Abstract
The ameliorative effect of medicarpin (MC) was investigated by animal behavioral experiments such as Morris water maze (MWM), Y-maze, and passive avoidance test (PAT), using scopolamine-induced cognitively impaired mice. The scopolamine (5 mg/kg), donepezil (5 mg/kg), and MC (5 and 15 mg/kg) were administered by intraperitoneal injection at a volume of 0.3 mL. In the MWM, the escape latency times of MC-treated groups were significantly decreased compared with the scopolamine-treated negative control, and times spent in the platform zone of MC-treated groups were increased dose-dependently. In the Y-maze, the zone alternations of the MC-treated group were increased to the level of the donepezil-treated positive control. In the PAT, the crossing times of MC-treated groups were significantly higher than those of the negative control with dose-dependency. On the other hand, the monoamine oxidase (MAO)-A, MAO-B, and acetylcholinesterase (AChE) activities, relating to cognitive functions, in hippocampus treated with MC were decreased. In addition, the AChE activity in SH-SY5Y cells was significantly decreased. In Western blots, phosphorylated cyclic adenosine monophosphate (cAMP) response element-binding protein (p-CREB), brain-derived neurotrophic factor (BDNF), phosphorylated protein kinase B (p-Akt), and dopamine D2 receptor (D2R) levels in the hippocampus were higher than those of the negative control. In addition, p-CREB, BDNF, p-Akt, and D2R levels in SH-SY5Y cells treated with MC were significantly increased. These results showed that MC ameliorated a cognitive function along with increased BDNF and D2R expressions, and they suggested that MC could be used for the treatment of neurological disorders such as Alzheimer’s disease and Parkinson’s disease.
1. Introduction
Alzheimer’s disease (AD) is one of the neurodegenerative diseases that mainly causes dementia [1]. According to a WHO update, the number of AD patients is expected to triple from 2010 by 2050. The incidence of AD increases by 5–8% for those aged about 65 or older, and it increases to 25–50% for those aged 85 or older [2]. Major symptoms of AD include memory and nervous loss, difficulties in speaking and problem-solving skills, and cognitive and behavioral changes [3]. Neurologically, AD is caused by the formation of nerve fiber tangles, an increase in extracellular nerve plaques, and high phosphorylation of tau proteins [4]. The aggregation of amyloid beta (Aβ) fragments is also known to be the main cause of AD [3,5]. Aβ fragments are produced by beta-site amyloid precursor protein clear enzyme 1 (β-secretase, BACE1) and γ- secretase. The Aβ fragments, consisting of 36–43 peptides, are aggregated to form insoluble oligomers. Among them, aggregated Aβ42 is a major toxic substance that delays brain cleaning and increases inflammation [6]. Therefore, BACE1 inhibitors can be used to reduce the Aβ42 formation. However, until now, studies of BACE1 inhibitors showed high failure rates for drug discovery for AD treatment [3]. Fortunately, aducanumab was reported to decrease Aβ fragment formation and was approved by the FDA in 2021 [7]. On the other hand, a reduction in cholinergic receptors was reported in AD patients, and cholinesterase (ChE) inhibitors have been attracted as candidates for AD treatment [8].
Parkinson’s disease (PD) is one of the neurodegenerative diseases, and it is characterized by motor and non-motor symptoms. PD occurs mainly in the elderly people, but it can appear in young people [9]. Typical symptoms of PD are memory loss, attention deficit, and cognitive function decline [10]. PD is caused by the necrosis of dopamine (DA) neurons located within the substance nigra, which is the brain region responsible for DA synthesis [11]. DA levels within the substantia nigra pars compacta (SNPC) neurons are maintained through the synthesis of DA, synaptic vesicle load, absorption from extracellular space, and catabolism [12]. In the cortex, DA signals are mediated by the activation of D1-like (D1 and D5) and D2-like (D2, D3, and D4) DA receptors [13]. In general, DA receptors have been reported to be associated with cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) and Ca2+ pathways through G-protein-mediated signaling. However, DA D2 receptor (D2R), the main receptor for most antipsychotic drugs, has been reported to be associated with protein kinase B (Akt)-GSK-3 (glycogen synthase kinase 3) signaling [14]. The DA action in these receptors has an important role in behavioral regulation and some cognitive functions [12]. In addition, cAMP response element-binding protein (CREB)-mediated gene damage was observed in the brain of AD patients, and the level of CREB-regulated brain-derived neurotrophic factor (BDNF) was decreased in the post-brain [15]. Furthermore, neurotransmitter disturbance can cause cognitive decline [16]. Therefore, D2R, CREB, and BDNF are mainly related to the cognitive function of the brain, and these factors have been extensively used for the study of cognitive and mood behavior changes by using an animal behavioral test [17,18,19]. On the other hand, the concentration of DA is associated with mitochondrial monoamine oxidase (MAO, EC 1.4.3.4) [20]. In addition, the cognitive impairment of PD is associated with neurotransmitter deficiency, especially cholinergic deficiency [21]. For example, neurocellular damage in nuclear bases and decreased cholinergic receptors were reported in PD patients [22,23].
In these respects, ChE and MAO enzymes are important in AD and PD patients. The ChE contains two types, acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). AChE specifically hydrolyzes acetylcholine (ACh), and BChE non-specifically hydrolyzes ACh and butyrylcholine (BCh), preferring BCh [24]. AChE shows therapeutic effects by increasing the concentration of ACh in the cerebral cortex of AD patients. BChE is also used in AD treatment as a serine hydrolysis enzyme that affects the hydrolysis of Ach [8,25]. ACh is the main neurotransmitter of the brain, functions in both the central and peripheral nervous systems, and is reduced by AChE to cause AD. Therefore, ChE inhibitors have been studied as important tools for the treatment of AD [8]. Tacrine was used as the first AChE inhibitor of AD treatment, but it has been withdrawn due to its severe hepatotoxicity [26]. Since then, donepezil, rivastigmine, and galantamine have been used as second-generation AChE inhibitors [25]. In addition, the N-methyl-D-aspartic acid (NMDA) antagonist memantin is also used to treat severe AD [27]. As for MAO, it exists in two forms, namely MAO-A and MAO-B, in the mitochondrial outer membrane [28]. In fact, selective MAO-A inhibitors have been used for antidepressant treatments, and selective MAO-B inhibitors have been targeted for AD and PD treatments. In addition, MAO was also reported to be associated with the aggregations of amyloid plaques, which is a major cause of AD [29].
Furthermore, a multi-targeted treatment has been developed to target MAO-B and AChE simultaneously, and it has been reported that multi-target inhibitors can increase both monoamine and choline ester levels, thereby improving the cognitive function of AD and alleviating symptoms [30,31,32,33].
Canavalia lineata (Thunb.) DC. is a vine plant living in Korea, Japan, China, and Taiwan, and its aged beans contain bioactive compounds. C. lineata has also been reported as a cause of miscarriage and traditionally has been used to prevent pregnancy [34,35]. In a previous study, we found that medicarpin (MC) and homopterocarpin isolated from C. lineata showed potent hMAO-B inhibition [36]. MC is a pterocarpan, which is a derivative of isoflavonoids. In other studies, MC was isolated from Pueraia lobata [37], Medicago truncatula [38], Abrus precatorius [39], Butea monosperma [40], roots of Ononis angustissima L. [41], Robinia pseudoacacia L. [42], Radix hedysari [43], and other traditional Chinese herbs [44]. In addition, MC has been reported for various physiological activities such as anti-inflammatory [45], anti-cancer [46], and antioxidant [42]. Recently, the neuroprotective effect of MC on AD with its therapeutic mechanisms was explored by using network proximity prediction and Western blotting for GSK-3β and MAPK14 [47], and the ameliorative effect of brain injury [44] and anti-depression effect [48] by MC were reported.
In this study, we investigated the molecular biological effects of MC including animal behavioral tests such as Morris water maize (MWM), Y-maize, and passive avoidance test (PAT). Specifically, first, the cognitive and memory function improvements were evaluated using scopolamine-induced mice treated with MC. Second, signaling pathways were analyzed in mouse hippocampus and neuroblastoma cells (SH-SY5Y cells) through protein expression using Western blotting for BDNF, D2R, Akt, and CREB. Third, MAO and AChE activities were analyzed for the samples.
2. Materials and Methods
2.1. Animals and Administration
Naïve male Institute of Cancer Research (ICR) mice (n = 8 per group; age, 5 weeks; weight, 25–30 g) were supplied by ORIENT BIO (Seongnam, Republic of Korea). Animals were housed in standard conditions: temperature at 25 ± 2 °C, relative humidity at 60 ± 10%, under a 12 h light (07:00–19:00)/12 h dark cycle [49,50]. Animals were randomly divided into 5 groups: control, negative (5 mg/kg scopolamine, Scop); positive (Scop + 5 mg/kg donepezil), low-dose MC (Scop + 5 mg/kg MC), and high-dose MC (Scop + 15 mg/kg MC). The saline, scopolamine, donepezil, and MC were administered by intraperitoneal injection (i.p.) at a volume of 0.3 mL. Experimental procedures for animals were performed according to protocols approved by the Sunchon National University Institutional Animal Care and Use Committee (SCNU IACUC, permit number: SCNU IACUC-2022-22) and the guidelines for the care and use of laboratory animals.
