An Effective Methanol-Blocking Cation Exchange Membrane Modified with Graphene Oxide Nanosheet for Direct Methanol Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide (GO)
2.3. Preparation of Grafted Cellulose Acetate/Graphene Oxide Composite Membranes (GCA/GO)
2.4. Measurements and Characterization of Membranes
3. Results and Discussion
3.1. Characterization of GO
3.2. Characterization of Nanocomposite Membranes
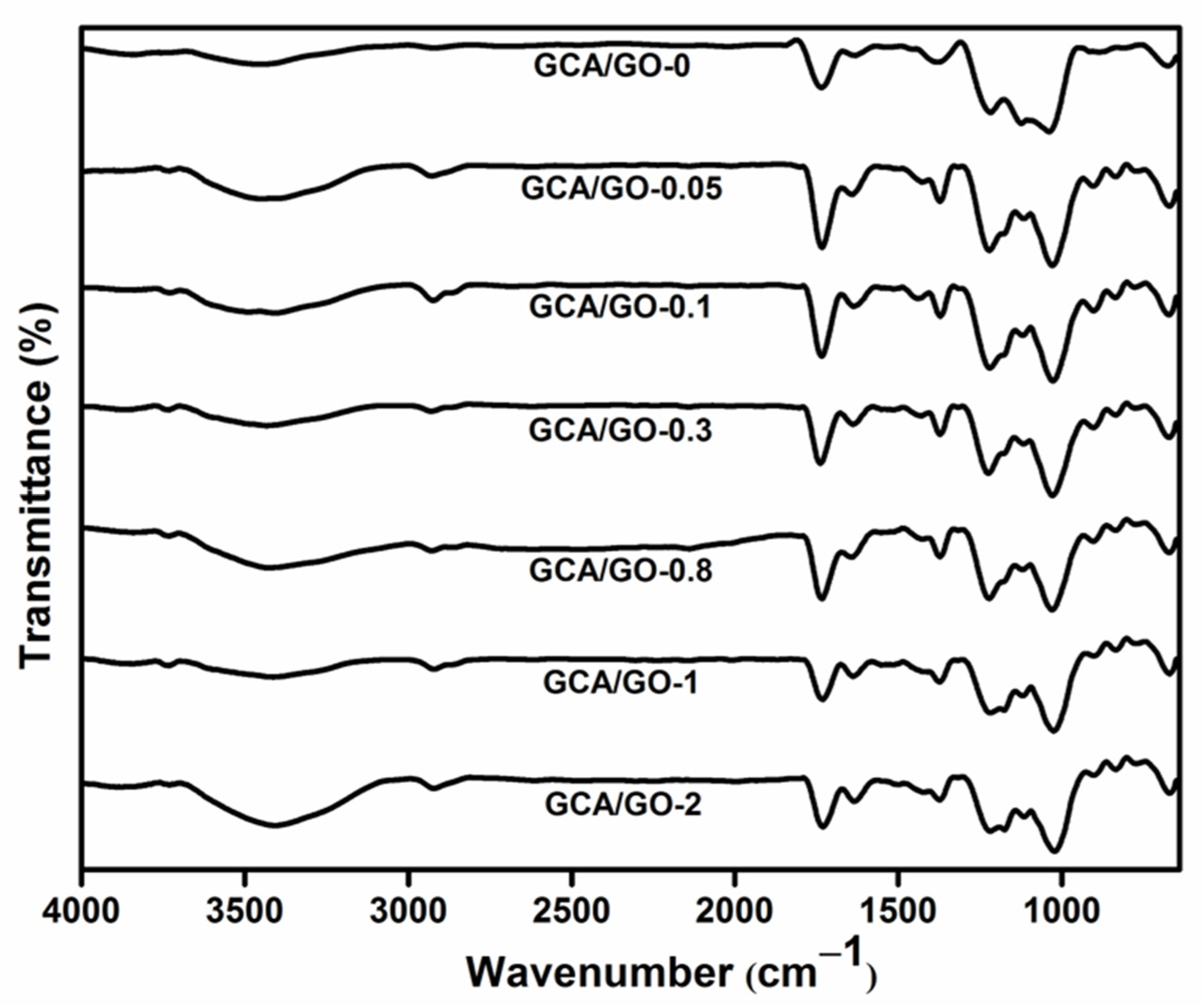
3.2.1. FT-IR Spectroscopy
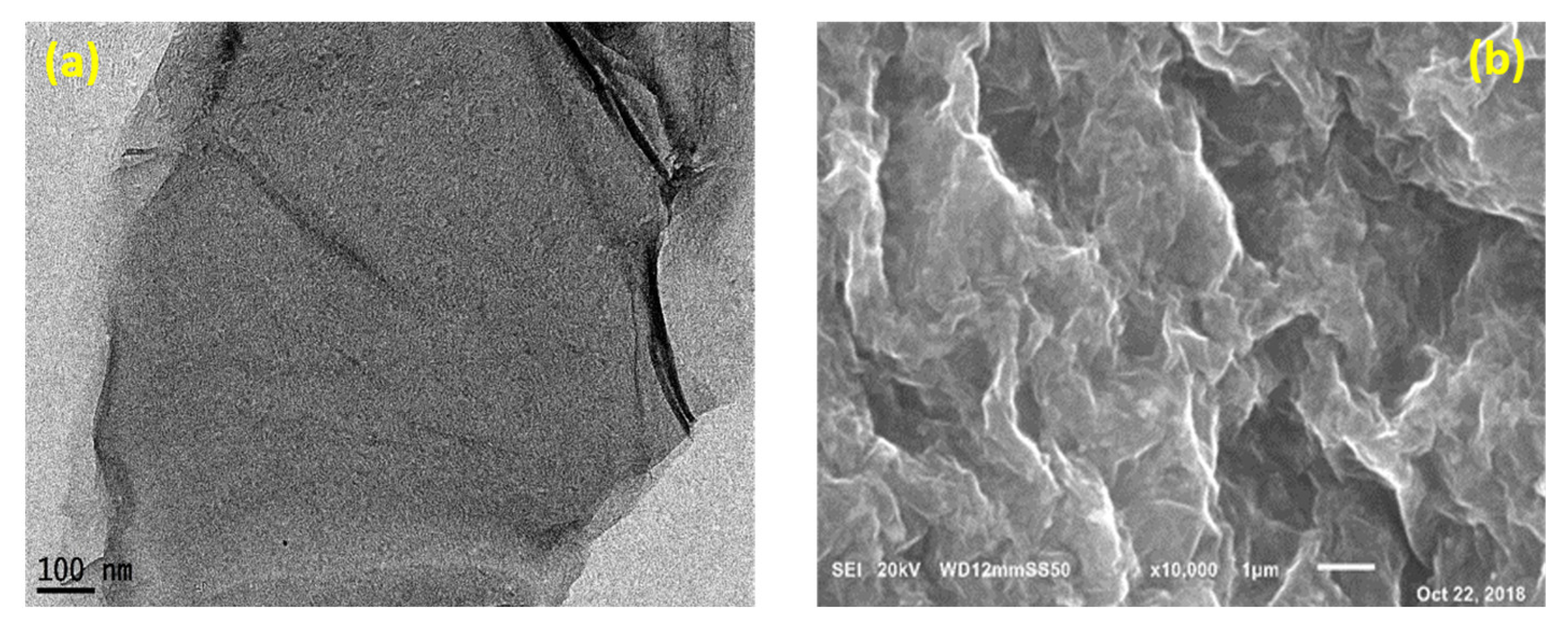
3.2.2. SEM
3.2.3. Thermogravimetric Analysis (TGA)
3.2.4. Differential Scanning Calorimetry (DSC)
3.2.5. Ion Exchange Capacity (IEC)
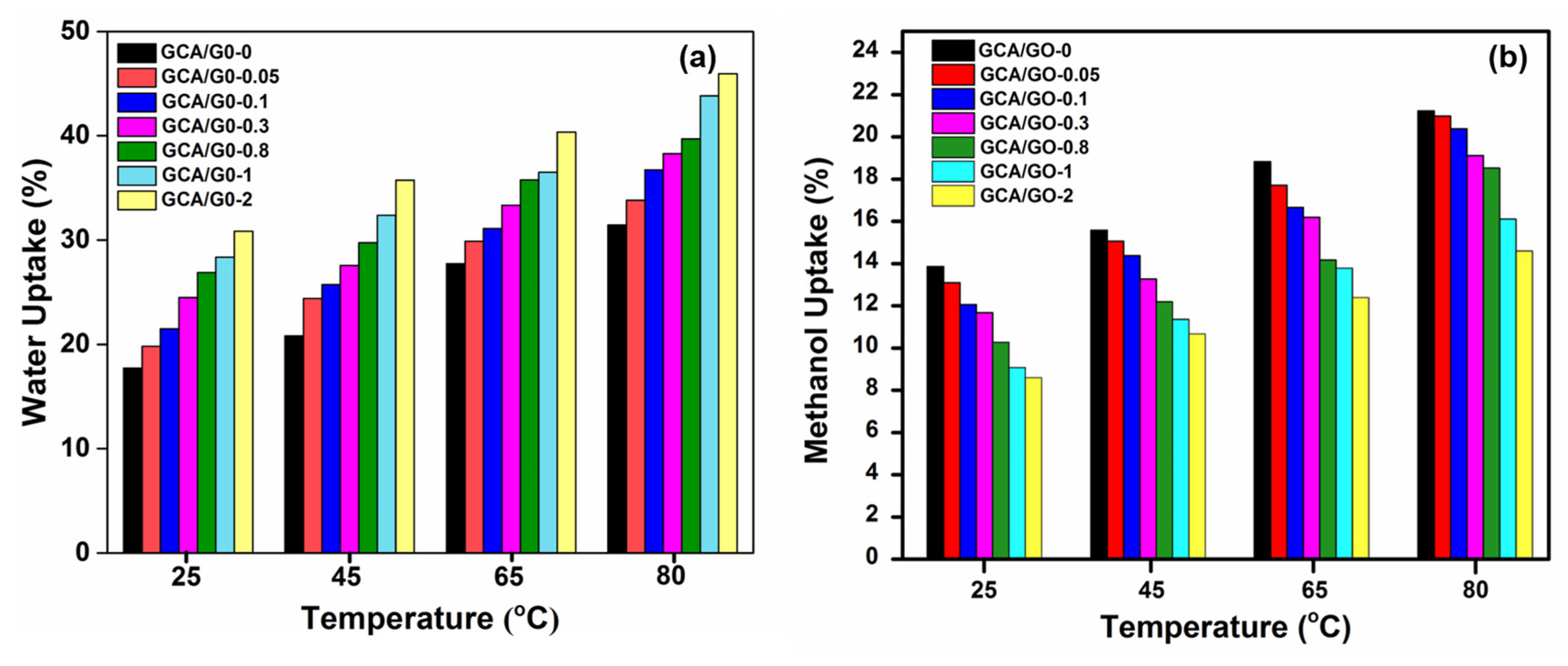
3.2.6. Liquid Uptake and Swelling Ratio
3.2.7. Contact Angle Measurement
3.2.8. Oxidative Stability
3.2.9. Mechanical Stability
3.2.10. Proton Conductivity
3.2.11. Methanol Permeability
3.2.12. Selectivity
3.2.13. Fuel Cell Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yogarathinam, L.T.; Jaafar, J.; Ismail, A.F.; Goh, P.S.; Bin Mohamed, M.H.; Radzi Hanifah, M.F.; Gangasalam, A.; Peter, J. Polyaniline decorated graphene oxide on sulfonated poly(ether ether ketone) membrane for direct methanol fuel cells application. Polym. Adv. Technol. 2022, 33, 66–80. [Google Scholar] [CrossRef]
- Choi, B.G.; Huh, Y.S.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Park, H. Enhanced transport properties in polymer electrolyte composite membranes with graphene oxide sheets. Carbon N. Y. 2012, 50, 5395–5402. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Hashem, A.E.; Tamer, T.M.; Omer, A.M.; Yossuf, M.E.; Sabet, M.M. Development of cross linked chitosan/alginate polyelectrolyte proton exchanger membranes for fuel cell applications. Int. J. Electrochem. Sci. 2017, 12, 3840–3858. [Google Scholar] [CrossRef]
- Vinodh, R.; Atchudan, R.; Kim, H.-J.; Yi, M. Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review. Polymers 2022, 14, 300. [Google Scholar] [CrossRef]
- Khalifa, R.E.; Omer, A.M.; Abd Elmageed, M.H.; Mohy Eldin, M.S. Titanium Dioxide/Phosphorous-Functionalized Cellulose AcetateNanocomposite Membranes for DMFC Applications: Enhancing Properties and Performance. ACS Omega 2021, 6, 17194–17202. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Abd Elmageed, M.H.; Omer, A.M.; Tamer, T.M.; Yossuf, M.E.; Khalifa, R.E. Novel proton exchange membranes based on sulfonated cellulose acetate for fuel cell applications: Preparation and characterization. Int. J. Electrochem. Sci. 2016, 11, 10150–10171. [Google Scholar] [CrossRef]
