Phenol Degradation Performance in Batch and Continuous Reactors with Immobilized Cells of Pseudomonas putida
Abstract
1. Introduction
2. Kinetic Model
2.1. Cell Growth in Batch Reactor
2.2. Phenol Degradation by Immobilized Cells
3. Materials and Methods
3.1. P. putida Cell Activation
3.2. Nutrient Medium
3.3. Preparation of Immobilized Cells
3.4. Batch Tests
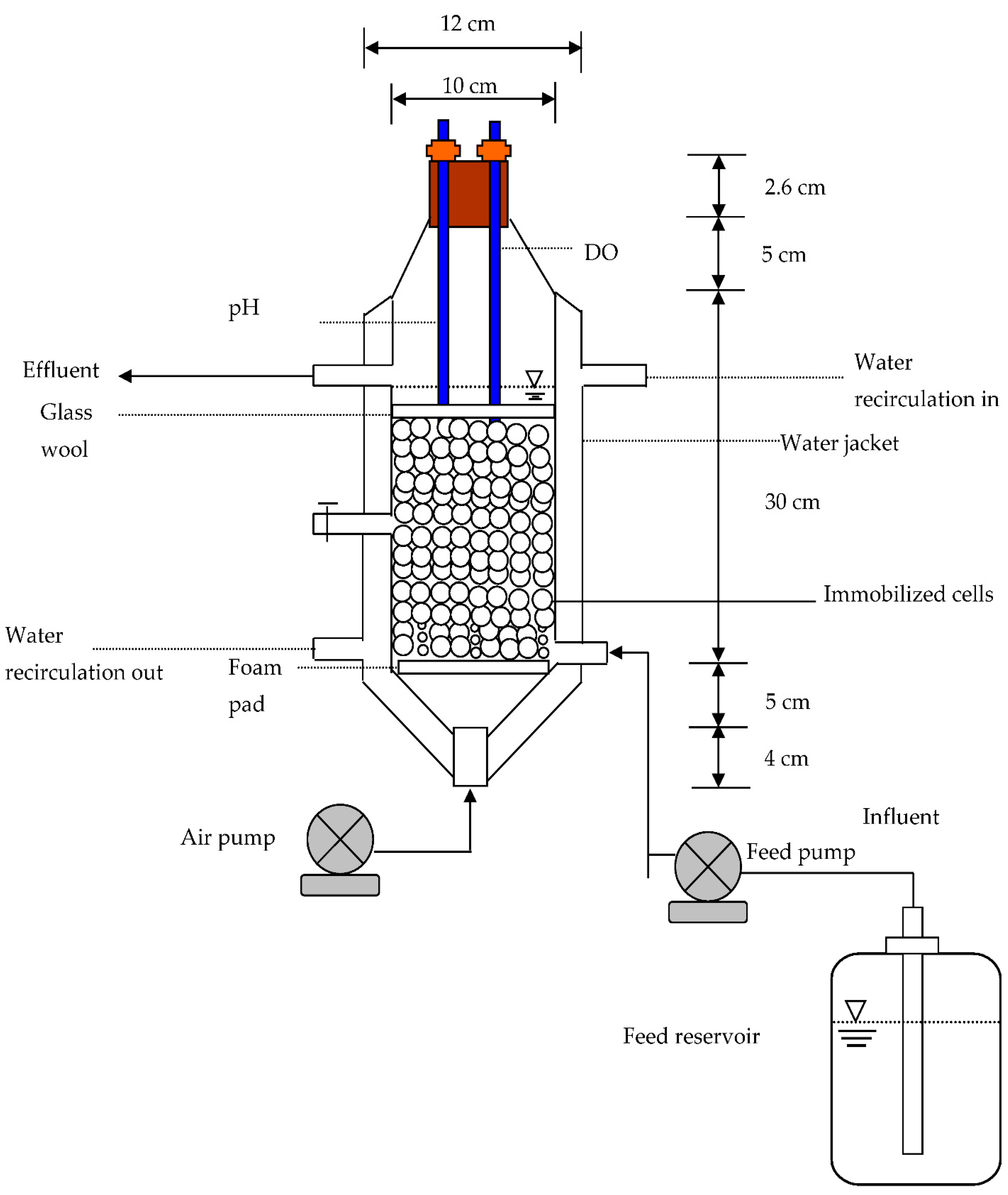
3.5. Column Tests
3.6. Analytical Techniques
4. Results and Discussion
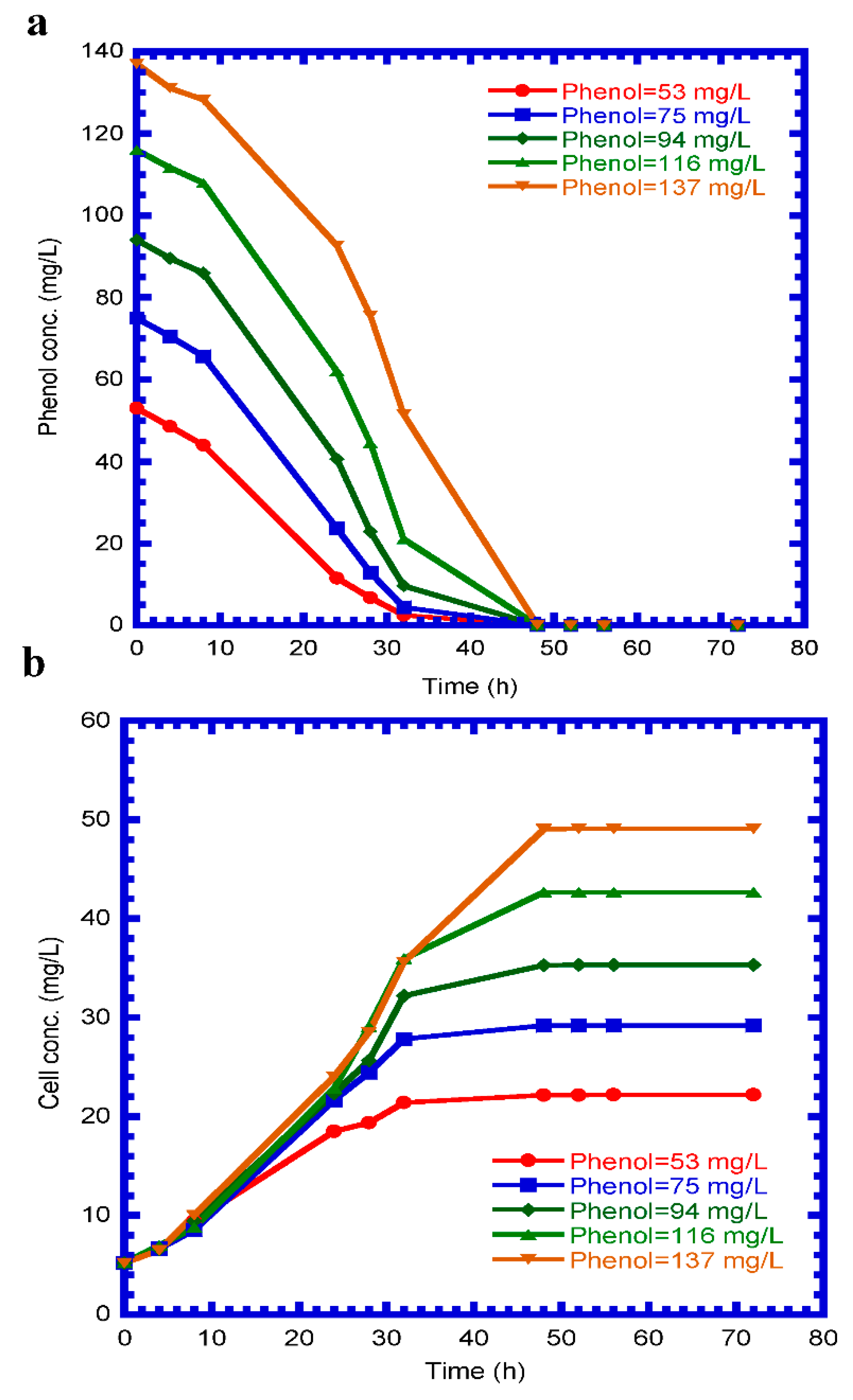
4.1. Phenol Degradation and Cell Growth in the Batch Mode
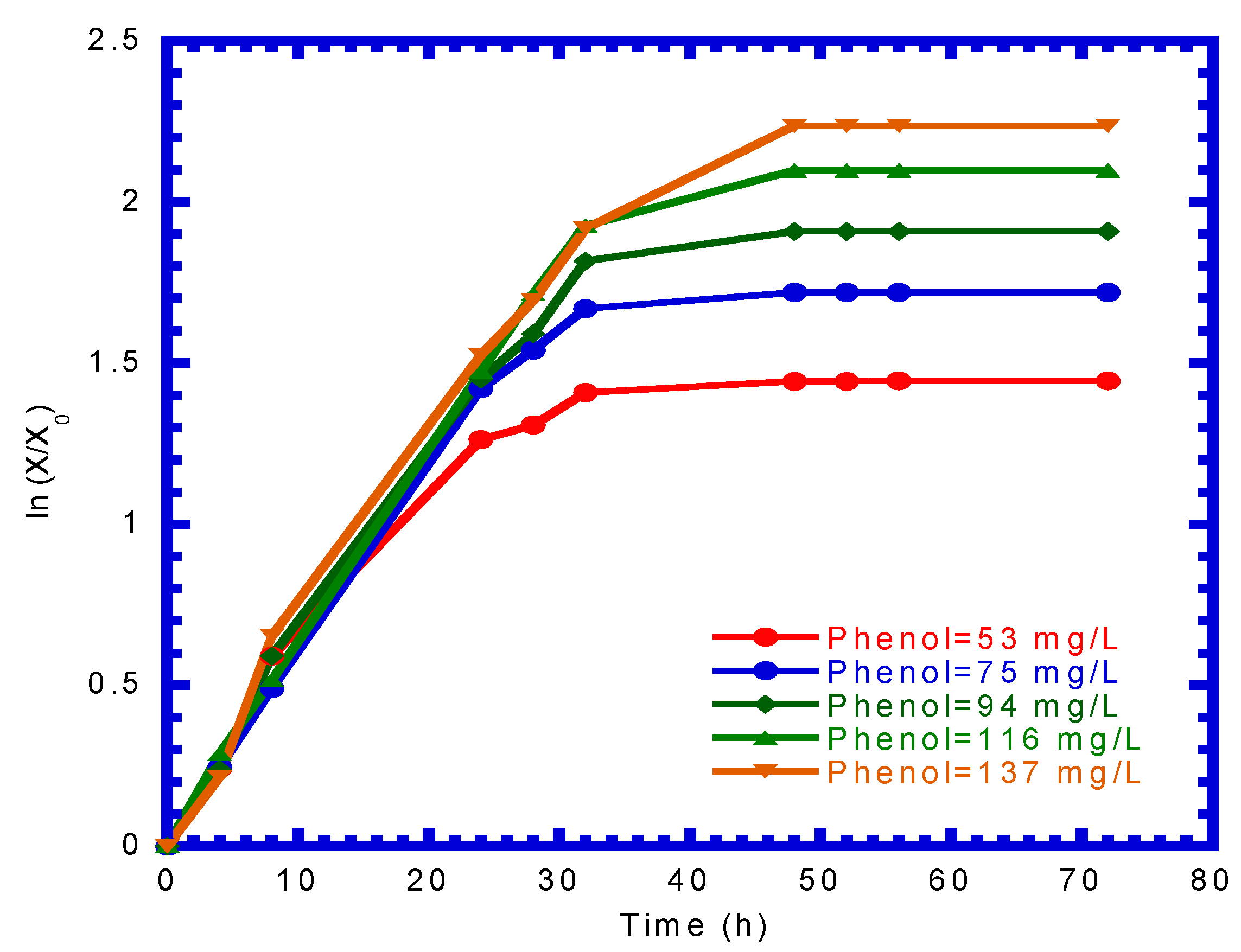
4.2. Determination of Kinetic Parameters
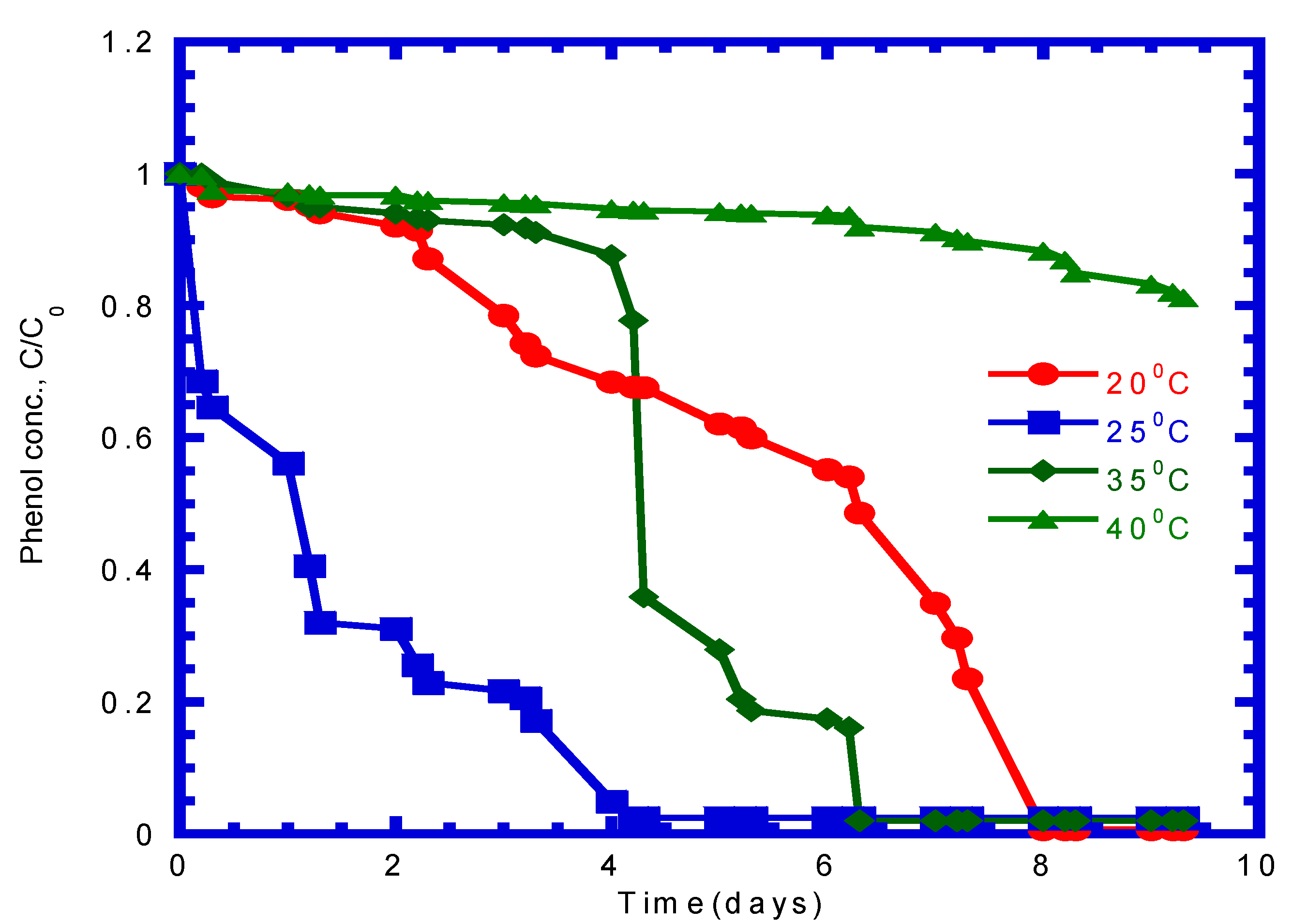
4.3. Influence of Temperature on Phenol Utilization in Batch Mode
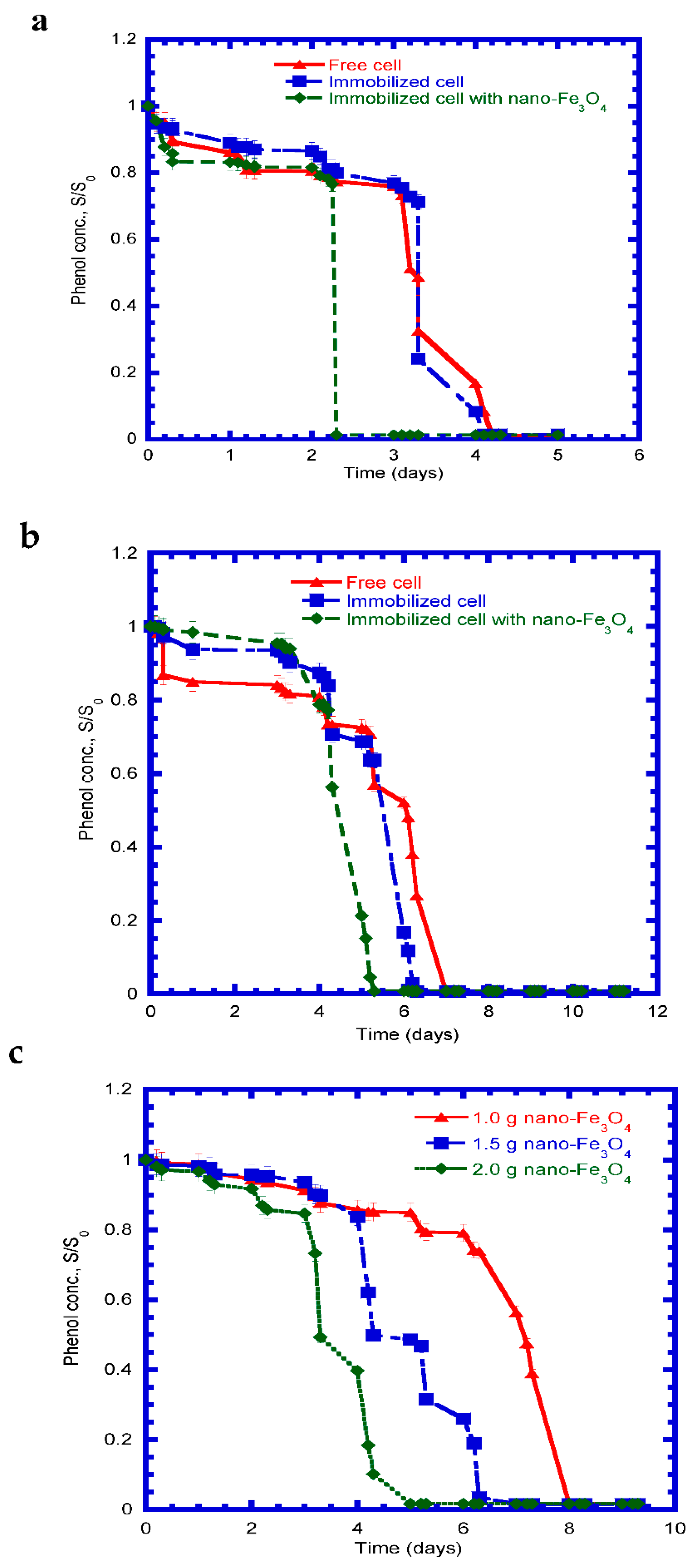
4.4. Phenol Degradation by Free and Immobilized Cells in Batch Mode
| Culture | Maximum Initial Phenol Conc. (mg/L) | μm (1/h) | KS (mg/L) | KI (mg/L) | Temp (°C)/pH | Reference |
|---|---|---|---|---|---|---|
| P. putida DSM 50222 | - | 0.39 | 1.1 | 903 | 30/6.6 | [30] |
| P. putida P 71 | - | 0.57 | 18.5 | 99.4 | 25/6.8 | [31] |
| P. putida DSM 548 | 100 | 0.44 | 6.19 | 54.1 | 26/6.9 | [32] |
| P. putida CCRC1 4365 | 1000 | 0.33 | 13.9 | 669 | 30/6.8 | [25] |
| P. putida F1 ATCC 700007 | 500 | 0.051 | 18 | 430.1 | 30/7.0 | [33] |
