Methods and Advances in the Design, Testing and Development of In Vitro Diagnostic Instruments
Abstract
1. Introduction
2. Development of In Vitro Diagnostic Methods and Instruments
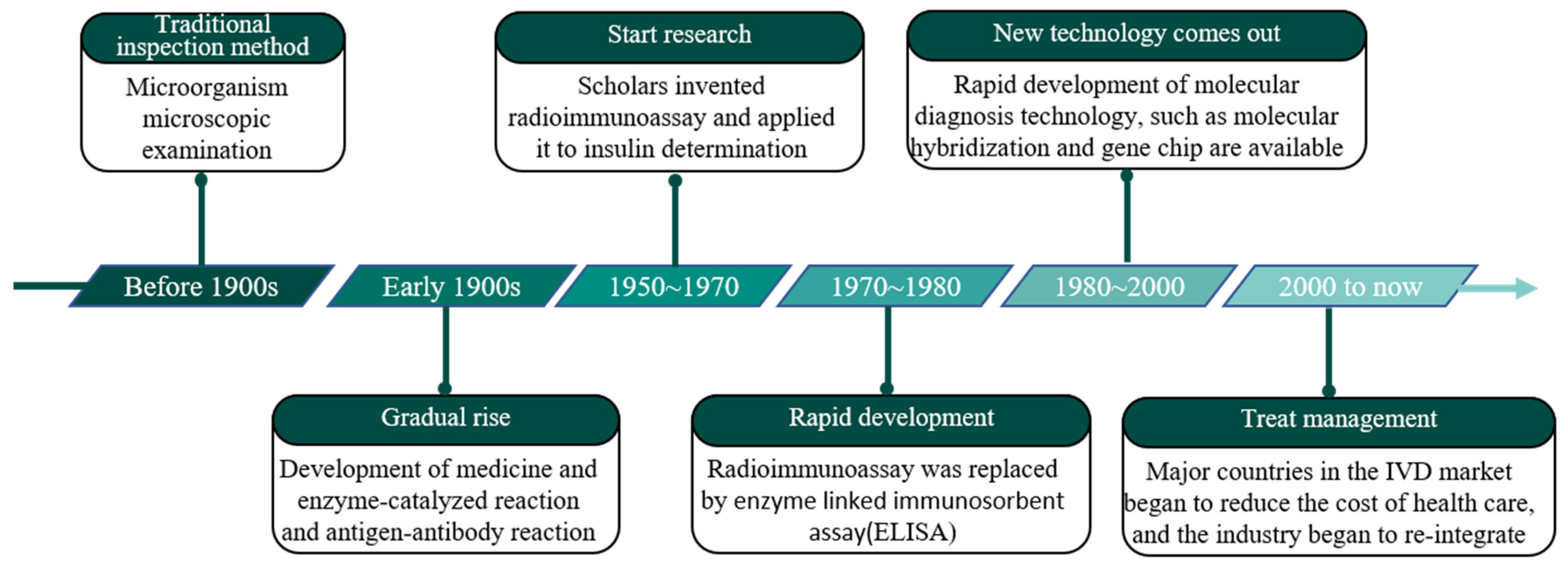
2.1. Development of In Vitro Diagnostic Methodology
2.2. Technologies Related to In Vitro Diagnostic Devices and Their Applications
2.2.1. Electrochemical Analysis Technology
2.2.2. Spectrum Analysis Technology
2.2.3. Chromatography Analysis Technology
2.2.4. Mass Spectrum Analysis Technology
2.2.5. Electrophoresis Analysis Technology
2.2.6. Flow Cytometry Analysis Technology
2.2.7. Labeled Immunization Analysis Technology
2.2.8. Molecular Biology Analysis Technology
2.3. Classification of In Vitro Diagnostic Instruments
2.4. Systematic Design, Testing and Development Process of In Vitro Diagnostic Instruments
2.4.1. Product Design
Overall Solution Design and Evaluation
Form Design and Evaluation
Software System Design and Evaluation
Hardware System Design and Evaluation
Core Module Design and Evaluation
2.4.2. Product Prototype Development
Hardware System Detailed Design and Evaluation
Software System Detailed Design and Evaluation
Design Engineering
Whole Instrument Design and Verification
2.4.3. Product Finalization Development
Module Improvement and Mold Development
Module Validation
Whole Instrument Improvement
Whole Instrument Validation
2.4.4. Product Receiving Inspection
3. Market Trends of In Vitro Diagnostic Instruments
3.1. Chemiluminescence
3.2. Gene Sequencing
3.3. POCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECLIA | Electro-chemiluminescence immunoassay |
| EMC | Electromagnetic compatibility |
| FCM | Flow cytometry |
| HPLC | High performance liquid chromatography |
| IVD | In vitro diagnostic |
| LAN | Local area network |
| LC-MS | Liquid chromatography-mass spectrometry |
| MS | Mass spectrum |
| PCR | Polymerase chain reaction |
| POCT | Point of care testing |
| WAN | Wide area network |
References
- Peng, P.; Liu, C.; Li, Z.D.; Xue, Z.R.; Mao, P.; Hu, J.; Xu, F.; Yao, C.Y.; You, M.L. Emerging ELISA derived technologies for in vitro diagnostics. TrAC Trends Anal. Chem. 2002, 152, 116605. [Google Scholar] [CrossRef]
- Müller, H.; Holzinger, A.; Plass, M.; Brcic, L.; Stumptner, C.; Zatloukal, K. Explainability and causability for artificial intelligence-supported medical image analysis in the context of the European In Vitro Diagnostic Regulation. New Biotechnol. 2022, 70, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; He, L.; Lindner, E.; Wu, D.Y. Highly porous poly(l-lactic) acid nanofibers as a dual-signal paper-based bioassay platform for in vitro diagnostics. Appl. Surf. Sci. 2021, 542, 148732. [Google Scholar] [CrossRef]
- Cao, S.; Xie, Z.; Xiao, G.; Sun, X.; Diao, H.; Zhou, X.; Yue, Z. Photoelectrochemical sensors based on heterogeneous nanostructures for in vitro diagnostics. Biosens. Bioelectron. X 2022, 11, 100200. [Google Scholar]
- Iglesia, T.B.; Yañez-Vico, R.M.; Iglesias-Linares, A. Diagnostic performance of cone-beam computed tomography to diagnose in vivo/in vitro root resorption: A systematic review and meta-analysis. J. Evid.-Based Dent. Pract. 2022, 101803. [Google Scholar] [CrossRef]
- Guliy, O.I.; Evstigneeva, S.S.; Bunin, V.D. Electrical sensor system for in vitro bacteria biofilm diagnostics. Biosens. Bioelectron. X 2022, 11, 100174. [Google Scholar] [CrossRef]
- Geraldi, A.; Giri-Rachman, E.A. Synthetic biology-based portable in vitro diagnostic platforms. Alex. J. Med. 2018, 54, 423–428. [Google Scholar] [CrossRef]
- Chi, X.; Huang, D.; Zhao, Z.; Zhou, Z.; Yin, Z.; Gao, J. Nanoprobes for in vitro diagnostics of cancer and infectious diseases. Biomaterials 2012, 33, 189–206. [Google Scholar] [CrossRef]
- Takazawa, T.; Sabato, V.; Ebo, D.G. In vitro diagnostic tests for perioperative hypersensitivity, a narrative review: Potential, limitations, and perspectives. Br. J. Anaesth. 2019, 123, e117–e125. [Google Scholar] [CrossRef]
