A Study on the Corrosion Behaviour of Laser Textured Pure Aluminium in Saltwater
Abstract
1. Introduction
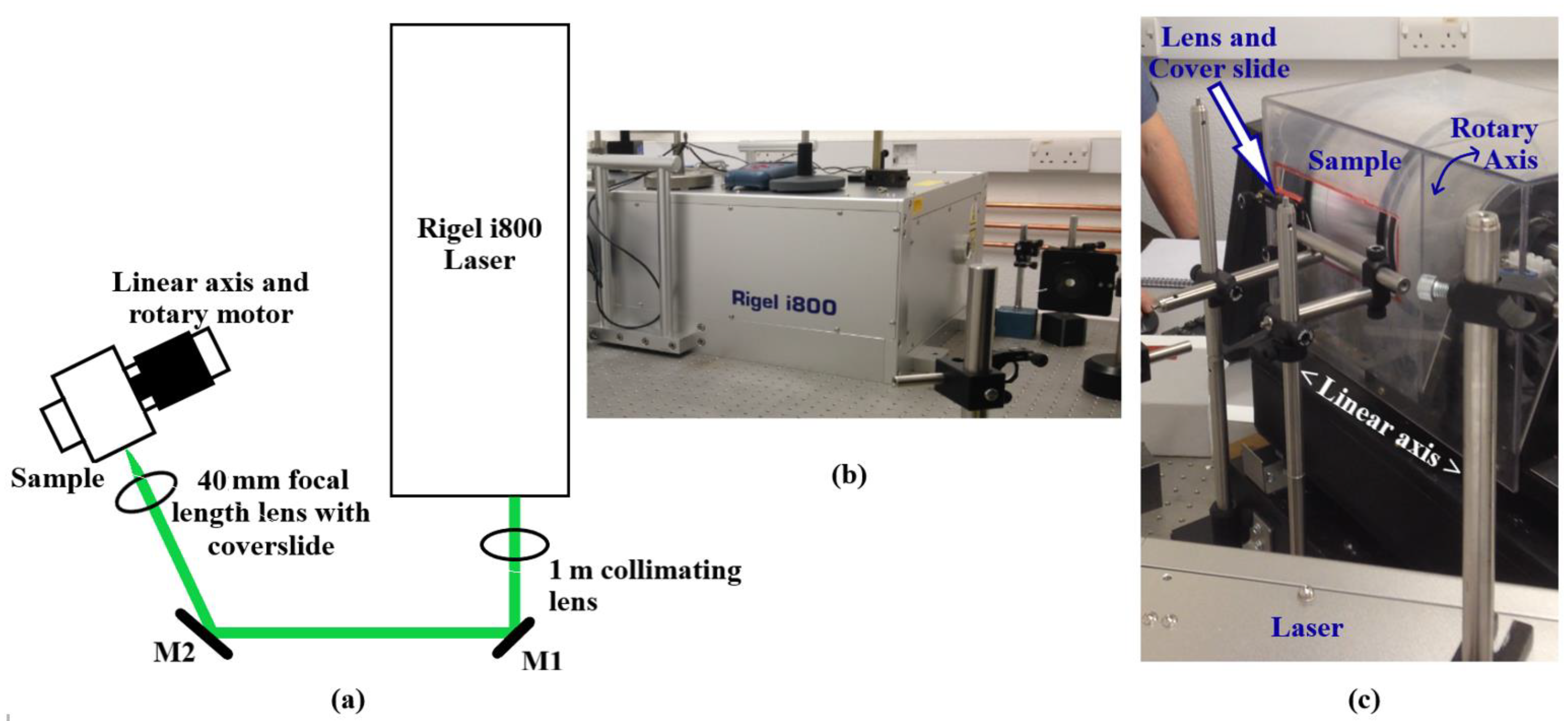
2. Experimental Setup
2.1. Materials
2.2. Laser Parameters
2.3. Electrochemical Analyses
3. Results
Electrochemical Assessments
3.1.1. Open Circuit Potential and Zero Resistance Ammeter (Electrochemical Noise), Passive Corrosion Testing
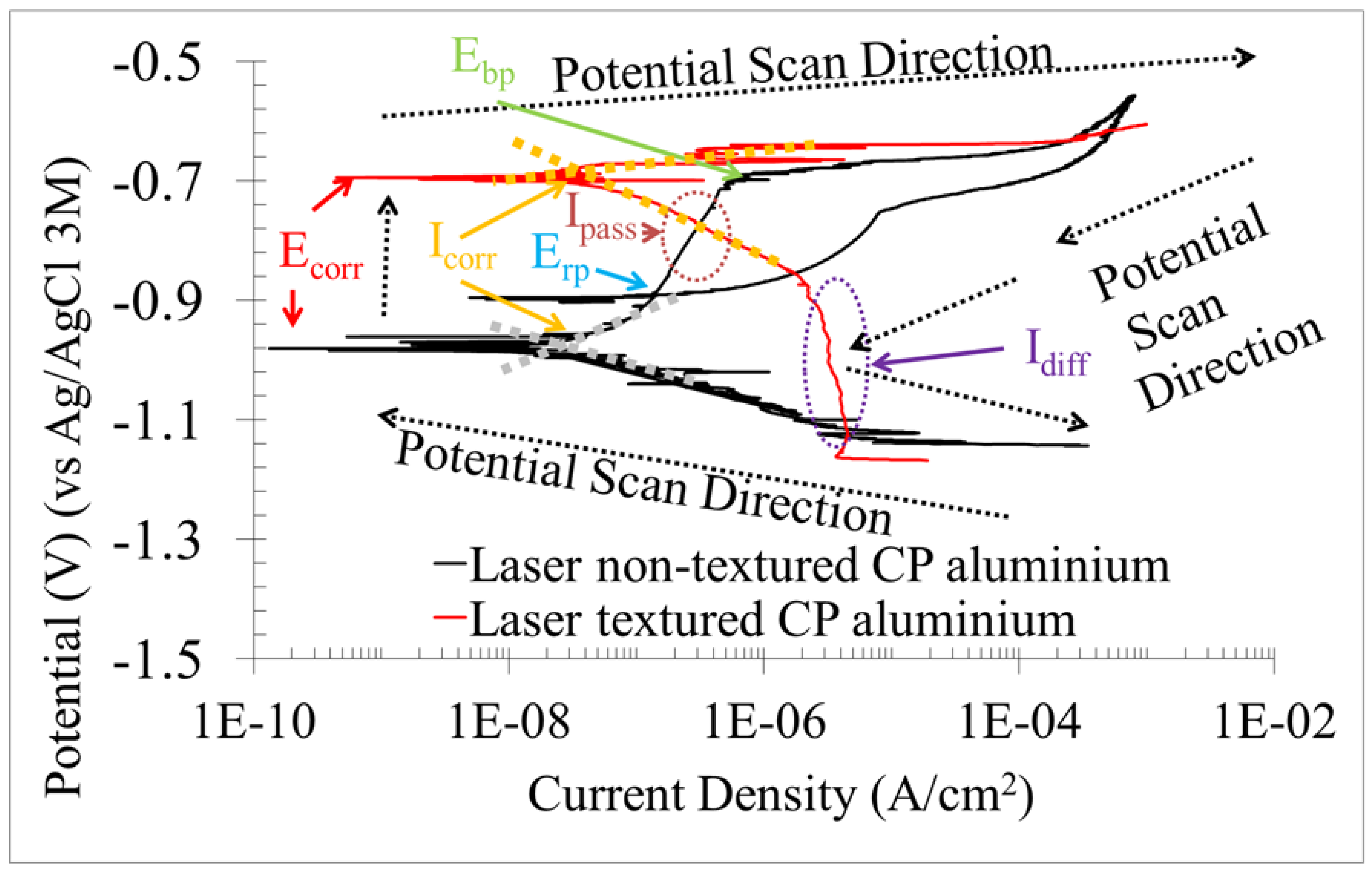
3.1.2. Potentiodynamic Polarisation Curve (PPC), Active Corrosion Testing of Direct Current
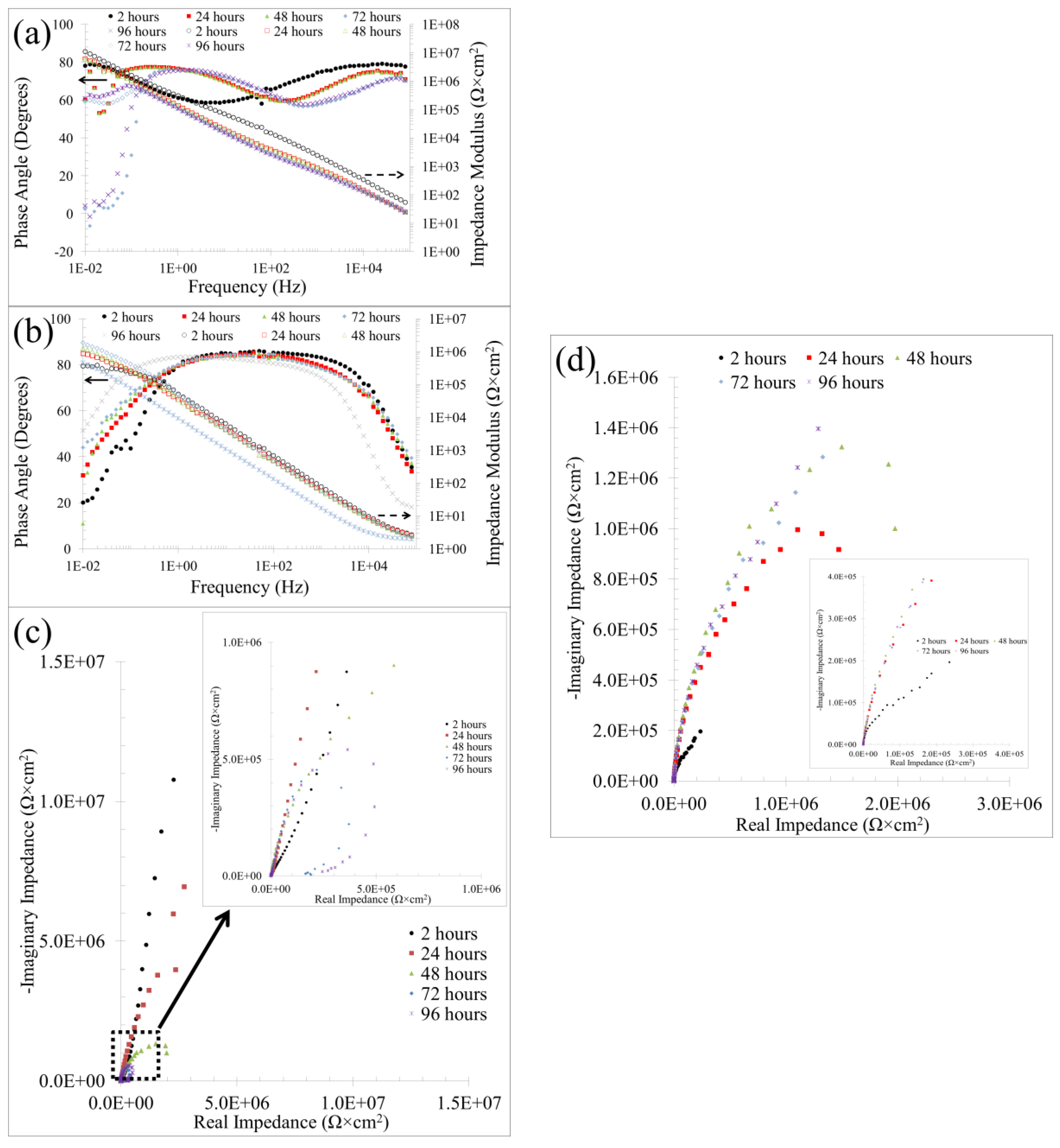
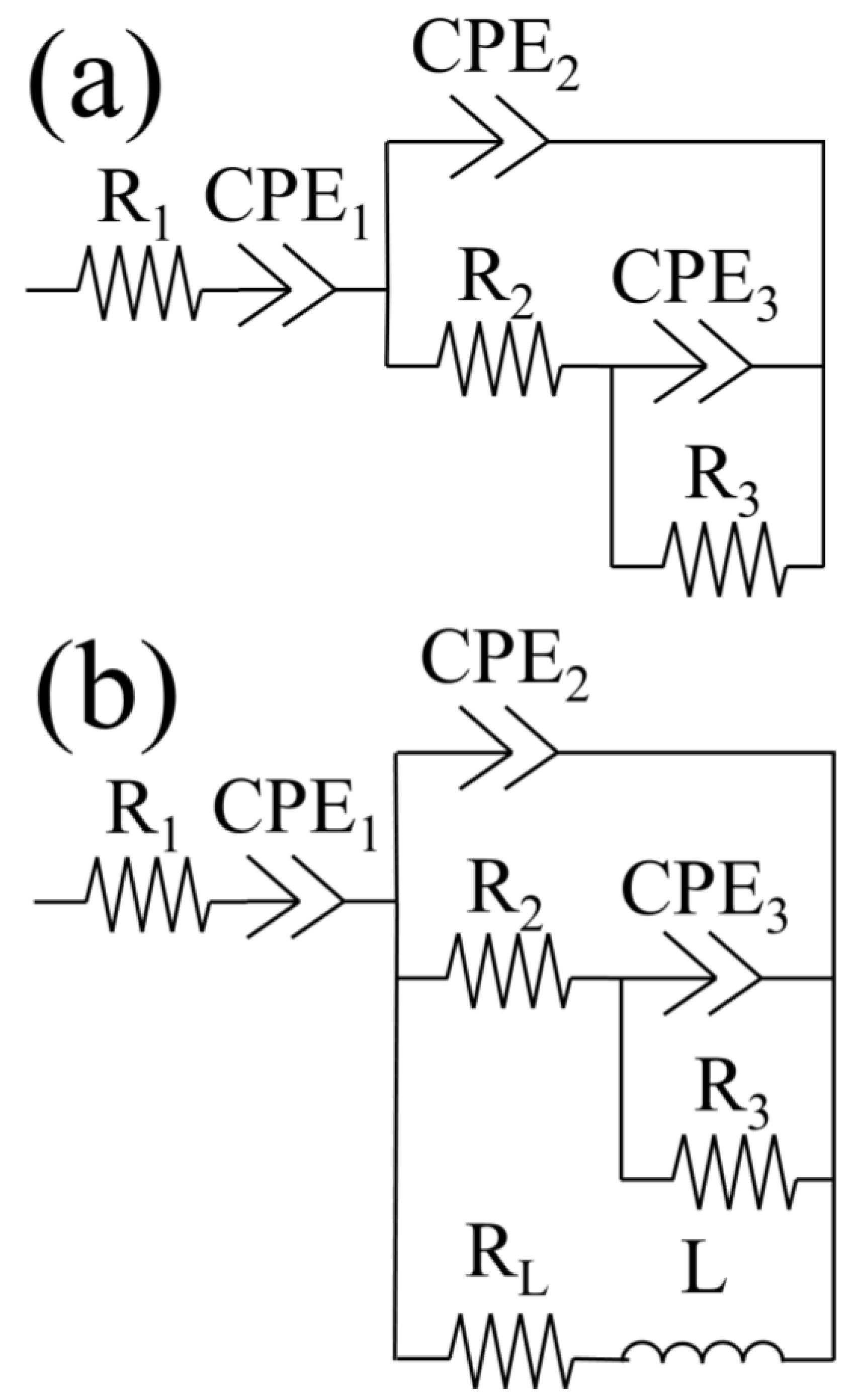
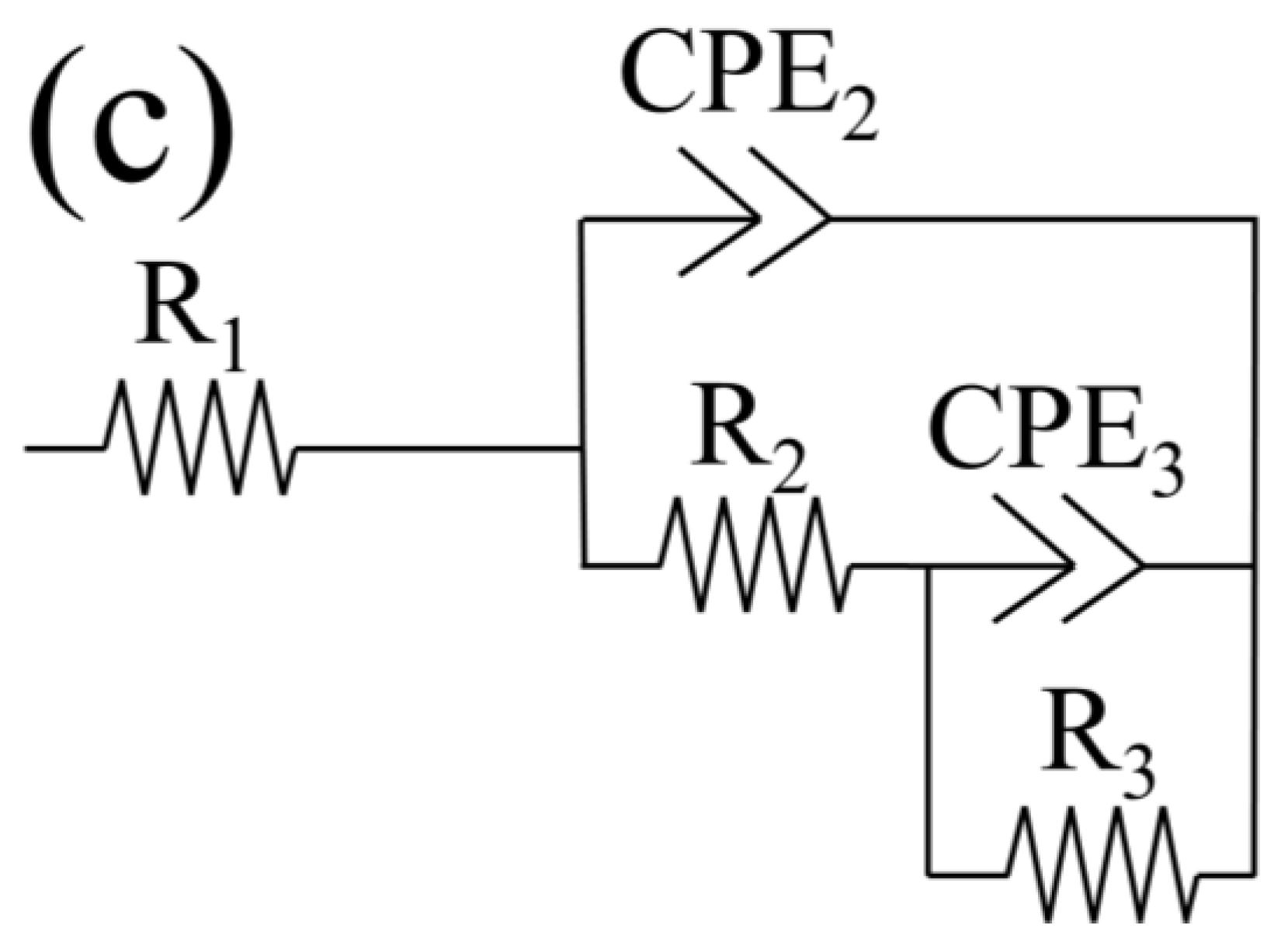
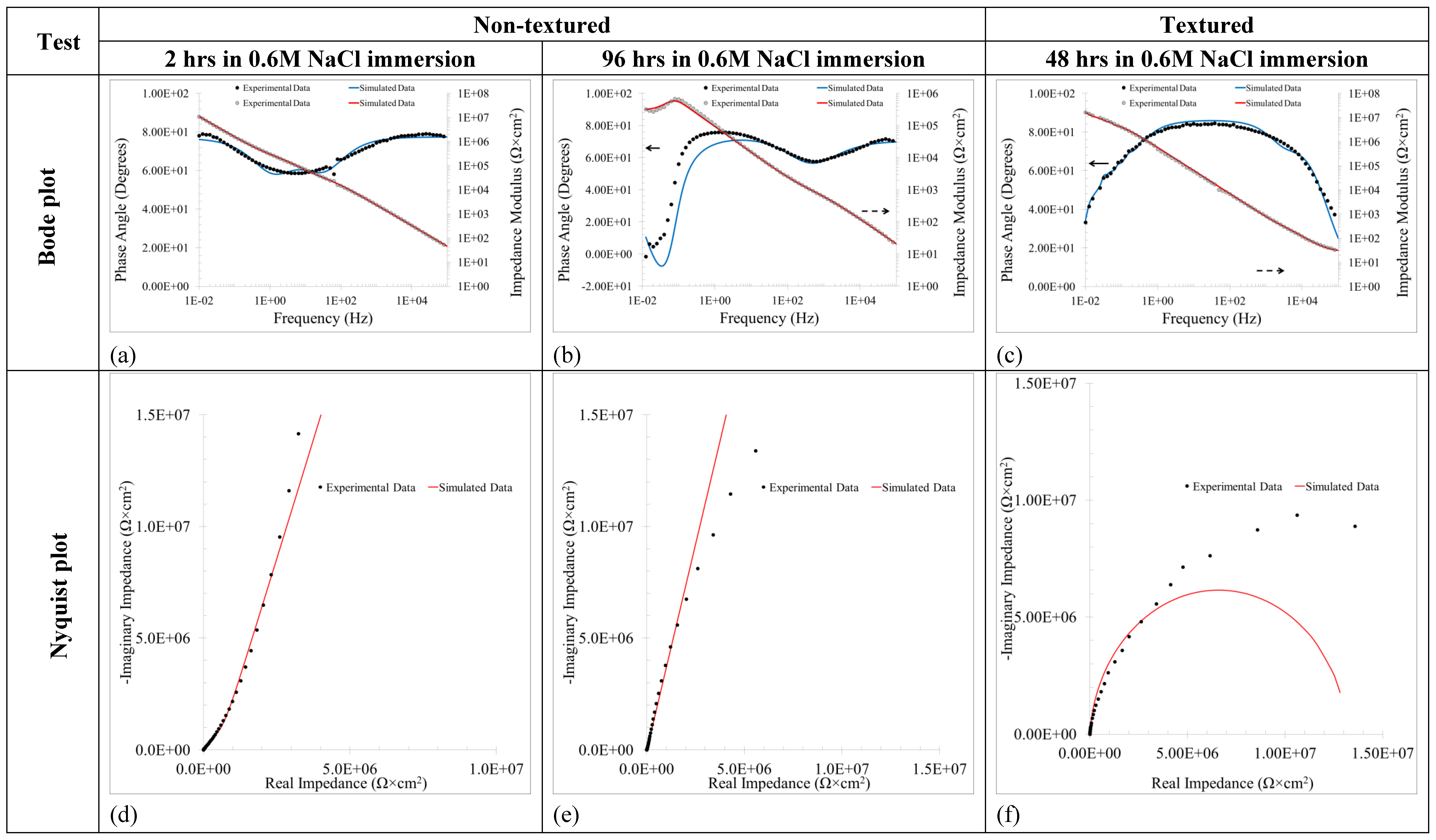
3.1.3. Electrochemical Impedance Spectroscopy (EIS), Active Corrosion Testing of Alternating Current
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, J.R. Aluminum and Aluminum Alloys: ASM International; ASM International: Novelty, OH, USA, 1993. [Google Scholar]
- Aamir, M.; Giasin, K.; Tolouei-Rad, M.; Vafadar, A. A review: Drilling performance and hole quality of aluminium alloys for aerospace applications. J. Mater. Res. Technol. 2020, 9, 12484–12500. [Google Scholar] [CrossRef]
- Mariglis, M. The Influence of Inter-anneal and Cold Rolling Processes on Key Lithographic Sheet Properties. Ph.D Thesis, The University of Manchester, Manchester, UK, 2012. [Google Scholar]
- Sverdlin, A. Properties of pure aluminum. In Handbook of Aluminum; Totten, G.E., MacKenzie, D.S., Eds.; CRC Press: Boca Raton, FL, USA, 2003; Volume 1, pp. 33–59. [Google Scholar]
