Preparation of Polymer Solution for Profile Control and Displacement Using Wastewater with High Ca2+/Mg2+ and Fe2+ Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Experimental Methods and Contents
- (1)
- Preparation of polymer solution
- (2)
- Effect of Fe2+ on the viscosity of the polymer solution
- (3)
- Measurement of Fe2+ concentration in the polymer solution
- (4)
- Determination of the basic physical property parameters of the polymers
- (a)
- Characteristic viscosity and polymer molecular weight: The characteristic viscosity number of the polymer and weak gel was obtained by measurement of the Uhler vissimeter, and the average molecular weight of the polymer and weak gel was calculated using the formulawhere and are empirical constants, = 6.31 × 10−3, and = 0.8 in the present paper.
- (b)
- Degree of hydrolysis: The degree of hydrolysis of the partially hydrolyzed polyacrylamide was determined by the method described in GB/T 12005.6-1989.
- (5)
- Polymer characterization
- (6)
- Performance evaluation of the PAM-IR solutionWe prepared a 0.5% PAM-IR solution using simulated brine solutions with various concentrations of NaCl, Ca2+, Mg2+, and Fe2+. We determined the state of the polymer in the solution at 3 h and measured its apparent viscosity to assess the resistance of the polymer solution to salinity and Fe2+. The apparent viscosity was measured using the MCR302 rheometer at 40 °C and a shear rate of 10 s−1. The experiments are performed on:
- (a)
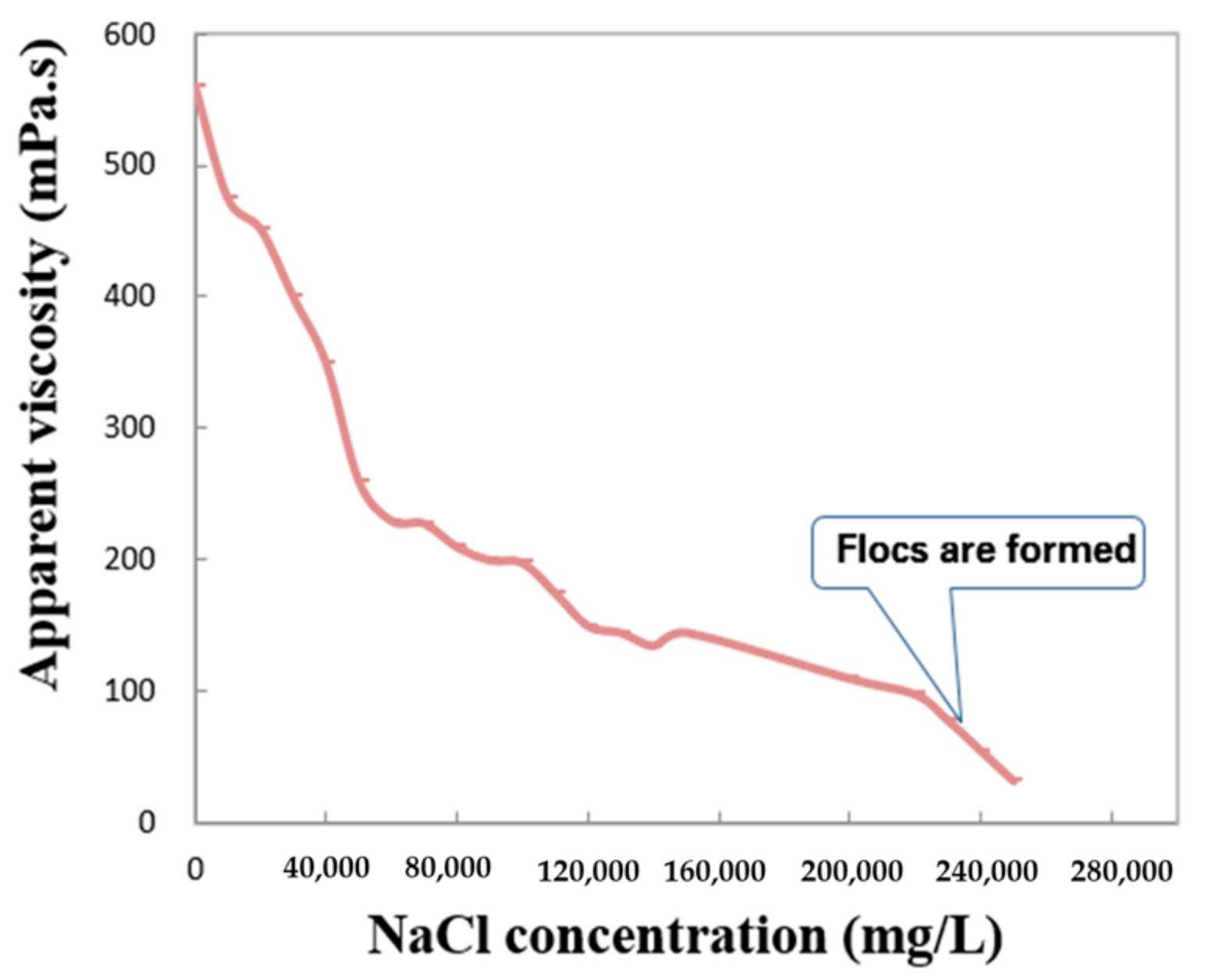
- Salt sensitivity: various concentrations of NaCl were added to distilled water to form the simulated brines, which were then used to prepare polymer solutions.
- (b)
- Ca2+ influence: various concentrations of Ca2+ were added to a 100 × 103 mg/L NaCl aqueous solution to form simulated brines, which were then used to prepare polymer solutions.
- (c)
- Mg2+ influence: various concentrations of Mg2+ were added to a 100 × 103 mg/L NaCl aqueous solution to form simulated brines, which were then used to prepare polymer solutions.