2.2. Chemicals and Enzymes
Donepezil, scopolamine, dimethyl sulfoxide (DMSO), acetylthiocholine iodide (ATCI), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), benzylamine, kynuramine, glycine, sodium dodecyl sulfate (SDS), and trizma base were purchased from Sigma-Aldrich (St. Louis, MO, USA). MC was purchased from ChemFaces (CFN98411, Wuhan, China). Phosphate-buffered saline (PBS), radioimmunoprecipitation (RIPA) lysis and extraction buffer, nitrocellulose membranes, tris-buffered saline containing 20% tween 20 (TBS-T), primary antibodies such as BDNF, CREB, p-CREB, D2R, Akt, p-Akt, horseradish-peroxidase-conjugated goat anti-rabbit IgG (H+L) secondary antibody, and chemiluminescence kit were purchased from Thermo Fisher Scientific Inc. (Rockford, IL, USA). Skim milk for membrane blocking was purchased from Becton Dickinson and company (Franklin lakes, New jersey, USA). All other chemicals were of reagent grade.
2.3. Animal Behavioral Experiments
2.3.1. Morris Water Maze (MWM)
An MWM test was performed in a circular pool (60 cm in diameter), and mice were trained to escape from the pool by arriving on a hidden platform (7 cm in diameter) as described previously [47,51,52] with slight modification. The pool was separated into quarters and filled with water until a platform in the pool was submerged 1 cm below the surface. The test was performed for 7 days. From the 1st to the 6th day, the mice learned to find to a hidden platform in the pool for 90 s, and the arrival time and the movement path to the hidden platform were recorded. If the mice arrived at the hidden platform and stayed for 5 s, they are judged to have escaped from the pool. On the 7th day, the mice were tested in the pool, where the hidden platform was removed and the time spent in the target zone was recorded.
2.3.2. Passive Avoidance Test (PAT)
The PAT was carried out using the GEMINI avoidance system (Gemini, San Diego, CA, USA), consisting of two rooms and equipped with a device capable of giving an electric shock to the floor as described previously [53,54] with slight modification. On the first day of training, the mice were placed in a separate dark room and adapted for 10 s. Then, the separation door was opened, and the light at the starting room was turned on, and then, the time of movement to the opposite dark room was recorded. When the mice moved the opposite room, the electric foot shock stress of five seconds was given to them. On the second days, the same procedure was applied except for no electric shock stress. When the mice did not move to the opposite room for 3 min, the mice had escaped from the test instrument.
2.3.3. Y-Maze
The memory of the mice was measured using a Y-maze instrument (arm length: 36 cm, bottom width: 5 cm, and arm height: 13.5 cm) as described previously [55,56] with slight modification. The mice were located at the center point, and the total numbers and alternations in each arm were recorded for 8 min. The memory was evaluated as correct alterations/total arm entries.
2.4. Enzyme Assays
The hippocampus of collected experimental animals was analyzed using a RIPA lysis and extraction buffer to measure MAO-A, MAO-B, and AChE enzyme activities [57,58]. MAO-A and MAO-B activities were determined using kynuramine and benzylamine, respectively [57]. AChE activity was assayed using 0.5 mM ATCI and color reagent (DTNB) [58]. All absorbance measurements were continuously assayed.
2.5. Cell Culture
SH-SY5Y (neuroblastoma) cells were cultured in MEM and supplemented with heat-inactivated 10% FBS, 100 U/mL of penicillin/streptomycin solution, and 50 μM of 2-mercaptoethanol in a humidified atmosphere at 37 °C with 5% CO2, and media was changed when it was 80% confluent every 2 days, using 75 cm2 T-flask [59]. The passage numbers used were 10 to 30. Following trypsinization, SH-SY5Y cells were treated with 3 and 10 µM of MC. SH-SY5Y cells were obtained from the Korean Cell Line Bank (Seoul, Republic of Korea).
2.6. Western Blotting
The proteins were separated using 11% SDS polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked in TBS-T containing 5% skim milk for 2 h at room temperature and attached at 4 °C overnight with the primary antibodies as follows: CREB (#701120, 1:2500), p-CREB (#44-298G, 1:1000), BDNF (#MA5-31039, 1:500), Akt (#4691, 1:1000), p-Akt (#4060, 1:2000), D2R (D2R-212AP, 1:1000), and beta-actin (#PA1-183, 1:500). The membrane with the primary antibody was mixed with the horseradish-peroxidase-conjugated goat anti-rabbit IgG (H+L) secondary antibody (#31460, 1:10,000) for 1 h at room temperature [50]. The membrane was detected using MicroChemi 4.2 (DNR Bio-Imaging Systems Ltd., Neve Yamin, Israel) with the chemiluminescence solution.
2.7. Statistical Analysis
An analysis of variance (ANOVA) test was used to determine the significances of differences between protein expression levels in control, negative control (scopolamine), positive control (scopolamine + donepezil), and two dose-dependent groups (low and high concentration of MC) using IBM SPSS Statistics 27 (IBM Corporation, Armonk, NY, USA) [49]. ANOVA was applied to analyze differences for the parameters between each group, which was followed by Bonferroni’s significant post hoc test for multiple comparisons. Probability values less than 0.05 were considered significant.
3. Results
3.1. Animal Behavioral Test
3.1.1. Animal Experiment Plan
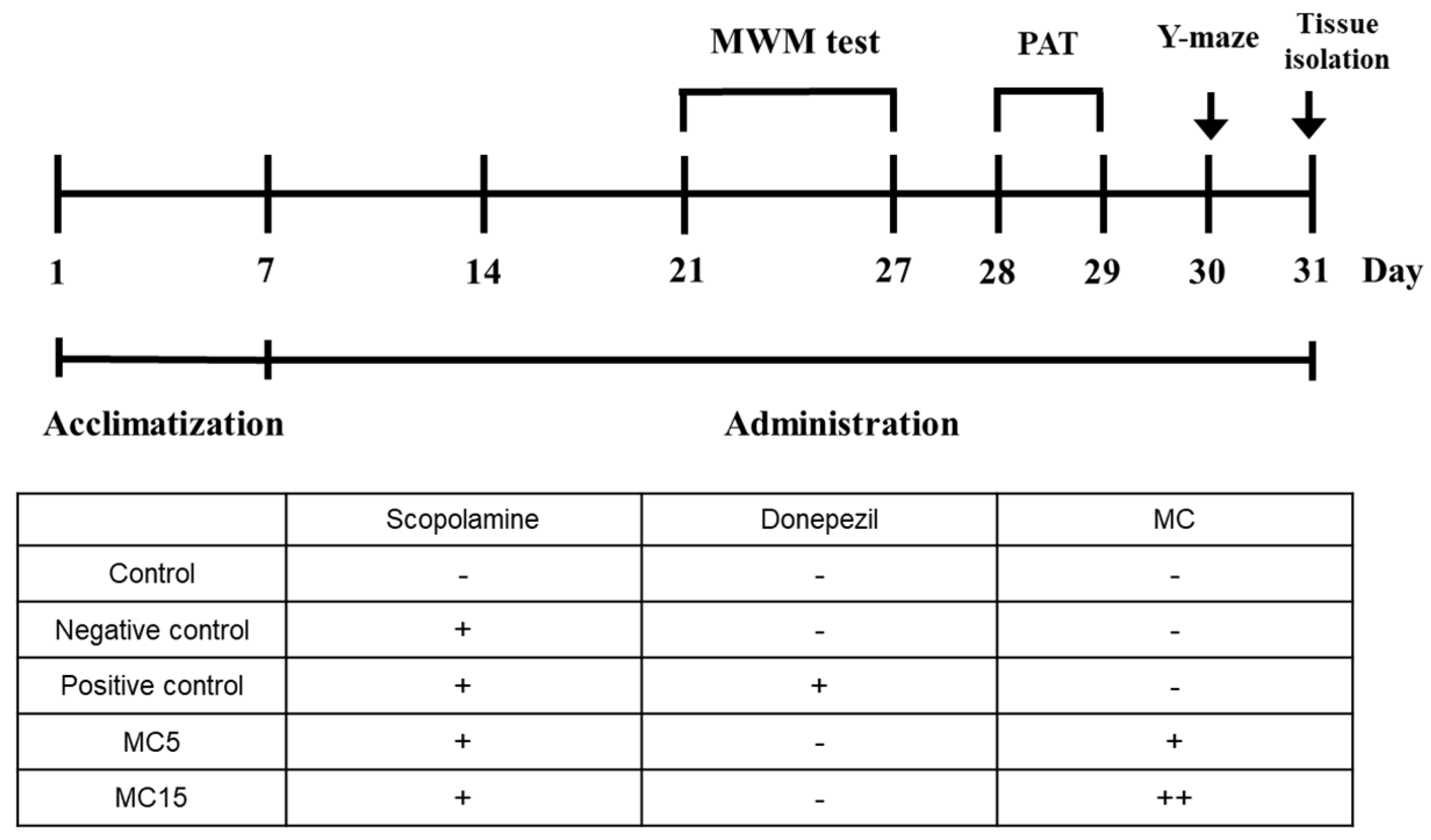
After acclimatization of the mice for 1 week, scopolamine was treated to the end of 31st day for negative control, and the donepezil and MC were also administered for positive control and dosed groups, respectively. Animal behavioral experiments of MWM, PAT, and Y-maze were performed for 7, 2, and 1 day(s), respectively, after the drug treatments for two weeks. After the completion of animal behavioral experiments, the mice were sacrificed, and hippocampus tissues were isolated (Figure 1).