- Yuan, T.; Pu, L.; Huang, Q.; Zhang, H.; Li, X.; Yang, H. An effective methanol-blocking membrane modified with graphene oxide nanosheets for passive direct methanol fuel cells. Electrochim. Acta 2014, 117, 393–397. [Google Scholar] [CrossRef]
- Lin, C.W.; Lu, Y.S. Highly ordered graphene oxide paper laminated with a Nafion membrane for direct methanol fuel cells. J. Power Sources 2013, 237, 187–194. [Google Scholar] [CrossRef]
- Kamjornsupamitr, T.; Sangthumchai, T.; Youngme, S.; Martwiset, S. Proton conducting composite membranes from crosslinked poly(vinyl alcohol) and poly(styrene sulfonic acid)-functionalized silica nanoparticles. Int. J. Hydrogen Energy 2018, 43, 11190–11201. [Google Scholar] [CrossRef]
- Subel, B.L.; Wesdemiotis, C. Mass spectrometry analysis of poly(styrene sulfonate sodium salt), a polyanionic electrolyte. Int. J. Mass Spectrom. 2011, 301, 195–201. [Google Scholar] [CrossRef]
- Interial, L.G.D.; Benavides, R.; Morales-Acosta, D.; Francisco-Vieira, L.; da Silva, L. Development of Polymeric Electrolytes for Fuel Cells: Synthesis and Characterization of New Sulfonated Polystyrene-Co-Acrylonitrile-Co-Butyl Acrylate Terpolymers. Solid State Ion. 2022, 387, 116066. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, D.; Yan, L.; Yue, B.; Pan, T.; Hu, Y.; He, S.; Zhao, H.; Zhang, J. Design and Synthesis of Side-Chain Optimized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-g-Poly(Styrene Sulfonic Acid) as Proton Exchange Membrane for Fuel Cell Applications: Balancing the Water-Resistance and the Sulfonation Degree. Int. J. Hydrogen Energy 2021, 46, 20664–20677. [Google Scholar] [CrossRef]
- Mesbah, F.; el Gayar, D.; Farag, H.; Tamer, T.M.; Omer, A.M.; Mohy-Eldin, M.S.; Khalifa, R.E. Development of Highly Ionic Conductive Cellulose Acetate-g-Poly (2-Acrylamido-2-Methylpropane Sulfonic Acid-Co-Methyl Methacrylate) Graft Copolymer Membranes. J. Saudi Chem. Soc. 2021, 25, 101318. [Google Scholar] [CrossRef]