- Alam, T.; Ansari, A.H. Review on Aluminium and Its Alloys for automotive applications. Int. J. Adv. Technol. Eng. Sci. 2017, 5, 278–294. [Google Scholar]
- Nurul, M.; Syahrullail, S. Surface texturing and alternative lubricant: Tribological study of tapered die sliding contact surface in cold extrusion process. Tribol. Trans. 2017, 60, 176–186. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Patané, S.; Proverbio, E. Effect of Chemical Surface Texturing on the Superhydrophobic Behavior of Micro–Nano-Roughened AA6082 Surfaces. Materials 2021, 14, 7161. [Google Scholar] [CrossRef] [PubMed]
- Ahuir-Torres, J.I.; Arenas, M.; Perrie, W.; Dearden, G.; De Damborenea, J. Surface texturing of aluminium alloy AA2024-T3 by picosecond laser: Effect on wettability and corrosion properties. Surf. Coat. Technol. 2017, 321, 279–291. [Google Scholar] [CrossRef]
- Ansari, I.A.; Watkins, K.G.; Sharp, M.C.; Hutchinson, R.; Potts, R. Modification of Aluminium Surface Using Picosecond Laser for Printing Applications. MRS Online Proc. Libr. 2009, 1230, 714. [Google Scholar] [CrossRef]
- Ansari, I.; Watkins, K.; Sharp, M.; Hutchinson, R.; Potts, R.; Clowes, J. Modification of anodised aluminium surfaces using a picosecond fibre laser for printing applications. J. Nanosci. Nanotechnol. 2012, 12, 4946–4950. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Alok, A.; Das, M. Surface texturing by electrochemical micromachining: A review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Renk, T.J.; Provencio, P.P.; Shlapakovski, A.; Petrov, A.; Yatsui, K.; Jiang, W.; Suematsu, H. Materials modification using intense ion beams. Proc. IEEE 2004, 92, 1057–1081. [Google Scholar] [CrossRef]
