Abstract
Cellulose acetate (CA) is a semisynthetic, biodegradable polymer. Due to its characteristics, CA has several applications, including water membranes, filament-forming matrices, biomedical nanocomposites, household tools, and photographic films. This review deals with topics related to the CA membranes, which are prepared using different techniques, such as the phase inversion technique. CA membranes are considered very important since they can be used as microfiltration membranes (MF), ultrafiltration membranes (UF), nanofiltration membranes (NF), reverse osmosis (RO) membranes, and forward osmosis (FO) membranes. Membrane fouling results from the accumulation of materials that the membrane rejects on the surface or in the membrane’s pores, lowering the membrane’s flux and rejection rates. There are various forms of CA membrane fouling, for instance, organic, inorganic, particulate fouling, and biofouling. In this review, strategies used for CA membrane antifouling are discussed and summarized into four main techniques: feed solution pretreatment, cleaning of the membrane surface, membrane surface modification, which can be applied using either nanoparticles, polymer reactions, surface grafting, or surface topography, and surface coating.
1. Introduction
Due to their functionality, processability, affordability, and lightweight, traditional materials have been substituted with polymers in the application of packaging, such as paper, glass, and metal [1]. However, despite their wide range of applications, polymers are nonbiodegradable materials with adverse environmental effects [2]. They cause air and groundwater pollution and contribute to the “greenhouse effect,” which is dangerous for human and animal life [3]. As a result, nonbiodegradable polymers are now being replaced with biodegradable polymers of natural and manufactured origin [2]. Cellulose acetate (CA), a safer substitute for cellulose nitrate, began at the start of the twentieth century [4].
CA is a semisynthetic biodegradable polymer produced by a chemical reaction between cellulose, one of the most prevalent polysaccharides on earth, acetic anhydride, and acetic acid in the presence of sulfuric acid (H2SO4)–Figure 1. CA is a vital cellulose ester [4,5,6].
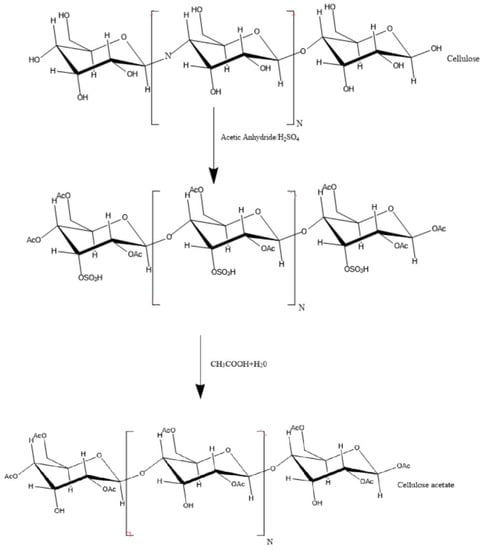
Figure 1.
Scheme for the preparation of cellulose acetate.
CA is an insoluble cellulose derivative that is considered a nontoxic, nonirritant, and biodegradable substance. CA is partially acetylated, with an acetyl concentration ranging from 29% to 44.8%, corresponding to mono-, di-, and triacetate [7].
Thermal stability, low weathering, good toughness, heat and chemical resistance, insolubility in water, nontoxicity, good mechanical properties, good hydrolytic stability, biocompatibility, biodegradability, high affinity, relatively low cost, comfort, natural feel, softness, luster, excellent chemical resistance, draping quality, and dimensional stability are all characteristics of CA [8,9,10,11]. Its properties depend on the extent of esterification, which is measured by the number of acetate groups that substitute OH groups and defines whether the final compound is acetate, diacetate, or triacetate [4]. The average amount of acetyl groups that substitute hydroxyl groups is called the degree of substitution (DS). CA with a DS higher than 0.8 is soluble in acetic acid; when DS is between 2.0 and 2.5, CA is soluble in methyl acetate, acetone, and dioxane. Higher acetylated forms of CA are soluble in dichloromethane [5,12].
One of the most prevalent biopolymers that degrades after disposal is cellulose. However, its ability to be made into membranes on an industrial scale is constrained because it is only soluble in a small number of solvents. One of the most alluring uses for cellulose membranes is organic solvent nanofiltration (OSN), which aims to get over this barrier. It is an emerging technique between cellulose, a plant-based material, and other materials, such as date-seed biomass [13], sacrificial acetate groups as electron donors or hole scavengers [14], or chitosan [15], which is obtained from shrimp farming waste and used as biomass material. These materials were investigated for the fabrication of oil- and solvent-resistant nanofiltration membranes and to increase the stability separation performance of CA membranes [13,15].
CA has several other applications, including biomedical nanocomposites, biomedical separation, antimicrobial membranes, affinity membranes, and filament-forming matrices [10]. It has also been utilized in various household items, including fabrics, children’s toys, eyeglass frames, and tool handles, although its primary use is as a basis for photographic film [4]. In addition, cellulose derivatives such as the butyrate derivative can be used for drug-releasing purposes in capsules [16].
This review highlights the main aspects of cellulose acetate as membranes, including fouling types and antifouling strategies, since CA is one of the most used polymeric materials that has been used in so many different types of membranes. The following sections elaborate on the importance of this polymer as a membrane compared to other types of polymers, in addition to its fouling issues, which, if handled properly, would maximize the use of its benefits.
2. CA Membranes, among Other Polymeric Membranes
Other than cellulose acetate, membranes are composed of different polymeric materials, such as polyamide (PA) polyvinylidene fluoride (PVDF), polyvinyl alcohol (PVA), polypropylene (PP), polysulfone (PSF), polyethersulfone (PES), and polyethylene (PE), however efficient biodegradable polymers are of great interest in science and technology, making CA of particular interest to researchers since it is a biodegradable polymer that has hydrophilic nature, and has high chemical and mechanical stability, nontoxicity, superior transport properties, excellent film-forming property, ease of availability, environmentally friendly, biocompatible, renewability, cost-effective, and a proper substrate for developing nanocomposites with novel material properties. Due to such attractive properties, CA has been used in the preparation of different types of membranes, such as gas separation, reverse osmosis (RO), nanofiltration (NF), ultrafiltration (UF), microfiltration (MF), pervaporation, etc. [17,18].
While CA membrane consists of only one polymeric material with a very thin asymmetric structure, another type of membrane, a thin film composite (TFC) membrane, consists of at least two distinct layers; a top active, selective layer and a porous substrate, allowing for sufficient water flux. The most popular TFC membranes are based on the PA selective layer [19]. Commercial RO membranes were initially obtained from PA and CA membranes. Additionally, TFC membranes are used for nanofiltration (NF) processes due to their thin selective layer and high mechanical strength, resulting in high flux [20]. Compared to CA membranes, TFCs show better salt rejection, higher water flux, and higher resistance to biological attacks, in addition to working at a broader range of temperatures and pH. However, TFC has the disadvantage of having a low resistance to chlorine, and since chlorine is usually present in chlorinated waters, this is considered a performance inhibitor [21]. On the other hand, CA membranes exhibit higher chlorine resistance, rejection rate, and durability [22]. To make use of the advantages of both types of membranes, Ounifi et al. (2021) deposed a PA thin film over a CA support by the interfacial polymerization method, leading to solid adhesion between PA and CA, which was explained by the formation of the polyamide film on the substrate surface and inside the pores [19].
3. CA Membranes: Types and Applications
Membrane processes are divided into categories based on various factors, such as driving force, membrane materials, membrane configuration, the size range of components eliminated, and separation mechanisms [23]. The pores’ dimensions in a membrane classify membranes. The smaller the pores, the more deriving force is required during the processes. Numerous factors influence membrane porosity, including the polymer solution’s temperature, the solvent/coagulant used, the cast polymer film’s thickness, the environment or the coagulation bath, the polymer solution’s composition, the salt addition, air moisture time, the support material (nonwoven, glass, metal, and polymer), and the nonsolvents used [24,25]. Brown used CA for the first time as a membrane in the 1910s. CA membranes are prepared using different techniques, and each has some advantages and disadvantages, such as (1) evaporation, (2) thermally induced phase separation, (3) vapor-induced phase separation, and (4) immersion precipitation [26,27]. The phase inversion technique is widely used to produce CA membranes [16]. CA can be used as microfiltration membranes (MF) [27], ultrafiltration membranes (UF) [28], nanofiltration membranes (NF) [29], reverse osmosis (RO) membranes [30], and forward osmosis (FO) membranes. Table 1 includes examples from the literature of materials used in the preparation of different types of CA membranes.

Table 1.
Examples from the literature for materials used in different CA membrane types.
3.1. Cellulose Acetate MF Membrane
Due to the comparatively wide pore dimension (0.1–1.0 µm), MF is categorized as a low-pressure (<2 bar) process. Using the physical separation principle, MF can eliminate significant pathogens, macrometer-sized particles, microscopic bacteria, yeast cells, and proteins, as well as organic colloids [56,57]. MF CA membranes are used for different purposes, such as membrane bioreactors, wastewater treatment, enzyme filtration, protein filtration, diagnostic cytology, biological fluid filtration, and more [58].
The casting solutions are usually composed of CA with acetone [32,33] or a mixture of formamide and acetone as solvent [59]. In general, a mixture of different compositions can be prepared. First, air bubbles are removed by leaving the solution to stand for some time in an airtight atmosphere. Then, after partial evaporation in the atmosphere for a specific time that can range between a few seconds and a few minutes, it is cast on a plate gently submerged in water. When the membrane is produced, it is rinsed with deionized water for many hours after complete precipitation to remove all solvents and additives [32,33,59].
The biomedical industry may use commercially treated CA MF membranes with antibacterial properties for venting and safeguarding medical equipment and the ventilation of operating and intensive care rooms [60]. Additionally, the CA MF membranes have been used for the separation of oil and water [61,62], whey and fruit juice filtration [33], and adsorption techniques [63].
3.2. Cellulose Acetate UF Membrane
UF is also classified as a low-pressure (2–5 bar) process. UF membranes generally operate by sieving. However, they have a more extensive separation range than MF membranes (typically between 0.01 and 0.1 µm). They can eliminate colloids, particles, pathogens, and viruses [23]. The ultrafiltration process can successfully separate proteins and other substances with high molecular weights from contaminated water. It has been intensively used in the food, biotechnological, pharmaceutical, electrical, chemical, and electrocoating industries for product recovery and pollution management [64].
Phase inversion is typically used to manufacture CA-UF membranes. The solution for casting is usually composed of CA and N,N-dimethyl formamide (DMF), dimethylacetamide (DMAc), and acetone:
- The casting solution is mixed until it becomes homogenous.
- The solution is degassed to clear any air bubbles before casting. The solution is then cast, and following that, the evaporation time during membrane formation spans from 2 sec to 120 sec.
- A deionized water bath is gradually filled with the cast film.
- When the inversion process is completed, for further characterization, the membrane is kept in a deionized water bath [28,65,66,67].
CA membranes are now the go-to solution for avoiding the fouling of proteins [65,68,69]. However, due to the denser top layer and low porosity sublayer of CA-UF membranes, water flux during the separation process is low, which limits their applications. Therefore, in order to broaden the range of applications for cellulose acetate ultrafiltration (CA-UF) membranes and increase their efficiency, CA must be modified to fulfill the demands of an antifouling-high-water flux membrane system [66,70]. CA-UF membrane properties can be improved in several ways. One of these is to alter the solvent in the casting solution. For instance, mixed solvents of acetone, DMF, and/or 2-propanol were utilized on a trial basis to prepare CA casting solution [71,72,73].
