Coupling of Anammox Activity and PAH Biodegradation: Current Insights and Future Directions
Abstract
1. Introduction
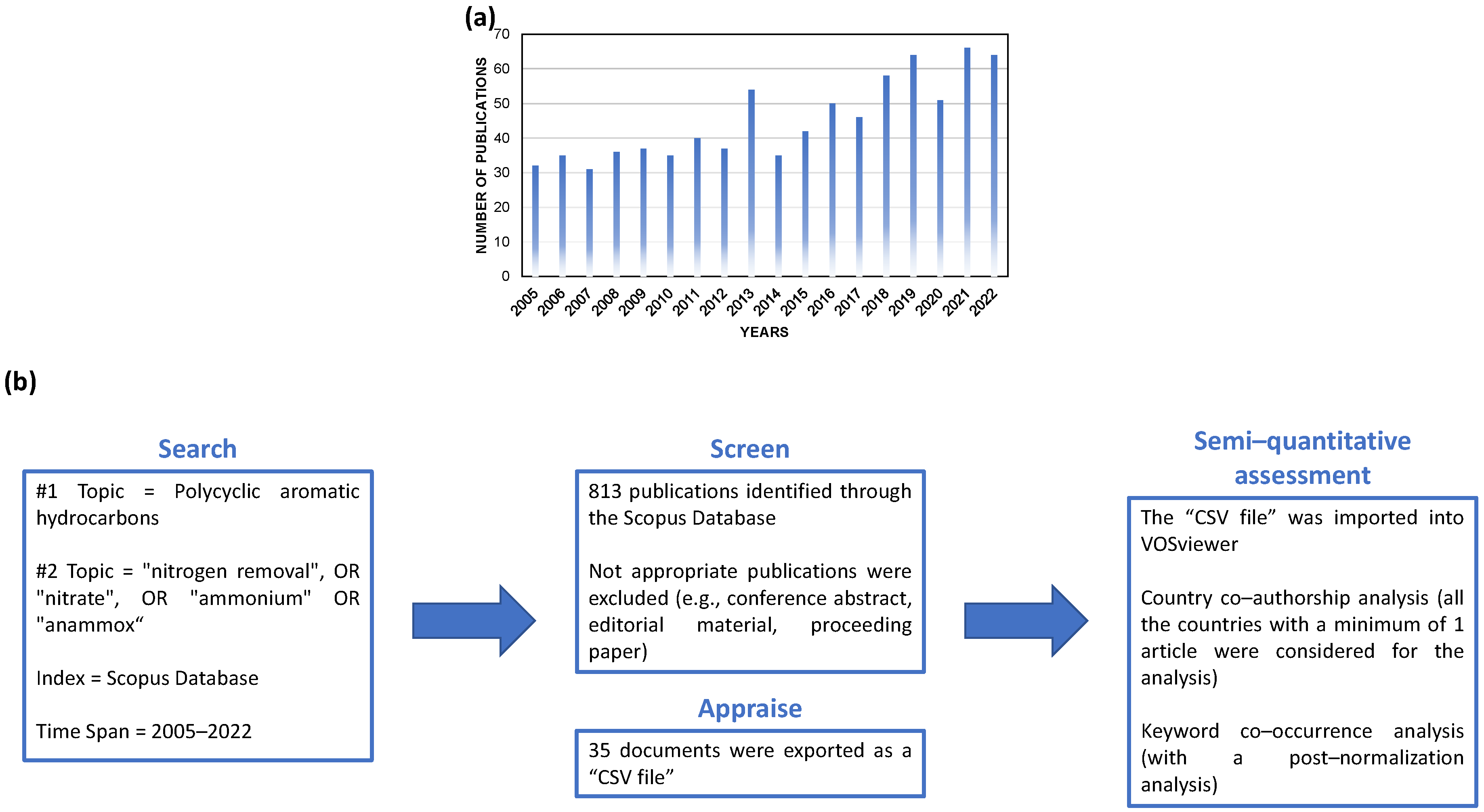
2. Data Collection and Extraction
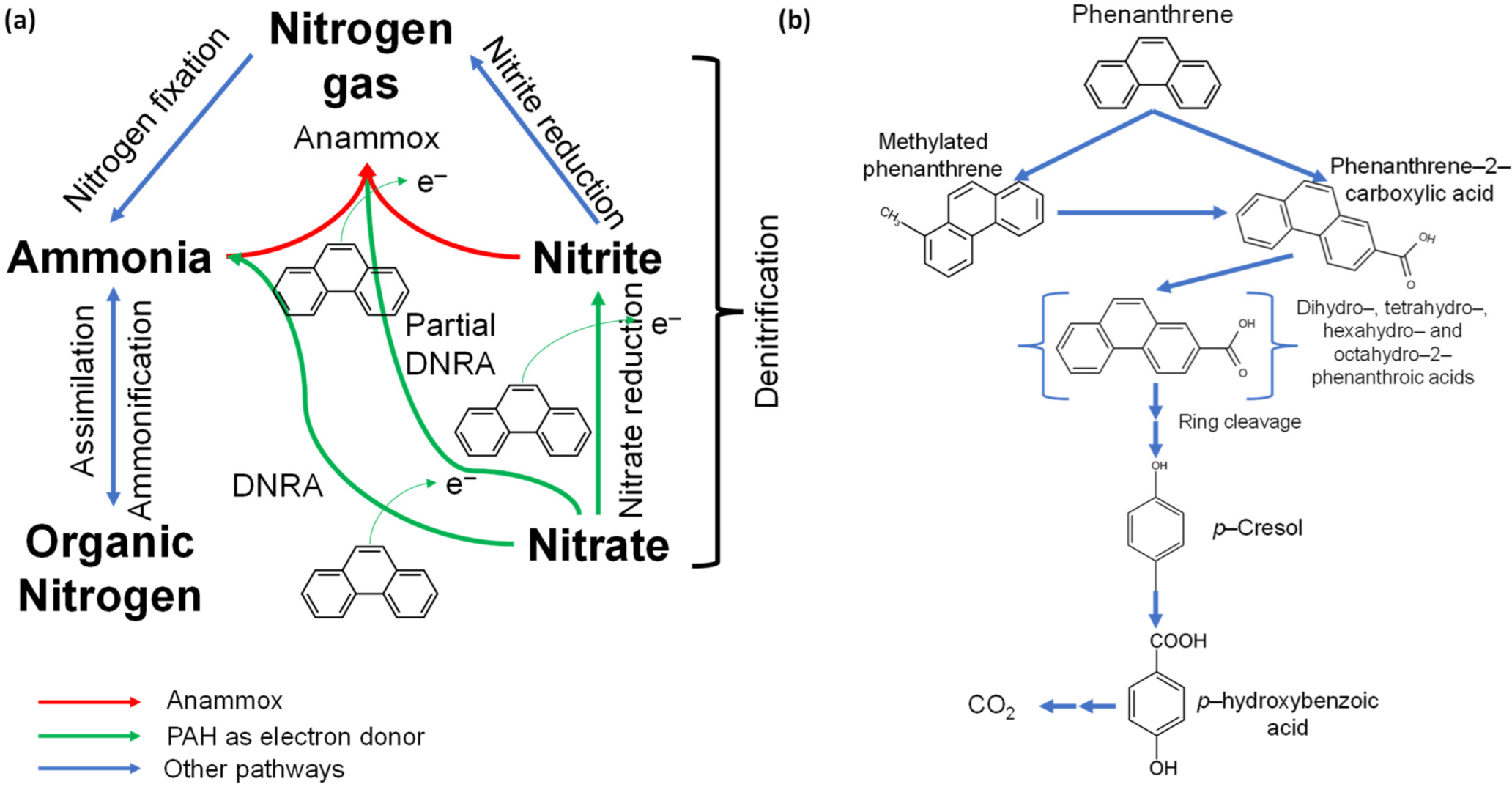
3. Nitrogen Biogeochemistry and the Anammox Process
4. Insights Regarding Anoxic PAH Degradation during the Anammox Process
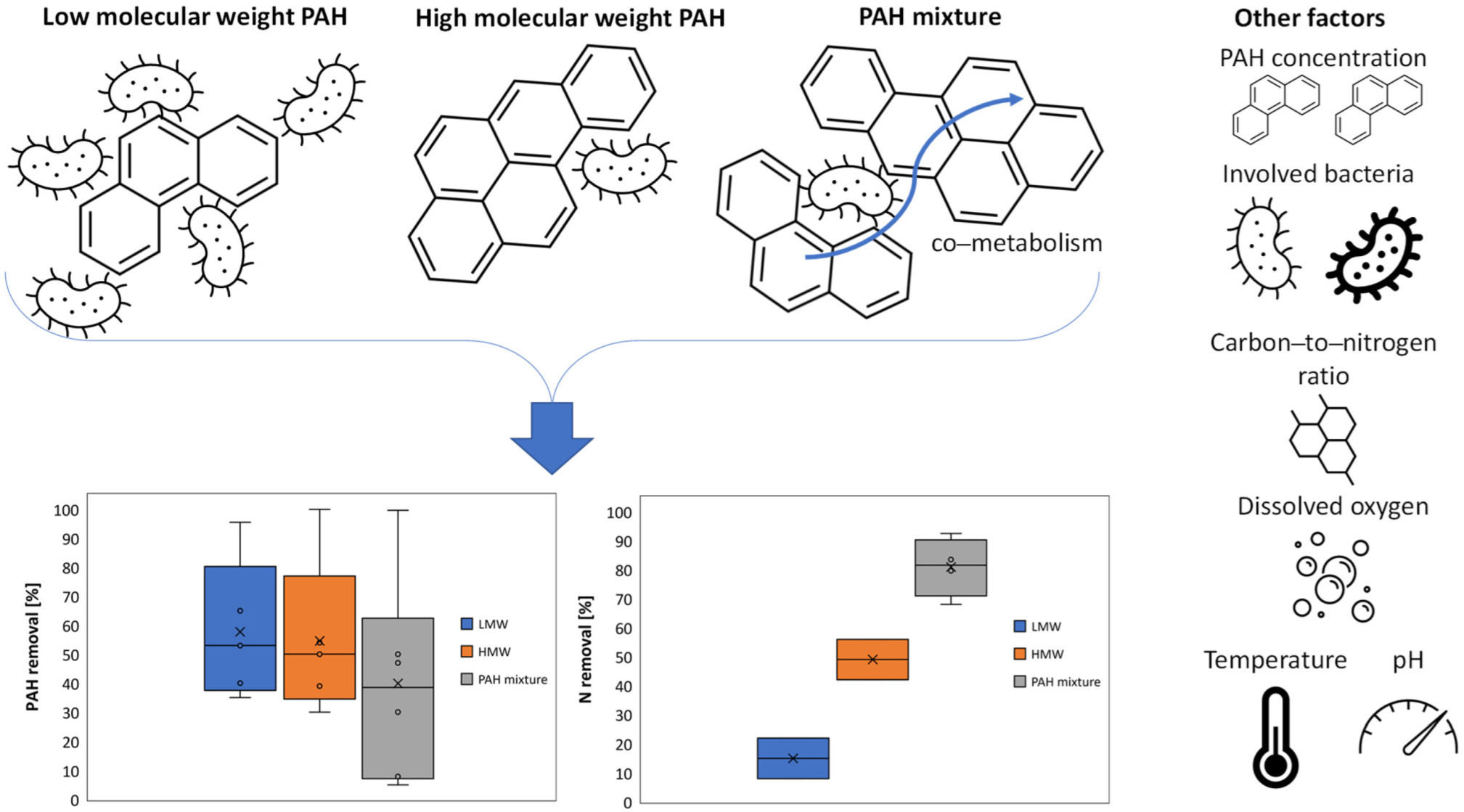
5. Biodegradation Efficiencies of PAHs and Their Impact on Nitrogen Removal
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, A.D.; Cabezas, A.; Etchebehere, C.; Chernicharo, C.A.D.L.; de Araújo, J.C. Microbial Communities in Anammox Reactors: A Review. Environ. Technol. Rev. 2017, 6, 74–93. [Google Scholar] [CrossRef]
- Thamdrup, B. New Pathways and Processes in the Global Nitrogen Cycle. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 407–428. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Van Niftrik, L.; Strous, M.; Kartal, B.; Keltjens, J.T.; Op Den Camp, H.J.M. Biochemistry and Molecular Biology of Anammox Bacteria. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 65–84. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y.; Ma, B.; Wang, S.; Zhu, G. Anaerobic Ammonium Oxidation in Traditional Municipal Wastewater Treatment Plants with Low-Strength Ammonium Loading: Widespread but Overlooked. Water Res. 2015, 84, 66–75. [Google Scholar] [CrossRef]
- Du, R.; Cao, S.; Li, B.; Wang, S.; Peng, Y. Simultaneous Domestic Wastewater and Nitrate Sewage Treatment by DEnitrifying AMmonium OXidation (DEAMOX) in Sequencing Batch Reactor. Chemosphere 2017, 174, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Khin, T.; Annachhatre, A.P. Novel Microbial Nitrogen Removal Processes. Biotechnol. Adv. 2004, 22, 519–532. [Google Scholar] [CrossRef]
- Liu, S.; Yang, F.; Meng, F.; Chen, H.; Gong, Z. Enhanced Anammox Consortium Activity for Nitrogen Removal: Impacts of Static Magnetic Field. J. Biotechnol. 2008, 138, 96–102. [Google Scholar] [CrossRef]
- Monballiu, A.; Desmidt, E.; Ghyselbrecht, K.; De Clippeleir, H.; Van Hulle, S.W.H.; Verstraete, W.; Meesschaert, B. Enrichment of Anaerobic Ammonium Oxidizing (Anammox) Bacteria from OLAND and Conventional Sludge: Features and Limitations. Sep. Purif. Technol. 2013, 104, 130–137. [Google Scholar] [CrossRef]
- Kampschreur, M.J.; Poldermans, R.; Kleerebezem, R.; Van Der Star, W.R.L.; Haarhuis, R.; Abma, W.R.; Jetten, M.S.M.; Van Loosdrecht, M.C.M. Emission of Nitrous Oxide and Nitric Oxide from a Full-Scale Single-Stage Nitritation-Anammox Reactor. Water Sci. Technol. 2009, 60, 3211–3217. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, H.C.; Pan, Y.; Shyla, F.S.; Tam, N.F.Y. Effects of Polybrominated Diphenyl Ethers and Plant Species on Nitrification, Denitrification and Anammox in Mangrove Soils. Sci. Total Environ. 2016, 553, 60–70. [Google Scholar] [CrossRef]