- Grifa, R.A.; Pozzoli, G. Performance evaluation of in vitro diagnostic medical devices: Methodology and differences compared to studies on other medical devices. Microchem. J. 2018, 136, 279–282. [Google Scholar] [CrossRef]
- Konkol, N.R.; McNamara, C.J.; Hellman, E.; Mitchell, R. Early detection of fungal biomass on library materials. J. Cult. Herit. 2012, 13, 115–119. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, R.; Liu, R.; Tian, Y.; Yang, Z. Application of phage-display developed antibody and antigen substitutes in immunoassays for small molecule contaminants analysis: A mini-review. Food Chem. 2021, 339, 128084. [Google Scholar] [CrossRef]
- Neidle, S. Beyond the double helix: DNA structural diversity and the PDB. J. Biol. Chem. 2021, 296, 100553. [Google Scholar] [CrossRef]
- Siegel, D.L. Recombinant monoclonal antibody technology. Transfus. Clin. Biol. 2002, 9, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Jones, A.T.; Stephens, D.J. Intracellular trafficking pathways and drug delivery: Fluorescence imaging of living and fixed cells. Adv. Drug Deliv. Rev. 2005, 57, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Ioannidis, J.P.A.; Salem, D.; Chew, P.W.; Lau, J. Accuracy of biomarkers to diagnose acute cardiac ischemia in the emergency department: A meta-analysis. Ann. Emerg. Med. 2001, 37, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Qi, T.; Wu, Z.; He, Y.; Guan, S.; Luo, S.; Zhang, Q.; Luo, W.; Xiao, W.; Situ, B.; et al. A portable smartphone-based hemoglobin point-of-care testing platform for accurate anemia diagnostics. Biosens. Bioelectron. 2022, 217, 114711. [Google Scholar] [CrossRef]
- Gurramkonda, C.; Zahid, M.; Nemani, S.K.; Adnan, A.; Gudi, S.K.; Khanna, N.; Ebensen, T.; Lünsdorf, H.; Guzmán, C.A.; Rinas, U. Purification of hepatitis B surface antigen virus-like particles from recombinant Pichia pastoris and in vivo analysis of their immunogenic properties. J. Chromatogr. B 2013, 940, 104–111. [Google Scholar] [CrossRef]
- Gossen, P.D.; MacGregor, J.F. On-Line Particle Diameter for Poly(Vinyl Acetate) Latex Using Specific Turbidity Method. J. Colloid Interface Sci. 1993, 160, 24–38. [Google Scholar] [CrossRef]
- Cao, Y.; Dai, Y.; Zhang, L.; Wang, D.; Yu, Q.; Hu, W.; Wang, X.; Yu, P.; Ping, Y.; Sun, T.; et al. Serum oncostatin M is a potential biomarker of disease activity and infliximab response in inflammatory bowel disease measured by chemiluminescence immunoassay. Clin. Biochem. 2022, 100, 35–41. [Google Scholar] [CrossRef]
- Thumvijit, T.; Supawat, B.; Wattanapongpitak, S.; Kothan, S.; Tungjai, M. Effect of iodinated radiographic contrast media on radioimmunoassay for measuring thyroid hormones. Appl. Radiat. Isot. 2022, 185, 110261. [Google Scholar] [CrossRef] [PubMed]
- Steininger, C.; Kundi, M.; Aberle, S.W.; Aberle, J.H.; Popow-Kraupp, T. Effectiveness of reverse transcription-pcr, virus isolation, and enzyme-linked immunosorbent assay for diagnosis of influenza a virus infection in different age groups. J. Clin. Microbiol. 2002, 40, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Song, L.W.; Wang, Y.B.; Fang, L.L.; Wu, Y.; Yang, L.; Chen, J.Y.; Xia, N.S. Rapid fluorescent lateral-flow immunoassay for hepatitis B virus genotyping. Anal. Chem. 2015, 87, 5173–5180. [Google Scholar] [CrossRef] [PubMed]
- Leivo, J.; Kivimäki, L.; Juntunen, E.; Pettersson, K.; Lamminmäki, U. Development of anti-immunocomplex specific antibodies and non-competitive time-resolved fluorescence immunoassay for the detection of estradiol. Anal. Bioanal. Chem. 2019, 411, 5633–5639. [Google Scholar] [CrossRef]
- Figuero, E.; Sánchez-Beltrán, M.; Cuesta-Frechoso, S.; Tejerina, J.M.; del Castro, J.A.; Gutiérrez, J.M.; Sanz, M. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J. Periodontol. 2011, 82, 1469–1477. [Google Scholar] [CrossRef]
- Cui, C.; Shu, W.; Li, P. Fluorescence in situ hybridization: Cell-based genetic diagnostic and research applications. Front. Cell Dev. Biol. 2016, 4, 89. [Google Scholar] [CrossRef]
