Abstract
Neurodegenerative diseases (NDs), such as Parkinson’s Disease (PD), Alzheimer’s Disease (AD), Multiple Sclerosis (MS) and amyotrophic lateral sclerosis (ALS), are characterized by progressive loss of structure or function of neurons. Current therapies for NDs are only symptomatic and long-term ineffective. This challenge has promoted the development of new therapies against relevant targets in these pathologies. In this review, we will focus on the most promising therapeutic approaches based on dendrimers (DDs) specially designed for the treatment and diagnosis of NDs. DDs are well-defined polymeric structures that provide a multifunctional platform for developing different nanosystems for a myriad of applications. DDs have been proposed as interesting drug delivery systems with the ability to cross the blood–brain barrier (BBB) and increase the bioavailability of classical drugs in the brain, as well as genetic material, by reducing the synthesis of specific targets, as β-amyloid peptide. Moreover, DDs have been shown to be promising anti-amyloidogenic systems against amyloid-β peptide (Aβ) and Tau aggregation, powerful agents for blocking α-synuclein (α-syn) fibrillation, exhibit anti-inflammatory properties, promote cellular uptake to certain cell types, and are potential tools for ND diagnosis. In summary, DDs have emerged as promising alternatives to current ND therapies since they may limit the extent of damage and provide neuroprotection to the affected tissues.
1. Introduction
Neurodegenerative diseases (NDs), such as Parkinson′s disease (PD), Alzheimer’s disease (AD), multiple sclerosis (MD) and amyotrophic lateral sclerosis (ALS), are a group of progressive and chronic pathological conditions derived from neuronal loss. There are several common features of these syndromes. On the one hand, the cause of these disorders seems to be a combination of genetic factors and environmental conditions, such as aging. On the other hand, these pathologies are characterized by neuroinflammation and the accumulation of aberrant proteins in toxic aggregates responsible for clinical symptoms [1]. Moreover, the prevalence of these disorders is expected to increase in the coming years due to the lack of effective therapies and the increasing life expectancy of the population. Currently, the treatment available for these disorders is symptomatic. Pharmacological and non-pharmacological approaches do not modify the pathological causes of these diseases or their progression, resulting in a loss of effectiveness and clinical benefits over time. Therefore, NDs represent one of the world’s greatest health challenges and claim the development of new therapeutic approaches. New emerging strategies for the treatment of NDs are known as disease-modifying therapies (DMT), specially designed to stop or slow down the pathological course of these diseases and improve the quality of life of patients. DMT includes small molecules and non-drug therapies, such as gene therapy approaches, neurotrophic factors or cell-base treatments, which are in different phases of preclinical and clinical trials, extensively reviewed (for PD [2,3,4] for AD [5,6,7,8] and for MS [9,10]).
Dendrimers (DDs) are well-defined polymeric structures that offer a multifunctional platform for developing different nanosystems with multiple applications. They have been proposed as interesting drug delivery systems with the ability to cross the blood–brain barrier (BBB) and increase the bioavailability of classical drugs in the brain. In addition, DDs have demonstrated their efficacy in the delivery of genetic material by reducing the expression of specific target proteins. Moreover, DDs have shown promise as anti-amyloidogenic systems, exhibit anti-inflammatory properties, promote cellular uptake to certain cell types, and are potential tools for the diagnosis of ND. Finally, DDs have demonstrated greater aqueous solubility, biocompatibility, polyvalence and precise molecular weight than traditional polymers, making them suitable for targeted drug delivery to the brain [11,12,13,14,15,16,17].
In this review, we will focus on different therapeutic approaches based on DDs specially designed for the treatment and diagnosis of NDs. We will describe the most promising dendrimer nanoparticles currently employed as gene delivery systems, drug carriers or DDs with inherent anti-inflammatory properties, and finally, we will also summarize their use as diagnostic tools.