- Lawal, A.T. Polycyclic Aromatic Hydrocarbons. A Review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Soclo, H.H.; Garrigues, P.; Ewald, M. Origin of Polycyclic Aromatic Hydrocarbons (PAHs) in Coastal Marine Sediments: Case Studies in Cotonou (Benin) and Aquitaine (France) Areas. Mar. Pollut. Bull. 2000, 40, 387–396. [Google Scholar] [CrossRef]
- Munoz, B.; Albores, A. DNA Damage Caused by Polycyclic Aromatic Hydrocarbons: Mechanisms and Markers. In Selected Topics in DNA Repair; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Habe, H.; Omori, T. Genetics of Polycyclic Aromatic Hydrocarbon Metabolism in Diverse Aerobic Bacteria. Biosci. Biotechnol. Biochem. 2003, 67, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Ambrosoli, R.; Petruzzelli, L.; Minati, J.L.; Marsan, F.A. Anaerobic PAH Degradation in Soil by a Mixed Bacterial Consortium under Denitrifying Conditions. Chemosphere 2005, 60, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; He, Z.; Zhang, Q.; Liu, J.; Guo, J.; Sun, G.; Zhou, J. Responses of Aromatic-Degrading Microbial Communities to Elevated Nitrate in Sediments. Environ. Sci. Technol. 2015, 49, 12422–12431. [Google Scholar] [CrossRef]
- Duran, R.; Cravo-Laureau, C. Role of Environmental Factors and Microorganisms in Determining the Fate of Polycyclic Aromatic Hydrocarbons in the Marine Environment. FEMS Microbiol. Rev. 2016, 40, 814–830. [Google Scholar] [CrossRef]
- Semple, K.T.; Morriss, A.W.J.; Paton, G.I. Bioavailability of Hydrophobic Organic Contaminants in Soils: Fundamental Concepts and Techniques for Analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Bianco, F.; Race, M.; Papirio, S.; Oleszczuk, P.; Esposito, G. Coupling of Desorption of Phenanthrene from Marine Sediments and Biodegradation of the Sediment Washing Solution in a Novel Biochar Immobilized–Cell Reactor. Environ. Pollut. 2022, 308, 119621. [Google Scholar] [CrossRef]
- Bianco, F.; Marcińczyk, M.; Race, M.; Papirio, S.; Esposito, G.; Oleszczuk, P. Low Temperature-Produced and VFA-Coated Biochar Enhances Phenanthrene Adsorption and Mitigates Toxicity in Marine Sediments. Sep. Purif. Technol. 2022, 296, 121414. [Google Scholar] [CrossRef]
- Mu, J.; Chen, Y.; Song, Z.; Liu, M.; Zhu, B.; Tao, H.; Bao, M.; Chen, Q. Effect of Terminal Electron Acceptors on the Anaerobic Biodegradation of PAHs in Marine Sediments. J. Hazard. Mater. 2022, 438, 129569. [Google Scholar] [CrossRef]