| P. putida MTCC 1194 | 1000 | 0.305 | 36.33 | 129.79 | 29.9 ± 0.3/- | [34] |
| P. putida 17484 | 137 | 0.15 | 87.5 | 111.6 | 30/7.0 | This work |
4.5. Phenol Degradation under Salinity in Batch Mode
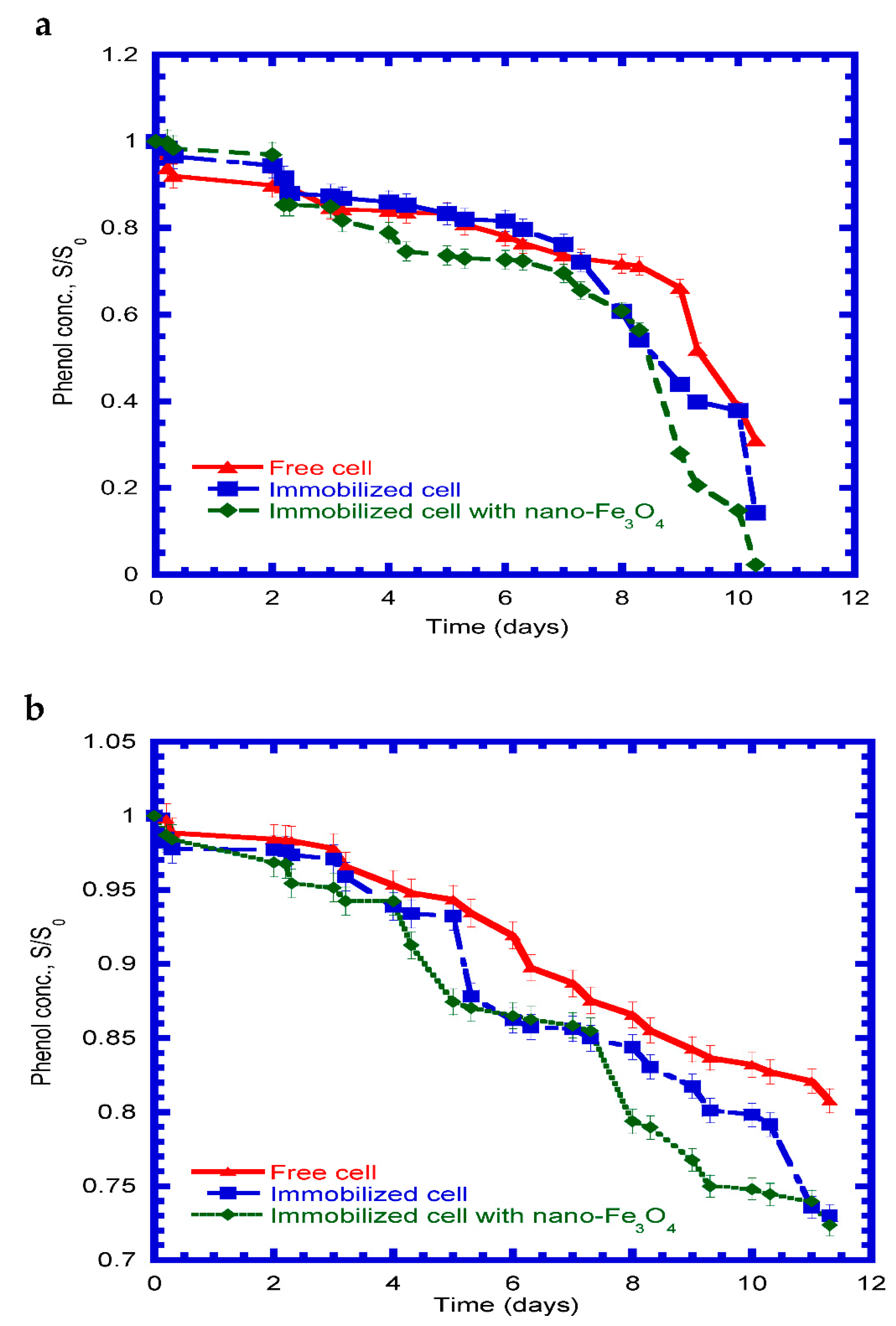
4.6. Phenol Degradation in Continuous Mode
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Li, Y.; Yu, Z.; Chen, X. Effects of 4-chlorophenol wastewater treatment on sludge acute toxicity, microbial diversity and functional genes expression in an activated sludge process. Bioresour. Technol. 2018, 265, 39–44. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Lu, Y.; Hu, Z.; Chen, S.; Zeng, R.J. The performance and microbial communities of an anaerobic membrane bioreactor for treating fluctuating 2-chlorophenol wastewater. Bioresour. Technol. 2020, 317, 124001. [Google Scholar] [CrossRef]
- Liu, Q.S.; Zheng, T.Z.; Wang, P.; Jiang, J.P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Arora, P.K.; Bae, H. Bacterial degradation of chlorophenols and their derivatives. Microb. Cell Factories 2014, 13, 31. [Google Scholar] [CrossRef]
- Kayan, I.; Oz, N.A.; Kantar, C. Coupling pyrite-Fenton process with aerobic biodegradation for the treatment of 2-chlorophenol. Water Air Soil Pollut. 2020, 231, 463. [Google Scholar] [CrossRef]