- Ryu, S.K.; Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Yoo, D.J. Enhancing physicochemical properties and single cell performance of sulfonated poly(Arylene ether) (spae) membrane by incorporation of phosphotungstic acid and graphene oxide: A potential electrolyte for proton exchange membrane fuel cells. Polymers 2021, 13, 2364. [Google Scholar] [CrossRef] [PubMed]
- Špitalisky, Z.; Danko, M.; Mosnacek, J. Preparation of functionalized graphene sheets. Curr. Org. Chem. 2011, 15, 1133–1150. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Seo, D.C.; Jeon, I.; Jeong, E.S.; Jho, J.Y. Mechanical properties and chemical durability of nafion/sulfonated graphene oxide/cerium oxide composite membranes for fuel-cell applications. Polymers 2020, 12, 1375. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kim, N.H.; Jung, D.; Lee, J.H. Enhanced Mechanical Properties and Proton Conductivity of Nafion-SPEEK-GO Composite Membranes for Fuel Cell Applications. J. Membr. Sci. 2014, 458, 128–135. [Google Scholar] [CrossRef]
- Shabani, M.; Younesi, H.; Rahimpour, A.; Rahimnejad, M. Upgrading the Electrochemical Performance of Graphene Oxide-Blended Sulfonated Polyetheretherketone Composite Polymer Electrolyte Membrane for Microbial Fuel Cell Application. Biocatal. Agric. Biotechnol. 2019, 22, 101369. [Google Scholar] [CrossRef]
- Madih, K.; El-Shazly, A.H.; Elkady, M.F.; Aziz, A.N.; Youssef, M.E.; Khalifa, R.E. A facile synthesis of cellulose acetate reinforced graphene oxide nanosheets as proton exchange membranes for fuel cell applications. J. Saudi Chem. Soc. 2022, 26, 101435. [Google Scholar] [CrossRef]
- Shalaby, A.A.; Abd Elmageed, M.H.; Malash, G.F.; Tamer, T.M.; Omer, A.M.; Mohy-Eldin, M.S.; Khalifa, R.E. Development of novel cellulose acetate-g-poly(sodium 4-styrenesulfonate) proton conducting polyelectrolyte polymer. J. Saudi Chem. Soc. 2021, 25, 101327. [Google Scholar] [CrossRef]
- Umsarika, P.; Changkhamchom, S.; Paradee, N.; Sirivat, A.; Supaphol, P.; Hormnirun, P. Proton exchange membrane based on sulfonated poly (aromatic imide-co-aliphatic imide) for direct methanol fuel cell. Mater. Res. 2018, 21, e20170823. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Cai, W.; Xiong, J.; Ma, L.; Yang, Z.; Huang, Y.; Cheng, H. Non-destructive modification on Nafion membrane via in-situ inserting of sheared graphene oxide for direct methanol fuel cell applications. Electrochim. Acta 2018, 282, 362–368. [Google Scholar] [CrossRef]