- Bianco, F.; Race, M.; Papirio, S.; Esposito, G. A Critical Review of the Remediation of PAH- Polluted Marine Sediments: Current Knowledge and Future Perspectives. Resour. Environ. Sustain. 2022, 11, 100101. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth ’ s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Kartal, B.; Van Niftrik, L.; Rattray, J.; Van De Vossenberg, J.L.C.M.; Schmid, M.C.; Sinninghe Damstà ©, J.; Jetten, M.S.M.; Strous, M. Candidatus ‘Brocadia fulgida’: An autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiol. Ecol. 2008, 63, 46–55. [Google Scholar] [CrossRef]
- Francis, C.A.; Beman, J.M.; Kuypers, M.M.M. New Processes and Players in the Nitrogen Cycle: The Microbial Ecology of Anaerobic and Archaeal Ammonia Oxidation. ISME J. 2007, 1, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G. Earth’ s Biogeochemical Cycles. Science 2012, 320, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Huy Quoc Anh, D.; Tantayotai, P.; Cheenkachorn, K.; Sriariyanun, M. Anammox Process: The Principle, the Technological Development and Recent Industrial Applications. Appl. Sci. Eng. Prog. 2015, 8, 237–244. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, H.; Sun, J.; Wang, H. Investigation of Anaerobic Phenanthrene Biodegradation by a Highly Enriched Co-Culture, PheN9, with Nitrate as an Electron Acceptor. J. Hazard. Mater. 2020, 383, 121191. [Google Scholar] [CrossRef]
- Urakawa, H.; Rajan, S.; Feeney, M.E.; Sobecky, P.A.; Mortazavi, B. Ecological Response of Nitrification to Oil Spills and Its Impact on the Nitrogen Cycle. Environ. Microbiol. 2019, 21, 18–33. [Google Scholar] [CrossRef]
- Kartal, B.; Maalcke, W.J.; De Almeida, N.M.; Cirpus, I.; Gloerich, J.; Geerts, W.; Op Den Camp, H.J.M.; Harhangi, H.R.; Janssen-Megens, E.M.; Francoijs, K.J.; et al. Molecular Mechanism of Anaerobic Ammonium Oxidation. Nature 2011, 479, 127–130. [Google Scholar] [CrossRef]
- Kartal, B.; Keltjens, J.T.; Jetten, M.S.M. Metabolism and Genomics of Anammox Bacteria. In Nitrification; ASM Press: Washington, DC, USA, 2014; pp. 179–200. [Google Scholar]
- Rattray, J.E.; van de Vossenberg, J.; Jaeschke, A.; Hopmans, E.C.; Wakeham, S.G.; Lavik, G.; Kuypers, M.M.M.; Strous, M.; Jetten, M.S.M.; Schouten, S.; et al. Impact of Temperature on Ladderane Lipid Distribution in Anammox Bacteria. Appl. Environ. Microbiol. 2010, 76, 1596–1603. [Google Scholar] [CrossRef]