- (d)
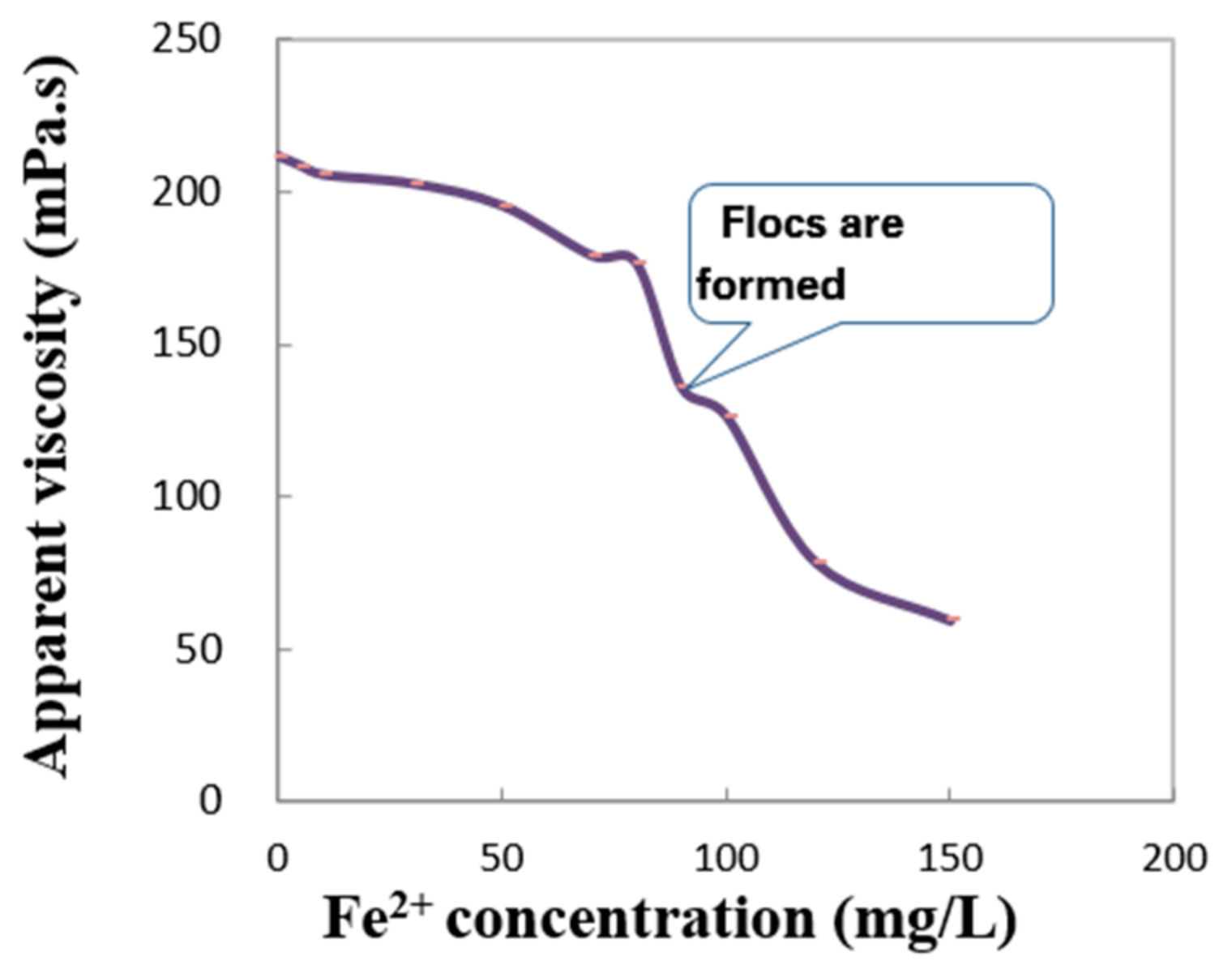
- Fe2+ influence: various concentrations of Fe2+ were added to a 100 × 103 mg/L NaCl aqueous solution to form simulated brines, which were then used to prepare polymer solutions.
- (7)
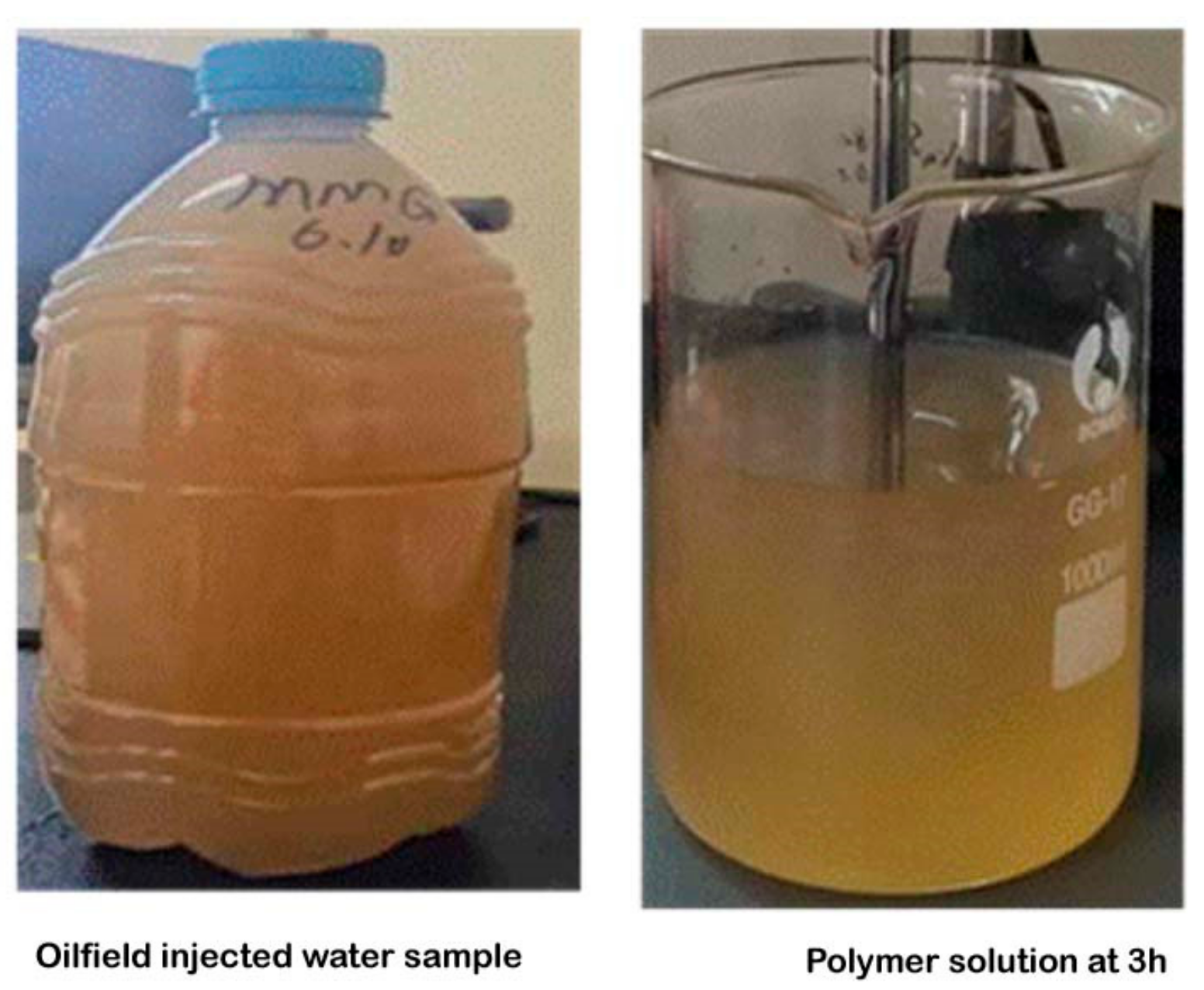
- Verification experiment with wastewater
- (8)
- Resistance factor and residual resistance factor test
3. Results and Discussion
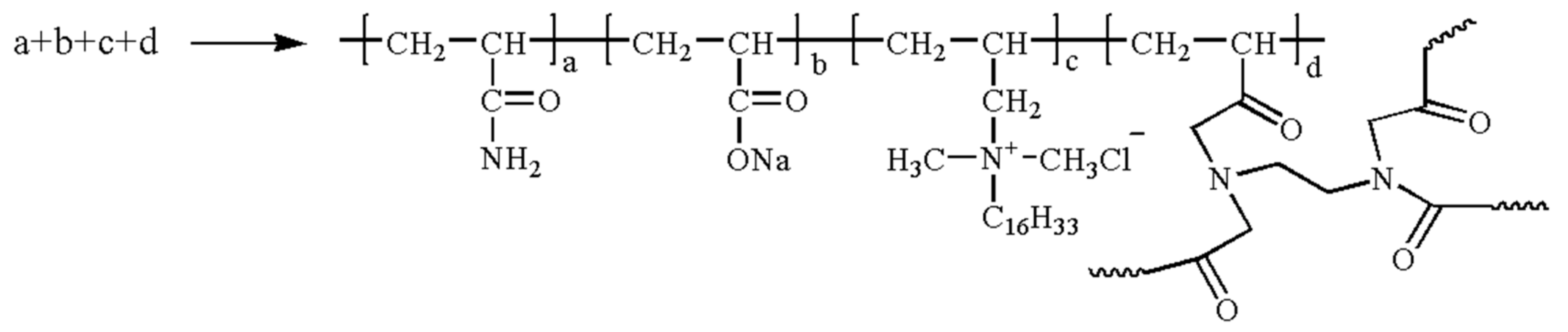
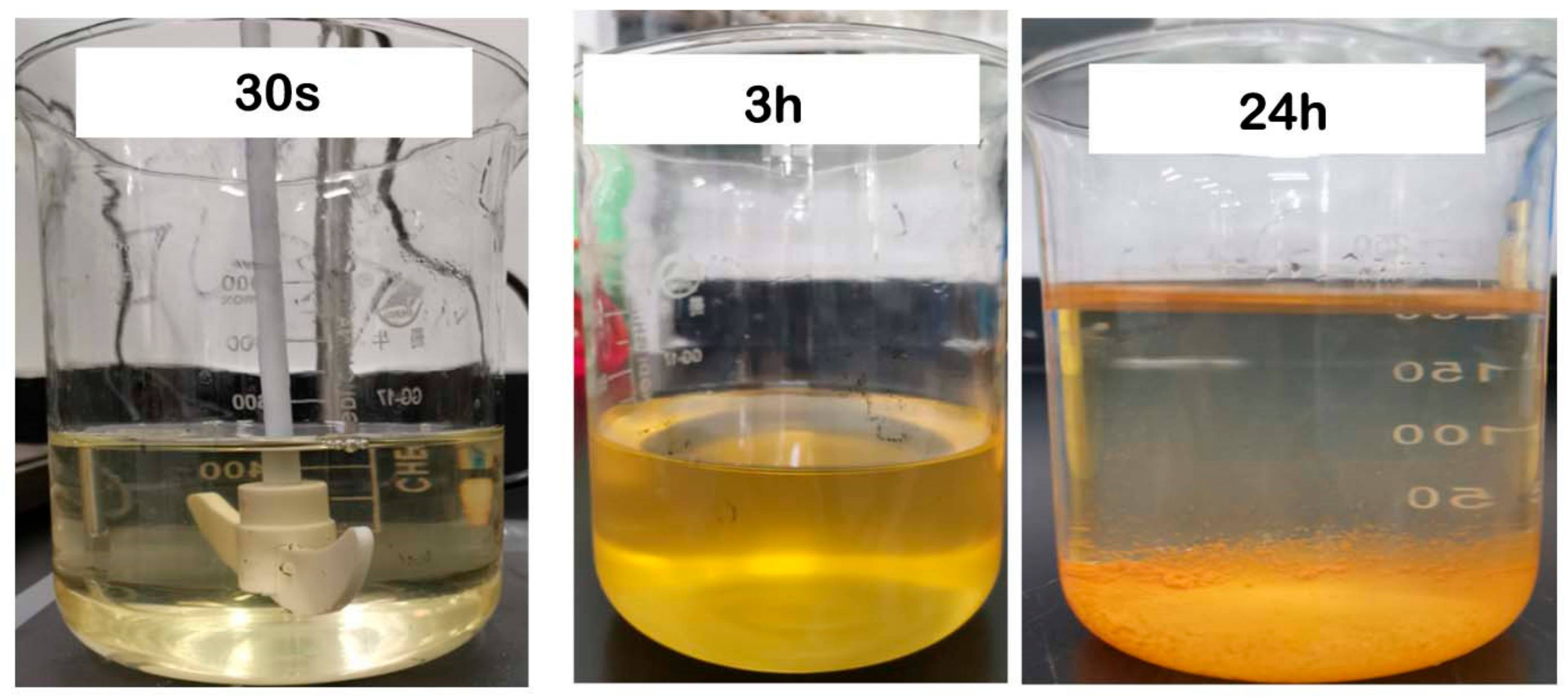