Furthermore, additives or pore-forming agents significantly improve the CA membranes’ performance. For example, Arthanareeswaran et al. prepared CA-UF membranes with an additive of polyethylene glycol 600 [1]. Additionally, Sivakumar increased the water flux of CA/polyurethane UF membranes by utilizing an agent for pore-forming; polyvinylpyrrolidone [68].
The CA UF membranes have been used in many disciplines, including removing heavy metal [74,75], treatment of dye wastewater [76], separating oil and water [77], BSA filtration [78,79], separation of protein [80], and blood hemodialysis [81,82].
3.3. Cellulose Acetate NF Membrane
NF is classified as a high-pressure process. NF membranes were created with the primary goal of simultaneously removing and softening organics. The pore size of the NF membranes is between 1 nm and 5 nm, which enables certain monovalent salts, such as Cl-, and low-sized solutes to pass through. Utilizing NF membrane technology in the treatment of wastewater, drinking water, and process water generation has sparked attention in the previous decade [23,83].
The first polymer utilized to make NF membranes was CA. CA is resistant to chlorine and solvents, which makes it a suitable material for membranes [72]. For CA-NF membrane preparation, a mixture of acetone and formamide [39,43,84] or NMP [42] is utilized in the process of preparing the casting solution. First, the solution is stirred to dissolve the CA polymer. After degassing, the solution of CA is cast on a glass plate and then instantly immersed in a cold-water bath for a few hours. Following that, the membranes are submerged in a hot water bath for a few minutes so that any remaining solvent is removed [39,43,84].
However, a very low flux and rejection ratio result from the low-porosity sublayer and dense skin layer (Figure 2). As a result, many researchers focused on using additives in the CA membrane casting solution to improve the membranes’ properties. Polyvinylpyrrolidone (PVP) has been introduced to the CA casting solution as a pore former, while hydrophilic PEG 600 has been added in order to change the membrane’s porosity [24,43,85].
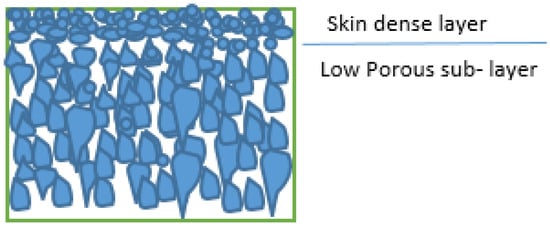
Figure 2.
Nanofiltration membrane layers.
The CA-NF membranes have been used in many disciplines, including desalination [86,87], removal of heavy metals [84], treatment of dye wastewater [87], nitroaromatic rejection from water contaminated by dye, pesticide, and explosives [88,89], domestic wastewater treatment [90], and removal of micropollutants from water [91].
3.4. Cellulose Acetate RO Membrane
RO is also a high-pressure process (up to 80 bar). RO membranes have a homogenous polymer layer that permits water to pass through them on top of a hierarchically structured polymer support. The application pressure must exceed the solution’s osmotic pressure. Most dissolved materials are rejected by the RO’s very selective membranes [23,92,93,94].
In the 1950s, the symmetrical CA membrane had been reported to retain salt successfully, attaining a rejection rate of 98 percent, but the permeate flux was poor (<10 mL m−2 h−1) [95]. Then, a 200-nm-dense layer was created on top of a microporous layer to create a CA asymmetric membrane. The water flux was much more significant with this new morphology than with the original symmetric membrane [96]. In today’s world, RO is the most extensively utilized desalination method because of its high rejection of salts; sodium chloride is rejected by 99.5–99.8% of commercial seawater RO membranes. Despite their high water permeability, RO membranes of brackish water routinely employed for potable reuse reject approximately 99.5% of salt [23,97].
The casting solution used in RO preparation is composed of CA, with acetone, DMA, and DMF-NMP as solvents used solely or in mixtures. First, casting is performed on a glass plate, and the solvent is allowed to evaporate, forming a semisolid mass, and then immersed in a water bath. Then, the resulting membrane is heated for annealing. Finally, the resulting RO membrane is stored in water for further uses [26,44,45,46,47,48,98].
The CA-RO membranes have been used for desalination [46,48,99,100], to remove ammonium from aqueous solutions [101], a trace of organic compounds and micropollutants are rejected from water more effectively by the CA-RO membranes [102], and fermentation broth separation [103].
3.5. Cellulose Acetate FO Membrane
Due to its potential for lower energy usage, high rejections to pollutants, and low membrane fouling, the CA forward osmosis (FO) membrane has received much attention in the fields of water desalination and water recovery [104,105].
As an affordable alternative to CA-RO, CA-FO technologies that run at little or no pressure can desalinate water. Similar to CA-RO, CA-FO separates water from dissolved solutes using a semipermeable membrane [106]. Small molecules, such as water, can pass across the semipermeable membrane, whereas larger molecules are blocked. This technique involves circulating a concentrated solution on the membrane’s permeate side with a higher osmotic pressure than the feed. The osmotic pressure gradient across the membranes promotes the flow of pure water through the membrane. Reduced pressures, high dissolved solute rejection, and low fouling propensity are all characteristics of CA-FO operation [107,108]. The CA-FO membrane displays minimal or reversible membrane fouling and runs at zero or low operational pressure [109].
The CA-FO membrane casting solution consists of CA with DMA, DMAc, or NMP as solvents. After casting, the solvent is allowed to evaporate for a few seconds, and then the casting plate is immersed in a water bath for a specific time. Finally, the prepared membrane is peeled off the plate and stored in a deionized water bath [52,53,54,55].
The CA-NF membranes have been used in many disciplines, including desalination [110], desalination using MgSO4 draw solution [108], salt rejection [111,112], treated sewage effluent [113], and recovery of water and nutrients from wastewater [114].
4. Cellulose Acetate (CA) Membrane Fouling
Fouling arises through the accumulation of materials rejected by the membrane within its pores or on its surface [115]. The particle dimensions of the pollutants present in the feed solution significantly influence the fouling mechanism. Contaminants may be adsorbed at the internal pore surface, leading to pore plugging, if the foulant size is similar to or lower than the membrane’s pore diameter. However, when the foulant has more significant dimensions than the membrane’s pores, a cake layer is formed on its surface [116,117]. The CA membrane has more significant fouling problems than other frequently used polymeric membranes due to its low surface hydrophilicity.
In general, the classification of membrane fouling is based on foulant type, with four main foulant types: particulate, organic, inorganic, and biological micro-organisms. In addition, another classification is based on the fouling location, internal or external fouling [118].
Internal membrane fouling refers to accumulating both colloidal particles and solutes in the pores. In contrast, external membrane fouling refers to the deposition of colloids, particles, and macromolecules on their surface, creating a fouling layer over the top of the surface, where it could be a gel layer formed due to the pressure variance between the permeate and the feed, or it could be a cake layer formed as a result of solids accumulation on the membrane surface [119]. It also can be classified according to the degree of foulant removal, where it could be removed by physical cleaning (reversible), or it could be irreversible, thus, requiring the chemical cleaning to be removed [120]. The following subsection briefly describes the four primary fouling types based on dominant foulants.
4.1. Types of CA Membrane Fouling Based on Foulant Types
In general, the membrane material or membrane type may affect the fouling type due to different surface morphology or different modes of filtration (i.e., pressure driven). However, the main affecting factor is the solution chemistry [121], and besides the foulant properties, the foulant layer structure and interaction membrane surface, the foulant controls the fouling type and behavior [122].
4.1.1. Organic Fouling
Organic fouling is the accumulation of dense organic materials mainly on the surface of the membrane [118,123]. Natural and soluble organic matter and synthetic compounds are all examples of dissolved organic matter [118,123]. Organic fouling occurs by various mechanisms, mainly: adsorption, attachment, pore-clogging, and the development of a gel or cake layer. Several parameters affect the occurrence of these mechanisms, such as organic matter characteristics (charge, hydrophobicity, size), membrane characteristics (surface charge, pore size, hydrophobicity, surface roughness), feedwater chemistry (pH, concentration of divalent cation, and ionic strength); and operating conditions (permeate flux, pressure, temperature, and crossflow velocity) [124,125,126].
Membrane surface characteristics have a substantial role in organic matter adsorption, which is the dominant organic fouling mechanism. Thus, reducing the effect of organic matter adsorption can control such fouling. For example, it was reported that surface roughness reduction and membrane hydrophilicity increase have the potential to decrease and control organic fouling [126,127].
4.1.2. Inorganic (Scaling) Fouling
Inorganic fouling occurs when the feed water contains inorganic compounds, such as hydroxides, salts, and minerals that precipitate out of the solution once the solution’s concentration becomes higher than its saturation point (equilibrium solubility), resulting in its deposition and accumulation on the membrane surface or in between its pores, where it leads to the development of a cake layer or clogging of the pores [118,128,129]. Several researchers have proved that inorganic fouling is likely to happen if the feed water contains high calcium or magnesium concentrations [3].
Inorganic fouling occurs through two mechanisms: crystallization, which includes ion precipitation, and then deposition on the surface. In addition to particulate fouling that occurs through convective particulates transport from the solution to the surface [128,130] since the concentration of the inorganic compounds at the surface is higher than that in the solution [129].
4.1.3. Particulate/Colloidal Fouling
Particulate/colloidal fouling is produced by a buildup of large macromolecules (aggregates), including biological, organic, and inorganic material on the membrane surface [129,131], creating a cake layer that blocks water from going through pores of the membrane resulting in pores clogging [132]. This affects energy consumption, where the pressure required for operation should increase to enforce the water passing through the membrane [133].
The particulate fouling occurs first by pore-plugging, which arises due to particles building up around the pore opening, causing the aperture to be narrowed and restricted. Over time, this led to deposition accumulation on the initial ply, resulting in the closing of the membranes’ pores. Then a thick ‘cake layer’ is produced due to particle deposition increase. Such a layer affects the membrane’s efficiency as the hydraulic resistance increases and permeate flux decreases [129].
In general, smooth and low surface charge membranes with improved hydrophilic properties are less likely to be exposed to particulate fouling. In contrast, inappropriate operating conditions such as high flux rates of membrane and low crossflow velocity can result in severe membrane fouling [118,134].
4.1.4. Biological Fouling (Biofouling)
Biofouling occurs due to the growth and buildup of biological pollutants (microorganisms) in the pores of the membranes or on their surfaces. It includes microorganisms, plants, algae, and others, and it restricts the flowing of water from one side to the other. Such foulants are present, grow, and multiply in warm nature with low flow rates and produce a protective material known as extracellular polymeric substance (EPS); thus, the microorganisms and EPS together create a layer called biofilm that resists the backwashing cleaning technique [118]. The EPS is mainly composed of polysaccharides and proteins as well as macromolecule sources, including heteropolysaccharides, lipoproteins, and glycoproteins, and it induces the micro-organism’s growth as it creates the favorable conditions for bacteria to grow close to each other [131].
The biofouling mechanism involves three stages; the accumulation of soluble and suspended EPS on the surface and in the pores of membranes; the clocking of membrane pores via cell debris and tiny colloidal particles; and the sludge cake adhesion on the surface of the membrane [135]. Biofouling control is critical, especially for CA membranes, since it is more vulnerable to a microbial attack where the microorganisms can degrade the membrane surface and utilize cellulase enzymes [127,130,136]. Maddah et al. [137] have identified the most common microorganisms that attack the membranes, including bacteria (Mycobacterium, Pseudomonas, Bacillus, … etc.) and fungi (Penicillium, Mucor, Aspergillus, … etc.).