- Yin, G.; Bie, S.; Gu, H.; Shu, X.; Zheng, W.; Peng, K.; Jiang, M. Application of gene chip technology in the diagnostic and drug resistance detection of Helicobacter pylori in children. J. Gastroenterol. Hepatol. 2020, 35, 1331–1339. [Google Scholar] [CrossRef]
- Palomaki, G.E.; Deciu, C.; Kloza, E.M.; Lambert-Messerlian, G.M.; Haddow, J.E.; Neveux, L.M.; Canick, J.A. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: An international collaborative study. Genet. Med. 2012, 14, 296–305. [Google Scholar] [CrossRef]
- Banu, S.; Rahman, S.M.; Khan, M.S.R.; Ferdous, S.S.; Ahmed, S.; Gratz, J.; Houpt, E.R. Discordance across several methods for drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates in a single laboratory. J. Clin. Microbiol. 2014, 52, 156–163. [Google Scholar] [CrossRef]
- Camesano, T.A.; Natan, M.J.; Logan, B.E. Observation of changes in bacterial cell morphology using tapping mode atomic force microscopy. Langmuir 2000, 16, 4563–4572. [Google Scholar] [CrossRef]
- Budding, A.E.; Hoogewerf, M.; Vandenbroucke-Grauls, C.M.; Savelkoul, P.H. Automated broad-range molecular detection of bacteria in clinical samples. J. Clin. Microbiol. 2016, 54, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.N. Classification of blood types by microscope color images. Int. J. Mach. Learn. Comput. 2013, 3, 376. [Google Scholar] [CrossRef]
- Acharya, V.; Kumar, P. Identification and red blood cell automated counting from blood smear images using computer-aided system. Med. Biol. Eng. Comput. 2018, 56, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Szczepański, T.; van der Velden, V.H.; van Dongen, J.J. Flow-cytometric immunophenotyping of normal and malignant lymphocytes. Clin. Chem. Lab. Med. 2006, 44, 775–796. [Google Scholar] [CrossRef]
- Hu, J.; Cui, X.; Gong, Y.; Xu, X.; Gao, B.; Wen, T.; Xu, F. Portable microfluidic and smartphone-based devices for monitoring of cardiovascular diseases at the point of care. Biotechnol. Adv. 2016, 34, 305–320. [Google Scholar] [CrossRef]
- Zhang, Z.; Ohta, S.; Shiba, S.; Niwa, O. Nanocarbon film electrodes for electro-analysis and electrochemical sensors. Curr. Opin. Electrochem. 2022, 35, 101045. [Google Scholar] [CrossRef]
- Shahid, M.; Sami, H.; Sharma, S.; Kumar, A.; Khan, P.A.; Khan, H.M. Comparative analysis of Electro-Chemiluminescence Immunoassay (ECLIA), ELISA and Rapid Diagnostic Test (RDT) for detection of Hepatitis B Surface Antigen (HBSAG). Pathology 2020, 52, S126–S127. [Google Scholar] [CrossRef]
- Topal, S.; Şaylan, M.; Zaman, B.T.; Bakırdere, S. Determination of trace cadmium in saliva samples using spray assisted droplet formation-liquid phase microextraction prior to the measurement by slotted quartz tube-flame atomic absorption spectrophotometry. J. Trace Elem. Med. Biol. 2021, 68, 126859. [Google Scholar] [CrossRef]
- Erarpat, S.; Bodur, S.; Günkara, O.T.; Bakırdere, S. Combination of high performance liquid chromatography and flame atomic absorption spectrophotometry using a novel nebulizer interface supported T shaped slotted quartz tube for the determination of Vitamin B12. J. Pharm. Biomed. Anal. 2022, 217, 114855. [Google Scholar] [CrossRef]
- Strzelak, K.; Koncki, R. Nephelometry and turbidimetry with paired emitter detector diodes and their application for determination of total urinary protein. Anal. Chim. Acta 2013, 788, 68–73. [Google Scholar] [CrossRef]
- Hui, X.; Tian, J.; Wang, X.; Zhang, Z.; Zhao, Y.; Gao, W.; Li, H. Overall analyses of the reactions catalyzed by acetohydroxyacid synthase/acetolactate synthase using a precolumn derivatization-HPLC method. Anal. Biochem. 2023, 660, 114980. [Google Scholar] [CrossRef] [PubMed]
- Theiner, S.; Schoeberl, A.; Schweikert, A.; Keppler, B.K.; Koellensperger, G. Mass spectrometry techniques for imaging and detection of metallodrugs. Curr. Opin. Chem. Biol. 2021, 61, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Jain, B.; Chauhan, V.; Deswal, B.; Kaur, S.; Sharma, S.; Abourehab, M.A.S. Simple determination of dichlorvos in cases of fatal intoxication by gas Chromatography-Mass spectrometry. J. Chromatogr. B 2023, 1215, 123582. [Google Scholar] [CrossRef] [PubMed]