- Aziz, A.; Hassab, M.; Youssef, M.E.; El-Maghlany, W.M. Performance Analysis of DMFC and PEMFC Numerically with Models Validation. Int. J. Adv. Electron. Comput. Sci. 2018, 5, 44–47. [Google Scholar]
- Gouda, M.H.; Elnouby, M.; Aziz, A.N.; Youssef, M.E.; Santos, D.M.F.; Elessawy, N.A. Green and Low-Cost Membrane Electrode Assembly for Proton Exchange Membrane Fuel Cells: Effect of Double-Layer Electrodes and Gas Diffusion Layer. Front. Mater. 2020, 6, 337. [Google Scholar] [CrossRef]
- Aziz, A.N.; Rabhy, O.O.; Youssef, M.E. Modeling and Experimental investigation for PEMFC to achieve high Fuel Cell performance. In Proceedings of the International Conference on New Trends for Sustainable Energy-ICNTSE, Madrid, Spain, 23–26 May 2016; pp. 228–231. [Google Scholar]
- Cullity, B.D.; Bernard, D. Elements of X-Ray Diffraction; Addison-Wesley Publishing Company, Inc.: Boston, MA, USA, 1978; ISBN 0201011743. [Google Scholar]
- Surekha, G.; Krishnaiah, K.V.; Ravi, N.; Padma Suvarna, R. FTIR, Raman and XRD Analysis of Graphene Oxide Films Prepared by Modified Hummers Method. J. Phys. Conf. Ser. 2020, 1495, 012012. [Google Scholar] [CrossRef]
- Sudesh; Kumar, N.; Das, S.; Bernhard, C.; Varma, G.D. Effect of Graphene Oxide Doping on Superconducting Properties of Bulk MgB2. Supercond. Sci. Technol. 2013, 26, 095008. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Omer, A.M.; Khalifa, R.E.; Eltaweil, A.S. Floatable cellulose acetate beads embedded with flower-like zwitterionic binary MOF/PDA for efficient removal of tetracycline. J. Colloid Interface Sci. 2022, 620, 333–345. [Google Scholar] [CrossRef]
- Huang, B.; Dai, Y.; Liu, Y.; Zhang, X.; Qin, X.; Wang, J.; Cheng, H. Electronic Supplementary Information (ESI) Crystal Facets Controlled Synthesis of Graphene @TiO2 Nanocomposites by One-Pot Hydrothermal Process. CrystEngComm. 2012, 14, 1687. [Google Scholar] [CrossRef]
- Ramya, A.V.; Manoj, B.; Mohan, A.N. Extraction and Characterization of Wrinkled Graphene Nanolayers from Commercial Graphite. Asian J. Chem. 2016, 28, 1031–1034. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Omer, A.M.; Tamer, T.M.; Abd Elmageed, M.H.; Youssef, M.E.; Khalifa, R.E. Novel aminated cellulose acetate membranes for direct methanol fuel cells (DMFCs). Int. J. Electrochem. Sci. 2017, 12, 4301–4318. [Google Scholar] [CrossRef]