4.2. CA Fouling Influencing Factors
Several researchers have studied the influencing factors of CA fouling in several types of membranes. For instance, Xinghua Sun et al. [138] have investigated fouling using Bovine Serum Albumin (BSA) at constant permeate flux during the dead-end microfiltration, taking into consideration; the effect of the protein’s aggregates size, blocking, and constriction of membrane pores, cake formation and the solution’s pH on fouling of the membrane. They stated that the formed cake layer resists the front-washing cleaning strategy and cannot be removed. However, it can be removed easily at a pH of 6.9, proving the CA membrane’s reversibility and pH-dependent nature. They also reported that the main reason for fouling was the cake layer formed due to large BSA aggregates since it significantly affected membrane fouling compared to small aggregates. While Winfield [139] investigated Cellulose Acetate-Reverse Osmosis (CA-RO) membranes fouling by sewage effluent, he stated that membrane fouling is dominated by dissolved organic matter than large suspended particles.
Another study by Masatoshi Hashino et al. [140] examined the behavior of the fouling of the cellulose acetate butyrate (CAB) membrane using three kinds of natural organic matter (NOM), including sodium alginate, BSA, and humic acid. They observed the blocking of the pores and the development of a cake layer that consisted of substances with a size of more than 0.1 µm.—which is considered high molecular weight—when using the sodium alginate solution, thus leading to a more significant decline in permeate flux than in humic acid and BSA. However, a progressive reduction in permeate flux was detected when using the BSA solution after 240 min of filtration due to BSA adsorption to the CAB membrane, resulting in a lower permeate flux than humic acid.
Moreover, Xunyao Fu et al. [141] investigated the surface morphology effect on the fouling of cellulose acetate butyrate (CAB) membranes using a humic acid solution where they prepared different surface structures. They observed a fast decline in the membrane’s relative permeability due to fouling by the solution of humic acid, which penetrates the membrane and deposits onto its pores, resulting in lower rejection of humic acid and consequently leading to substantial flux decline.
Furthermore, Dwi Indarti et al. [142] have explored the fouling behavior of CA UF membrane using Tempe wastewater with high protein content. They found that both the exerted pressure and protein concentration affect the membrane fouling, where high protein molecules concentration in the feed water results in membrane fouling while the high exerted pressure leads to enhancing the permeate flux and thus enhancing fouling resistance. In other words, the higher the protein molecules concentration in the feed water and the lower the exerted pressure, which strongly affects the permeate flux, the more significant the fouling. The fouling resistance of the CA UF membrane has also been studied by Andrea Iris SCHÄFER [143], where they investigated the temperature impact on water flux by adjusting the temperature between 15 °C and 40 °C and measuring the variation of flux as well as variation of dynamic viscosity with temperature. An enhancement in permeate flux was detected as the viscosity decreased, thus indicating a higher permeability and fouling resistance at a higher temperature.
Additionally, the impact of the ionic strength and the colloid concentration of the feed water on fouling was also explored. Zhu and Elimelech [144] have examined the fouling of CA-RO membranes by colloidal silica. It was found that the membrane fouling is negatively affected by the high solution’s ionic strength and high concentration of feed colloids. This is because the particles will be less negative in higher ionic strength solutions due to double layer compression and stern layer charge reduction [145]; because equivalently charged ions repel negatively charged particles, membrane fouling is delayed [146]. It is worth mentioning that the researchers have conducted the same experiment using an aromatic polyamide composite RO membrane, and they found that the flux across the CA-RO membrane decreased at a lower rate compared to the composite RO membrane due to the differences in the roughness of the membranes surface, where the CA membrane surface is considered smooth, confirming that the surface morphology has significant effects on the colloidal fouling. This was also confirmed by Gu et al. [121], who studied the organic fouling of two membranes and reported that cellulose triacetate membrane had less fouling at early stages compared to polyamide membranes which have rougher membrane surface as it offers a larger surface area than smooth membrane surface that induces the interaction between particles [147] and leads to particle accumulation, resulting in severe flux decline [148].
The hydrophilicity of the membrane’s surface is known to enhance antifouling properties. Different studies in the literature studied enhancement hydrophilicity and, consequently, enhancement of the antifouling properties. Khan et al. [149] introduced an organic acid to the casting solution of CA membranes preparation, which caused asymmetry in the membrane structure, high hydrophilic properties, and the smoothness of the surface, leading to better antifouling performance. In comparison, another study was performed on CTA membranes by Naseem et al. [150], who introduced TiO2/GO nanoparticles into the membrane matrix, which enhanced membrane hydrophilicity and antifouling performance. No study was found to compare CA and CTA fouling behavior.
The efficiency of CA membranes is strongly influenced by the acetylation degree. An increase in acetylation produced a more excellent salt rejection ratio, but the flux values were shallow [151]. Depending upon the number of hydroxyl groups in the repeat unit of cellulose that are replaced by acetyl groups, three different forms of cellulose acetates with varying degrees of acetylation can be produced: cellulose monoacetate, cellulose diacetate, and cellulose triacetate, the average number of acetyl groups per repeat unit of the polymer, which determines the degree of acetylation, ranges from 1 to 3 [152,153]. Hydrophobic acetyl groups replace the hydrophilic hydroxyl groups on the cellulose surface throughout the process of acetylation reactions, and the molar ratio of acetic anhydride to acetic acid causes an increase in the degree of acetylation. Increasing the acetylation degree results in an increase in the hydrophobic properties of the cellulose. The acetylation degree effects on cellulose acetate’s hydrophobic characteristics are believed to be significant for membranes, filters, scaffolds, and textile applications [154].
Zhu and Elimelech [144] examined the effect of permeate water flux by using various permeation rates (3.31 × 10−6, 5.27 × 10−6, and 1.10 × 10−5 m/s) on membrane fouling at similar solution chemical composition (0.01MNaCl and 30 mg/L silica colloids), and found that the amount of deposited colloids increases as the rate of particle transport increases, thus fouling is more significant at high permeation rates [144].
They also examined the fouling of CA-RO membranes by aluminum oxide colloids. The experiments were conducted at different concentrations of NaCl, mainly: 0.1, 0.01, and 0.001 M, at a fixed transmembrane pressure of 400 psi and pH = 5.6 to 6. In addition, they used a feed concentration of 100 mg/L.
The CA-RO colloidal fouling was substantial at high ionic strength (0.1 M). That was because the double layer’s repulsive forces decreased, and particles interacted with previously retained particles and deposited onto their surface instead of on the membrane surface, producing a thick fouling layer that increases the water flow resistance and consequently decreases the water flux. Humic substances’ effects on colloidal fouling have also been explored since most of the feed water selected to be treated by RO membrane has a considerable amount of dissolved organic matter with a considerable fraction of humic substances. Therefore, a series of experiments were performed without colloidal particles and at different concentrations (0, 10, 30, and 100 mg/L) with humic substances available at 0.6 mg/L total organic carbon (TOC) and 0.1 M NaCl. It was found that the higher the particle concentrations, the more severe the membrane fouling. Moreover, it was detected that the utilized concentration of humic substances would not cause fouling to the membrane, as can be confirmed by the constant water flux through the membrane in the absence of colloidal particles [144].
According to all the studies mentioned above, it is evident that improving the performance of CA membranes is necessary. Therefore, several studies have investigated CA membrane’s fouling remediation and cleaning techniques, as described in the following section, where a substantial enhancement was accomplished.
5. Cellulose Acetate (CA) Membranes Antifouling Strategies
Strategies for CA membrane antifouling can be achieved using different techniques (Figure 3):
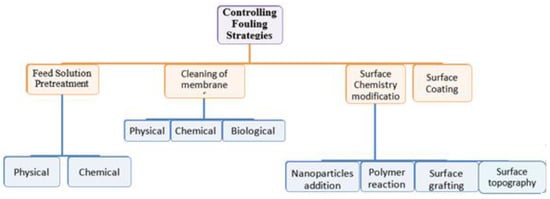
Figure 3.
Different techniques of membrane antifouling.
5.1. Feed Solution Pretreatment
This technique is often used as a first step to prevent quick membrane fouling by preventing the solute from settling on the membrane’s surface or in between the pores. This technique generally employs physical or chemical means. A pre-filtration or adsorption is typically employed in the physical method to eliminate foulants that are outcast due to their fouling proclivity [116]. Antiscalants or disinfectants are included in the solution for the chemical pretreatment to help the particles in the feed precipitate, coagulate, or flocculate [155].
5.2. Cleaning of the Membrane Surface
Methods of cleaning membranes are divided into physical, chemical, and biological methods [155]. Cleaning by simple physical methods can remove a small number of foulants, but the only way to remove a high amount of foulants is to use aggressive chemical cleaning, which causes membrane matrix materials to degrade [156]. In biological cleaning methods, a cleaning solution containing bioactive agents is used to catalytically decompose foulants and lower the probability of fouling [155]. Enzymes, antibodies, peptides, nucleic acids, and polysaccharides are the bioactive agents used in biological cleaning methods [157,158].
5.3. Surface Modification
Foulants’ interactions with the membrane surface are influenced by surface chemistry. According to Whiteside and coworkers, surfaces that resist fouling have three common characteristics: they are electrically neutral, hydrophilic, and capable of establishing hydrogen bonds [159].
A pure water layer can quickly form on a very hydrophilic surface, inhibiting the hydrophobic foulants from adhering to and depositing on the membrane’s surface, thereby decreasing fouling [160].
5.3.1. Surface Chemistry Modification Using Nanoparticles (NPS)
The surface can be chemically modified either by incorporating nanoparticles inside the polymer matrix or by changing the chemistry of the polymer matrix using different reactions.
Metal-Based Nanoparticles
The influence of bioinspired polydopamine (PD) coating accompanied by in situ silver nanoparticles (AgNPs) immobilization on CA/UF membrane modification was studied by Saraswathi and coworkers. CA membrane’s hydrophilicity, antifouling, and anti-biofouling characteristics have all improved dramatically due to the treatments. The immobilized AgNPs offered outstanding anti-biofouling properties to the modified membrane, while the PD coating provided strong surface hydrophilicity and antifouling properties [161]. In another study, bactericidal CA nanofiltration composite membranes were prepared with three types: CA-based membranes with polyvinylpyrrolidone coated AgNPs, B-zeolite, and silver ion-exchanged B-zeolite. The results showed that adding AgNPs increases the hydraulic permeability of the membrane by three times while adding silver-ion loaded zeolite increases the permeability of the hydraulic by 56.3% [162]. Additionally, in 2015, AgNPs bonded with the CA polymer matrix showed improvement in the hydraulic permeabilities by Sonawane and coworkers [163].
The utilization of dispersed Au (0), Ag(0), or Pt(0) NPs in a 1-n-butyl-3-methylimidazolium bis(trifluoromethane sulfonyl)imide ionic liquid (IL) in combination with CA resulted in functionalized membrane films with good thermal, mechanical, and chemical stability as found by Scheeren and coworker. The CA/IL/NPs combination increased their ability to resist E. coli and S. aureus [164].
Metal Oxide-Based Nanoparticles
The phase inversion technique was used to make ZnO nanoparticles (NPs) modified ultrafiltration CA membranes (CA/ZnO NPs). Surface pore size, porosity, contact angle, and surface hydrophilicity were all impacted by varying concentrations of ZnO NPs in the polymeric membrane. The CA membranes’ permeability is reduced when polymer content is increased. The permeability of the blended membranes increased due to the increasing hydrophilicity and porosity of the membrane. Notably, a tiny amount of ZnO NP supplementation was adequate to improve mechanical characteristics. Due to a network of hydrogen connections between CA and ZnO NPs, the inclusion of ZnO NPs increased the surface hydrophilicity, allowing for acceptable wettability [165].