- Famiglini, G.; Palma, P.; Termopoli, V.; Cappiello, A. The history of electron ionization in LC-MS, from the early days to modern technologies: A review. Anal. Chim. Acta 2021, 1167, 338350. [Google Scholar] [CrossRef]
- Gugten, J.G. Tandem mass spectrometry in the clinical laboratory: A tutorial overview. Clin. Mass Spectrom. 2020, 15, 36–43. [Google Scholar] [CrossRef]
- Okai, G.G.; Cardozo, K.H.M.; Ferrer, C.M.; Goldman, M.M.; Clarke, N.J.; Vieira, J.G.H. LC-MS/MS reduces interference by high levels of 25(OH)D and its metabolites on measured 1,25(OH)2D. Steroids 2022, 187, 109095. [Google Scholar] [CrossRef]
- Bhimwal, R.; Rustandi, R.R.; Payne, A.; Dawod, M. Recent advances in capillary gel electrophoresis for the analysis of proteins. J. Chromatogr. A 2022, 1682, 463453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Sun, P.; Yu, J.; Pu, Q. A fully functional palmtop microchip electrophoresis analyzer with laser-induced fluorescence detection. Sens. Actuators B Chem. 2022, 372, 132645. [Google Scholar] [CrossRef]
- Devanesan, S.; AlQahtani, F.; AlSalhi, M.S.; Jeyaprakash, K.; Masilamani, V. Diagnosis of thalassemia using fluorescence spectroscopy, auto-analyzer, and hemoglobin electrophoresis—A prospective study. J. Infect. Public Health 2019, 12, 585–590. [Google Scholar] [CrossRef]
- Farmer, J.R.; DeLelys, M. Flow Cytometry as a Diagnostic Tool in Primary and Secondary Immune Deficiencies. Clin. Lab. Med. 2019, 39, 591–607. [Google Scholar] [CrossRef]
- Sorigue, M.; Cañamero, E.; Miljkovic, M.D. Systematic review of staging bone marrow involvement in B cell lymphoma by flow cytometry. Blood Rev. 2021, 47, 100778. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mengel, K.; Friedberg, K.D. In vivo effects of fluconazole on lymphocyte subpopulations of the thymus and spleen in mice: Flow cytometry analysis. Int. J. Immunopharmacol. 1996, 18, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Calidonio, J.M.; Schifferli, K.H. Biophysical and biochemical insights in the design of immunoassays. Biochim. Biophys. Acta BBA Gen. Subj. 2023, 1867, 130266. [Google Scholar] [CrossRef]
- Marini, S.; Fasciglione, G.F.; Giardina, B. Production and characterization of monoclonal antibodies against l-carnitine: Radioimmunologic assays for l-carnitine determination. Clin. Chim. Acta 1996, 249, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Jiang, T.; Lu, T.; Jin, Q.; Xi, Y.; Zhang, W. Biomimetic-mineralized bifunctional nanoflowers for enzyme-free and colorimetric immunological detection of protein biomarker. Talanta 2022, 238, 123001. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Bai, M.; Wang, Y.; Liao, X.; Zhang, Y.; Wang, P.; Wei, J.; Zhang, H.; Wang, J.; et al. An ultrasensitive sandwich chemiluminescent enzyme immunoassay based on phage-mediated double-nanobody for detection of Salmonella Typhimurium in food. Sens. Actuators B Chem. 2022, 352, 131058. [Google Scholar] [CrossRef]
- Bastarache, J.A.; Koyama, T.; Wickersham, N.E.; Ware, L.B. Validation of a multiplex electrochemiluminescent immunoassay platform in human and mouse samples. J. Immunol. Methods 2014, 408, 13–23. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Fan, F.; Chen, S.; Zhang, Y.; Zhang, Y.; Meng, X.; Lin, J. Present status of microfluidic PCR chip in nucleic acid detection and future perspective. TrAC Trends Anal. Chem. 2022, 157, 116737. [Google Scholar] [CrossRef]
- Pearson, L.A.; Agostino, P.M.; Neilan, B.A. Recent developments in quantitative PCR for monitoring harmful marine microalgae. Harmful Algae 2021, 108, 102096. [Google Scholar] [CrossRef]
- Tillati, S.; Pati, I.; Delle Donne, M. Horiba Micros ES 60 Blood Cell Analyzer in Blood Donor Eligibility: A Validation Study. Diagnostics 2022, 12, 2586. [Google Scholar] [CrossRef]
