Phytochemical Composition and Insight into Antibacterial Potential of Origanum vulgare Essential Oil from Saudi Arabia Using In Vitro and In Silico Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Essential Oil Extraction
2.3. Gas Chromatographic Analysis
2.4. Antimicrobial Activity Determination
2.4.1. Micro-Organism Tested
2.4.2. Preparation of Culture Media
2.4.3. Inoculum Preparation
2.4.4. Disk Diffusion Assay
2.5. Molecular Docking
2.5.1. Preparation of the Ligand
2.5.2. Preparation of the Receptors
2.5.3. Docking Simulation
2.5.4. Docking Validation Protocol
3. Results and Discussion
3.1. Phytochemical Analysis of Origanum vulgare
3.2. Antimicrobial Activity
3.3. Docking Validation
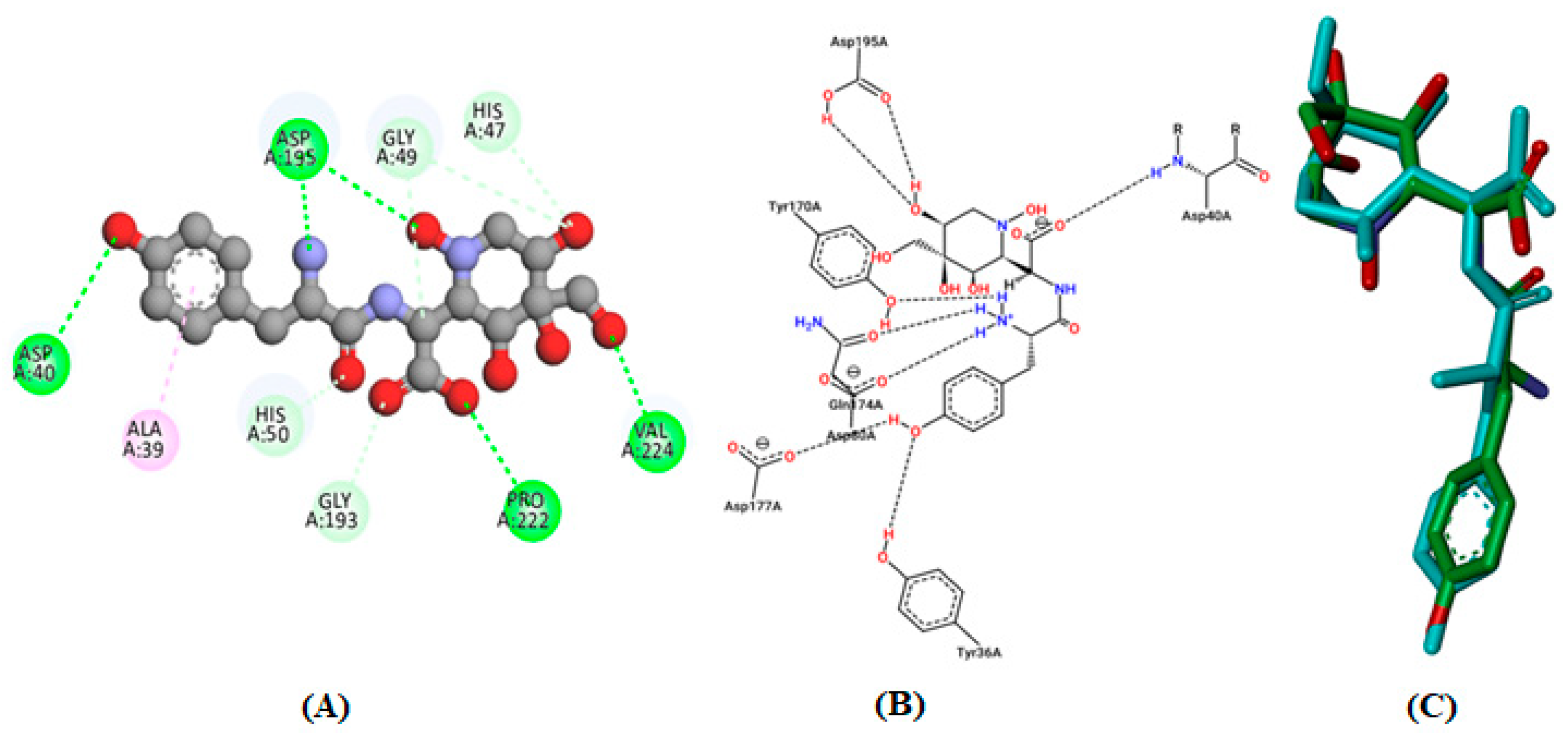
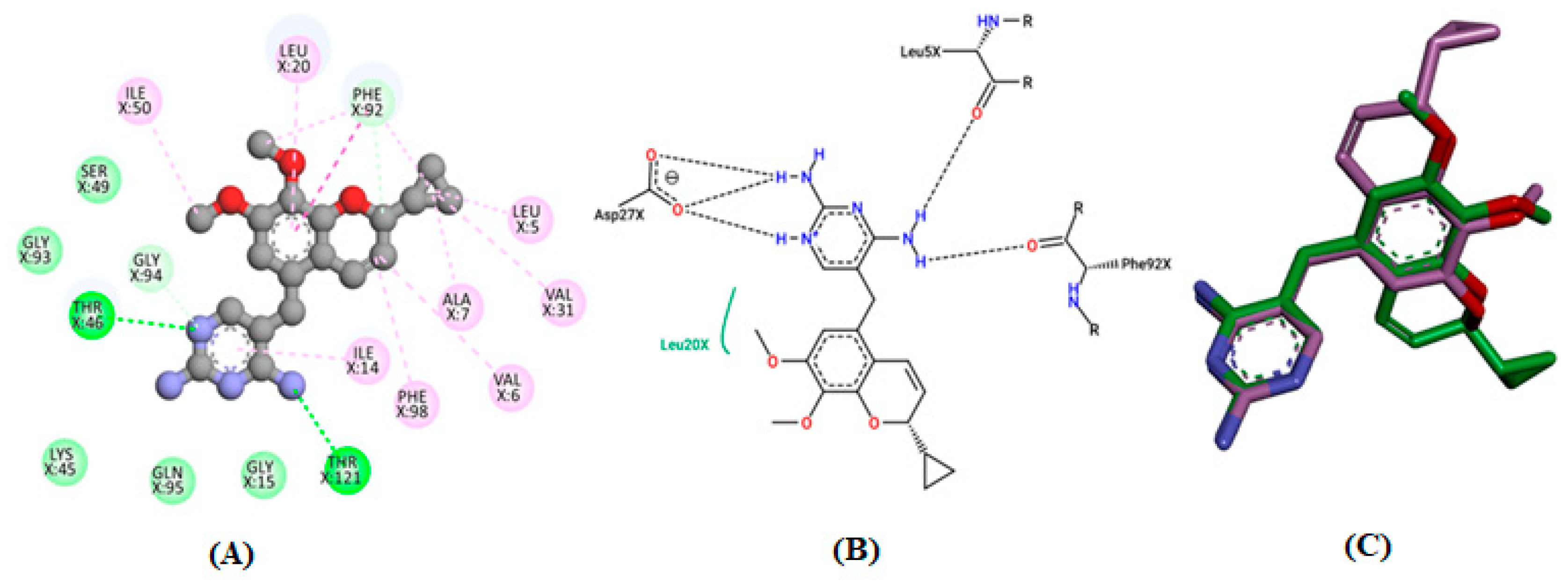
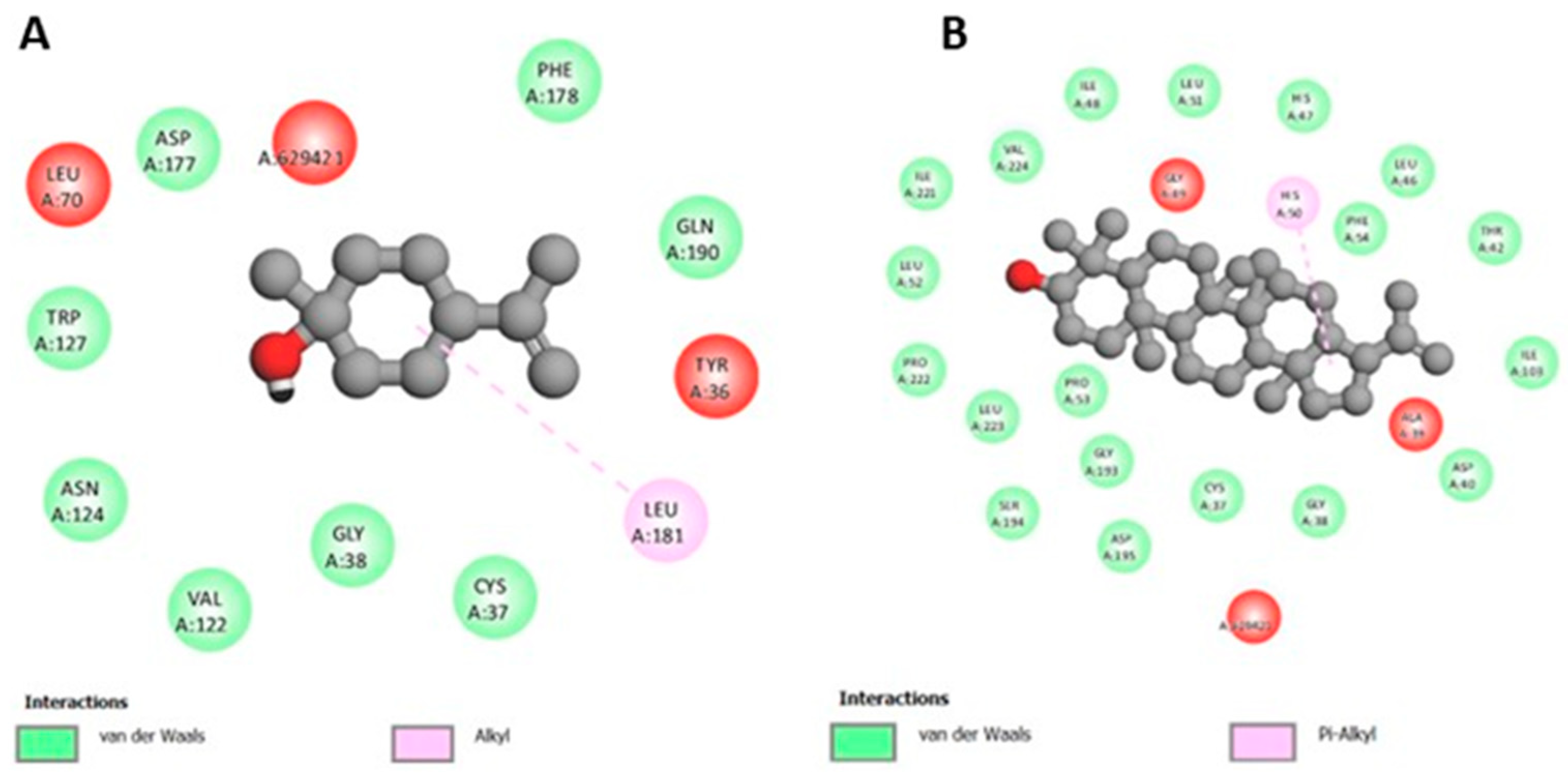