- Yang, M.; Huang, Y.; Cao, H.; Lin, Y.; Cheng, X.; Wang, X. A novel polymeric adsorbent by a self-doped manner: Synthesis, characterization, and adsorption performance to phenol from aqueous solution. Polym. Bull. 2016, 73, 2321–2341. [Google Scholar] [CrossRef]
- Xue, J.; Wu, Y.; Shi, K.; Xiao, X.; Gao, Y.; Li, L.; Qiao, Y. Study on the degradation performance and kinetics of immobilized cells in straw-alginate beads in marine environment. Bioresour. Technol. 2019, 280, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Zhang, Y.; Liu, G.; Ye, Z.; Chu, P.K. Treatment of heavy oil wastewater by a conventional activated sludge process coupled with an immobilized biological filter. Int. Biodeterior. Biodegrad. 2013, 84, 65–71. [Google Scholar] [CrossRef]
- Li, S.; Ma, B.; She, Z.; Guo, L.; Zhao, Y.; Jin, C.; Gao, M. Effect of norfloxacin on performance, microbial enzymatic activity and microbial community of a sequencing batch reactor. Environ. Technol. Innov. 2020, 18, 100726. [Google Scholar] [CrossRef]
- González, G.; Herrera, M.G.; García, M.T.; Peña, M.M. Biodegradation of phenol in a continuous process: Comparative study of stirred tank and fluidized-bed bioreactors. Bioresour. Technol. 2001, 76, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Bergero, M.F.; Lucchesi, G.I. Immobilization of Pseudomonas putida A (ATCC 12633) cells: A promising tool for effective degradation of quaternary ammonium compounds in industrial effluents. Int. Biodeterior. Biodegrad. 2015, 100, 38–43. [Google Scholar] [CrossRef]