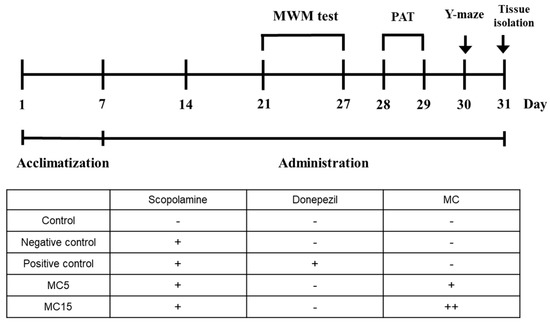
Figure 1.
The schedule of animal behavioral experiments. MWM, Morris water maze; PAT, passive avoidance test. The injection volume used was 300 μL. Scopolamine and donepezil were used at 5 mg/kg concentration. MC was used at 5 mg/kg (MC5) and 15 mg/kg (MC15). All solutions were made with PBS.
3.1.2. MWM
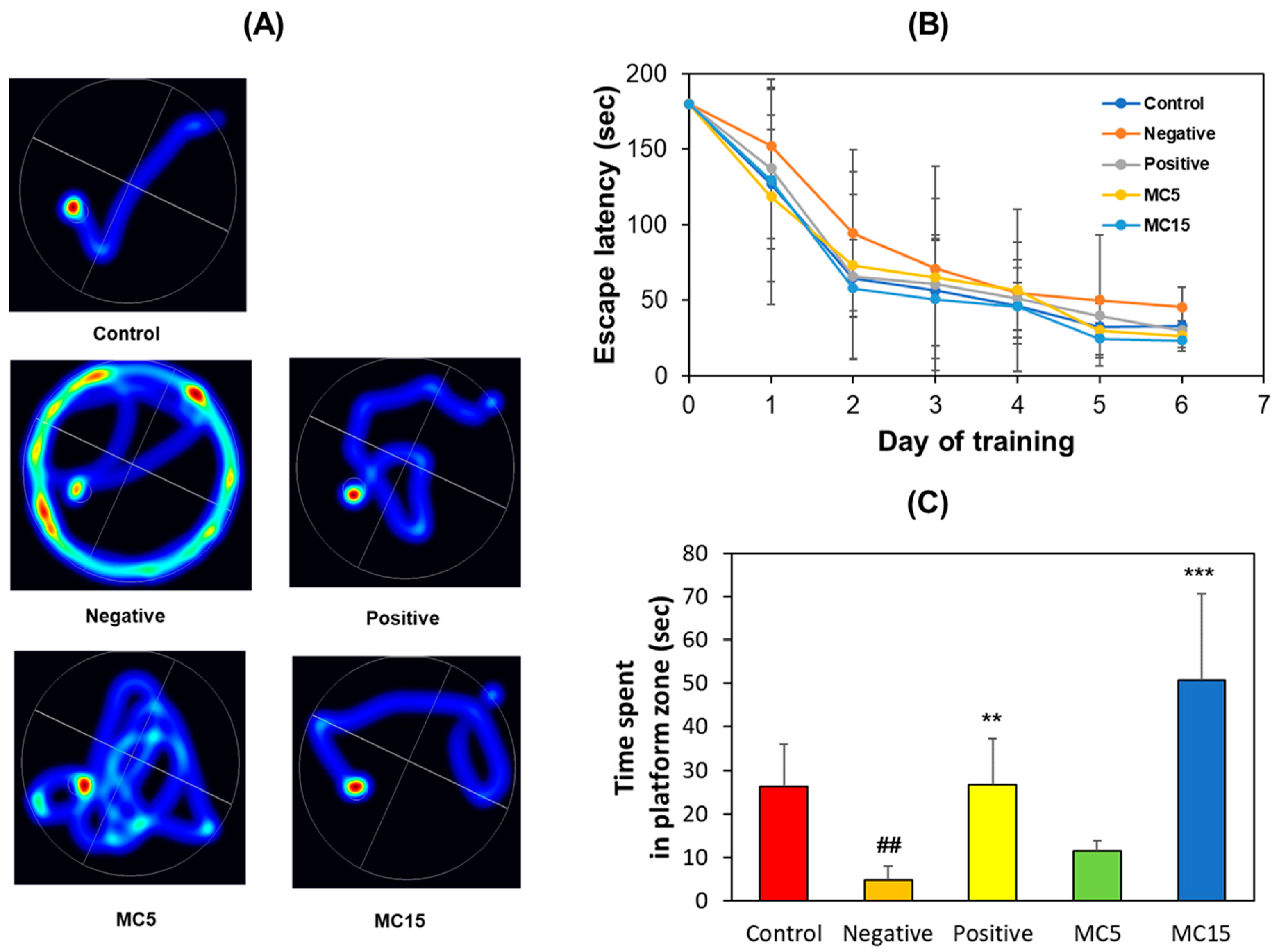
An MWM test was performed on scopolamine-induced mice to evaluate memory deficiency and cognitive improvement effect of MC using two doses, i.e., MC5 and MC15 groups treated with 5 and 15 mg/kg, respectively. The scopolamine-treated group (model mice, negative control) showed an aimless swimming pathway, but the donepezil-treated group (5 mg/kg, positive control) showed a decrease in travel distance, and also, the MC-treated groups showed a concentration-dependent decrease in travel distance (Figure 2A). In addition, the scopolamine-treated group showed a long escape time as opposed to the donepezil- and MC-treated groups (Figure 2B). On the 7th day of the experiment, when each mouse performed free swimming in the pool in which the platform was removed, the swimming time in the target zone, i.e., the platform zone, was long for the donepezil-treated group and for the MC15 group, but it was short for the scopolamine-treated group and the MC5 group (Figure 2C). These results suggest that the high concentration of MC in MC15 restored the cognitive function of mice to the level of the donepezil-treated group.
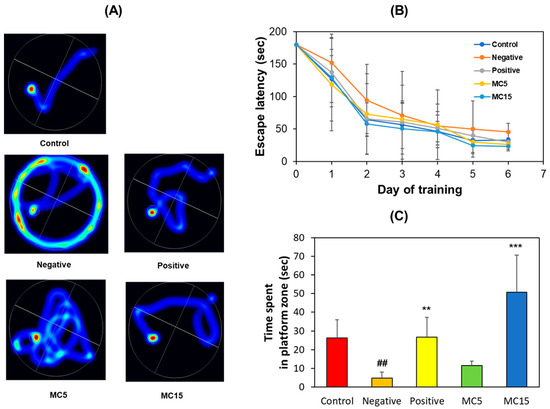
Figure 2.
Recovery of long-term memory by MC on MWM test. (A) Swimming paths; (B) Escape latency for 6 consecutive days; (C) Time spent in platform zone in which the platform was removed. Data are presented as mean ± SEM. ## p < 0.01, compared to the control group; ** p < 0.01 and *** p < 0.001, compared to the scopolamine-treated group (n = 8).
3.1.3. Y-Maze and PAT
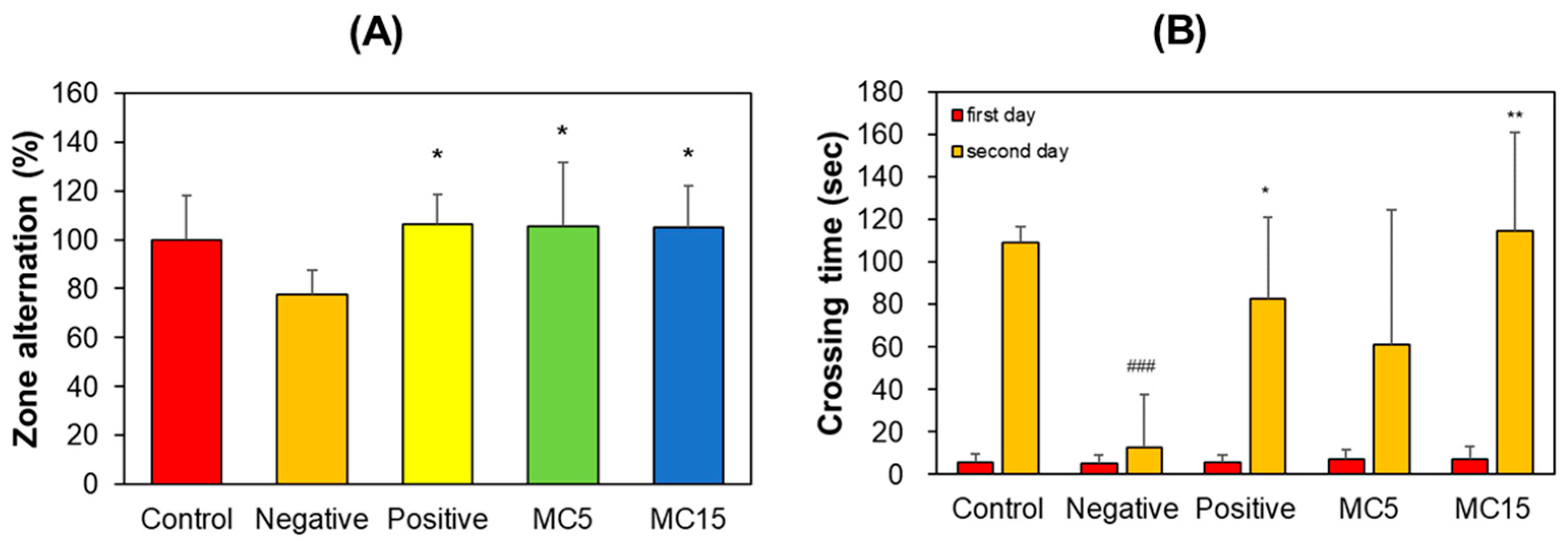
The scopolamine-treated group showed a decrease in correct zone alternation in the Y-maze teat; however, MC5 and MC15 showed restored values in zone alternation to the level of the donepezil-treated group (Figure 3A). In PAT, the crossing times of all five groups on the first day were low and very similar to each other. However, that of the control on the second day increased to 109 s, but that of the scopolamine-treated negative control decreased to 13 s. Effectively, MC5 and MC15 showed restored crossing times to the level of the donepezil-treated positive control (Figure 3B). In addition, the recovery degrees of MC5 and MC15 were concentration-dependent (Figure 3B).