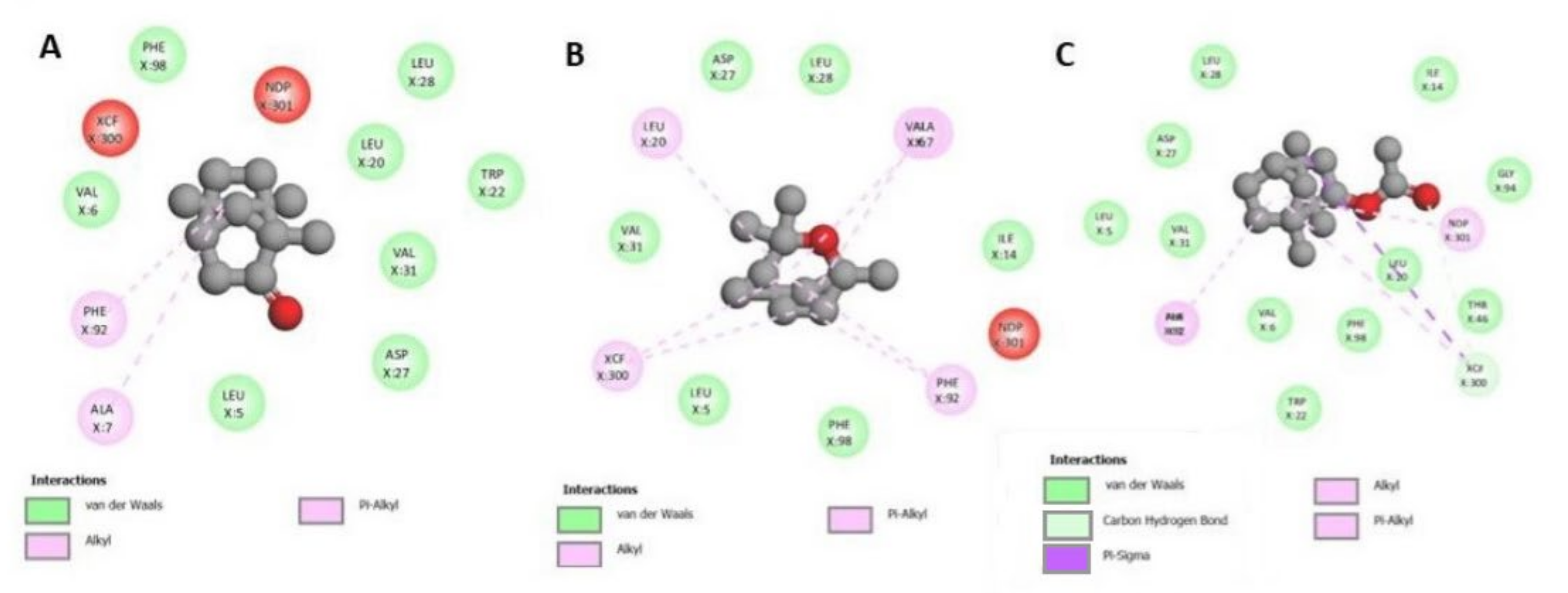
3.4. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| GC-MS | Gas chromatography–mass spectrometry |
| TyrRS | Tyrosyl-tRNA synthetase |
| DHFR | Dihydrofolate reductase |
| EO | Essential oil |
| DMSO | Dimethyl sulfoxide |
| CFU | Colony forming unit |
| PDB | Protein data bank |
| RT | Retention time |
| mm | Millimeter |
References
- Aljuraifani, A. Antimicrobial Activity of Some Medicinal Plants Used in Saudi Arabia. Can. J. Pure Appl. Sci. 2011, 5, 1509–1512. [Google Scholar]
- Cohen, M.L. Epidemiology of Drug Resistance: Implications for a Post—Antimicrobial Era. Science 1992, 257, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, M.D.; Rodríguez-Baño, J.; Martínez-Martínez, L.; Pascual, A.; Pérez-Canoa, R.; Perea, E.J.; Muniain, M.A. Epidemiology, clinical features