- Liu, C.; Yamamoto, T.; Nishiyama, T.; Fujii, T.; Furukawa, K. Effect of Salt Concentration in Anammox Treatment Using Non Woven Biomass Carrier. J. Biosci. Bioeng. 2009, 107, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Sonthiphand, P.; Hall, M.W.; Neufeld, J.D. Biogeography of Anaerobic Ammonia-Oxidizing (Anammox) Bacteria. Front. Microbiol. 2014, 5, 1–15. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Strous, M.; van de Pas-Schoonen, K.T.; Schalk, J.; van Dongen, U.G.J.M.; van de Graaf, A.A.; Logemann, S.; Muyzer, G.; van Loosdrecht, M.C.M.; Kuenen, J.G. The Anaerobic Oxidation of Ammonium. FEMS Microbiol. Rev. 1998, 22, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Woebken, D.; Lam, P.; Kuypers, M.M.M.; Naqvi, S.W.A.; Kartal, B.; Strous, M.; Jetten, M.S.M.; Fuchs, B.M.; Amann, R. A Microdiversity Study of Anammox Bacteria Reveals a Novel Candidatus Scalindua Phylotype in Marine Oxygen Minimum Zones. Environ. Microbiol. 2008, 10, 3106–3119. [Google Scholar] [CrossRef]
- Van Niftrik, L.A.; Fuerst, J.A.; Sinninghe Damsté, J.S.; Kuenen, J.G.; Jetten, M.S.M.; Strous, M. The Anammoxosome: An Intracytoplasmic Compartment in Anammox Bacteria. FEMS Microbiol. Lett. 2004, 233, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Luan, X.; Dang, H.; Zhou, H.; Zhao, Y.; Liu, H.; Zhang, Y.; Dai, L.; Ye, Y.; Klotz, M.G. Deep-Sea Methane Seep Sediments in the Okhotsk Sea Sustain Diverse and Abundant Anammox Bacteria. FEMS Microbiol. Ecol. 2014, 87, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.L.; Shen, L.D.; Xu, X.Y.; Zheng, P. Anaerobic Ammonium Oxidation (Anammox) in Different Natural Ecosystems. Biochem. Soc. Trans. 2011, 39, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Kimura, Y.; Otsubo, Y.; Suwa, Y.; Tsuneda, S.; Isaka, K. Temperature Dependence for Anammox Bacteria Enriched from Freshwater Sediments. J. Biosci. Bioeng. 2012, 114, 429–434. [Google Scholar] [CrossRef]
- Cao, W.; Yang, J.; Li, Y.; Liu, B.; Wang, F.; Chang, C. Dissimilatory Nitrate Reduction to Ammonium Conserves Nitrogen in Anthropogenically Affected Subtropical Mangrove Sediments in Southeast China. Mar. Pollut. Bull. 2016, 110, 155–161. [Google Scholar] [CrossRef]
- Sun, P.; Bai, J.; Li, K.; Zhao, Y.; Tian, W.; Bai, X.; Tian, Y. Impact of Phenanthrene on Denitrification Activity and Transcription of Related Functional Genes in Estuarine and Marine Sediments. J. Ocean Univ. China 2020, 19, 124–134. [Google Scholar] [CrossRef]
- Suszek-Łopatka, B.; Maliszewska-Kordybach, B.; Klimkowicz-Pawlas, A.; Smreczak, B. The Drought and High Wet Soil Condition Impact on PAH (Phenanthrene) Toxicity towards Nitrifying Bacteria. J. Hazard. Mater. 2019, 368, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, L.; Zhang, D.; Lu, P.; Zhang, X.; He, Q. Denitrification Synergized with ANAMMOX for the Anaerobic Degradation of Benzene: Performance and Microbial Community Structure. Appl. Microbiol. Biotechnol. 2017, 101, 4315–4325. [Google Scholar] [CrossRef]