- Kureel, M.K.; Geed, S.R.; Giri, B.S.; Shukla, A.K.; Rai, B.N.; Singh, R.S. Removal of aqueous benzene in the immobilized batch and continuous packed bed bioreactor by isolated bacillus, sp. m1. Resour.-Effic. Technol. 2016, 2, S87–S95. [Google Scholar] [CrossRef]
- Parameswarappa, S.; Karigar, C.; Nagenahalli, M. Degradation of ethylbenzene by free and immobilized Pseudomonas fluorescens-cs2. Biodegradation 2008, 19, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Stelting, S.; Burns, R.G.; Sunna, A.; Visnovsky, G.; Bunt, C.R. Immobilization of Pseudomonas sp. strain ADP: A stable inoculant for the bioremediation of atrazine. App. Clay Sci. 2012, 64, 90–93. [Google Scholar] [CrossRef]
- Isaka, K.; Udagawa, M.; Kimura, Y.; Sei, K.; Ike, M. Biological wastewater treatment of 1,4-dioxane using polyethylene glycol gel carriers entrapping Afipia sp. D1. J. Biosci. Bioeng. 2016, 121, 203–208. [Google Scholar] [CrossRef]
- Wang, X.; Gai, Z.; Yu, B.; Feng, J.; Xu, C.; Yuan, Y.; Lin, Z.; Xu, P. Degradation of carbazole by microbial cells immobilized in magnetic gellan gum gel beads. Appl. Environ. Microbiol. 2007, 73, 6421–6428. [Google Scholar] [CrossRef]