- Tang, S.S.; Liang, C.H.; Liu, Y.L. Intermittent hypoxia is involved in gut microbial dysbiosis in type 2 diabetes mellitus and obstructive sleep apnea-hypopnea syndrome. World J. Gastroenterol. 2022, 28, 2320. [Google Scholar] [CrossRef]
- Altunok, İ.; Aksel, G.; Eroğlu, S.E. Correlation between sodium, potassium, hemoglobin, hematocrit, and glucose values as measured by a laboratory autoanalyzer and a blood gas analyzer. Am. J. Emerg. Med. 2019, 37, 1048–1053. [Google Scholar] [CrossRef]
- Karadag Gecgel, S. Comparison of HBV-DNA Levels with Biochemical and Microbiological Parameters for Chronic Hepatitis Evaluation, Bursa, Turkey. J. Med. Microbiol. Infect. Dis. 2021, 9, 17–24. [Google Scholar]
- Hu, F.; Li, J.; Zhang, Z. Smartphone-based droplet digital LAMP device with rapid nucleic acid isolation for highly sensitive point-of-care detection. Anal. Chem. 2019, 92, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Barbé, B.; Corsmit, E.; Jans, J. Pilot Testing of the “Turbidimeter”, a Simple, Universal Reader Intended to Complement and Enhance Bacterial Growth Detection in Manual Blood Culture Systems in Low-Resource Settings. Diagnostics 2022, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Badawi, R.D.; Shi, H.; Hu, P. First human imaging studies with the EXPLORER total-body PET scanner. J. Nucl. Med. 2019, 60, 299–303. [Google Scholar] [CrossRef]
- Higuchi, S.; Kamishiro, Y.; Ishihara, M. Evaluation of a radon air monitor in the measurement of radon concentration in water in comparison with a liquid scintillation counter. Radiat. Prot. Dosim. 2019, 184, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.O.; Kim, Y. Small red blood cell fraction on the UF-1000i urine analyzer as a screening tool to detect dysmorphic red blood cells for diagnosing glomerulonephritis. Ann. Lab. Med. 2019, 39, 271. [Google Scholar] [CrossRef]
- Yao, Q.; Li, Y.; Tang, X. Separation of petroleum ether extracted residue of low temperature coal tar by chromatography column and structural feature of fractions by TG-FTIR and PY-GC/MS. Fuel 2019, 245, 122–130. [Google Scholar] [CrossRef]
- Masindi, V.; Fosso-Kankeu, E.; Mamakoa, E. Emerging remediation potentiality of struvite developed from municipal wastewater for the treatment of acid mine drainage. Environ. Res. 2022, 210, 112944. [Google Scholar] [CrossRef]
- Hooghvorst, I.; Torrico, O.; Nogués, S. Doubled Haploid Parthenogenetic Production of Melon ‘Piel de Sapo’ Doubled Haploid Technology; Humana: New York, NY, USA, 2021; pp. 87–95. [Google Scholar]
- Hermilasari, R.D.; Rizal, D.M.; Wirohadidjojo, Y.W. Effect of Platelet-Rich Plasma (PRP) on testicular damage in streptozotocin-induced diabetic rats. Bali Med. J. 2020, 9, 351–355. [Google Scholar] [CrossRef]
- Fadeyi, E.A.; Wagner, S.J.; Goldberg, C. Fatal sepsis associated with a storage container leak permitting platelet contamination with environmental bacteria after pathogen reduction. Transfusion 2021, 61, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Chizh, N.A. Cryogenic Equipment in Minimally Invasive Surgery. Probl. Cryobiol. Cryomed. 2018, 28, 200–211. [Google Scholar] [CrossRef][Green Version]
- Weaver, D.T.; McElvany, B.D.; Gopalakrishnan, V. UV decontamination of personal protective equipment with idle laboratory biosafety cabinets during the COVID-19 pandemic. PLoS ONE 2021, 16, e0241734. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Qian, S.; He, Z.; Zheng, J.; Zheng, F.; Jiang, X. Clinical Laboratory Instruments and Technologies; People’s Medical Publishing House: Beijing, China, 2015; pp. 1–5. [Google Scholar]
- Seger, C.; Salzmann, L. After another decade: LC–MS/MS became routine in clinical diagnostics. Clin. Biochem. 2020, 82, 2–11. [Google Scholar] [CrossRef]
- Lan, T.; Zhang, J.; Lu, Y. Transforming the blood glucose meter into a general healthcare meter for in vitro diagnostics in mobile health. Biotechnol. Adv. 2016, 34, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Lough, F.; Perry, J.D.; Stanforth, S.P.; Dean, J.R. Detection of exogenous VOCs as a novel in vitro diagnostic technique for the detection of pathogenic bacteria. TrAC Trends Anal. Chem. 2017, 87, 71–81. [Google Scholar] [CrossRef]