and prognosis of infections due to Stenotrophomonas maltophilia. Enferm. Infecc. Microbiol. Clin. 2006, 24, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.A.; Brody, S.L.; Kollef, M.H. The Epidemiology, Pathogenesis and Treatment of Pseudomonas Aeruginosa Infections. Drugs 2007, 67, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, Phytotoxic and Insecticidal Properties of Essential Oil Isolated from Turkish Origanum Acutidens and Its Three Components, Carvacrol, Thymol and p-Cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial Activity of the Essential Oils of Salvia Officinalis L. and Salvia Triloba L. Cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Seydim, A.C.; Sarikus, G. Antimicrobial Activity of Whey Protein Based Edible Films Incorporated with Oregano, Rosemary and Garlic Essential Oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Imtara, H.F.I. Aromiels Du Maroc et de Palestine. Ph.D. Thesis, University of Sidi Mohamed Ben Abdullah, Fez, Morocco, 2018. [Google Scholar]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Högberg, L.D.; Heddini, A.; Cars, O. The Global Need for Effective Antibiotics: Challenges and Recent Advances. Trends Pharmacol. Sci. 2010, 31, 509–515. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Couic-Marinier, F.; Lobstein, A. Composition chimique des huiles essentielles. Actual. Pharm. 2013, 52, 22–25. [Google Scholar] [CrossRef]
- Manuel des Corps Gras: 2 Volumes Inséparables KARLESKIND Alain. Available online: https://www.lavoisier.fr/livre/agro-alimentaire/manuel-des-corps-gras-2-volumes-inseparables/karleskind/descriptif-9782852066625 (accessed on 26 January 2023).