- Shi, S.; Qu, Y.; Ma, F.; Zhou, J. Bioremediation of coking wastewater containing carbazole, dibenzofuran and dibenzothiphene by immobilized naphthalene-cultivated Arthrobacter sp. W1 in magnetic gellan gum. Bioresour. Technol. 2014, 166, 79–86. [Google Scholar] [CrossRef]
- Leilei, Z.; Mingxin, H.; Suiyi, Z. Biodegradation of p-nitrophenol by immobilized Rhodococcus sp. strain Y-1. Chem. Biochem. Eng. Q. 2012, 26, 137–144. [Google Scholar]
- Hou, J.; Liu, F.; Wu, N.; Ju, J.; Yu, B. Efficient biodegradation of chlorophenols in aqueous phase by magnetically immobilized aniline-degrading Rhodococcus rhodochrous strain. J. Nanobiotechnol. 2016, 14, 5. [Google Scholar] [CrossRef]
- Bhunia, B.; Basak, B.; Bhattacharya, P.; Dey, A. Kinetic studies of alkaline protease from Bacillus licheniformis NCIM-2042. J. Microbiol. Biotechnol. 2012, 22, 1758–1766. [Google Scholar] [CrossRef]
- Liu, J.; Jia, X.; Wen, J.; Zhou, Z. Substrate interactions and kinetics study of phenolic compounds biodegradation by Pseudomonas sp. cbp 1-3. Biochem. Eng. J. 2012, 67, 156–166. [Google Scholar] [CrossRef]
- Massalha, N.; Shaviv, A.; Sabbah, I. Modeling the effect of immobilization of microorganisms on the rate of biodegradation of phenol under inhibitory conditions. Water Res. 2010, 44, 5252–5259. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Loh, K.C. Modeling the role of metabolic intermediates in kinetics of phenol biodegradation. Enzyme Microb. Technol. 1999, 25, 177–184. [Google Scholar] [CrossRef]