2. Nanomedicine
Nanotechnology is an innovative branch of science and engineering that involves the design, synthesis, characterization and application of materials and devices at atomic, molecular and macromolecular scales with nanometric size (10−9 to 10−6 m). It is a multidisciplinary field that integrates physical, chemical and biological sciences with engineering. It is a recent cutting-edge technology with high potential in several areas, such as electronic, chemical engineering, environmental and food industry, pharmacology, and medicine, due to the possibility of creating novel products with new features. One of the most attractive fields of application of nanotechnology is nanomedicine, which aims to prevent, diagnose and treat different diseases [11]. Nanomedicine has been persistently and exponentially growing over the last decades. The role of nanotechnology in biomedical sciences is mainly focused on improving the properties of diagnostic or therapeutic approaches by means of nano-size compounds, also called nanoparticles (NPs), which are used for a variety of purposes. For example, NPs are excellent drug carriers with the ability to increase the bioavailability of therapeutic compounds by different mechanisms, such as increasing water solubility [12], targeting the desired tissue [13], increasing blood circulation time [14], avoiding enzymatic degradation, evading immune system [15] or allowing more efficient administration routes. This is the case with intranasal delivery instead of oral intake for the treatment of NDs [16]. Moreover, NPs are also an excellent tool for delivering genetic material [17] with the ability to protect it from degradative enzymes inside the human body [18]. On the other hand, NPs can be used as diagnostic compounds due to their chemical nature, such as magnetic nanoparticles (MNPs), which act as contrast agents for imaging [19] or as theragnostic compounds that function as both therapeutic and diagnostic tools [20,21]. There are several types of NPs with potential biological applications in nanomedicine, such as liposomes (LP), DDs, gold NPs, polymeric micelles, metallic NPs, fullerenes or quantum dots [22]. The particle size and surface area of these NPs are critical factors that determine their interaction with biological systems. Apparently, as the size of the materials decreases, there is an exponential increase in surface area relative to volume, and more atoms are on the surface compared to those in the interior. This makes nanomaterials more chemically reactive. For this reason, particle size and surface area determine not only the biological function of NPs but also their toxicity [23] and biodistribution [24]. Despite their excellent properties, NPs inevitably bind to biomolecules in biological fluids, forming the “protein corona” when they are introduced into living systems [25]. In addition to the bio-nano interaction, NPs are also recognized as foreign substances by the human body, and they are usually sequestered, degraded and eliminated by the mononuclear phagocytic system, leading to rapid clearance, low efficiency, and high liver accumulation. As a consequence, few NPs have been included in phase 1 clinical trials due to the limited characterization of several aspects, such as pharmacokinetic parameters or immune response [26]. To overcome this issue and improve their pharmacokinetic profile, NPs are usually decorated on their surfaces. Different molecules, including polymers and biomolecules, have been linked to NPs to reduce their interactions with serum proteins and increase their availability in the bloodstream. For example, polyethylene glycol (PEG) addition is usually employed to increase blood clearance. However, these structural modifications may also affect the biological function of NPs and hinder their uptake by the target cells [27]. Therefore, an in-depth knowledge of the pharmacokinetic, pharmacodynamic and toxicity profile of functionalized NPs is required prior to their use in humans.
Nanotechnology has been widely applied for both diagnostic and therapeutic purposes in several pathologies, such as NDs [28], inflammatory diseases [29], cardiovascular problems [30] and infectious diseases [31]. Currently, the best known use of nanotechnology in the clinical setting is the mRNA-based COVID-19 vaccines, which encapsulate the mRNA of the SARS-CoV-2 spike protein into lipid-base nanoparticles (LNPs) and deliver it into host cells [32,33]. In addition, nanotechnology has been extended to the clinic for oncology therapies [34] and there are some nano-formulations approved for medical use, such as Vyxeos®, which consist of cytarabine plus daunorubicin encapsulated in LP for the treatment of acute myeloid leukemia [35]. NBTXR3, an NP coated with negatively charged phosphate groups, is currently being studied in a I-II phase clinical trial (NCT02805894) for the treatment of intermediate/high risk prostate cancer. Another example is Patisiran/ONPATTRO® formulation, a lipid-based delivery system approved for the treatment of hereditary transthyretin amyloidosis [36].
