Adsorption of Methylene Blue by Bentonite Supported Nano Zero Valent Iron (B-nZVI)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Bentonite Composite Nano Zero-Valent Iron (B-nZVI)
2.2.2. Material Characterization
2.2.3. Batch Equilibrium Test
3. Results and Discussion
3.1. Materials Characterization
3.2. Adsorption Materials
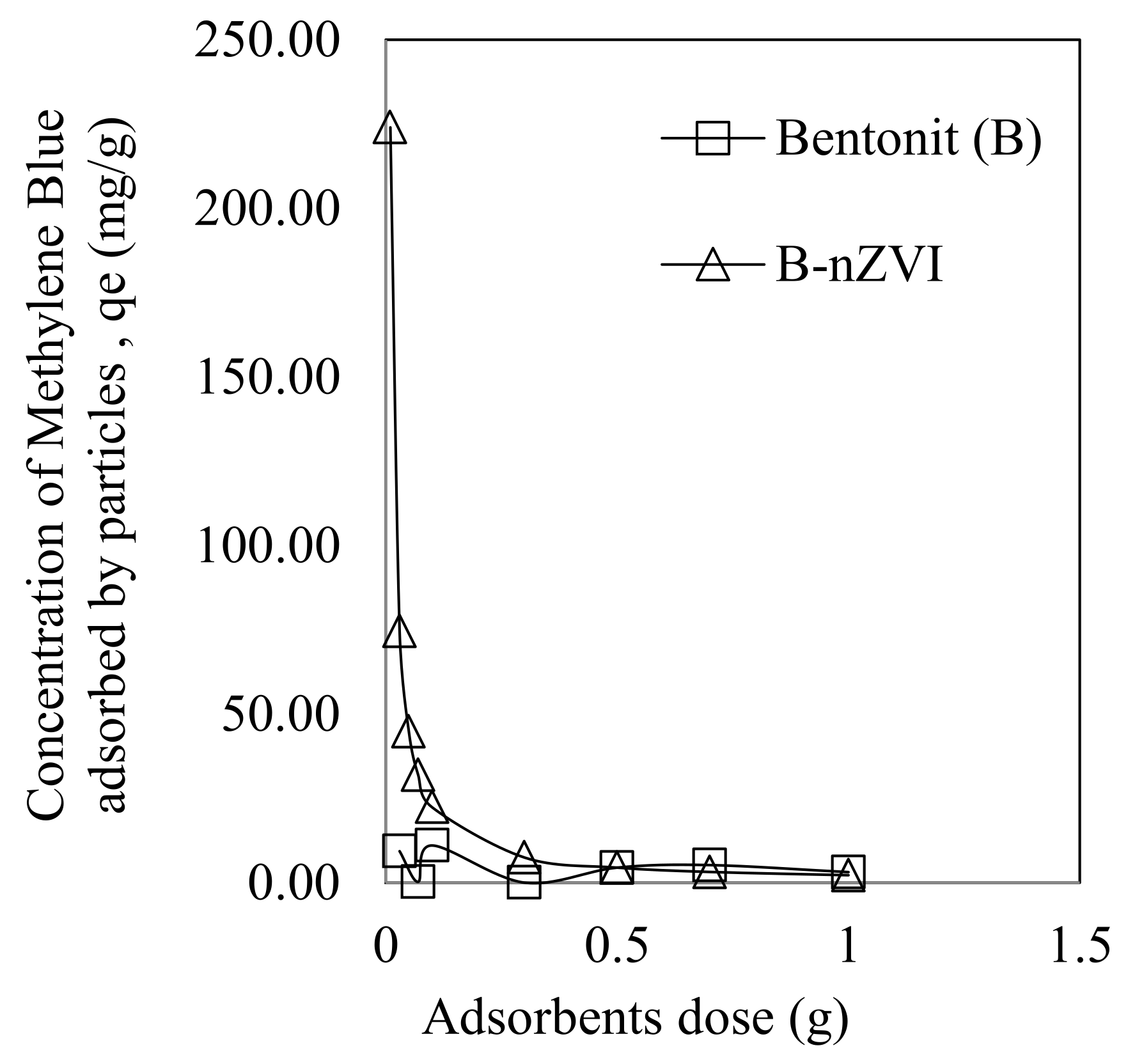
3.2.1. Dose Effect
3.2.2. Concentration Effect
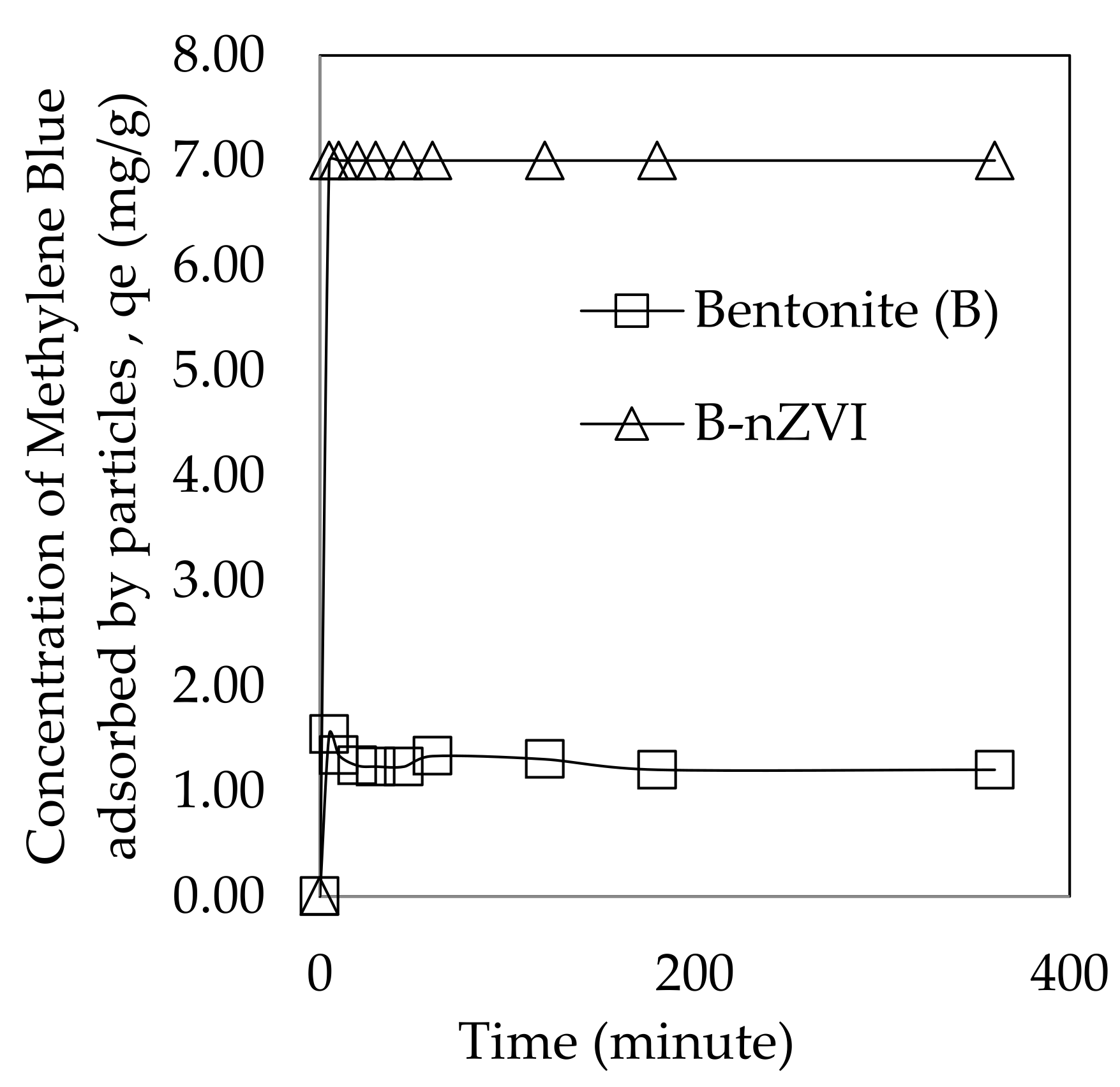
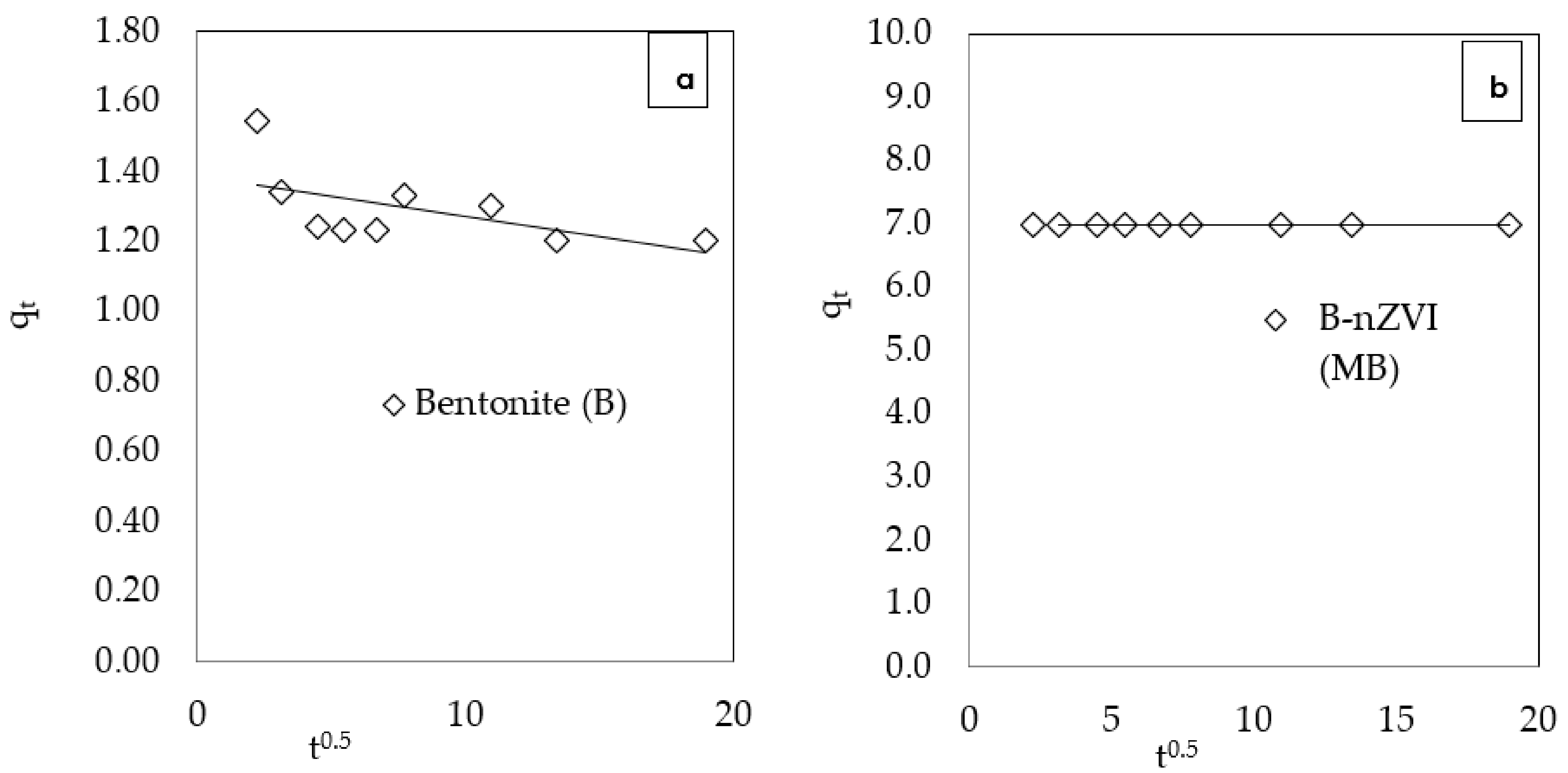
3.2.3. Kinetic Effect
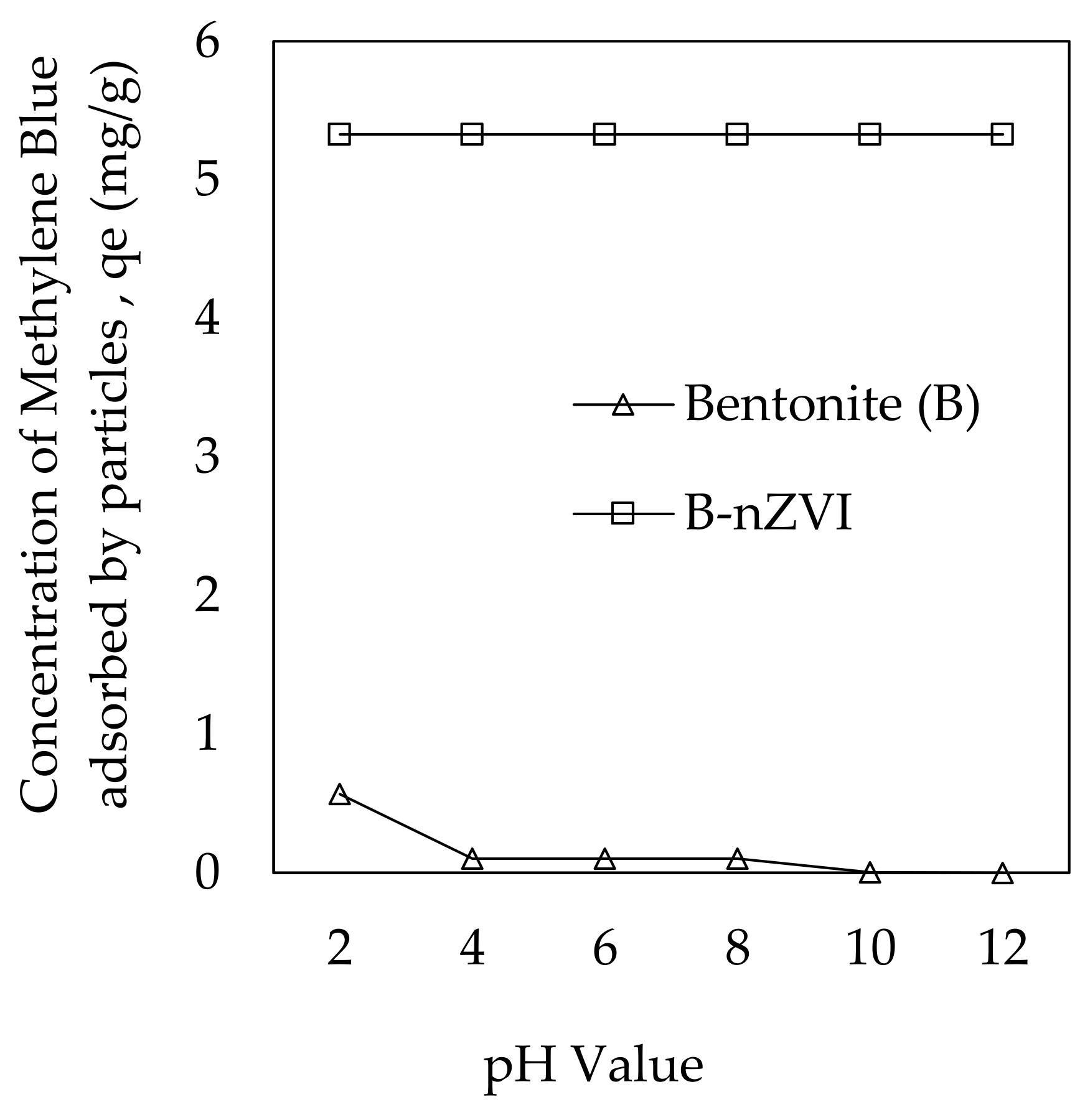
3.2.4. pH Effect
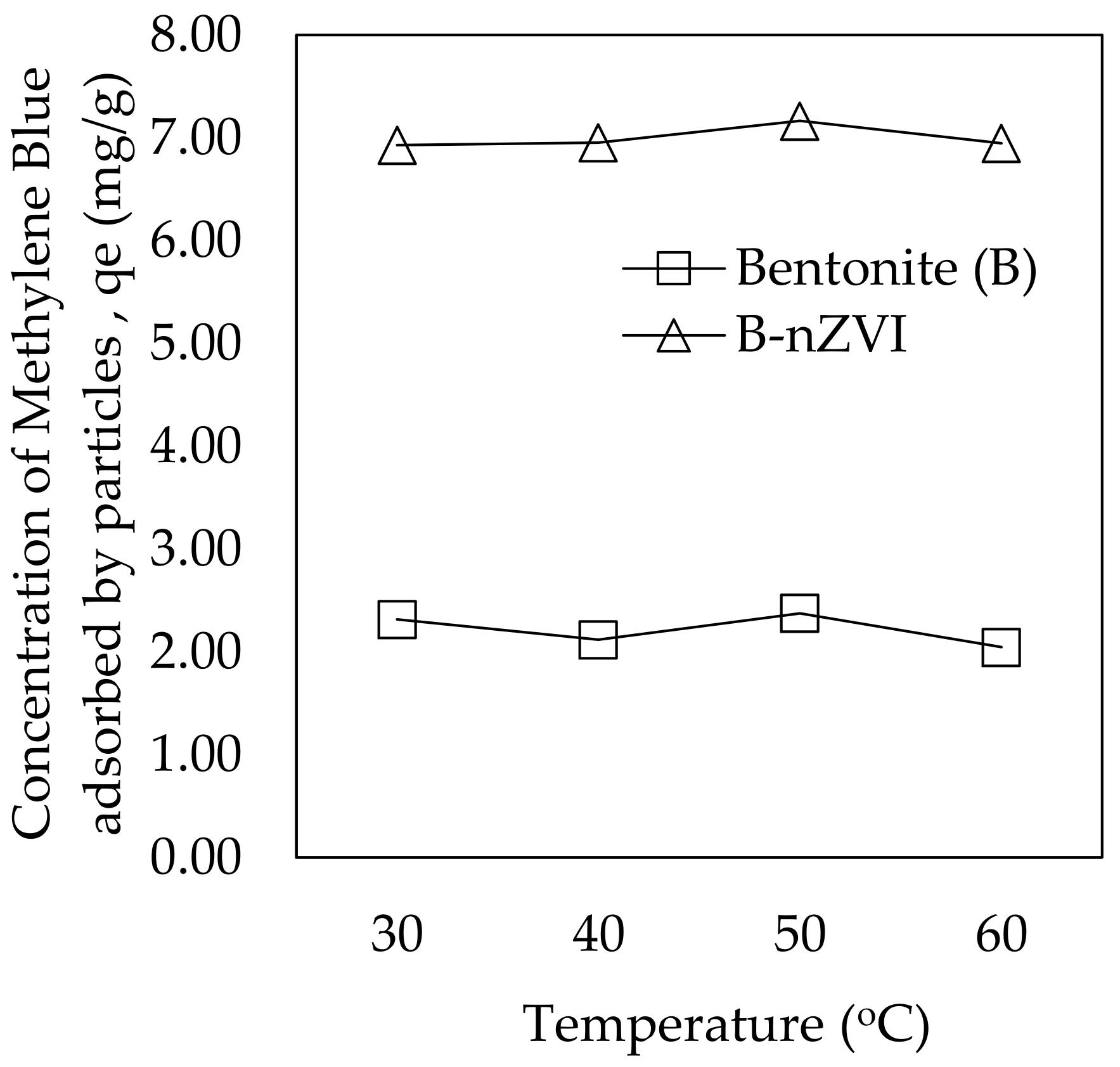
3.2.5. Temperature Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aminu, I.; Gumel, S.M.; Ahmad, W.A.; Idris, A.A. Adsorption Isotherms and Kinetic Studies of Congo-Red Removal from Waste Water Using Activated Carbon Prepared from Jujube Seed. Am. J. Anal. Chem. 2020, 11, 47–59. [Google Scholar] [CrossRef]
- Haddadian, Z.; Shavandi, M.A.; Zainal, Z.; Halim, M.; Ismail, S. Removal Methyl Orange from Aqueous Solutions Using Dragon Fruit (Hylocereusundatus ) Foliage. Chem. Sci. Trans. 2013, 2, 900–910. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Zhang, C.; Yue, Q.; Li, Y.; Li, C. Equilibrium and Kinetic Studies of Methyl Orange and Methyl Violet Adsorption on Activated Carbon Derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Shahid, M.; Farooqi, Z.H.; Begum, R.; Arif, M.; Wu, W.; Irfan, A. Hybrid Microgels for Catalytic and Photocatalytic Removal of Nitroarenes and Organic Dyes From Aqueous Medium: A Review. Crit. Rev. Anal. Chem. 2020, 50, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Ghosh, A.; Satpathi, S.; Saha, R. Modified synthesis of nanoscale zero-valent iron and its ultrasound-assisted reactivity study on a reactive dye and textile industry effluent. Desalin. Water Treat. 2015, 57, 19321–19332. [Google Scholar] [CrossRef]