- Vishnoi, M.; Kumar, P.; Murtaza, Q. Surface texturing techniques to enhance tribological performance: A review. Surf. Interfaces 2021, 27, 101463. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Synergistic effect of superhydrophobicity and oxidized layers on corrosion resistance of aluminum alloy surface textured by nanosecond laser treatment. ACS Appl. Mater. Interfaces 2015, 7, 19500–19508. [Google Scholar] [CrossRef]
- Misyura, S.; Feoktistov, D.; Morozov, V.; Orlova, E.; Kuznetsov, G.; Islamova, A. Effect of heat treatment on corrosion of laser-textured aluminum alloy surfaces. J. Mater. Sci. 2021, 56, 12845–12863. [Google Scholar] [CrossRef]
- Alvi, M.H.; Cheong, S.W.; Weiland, H.; Rollett, A.D. Recrystallization and texture development in hot rolled 1050 aluminum. In Materials Science Forum; Trans Tech Publications: Zurich, Switzerland, 2004. [Google Scholar]
- Sharp, M.C.; Rosowski, A.P.; French, P.W. Nanosecond laser texturing of aluminium for control of wettability. In Industrial Laser Applications Symposium (ILAS 2015); SPIE: Bellingham, WA, USA, 2015; Volume 9657, pp. 129–135. [Google Scholar]
- Xia, D.-H.; Song, S.; Behnamian, Y.; Hu, W.; Cheng, Y.F.; Luo, J.-L.; Huet, F. Electrochemical noise applied in corrosion science: Theoretical and mathematical models towards quantitative analysis. J. Electrochem. Soc. 2020, 167, 081507. [Google Scholar] [CrossRef]
- Meng, G.; Wei, L.; Zhang, T.; Shao, Y.; Wang, F.; Dong, C.; Li, X. Effect of microcrystallization on pitting corrosion of pure aluminium. Corros. Sci. 2009, 51, 2151–2157. [Google Scholar] [CrossRef]