The electrospinning method was used to produce hybrid membranes made of CA and titanium oxide nanoparticles (TiO2NPs)/ (CA-TiO2) hybrid membranes. The CA–TiO2 hybrid membranes had a structure similar to that of a pristine CA membrane. However, the network linking the fiber structures was enhanced by the deposition of NPs on the membrane fibers’ surfaces. Characterization investigations confirmed the presence of NPs in the CA electrospun membrane matrix, and the pristine CA membrane was successfully modified. Increasing the TiO2 amount in the solution of CA improved the positively charged properties of hybrid membranes, and it led to an increase in the roughness of the surface and an improvement the tangles and the network connecting the fibers. Furthermore, considerable amounts of agglomerations were noticed on the upper layer of the membrane for higher concentrations of TiO2NPs (4.5 and 6.5 wt%), and a portion of the fibers exhibited a change in shape from round to flat. As a result, 1.0 and 2.5 wt% of hybrid membranes were determined to have the optimal morphological structures [166].
Merging silicon oxide nanoparticles (SiO2NPs) and poly(3,4-ethylene dioxythiophene) (PEDOT) into the CA matrix resulted in the construction of new antifouling UF nanocomposite membranes. The nanocomposite membranes outperform the pristine membrane in terms of hydrophilicity, porosity, bovine serum albumin (BSA) rejection, and flux, using the abundance of surface-terminating groupings on CA, SiO2NPs, and PEDOT. Molecular modeling was utilized to theoretically and experimentally examine the properties of the membranes and synthesized materials. The hypothesized membrane structure was moderately positive and demonstrated a preference for electrophilic addition reactions [37].
Metal-Organic Frameworks (MOFs)
Another nanoparticle that can be used for enhancing antifouling is metal-organic frameworks (MOFs), which were found to increase the hydrophilicity of membrane surfaces, leading to the enhancement of antifouling properties [167]. Vantopour et al. [168] introduced zeolitic imidazole framework-8 (ZIF-8) particles into CA-NF membranes. This resulted in an increase in flux recovery, a decrease in contact angle, and a decrease in fouling. They concluded that the ZIF-8 MOF enhanced the hydrophilicity of the membrane surface, leading to a compact water barrier and decreasing the interactions between the contaminant and the surface of the membrane. In another study, Yang et al. [36] synthesized MOF-Lignocellulose nanofibrils composed of HKUST-1@ LCNFs, a hydrophilic filler, and blended them with CA-UF membranes, which resulted in improved antifouling properties. Ee et al. [169] used a composite of cellulose nanocrystals (CNC) with UiO66-NH2 as an electrospray coating for CA membranes, which led to higher biofouling resistance.
Clay-Based Nanoparticles
The wet-phase inversion technique was used to make CA/nanoclay mixed matrix membranes. Mixing nanoclay with the CA polymer solution enhanced the rate of demixing, resulting in the creation of macrovoids. Two different forms of nanoclay were used: Na- montmorillonite and organically modified montmorillonite. CA organo-montmorillonite outperformed virgin CA and CA sodium montmorillonite in terms of membrane performance and antifouling characteristics. In addition, the membrane pore size, porosity, and hydrophilicity improved when organo-montmorillonite was embedded in the CA matrix compared to sodium montmorillonite [170].
Carbon-Based
To enhance the UF/CA membrane efficiency, various amounts of virgin multiwalled carbon nanotubes were blended with the CA membrane to enhance the UF/CA membrane efficiency and produce asymmetric flat-sheet composite membranes for methylene blue removal. Due to the high hydrophilicity of the surface supplied by the CNTs, the composite membranes had the best antifouling capabilities and showed an improvement in methylene blue removal [171].
De Faria and coworkers reported the development of functional materials that can resist bacterial colonization by combining graphene oxide-silver (GO-Ag) nanocomposites with CA membranes. S. aureus and E. coli strains demonstrated promising antibacterial activity when CA membranes included graphene oxide-silver nanocomposites. The treated membranes showed extraordinary resistance to E. coli adhesion and proliferation. In the (GO-Ag) nanocomposites membranes, surface pores were larger, and pure water flux was higher than in virgin membranes [172].
Others
Using the phase-inversion process, asymmetric RO membranes of CA were produced in the presence of chitosan NPs as an anti-biofouling agent. According to the observations, the inclusion of chitosan NPs boosted the salt rejection and water flux. In addition, the static adhesion test revealed that the membrane incorporating chitosan nanoparticles had improved fouling resistance to bacterial activity [15].
Hydrophilic polydopamine-sulfobetaine methacrylate—PDA/SBMA—De Guzman and coworkers synthesized NPs. Increased demixing rates were achieved by adding hydrophilic PDA/SBMA NPs to the CA solution. As a result, membranes become more porous, hydrophilic, and thick. However, incorporating smaller NPs into the CA matrix did not always result in greater efficiency. PDA/SBMA nanoparticles of sufficient net charge and size must be introduced to achieve optimal membrane characteristics and performance. It had good antifouling characteristics as well. Furthermore, it was effective in treating a variety of oily feed combinations comprising various forms of emulsified oil [77].
5.3.2. Surface Chemistry Modification Using Polymer Reaction
A one-step reaction recently synthesized PA with greater hydrophilicity and oleophobicity characteristics than CA by esterification between perfluoroalkyl polyethoxy acetic acid and the hydroxyl groups. A blend membrane created by combining CA and PA in a 4:1 ratio demonstrated better membrane characteristics. Furthermore, the results showed that the membranes were antifoulants because they had excellent hydrophilicity and superoleophobicity because the fluorinated group introduced in PA was built using both oleophobic and hydrophilic perfluoroalkyl groups [173].
In another study, a two-step procedure that included chlorination and subsequent substitution reactions produced a new kind of antifouling dopamine-modified CA membrane. The results showed enhancements in the permeability and hydrophilicity of modified membranes compared to those of the nonmodified membranes. In addition, the CA membrane modified with dopamine had excellent durability and a broader range of applications in water treatment [174].
In 2014, Kanagaraj and coworkers synthesized charged surface-modifying macromolecules (cSMM) mixed at different concentrations to prepare CA membranes using solution casting. The additive content of cSMM increased surface hydrophilicity and the pore dimensions, causing higher pure water. The average pore size, porosity, and molecular weight cut-off value of a cSMM blended membrane rise dramatically as the content of cSMM rises. The enhanced hydrophilicity resulted in resisting protein adsorption at the surfaces of the membrane and consequently improved antifouling characteristics [175].
5.3.3. Surface Grafting
Surface grafting is a chemical modification, e.g., covalent bonding between the surface and a substance, leading to a change in the surface’s properties [176]. For example, Tyrka et al. [176] examined the potentiality of covalent bonding, an active substance covalently bonding 3-aminopropyl (diethoxy)methylsilane to the CA membrane surface in scCO2 to obtain anti-biofouling properties. Grafting increased material hydrophilicity, as supported by contact angle results, and showed anti-biofouling solid properties. In another study, CA membrane surfaces were grafted with epoxy propyl dimethyl dodecyl ammonium chloride [177]. The antibacterial, antifouling, and antibioadhesion properties of the CA membrane were enhanced due to grafting.
5.3.4. Surface Topography
In order to gain optimum surface area interactions and protection, micro-organisms head to set in zones that are somewhat larger than themselves. Therefore, surface topography depends on limiting micro-organism settlement by forcing restrictions on size; as a result, developing a nano- or microstructure on the surface top limits the attachment options numbers, decreasing fouling adherence and making fouling removal more accessible in the event of settling [83]. Additionally, fouling particles prefer to be trapped by a rougher surface than by a smoother one; hence, a smoother membrane surface is predicted to have faceless fouling [178].
The function of the morphology of the membrane surface in colloidal fouling of CA and thin-film composite polyamide membranes was examined by Elimelech and coworkers. Compared to the CA membranes, the thin-film composite membranes had a greater fouling rate [179]. Hence, decreasing the membrane’s surface roughness might increase its antifouling characteristics, but on the other hand, it could adversely affect the membrane flux [180].
A hydrophilic CA-graft-(glycidylmethacrylate-g-polyethylene glycol) (CA-g-(GMA-g-PEG)) was developed and inserted into acetylated methyl cellulose (AMC). Increased CA-g-(GMA-g-PEG) concentration in the AMC matrix, decreased macrovoids and caused sponge-like structures all over the membrane cross-section. Treating membranes with AMC resulted in a smoother surface and increased hydrophilicity, which resulted in a lower fouling tendency in the membrane compared to untreated membranes [181].
5.4. Surface Coating
The membrane surface is coated with a thin layer to enhance its surface charge, hydrophilicity, and smoothness. However, this is only applied to polymers with a high molecular weight. Another problem is instability caused by a weak surface-layer interaction (also known as interfacial incompatibility) [182]. The electrostatic repulsive force reduces fouling production when both the foulant and the membrane have similar surface charges [183]. Poly (ethylene glycol) and other hydrophilic polymers (polysaccharides, polyacrylates) were used to make antifouling coatings, but attention to these materials has lately switched to zwitterionic polymers [184].
The surface of the electrospun CA fiber networks was efficiently covered with chitin nanocrystals, resulting in a new and exceptionally effective surface treatment approach for antifouling membrane fabrication. In addition, hydrophobic CA was used to make superhydrophilic membranes with a 0° contact angle [185].
To increase the hydrophilicity and lipophilicity of cellulose nanocrystals (CNCs), dopamine (DA) was self-polymerized in water, resulting in a thin coating of polydopamine (PDA). The PDA-coated CNCs (PDA@CNCs) were combined with cellulose acetate and a pore-forming agent (CA). The filtration capacity, antifouling performance, and tensile strength of the CA membrane were significantly improved by adding a small amount of PDA@CNCs. Additionally, adding PDA@CNCs enhanced the lipophilicity and dispersion of CNCs, increased the binding force between CNCs filler and CA matrix, and enhanced the performance of CA composite membranes, expanding the industrial application of CA materials and opening up a wide range of new application possibilities in the fields of biological separation membranes and water [186].
Another process is used to enhance the characteristics of the membrane, which is created by covering the porous electrospun polyacrylonitrile (ePAN) substrate with negatively charged (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO)-oxidized cellulose nanofibers (CNF). As a result, the morphology, pore size distribution, hydrophilicity, and zeta potential of CN membranes showed great permeation flux, high rejection ratios, good antifouling tendencies, and high practical antifouling self-cleaning, which have all been significantly impacted by this advancement. In addition, the outcomes demonstrated the viability of utilizing charged CNF as a barrier layer for ultrafiltration membranes used in wastewater treatment to prevent fouling [187].
6. Conclusions and Future Prospective
Since CA has many characteristics, such as being biodegradable, biocompatible, insoluble in water, nontoxic, having excellent chemical resistance, and highly stable, it has been intensively used in different applications. However, CA membranes, which can be used as MF, UF, NF, RO, and FO membranes, are susceptible to fouling, affecting the membrane performance. This review highlights different strategies used for antifouling. It summarizes them into four main techniques:
- Feed solution pretreatment, which can be achieved physically or chemically.
- Cleaning of the membrane surface, which can be achieved physically, chemically, or biologically.
- Surface modification can be achieved either by using nanoparticles (metal or metal oxide-based, clay-based, or carbon-based nanoparticles) or by using polymer reactions, or through surface grafting or surface topography.
- Surface coating.
As CA has been used in numerous applications in water filtration, finding solutions for its antifouling issues would maximize the use of its benefits when compared to other polymeric membrane systems. Some points to be considered in future work are examining the difference in antifouling behavior between cellulose acetate and cellulose triacetate, as this might be an interesting aspect that should have been studied more deeply in the literature.
Another suggestion is to examine the effect of preparation techniques, such as solution casting, electrospinning, and spin coating, since this is expected to affect the morphology and structure of the membrane.