- Sulaiman, S.M.; Al-Jabari, M.H. Enhanced adsorptive removal of diclofenac sodium from aqueous solution by bentonite-supported nanoscale zero-valent iron. Arab J. Basic Appl. Sci. 2021, 28, 51–63. [Google Scholar] [CrossRef]
- Medina-Pérez, G.; Fernández-Luqueño, F.; Vazquez-Nuñez, E.; López-Valdez, F.; Prieto-Mendez, J.; Madariaga-Navarrete, A.; Miranda-Arámbula, M. Remediating Polluted Soils Using Nanotechnologies: Environmental Benefits and Risks. Pol. J. Environ. Stud. 2019, 28, 1013–1030. [Google Scholar] [CrossRef]
- Sombra, F.; Lago, F.R.; Yokoyama, L. Synthesis and characterization of zero-valent iron nanoparticles supported on SBA-15. J. Mater. Res. Technol. 2016, 6, 178–183. [Google Scholar] [CrossRef]
- Arif, M. Complete life of cobalt nanoparticles loaded into cross-linked organic polymers: A review. RSC Adv. 2022, 12, 15447–15460. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdo, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behaviour, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Tai, C.; Zhou, Q.; Hu, J.; Jiang, G. Rapid decolorization of water soluble azo-dyes by nanosized zero-valent iron immobilized on the exchange resin. Sci. China Ser. B Chem. 2008, 51, 186–192. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, X.; Chen, Z.; Megharaj, M.; Naidu, R. Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J. Colloid Interface Sci. 2011, 363, 601–607. [Google Scholar] [CrossRef]
- Tomašević, D.D.; Kozma, G.; Kerkez, D.V.; Dalmacija, B.D.; Dalmacija, M.B.; Bečelić-Tomin, M.R.; Kukovecz, Á.; Kónya, Z.; Rončević, S. Toxic metal immobilization in contaminated sediment using bentonite- and kaolinite-supported nano zero-valent iron. J. Nanoparticle Res. 2014, 16, 2548. [Google Scholar] [CrossRef]