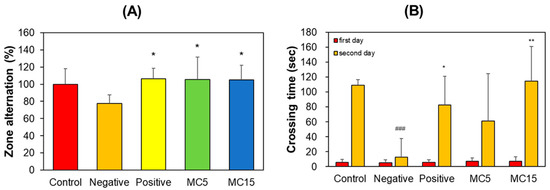
Figure 3.
Effect of MC on short-term memory function. (A) Y-maze test; (B) PAT. Zone alternation and crossing time were measured by Y-maze and PAT, respectively. Data are presented as mean ± SEM. ### p < 0.001, compared to the control group; * p < 0.05 and ** p < 0.01, compared to the scopolamine-treated group (n = 8).
3.2. Enzyme Assays in Hippocampus Tissues
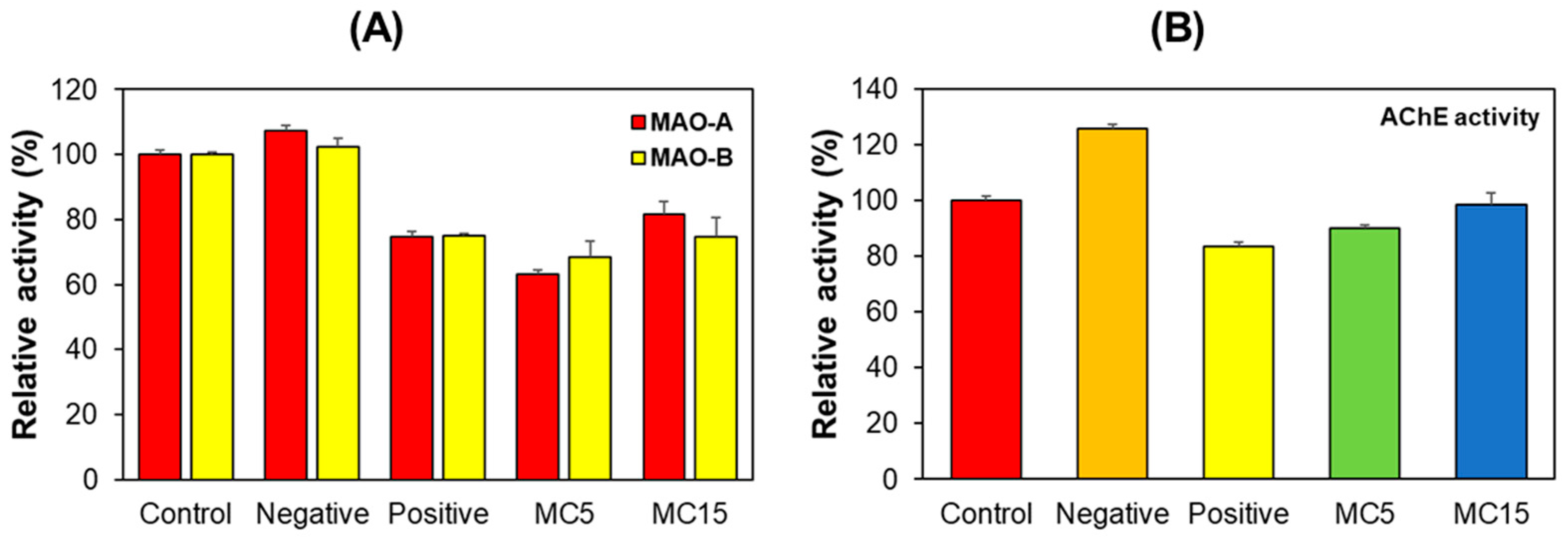
MAO-A, MAO-B, and AChE activities were measured for hippocampus tissues collected after the behavioral experiments. The negative control group showed similar MAO-A and MAO-B activities to the control group. However, MAO-A and MAO-B activities in the positive control and MC5 or MC15 groups were lower than those in the control and the negative control (Figure 4A). On the other hand, in the case of AChE, the negative control group showed higher activity than the control group, but the MC5 or MC15 groups showed similar activity to the positive control (Figure 4B). These results suggested that MC was involved in MAOs and AChE activities, which regulate neurotransmitter concentrations in mice hippocampus tissues.
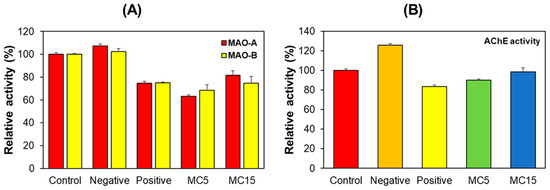
Figure 4.
Effect of MC on MAO-A, MAO-B, and AChE activities relating to neurotransmitters in hippocampus tissues. Results are expressed as mean ± SEM from triplicate experiments. (A) MAO-A and MAO-B activities; (B) AChE activity.
3.3. Enzyme Assays in SH-SY5Y Cells
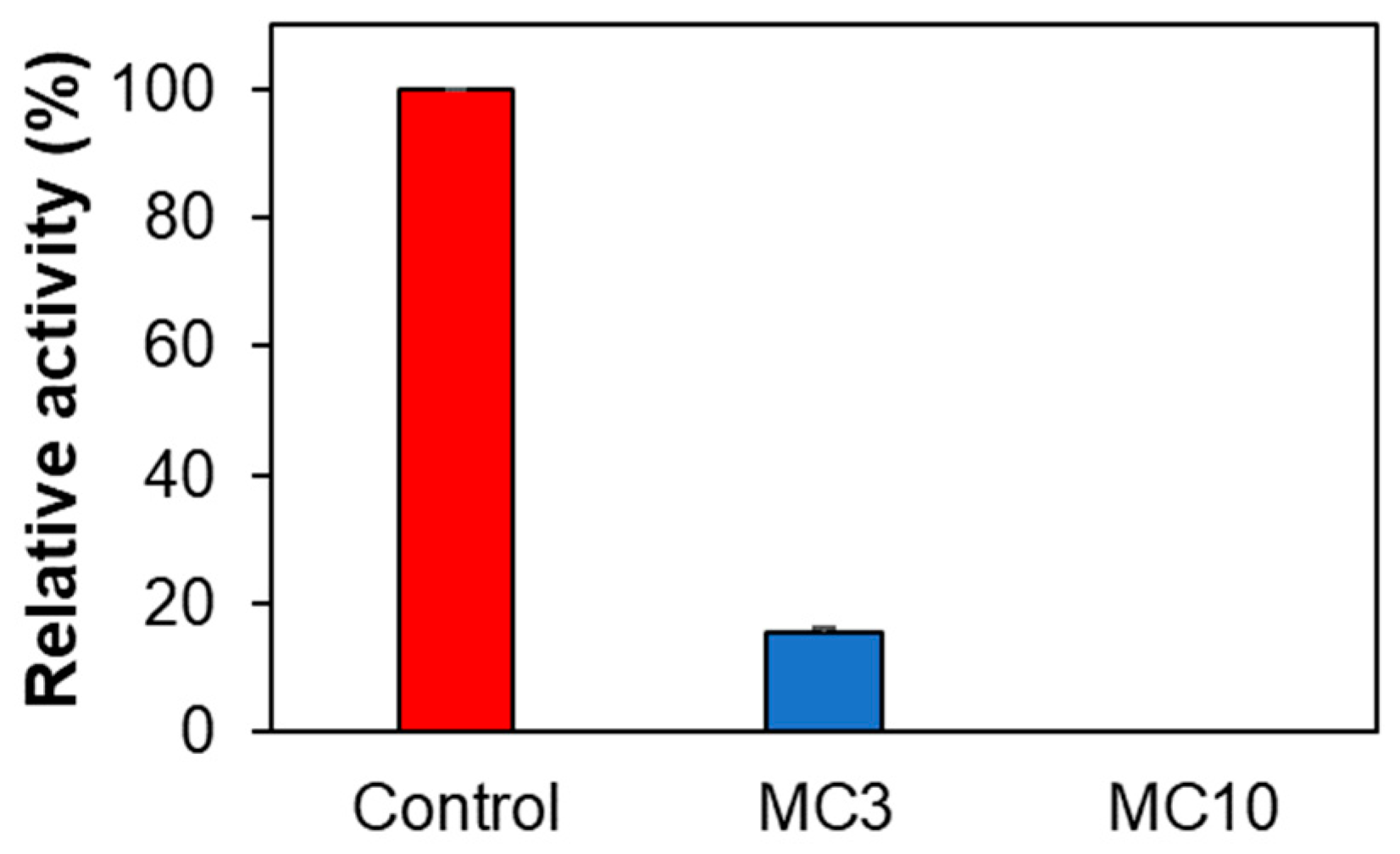
When SH-SY5Y cells were treated with low concentrations of MC (i.e., MC3), AChE activity was significantly reduced to 15.5%, and when it was treated with high concentrations of MC (i.e., MC10), AChE activity was not detected (Figure 5). These results suggested that MC could inhibit AChE in SH-SY5Y cells, although it weakly inhibited AChE in enzyme and inhibitor assay (19.4% inhibition at 10 µM of MC) [36]. MAO-A and MAO-B activities for control, MC3, and MC10 were not detected in SH-SY5Y cells.