Author Contributions
Conceptualization, R.A.-Z.; Data collection and investigation, N.A., A.A.I. and G.H.; writing—original draft preparation, N.A., A.K., A.A.I. and G.H.; writing—review and editing, R.A.-Z.; supervision, R.A.-Z.; project administration, R.A.-Z. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AgNPs | Silver Nanoparticles |
| BSA | Bovine Serum Albumin |
| CA | Cellulose Acetate |
| CAB | Cellulose Acetate Butyrate |
| CA-g-(GMA-g-PEG) | CA-graft-(glycidylmethacrylate-g-polyethylene glycol) |
| CAP | Cellulose Acetate Propionate |
| CA-RO | Cellulose Acetate Reverse Osmosis |
| CA-TiO2 | CA and titanium oxide |
| CA-UF | Cellulose Acetate Ultrafiltration |
| CDA | Cellulose Diacetate- |
| CNCs | Cellulose Nanocrystals |
| CNF | Cellulose Nanofibers |
| DA | Dopamine |
| DMA | N, N-Dimethylacetamide |
| DMAc | Cellulose Triacetate Forward Osmosis |
| DMF | N,N-Dimethyl Formamide |
| DS | Degree of Substitution |
| ePAN | Electrospun Polyacrylonitrile |
| EPS | Extracellular Polymetric Substance |
| FO | Forward Osmosis |
| GO | Graphene oxide |
| GO-Ag | Graphene Oxide-Silver |
| GVL | γ-Valerolactone |
| MAC | Methyl Acetate |
| 2ME-THF | Methyltetrahydrofuran |
| MF | Microfiltration membrane |
| MOFs | Metal Organic Frameworks |
| NF | Nanofiltration |
| NMP | 1–Methyl–2–Pyrrolidone |
| NOM | Natural Organic Matter |
| NPs | Nanoparticles |
| OSN | Organic Solvent Nanofiltration |
| PA | Polyamide |
| PDA | Polydopamine |
| PDA/SBMA | Polydopamine-Sulfobetaine Methacrylate |
| PE | Polyethylene |
| PEDOT | Poly(3,4-Ethylene Dioxythiophene) |
| PES | Polyethersulfone |
| PP | Polypropylene |
| PSF | Polysulfone |
| PVA | Polyvinyl Alcohol |
| PVDF | Polyvinylidene Fluoride |
| PVP | Polyvinylpyrrolidone |
| RO | Reverse Osmosis |
| SiO2NPs | Silicon Oxide Nanoparticles |
| SMM | Surface Modifying Macromolecule |
| TEMPO | 2,2,6,6-Tetramethylpiperidin-1-yl)oxyl |
| TFC | Thin Film Composite |
| TiO2NPs | Titanium Oxide Nanoparticles |
| TOC | Total organic carbon |
References
- Risch, S.J. Food Packaging History and Innovations. J. Agric. Food Chem. 2009, 57, 8089–8092. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable Polymers-A Review on Recent Trends and Emerging Perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Yokesahachart, C.; Yoksan, R. Effect of amphiphilic molecules on characteristics and tensile properties of thermoplastic starch and its blends with poly(lactic acid). Carbohydr. Polym. 2011, 83, 22–31. [Google Scholar] [CrossRef]
- Schilling, M.; Bouchard, M.; Khanjian, H.; Learner, T.; Phenix, A.; Rivenc, R. Application of Chemical and Thermal Analysis Methods for Studying Cellulose Ester Plastics. Acc. Chem. Res. 2010, 43, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and Applications of Cellulose Acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Rimdusit, S.; Jingjid, S.; Damrongsakkul, S.; Tiptipakorn, S.; Takeichi, T. Biodegradability and property characterizations of Methyl Cellulose: Effect of nanocompositing and chemical crosslinking. Carbohydr. Polym. 2008, 72, 444–455. [Google Scholar] [CrossRef]
- Kaur, G.; Grewal, J.; Jyoti, K.; Jain, U.K.; Chandra, R.; Madan, J. Chapter 15—Oral controlled and sustained drug delivery systems: Concepts, advances, preclinical, and clinical status. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Grumezescu, A.M., Ed.; William Andrew Publishing: Burlington, MA, USA, 2018; pp. 567–626. [Google Scholar] [CrossRef]
- Cobo, F.N.; Faria-Tisher, P.C.S.; Duarte, J.L.; Carvalho, G.M. Preparation and characterization of microporous cellulose acetate films using breath figure method by spin coating technique. Cellulose 2017, 24, 4981–4995. [Google Scholar] [CrossRef]
- Vallejos, M.; Peresin, M.S.; Rojas, O.J. All-Cellulose Composite Fibers Obtained by Electrospinning Dispersions of Cellulose Acetate and Cellulose Nanocrystals. J. Polym. Environ. 2012, 20, 1075–1083. [Google Scholar] [CrossRef]
- K Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef]
- Park, H.-M.; Misra, M.; Drzal, L.T.; Mohanty, A.K. “Green” Nanocomposites from Cellulose Acetate Bioplastic and Clay: Effect of Eco-Friendly Triethyl Citrate Plasticizer. Biomacromolecules 2004, 5, 2281–2288. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Alammar, A.; Hardian, R.; Szekely, G. Upcycling agricultural waste into membranes: From date seed biomass to oil and solvent-resistant nanofiltration. Green Chem. 2022, 24, 365–374. [Google Scholar] [CrossRef]
- Thi, H.Y.N.; Kim, S.; Nguyen, B.T.D.; Lim, D.; Kumar, S.; Lee, H.; Szekely, G.; Kim, J.F. Closing the Sustainable Life Cycle Loop of Membrane Technology via a Cellulose Biomass Platform. ACS Sustain. Chem. Eng. 2022, 10, 2532–2544. [Google Scholar] [CrossRef]
- El-Ghaffar, M.A.A.; Elawady, M.M.; Rabie, A.M.; Abdelhamid, A.E. Enhancing the RO performance of cellulose acetate membrane using chitosan nanoparticles. J. Polym. Res. 2020, 27, 33. [Google Scholar] [CrossRef]
- Hoenich, N. Cellulose for medical applications: Past, present, and future. Bioresources 2006, 1, 270–280. [Google Scholar] [CrossRef]
- Vatanpour, V.; Yuksekdag, A.; Ağtaş, M.; Mehrabi, M.; Salehi, E.; Castro-Muñoz, R.; Koyuncu, I. Zeolitic imidazolate framework (ZIF-8) modified cellulose acetate NF membranes for potential water treatment application application. Carbohydr. Polym. 2023, 299, 120230. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Al-Rosan, S.A.; Albiss, B.A. Photocatalytic Degradation of Methylene Blue Using Polymeric Membranes Based on Cellulose Acetate Impregnated with ZnO Nanostructures. Polymers 2021, 13, 3451. [Google Scholar] [CrossRef]
- Ounifi, I.; Guesmi, Y.; Ursino, C.; Castro-Muñoz, R.; Agougui, H.; Jabli, M.; Hafiane, A.; Figoli, A.; Ferjani, E. Synthesis and Characterization of a Thin-Film Composite Nanofiltration Membrane Based on Polyamide-Cellulose Acetate: Application for Water Purification. J. Polym. Environ. 2022, 30, 707–718. [Google Scholar] [CrossRef]
- Tashvigh, A.A.; Elshof, M.G.; Benes, N.E. Development of Thin-Film Composite Membranes for Nanofiltration at Extreme pH. ACS Appl. Polym. Mater. 2021, 3, 5912–5919. [Google Scholar] [CrossRef]
- Krishnan, J.N.; Venkatachalam, K.R.; Ghosh, O.; Jhaveri, K.; Palakodeti, A.; Nair, N. Review of Thin Film Nanocomposite Membranes and Their Applications in Desalination. Front. Chem. 2022, 10, 781372. [Google Scholar] [CrossRef]
- Liu, S.; Hu, L.F.; Zhang, W.C.; Ma, H.Y. Cellulose Acetate Reverse Osmosis Membranes for Desalination: A Short Review. Non-Met. Mater. Sci. 2019, 1, 15–25. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, N.; Madaeni, S.S.; Alizadeh, A.; Daraei, P.; Zinatizadeh, A.A.; Rahimpour, F. Separation of nitrophenols using cellulose acetate nanofiltration membrane: Influence of surfactant additives. Sep. Purif. Technol. 2012, 85, 147–156. [Google Scholar] [CrossRef]
- Barth, C.; Gonçalves, M.; Pires, A.; Roeder, J.; Wolf, B. Asymmetric polysulfone and polyethersulfone membranes: Effects of thermodynamic conditions during formation on their performance. J. Membr. Sci. 2000, 169, 287–299. [Google Scholar] [CrossRef]
- Shibata, T. 5.6 Cellulose acetate in separation technology. Macromol. Symp. 2004, 208, 353–370. [Google Scholar] [CrossRef]
- Sossna, M.; Hollas, M.; Schaper, J.; Scheper, T. Structural development of asymmetric cellulose acetate microfiltration membranes prepared by a single-layer dry-casting method. J. Membr. Sci. 2007, 289, 7–14. [Google Scholar] [CrossRef]
- Yang, S.; Zou, Q.; Wang, T.; Zhang, L. Effects of GO and MOF@GO on the permeation and antifouling properties of cellulose acetate ultrafiltration membrane. J. Membr. Sci. 2019, 569, 48–59. [Google Scholar] [CrossRef]
- He, Y.; Li, G.-M.; Wang, H.; Jiang, Z.-W.; Zhao, J.-F.; Su, H.-X.; Huang, Q.-Y. Experimental study on the rejection of salt and dye with cellulose acetate nanofiltration membrane. Festschr. Issue Honor Profr. Yi Hua Ma 2009, 40, 289–295. [Google Scholar] [CrossRef]
- Kimura, S.; Sourirajan, S. Analysis of data in reverse osmosis with porous cellulose acetate membranes used. AIChE J. 1967, 13, 497–503. [Google Scholar] [CrossRef]
- Takao, S.; Rajabzadeh, S.; Otsubo, C.; Hamada, T.; Kato, N.; Nakagawa, K.; Shintani, T.; Matsuyama, H.; Yoshioka, T. Preparation of Microfiltration Hollow Fiber Membranes from Cellulose Triacetate by Thermally Induced Phase Separation. ACS Omega 2022, 7, 33783–33792. [Google Scholar] [CrossRef]
- Redha, Z.M.; Bu-Ali, Q.; Saeed, Y.A.; Ali, A.M. Optimization of the Asymmetric Cellulose Acetate Membrane Synthesis Variables for Porosity and Pure Water Permeation Flux Using Response Surface Methodology: Microfiltration Application. Arab. J. Sci. Eng. 2021, 46, 6593–6607. [Google Scholar] [CrossRef]
- Battirola, L.C.; Andrade, P.F.; Marson, G.V.; Hubinger, M.D.; Gonçalves, M.D.C. Cellulose acetate/cellulose nanofiber membranes for whey and fruit juice microfiltration. Cellulose 2017, 24, 5593–5604. [Google Scholar] [CrossRef]
- Lin, J.; Fu, C.; Zeng, W.; Wang, D.; Huang, F.; Lin, S.; Cao, S.; Chen, L.; Ni, Y.; Huang, L. Regulating the structure of cellulose-based ultrafiltration membrane to improve its performance for water purification. Ind. Crop. Prod. 2023, 192, 116082. [Google Scholar] [CrossRef]
- Lee, H.; Lee, W.; Chung, J.W.; Kwak, S.-Y. Enhanced permeability of cellulose acetate ultrafiltration membrane by incorporation of cellulose graft copolymer. Cellulose 2022, 29, 9753–9775. [Google Scholar] [CrossRef]
- Yang, S.; Tang, R.; Dai, Y.; Wang, T.; Zeng, Z.; Zhang, L. Fabrication of cellulose acetate membrane with advanced ultrafiltration performances and antibacterial properties by blending with HKUST-1@LCNFs. Sep. Purif. Technol. 2021, 279, 119524. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdel-Karim, A.; Radwan, E.K.; Sharmoukh, W.; Wassel, A.R.; Bayoumy, A.M.; Ibrahim, M. Experimental and theoretical investigations on fouling resistant cellulose acetate/SiO2 NPs/PEDOT ultrafiltration nanocomposite membranes. J. Clean. Prod. 2021, 324, 129288. [Google Scholar] [CrossRef]