- Quézel, P.; Santa, S. Nouvelle Flore de L’algérie et des Régions Désertiques Méridionales; Editions du Centre National de la Recherche Scientifique: Paris, France, 1962. [Google Scholar]
- Kintzios, S.E. (Ed.) Oregano: The Genera Origanum and Lippia; CRC Press: London, UK, 2004; ISBN 978-0-429-21897-2. [Google Scholar]
- Yan, F.; Azizi, A.; Janke, S.; Schwarz, M.; Zeller, S.; Honermeier, B. Antioxidant Capacity Variation in the Oregano (Origanum Vulgare L.) Collection of the German National Genebank. Ind. Crops Prod. 2016, 92, 19–25. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J.L.D. Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an in Vitro Model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed]
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical Composition and Biological Activity of Essential Oils of Origanum Vulgare L. Subsp. Vulgare L. under Different Growth Conditions. Molecules 2013, 18, 14948–14960. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic Activity of Origanum Vulgare L. on Hepatocellular Carcinoma Cell Line HepG2 and Evaluation of Its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Shawir, M.S.; Abou-Taleb, H.K. Chemical Composition, Insecticidal and Biochemical Effects of Essential Oils of Different Plant Species from Northern Egypt on the Rice Weevil, Sitophilus Oryzae L. J. Pest. Sci. 2016, 89, 219–229. [Google Scholar] [CrossRef]
- De Mastro, G.; Fracchiolla, M.; Verdini, L.; Montemurro, P. Oregano and Its Potential Use as Bioherbicide. In Proceedings of the I International Symposium on the Labiatae: Advances in Production, Biotechnology and Utilisation, Sanremo, Italy, 22 February 2006; Volume 723. [Google Scholar]
- Barbosa, P.; Faria, J.M.S.; Mendes, M.D.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Bioassays against Pinewood Nematode: Assessment of a Suitable Dilution Agent and Screening for Bioactive Essential Oils. Molecules 2012, 17, 12312–12329. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Rostro-Alanis, M.d.J.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical Composition and Biological Activities of Oregano Essential Oil and Its Fractions Obtained by Vacuum Distillation. Molecules 2019, 24, 1904. [Google Scholar] [CrossRef]
- Stoilova, I.; Bail, S.; Buchbauer, G.; Krastanov, A.; Stoyanova, A.; Schmidt, E.; Jirovetz, L. Chemical Composition, Olfactory Evaluation and Antioxidant Effects of an Essential Oil of Origanum Vulgare L. from Bosnia. Nat. Prod. Commun. 2008, 3, 1934578X0800300702. [Google Scholar] [CrossRef]
- Imtara, H.; Al-Waili, N.; Aboulghazi, A.; Abdellaoui, A.; Al-Waili, T.; Lyoussi, B. Chemical Composition and Antioxidant Content of Thymus Vulgaris Honey and Origanum Vulgare Essential Oil; Their Effect on Carbon Tetrachloride-Induced Toxicity. Vet. World 2021, 14, 292–301. [Google Scholar] [CrossRef]
- Khalil, M.I.M.; Ibrahim, M.M.; El-Gaaly, G.A.; Sultan, A.S. Trigonella Foenum (Fenugreek) Induced Apoptosis in Hepatocellular Carcinoma Cell Line, HepG2, Mediated by Upregulation of P53 and Proliferating Cell Nuclear Antigen. BioMed Res. Int. 2015, 2015, 914645. [Google Scholar] [CrossRef]
- Sparkman, O.D. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef]
- Wiley/NBS. Registry of Mass Spectral Data. Available online: https://analyticalscience.wiley.com/do/10.1002/sepspec.9780471628866 (accessed on 25 November 2022).