- Xu, Z.; Bando, Y.; Liu, L.; Wang, W.; Bai, X.; Golberg, D. Electrical Conductivity, Chemistry, and Bonding Alternations under Graphene Oxide to Graphene Transition as Revealed by in Situ TEM. ACS Nano 2011, 5, 4401–4406. [Google Scholar] [CrossRef] [PubMed]
- Muthumeenal, A.; Saraswathi, M.S.A.; Rana, D.; Nagendran, A. Fabrication and electrochemical properties of highly selective SPES/GO composite membranes for direct methanol fuel cells. J. Environ. Chem. Eng. 2017, 5, 3828–3833. [Google Scholar] [CrossRef]
- Suhaimin, N.S.; Jaafar, J.; Aziz, M.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Nanocomposite membrane by incorporating graphene oxide in sulfonated polyether ether ketone for direct methanol fuel cell. Mater. Today Proc. 2021, 46, 2084–2091. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Q.; Li, D.; Tong, J.; Li, X. Properties improvement of SPEEK based proton exchange membranes by doping of ionic liquids and Y2O3. Prog. Nat. Sci. Mater. Int. 2012, 22, 26–30. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Sulfonated poly(ether ether ketone)/polyaniline composite proton-exchange membrane. J. Memb. Sci. 2006, 280, 389–396. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, S.; Liu, Y.; Wang, Y.; Pang, J.; Wang, Q.; Wang, G. A novel crosslinking organic-inorganic hybrid proton exchange membrane based on sulfonated poly(arylene ether sulfone) with 4-amino-phenyl pendant group for fuel cell application. J. Memb. Sci. 2013, 434, 161–170. [Google Scholar] [CrossRef]
- Shukla, A.; Dhanasekaran, P.; Sasikala, S.; Nagaraju, N.; Bhat, S.D.; Pillai, V.K. Covalent grafting of polystyrene sulfonic acid on graphene oxide nanoplatelets to form a composite membrane electrolyte with sulfonated poly(ether ether ketone) for direct methanol fuel cells. J. Memb. Sci. 2020, 595, 117484. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Evaluation of Quaternized polyvinyl alcohol/graphene oxide-based membrane towards improving the performance of air-breathing passive direct methanol fuel cells. Int. J. Energy Res. 2020, 44, 8988–9000. [Google Scholar] [CrossRef]
- Prapainainar, P.; Pattanapisutkun, N.; Prapainainar, C.; Kongkachuichay, P. Incorporating graphene oxide to improve the performance of Nafion-mordenite composite membranes for a direct methanol fuel cell. Int. J. Hydrogen Energy 2019, 44, 362–378. [Google Scholar] [CrossRef]