- Smialowska, Z.S. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Speckert, L.; Burstein, G. Combined anodic/cathodic transient currents within nucleating pits on Al–Fe alloy surfaces. Corros. Sci. 2011, 53, 534–539. [Google Scholar] [CrossRef]
- Kakinuma, H.; Muto, I.; Oya, Y.; Momii, T.; Sugawara, Y.; Hara, N. Morphological Change and Open-circuit Potential of Single Metastable Pit on AA1050 Aluminum in NaCl Solution. J. Electrochem. Soc. 2021, 168, 021504. [Google Scholar] [CrossRef]
- García-Ochoa, E.; González-Sánchez, J.; Corvo, F.; Usagawa, Z.; Dzib-Pérez, L.; Castañeda, A. Application of electrochemical noise to evaluate outdoor atmospheric corrosion of copper after relatively short exposure periods. J. Appl. Electrochem. 2008, 38, 1363–1368. [Google Scholar] [CrossRef]
- Delijić, K.; Markoli, B.; Naglič, I. The influence of the chemical composition on the corrosion performances of some Al-Fe-Si, Al-Mg-Si and Al-Mg-Mn type of alloys. Metall. Mater. Eng. 2014, 20, 217–234. [Google Scholar] [CrossRef]
- Šeruga, M.; Hasenay, D. Electrochemical and surface properties of aluminium in citric acid solutions. J. Appl. Electrochem. 2001, 31, 961–967. [Google Scholar] [CrossRef]
- Mansfeld, F.; Sun, Z. Localization index obtained from electrochemical noise analysis. Corrosion 1999, 55, 915–918. [Google Scholar] [CrossRef]
- Song, G.; Johannesson, B.; Hapugoda, S.; StJohn, D. Galvanic corrosion of magnesium alloy AZ91D in contact with an aluminium alloy, steel and zinc. Corros. Sci. 2004, 46, 955–977. [Google Scholar] [CrossRef]
