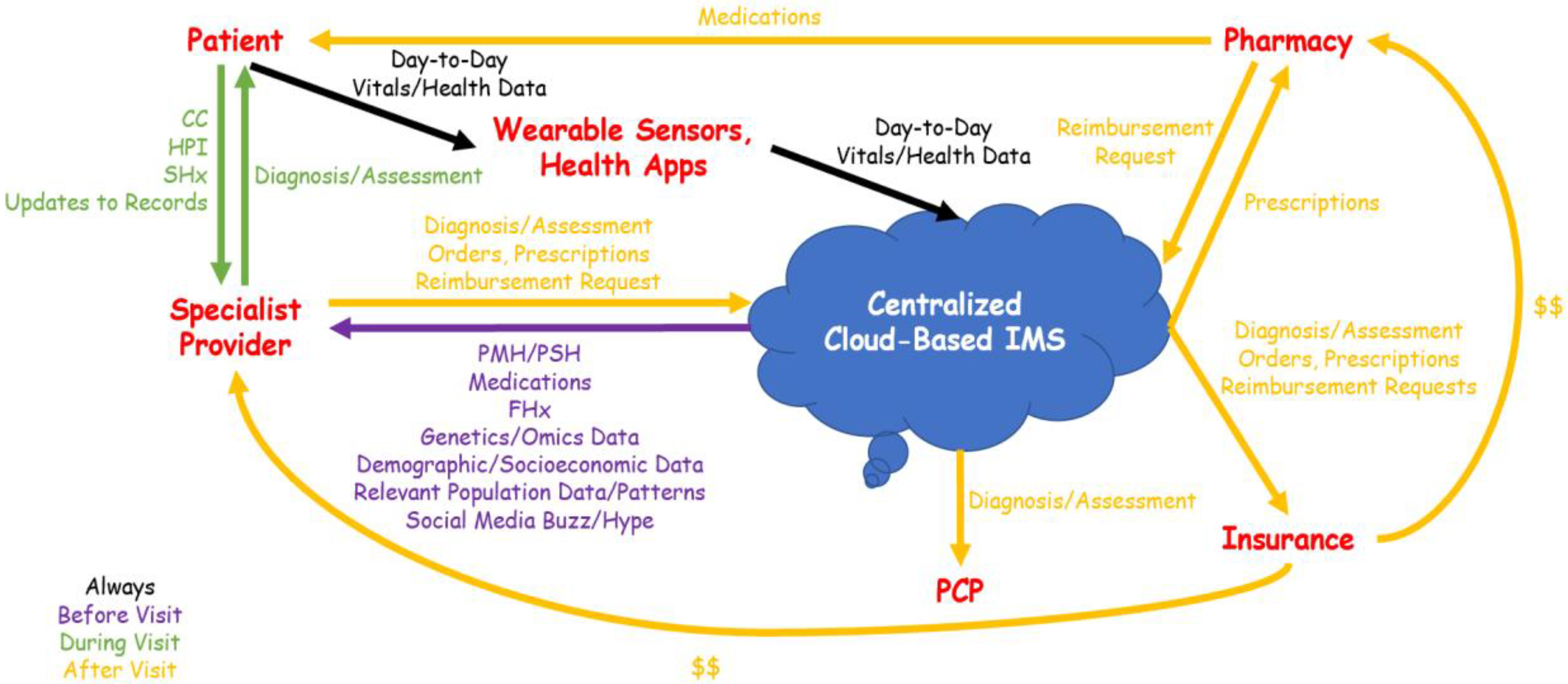
Despite numerous recent initiatives advancing precision medicine and personalized care, current clinical workflows remain archaic and incompatible with big data-driven approaches to healthcare. A typical clinical workflow is depicted in
Figure 3. Prior to a visit with a specialist provider, the patient gathers relevant past medical records, which may include previous diagnoses and therapeutic regimens, medical images on various media (e.g., CDs, USBs, patient portals), and day-to-day health data from wearable sensors and health-related applications. This is a time-consuming process that may involve requesting records from previous primary care and specialist providers, and some pertinent records may be lost, ignored, or otherwise overlooked.
During the encounter itself, the specialist provider must assess and account for these records, in addition to eliciting all aspects of the medical history from the patient (chief complaint—CC, history of present illness—HPI, past medical history—PMH/past surgical history—PSH, medications, family history—FHx, social history—SHx). Due to the limited time allocated to each encounter, much of this information may again be ignored or overlooked. Moreover, given the immense amount of information that must be exchanged and documented, precision medicine data (e.g., ‘omics’, demographics, socioeconomics)—even if available—may not be accounted for.
After the encounter, the specialist provider documents the diagnosis, assessment, and plan in their EHR system, communicates any prescriptions to a pharmacy, and requests insurance reimbursement. The patient, apprised of the diagnosis and assessment, can update their medical records with their primary care physician (PCP) if they desire.
Although clinical workflows of this nature are the norm in modern healthcare, it is immediately obvious that they are inefficient and hinder the implementation of precision medicine. As different healthcare providers utilize disparate, non-interoperable EHRs, the patient serves as their own de facto health information exchange (HIE). This approach has several significant disadvantages. Firstly, it is difficult for any person to not only account for all of their health records, but assess them for pertinence, prior to every specialist visit. This will inevitably lead to important data points being overlooked; as such, diagnostics and therapeutics will not be “precise”—they do not utilize all available data and therefore cannot be completely individualized. Moreover, when the patient serves as the custodian of their health information, a specialist provider must spend a substantial portion of the encounter eliciting the complete medical history—a significant time overhead for both provider and patient. Finally, due to disparate EHR systems, PCPs may remain unaware of diagnoses and treatment plans made by specialists unless told by patients. These deficiencies lead to missed clinical opportunities and lost revenues.