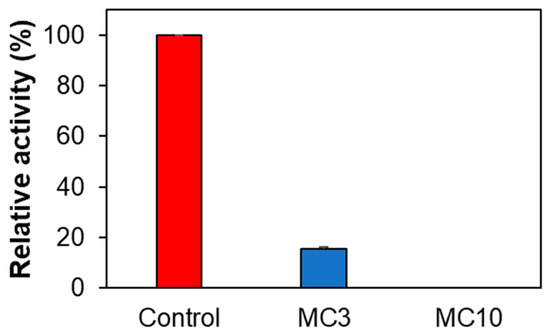
Figure 5.
Effect of MC on AChE activity in SH-SY5Y cells. Results are expressed as mean ± SEM from triplicate experiments. MC3, 3 µM; MC10, 10 µM of MC.
3.4. Western Blotting of Hippocampus Tissues and SH-SY5Y Cells
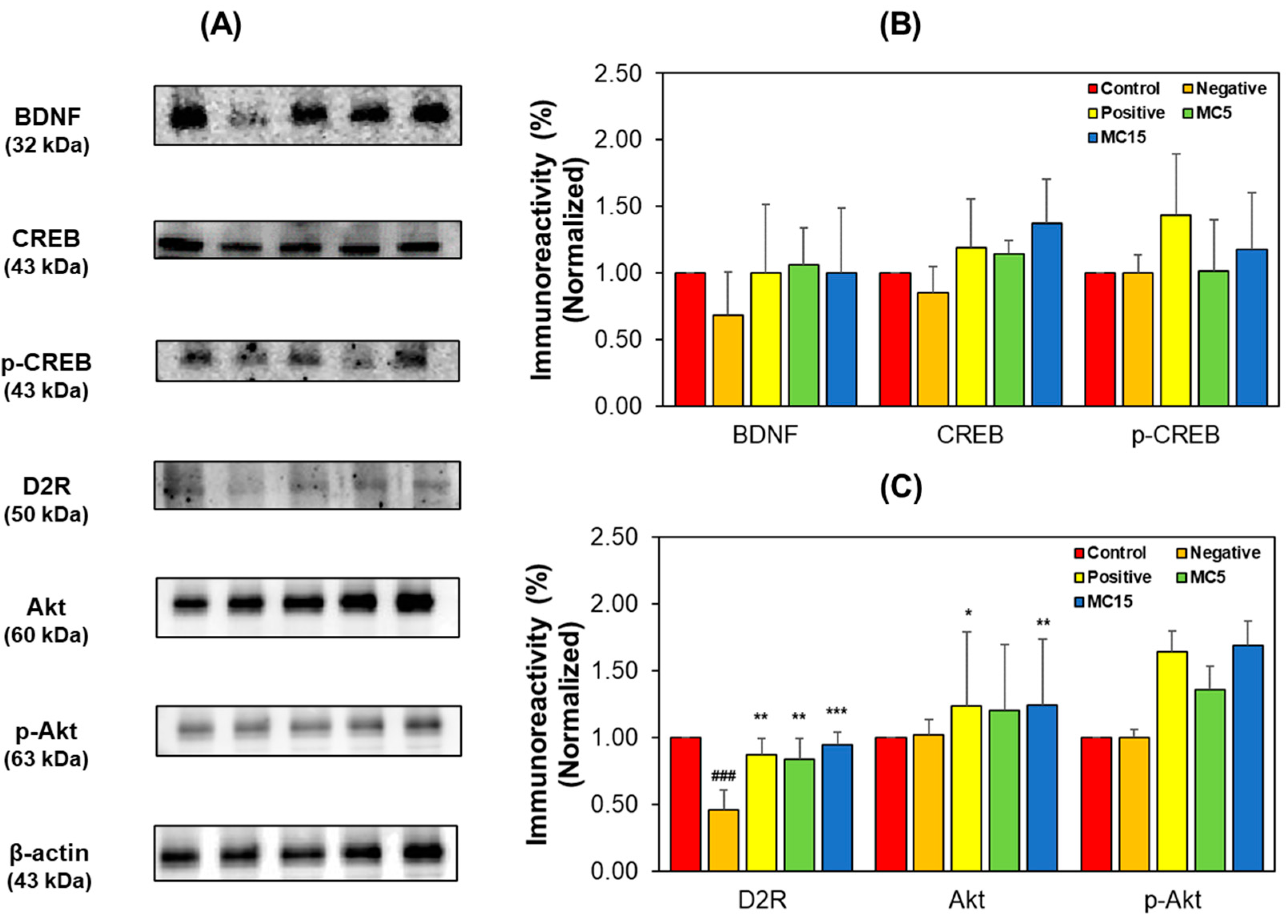
Factors related to cognitive function and DA receptor were analyzed through Western blotting for hippocampus tissues and SH-SY5Y cells by measuring, the expression levels of BDNF-CREB and D2R signaling pathway factors. In hippocampus tissues, the expression levels of BDNF, CREB, and p-CREB in the MC-treated groups increased to the levels of the donepezil-treated positive group (Figure 6A,B). BDNF and CREB levels decreased in the scopolamine-treated negative group, however, the p-CREB level of the negative group was not lower than that of the control—instead, it was observed to be lower than that of the positive group. Specially, p-CREB expression in the MC-treated groups showed a dose-dependent tendency. Additionally, in the D2R signaling pathway, the expression level of D2R decreased in the negative group; however, the D2R level in the MC-treated group were also restored to the levels in the positive group (Figure 6A,C). The Akt and p-Akt levels of the negative groups were not lower than those of the control groups; instead, those were observed to be lower than those of the positive group.
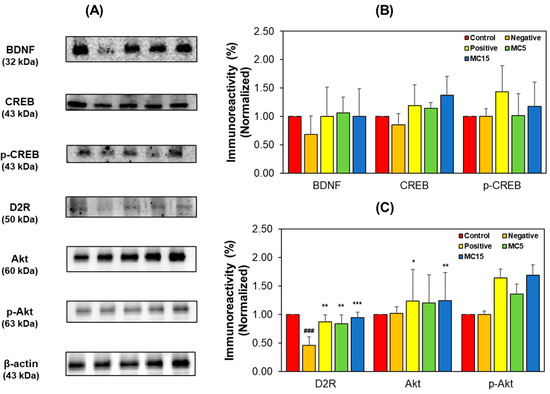
Figure 6.
Western blotting of CREB, p-CREB, BDNF, Akt, p-Akt, and D2R in the hippocampus tissues. Western blots (A), and levels of immunoreactivities for BDNF, CREB, and p-CREB (B) and for D2R, Akt, and pAkt (C). C, control; N, negative control (scopolamine-treated); P, positive control (scopolamine + donepezil treated); MC5 and MC15, doses of the compound MC (scopolamine + 5 and 15 mg/kg of MC, respectively). Data are presented as mean ± SEM. ### p < 0.001, compared to the control group; * p < 0.05, ** p < 0.01 and *** p < 0.001, compared to the scopolamine-treated group.