- Vashist, S.K.; Schneider, E.M.; Luong, J.H.T. Rapid sandwich ELISA-based in vitro diagnostic procedure for the highly-sensitive detection of human fetuin A. Biosens. Bioelectron. 2015, 67, 73–78. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, L.; Duan, H. Nanomaterial based electrochemical sensors for in vitro detection of small molecule metabolites. Biotechnol. Adv. 2016, 34, 234–249. [Google Scholar] [CrossRef]
- Kumánovics, A.; Akha, A.A.S. Flow cytometry for B-cell subset analysis in immunodeficiencies. J. Immunol. Methods 2022, 509, 113327. [Google Scholar] [CrossRef]
- Song, H.; Zhu, Y. The in vitro diagnostics industry in China. View 2020, 1, e5. [Google Scholar] [CrossRef]
- Pandith, A.; Kim, H.; Shin, T.; Seo, Y.J. Unprecedented green-emissive boranyl-hydrazone supramolecular assemblies and their in vitro diagnostic applications. J. Photochem. Photobiol. B Biol. 2019, 197, 111553. [Google Scholar] [CrossRef]
- Perruca-Foncillas, R.; Davidsson, J.; Carlquist, M.; Gorwa-Grauslund, M.F. Assessment of fluorescent protein candidates for multi-color flow cytometry analysis of Saccharomyces cerevisiae. Biotechnol. Rep. 2022, 34, e00735. [Google Scholar] [CrossRef] [PubMed]
- Dundon, W.G.; Settypalli, T.B.K.; Spiegel, K.; Steinrigl, A.; Revilla-Fernández, S.; Schmoll, F.; Naletoski, I.; Lamien, C.E.; Cattoli, G. Comparison of eleven in vitro diagnostic assays for the detection of SARS-CoV-2 RNA. J. Virol. Methods 2021, 295, 114200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Koo, B.; Liu, H.; Jin, C.E.; Shin, Y. A single-tube approach for in vitro diagnostics using diatomaceous earth and optical sensor. Biosens. Bioelectron. 2018, 99, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Akizawa, H.; Zhao, S.; Takahashi, M.; Nishijima, K.; Kuge, Y.; Tamaki, N.; Seki, K.; Ohkura, K. In vitro and in vivo evaluations of a radioiodinated thymidine phosphorylase inhibitor as a tumor diagnostic agent for angiogenic enzyme imaging. Nucl. Med. Biol. 2010, 37, 427–432. [Google Scholar] [CrossRef]
- Seo, S.B.; Hwang, J.; Kim, E.; Kim, K.; Roh, S.; Lee, G.; Lim, J.; Kang, B.; Jang, S.; Son, S.U.; et al. Isothermal amplification-mediated lateral flow biosensors for in vitro diagnosis of gastric cancer-related microRNAs. Talanta 2022, 246, 123502. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Kozlov, S.V.; Brovko, F.A.; Fursova, K.K.; Shardin, V.V.; Fomin, A.S.; Gabalov, K.P.; Soldatov, D.A.; Zhnichkova, E.G.; Dykman, L.A.; et al. Phage antibodies against heat shock proteins as tools for in vitro cancer diagnosis. Biosens. Bioelectron. X 2022, 11, 100211. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Tian, T.; Liu, Y.; Zhang, J.; Qian, K. Advanced on-site and in vitro signal amplification biosensors for biomolecule analysis. TrAC Trends Anal. Chem. 2022, 149, 116565. [Google Scholar] [CrossRef]
- Nusbaum, K.B.; Korman, A.M.; Tyler, K.; Kaffenberger, J.; Trinidad, J.; Kaffenberger, B.H. In vitro diagnostics for the medical dermatologist. Part I: Autoimmune tests. J. Am. Acad. Dermatol. 2021, 85, 287–298. [Google Scholar] [CrossRef]
- Yamamoto, S.; Whyte, T.; Toen, C.V.; Melnyk, A.; Shewchuk, J.; Street, J.; Cripton, P.; Oxland, T.R. The diagnostic precision of computed tomography for traumatic cervical spine injury: An in vitro biomechanical investigation. Clin. Biomech. 2022, 92, 105529. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, S.; Mishra, P. Next generation biosensors employing molecularly imprinted polymers as sensing elements for in vitro diagnostics. Biosens. Bioelectron. X 2022, 11, 100201. [Google Scholar] [CrossRef]
- Braga, F.; Pasqualetti, S.; Panteghini, M. The role of external quality assessment in the verification of in vitro medical diagnostics in the traceability era. Clin. Biochem. 2018, 57, 23–28. [Google Scholar] [CrossRef]