- Juang, R.S.; Tsai, S.Y. Growth kinetics of Pseudomonas putida in the biodegradation of single and mixed phenol and sodium salicylate. Biochem. Eng. J. 2006, 31, 133–140. [Google Scholar] [CrossRef]
- Chung, T.P.; Tseng, H.Y.; Juang, R.S. Mass transfer effect and intermediate detection for phenol degradation in immobilized Pseudomonas putida systems. Process Biochem. 2003, 38, 1497–1507. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, T.; Shang, Y.; Yang, K.; Wang, H. Biodegradation of phenol by entrapped cell of Debaryomyces sp. with nano-Fe3O4 under hypersaline conditions. Int. Biodeterior. Biodegrad. 2017, 123, 37–45. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, M.S.; Siddavattam, D.; Karegoudar, T.B. Generation of continuous packed bed reactor with PVA-alginate blend immobilized Ochrobactrum sp. DGVK1 cells for effective removal of N,N-dimethylformamide from industrial effluents. J. Hazard. Mater. 2012, 199–200, 58–63. [Google Scholar] [CrossRef]
- Ke, Q.; Zhang, Y.; Wu, X.; Su, X.; Wang, Y.; Lin, H.; Mei, R.; Zhang, Y.; Hashmi, M.Z.; Chen, C.; et al. Sustainable Biodegradation of phenol by immobilized Bacillus sp. SAS19 with porous carbonaceous gels as carriers. J. Environ. Manag. 2018, 222, 185–189. [Google Scholar] [CrossRef]