- Kim, K.R.; Lee, B.T.; Kim, K.W. Arsenic stabilization in mine tailings using nano-sized magnetite and zero valent iron with the enhancement of mobility by surface coating. J. Geochemical Explor. 2012, 113, 124–129. [Google Scholar] [CrossRef]
- Luo, S.; Qin, P.; Shao, J.; Peng, L.; Zeng, Q.; Gu, J.D. Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for Orange II removal. Chem. Eng. J. 2013, 223, 1–7. [Google Scholar] [CrossRef]
- Xi, Y.; Megharaj, M.; Naidu, R. Dispersion of zerovalent iron nanoparticles onto bentonites and use of these catalysts for orange II decolourisation. Appl. Clay Sci. 2011, 53, 716–722. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye using activated carbon prepared from oil palm shell: Batch and fixed bed studies. Desalination 2008, 225, 13–28. [Google Scholar] [CrossRef]
- Rashmi, S.; Madhu, G.; Kittur, A.; Suresh, R. Synthesis, characterization and application of zero valent iron nanoparticles for the removal of toxic metal hexavalent chromium from aqueous solution. Int. J. Curr. Eng. Technol. 2013, 1, 37–42. [Google Scholar]
- Yaacob, W.Z.W.; How, H.K. Synthesis and Characterization of Marine Clay-Supported Nano Zero Valent Iron. Am. J. Environ. Sci. 2015, 11, 115–124. [Google Scholar] [CrossRef]
- Roy, W.; Krapac, I.; Chou, S.; Griffin, R. Batch-Type Procedures for Estimating Soil Adsorption of Chemicals. EPA/530/SW- 87/006-F; United States Environmental Protection Agency: Washington, DC, USA, 1992. [Google Scholar]
- Shahid, M.; Farooqi, Z.H.; Begum, R.; Arif, M.; Irfan, A.; Azam, M. Extraction of cobalt ions from aqueous solution by microgels for in-situ fabrication of cobalt nanoparticles to degrade toxic dyes: A two fold-environmental application. Chem. Phys. Lett. 2020, 754, 137645. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gasses on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1382. [Google Scholar] [CrossRef]