- Urzua, R.; Siqueiros, J.; Morales, L.; Rosales, I.; Uruchurtu, J. On-line corrosion monitoring of 70 Cu 30 Ni alloy in a LiBr solution under absorption heat pump flow conditions. Port. Electrochim. Acta 2009, 27, 127–142. [Google Scholar] [CrossRef]
- Cottis, R.A. Electrochemical noise for corrosion monitoring. Tech. Corros. Monit. 2021, 2, 99–122. [Google Scholar] [CrossRef]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Seong-Jong, K.; Seok-Ki, J.; Min-Su, H.; Jae-Cheul, P.; Jeong, J.-Y.; Chong, S.-O. Mechanical and electrochemical characteristics in sea water of 5052-O aluminum alloy for ship. Trans. Nonferrous Met. Soc. China 2013, 23, 636–641. [Google Scholar]
- Anantharaj, S.; Noda, S.; Driess, M.; Menezes, P.W. The pitfalls of using potentiodynamic polarization curves for tafel analysis in electrocatalytic water splitting. ACS Energy Lett. 2021, 6, 1607–1611. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- Kruger, J.; Revie, R.W. Passivity. In Uhlig’s Corrosion Handbook, 3rd ed; ECS-The Electrochemical Society: Ontario, CA, USA, 2011; pp. 151–155. [Google Scholar]
- Kakaei, K.; Esrafili, M.D.; Ehsani, A. Graphene and anticorrosive properties. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–337. [Google Scholar]
- Cesiulis, H.; Tsyntsaru, N.; Ramanavicius, A.; Ragoisha, G. The study of thin films by electrochemical impedance spectroscopy. In Nanostructures and Thin Films for Multifunctional Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 3–42. [Google Scholar]