- Ali, M.; Chai, L.-Y.; Tang, C.-J.; Zheng, P.; Min, X.-B.; Yang, Z.-H.; Xiong, L.; Song, Y.-X. The Increasing Interest of ANAMMOX Research in China: Bacteria, Process Development, and Application. Biomed Res. Int. 2013, 2013, 134914. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Niu, J.; Liu, Y.; Zhao, J.; Yin, Z. Effects of Polycyclic Aromatic Hydrocarbons on Sludge Performance for Denitrification and Phosphorus Removal. Chem. Eng. J. 2020, 397, 125552. [Google Scholar] [CrossRef]
- Ribeiro, H.; Mucha, A.P.; Azevedo, I.; Salgado, P.; Teixeira, C.; Almeida, C.M.R.; Joye, S.B.; Magalhães, C. Differential Effects of Crude Oil on Denitrification and Anammox, and the Impact on N2O Production. Environ. Pollut. 2016, 216, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Lu, H.; Zhang, J.; Zhu, S.; Wang, Y.; Lei, Y.; Zhang, R.; Song, L. Simultaneous Heterotrophic Nitrification and Aerobic Denitrification (SND) for Nitrogen Removal: A Review and Future Perspectives. Environ. Adv. 2022, 9, 100254. [Google Scholar] [CrossRef]
- Han, X.; Peng, S.; Zhang, L.; Lu, P.; Zhang, D. The Co-Occurrence of DNRA and Anammox during the Anaerobic Degradation of Benzene under Denitrification. Chemosphere 2020, 247, 125968. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Ma, Z.; Wan, Y. Nitrogen Biogeochemistry of Anaerobic Biodegradation of Naphthalene. Water Air Soil Pollut. 2019, 230, 222. [Google Scholar] [CrossRef]
- Ribeiro, H.; de Sousa, T.; Santos, J.P.; Sousa, A.G.G.; Teixeira, C.; Monteiro, M.R.; Salgado, P.; Mucha, A.P.; Almeida, C.M.R.; Torgo, L.; et al. Potential of Dissimilatory Nitrate Reduction Pathways in Polycyclic Aromatic Hydrocarbon Degradation. Chemosphere 2018, 199, 54–67. [Google Scholar] [CrossRef]
- Castro-Barros, C.M.; Jia, M.; van Loosdrecht, M.C.M.; Volcke, E.I.P.; Winkler, M.K.H. Evaluating the Potential for Dissimilatory Nitrate Reduction by Anammox Bacteria for Municipal Wastewater Treatment. Bioresour. Technol. 2017, 233, 363–372. [Google Scholar] [CrossRef]
- Zhang, D.; Han, X.; Zhou, S.; Yuan, S.; Lu, P.; Peng, S. Nitric Oxide-Dependent Biodegradation of Phenanthrene and Fluoranthene: The Co-Occurrence of Anaerobic and Intra-Aerobic Pathways. Sci. Total Environ. 2021, 760, 144032. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.P.; Lee, S.-H.; Jeong, G.; Yoon, H.; Park, H.-D. Recent Developments of the Mainstream Anammox Processes: Challenges and Opportunities. J. Environ. Chem. Eng. 2021, 9, 105583. [Google Scholar] [CrossRef]