- Garcí, E.R.; Medina, R.L.; Lozano, M.M.; Pérez, I.H.; Valero, M.J.; Franco, A.M.M. Adsorption of Azo-Dye Orange II from Aqueous Solutions Using a Metal-Organic Framework Material: Iron- Benzenetricarboxylate. Materials 2014, 12, 8037–8057. [Google Scholar] [CrossRef] [PubMed]
- Vadi, M.; Abbasi, M.; Zakeri, M.; Yazdi, B.J. Application of the Freundlich, Langmuir, Temkin and Harkins-Jura Adsorption Isotherms for Some Amino Acids and Amino Acids Complexation with Manganese Ion ( II ) on Carbon Nanotube. 2010 Int. Conf. Nanotechnol. Biosens. IPCBEE 2011, 2, 117–119. [Google Scholar]
- Yu, C.; Shao, J.C.; Cai, X.Q.; Yu, X.N. Research on preparation of nanoscale iron supported by bentonite and its remediation of lead ions. Dig. J. Nanomater. Biostructures 2018, 13, 31–38. [Google Scholar]
- Zarime, N.A.; Yaacob, W.Z.W.; Jamil, H. Degradation of anionic dye (acid orange II) by bentonite supported nano-zero valent iron. Asian J. Chem. 2019, 31. [Google Scholar] [CrossRef]
- Wang, X.W.W.; Zhou, M.; Mao, Q.; Yue, J. Novel NaY zeolite-supported nanoscale zero-valent iron as an efficient heterogeneous Fenton catalyst. Catal. Commun. 2010, 11, 937–941. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, Z.; Liu, Y.; Yang, G. Preparation of organosolv lignin-stabilized nano zero-valent iron and its application as granular electrode in the tertiary treatment of pulp and paper wastewater. Chem. Eng. J. 2018, 331, 317–325. [Google Scholar] [CrossRef]
- Zarime, N.A.; Yaacob, W.Z.W.; Jamil, H. Removal of heavy metals using bentonite supported nano-zero valent iron particles. 2017 UKM FST Postgrad. Colloq. AIP Conf. Proc. Am. Inst. Phys. 2018, 1940, 020029-1–020029-7. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S.; Chaudhary, M.; Tyagi, I. Removal of Ni (II) ions from water using scrap tire. J. Mol. Liq. 2014, 190, 215–222. [Google Scholar] [CrossRef]