In the interest of improving healthcare quality and efficiency while reducing cost, the healthcare fusion model can be applied to optimize clinical workflows. A fused healthcare system utilizes a centralized, cloud-based IMS as its one-stop HIE for all parties. Consequently, the burden of health record management shifts from individual healthcare providers and patients to a centralized system that contains patient and population data.
Let us reconsider the patient’s visit to a specialist provider in the context of a clinical workflow designed for a fused healthcare system (
Figure 4). In this workflow, no record gathering is required from the patient prior to the visit, as the patient’s complete medical records are stored in the IMS. Instead, before the visit, the specialist provider retrieves the patient’s medical profile from the IMS and can consider not only clinical records, but any available family, genetic, omics, demographic, and socioeconomic data, as well as population-level patterns and internet “buzz” that the patient may be exposed to. This enables the provider to devote more time during the encounter itself to problem-solving, as only a few aspects of the history (CC, HPI, FHx, SHx, and any record updates) must be elicited. More importantly, diagnoses and therapeutic plans can be precisely tailored to the patient’s individual medical, demographic, and socioeconomic context. After the encounter, relevant data are uploaded to the IMS and made available to insurance companies, pharmacies, and PCPs as needed; health records are therefore updated without any need for action by the patient.
To recap, the key elements in this optimized workflow are the following: centralized cloud-based IMS, patient information, and the interconnectivity of stakeholders (PCP, specialist provider, pharmacy, and insurance companies).
Many advantages can be realized through the implementation of a fused healthcare system with streamlined clinical workflows (
Figure 5). The benefits of ROBIN-ization can be enumerated in the acronym FATEMA:
Functionality of workflow: redundancies present in current workflows are minimized;
Accuracy of diagnosis: providers have access to complete patient datasets, enabling more accurate and individualized diagnoses;
Transfer between providers: since all patient data are stored in the IMS, patients can seamlessly transition between different providers—all of whom can access the same data;
Efficiency of visits: providers need not take a full history, as much of it can be retrieved (in extensive detail) from the IMS;
Management of data: the IMS reduces data-related overheads for patients and providers;
Accessibility of medication: prescriptions can be immediately communicated between providers and pharmacies via the IMS, reducing patient wait times.
Additionally, there are four key deliverables, enumerated within the acronym HARP:
The ramification of such optimized clinical workflow is high-value healthcare that is defined as “providing the best care possible, efficiently using resources, and achieving optimal results for each patient”.
7.2. Case Study 2: Precision Prescription—OTC Purchase and Ovarian Cancer
Here we will discuss a concrete example of how health fusion work in a clinical workflow is valuable. Moreover, how fused data can be leveraged showing its usefulness and being impactful. In a recently published observational case-control study, tracking what over-the-counter (OTC) shoppers buy, via their loyalty-card data, can potentially help identify those with early signs of ovarian cancer [
12].
Ovarian cancer is often diagnosed late, as when it first develops, it might not cause any noticeable symptoms. Additionally, no reliable screening test is available. Ovarian cancer symptoms may include pain, discomfort, bloating, indigestion, bowel changes, and frequent urination. Typical treatment involves surgery, chemotherapy, and other types of cancer therapy. Undoubtedly, early diagnosis improves the chance of successful treatment.
The UK study partnered with two big pharmacy retailers and 283 female customers, who agreed to share their shopping data going back over six years. These females frequently used OTC medication to self-care for non-specific ovarian cancer symptoms (pain and indigestion drugs) prior to their confirmed diagnosis. As the lead investigator concluded: “Using shopping data, our study found a noticeable increase in purchases of pain and indigestion medications among women with ovarian cancer, up to eight months before diagnosis, compared with women without ovarian cancer. This suggests that long before women have recognized their symptoms as alarming enough to go to the physician, they may be treating them at home”.
Final thought, if such data were available and fused in an IMS system including various processes/controls which is really possible under the NHS organization, and where qualitative and quantitative healthcare data from stakeholders is accessible, such disease early detection could become routine with benefit to both the patient and cost saving to the healthcare services.
8. Conclusions
For decades to come, the constant rise of new medical technologies will continue to shift the healthcare field towards a consolidation of healthcare data and business. Health-related data and capital investment from stakeholders constitute the two inputs of the fused macrocosm. On the data side, big data analytics systems, optimized through our proposed healthcare fusion framework and ROBIN criteria, efficiently analyze quantitative medical data, the products of which are combined with qualitative knowledge to facilitate outcomes in individual- and population-level healthcare. To facilitate and realize profits from such outcomes, stakeholders should invest to improve the “ROBIN-ness” of big data systems. In summary, the healthcare fusion framework unifies all stakeholders in healthcare under a single umbrella to provide an overarching approach to healthcare delivery and, by illustration here, optimize clinical workflow in a diverse world.
We believe that we ought to depend on advances in data sciences in order to expand on what is for the moment a fairly limited repertoire of computational solutions for things such as polygenic risk scores for diseases definition for instance. Future work ought to center on building an ecosystem that permits clinical data integration, clinical information flow, and leverages AI power, and further, envisions processes that accelerate data-driven health care. A clinical/patient perspective is critical rather than just an IT perspective to optimize future clinical workflow.
It is said that it takes a village to deliver precision medicine and health.