3.1. PAM-IR Synthesis
3.2. Results of Basic Physical Properties Determination
3.3. Characterization of the New Polymer
- (1)
- FTIR analysis results
- (2)
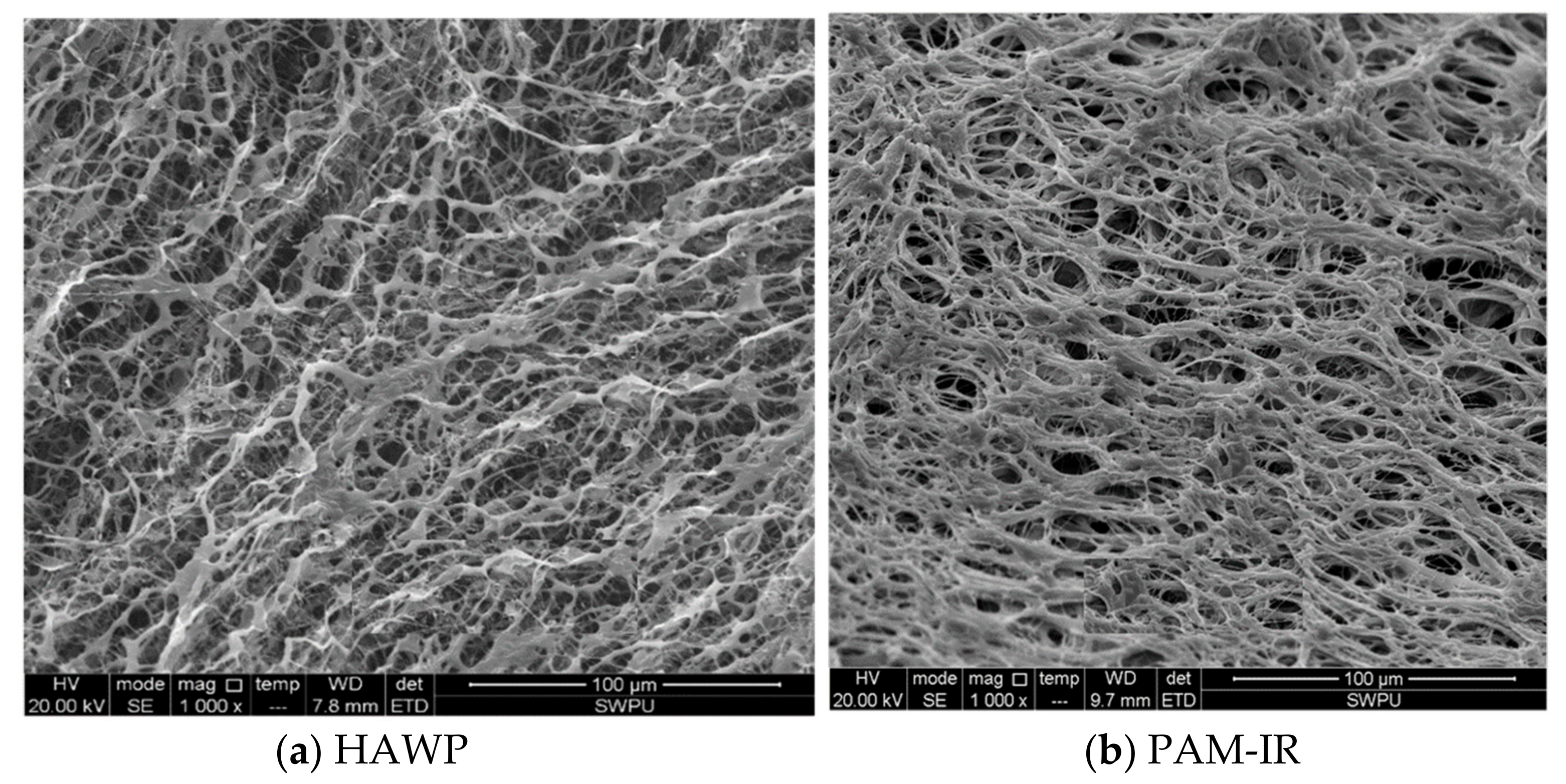
- SEM characterization results
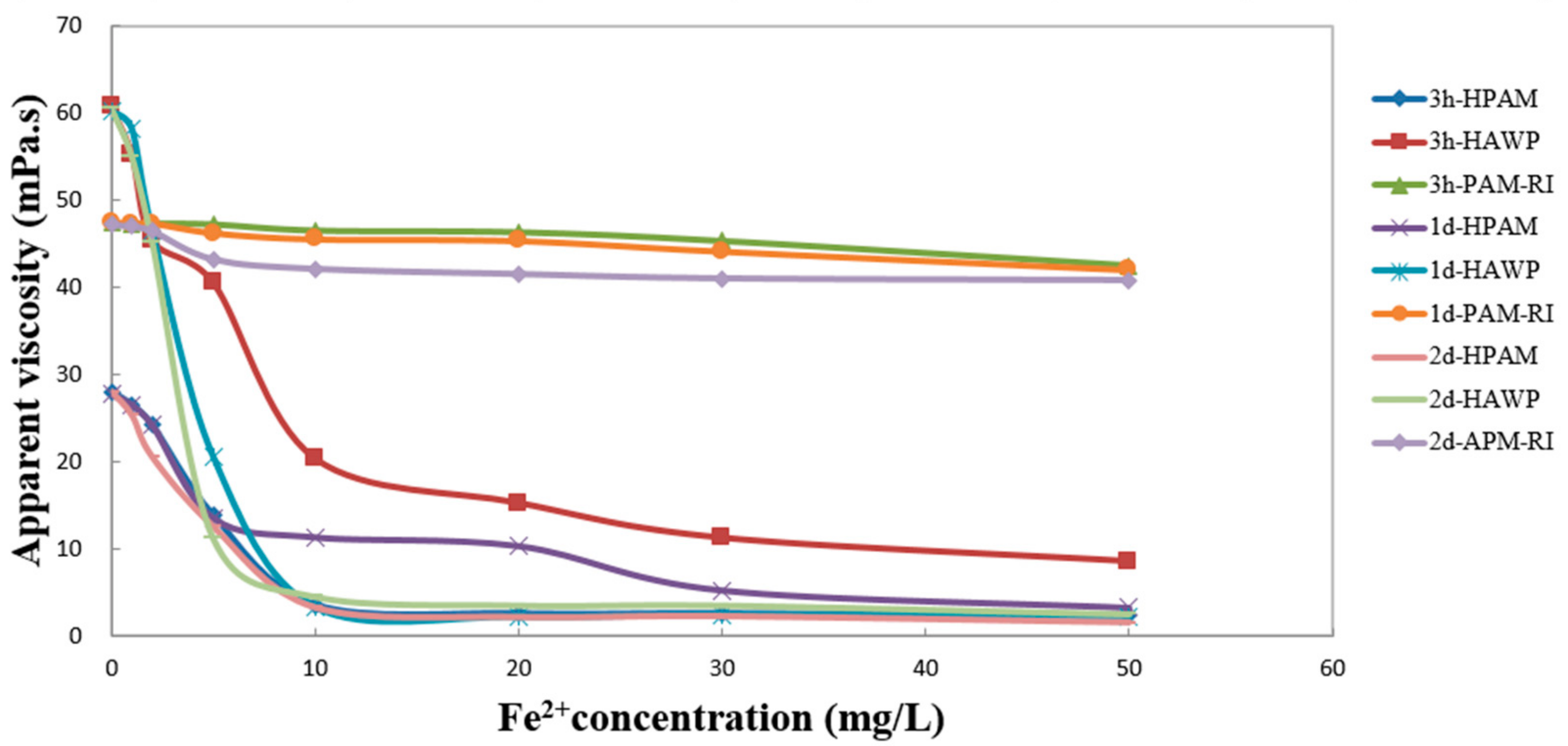
3.4. Effect of Fe2+ on the Apparent Viscosity of the Polymer Solution
3.5. Fe2+ Concentration of the Polymer Solution
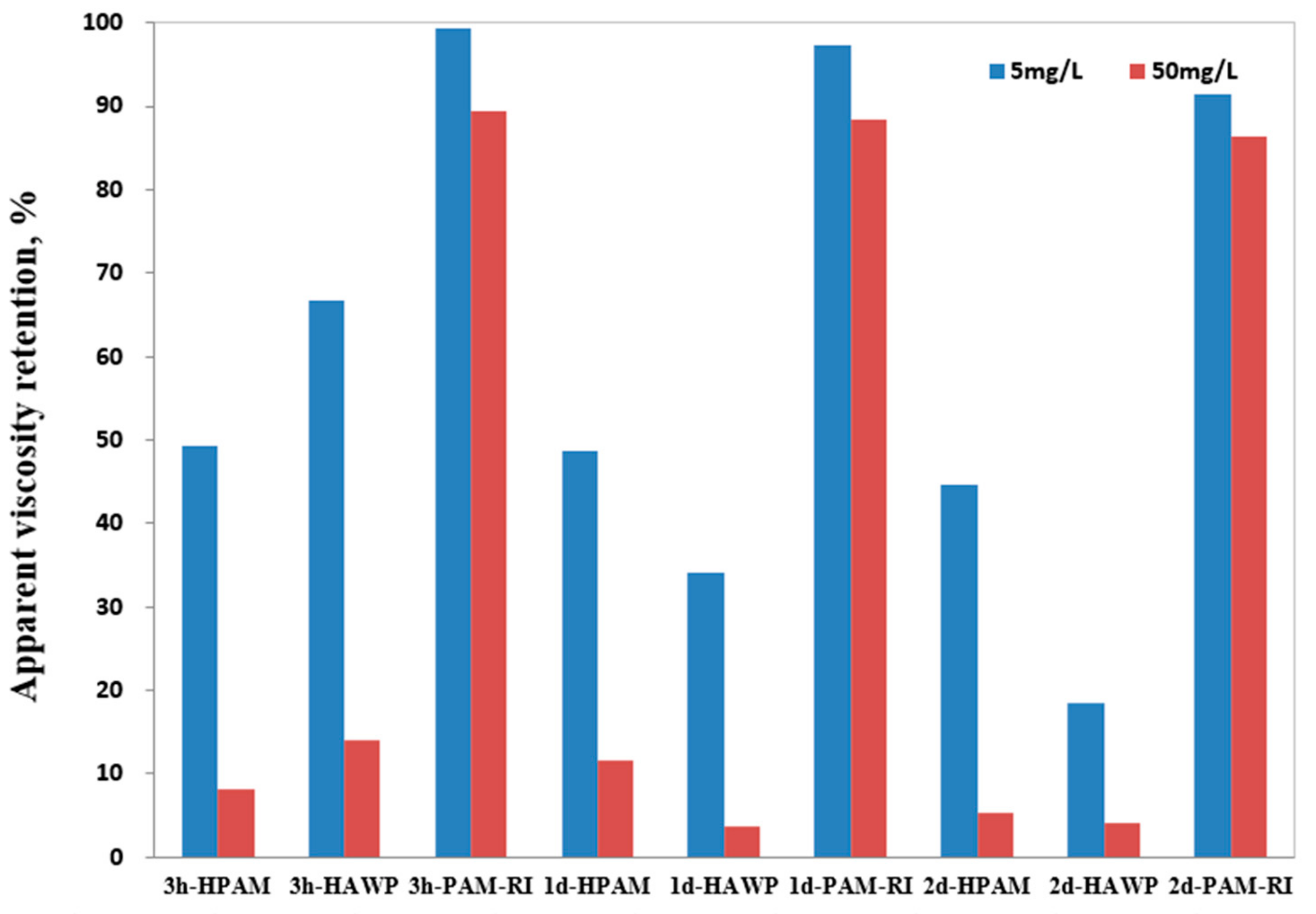
3.6. Evaluation of the Fe2+-Resistant Polymers