- Ayyaru, S.; Ahn, Y.H. Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J. Memb. Sci. 2017, 525, 210–219. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N. Influence of Graphene Oxide on the Ethanol Permeability and Ionic Conductivity of QPVA-Based Membrane in Passive Alkaline Direct Ethanol Fuel Cells. Nanoscale Res. Lett. 2019, 14, 28. [Google Scholar] [CrossRef]
- He, Y.; Tong, C.; Geng, L.; Liu, L.; Lü, C. Enhanced performance of the sulfonated polyimide proton exchange membranes by graphene oxide: Size effect of graphene oxide. J. Memb. Sci. 2014, 458, 36–46. [Google Scholar] [CrossRef]
- Pan, J.; Chen, C.; Li, Y.; Wang, L.; Tan, L.; Li, G.; Tang, X.; Xiao, L.; Lu, J.; Zhuang, L. Constructing ionic highway in alkaline polymer electrolytes. Energy Environ. Sci. 2014, 7, 354–360. [Google Scholar] [CrossRef]
- Kreuer, K.-D. Conduction mechanisms in materials with volatile molecules. In Proton Conductors; Cambridge University Press: Cambridge, UK, 2010; pp. 474–486. Available online: https://www.cambridge.org/core/books/abs/proton-conductors/conduction-mechanisms-in-materials-with-volatile-molecules/61EAD073106A6589ACCD2C7D73E57EFC (accessed on 4 May 2022). [CrossRef]
- Cai, W.; Fan, K.; Li, J.; Ma, L.; Xu, G.; Xu, S.; Ma, L.; Cheng, H. A bi-functional polymeric nano-sieve Nafion composite membrane: Improved performance for direct methanol fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 17102–17111. [Google Scholar] [CrossRef]
- Kumar, R.; Mamlouk, M.; Scott, K. A Graphite Oxide Paper Polymer Electrolyte for Direct Methanol Fuel Cells. Int. J. Electrochem. 2011, 2011, 434186. [Google Scholar] [CrossRef]
- Karim, M.R.; Hatakeyama, K.; Matsui, T.; Takehira, H.; Taniguchi, T.; Koinuma, M.; Matsumoto, Y.; Akutagawa, T.; Nakamura, T.; Noro, S.I.; et al. Graphene oxide nanosheet with high proton conductivity. J. Am. Chem. Soc. 2013, 135, 8097–8100. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Omer, A.M.; Tamer, T.M.; Abd Elmageed, M.H.; Youssef, M.E.; Khalifa, R.E. Development of Novel Phosphorylated Cellulose Acetate Polyelectrolyte Membranes for Direct Methanol Fuel Cell Application. Int. J. Electrochem. Sci. 2016, 11, 3467–3491. [Google Scholar] [CrossRef]