- Cho, S.; Kambey, C.; Nguyen, V. Performance of Anammox Processes for Wastewater Treatment: A Critical Review on Effects of Operational Conditions and Environmental Stresses. Water 2019, 12, 20. [Google Scholar] [CrossRef]
- Xu, G.; Zhou, Y.; Yang, Q.; Lee, Z.M.-P.; Gu, J.; Lay, W.; Cao, Y.; Liu, Y. The Challenges of Mainstream Deammonification Process for Municipal Used Water Treatment. Appl. Microbiol. Biotechnol. 2015, 99, 2485–2490. [Google Scholar] [CrossRef]
- Laureni, M.; Weissbrodt, D.G.; Villez, K.; Robin, O.; de Jonge, N.; Rosenthal, A.; Wells, G.; Nielsen, J.L.; Morgenroth, E.; Joss, A. Biomass Segregation between Biofilm and Flocs Improves the Control of Nitrite-Oxidizing Bacteria in Mainstream Partial Nitritation and Anammox Processes. Water Res. 2019, 154, 104–116. [Google Scholar] [CrossRef]
- Agrawal, N.; Kumar, V.; Shahi, S.K. Biodegradation and Detoxification of Phenanthrene in in Vitro and in Vivo Conditions by a Newly Isolated Ligninolytic Fungus Coriolopsis Byrsina Strain APC5 and Characterization of Their Metabolites for Environmental Safety. Environ. Sci. Pollut. Res. 2022, 29, 61767–61782. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, H. Activated Carbon from Sawdust for Naphthalene Removal from Contaminated Water. Environ. Technol. Innov. 2020, 20, 101080. [Google Scholar] [CrossRef]
- Yu, C.C.; Chang, T.C.; Liao, C.S.; Chang, Y.T. A Comparison of the Microbial Community and Functional Genes Present in Free-Living and Soil Particle-Attached Bacteria from an Aerobic Bioslurry Reactor Treating High-Molecular-Weight PAHs. Sustainability 2019, 11, 1088. [Google Scholar] [CrossRef]
- Khandelwal, A.; Sugavanam, R.; Ramakrishnan, B.; Dutta, A.; Varghese, E.; Banerjee, T.; Nain, L.; Singh, S.B.; Singh, N. Bio-Polysaccharide Composites Mediated Degradation of Polyaromatic Hydrocarbons in a Sandy Soil Using Free and Immobilized Consortium of Kocuria Rosea and Aspergillus Sydowii. Environ. Sci. Pollut. Res. 2022, 29, 80005–80020. [Google Scholar] [CrossRef]
- Lu, J.; Guo, Z.; He, M.; Hu, Z.; Wu, H.; Zhuang, L.; Kong, Q.; Zhang, J. Highly Enhanced Removal of Nutrients and Benzo[a]Pyrene in a Siphon Constructed Wetland with Magnetite: Performance and Mechanisms. Chem. Eng. J. 2022, 446, 136895. [Google Scholar] [CrossRef]