- Shi, Y.; Pan, Q.; Li, M.; Huang, X.; Li, B. Effect of Sc and Zr additions on corrosion behaviour of Al–Zn–Mg–Cu alloys. J. Alloy. Compd. 2014, 612, 42–50. [Google Scholar] [CrossRef]
- Fernandes, J.; Ferreira, M.; Rangel, C. Electrochemical impedance studies on pure aluminium in carbonate solution. J. Appl. Electrochem. 1990, 20, 874–876. [Google Scholar] [CrossRef]
- Oh, H.-J.; Jang, K.-W.; Chi, C.-S. Impedance characteristics of oxide layers on aluminium. Bull. Korean Chem. Soc. 1999, 20, 1340–1344. [Google Scholar]
- Tiwari, S.; Balasubramaniam, R.; Gupta, M. Corrosion behavior of SiC reinforced magnesium composites. Corros. Sci. 2007, 49, 711–725. [Google Scholar] [CrossRef]
- Tighiouaret, S.; Hanna, A.; Azzeddine, H.; Rabahi, L.; Dakhouche, A.; Brisset, F.; Helbert, A.L.; Baudin, T.; Bradai, D. On the evolution of microstructure, texture and corrosion behavior of a hot-rolled and annealed AZ31 alloy. Mater. Chem. Phys. 2021, 267, 124598. [Google Scholar] [CrossRef]

| Element | Si | Fe | Cu | Mn | Mg | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| Mass (%) | 0.080 | 0.310 | 0.003 | 0.036 | 0.004 | 0.009 | 0.008 | 99.54 |
| Nr. | Parameter | Value | Units |
|---|---|---|---|
| 1 | Average Power | 800 | W |
| 2 | Pulse Repetition Rate | 10,000 | Hz |
| 3 | Pulse Energy (calculated from 1 and 2) | 80 | mJ |
| 4 | Pulse Length | 60 | ns |
| 5 | Beam Diameter at Lens | 21 | mm |
| 6 | Focal length | 40 | mm |
| 7 | Defocus | 4 | mm |
| 8 | Estimated spot size at sample (from 5, 6, 7) | 2.1 | mm |
| 9 | Rotary Speed of 170 mm cylinder | 963 | rpm |