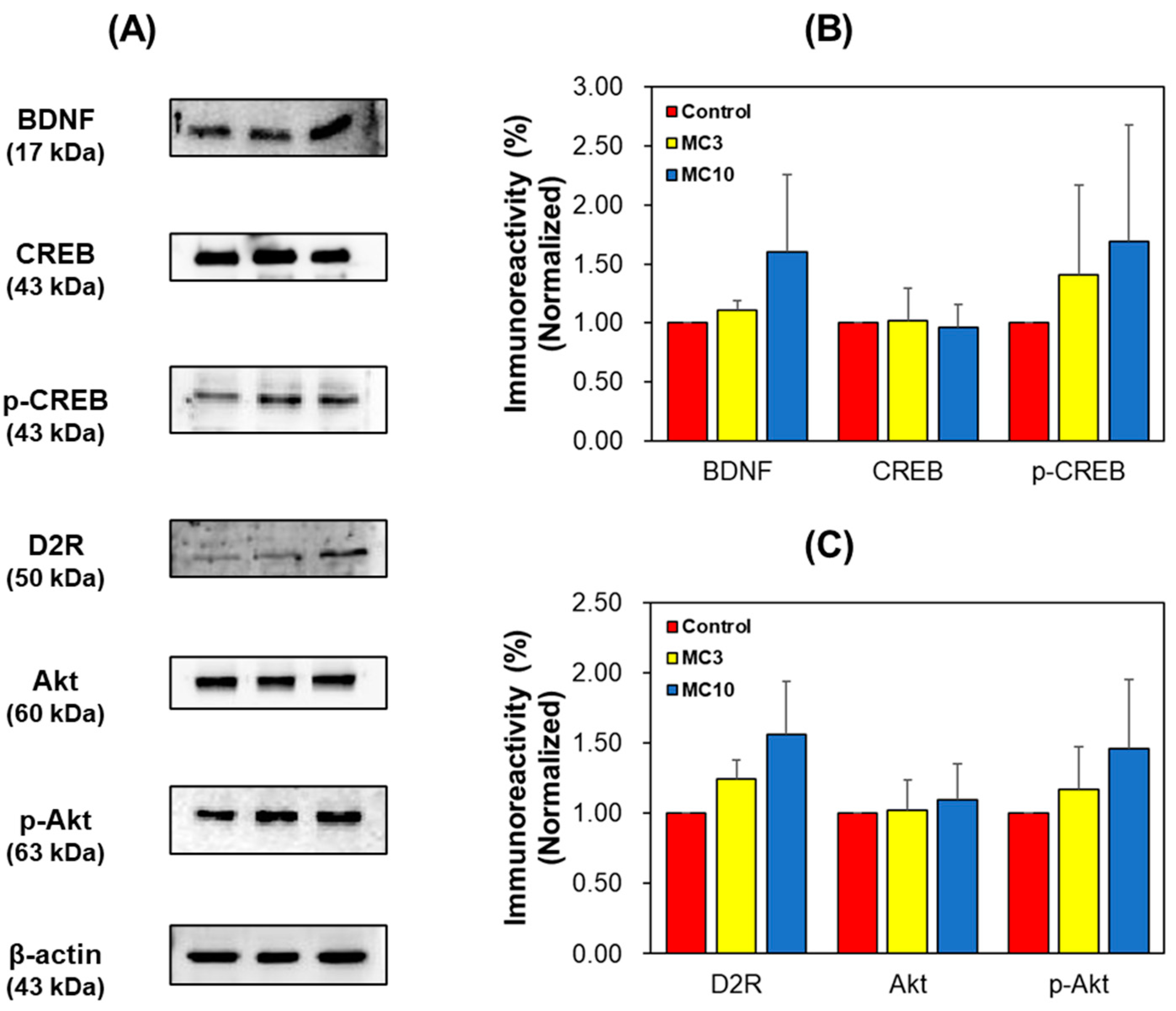
In SH-SY5Y cells, the expression levels of BDNF, p-CREB, D2R, and p-Akt in the MC-treated groups increased dose-dependently (Figure 7). These results showed that BDNF and D2R levels reduced by scopolamine treatment were recovered by MC administration to the similar to or higher levels than the levels by donepezil treatment. In addition, CREB and Akt were activated by BDNF and D2R.
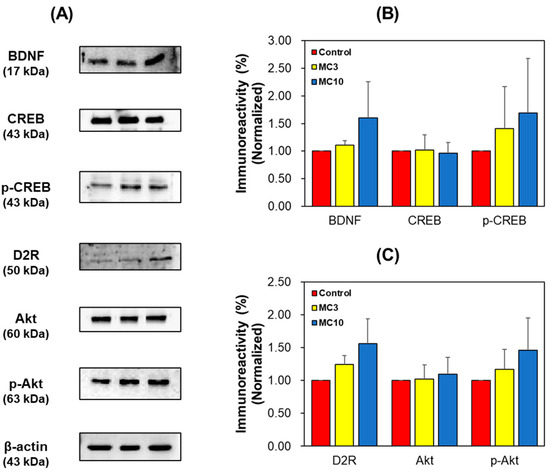
Figure 7.
Western blotting of CREB, p-CREB, BDNF, Akt, p-Akt, and D2R in the SH-SY5Y cells. Western blots (A), and levels of immunoreactivities for BDNF, CREB, and p-CREB (B) and for D2R, Akt, and pAkt (C). C, control; MC3 and MC10, doses of the compound MC (scopolamine + 3 and 10 μM of MC, respectively). Data are presented as mean ± SEM.
These results suggested that MC increased the levels of those factors in hippocampus tissues and SH-SY5Y cells and might contribute to amelioration of the cognitive impairment.
4. Discussion
In this study, the potential therapeutic effect of MC on AD and PD was evaluated through animal behavioral experiments and brain hippocampus tissue analysis. In addition, changes in enzymes and proteins related to neurotransmission in accordance with MC administration were evaluated by using enzyme assays and Western blotting.
In the animal behavioral experiments, the scopolamine-treated negative control group showed an increase in aimless movement in MWM, which is used to evaluate long-term memory, but the MC-administered group showed recovered values to the values of the donepezil-treated positive control group in a dose-dependent manner. This result was similar to the observation in another study previously reported [47]. Additionally, we found that the MC group recovered short-term memory similar to the donepezil-treated positive group, and especially, dose-dependence recovery was observed in PAT. These results indicate that MC is involved in both long-term and short-term memory based on Y-maze and PAT results for short-term memory improvement and MWM results for long-term memory improvement.
In previous study, it was reported that MC affected the cholinergic system, neuronal apoptosis, and synaptic function using the experiments such as AChE assay, GSK-3β and MAPK14 blotting and an in silico network-based approach [47]. In this study, we carried out other experiments such as measurements of MAO-A, MAO-B, and AChE activities in mouse brain hippocampus tissue and SH-SY5Y cells. In addition, we analyzed the expression levels of BDNF and D2R related to cognitive function through the signaling pathway via Western blot. In this study, MAO-A, MAO-B, and AChE were reduced in the mouse hippocampus when MC was administered. AChE was also significantly reduced in SH-SY5Y cells when treated with MC. Interestingly, it was observed that MC potently inhibited MAO-B (95.83% at 10 µM of MC, IC50 = 0.45 µM); however, it weakly inhibited AChE (19.4% inhibition at 10 µM of MC) when assayed using respective purified enzymes [36]. These results might suggest that MAO-B and AChE can interact in hippocampus tissues during the binding of MC to the enzymes and AChE can be inhibited more than expected with in vitro experiment; concentrations of neurotransmitters by MAO or AChE can be adjusted along with the choline receptor.
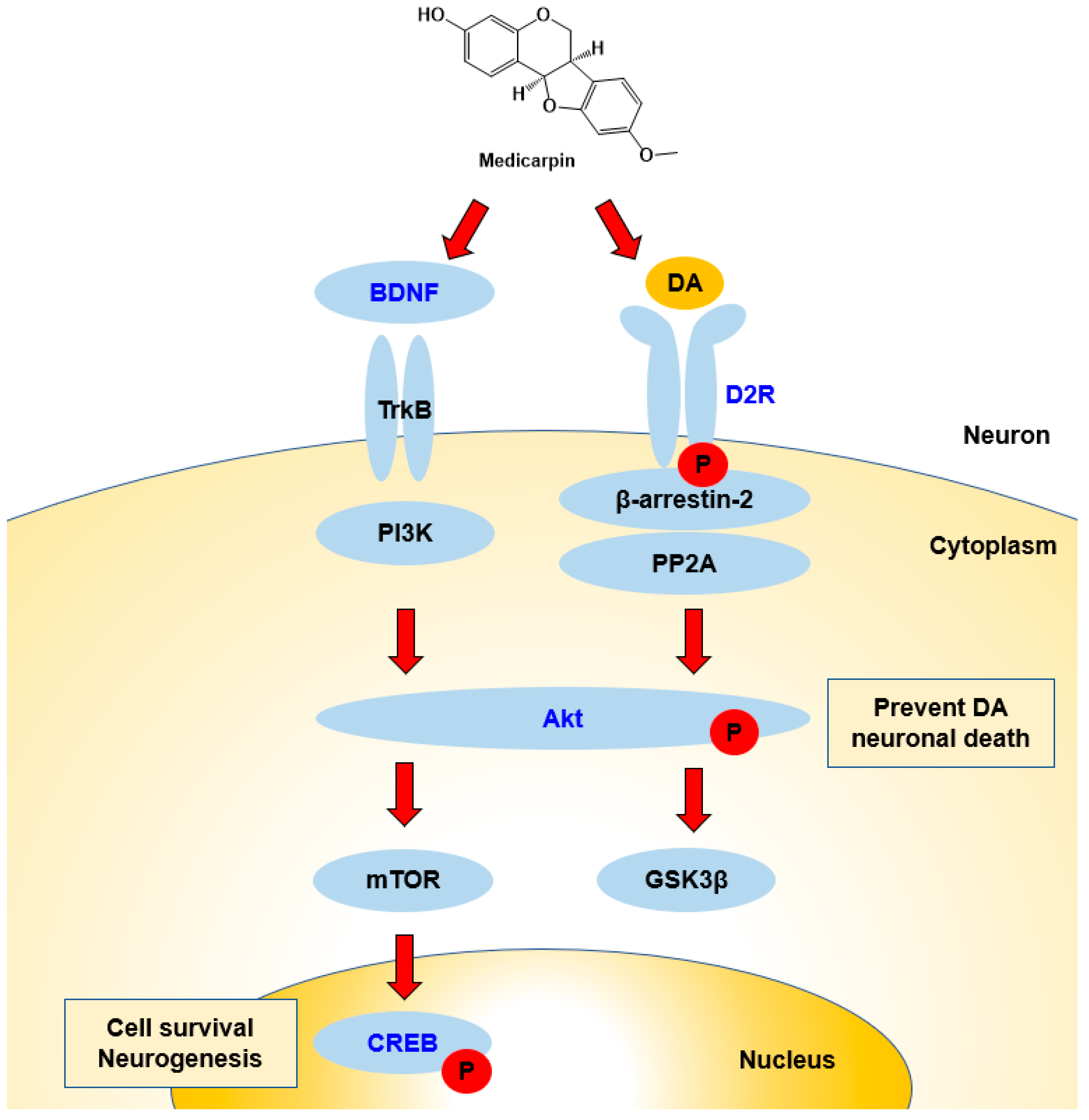
In Western blotting, MC increased BDNF and D2R expression levels as well as the phosphorylation of CREB and/or Akt proteins. This increased BDNF expression was similar to the observation in other studies previously reported [44,47]. From these results, it can be proposed that the signaling pathways proceed via Akt and CREB proteins (Figure 8). Increased BDNF expressions have been observed when various chemicals or extracts were treated [49,60]. D2R is the main receptor for most antipsychotic drugs and is associated with Akt and GSK-3 signaling [14]. In this study, levels of BDNF and D2R increased with MC administration, and these results suggest that MC can be used for the treatments of AD and PD through cognitive function improvement and dopamine receptor expression.