- Huang, R.Y.; Tian, W.J.; Liu, Q.; Yu, H.B.; Jin, X.; Zhao, Y.G. Enhanced biodegradation of pyrene and indeno(1,2,3-cd)pyrene using immobilized in cinder beads in estuarine wetlands. Mar. Pollut. Bull. 2016, 102, 128–133. [Google Scholar] [CrossRef]
- Hutchinson, D.H.; Robinson, C.W. Kinetics of simultaneous batch degradation of cresol and phenol by Pseudomonas putida. Appl. Microbiol. Biotechnol. 1988, 25, 599–604. [Google Scholar] [CrossRef]
- Beyenal, H.S.; Tanyolac, S.A.; Salih, B. Diffusion coefficients of phenol and oxygen in a biofilm of Pseudomonas putida. AIChE J. 1997, 43, 243–250. [Google Scholar] [CrossRef]
- Monteiro, A.A.M.G.; Boaventura, R.A.R.; Rodrigues, A.E. Phenol biodegradation by Pseudomonas putida DSM 548 in a batch reactor. Biochem. Eng. J. 2000, 6, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Abuhamed, T.; Bayrakter, E.; Mehmetoglu, T.; Mehmetoglu, U. Kinetics model for growth of Pseudomonas putida F1 during benzene, toluene and phenol biodegradation. Process Biochem. 2004, 39, 983–988. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Kumar, S. Biodegradation kinetics of phenol and catechol using Pseudomonas putida MTCC 1194. Biochem. Eng. J. 2005, 22, 151–159. [Google Scholar] [CrossRef]




| Kinetic Parameters | Value |
|---|---|
| Maximum specific degradation rate, k (mg cell/mg phenol‒d) | 11.39 |
| Saturation constant, KS (mg/L) | 87.5 |