Dendrimers
The word “dendrimer” was created by Prof. Donald Tomalia from the Greek words δέντρo (dendro) and μέροσ (meros), which translates to “tree and “part,” respectively [37]. DDs are highly branched, three-dimensional synthetic macromolecules with a well-defined structure. They are synthesized with precise control of their size, shape and terminal group functionality [38]. In their basic structure, DDs contain three different parts: a central core (either a single atom or a group of atoms having two or more identical chemical functionalities), the external branches emanating from the core as repetition units and the terminal groups located on the surface of the NP. The assembly of units is organized in a geometric progression from the core, which results in a series of radially concentric layers. The sequential growth of DDs is identified with a specific “generation number” (G) and each generation is classified in terms of size, shape, molecular weight and number of surface functional groups. The core is defined as Generation 0 (G0), while the successive addition of branching units gives rise to higher generations such as G1, G2 or G3, e.g., (Figure 1) [38]. The core and the layers of the branching units (generations) affect DD morphology, specifically in the three-dimensional shape. Instead, the internal part determines the hostguess properties of the DDs. Finally, the external charge, the flexibility of the branches and the chemical nature of terminal groups determine their functionality and their interaction with solvents, other molecules or cell structures [39]. Lower generations of DDS are more flexible and have more open and amorphous structures than higher generation DDs, which can adopt spherical conformations. This is known as the “dendritic effect” and is recognized as a unique property dependent on the architecture and size of the DD [40]. The structure of DDs can be precisely controlled and reproduced during their stepwise synthesis by divergent or convergent strategies, hypercore and branched synthesis, “click chemistry,” lego chemistry and double and mixed exponential growth methods [41]. Divergent and convergent methods are the most commonly used. In divergent synthesis, the DD structure initiates at the central core and monomers are added sequentially to create the desired DD generation. However, in the convergent method, DDs start at the periphery and grow to the central core [42]. Consequently, DDs are compounds with well-defined structures, narrow polydispersity and multivalent properties [38]. Unlike other NPs, DDs are characterized by low cytotoxicity, high efficiency to penetrate into the cells and intrinsic fluoresce [43]. Moreover, DDs present additional advantages, such as high stability [44] and the possibility of including a large number of active therapeutic drugs covalently [45]. On the other hand, DD properties can be easily tuned by replacing their terminal groups and modifying the external electrical charge. Consequently, three different families of DDs can be distinguished: cationic, anionic and neutral. Cationic DDs have tertiary amines as terminal functions, which are subsequently protonated, providing a net positive charge to the molecule. Instead, negatively charged DDs are classically obtained by grafting carboxylic acids as terminal functions from which sodium salts are easily obtained. In marked contrast, the use of neutral dendrimers has been poorly explored [39].
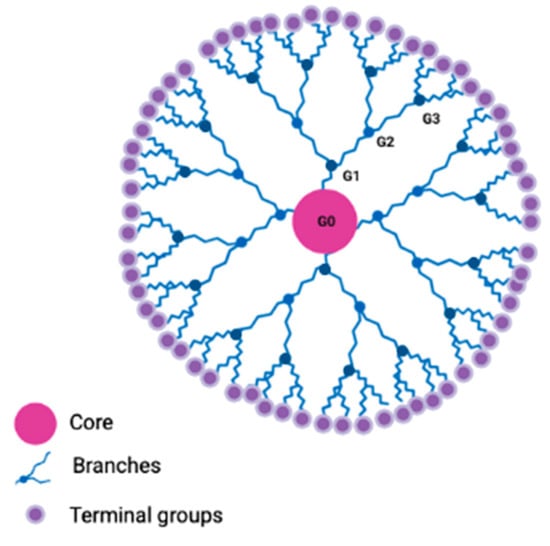
Figure 1.
Dendrimer basic structure. DD architecture presents three well-differentiated parts: the central core (in pink), the branching units (in blue), and the terminal groups (in purple). Generation number (G) is indicated as G0, G1, G2 and G3.