3.7. Verification with the Field Water Sample
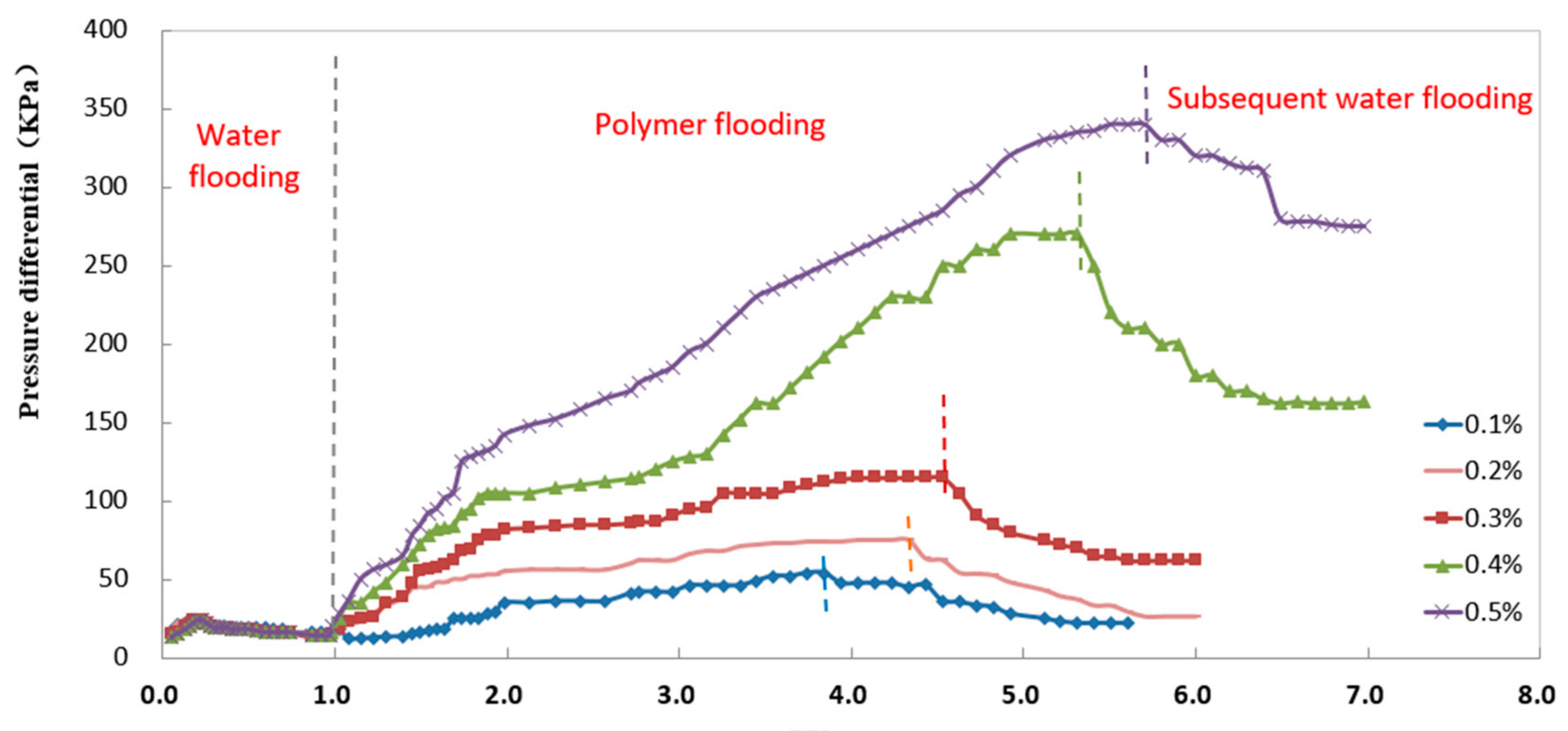
3.8. Resistance Factor and Residual Resistance Factor Tests
4. Conclusions
- (1)
- The new polymer, PAW-IR, exhibits a relatively regular three-dimensional network morphology, and has a good solubility and a favorable thickening performance with regard to oilfield wastewater. It can be completely dissolved in oilfield wastewater within 180 min. It can tolerate an NaCl concentration of up to 23 × 104 mg/L, a Ca2+ concentration of up to 1 × 104 mg/L, an Mg2+ concentration of up to 0.9 × 104 mg/L, and a Fe2+ concentration of up to 90 mg/L, demonstrating favorable thickening performance and resistance to salt and Fe2+.
- (2)
- PAM-IR is prepared by grafting the Fe2+-resistant groups onto the branch chains of the traditional hydrophobically associating polymers synthesized to avoid the viscosity reduction in the polymer solution while capturing the iron ions. Compared with traditional physical methods (e.g., nitrogen production and oxygen isolation, and better design of fluid transfer) and chemical methods (e.g., the precipitation of Fe2+ by the addition of alcohol, formaldehyde, and sodium borohydride), the preparation of PAM-IR does not require additional investment and complex process design, which will improve the overall efficiency of polymer flooding in reservoirs with high concentrations of Fe2+ and Ca2+/Mg2+ in the formation water.