- Treangen, T.J.; Maybank, R.A.; Enke, S.; Friss, M.B.; Diviak, L.F.; Karaolis, D.K.R.; Koren, S.; Ondov, B.; Phillippy, A.M.; Bergman, N.H.; et al. Complete Genome Sequence of the Quality Control Strain Staphylococcus Aureus Subsp. Aureus ATCC 25923. Genome Announc. 2014, 2, e01110-14. [Google Scholar] [CrossRef]
- Minogue, T.D.; Daligault, H.A.; Davenport, K.W.; Bishop-Lilly, K.A.; Broomall, S.M.; Bruce, D.C.; Chain, P.S.; Chertkov, O.; Coyne, S.R.; Freitas, T.; et al. Complete Genome Assembly of Escherichia Coli ATCC 25922, a Serotype O6 Reference Strain. Genome Announc. 2014, 2, e00969-14. [Google Scholar] [CrossRef]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Honey Antibacterial Effect Boosting Using Origanum Vulgare L. Essential Oil. Evid.-Based Complement. Altern. Med. 2018, 2018, e7842583. [Google Scholar] [CrossRef]
- Daraghmeh, J.; Imtara, H. In Vitro Evaluation of Palestinian Propolis as a Natural Product with Antioxidant Properties and Antimicrobial Activity against Multidrug-Resistant Clinical Isolates. J. Food Qual. 2020, 2020, e8861395. [Google Scholar] [CrossRef]
- Sadeq, O.; Mechchate, H.; Es-safi, I.; Bouhrim, M.; Jawhari, F.Z.; Ouassou, H.; Kharchoufa, L.; AlZain, M.N.; Alzamel, N.M.; Al kamaly, M.O.; et al. Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria Fruticosa, Achillea Fragrantissima, and Phoenix Dactylifera. Plants 2021, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Aref, H.L.; Karima, B.H.S.; Fekih, A.; Chemli, R.; Mars, M.; Aouni, M.; Chaumon, J.P.; Said, K. Variability in Antimicrobial Activity of Latex from Two Varieties of Ficus Carica. AJMR 2011, 5, 1361–1367. [Google Scholar] [CrossRef]
- El Fadili, M.; Er-rajy, M.; Imtara, H.; Kara, M.; Zarougui, S.; Altwaijry, N.; Al Kamaly, O.; Al Sfouk, A.; Elhallaoui, M. 3D-QSAR, ADME-Tox In Silico Prediction and Molecular Docking Studies for Modeling the Analgesic Activity against Neuropathic Pain of Novel NR2B-Selective NMDA Receptor Antagonists. Processes 2022, 10, 1462. [Google Scholar] [CrossRef]
- El Fadili, M.; Er-Rajy, M.; Kara, M.; Assouguem, A.; Belhassan, A.; Alotaibi, A.; Mrabti, N.N.; Fidan, H.; Ullah, R.; Ercisli, S.; et al. QSAR, ADMET In Silico Pharmacokinetics, Molecular Docking and Molecular Dynamics Studies of Novel Bicyclo (Aryl Methyl) Benzamides as Potent GlyT1 Inhibitors for the Treatment of Schizophrenia. Pharmaceuticals 2022, 15, 670. [Google Scholar] [CrossRef] [PubMed]
- Busatta, C.; Mossi, A.J.; Rodrigues, M.R.A.; Cansian, R.L.; Oliveira, J.V. De Evaluation of Origanum Vulgare Essential Oil as Antimicrobial Agent in Sausage. Braz. J. Microbiol. 2007, 38, 610–616. [Google Scholar] [CrossRef]
- Hayani, M.; Bencheikh, N.; Ailli, A.; Bouhrim, M.; Elbouzidi, A.; Ouassou, H.; Kharchoufa, L.; Baraich, A.; Atbir, A.; Ayyad, F.Z.; et al. Quality Control, Phytochemical Profile, and Antibacterial Effect of Origanum Compactum Benth. Essential Oil from Morocco. IJPB 2022, 13, 546–560. [Google Scholar] [CrossRef]