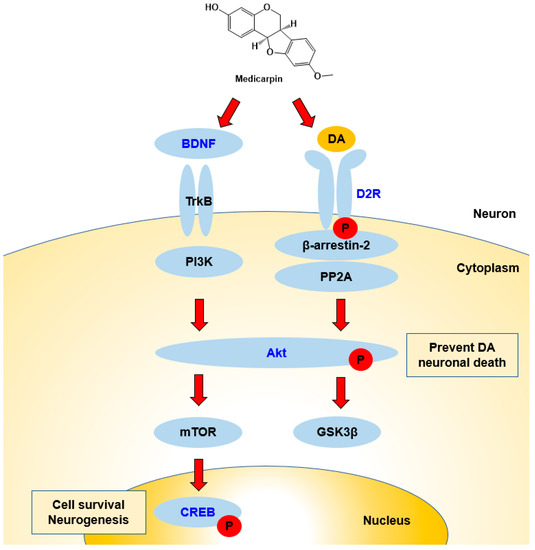
Figure 8.
Possible responses of BDNF/Akt/CREB and D2R signaling pathways during interactions with MC. BDNF activates Akt, and next, Akt activates CREB, which is related to cell survival and neurogenesis. D2R activates the Akt–GSK3 pathway, and the activation of Akt prevents DA neuronal death. Names in blue color represent the factors tested in this study.
Collectively, our results showed that MC can be used for the treatment of AD by improving cognitive function, such as long-term and short-term memories. In addition, the increased expressions of BDNF and D2R through increased levels of p-CREB and p-Akt in the mouse hippocampus and neuron cells showed improved signaling cognitive function. It is interesting that MC showed potent MAO-B inhibition with weak AChE inhibition in the cuvette assays; however, it showed extreme AChE inhibition in the cells and the in vivo experiments. These findings suggest that MC can be a promising candidate agent for the treatment of PD as well as AD.
5. Conclusions
MC significantly ameliorated long-term memory in a MWM experiment using scopolamine-induced cognitively impaired mice. In addition, short-term memory in Y-maze and PAT was significantly recovered. In hippocampus tissues, MAO-A, MAO-B, and AChE activities were decreased when MC was administered. AChE activity was also decreased in SH-SY5Y cells. Western blots of proteins in hippocampus tissues showed that expression levels of p-CREB, BDNF, p-Akt, and D2R were increased. In SH-SY5Y cells, expression levels of the four factors were also increased. These results suggest that MC can be used to treat AD and PD through improved cognitive and memory function and increased expression of the DA receptor.
Author Contributions
Conceptualization, H.K.; animal behavioral experiments, J.M.O. and J.E.P.; enzyme assay, J.M.O.; cell culture, S.-K.M.; Western blot, J.M.O.; data curation, J.M.O.; writing—original draft preparation, J.M.O.; writing—review and editing, H.K.; supervision, S.-T.Y. and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by of the Sunchon National University Promotion Project, 2022.
Institutional Review Board Statement
The animal study protocol was approved by the Sunchon National University Institutional Animal Care and Use Committee (SCNU IACUC, permit number: SCNU IACUC-2022-22).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Se Thoe, E.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Moussa-Pacha, N.M.; Abdin, S.M.; Omar, H.A.; Alniss, H.; Al-Tel, T.H. BACE1 inhibitors: Current status and future directions in treating Alzheimer’s disease. Med. Res. Rev. 2020, 40, 339–384. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Dubey, R. Target enzyme in Alzheimer’s disease: Acetylcholinesterase inhibitors. Curr. Top Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Ali, S.; Asad, M.H.H.B.; Maity, S.; Zada, W.; Rizvanov, A.A.; Iqbal, J.; Babak, B.; Hussain, I. Fluoro-benzimidazole derivatives to cure Alzheimer’s disease: In-silico studies, synthesis, structure-activity relationship and in vivo evaluation for β secretase enzyme inhibition. Bioorg. Chem. 2019, 88, 102936. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Anand, P.; Singh, B. A Review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Marino, B.L.B.; de Souza, L.R.; Sousa, K.P.A.; Ferreira, J.V.; Padilha, E.C.; da Silva, C.H.T.P.; Taft, C.A.; Hage-Melim, L.I.S. Parkinson’s disease: A review from pathophysiology to treatment. Mini Rev. Med. Chem. 2020, 20, 754–767. [Google Scholar] [CrossRef]
- Masato, A.; Plotegher, N.; Boassa, D.; Bubacco, L. Impaired dopamine metabolism in Parkinson’s disease pathogenesis. Mol. Neurodegener. 2019, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.-M.; Gainetdinov, R.R.; Caron, M.G. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol. Sci. 2007, 28, 166–172. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331–341. [Google Scholar]
- Yan, L.; Xu, X.; He, Z.; Wang, S.; Zhao, L.; Qiu, J.; Wang, D.; Gong, Z.; Qiu, X.; Huang, H. Antidepressant-like effects and cognitive enhancement of coadministration of chaihu shugan san and fluoxetine: Dependent on the BDNF-ERK-CREB signaling pathway in the hippocampus and frontal cortex. Biomed. Res. Int. 2020, 2020, 2794263. [Google Scholar] [CrossRef]
- Lian, W.-W.; Zhou, W.; Zhang, B.-Y.; Jia, H.; Xu, L.-J.; Liu, A.-L.; Du, G.-H. DL0410 ameliorates cognitive disorder in SAMP8 mice by promoting mitochondrial dynamics and the NMDAR-CREB-BDNF pathway. Acta Pharmacol. Sin. 2021, 42, 1055–1068. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Y.; Fang, M.-S.; Li, Y.; Li, W.; Mahaman, Y.A.R.; Zeng, K.; Xia, Y.; Ke, D.; Liu, R.; et al. ω-3PUFAs improve cognitive impairments through Ser133 phosphorylation of CREB upregulating BDNF/TrkB signal in Schizophrenia. Neurotherapeutics 2020, 17, 1271–1286. [Google Scholar] [CrossRef]
- Calabresi, P.; Picconi, B.; Parnetti, L.; Di Filippo, M. A convergent model for cognitive dysfunctions in Parkinson’s Disease: The critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 2006, 5, 974–983. [Google Scholar] [CrossRef]
- Emre, M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003, 2, 229–237. [Google Scholar] [CrossRef]
- Nakano, I.; Hirano, A. Parkinson’s disease: Neuron loss in the nucleus basalis without concomitant Alzheimer’s disease. Ann. Neurol. 1984, 15, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Curtis, M.; Dick, D.J.; Candy, J.M.; Atack, J.R.; Bloxham, C.A.; Blessed, G.; Fairbairn, A.; Tomlinson, B.E.; Perry, R.H. Cholinergic correlates of cognitive impairment in Parkinson’s disease: Comparisons with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1985, 48, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.; Guillozet, A.; Shaw, P.; Quinn, B. Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiol. Dis. 2002, 9, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Manning, F.C. Tacrine therapy for the dementia of Alzheimer’s disease. Am. Fam. Physician 1994, 50, 819–826. [Google Scholar]
- Al Mamun, A.; Uddin, M.S. KDS2010: A potent highly selective and reversible MAO-B inhibitor for Alzheimer’s disease. Comb. Chem. High Throughput Screen. 2020, 23, 836–841. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Albreht, A. Kinetics, mechanism, and inhibition of monoamine oxidase. J. Neural Transm. 2018, 125, 1659–1683. [Google Scholar] [CrossRef]
- Schedin-Weiss, S.; Inoue, M.; Hromadkova, L.; Teranishi, Y.; Yamamoto, N.G.; Wiehager, B.; Bogdanovic, N.; Winblad, B.; Sandebring-Matton, A.; Frykman, S.; et al. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with γ-secretase and regulates neuronal amyloid β-peptide levels. Alzheimers Res. Ther. 2017, 9, 57. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Gabr, M.T. Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2019, 14, 437–440. [Google Scholar] [PubMed]