- Kakavandi, B.; Kalantary, R.R.; Farzadkia, M.; Mahvi, A.H.; Esrafili, A.; Azari, A.; Yari, A.R.; Javid, A.B. Enhanced chromium (VI) removal using activated carbon modified by zero valent iron and silver bimetallic nanoparticles. J. Environ. Health Sci. Eng. 2014, 12, 115. [Google Scholar] [CrossRef]
- Wan Zuhairi, W.Y.; Rahim, S.A. Sorption Parameters of Pb and Cu on Natural Clay Soils from Selangor, Malaysia. Sains Malaysiana 2007, 36, 149–157. [Google Scholar]
- Pourfadakari, S.; Mahvi, A.H. Kinetics and Equilibrium Studies for Removal of Reactive Red 198 From Aqueous Solutions Using Zero Valent Iron powder. Health Scope 2014, 3, 14883. [Google Scholar] [CrossRef]
- Alemayehu, E.; Lennartz, B. Virgin volcanic rocks: Kinetics and equilibrium studies for the adsorption of cadmium from water. J. Hazard. Mater. 2009, 169, 395–401. [Google Scholar] [CrossRef]
- Shaibu, S.E.; Adekola, F.A.; Adegoke, H.I.; Ayanda, O.S. A comparative study of the adsorption of methylene blue onto synthesized nanoscale zero-valent iron-bamboo and manganese-bamboo composites. Materials 2014, 7, 4493–4507. [Google Scholar] [CrossRef]
- Chikri, R.; Elhadiri, N.; Benchanaa, M.; El maguana, Y. Efficiency of Sawdust as Low-Cost Adsorbent for Dyes Removal. J. Chem. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Arif, M.; Shahid, M.; Irfan, A.; Nisar, J.; Wang, X.; Batool, N.; Ali, M.; Farooqi, Z.H.; Begum, R. Extraction of copper ions from aqueous medium by microgel particles for in-situ fabrication of copper nanoparticles to degrade toxic dyes. Zeitschrift fur Phys. Chemie 2022, 236, 1219–1241. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; Issa, A.A.; Khraisheh, M.A.; Walker, G.M. Sorption of Zn(II), Pb(II), and Co(II) using natural sorbents: Equilibrium and kinetic studies. Water Res. 2006, 40, 2645–2658. [Google Scholar] [CrossRef]