- Mastro, G.D.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential Oil Diversity of Origanum Vulgare L. Populations from Southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical Composition and Bioactivity of Different Oregano (Origanum Vulgare) Extracts and Essential Oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Stešević, D.; Jaćimović, Ž.; Šatović, Z.; Šapčanin, A.; Jančan, G.; Kosović, M.; Damjanović-Vratnica, B. Chemical Characterization of Wild Growing Origanum Vulgare Populations in Montenegro. Nat. Prod. Commun. 2018, 13, 1934578X1801301031. [Google Scholar] [CrossRef]
- Perry, N.B.; Anderson, R.E.; Brennan, N.J.; Douglas, M.H.; Heaney, A.J.; McGimpsey, J.A.; Smallfield, B.M. Essential Oils from Dalmatian Sage (Salvia Officinalis L.): Variations among Individuals, Plant Parts, Seasons, and Sites. J. Agric. Food Chem. 1999, 47, 2048–2054. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical Constituents, in Vitro Antibacterial and Antifungal Activity of Mentha×Piperita L. (Peppermint) Essential Oils. J. King Saud. Univ.-Sci. 2019, 31, 528–533. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.-M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum Vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of Antioxidant and Antibacterial Properties on Meat Homogenates of Essential Oils Obtained from Four Thymus Species Achieved from Organic Growth. Foods 2017, 6, 59. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Interactions of Cyclic Hydrocarbons with Biological Membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of Action of Melaleuca Alternifolia (Tea Tree) Oil on Staphylococcus Aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef]
- Dorman, H.J.; Deans, S.G. Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-Ol as an Antibacterial and Antibiofilm Agent against Staphylococcus Aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Rúa, J.; del Valle, P.; de Arriaga, D.; Fernández-Álvarez, L.; García-Armesto, M.R. Combination of Carvacrol and Thymol: Antimicrobial Activity Against Staphylococcus Aureus and Antioxidant Activity. Foodborne Pathog. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Saghrouchni, H.; El Barnossi, A.; Salamatullah, A.M.; Bourhia, M.; Alzahrani, A.; Alkaltham, M.S.; Alyahya, H.K.; Tahiri, N.E.H.; Imtara, H.; Var, I. Carvacrol: A Promising Environmentally Friendly Agent to Fight Seeds Damping-Off Diseases Induced by Fungal Species. Agronomy 2021, 11, 985. [Google Scholar] [CrossRef]
- He, J.; Qiao, W.; An, Q.; Yang, T.; Luo, Y. Dihydrofolate Reductase Inhibitors for Use as Antimicrobial Agents. Eur. J. Med. Chem. 2020, 195, 112268. [Google Scholar] [CrossRef] [PubMed]
- Eakin, A.E.; Green, O.; Hales, N.; Walkup, G.K.; Bist, S.; Singh, A.; Mullen, G.; Bryant, J.; Embrey, K.; Gao, N.; et al. Pyrrolamide DNA Gyrase Inhibitors: Fragment-Based Nuclear Magnetic Resonance Screening to Identify Antibacterial Agents. Antimicrob. Agents Chemother. 2012, 56, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- J, S.; Pc, L.; Hl, Z. Tyrosyl-TRNA Synthetase Inhibitors: A Patent Review. Expert Opin. Ther. Pat. 2017, 27. [Google Scholar] [CrossRef]