- (3)
- PAW-IR has a good injection performance and can establish high resistance factor and residual resistance factor, indicating a satisfactory plugging performance. It is promising for enhancing oil recovery by flooding with high-salinity and high-Fe2+ polymers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alfazazi, U.; AlAmeri, W.; Hashmet, M.R. Screening of New HPAM Base Polymers for Applications in High Temperature and High SalinityCarbonate Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 12–15 November 2018. SPE-192805-MS. [Google Scholar] [CrossRef]
- Fan, J.; Wei, L.; Luo, W.L. Influencing Factors of the Viscosity Decrease on Polymer Sewage Solution. Oilfield Chem. 2011, 28, 251–253. [Google Scholar]
- Chen, Y.; Li, D.; Song, H.; Song, Y. Research progress of the effect of metal cations on the viscosity of polyacrylamide solution. J. Chem. Ind. Eng. 2013, 34, 36–41. [Google Scholar]
- Li, M.R.; Liu, Z.; Song, X.W.; Ma, B.D.; Zhang, W. Effect of metal ions on the viscosity of polyacrylamide solution and the mechanism of viscosity degradation. J. Fuel Chem. Technol. 2012, 40, 43–47. [Google Scholar]
- YU, Y.; Sun, C.; Huang, J.; Yan, Y.; Li, X.; Xu, W.; Xing, L.; Wu, X.; Wang, J. Reconstruction Project of Iron Removal Process from Oilfield Produced Wastewater for Polymer Injection. China Water Wastewater 2012, 10, 66–69. [Google Scholar]
- Wang, D.; Seright, R.S. Examination of literature on colloidal dispersion gels for oil recovery. Pet. Sci. 2021, 18, 1097–1114. [Google Scholar] [CrossRef]
- Zaitoun, A.; Dupuis, G. Conformance control using SMG microgels: Laboratory evaluation and first field results. In Proceedings of the SPE Europec Featured at 79th EAGE Conference and Exhibition, Paris, France, 12–15 June 2017. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, D. Analysis on Influence Factors of Formulating Polymer Solution with Sewage and Thickening Effect. Contemp. Chem. Ind. 2016, 45, 272–275. [Google Scholar]
- Kang, W.L.; Meng, L.W.; Niu, J.G. Mechanism of the Effect of Salinity on HPAM Solution Viscosity. Polym. Mater. Sci. Eng. 2006, 22, 175–181. [Google Scholar]
- Bin, C.; Xiaoyan, W.; Shanshan, W.; Shijia, C.; Qingquan, Z. Effect of Fe2+ on the apparent viscosity of polymer solution and controlling methods. Chem. Eng. Oil Gas 2014, 43, 168–173. [Google Scholar]
- Wang, Z.; Wang, F.; Zong, H. Influence of iron ions on viscosity of polymer solution and its removal. Ind. Water Wastewater 2017, 48. [Google Scholar]
- Ding, Y.; Zhang, J.; Ma, B.; Zhou, M.; Huang, M.; Li, M.; Hao, Q. Tackification Measures and Mechanism of Polymer Solution Prepared by Sewage. Oilfield Chem. 2013, 32, 123–127. [Google Scholar]
- Yang, H.J.; Luo, P.Y. Factors for and Control of Polymer Degradation in Recycled Produced Water Solutions. Oilfield Chem. 2005, 22, 158–162. [Google Scholar]
- Wang, Z.; Wang, Z. Discussion on Effective Reinjection Method of Produced Wastewater in Polymer Flooding. Environ. Prot. Oil Gas Fields 2018, 22, 40–42. [Google Scholar]
- Yao, C.; Xu, X.; Wang, D.; Lei, G.; Xue, S.; Hou, J.; Steenhuis, T.S. Research and application of micron-size polyacrylamide elastic microspheres as a smart sweep improvement and profile modification agent. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar] [CrossRef]
- Xing, E.; Tao, X.; Jin, L. Determination and Influence Factors of Resistance Coefficient and Residual Resistance Coefficient. Liaoning Chem. Ind. 2016, 45, 284–287. [Google Scholar]
- Sagyndikov, M.; Salimgrayev, I.; Ogay, E.; KSeright, R.S.; Kudaibergnov, S.E. Assessing polyacrylamide solution chemical stability during a polymer flood in the Kalamkas field, Western Kazakhstan. Bull. Univ. Kargnda-Chem. 2022, 105, 99–112. [Google Scholar] [CrossRef]
- Temizel, C.; Putra, D.; Zhang, M.; Moreno, R. Smart nanoparticles for conformance improvement in waterflooding. In Proceedings of the SPE Annual Caspian Technical Conference and Exhibition, Baku, Azerbaijan, 1–3 November 2017. [Google Scholar] [CrossRef]
- Xiong, Z.; Deng, T.; Xu, H.; Jia, F.; Yu, X.; Ma, C. Performance evaluation on a hydrophobically associating polymer. J. Guangdong Univ. Petrochem. Technol. 2022, 32, 24–30. [Google Scholar]
- Shirazi, M.; Kord, S.; Jamialahmadi, M.; Tamsilian, Y. Polymer Enhanced Oil Recovery Process: An Updated, Narrowed Review. Acad. J. Polym. Sci. 2018, 1, 555572. [Google Scholar] [CrossRef]
- Mohsenatabar Firozjaii, A.; Zargar, G.; Kazemzadeh, E. An investigation into polymer flooding in high temperature and high salinity oil reservoir using acrylamide based cationic co-polymer: Experimental and numerical simulation. Orig. Pap.-Prod. Eng. 2019, 9, 1485–1494. [Google Scholar] [CrossRef]
- Rellegadla, S.; Prajapat, G.; Agrawal, A. Polymers for enhanced oil recovery: Fundamentals and selection criteria. Appl. Microbiol. Biotechnol. 2017, 101, 4387–4402. [Google Scholar] [CrossRef]