- Pan, M.; Lin, X.; Xie, J.; Huang, X. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskite nano-composites. RSC Adv. 2017, 7, 4492–4500. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhao, R.; Li, Y.; Li, C.; Zhang, C. Adsorption of Pb(II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour. Technol. 2010, 101, 5808–5814. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study. South African J. Chem. Eng. 2020, 32, 39–55. [Google Scholar] [CrossRef]
- Kerkez, D.V.; Tomašević, D.D.; Kozma, G.; Bečelić-Tomin, M.R.; Prica, M.D.; Rončević, S.D.; Kukovecz, A.; Dalmacija, B.D.; Kónya, Z. Three different clay-supported nanoscale zero-valent iron materials for industrial azo dye degradation: A comparative study. J. Taiwan Inst. Chem. Eng. 2014, 45, 2451–2461. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, M.; Sharma, S. Process Development for the Removal of Lead and Chromium from Aqueous Solutions using Red Mud, an Aluminium Industry Waste. Wat. Res. 2001, 35, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.R.; Samadi, M.T.; Shokoohi, R.; Nasab, H.Z. Monitoring of pH, Oxidation-Reduction Potential and Dissolved Oxygen to Improve the Performance of Dimethyl Phthalate Removal From Aqueous Solutions. Avicenna J. Environ. Health Eng. 2015. In Press. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Loizidou, M.M.; Grigoropoulou, H.P. Ion exchange studies on natural and modified zeolites and the concept of exchange site accessibility. J. Colloid Inteface Sci. 2004, 275, 570–576. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Arif, M.; Farooqi, Z.H.; Irfan, A.; Begum, R. Gold nanoparticles and polymer microgels: Last five years of their happy and successful marriage. J. Mol. Liq. 2021, 336, 116270. [Google Scholar] [CrossRef]