- Cai, H.; Shao, K.; Zhong, S.; Zhao, C.; Zhang, G.; Li, X.; Na, H. Properties of composite membranes based on sulfonated poly(ether ether ketone)s (SPEEK)/phenoxy resin (PHR) for direct methanol fuel cells usages. J. Memb. Sci. 2007, 297, 162–173. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Wang, Y. Sulfonated poly(ether ether ketone) membranes for direct methanol fuel cell. J. Memb. Sci. 2003, 226, 159–167. [Google Scholar] [CrossRef]
- Lei, L.; Zhu, X.; Xu, J.; Qian, H.; Zou, Z.; Yang, H. Highly stable ionic-covalent cross-linked sulfonated poly(ether ether ketone) for direct methanol fuel cells. J. Power Sources 2017, 350, 41–48. [Google Scholar] [CrossRef]
- Rambabu, G.; Nagaraju, N.; Bhat, S.D. Functionalized fullerene embedded in Nafion matrix: A modified composite membrane electrolyte for direct methanol fuel cells. Chem. Eng. J. 2016, 306, 43–52. [Google Scholar] [CrossRef]







| Wave Number (cm−1) | Functional Group |
|---|---|
| 3431 | -H |
| 2920 | CH2 |
| 2856 | CH2 |
| 1633 | C=O |
| 1556 | C=C |
| 1408 | C-O |
| 1238 | C-O-C |
| 1050 | COOH |
| 831 | C-H |
| 671 | C-H |
| 565 | C-H |
| 463 | C-H |
| Membranes | T20% | T50% | Weight Loss (%) at Ambient Temperature (0–120 °C) |
|---|---|---|---|
| GCA/GO-0 | 221.54 | 291.95 | 13.040 |
| GCA/GO-0.05 | 262.69 | 303.85 | 4.8700 |
| GCA/GO-0.1 | 265.34 | 338.40 | 5.2300 |
| GCA/GO-0.3 | 244.18 | 355.47 | 8.8400 |
| GCA/GO-0.8 | 210.54 | 398.24 | 12.520 |
| GCA/GO-1 | 220.00 | 408.82 | 12. 060 |
| GCA/GO-2 | 235.00 | 426.17 | 11.220 |
| Membranes | Dimension Changes (%) in Water | Dimension Changes (%) in Methanol | ||||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 45 °C | 65 °C | 80 °C | 25 °C | 45 °C | 65 °C | 80 °C | |
| GCA/GO-0 | 6.68 | 6.77 | 7.35 | 7.83 | 6.97 | 7.69 | 8.92 | 8.47 |
| GCA/GO-0.05 | 5.55 | 5.79 | 5.82 | 5.89 | 5.17 | 6.12 | 7.32 | 7.27 |
| GCA/GO-0.1 | 5.52 | 5.84 | 5.89 | 5.97 | 5.88 | 6.25 | 6.52 | 6.38 |
| GCA/GO-0.3 | 5.81 | 5.95 | 6.17 | 6.19 | 5.88 | 6.52 | 6.66 | 7.14 |
| GCA/GO-0.8 | 5.97 | 5.97 | 6.16 | 6.23 | 6.38 | 6.52 | 6.66 | 6.70 |
| GCA/GO-1 | 6.00 | 6.28 | 6.59 | 6.63 | 5.70 | 5.70 | 5.8 | 6.00 |
| GCA/GO-2 | 6.22 | 6.26 | 6.46 | 6.80 | 5.80 | 5. 80 | 6.00 | 6.00 |
| Membrane | Time (h) | Retained Weight (%) | Contact Angle (°) |
|---|---|---|---|
| GCA/GO-0 | 60 | 98.5 | 60.98 |