| 10 | Tangential speed (“scan speed”) | 8.6 | m/s |
| 11 | Linear translation speed | 0.81 | m/min |
| 12 | Hatch spacing (calculated from 9, 11) | 0.84 | mm |
| 13 | Coverage rate (calculated from 10, 11, 12) | 0.43 | m2/min |
| Sample | σE (mV) | σI (μA/cm2) | IR.M.S (μA/cm2) | C.R.AEN (μm/Year) | L.I. | RAEN (kΩ × cm2) |
|---|---|---|---|---|---|---|
| Non-textured | 11.340 | 0.331 | 3.194 | 34.314 | 0.09 | 34.221 |
| Textured | 7.676 | 0.466 | 12.281 | 131.954 | 0.04 | 16.481 |
| Factor | Non-Textured Aluminium | Textured Aluminium |
|---|---|---|
| Ecorr (V) | −0.988 | −0.695 |
| Icorr (nA/cm2) | 47.085 | 338.101 |
| C.R.corr (μm/year) | 0.506 | 3.633 |
| βc (mV/decade) | −0.035 | −0.012 |
| βa (mV/decade) | 0.150 | 0.001 |
| Rp (kΩ*cm2) | 421 | 0.941 |
| Ipass (μA/cm2) | 0.110 | - |
| C.R.pass (μm/year) | 1.182 | - |
| Idiff (A/cm2) | - | 3.673 |
| C.R.diff (μm/year) | - | 39.463 |
| Epb (V) | −0.698 | - |
| Erp (V) | −0.896 | - |
| Non-Textured Samples | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Ra-p (μΩ) | CPEa-p (μS × sn/cm2) | nl | Rf (kΩ × cm2) | CPEf (μS × sn/cm2) | nf | Rct (kΩ × cm2) | CPEdl (μS × sn/cm2) | ndl | Rad-de (kΩ × cm2) | Lad-de (kH × cm2) | X2 (10−3) | ||
| 2 | 0.065 | 0.95 | 0.85 | 146.0 | 0.747 | 0.85 | 15.80 | 0.48 | 0.87 | - | - | 1.62 | ||
| 24 | 136.0 | 1.38 | 0.83 | 4.1 | 361.0 | 1.00 | 8.46 | 1.33 | 0.81 | - | - | 1.52 | ||
| 48 | 1752.0 | 1.53 | 0.84 | 2.0 | 0.832 | 0.95 | 0.78 | 1.96 | 0.82 | 123.0 | 0.23 | 2.16 | ||
| 72 | 2924.0 | 88.5 | 0.68 | 83.2 | 2.682 | 0.79 | 0.41 | 1.67 | 0.81 | 225.0 | 225.0 | 5.07 | ||