| Cell yield, Y (mg cell/mg phenol) | 0.316 |
| Effective diffusivity of phenol, De (cm2/d) | 0.965 |
| Mass transfer coefficient, kf (cm/d) | 92.52 |
| Total surface area of beads, At (cm2) | 2.053 × 104 |
| Porosity, ε (dimensionless) | 0.72 |
| Influent phenol concentration, Sb0 (mg/L) | 137 |
| Influent flow rate, Q (L/d) | 7.2 |
| Working volume, V (L) | 1.568 |
| Initial cell concentration in the bead, Xe0 (mg/L) | 5.24 |
| Inhibition constant, KI (mg/L) | 111.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Gu, Y.-J. Phenol Degradation Performance in Batch and Continuous Reactors with Immobilized Cells of Pseudomonas putida. Processes 2023, 11, 739. https://doi.org/10.3390/pr11030739
Lin Y-H, Gu Y-J. Phenol Degradation Performance in Batch and Continuous Reactors with Immobilized Cells of Pseudomonas putida. Processes. 2023; 11(3):739. https://doi.org/10.3390/pr11030739
Chicago/Turabian StyleLin, Yen-Hui, and Yi-Jie Gu. 2023. "Phenol Degradation Performance in Batch and Continuous Reactors with Immobilized Cells of Pseudomonas putida" Processes 11, no. 3: 739. https://doi.org/10.3390/pr11030739
APA StyleLin, Y.-H., & Gu, Y.-J. (2023). Phenol Degradation Performance in Batch and Continuous Reactors with Immobilized Cells of Pseudomonas putida. Processes, 11(3), 739. https://doi.org/10.3390/pr11030739