| Sample | BET Surface Area (m²/g) | Pore Volume (cm³/g) | Pore Size (Å) |
|---|---|---|---|
| Bentonite (B) | 74.7755 | 0.0994 | 53.1595 |
| B-nZVI | 5.6418 | 0.0226 | 160.2891 |
| Sample | Test | Element K | Element Mg | Element Ca | Element Na | CEC Value (meq/100 g) | Average (meq/100 g) | Standard Deviation | % RSD |
|---|---|---|---|---|---|---|---|---|---|
| Bentonite (B) | 1 | 0.81 | 24.52 | 19.11 | 0.18 | 44.61 | 48.35 | 3.24 | 6.70 |
| 2 | 0.87 | 26.00 | 21.01 | 0.04 | 47.91 | ||||
| 3 | 0.64 | 26.50 | 25.23 | 0.14 | 52.51 | ||||
| B-nZVI | 1 | 3.03 | 22.90 | 5.92 | 0.01 | 31.85 | 30.81 | 1.03 | 3.33 |
| 2 | 2.87 | 20.93 | 5.61 | 0.01 | 29.41 | ||||
| 3 | 3.24 | 21.74 | 6.15 | 0.02 | 31.16 |
| Element (%) | Sample | |
|---|---|---|
| Bentonite | B-nZVI | |
| SiO2 | 48.65 | 17.36 |
| Al2O3 | 15.44 | 5.27 |
| Fe2O3 | 16.39 | 34.14 |
| Na2O | 1.94 | 20.76 |
| Cl | 0.62 | 0.1 |
| Cr2O3 | 0.01 | 0.01 |
| K2O | 0.19 | 0.08 |
| TiO2 | 1.76 | 0.97 |
| MgO | 2.79 | 0.88 |
| CaO | 1.49 | 0.71 |
| P2O5 | 0.15 | 0.07 |
| SO3 | 0.17 | 0.01 |
| ZrO2 | 0.02 | 0.01 |
| MnO | 0.08 | 0.05 |
| V2O5 | 0.06 | 0.03 |
| ZnO | 0.02 | 0.01 |
| CuO | 0.02 | 0.01 |
| SrO | 0.02 | 0.01 |
| NiO | 0.01 | - |
| LOI | 9.85 | 19.75 |
| Total (%) | 99.68 | 100.23 |
| Sample | Linear Equation | Langmuir Equation | Freundlich Equation | |||||
|---|---|---|---|---|---|---|---|---|
| Kd (L/g) | R2 | KL (L/g) | Am (mg/g) | R2 | KF | 1/n | R2 | |
| Bentonite (B) | 0.0219 | 0.8892 | 0.0206 | 0.6836 | 0.6105 | 20.1976 | 0.5159 | 0.3861 |
| B-nZVI | 12.3610 | 0.2740 | 30,314.0536 | 0.1516 | 1.0000 | 256.7438 | 3.3268 | 0.8111 |