| 96 | 1056.0 | 116.0 | 1.00 | 1076.0 | 2.370 | 0.81 | 0.57 | 2.93 | 0.76 | 428.0 | 2034.0 | 3.40 | ||
| Textured Samples | ||||||||||||||
| Time (h) | Rs (Ω × cm2) | Rf (Ω × cm2) | CPEf (μS × sn/cm2) | nf | Rct (kΩ × cm2) | CPEdl (μS × sn/cm2) | ndl | X2 (10−3) | ||||||
| 2 | 2.566 | 11.2 | 1.53 | 0.95 | 1750 | 0.90 | 0.92 | 1.58 | ||||||
| 24 | 2.590 | 22.1 | 2.32 | 0.94 | 5830 | 1.40 | 0.91 | 1.37 | ||||||
| 48 | 2.605 | 96.0 | 2.52 | 0.94 | 611 | 0.60 | 1.00 | 3.07 | ||||||
| 72 | 2.331 | 75.5 | 2.19 | 0.95 | 560 | 1.05 | 0.96 | 5.77 | ||||||
| 96 | 2.117 | 64.0 | 2.24 | 0.95 | 603 | 1.10 | 0.95 | 7.21 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahuir-Torres, J.I.; Kotadia, H.R.; Öpoz, T.T.; Sharp, M.C. A Study on the Corrosion Behaviour of Laser Textured Pure Aluminium in Saltwater. Processes 2023, 11, 721. https://doi.org/10.3390/pr11030721
Ahuir-Torres JI, Kotadia HR, Öpoz TT, Sharp MC. A Study on the Corrosion Behaviour of Laser Textured Pure Aluminium in Saltwater. Processes. 2023; 11(3):721. https://doi.org/10.3390/pr11030721
Chicago/Turabian StyleAhuir-Torres, Juan Ignacio, Hiren Ramniklal Kotadia, Tahsin Tecelli Öpoz, and Martin Charles Sharp. 2023. "A Study on the Corrosion Behaviour of Laser Textured Pure Aluminium in Saltwater" Processes 11, no. 3: 721. https://doi.org/10.3390/pr11030721
APA StyleAhuir-Torres, J. I., Kotadia, H. R., Öpoz, T. T., & Sharp, M. C. (2023). A Study on the Corrosion Behaviour of Laser Textured Pure Aluminium in Saltwater. Processes, 11(3), 721. https://doi.org/10.3390/pr11030721