- Chowdhury, S.; Kumar, S. Inhibition of BACE1, MAO-B, Cholinesterase enzymes, and anti-amyloidogenic potential of selected natural phytoconstituents: Multi-target-directed ligand approach. J. Food Biochem. 2021, 45, e13571. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Tipton, K.F. Assessment of Enzyme Inhibition: A review with examples from the development of monoamine oxidase and cholinesterase inhibitory drugs. Molecules 2017, 22, 1192. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Parambi, D.G.T.; Mathew, G.E.; Uddin, M.S.; Inasu, S.T.; Kim, H.; Marathakam, A.; Unnikrishnan, M.K.; Carradori, S. Emerging therapeutic potentials of dual-acting MAO and AChE inhibitors in Alzheimer’s and Parkinson’s diseases. Arch. Pharm. 2019, 352, e1900177. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.-J.; Lee, H.-J.; Yoo, E.-S.; Jung, D.-S.; Riu, K.-Z.; Lee, S.-J. Antioxidant effects and inhibitory effect on NO synthesis by extracts of Canavalia lineata. Kor. J. Pharmacogn. 2004, 35, 338–345. [Google Scholar]
- Hong, S.-J.; Kwon, O.-K.; Hwang, D.; Goo, S.H.; Kim, D.-Y.; Kim, M.H.; Kim, S.-Y.; Jang, H.-J.; Oh, S.-R. Anti-inflammatory activity of cajanin, an isoflavonoid derivative isolated from Canavalia lineata pods. Int. J. Mol. Sci. 2022, 23, 9492. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.-J.; Kang, M.-G.; Mun, S.-K.; Park, D.; Hong, S.-J.; Kim, M.H.; Kim, S.-Y.; Yee, S.-T.; Kim, H. Medicarpin and homopterocarpin isolated from Canavalia lineata as potent and competitive reversible inhibitors of human monoamine oxidase-B. Molecules 2022, 28, 258. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, Y.; Cao, Y.; Shan, M.; Song, D.; Ye, C.; Zhu, D. Studies on chemical composition of Pueraria lobata and its anti-tumor mechanism. Molecules 2022, 27, 7253. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Sanz, A.; Cifuentes, A.; Ibánez, E.; Paape, T.; Lucas, M.M.; Pueyo, J.J. Flavonoid accumulation varies in Medicago truncatula in response to mercury stress. Front. Plant Sci. 2022, 13, 933209. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.W.; Tan, H.B.; Li, B.L.; Qiu, S.X. Three new pterocarpans from the aerial parts of Abrus precatorius. Nat. Prod. Res. 2020, 34, 1836–1844. [Google Scholar] [CrossRef]
- Dixit, M.; Raghuvanshi, A.; Gupta, C.P.; Kureel, J.; Mansoori, M.N.; Shukla, P.; John, A.A.; Singh, K.; Purohit, D.; Awasthi, P.; et al. Medicarpin, a natural pterocarpan, heals cortical bone defect by activation of notch and wnt canonical signaling pathways. PLoS ONE 2015, 10, e0144541. [Google Scholar] [CrossRef]
- Ghribi, L.; Waffo-Téguo, P.; Cluzet, S.; Marchal, A.; Marques, J.; Mérillon, J.M.; Ben Jannet, H. Isolation and structure elucidation of bioactive compounds from the roots of the Tunisian Ononis angustissima L. Bioorganic Med. Chem. Lett. 2015, 25, 3825–3830. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, D.-M.; Cho, Y.-J.; Hyun, J.-W.; Ahn, M.-J. Medicarpin increases antioxidant genes by inducing NRF2 transcriptional level in HeLa cells. Antioxidants 2022, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.B.; Zhao, Y.Y.; Wang, B.; Zhang, Q.Y.; Zhang, L.; Tu, P. Quantification and stability studies on the flavonoids of Radix hedysari. J. Agric. Food Chem. 2006, 54, 6634–6639. [Google Scholar] [CrossRef] [PubMed]
- Chern, C.M.; Lu, C.K.; Liou, K.T.; Wang, Y.H.; Tsai, K.C.; Chang, C.L.; Chang, C.C.; Shen, Y.C. Medicarpin isolated from Radix hedysari ameliorates brain injury in a murine model of cerebral ischemia. J. Food Drug Anal. 2021, 29, 581–605. [Google Scholar] [CrossRef]
- Mansoori, M.N.; Raghuvanshi, A.; Shukla, P.; Awasthi, P.; Trivedi, R.; Goel, A.; Singh, D. Medicarpin Prevents arthritis in post-menopausal conditions by arresting the expansion of TH17 cells and pro-inflammatory cytokines. Int. Immunopharmacol. 2020, 82, 106299. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Maurya, R.; Mishra, D.P. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014, 5, e1465. [Google Scholar] [CrossRef]
- Li, D.; Cai, C.; Liao, Y.; Wu, Q.; Ke, H.; Guo, P.; Wang, Q.; Ding, B.; Fang, J.; Fang, S. Systems pharmacology approach uncovers the therapeutic mechanism of medicarpin against scopolamine-induced memory loss. Phytomedicine 2021, 91, 153662. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Zhang, J.; Zhou, Q.; Liu, X.; Zhou, G. Medicarpin improves depressive-like behaviors in a chronic unpredictable mild stress-induced mouse model of depression by upregulating liver X receptor β expression in the amygdala. Neurotox. Res. 2022, 40, 1937–1947. [Google Scholar] [CrossRef]
- Oh, J.M.; Ji, M.; Lee, M.-J.; Jeong, G.S.; Paik, M.-J.; Kim, H.; Suh, J.-W. Antidepressant-like effects of ethanol extract of Ziziphus jujuba Mill seeds in Mice. Appl. Sci. 2020, 10, 7374. [Google Scholar] [CrossRef]
- Oh, J.M.; Lee, H.-S.; Baek, S.C.; Lee, J.P.; Jeong, G.S.; Paik, M.-J.; Kim, H. Antidepressant-like activities of hispidol and decursin in mice and analysis of neurotransmitter monoamines. Neurochem. Res. 2020, 45, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, C.; Zhang, X.; Chen, H.; Dong, J.; Lu, W.; Song, Z.; Zhou, W. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J. Neuroinflammation 2018, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Sunyer, B.; Höger, H.; Lubec, G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the barnes maze, the multiple T-maze and in the Morris water maze. Behav. Brain Res. 2009, 198, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Zamansoltani, F.; Javadi, A.; Ganjvar, M. The effects of rutin on a passive avoidance test in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, F.; Arab, F.; Zarghami, A.; Bijani, A.; Kazemi, S.; Moghadamnia, A.A. The Effect of lamotrigine on learning in mice using the passive avoidance model. Epilepsy Behav. 2017, 69, 1–6. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Asai, M.; Hara, T. High-fat diet enhances working memory in the Y-maze test in male C57BL/6J mice with less anxiety in the elevated plus maze test. Nutrients 2020, 12, 2036. [Google Scholar] [CrossRef]
- Rao, S.S.; Lago, L.; Volitakis, I.; Shukla, J.J.; McColl, G.; Finkelstein, D.I.; Adlard, P.A. Deferiprone treatment in aged transgenic tau mice improves Y-maze performance and alters tau pathology. Neurotherapeutics 2021, 18, 1081–1094. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.-J.; Kim, W.J.; Kang, M.-G.; Baek, S.C.; Lee, J.P.; Park, D.; Oh, S.-R.; Kim, H. Calycosin and 8-O-methylretusin isolated from Maackia amurensis as potent and selective reversible inhibitors of human monoamine oxidase-B. Int. J. Biol. Macromol. 2020, 151, 441–448. [Google Scholar] [CrossRef]
- Lee, J.P.; Kang, M.-G.; Lee, J.Y.; Oh, J.M.; Baek, S.C.; Leem, H.H.; Park, D.; Cho, M.-L.; Kim, H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorg. Chem. 2019, 89, 103043. [Google Scholar] [CrossRef]
- Park, J.E.; Mun, S.-K.; Yee, S.-T.; Kim, H. Evaluation of inhibitory activities of Sophora flavescens and Angelica gigas Nakai root extracts against monoamine oxidases, cholinesterases, and beta-Secretase. Processes 2022, 10, 880. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Wu, Z.; Yu, X.; Yin, Y.; Qian, S.; Wang, Z.; Huang, J.; Wang, W.; Liu, T.; et al. Quercitrin rapidly alleviated depression-like behaviors in lipopolysaccharide-treated mice: The involvement of PI3K/AKT/NF-κB signaling suppression and CREB/BDNF signaling restoration in the hippocampus. ACS Chem. Neurosci. 2021, 12, 3387–3396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).