| Sample | Contaminants | Type of Solution | Initial Concentration, Co (mg/L) | qe Experimental (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | Interparticle Diffusion Model | Intraparticle Diffusion Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 (1/min) | qe Calculation (mg/g) | R2 | K2 (1/min) | qe Calculation (mg/g) | R2 | Kf (m2 g−1 min L−1) | R2 | Kd (mg g−1 min−0.5) | C | R2 | |||||
| Bentonite | MB | Single | 50 | 1.6572 | 0.0007 | 0.3571 | 0.0219 | 0.4114 | 1.2038 | 0.9993 | 0.0002 | 0.6253 | 0.2886 | 0.3214 | 0.6026 |
| B-nZVI | MB | Single | 50 | 7.4667 | 0.0006 | 0.5272 | 0.0668 | 0.0204 | 6.9979 | 1.0000 | 0.8645 | 0.9000 | 2.3829 | 0.3787 | 0.9189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarime, N.‘A.; Solemon, B.; Wan Yaacob, W.Z.; Jamil, H.; Che Omar, R.; Rafek, A.G.; Roslan, R. Adsorption of Methylene Blue by Bentonite Supported Nano Zero Valent Iron (B-nZVI). Processes 2023, 11, 788. https://doi.org/10.3390/pr11030788
Zarime N‘A, Solemon B, Wan Yaacob WZ, Jamil H, Che Omar R, Rafek AG, Roslan R. Adsorption of Methylene Blue by Bentonite Supported Nano Zero Valent Iron (B-nZVI). Processes. 2023; 11(3):788. https://doi.org/10.3390/pr11030788
Chicago/Turabian StyleZarime, Nur ‘Aishah, Badariah Solemon, Wan Zuhairi Wan Yaacob, Habibah Jamil, Rohayu Che Omar, Abdul Ghani Rafek, and Rasyikin Roslan. 2023. "Adsorption of Methylene Blue by Bentonite Supported Nano Zero Valent Iron (B-nZVI)" Processes 11, no. 3: 788. https://doi.org/10.3390/pr11030788
APA StyleZarime, N. ‘A., Solemon, B., Wan Yaacob, W. Z., Jamil, H., Che Omar, R., Rafek, A. G., & Roslan, R. (2023). Adsorption of Methylene Blue by Bentonite Supported Nano Zero Valent Iron (B-nZVI). Processes, 11(3), 788. https://doi.org/10.3390/pr11030788