- Vatanpour, V.; Faghani, S.; Keyikoglu, R.; Khataee, A. Enhancing the permeability and antifouling properties of cellulose acetate ultrafiltration membrane by incorporation of ZnO@graphitic carbon nitride nanocomposite. Carbohydr. Polym. 2021, 256, 117413. [Google Scholar] [CrossRef]
- Mahto, A.; Halakarni, M.A.; Maraddi, A.; D’Souza, G.; Samage, A.A.; Thummar, U.G.; Mondal, D.; Nataraj, S. Upcycling cellulose acetate from discarded cigarette butts: Conversion of contaminated microfibers into loose-nanofiltration membranes for selective separation. Desalination 2022, 535, 115807. [Google Scholar] [CrossRef]
- Rasool, M.; Vankelecom, I. γ-Valerolactone as Bio-Based Solvent for Nanofiltration Membrane Preparation. Membranes 2021, 11, 418. [Google Scholar] [CrossRef]
- Rasool, M.A.; Vankelecom, I.F. Preparation of full-bio-based nanofiltration membranes. J. Membr. Sci. 2021, 618, 118674. [Google Scholar] [CrossRef]
- Batool, M.; Shafeeq, A.; Haider, B.; Ahmad, N. TiO2 Nanoparticle Filler-Based Mixed-Matrix PES/CA Nanofiltration Membranes for Enhanced Desalination. Membranes 2021, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Mejia, J.A.A.; Ricci, A.; Figueiredo, A.S.; Versari, A.; Cassano, A.; Parpinello, G.P.; De Pinho, M.N. Recovery of Phenolic Compounds from Red Grape Pomace Extract through Nanofiltration Membranes. Foods 2020, 9, 1649. [Google Scholar] [CrossRef] [PubMed]
- Mansor, E.S.; Abdallah, H.; Shalaby, M.; Shaban, A. Enhancement of reverse osmosis membranes for groundwater purification using cellulose acetate incorporated with ultrathin graphitic carbon nitride nanosheets. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100760. [Google Scholar] [CrossRef]
- Mansor, E.S.; Abdallah, H.; Shaban, A. Development of TiO2/polyvinyl alcohol-cellulose acetate nanocomposite reverse osmosis membrane for groundwater-surface water interfaces purification. Mater. Sci. Eng. B 2023, 289, 116222. [Google Scholar] [CrossRef]
- Koriem, O.A.; Showman, M.S.; El-Shazly, A.H.; Elkady, M.F. Cellulose acetate/polyvinylidene fluoride based mixed matrix membranes impregnated with UiO-66 nano-MOF for reverse osmosis desalination. Cellulose 2023, 30, 413–426. [Google Scholar] [CrossRef]
- Nakao, T.; Goda, S.; Miura, Y.; Yasukawa, M.; Ishibashi, M.; Nakagawa, K.; Shintani, T.; Matsuyama, H.; Yoshioka, T. Development of cellulose triacetate asymmetric hollow fiber membranes with highly enhanced compaction resistance for osmotically assisted reverse osmosis operation applicable to brine concentration. J. Membr. Sci. 2022, 653, 120508. [Google Scholar] [CrossRef]
- Ghaseminezhad, S.M.; Barikani, M.; Salehirad, M. Development of graphene oxide-cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination. Compos. Part B Eng. 2019, 161, 320–327. [Google Scholar] [CrossRef]
- Wibisono, Y.; Noviani, V.; Ramadhani, A.T.; Devianto, L.A.; Sulianto, A.A. Eco-friendly forward osmosis membrane manufacturing using dihydrolevoglucosenone. Results Eng. 2022, 16, 100712. [Google Scholar] [CrossRef]
- A Chandran, A.M.; Tayal, E.; Mural, P.K.S. Polycaprolactone-blended cellulose acetate thin-film composite membrane for dairy waste treatment using forward osmosis. Environ. Sci. Pollut. Res. 2022, 29, 86418–86426. [Google Scholar] [CrossRef]
- Jain, H.; Kumar, A.; Verma, A.K.; Wadhwa, S.; Rajput, V.D.; Minkina, T.; Garg, M.C. Treatment of textile industry wastewater by using high-performance forward osmosis membrane tailored with alpha-manganese dioxide nanoparticles for fertigation. Environ. Sci. Pollut. Res. 2022, 29, 80032–80043. [Google Scholar] [CrossRef]
- Jain, H.; Verma, A.K.; Dhupper, R.; Wadhwa, S.; Garg, M.C. Development of CA-TiO2-incorporated thin-film nanocomposite forward osmosis membrane for enhanced water flux and salt rejection. Int. J. Environ. Sci. Technol. 2021, 19, 5387–5400. [Google Scholar] [CrossRef]
- Jain, H.; Kumar, A.; Rajput, V.D.; Minkina, T.; Verma, A.K.; Wadhwa, S.; Dhupper, R.; Garg, M.C.; Joshi, H. Fabrication and characterization of high-performance forward-osmosis membrane by introducing manganese oxide incited graphene quantum dots. J. Environ. Manag. 2022, 305, 114335. [Google Scholar] [CrossRef]
- Li, T.; Cheng, C.; Zhang, K.; Yang, J.; Han, G.; Wang, X.; Wang, Z.; Wang, L. UiO-66-NH2 nanocomposites incorporated cellulose acetate for forward osmosis membranes of high desalination performance. Environ. Technol. 2022, 2022, 2099306. [Google Scholar] [CrossRef]
- Jain, H.; Dhupper, R.; Verma, A.K.; Garg, M.C. Development of titanium dioxide incorporated ultrathin cellulose acetate membrane for enhanced forward osmosis performance. Nanotechnol. Environ. Eng. 2021, 6, 67. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Microfiltration membrane processes: A review of research trends over the past decade. J. Water Process. Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- del Pino, M.P.; Durham, B. Wastewater reuse through dual-membrane processes: Opportunities for sustainable water resources. Desalination 1999, 124, 271–277. [Google Scholar] [CrossRef]
- Zizovic, I.; Tyrka, M.; Matyja, K.; Moric, I.; Senerovic, L.; Trusek, A. Functional Modification of Cellulose Acetate Microfiltration Membranes by Supercritical Solvent Impregnation. Molecules 2021, 26, 411. [Google Scholar] [CrossRef]
- Radiman, C.; Widyaningsih, S.; Sugesty, S. New applications of kenaf (Hibiscus cannabinus L.) as microfiltration membranes. J. Membr. Sci. 2008, 315, 141–146. [Google Scholar] [CrossRef]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Bose, J.; Dasgupta, J.; Adhikari, U.; Sikder, J. Tuning permeation characteristics of cellulose acetate membrane embedded with raw and amine-functionalized silicon carbide nanoparticle for oil-water separation. J. Water Process. Eng. 2021, 41, 102019. [Google Scholar] [CrossRef]
- Fazullin, D.D.; Mavrin, G.V. Modification of Microfiltration Membranes with Ultraviolet Radiation to Separate Oil-In-Water Emulsions. Int. J. Eng. Res. Technol. 2020, 13, 3559–3563. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, M.; Chen, Y.; He, G.; Wu, M.; Wang, J. Preparation and performance of cellulose acetate/polyethyleneimine blend microfiltration membranes and their applications. J. Membr. Sci. 2004, 235, 73–86. [Google Scholar] [CrossRef]
- Aliane, A.; Bounatiro, N.; Cherif, A.; Akretche, D. Removal of chromium from aqueous solution by complexation—ultrafiltration using a water-soluble macroligand. Water Res. 2001, 35, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Su, Y.; Wang, Y.; Ma, X.; Sun, Q.; Jiang, Z. Enhanced permeation performance of cellulose acetate ultrafiltration membrane by incorporation of Pluronic F127. J. Membr. Sci. 2007, 294, 68–74. [Google Scholar] [CrossRef]
- Mu, K.; Zhang, D.; Shao, Z.; Qin, D.; Wang, Y.; Wang, S. Enhanced permeability and antifouling performance of cellulose acetate ultrafiltration membrane assisted by l-DOPA functionalized halloysite nanotubes. Carbohydr. Polym. 2017, 174, 688–696. [Google Scholar] [CrossRef]
- Kutowy, O.; Sourirajan, S. Cellulose acetate ultrafiltration membranes. J. Appl. Polym. Sci. 1975, 19, 1449–1460. [Google Scholar] [CrossRef]
- Sivakumar, M.; Malaisamy, R.; Sajitha, C.; Mohan, D.; Mohan, V.; Rangarajan, R. Preparation and performance of cellulose acetate–polyurethane blend membranes and their applications–II. J. Membr. Sci. 2000, 169, 215–228. [Google Scholar] [CrossRef]
- Sivakumar, M.; Malaisamy, R.; Sajitha, C.; Mohan, D.; Mohan, V.; Rangarajan, R. Ultrafiltration application of cellulose acetate–polyurethane blend membranes. Eur. Polym. J. 1999, 35, 1647–1651. [Google Scholar] [CrossRef]
- Han, B.; Zhang, D.; Shao, Z.; Kong, L.; Lv, S. Preparation and characterization of cellulose acetate/carboxymethyl cellulose acetate blend ultrafiltration membranes. Desalination 2013, 311, 80–89. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P.; Srinivasn, K.; Mohan, D.; Rajendran, M. Synthesis, characterization and thermal studies on cellulose acetate membranes with additive. Eur. Polym. J. 2004, 40, 2153–2159. [Google Scholar] [CrossRef]
- Haddada, R.; Ferjani, E.; Roudesli, M.S.; Deratani, A. Properties of cellulose acetate nanofiltration membranes. Application to brackish water desalination. Desalination 2004, 167, 403–409. [Google Scholar] [CrossRef]
- Ye, S.H.; Watanabe, J.; Iwasaki, Y.; Ishihara, K. Novel cellulose acetate membrane blended with phospholipid polymer for hemocompatible filtration system. J. Membr. Sci. 2002, 210, 411–421. [Google Scholar] [CrossRef]
- Kumar, M.; Rao, T.S.; Isloor, A.M.; Ibrahim, G.S.; Inamuddin; Ismail, N.; Ismail, A.F.; Asiri, A.M. Use of cellulose acetate/polyphenylsulfone derivatives to fabricate ultrafiltration hollow fiber membranes for the removal of arsenic from drinking water. Int. J. Biol. Macromol. 2019, 129, 715–727. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Arockiasamy, D.L.; Nagendran, A.; Mohan, D. Separation of proteins and toxic heavy metal ions from aqueous solution by CA/PC blend ultrafiltration membranes. Sep. Purif. Technol. 2008, 62, 32–38. [Google Scholar] [CrossRef]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Andra, C.K.A.; Ang, M.B.M.Y.; Dizon, G.V.C.; Caparanga, A.R.; Huang, S.-H.; Lee, K.-R. Increased performance and antifouling of mixed-matrix membranes of cellulose acetate with hydrophilic nanoparticles of polydopamine-sulfobetaine methacrylate for oil-water separation. J. Membr. Sci. 2021, 620, 118881. [Google Scholar] [CrossRef]
- Nabe, A.; Staude, E.; Belfort, G. Surface modification of polysulfone ultrafiltration membranes and fouling by BSA solutions. J. Membr. Sci. 1997, 133, 57–72. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, G.; Zhang, H.; Yang, F. Exploration of permeability and antifouling performance on modified cellulose acetate ultrafiltration membrane with cellulose nanocrystals. Carbohydr. Polym. 2017, 174, 190–199. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Nagendran, A.; Rana, D.; Matsuura, T. Separation of macromolecular proteins and removal of humic acid by cellulose acetate modified UF membranes. Int. J. Biol. Macromol. 2016, 89, 81–88. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Rajesh, S.; Mohan, D.; Soundararajan, P. Preparation, characterization, and performance evaluation of poly (ether-imide) incorporated cellulose acetate ultrafiltration membrane for hemodialysis. Sep. Sci. Technol. 2013, 48, 66–75. [Google Scholar] [CrossRef]