- Liang, L.; Song, X.; Kong, J.; Shen, C.; Huang, T.; Hu, Z. Anaerobic Biodegradation of High-Molecular-Weight Polycyclic Aromatic Hydrocarbons by a Facultative Anaerobe Pseudomonas Sp. JP1. Biodegradation 2014, 25, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Weng, R.; He, Y.; Wang, J.; Zhang, Z.; Wei, Z.; Yang, Y.; Huang, M.; Zhou, G. Quantitative Characterization and Genetic Diversity Associated with N-Cycle Pathways in Urban Rivers with Different Remediation Techniques. Sci. Total Environ. 2022, 804, 150235. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, S.; Persson, Y.; Frankki, S.; van Bavel, B.; Lundstedt, S.; Haglund, P.; Tysklind, M. Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) in Contaminated Soils by Fenton’s Reagent: A Multivariate Evaluation of the Importance of Soil Characteristics and PAH Properties. J. Hazard. Mater. 2007, 149, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-R.; Cang, L.; Wang, Q.-Y.; Zhou, D.-M.; Cheng, J.-M.; Xu, H. Roles of Abiotic Losses, Microbes, Plant Roots, and Root Exudates on Phytoremediation of PAHs in a Barren Soil. J. Hazard. Mater. 2010, 176, 919–925. [Google Scholar] [CrossRef]
- Nzila, A. Update on the Cometabolism of Organic Pollutants by Bacteria. Environ. Pollut. 2013, 178, 474–482. [Google Scholar] [CrossRef]
- Struk-Sokołowska, J.; Kotowska, U.; Piekutin, J.; Laskowski, P.; Mielcarek, A. Analysis of 1H-Benzotriazole Removal Efficiency from Wastewater in Individual Process Phases of a Sequencing Batch Reactor SBR. Water Resour. Ind. 2022, 28, 100182. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, B.; Gong, X.; Liu, X.; Peng, Y. Anammox Bacteria Enrich Naturally in Suspended Sludge System during Partial Nitrification of Domestic Sewage and Contribute to Nitrogen Removal. Sci. Total Environ. 2021, 787, 147658. [Google Scholar] [CrossRef]
- Hu, B.; Shen, L.; Du, P.; Zheng, P.; Xu, X.; Zeng, J. The Influence of Intense Chemical Pollution on the Community Composition, Diversity and Abundance of Anammox Bacteria in the Jiaojiang Estuary (China). PLoS ONE 2012, 7, e33826. [Google Scholar] [CrossRef]
- Zhi, W.; Ji, G. Quantitative Response Relationships between Nitrogen Transformation Rates and Nitrogen Functional Genes in a Tidal Flow Constructed Wetland under C/N Ratio Constraints. Water Res. 2014, 64, 32–41. [Google Scholar] [CrossRef]
- Sheng, H.; Weng, R.; Zhu, J.; He, Y.; Cao, C.; Huang, M. Calcium Nitrate as a Bio-Stimulant for Anaerobic Ammonium Oxidation Process. Sci. Total Environ. 2021, 760, 143331. [Google Scholar] [CrossRef]
- Dhar, K.; Subashchandrabose, S.R.; Venkateswarlu, K.; Krishnan, K.; Megharaj, M. Anaerobic Microbial Degradation of Polycyclic Aromatic Hydrocarbons: A Comprehensive Review. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2019; Volume 251, pp. 25–108. [Google Scholar]