- Fatangare, A.; Glässner, A.; Sachs, B.; Sickmann, A. Future perspectives on in-vitro diagnosis of drug allergy by the lymphocyte transformation test. J. Immunol. Methods 2021, 495, 113072. [Google Scholar] [CrossRef]
- Vieira, P.; Jesus, V.; Cândido, M.A.; Pacheco-Soares, C.; Castilho, M.; Raniero, L. Specific nanomarkers fluorescence: In vitro analysis for EGFR overexpressed cells in triple-negative breast cancer and malignant glioblastoma. Photodiagnosis Photodyn. Ther. 2022, 39, 102997. [Google Scholar] [CrossRef] [PubMed]
- Juárez, M.J.; Ibáñez-Echevarria, E.; Rojas, D.H.F.; Maquieira, Á.; Morais, S. Multiplexed analytical approaches to beta-lactam allergy in vitro testing standardization. Anal. Chim. Acta 2021, 1173, 338656. [Google Scholar] [CrossRef]
- Caputo, D.; Cascone, C.; Santo, R.D.; Digiacomo, L.; Quagliarini, E.; Pozzi, D.; Coppola, R.; Caracciolo, G. Fine-Tuning of Nanoparticle-Protein Corona Composition: A New Strategy for the Development of Efficient in Vitro Diagnostic Testing for Early Pancreatic Cancer Detection. J. Am. Coll. Surg. 2020, 231, e34. [Google Scholar]
- Kim, K.; Kashefi-Kheyrabadi, L.; Joung, Y.; Kim, K.; Dang, H.; Chavan, S.G.; Lee, M.; Choo, J. Recent advances in sensitive surface-enhanced Raman scattering-based lateral flow assay platforms for point-of-care diagnostics of infectious diseases. Sens. Actuators B Chem. 2021, 329, 129214. [Google Scholar] [CrossRef]
- Sufianov, A.; Begliarzade, S.; Ilyasova, T.; Xu, X.; Beylerli, O. MicroRNAs as potential diagnostic markers of glial brain tumors. Non-Coding RNA Res. 2022, 7, 242–247. [Google Scholar] [CrossRef]
| Method | Principle | Technology | Applications |
|---|---|---|---|
| Biochemical diagnosis | Test the sample based on biochemical reaction | Dry Chemistry | Biochemical testing in the clinical emergency biochemical program testing [16] |
| Others | Blood test, urine test, liver function, kidney function, etc. [17] | ||
| Immunization diagnosis | Immunology-based test using the specific reaction of antigen and antibody binding to each other | Immune colloidal gold technique | Hepatitis B, HIV, female pregnancy, drug testing, etc. [18] |
| Latex turbidity | Specific body fluid protein testing [19] | ||
| Chemiluminescence | Infectious disease, endocrine, tumor, drug, blood group testing, etc. [20] | ||
| Radioimmunoassay | Hormone, trace protein, tumor marker and drug trace substance testing [21] | ||
| Enzyme immunization | Infectious disease, endocrine, tumor, drug, blood group testing, etc. [22] | ||
| Fluorescence immunization | Bacteria, virus, skin activity testing [23] | ||
| Time-resolved fluorescence | Hormones, viral hepatitis, tumor testing, etc. [24] | ||
| Molecular diagnosis | Test the changes of the structure or expression levels of genetic material in patients using molecular biology methods | Polymerase Chain Reaction (PCR) | Bacteria, virus testing [25] |
| Fluorescence original position crossbreeding | Genetic profiling, virus testing [26] | ||
| Gene chips | Drug screening, new drug development, disease diagnosis, etc. [27] | ||
| Gene sequencing | Genetic mapping, Down’s syndrome screening, etc. [28] | ||
| Microbiological diagnosis | Test the types and numbers of microorganisms by microscopy | Drug sensitivity test | Laboratory diagnosis [29] |
| Morphological observation | Bacteria, fungus testing [30] | ||
| Automated microbiological analysis | Bacteria, fungus testing [31] | ||
| Blood diagnosis | Test the blood components such as red blood cells, white blood cells and hemoglobin | Smear and microscopy | Blood type testing [32] |
| Blood cell image analysis | Red blood cell, white blood cell, platelet testing [33] | ||
| Flow cytometry | Lymphocyte subpopulation testing, immunophenotyping, etc. [34] | ||
| POCT | Test the samples using portable analytical instruments and supporting reagents in the sampling site | Point of care testing | Cardiovascular disease markers, metabolic disease markers, coagulation characteristics testing, etc. [35] |
| Classification | Representative Instruments |