- Skauge, T.; Ormehaug, P.A.; Alsumaiti, A.; Masalmeh, S.; Skauge, A. Polymer Stability at Harsh Temperature and Salinity Conditions. In Proceedings of the SPE Conference at Oman Petroleum & Energy Show, Muscat, Oman, 21–23 March 2022. [Google Scholar]
- Reichenbach-Klinke, R.; Langlotz, B.; Wenzke, B.; Spindler, C.; Brodt, G. Hydrophobic Associative Copolymer with Favorable Properties for the Application in Polymer Flooding. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Woodlands, TX, USA, 11–13 April 2011. [Google Scholar]
- Shi, L.; Chen, L.; Ye, Z.; Zhou, W.; Zhang, J.; Yang, J.; Jin, J. Effect of polymer solution structure on displacement efficiency. Pet. Sci. 2012, 9, 230–235. [Google Scholar] [CrossRef]
- Zhi, L.I.U. Thickening Mechanism of Hydrophobically Associating Polyacrylamide and Polyacrylamide. Acta Pet. Sin. (Pet. Process. Sect.) 2012, 28, 1037–1042. [Google Scholar]
- Wang, S.; Zhu, G.; Yu, Z.; Li, C.; Wang, D.; Cao, X. Ozonation Degradation of Fiber Water Catalyzed by Fe2+ Pyrophosphate Dodium Complex. Spec. Petrochem. 2020, 37, 15–21. [Google Scholar]
- Dong, Z.X.; Lin, M.Q.; Xin, J.; Li, M.Y. Influence of dissolved oxygen content on oxidative stability of linked polymer solution. Pet. Sci. 2009, 6, 421–425. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Wang, L. Flow law of polymer solution in porous media and mechanism of improving oil displacement efficiency. Daqing Pet. Geol. Dev. 2002, 21, 57–61. [Google Scholar]
- Xulong, C.A.O.; Yanfeng, J.I.; Yangwen, Z.H.U. Research advance and technology outlook of polymer flooding. Reserv. Eval. Dev. 2020, 10, 8–16. [Google Scholar]
- Sagyndikov, M.; Mukhambetov, B.; Orynbasar, Y.; Nurbulatov, A.; Aidarbayev, S. Evaluation of Polymer Flooding Efficiency at brownfield development stage of giant Kalamkas oilfield, Western Kazakhstan. In Proceedings of the SPE Annual Caspian Technical, Conference and Exhibition, Astana, Kazakhstan, 31 October–2 November 2018. SPE-192555-MS. [Google Scholar]
- Seright, R.S.; Wavrik, K.E.; Zhang, G.; AlSofi, A.M. Stability and Behavior in Carbonate Cores for New Enhanced-Oil-Recovery Polymers at Elevated Temperatures in Hard Saline Brines. SPE Reserv. Eval. Eng. 2003, 24, SPE-200324-PA. [Google Scholar] [CrossRef]
- RSeright, R.S.; Skjevrak, I. Effect of Dissolved Iron and Oxygen on Stability of Hydrolyzed Polyacrylamide Polymers. SPE J. 2015, 20, 433–441. [Google Scholar] [CrossRef]
- Jouenne, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]






| Polymer | Characteristic Viscosity (dL/g) | Viscose Average Molecular Weight (106) | Degree of Hydrolysis (mol%) |
|---|---|---|---|
| PAM-RI | 31.5 | 29.2 | 23.9 |
| Fe2+ (mg/L) | Viscosity (mPa·s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 h | 1 d | 2 d | |||||||
| HPAM | HAWP | PAM-IR | HPAM | HAWP | PAM-IR | HPAM | HAWP | PAM-IR | |
| 0 | 28.0 | 60.7 | 47.5 | 27.7 | 60.3 | 47.5 | 28.0 | 60.5 | 47.2 |
| 1 | 26.5 | 55.2 | 47.2 | 26.5 | 58.2 | 47.2 | 25.3 | 55 | 47 |
| 2 | 24.2 | 45.3 | 47.3 | 24.2 | 46.5 | 47.3 | 20.5 | 45.2 | 46.5 |
| 5 | 13.8 | 40.5 | 47.2 | 13.5 | 20.5 | 46.2 | 12.5 | 11.2 | 43.2 |
| 10 | 3.5 | 20.3 | 46.5 | 11.3 | 3.2 | 45.5 | 3.2 | 4.5 | 42.1 |
| 20 | 2.5 | 15.2 | 46.3 | 10.3 | 2.1 | 45.3 | 2.1 | 3.5 | 41.5 |
| 30 | 2.5 | 11.2 | 45.3 | 5.2 | 2.3 | 44.1 | 2.2 | 3.5 | 41 |
| 50 | 2.3 | 8.5 | 42.5 | 3.2 | 2.2 | 42 | 1.5 | 2.5 | 40.8 |
| Core | Gas Logging Permeability (mD) | Water Logging Permeability (mD) | Injected Polymer Concentration (%) | FR | FRR |
|---|---|---|---|---|---|
| 1# | 405.1 | 210.9 | 0.1 | 0.53 | 0.34 |
| 2# | 410.5 | 226.5 | 0.2 | 3.39 | 1.57 |
| 3# | 398.2 | 200.8 | 0.3 | 8.85 | 4.77 |
| 4# | 402.8 | 210.5 | 0.4 | 19.29 | 11.64 |
| 5# | 395.8 | 215.3 | 0.5 | 24.00 | 19.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xu, A.; Ma, M.; Liu, S.; Ni, J.; Zhao, L. Preparation of Polymer Solution for Profile Control and Displacement Using Wastewater with High Ca2+/Mg2+ and Fe2+ Concentrations. Processes 2023, 11, 325. https://doi.org/10.3390/pr11020325
Li X, Xu A, Ma M, Liu S, Ni J, Zhao L. Preparation of Polymer Solution for Profile Control and Displacement Using Wastewater with High Ca2+/Mg2+ and Fe2+ Concentrations. Processes. 2023; 11(2):325. https://doi.org/10.3390/pr11020325
Chicago/Turabian StyleLi, Xuanran, Anzhu Xu, Mengqi Ma, Shanglin Liu, Jun Ni, and Lun Zhao. 2023. "Preparation of Polymer Solution for Profile Control and Displacement Using Wastewater with High Ca2+/Mg2+ and Fe2+ Concentrations" Processes 11, no. 2: 325. https://doi.org/10.3390/pr11020325
APA StyleLi, X., Xu, A., Ma, M., Liu, S., Ni, J., & Zhao, L. (2023). Preparation of Polymer Solution for Profile Control and Displacement Using Wastewater with High Ca2+/Mg2+ and Fe2+ Concentrations. Processes, 11(2), 325. https://doi.org/10.3390/pr11020325