| PAH | Contaminated Matrix | Pollution Type | Microbial Community | PAH Degradation | Effect on Nitrogen | Reference |
|---|---|---|---|---|---|---|
| Mixture of LMW and HMW | Soil | Natural + artificial | Kocuria rosea + anammox | t1/2 values for naphthalene (1.76–2.00 d−1), fluorene (2.52–6.65 d−1), phenanthrene (4.61–6.37 d−1), anthracene (9.01–12.22 d−1), and pyrene (10.98–15.55 d−1) | – | [61] |
| Phenanthrene | Fresh medium | Artificial | Pseudomonas stutzeri + Candidatus Kuenenia | 53% | NO3− decreased by 1.57 mM | [28] |
| Naphthalene and phenanthrene | Wastewater | Synthetic | Shinella + Parcubacteria | 7.94–99.63% | TN removal of 79.59% | [46] |
| Phenanthrene and fluoranthene | Wastewater | Synthetic | Anammox sludge | t1/2 values for phenanthrene (0.19 and 0.23 d−1) and fluoranthene (0.08 and 0.11 d−1) | NO3− removal rate of 6.03–221.3 μM·gVSS−1·d−1 | [53] |
| Benzo[a]pyrene | Constructed wetlands | Synthetic | Pseudoxanthomonas + anammox | 39–54% | NH4+ removal of 55.95% | [62] |
| Pyrene | Slurry (i.e., soil + water) | Artificial | Planctomycetales | >99.99% | – | [60] |
| Phenanthrene, fluoranthene, and benzo[a]pyrene | Fresh medium | Artificial | Pseudomonas sp. JP1 | 5–47% | NO3− decreased from 7.5 to 2.4 mM | [63] |
| Naphthalene and fluoranthene | Sediments | Natural + artificial | Mixture of microorganisms | 35–40 and 30–50% | NO3− decreased by 1.37 and 2.65 mM | [51] |
| ∑PAHs | Sediments | Natural | Candidatus Jettenia + Kuenenia | – | N removal of 83.49–92.46% | [64] |
| Naphthalene | Groundwater | – | Acinetobacter + Pseudomonas | 65–95.54% | Consumption and accumulation of NO3−, NO2−, and NH4+ | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, F.; Ali Saeed Al-Gheethi, A.; Race, M. Coupling of Anammox Activity and PAH Biodegradation: Current Insights and Future Directions. Processes 2023, 11, 458. https://doi.org/10.3390/pr11020458
Bianco F, Ali Saeed Al-Gheethi A, Race M. Coupling of Anammox Activity and PAH Biodegradation: Current Insights and Future Directions. Processes. 2023; 11(2):458. https://doi.org/10.3390/pr11020458
Chicago/Turabian StyleBianco, Francesco, Adel Ali Saeed Al-Gheethi, and Marco Race. 2023. "Coupling of Anammox Activity and PAH Biodegradation: Current Insights and Future Directions" Processes 11, no. 2: 458. https://doi.org/10.3390/pr11020458
APA StyleBianco, F., Ali Saeed Al-Gheethi, A., & Race, M. (2023). Coupling of Anammox Activity and PAH Biodegradation: Current Insights and Future Directions. Processes, 11(2), 458. https://doi.org/10.3390/pr11020458