- Ali, M.; Jahan, Z.; Sher, F.; Niazi, M.B.K.; Kakar, S.J.; Gul, S. Nano architectured cues as sustainable membranes for ultrafiltration in blood hemodialysis. Mater. Sci. Eng. C 2021, 128, 112260. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Figoli, A.; Ursino, C.; Santoro, S.; Ounifi, I.; Chekir, J.; Hafiane, A.; Ferjani, E. Cellulose acetate nanofiltration membranes for cadmium remediation. J. Membr. Sci. Res. 2020, 6, 226–234. [Google Scholar]
- Sivakumar, M.; Mohan, D.R.; Rangarajan, R. Studies on cellulose acetate-polysulfone ultrafiltration membranes: II. Effect of additive concentration. J. Membr. Sci. 2006, 268, 208–219. [Google Scholar] [CrossRef]
- Shaaban, M.F.; El-Khateeb, M.A.; Saad, M. Water desalination using cellulosic nano-filtration membrane composed of nano-scale polytetraflouroethylene. Egypt. J. Chem. 2019, 62, 15–20. [Google Scholar]
- Yu, S.; Liu, M.; Ma, M.; Qi, M.; Lü, Z.; Gao, C. Impacts of membrane properties on reactive dye removal from dye/salt mixtures by asymmetric cellulose acetate and composite polyamide nanofiltration membranes. J. Membr. Sci. 2010, 350, 83–91. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Alizadeh, A.; Rajabi, H.; Daraei, P.; Falsafi, M. Effect of fatty acids on the structure and performance of cellulose acetate nanofiltration membranes in retention of nitroaromatic pesticides. Desalination 2012, 301, 26–41. [Google Scholar] [CrossRef]
- Etemadi, H.; Yegani, R.; Seyfollahi, M.; Babaeipour, V. Preparation and performance evaluation of cellulose acetate/nanodiamond nanocomposite membrane in the treatment of pharmaceutical wastewater by membrane bioreactor. Desalin Water Treat 2017, 76, 98–111. [Google Scholar] [CrossRef]
- Choi, J.-H.; Fukushi, K.; Yamamoto, K. A submerged nanofiltration membrane bioreactor for domestic wastewater treatment: The performance of cellulose acetate nanofiltration membranes for long-term operation. Sep. Purif. Technol. 2007, 52, 470–477. [Google Scholar] [CrossRef]
- Cano-Odena, A.; Spilliers, M.; Dedroog, T.; De Grave, K.; Ramon, J.; Vankelecom, I. Optimization of cellulose acetate nanofiltration membranes for micropollutant removal via genetic algorithms and high throughput experimentation. J. Membr. Sci. 2011, 366, 25–32. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Reid, C.E.; Breton, E.J. Water and ion flow across cellulosic membranes. J. Appl. Polym. Sci. 1959, 1, 133–143. [Google Scholar] [CrossRef]
- Turbak, A.F. (Ed.) Synthetic Membranes: Volume I Desalination; American Chemical Society: Washington, DC, USA, 1981; Volume 153. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Kunst, B.; Sourirajan, S. Development and performance of some porous cellulose acetate membranes for reverse osmosis desalination. J. Appl. Polym. Sci. 1970, 14, 2559–2568. [Google Scholar] [CrossRef]
- Xu, P.; Na, N. Study on Antibacterial Properties of Cellulose Acetate Seawater Desalination Reverse-Osmosis Membrane with Graphene Oxide. J. Coast. Res. 2020, 105, 246–251. [Google Scholar] [CrossRef]
- Abdellah Ali, S.F.; William, L.A.; Fadl, E.A. Cellulose acetate, cellulose acetate propionate and cellulose acetate butyrate membranes for water desalination applications. Cellulose 2020, 27, 9525–9543. [Google Scholar] [CrossRef]
- Bódalo, A.; Gómez, J.-L.; Gómez, E.G.; León, G.; Tejera, M. Ammonium removal from aqueous solutions by reverse osmosis using cellulose acetate membranes. Desalination 2005, 184, 149–155. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Nghiem, L.D. Rejection of trace organic chemicals by a hollow fibre cellulose triacetate reverse osmosis membrane. Reverse Osmosis 2015, 368, 69–75. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The Application of Cellulose Acetate Membranes for Separation of Fermentation Broths by the Reverse Osmosis: A Feasibility Study. Int. J. Mol. Sci. 2022, 23, 11738. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Holloway, R.W.; Childress, A.E.; Dennett, K.E.; Cath, T.Y. Forward osmosis for concentration of anaerobic digester centrate. Water Res. 2007, 41, 4005–4014. [Google Scholar] [CrossRef]
- Sairam, M.; Sereewatthanawut, E.; Li, K.; Bismarck, A.; Livingston, A. Method for the preparation of cellulose acetate flat sheet composite membranes for forward osmosis—Desalination using MgSO4 draw solution. Desalination 2011, 273, 299–307. [Google Scholar] [CrossRef]
- Qasim, M.; Darwish, N.A.; Sarp, S.; Hilal, N. Water desalination by forward (direct) osmosis phenomenon: A comprehensive review. Desalination 2015, 374, 47–69. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Wang, X.; Cheng, C.; Zhang, K.; Yang, J.; Han, G.; Wang, Z.; Wang, X.; Wang, L. Desalination Characteristics of Cellulose Acetate FO Membrane Incorporated with ZIF-8 Nanoparticles. Membranes 2022, 12, 122. [Google Scholar] [CrossRef]
- Su, J.; Yang, Q.; Teo, J.F.; Chung, T.-S. Cellulose acetate nanofiltration hollow fiber membranes for forward osmosis processes. J. Membr. Sci. 2010, 355, 36–44. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Hou, D.; Bai, Y.; Liu, H. Fabrication and performance of PET mesh enhanced cellulose acetate membranes for forward osmosis. J. Environ. Sci. 2016, 45, 7–17. [Google Scholar] [CrossRef]
- Alfahel, R.; Azzam, R.S.; Hafiz, M.; Hawari, A.H.; Pandey, R.P.; Mahmoud, K.A.; Hassan, M.K.; Elzatahry, A.A. Fabrication of fouling resistant Ti3C2Tx (MXene)/cellulose acetate nanocomposite membrane for forward osmosis application. J. Water Process. Eng. 2020, 38, 101551. [Google Scholar] [CrossRef]
- Lakra, R.; Balakrishnan, M.; Basu, S. Development of cellulose acetate-chitosan-metal organic framework forward osmosis membrane for recovery of water and nutrients from wastewater. J. Environ. Chem. Eng. 2021, 9, 105882. [Google Scholar] [CrossRef]
- Huck, P.; Sozański, M. Chemical Basis for Water Technology. Earth Syst. Environ. Sci. 2011, 3, 429–469. [Google Scholar] [CrossRef]
- Ehsani, M.; Doan, H.; Lohi, A. A comprehensive review of membrane fouling and cleaning methods with emphasis on ultrasound-assisted fouling control processes. Korean J. Chem. Eng. 2021, 38, 1531–1555. [Google Scholar] [CrossRef]
- Singh, R. Water and Membrane Treatment. In Membrane Technology and Engineering for Water Purification; Elsevier: Amsterdam, The Netherlands, 2015; pp. 81–178. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Du, X.; Shi, Y.; Jegatheesan, V.; Haq, I.U. A Review on the Mechanism, Impacts and Control Methods of Membrane Fouling in MBR System. Membranes 2020, 10, 24. [Google Scholar] [CrossRef]
- Etemadi, H.; Yegani, R.; Seyfollahi, M.; Rabiee, M. Synthesis, characterization, and anti-fouling properties of cellulose acetate/polyethylene glycol-grafted nanodiamond nanocomposite membranes for humic acid removal from contaminated water. Iran. Polym. J. 2018, 27, 381–393. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.-N.; Wei, J.; Tang, C.Y. Organic fouling of thin-film composite polyamide and cellulose triacetate forward osmosis membranes by oppositely charged macromolecules. Water Res. 2013, 47, 1867–1874. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Nakagawa, K.; Matsuba, M.; Okamoto, M.; Shintani, T.; Sasaki, Y.; Yoshioka, T.; Kamio, E.; Wickramasinghe, S.R.; Matsuyama, H. Comparison of Fouling Behavior in Cellulose Triacetate Membranes Applied in Forward and Reverse Osmosis. Ind. Eng. Chem. Res. 2022, 61, 15345–15354. [Google Scholar] [CrossRef]
- Doan, H.; Lohi, A. Fouling in Membrane Filtration and Remediation Methods. In Mass Transfer—Advances in Sustainable Energy and Environment Oriented Numerical Modeling; Nakajima, H., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kilduff, J.E.; Belfort, G. Modes of Natural Organic Matter Fouling during Ultrafiltration. Environ. Sci. Technol. 2003, 37, 1676–1683. [Google Scholar] [CrossRef]
- A Zularisam, A.; Ismail, A.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef]
- Chennamsetty, R.; Escobar, I. Effect of Ion Beam Irradiation on Two Nanofiltration Water Treatment Membranes. Sep. Sci. Technol. 2008, 43, 4009–4029. [Google Scholar] [CrossRef]
- Gullinkala, T.; Escobar, I. Study of the hydrophilic-enhanced ultrafiltration membrane. Environ. Prog. 2008, 27, 210–217. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- García, A.; Rodríguez, B.; Giraldo, H.; Quintero, Y.; Quezada, R.; Hassan, N.; Estay, H. Copper-modified polymeric membranes for water treatment: A comprehensive review. Membranes 2021, 11, 93. [Google Scholar] [CrossRef]
- Jiang, S.-X.; Li, Y.-N.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef]
- Sprick, C.G. Functionalization of Silver Nanoparticles on Membranes and its Influence on Biofouling. Master’s Thesis, University of Kentucky, Lexington, Kentucky, 2017. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. RO Membrane Fouling. In Reverse Osmosis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 189–220. [Google Scholar] [CrossRef]
- Ong, C.S.; Goh, P.; Lau, W.; Misdan, N.; Ismail, A. Nanomaterials for biofouling and scaling mitigation of thin film composite membrane: A review. Desalination 2016, 393, 2–15. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef]
- Liao, B.Q.; Bagley, D.; Kraemer, H.E.; Leppard, G.G.; Liss, S.N. A Review of Biofouling and its Control in Membrane Separation Bioreactors. Water Environ. Res. 2004, 76, 425–436. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2011, 19, 152–165. [Google Scholar] [CrossRef]
- Maddah, H.; Chogle, A. Biofouling in reverse osmosis: Phenomena, monitoring, controlling and remediation. Appl. Water Sci. 2017, 7, 2637–2651. [Google Scholar] [CrossRef]