|---|---|
| Hematology analysis instruments | Blood group analyzer, blood cell analyzer, blood cell morphology analyzer, coagulation analyzer, platelet analyzer, blood flow analyzer, red blood cell sedimentation analyzer, flow cytometry analyzer, etc. [60]. |
| Biochemical analysis instruments | Biochemistry analyzer, blood glucose analyzer, etc. [61]. |
| Electrolyte and blood gas analysis instruments | Electrolyte analyzer, blood gas analyzer, electrolyte blood gas analyzer, electrolyte blood gas test electrode, etc. [62]. |
| Immunoassay instruments | Enzyme-linked immunoassay analyzer, chemiluminescence immunoassay analyzer, fluorescence immunoassay analyzer, immunochromatographic analyzer, immunoblotter, immunoscattering turbidity analyzer, biochemical immunoassay analyzer, etc. [63]. |
| Molecular biology analysis instruments | Gene sequencer, sanger sequencer, nucleic acid amplification analyzer, nucleic acid amplification instrument, nucleic acid molecular hybridization instrument, etc. [64]. |
| Microbiological analysis instruments | Microbial turbidimeter, microbial culture monitor, microbial drug sensitivity culture monitor, microbial identification instrument (non-mass spectrometry), microbial mass spectrometry identification instrument, microbial identification drug sensitivity analyzer, bacterial endotoxin/fungal dextran detector, H. pylori analyzer, etc. [65]. |
| Scanning image analysis instruments | Medical microscopes, image scanners, image analyzers, etc. [66]. |
| Radionuclide specimen determination instruments | Radioimmune γ counting device, liquid scintillation counting device, radioactive level scanning instrument, etc. [67]. |
| Urine and other body fluid analysis instruments | Dry chemical urine analyzer, urine organic fraction analyzer, urine analyzer, stool analyzer, sperm analyzer, reproductive tract secretion analyzer, other body fluid analyzer, other body fluid morphology analyzer, etc. [68]. |
| Other medical analysis instruments | Flow dot meter, minor element analyzer, mass spectrometry monitoring system, liquid chromatography analyzer, chromatography column, osmotic pressure assayer, circulating tumor cell analyzer, biochip analyzer, cataphoresis meter, etc. [69]. |
| Sampling instruments | Automatic sampler, sample processing and spiking system [70]. |
| Sample pre-processing instruments for morphological analysis | Blood cell analysis sample pre-processor, pathology analysis sample pre-processor, flow cytometry sample lyser, etc. [71]. |
| Sample separation instruments | Medical centrifugal machine, nucleic acid extraction and purification instrument, etc. [72]. |
| Culture and incubation instruments | Medical culture/constant temperature oven, anaerobic incubator, incubator, platelet shaker, etc. [73]. |
| Inspection and other utility instruments | Automatic sample adding instrument, sample processing system, medical cryogenic equipment, medical refrigeration equipment, medical freezing equipment, etc. [74]. |
| Medical bioprotective instruments | Biological safety cabinets, clean working bench, etc. [75]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xu, W.; Wang, B.; Si, X.; Li, S. Methods and Advances in the Design, Testing and Development of In Vitro Diagnostic Instruments. Processes 2023, 11, 403. https://doi.org/10.3390/pr11020403
Wang L, Xu W, Wang B, Si X, Li S. Methods and Advances in the Design, Testing and Development of In Vitro Diagnostic Instruments. Processes. 2023; 11(2):403. https://doi.org/10.3390/pr11020403
Chicago/Turabian StyleWang, Lei, Wenchang Xu, Biao Wang, Xiaonan Si, and Shengyu Li. 2023. "Methods and Advances in the Design, Testing and Development of In Vitro Diagnostic Instruments" Processes 11, no. 2: 403. https://doi.org/10.3390/pr11020403
APA StyleWang, L., Xu, W., Wang, B., Si, X., & Li, S. (2023). Methods and Advances in the Design, Testing and Development of In Vitro Diagnostic Instruments. Processes, 11(2), 403. https://doi.org/10.3390/pr11020403