| Receptors | ID | Class |
|---|---|---|
| TyrRS | 1jij | Ligase |
| DNA gyrase | 1KZN | Isomerase |
| DHFR | 3fyv | Oxidoreductase |
| # | Compound Name | Chemical Formula | Molecular Weight (g/mol) | RT (min) | Area % |
|---|---|---|---|---|---|
| 1 | Beta-Terpinene | C10H16 | 136.23 | 6.73 | 2.94 |
| 2 | Beta-Myrcene | C10H16 | 136.23 | 6.99 | 0.53 |
| 3 | 2-Carene | C10H16 | 136.23 | 7.42 | 2.29 |
| 4 | O-Cymene | C10H14 | 134.22 | 7.55 | 2.21 |
| 5 | Gamma-Terpinene | C10H16 | 136.23 | 8.08 | 6.36 |
| 6 | Terpinolene | C10H16 | 136.23 | 8.55 | 1.31 |
| 7 | Beta-Terpineol | C10H18O | 154.25 | 8.75 | 16.97 |
| 8 | 2-Cyclohexen-1-ol, 1-methyl-4-(1-methylethyl)-, trans- | C10H18O | 154.25 | 9.1 | 1.71 |
| 9 | Cyclohexanol, 2-methyl-5-(1-methylethenyl)-, (1α,2α,5β)- | C10H18O | 154.25 | 9.38 | 0.85 |
| 10 | Terpinen-4-ol | C10H18O | 154.25 | 9.99 | 43.32 |
| 11 | Alpha-Terpineol | C10H18O | 154.25 | 10.16 | 3.86 |
| 12 | Linalyl anthranilate | C17H23NO2 | 273.37 | 10.98 | 0.48 |
| 13 | Caryophyllene | C15H24 | 204.35 | 13.36 | 1.65 |
| 14 | 1,5-Heptadiene, 2,5-dimethyl-3-methylene- | C10H16 | 136.23 | 14.33 | 0.53 |
| 15 | 9,17-Octadecadienal, (Z)- | C18H32O | 264.4 | 21.03 | 0.48 |
| 16 | 3-Epimoretenol | C30H50O | 426.7 | 25.07 | 11.84 |
| 17 | (8S,14) Cedran-diol, | C15H26O | 238.4 | 27.93 | 1.43 |
| 18 | Cycloeucalenyl acetate | C32H52O2 | 468.8 | 28.33 | 1.22 |
| Escherichia coli | Staphylococcus aureus | |
|---|---|---|
| Origanum vulgare essential oil | 6 mm | 8 mm |
| DMSO | - | - |
| Antibiotic resistant | CXM, AML, CTX, SXT, CIP | PG, FA |
| Affinities (Kcal/mol) | |||
|---|---|---|---|
| TyrRS | DNA Gyrase | DHFR | |
| Beta-terpineol | −4.4 | −6.1 | −5.7 |
| Terpinen-4-ol | No interaction | −6.6 | −6.1 |
| 3-epimoretenol | −8.3 | −7.4 | −9.2 |
| Clorobiocin | −8.2 | −9.1 | _ |
| SCHEMBL2181345 | _ | _ | −6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al kamaly, O.; Alanazi, A.S.; Conte, R.; Imtara, H. Phytochemical Composition and Insight into Antibacterial Potential of Origanum vulgare Essential Oil from Saudi Arabia Using In Vitro and In Silico Approaches. Processes 2023, 11, 650. https://doi.org/10.3390/pr11030650
Al kamaly O, Alanazi AS, Conte R, Imtara H. Phytochemical Composition and Insight into Antibacterial Potential of Origanum vulgare Essential Oil from Saudi Arabia Using In Vitro and In Silico Approaches. Processes. 2023; 11(3):650. https://doi.org/10.3390/pr11030650
Chicago/Turabian StyleAl kamaly, Omkulthom, Ashwag S. Alanazi, Raffaele Conte, and Hamada Imtara. 2023. "Phytochemical Composition and Insight into Antibacterial Potential of Origanum vulgare Essential Oil from Saudi Arabia Using In Vitro and In Silico Approaches" Processes 11, no. 3: 650. https://doi.org/10.3390/pr11030650
APA StyleAl kamaly, O., Alanazi, A. S., Conte, R., & Imtara, H. (2023). Phytochemical Composition and Insight into Antibacterial Potential of Origanum vulgare Essential Oil from Saudi Arabia Using In Vitro and In Silico Approaches. Processes, 11(3), 650. https://doi.org/10.3390/pr11030650