- Sun, X.; Kanani, D.M.; Ghosh, R. Characterization and theoretical analysis of protein fouling of cellulose acetate membrane during constant flux dead-end microfiltration. J. Membr. Sci. 2008, 320, 372–380. [Google Scholar] [CrossRef]
- Winfield, B. A study of the factors affecting the rate of fouling of reverse osmosis membranes treating secondary sewage effluents. Water Res. 1979, 13, 565–569. [Google Scholar] [CrossRef]
- Hashino, M.; Hirami, K.; Katagiri, T.; Kubota, N.; Ohmukai, Y.; Ishigami, T.; Maruyama, T.; Matsuyama, H. Effects of three natural organic matter types on cellulose acetate butyrate microfiltration membrane fouling. J. Membr. Sci. 2011, 379, 233–238. [Google Scholar] [CrossRef]
- Fu, X.; Maruyama, T.; Sotani, T.; Matsuyama, H. Effect of surface morphology on membrane fouling by humic acid with the use of cellulose acetate butyrate hollow fiber membranes. J. Membr. Sci. 2008, 320, 483–491. [Google Scholar] [CrossRef]
- Indarti, D.; Ali, B.T.I.; Mulyono, T. Filtration of Protein in Tempe Wastewater Using Cellulose Acetate Membrane. UNEJ E-Proceeding 2017, 282–284. [Google Scholar]
- Schafer, A. Natural Organics Removal Using Membranes; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar] [CrossRef]
- Zhu, X.; Elimelech, M. Colloidal Fouling of Reverse Osmosis Membranes: Measurements and Fouling Mechanisms. Environ. Sci. Technol. 1997, 31, 3654–3662. [Google Scholar] [CrossRef]
- Elimelech, M.; Gregory, J.; Jia, X. Particle Deposition and Aggregation: Measurement, Modelling and Simulation; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Koo, J.Y.; Kang, S.P.; Kim, J.E.; Hyung, H.; Kim, Y.H.; Yoon, S.; Kim, S.S. Fouling resistant reverse osmosis membranes. In ACS Annual Meeting; American Water: Camden, NJ, USA, 2002. [Google Scholar]
- Wang, Y.-N.; Järvelä, E.; Wei, J.; Zhang, M.; Kyllönen, H.; Wang, R.; Tang, C.Y. Gypsum scaling and membrane integrity of osmotically driven membranes: The effect of membrane materials and operating conditions. Desalination 2016, 377, 1–10. [Google Scholar] [CrossRef]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar] [CrossRef]
- Khan, B.; Zhan, W.; Lina, C. Cellulose acetate (CA) hybrid membrane prepared by phase inversion method combined with chemical reaction with enhanced permeability and good anti-fouling property. J. Appl. Polym. Sci. 2020, 137, 49556. [Google Scholar] [CrossRef]
- Naseem, S.; Wu, C.-M.; Xu, T.-Z.; Lai, C.-C.; Rwei, S.-P. Oil-Water Separation of Electrospun Cellulose Triacetate Nanofiber Membranes Modified by Electrophoretically Deposited TiO2/Graphene Oxide. Polymers 2018, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Sjahro, N.; Yunus, R.; Abdullah, L.C.; Rashid, S.A.; Asis, A.J.; Akhlisah, Z.N. Recent advances in the application of cellulose derivatives for removal of contaminants from aquatic environments. Cellulose 2021, 28, 7521–7557. [Google Scholar] [CrossRef]
- Kamide, K.; Saito, M. Cellulose and cellulose derivatives: Recent advances in physical chemistry. Biopolymers 1987, 83, 1–56. [Google Scholar]
- Puleo, A.; Paul, D.; Kelley, S. The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J. Membr. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, X.; White, K.L.; Lin, S.; Wu, H.; Cao, S.; Huang, L.; Chen, L. Effect of the degree of substitution on the hydrophobicity of acetylated cellulose for production of liquid marbles. Cellulose 2016, 23, 811–821. [Google Scholar] [CrossRef]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.; Nigmatullin, R. Methods Employed for Control of Fouling in MF and UF Membranes: A Comprehensive Review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef]
- Saraswathi, M.S.S.A.; Rana, D.; Alwarappan, S.; Gowrishankar, S.; Kanimozhi, P.; Nagendran, A. Cellulose acetate ultrafiltration membranes customized with bio-inspired polydopamine coating and in situ immobilization of silver nanoparticles. New J. Chem. 2019, 43, 4216–4225. [Google Scholar] [CrossRef]
- Beisl, S.; Monteiro, S.; Santos, R.; Figueiredo, A.S.; Sánchez-Loredo, M.G.; Lemos, M.A.; Lemos, F.; Minhalma, M.; de Pinho, M.N. Synthesis and bactericide activity of nanofiltration composite membranes–Cellulose acetate/silver nanoparticles and cellulose acetate/silver ion exchanged zeolites. Water Res. 2019, 149, 225–231. [Google Scholar] [CrossRef]
- Sonawane, S.H.; Terrien, A.; Figueiredo, A.S.; Gonçalves, M.C.; De Pinho, M.N. The role of silver nanoparticles on mixed matrix Ag/cellulose acetate asymmetric membranes. Polym. Compos. 2017, 38, 32–39. [Google Scholar] [CrossRef]
- Scheeren, C.W.; Hermes, V.; Bianchi, O.; Hertz, P.; Dias, S.P.; Dupont, J. Antimicrobial membrane cellulose acetate containing ionic liquid and metal nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 5114–5122. [Google Scholar] [CrossRef]
- Asiri, A.M.; Petrosino, F.; Pugliese, V.; Khan, S.B.; Alamry, K.A.; Alfifi, S.Y.; Marwani, H.M.; Alotaibi, M.M.; Algieri, C.; Chakraborty, S. Synthesis and Characterization of Blended Cellulose Acetate Membranes. Polymers 2022, 14, 4. [Google Scholar] [CrossRef]
- Das, C.; Gebru, K.A. Cellulose Acetate Modified Titanium Dioxide (TiO2) Nanoparticles Electrospun Composite Membranes: Fabrication and Characterization. J. Inst. Eng. India Ser. E 2017, 98, 91–101. [Google Scholar] [CrossRef]
- Kadhom, M.; Al-Furaiji, M.; Salih, S.; Al-Obaidi, M.A.; Abdullah, G.H.; Albayati, N. A review on UiO-66 applications in membrane-based water treatment processes. J. Water Process. Eng. 2023, 51, 103402. [Google Scholar] [CrossRef]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose acetate in fabrication of polymeric membranes: A review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef]
- Ee, L.Y.; Tan, R.P.W.; Li, S.F.Y. Facile electrospray fabrication of ultralow biofouling cellulose acetate desalination membrane with nanocellulose/UiO66-NH2 fillers. J. Membr. Sci. 2023, 666, 121200. [Google Scholar] [CrossRef]
- Ang, M.; Devanadera, K.; Duena, A.; Luo, Z.-Y.; Chiao, Y.-H.; Millare, J.; Aquino, R.; Huang, S.-H.; Lee, K.-R. Modifying Cellulose Acetate Mixed-Matrix Membranes for Improved Oil–Water Separation: Comparison between Sodium and Organo-Montmorillonite as Particle Additives. Membranes 2021, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Hilliou, L.; de Amorim, M.T.P. Fabrication of pristine-multiwalled carbon nanotubes/cellulose acetate composites for removal of methylene blue. Polym. Bull. 2020, 77, 623–653. [Google Scholar] [CrossRef]
- de Faria, A.F.; de Moraes, A.C.M.; Andrade, P.F.; da Silva, D.S.; Gonçalves, M.D.C.; Alves, O.L. Cellulose acetate membrane embedded with graphene oxide-silver nanocomposites and its ability to suppress microbial proliferation. Cellulose 2017, 24, 781–796. [Google Scholar] [CrossRef]
- Shen, S.-S.; Chen, H.; Wang, R.-H.; Ji, W.; Zhang, Y.; Bai, R. Preparation of antifouling cellulose acetate membranes with good hydrophilic and oleophobic surface properties. Mater. Lett. 2019, 252, 1–4. [Google Scholar] [CrossRef]
- Guo, H.; Peng, Y.; Liu, Y.; Wang, Z.; Hu, J.; Liu, J.; Ding, Q.; Gu, J. Development and investigation of novel antifouling cellulose acetate ultrafiltration membrane based on dopamine modification. Int. J. Biol. Macromol. 2020, 160, 652–659. [Google Scholar] [CrossRef]
- Kanagaraj, P.; Neelakandan, S.; Nagendran, A. Preparation, characterization and performance of cellulose acetate ultrafiltration membranes modified by charged surface modifying macromolecule. Korean J. Chem. Eng. 2014, 31, 1057–1064. [Google Scholar] [CrossRef]
- Tyrka, M.; Nowak, M.; Misic, D.; Półbrat, T.; Koter, S.; Trusek, A.; Zizovic, I. Cellulose Acetate Membranes Modification by Aminosilane Grafting in Supercritical Carbon Dioxide towards Antibiofilm Properties. Membranes 2022, 12, 33. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.; Zhang, Y.; Tan, L. Improvement of Antibacterial and Antifouling Properties of a Cellulose Acetate Membrane by Surface Grafting Quaternary Ammonium Salt. ACS Appl. Mater. Interfaces 2022, 14, 38358–38369. [Google Scholar] [CrossRef]
- Sagle, A.C.; Van Wagner, E.M.; Ju, H.; McCloskey, B.; Freeman, B.D.; Sharma, M.M. PEG-coated reverse osmosis membranes: Desalination properties and fouling resistance. J. Membr. Sci. 2009, 340, 92–108. [Google Scholar] [CrossRef]
- Elimelech, M.; Zhu, X.; Childress, A.E.; Hong, S. Role of membrane surface morphology in colloidal fouling of cellulose acetate and composite aromatic polyamide reverse osmosis membranes. J. Membr. Sci. 1997, 127, 101–109. [Google Scholar] [CrossRef]
- Al-Jeshi, S.; Neville, A. An investigation into the relationship between flux and roughness on RO membranes using scanning probe microscopy. Desalination 2006, 189, 221–228. [Google Scholar] [CrossRef]
- Jayalakshmi, A.; Kim, I.-C.; Kwon, Y.-N. Cellulose acetate graft-(glycidylmethacrylate-g-PEG) for modification of AMC ultrafiltration membranes to mitigate organic fouling. RSC Adv. 2015, 5, 48290–48300. [Google Scholar] [CrossRef]
- Zare, S.; Kargari, A. Membrane properties in membrane distillation. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–156. [Google Scholar]
- Wang, Y.-N.; Tang, C.Y. Protein fouling of nanofiltration, reverse osmosis, and ultrafiltration membranes—The role of hydrodynamic conditions, solution chemistry, and membrane properties. J. Membr. Sci. 2011, 376, 275–282. [Google Scholar] [CrossRef]
- Maan, A.M.C.; Hofman, A.H.; De Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Goetz, L.A.; Jalvo, B.; Rosal, R.; Mathew, A.P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J. Membr. Sci. 2016, 510, 238–248. [Google Scholar] [CrossRef]
- Yao, A.; Yan, Y.; Tan, L.; Shi, Y.; Zhou, M.; Zhang, Y.; Zhu, P.; Huang, S. Improvement of filtration and antifouling performance of cellulose acetate membrane reinforced by dopamine modified cellulose nanocrystals. J. Membr. Sci. 2021, 637, 119621. [Google Scholar] [CrossRef]
- Yang, M.; Hadi, P.; Yin, X.; Yu, J.; Huang, X.; Ma, H.; Walker, H.; Hsiao, B.S. Antifouling nanocellulose membranes: How subtle adjustment of surface charge lead to self-cleaning property. J. Membr. Sci. 2021, 618, 118739. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).