| GCA/GO-0.05 | 62 | 98.58 | 55.77 |
| GCA/GO-0.1 | 65 | 97.65 | 52.59 |
| GCA/GO-0.3 | 72 | 97.14 | 47.12 |
| GCA/GO-0.8 | 82 | 96.88 | 46.02 |
| GCA/GO-1 | 84 | 96.67 | 40.38 |
| GCA/GO-2 | 95 | 96.86 | 35.71 |
| Membranes | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| GCA/GO-0 | 46.97 | 5.00 |
| GCA/GO-0.05 | 51.35 | 5.30 |
| GCA/GO-0.1 | 57.14 | 5.76 |
| GCA/GO-0.3 | 63.34 | 6.00 |
| GCA/GO-0.8 | 69.40 | 6.60 |
| GCA/GO-1 | 74.55 | 7.56 |
| GCA/GO-2 | 79.56 | 8.71 |
| Membranes | Conductivity (S.cm−1 × 10−3) | Resistance (Ω × 10−3) | Methanol Permeability (cm2 · s−1 × 10−7) | Electrochemical Selectivity (Ss · cm−3 × 103) |
|---|---|---|---|---|
| CA | 0.035 | 14285.7 | 18.000 | 0.0194 |
| GCA/GO-0 | 4.770 | 104.82 | 5.514 | 8.651 |
| GCA/GO-0.05 | 4.790 | 104.38 | 5.320 | 9.004 |
| GCA/GO-0.1 | 4.900 | 102.04 | 3.650 | 13.424 |
| GCA/GO-0.3 | 5.110 | 97.84 | 3.980 | 12.839 |
| GCA/GO-0.8 | 5.520 | 90.57 | 1.470 | 37.551 |
| GCA/GO-1 | 5.840 | 85.61 | 1.330 | 43.910 |
| GCA/GO-2 | 5.990 | 83.47 | 1.120 | 26.390 |
| Nafion 212 | 6.940 | 72.05 | 24.900 | 2.7870 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalaby, A.A.; Aziz, A.N.; Špitalský, Z.; Omer, A.M.; Mohy-Eldin, M.S.; Khalifa, R.E. An Effective Methanol-Blocking Cation Exchange Membrane Modified with Graphene Oxide Nanosheet for Direct Methanol Fuel Cells. Processes 2023, 11, 353. https://doi.org/10.3390/pr11020353
Shalaby AA, Aziz AN, Špitalský Z, Omer AM, Mohy-Eldin MS, Khalifa RE. An Effective Methanol-Blocking Cation Exchange Membrane Modified with Graphene Oxide Nanosheet for Direct Methanol Fuel Cells. Processes. 2023; 11(2):353. https://doi.org/10.3390/pr11020353
Chicago/Turabian StyleShalaby, Asmaa Attya, Andrew N. Aziz, Zdenko Špitalský, Ahmed Mohamed Omer, Mohamed Samir Mohy-Eldin, and Randa Eslah Khalifa. 2023. "An Effective Methanol-Blocking Cation Exchange Membrane Modified with Graphene Oxide Nanosheet for Direct Methanol Fuel Cells" Processes 11, no. 2: 353. https://doi.org/10.3390/pr11020353
APA StyleShalaby, A. A., Aziz, A. N., Špitalský, Z., Omer, A. M., Mohy-Eldin, M. S., & Khalifa, R. E. (2023). An Effective Methanol-Blocking Cation Exchange Membrane Modified with Graphene Oxide Nanosheet for Direct Methanol Fuel Cells. Processes, 11(2), 353. https://doi.org/10.3390/pr11020353