DD cytotoxicity depends on the structure and type of terminal groups [46]. Interestingly, the polyvalency or the addition of several chemically different end groups provides versatile functionalization that might produce multiple interactions with specific biological receptor domains. Moreover, the structure of DDs can be easily modified on their surface to provide them with additional multifunctional properties [47]. Functionalization of the periphery by the addition of diverse functional groups can improve interesting properties, such as pharmacological and pharmacokinetic profiles, selectivity for the target, and physicochemical features of the drugs, such as viscosity, solubility and stability [48]. For instance, DD decoration with transferrin facilitates the penetration of the BBB by interacting with specific receptors located on the surface of endothelial cells [49]. Other examples of surface modifications are PEGylation [50] or carbohydrate coupling, which reduce DD toxicity and increase stability in biological media [51,52].
To date, more than 200 kinds of DDs have been synthesized and grouped into different families according to their structure, including poly(amidoamine) (PAMAM), poly(propyleneimine) (PPI), poly(L-lysine) (PLL), poly(carbosilane) (CBS) and phosphorous-containing DDs [53].
The PAMAM family of DDs is one of the most widely used nanomedicines due to its hydrophilic profile and biocompatible properties [41]. This type of NP was defined by Tomalia and co-workers in 1985 [37] and is clearly recognized by a symmetrical structure. They consist of a core, synthesized by the addition of ethylenediamine, diaminododecane, diaminoexane and diaminobutane, followed by the internal branches of methyl acrylate and ethylenediamine and the final amide or carboxilic end-groups [54].
PPI DDs, the oldest class of DDs, were produced by Voegel in 1978. PPI DDs are synthesized by the divergent method by the addition of acrylonitrile, diamine or diaminobutane [55,56]. The most important advantages of PPI DDs include high biocompatibility, high surface area and fast electron transfer rate [57].
PLL DDs are polyaminoacidic structures of PLL organized in linear or cyclic structures. They have gained attention due to their high biocompatibility and easy functionalization of terminal groups [58].
CBS DDs are silicon-containing DDS, first defined in 1992. They are dendrimeric structures containing Si-C bonds at the branches, which provide high stability, flexibility and low polarity to the compounds. Several types of CBS DDS have been synthesized using the divergent method [59].
Phosphorous-containing DDs were defined in 1990. The first example of phosphorous DDs built with phosphonium at each branching point was reported by Engel and co-workers. However, it has never been used for biological purposes [60]. In 1994, Majoral and his group described the first method of synthesis for large phosphorous DDs, which is the most widely used [61]. There are three different families of phosphoDD based on the surface charges: cationic, anionic and neutral. Positively charged DDs were found to be more toxic than negatively charged or neutral DDs [40].
Among their biological applications, DDs are emerging as promising candidates for many applications in nanomedicine and deserve attention as solubility enhancers, carriers for drugs and biological entities and imaging agents [47]. In particular, DDs are interesting drug delivery systems [62,63] in cancer [64,65], NDs [66], ocular problems [67], infectious diseases [68,69] and fungi contamination [70]. DDs are efficient transfection agents for genetic materials, with special attention as carriers in the nervous system. Different ligands can be coupled to DDs to use as transfection reagents. For example, DDs are able to transport small interfering ribonucleic acid (siRNA), deoxyribonucleic acid (DNA) or plasmids into different cell types [64,71,72,73]. The transfection efficiency of DDs depends on the chemical nature of the terminal groups and their size, with the G3, G4 and G5 generations being the most efficient. Similar to other NPs, DDs can be easily modified on their surfaces to improve their therapeutic efficacy [74]. Apart from its use as a gene delivery system, DDs are powerful bioimaging and diagnostic tools for cancer [75,76]. Moreover, DDs offer additional applications in nanomedicine due to their versatility. Several studies have demonstrated its potential as drugs per se as promising anti-cancer agents or powerful anti-inflammatory molecules [29,77], even as key components included in the formulation of biomaterials, such as biochips [78] and vaccines [74].
3. Use of Dendrimers in Neurodegenerative Diseases
3.1. Alzheimer’s Disease
Since Alois Alzheimer described AD in 1907, it has been considered the most prevalent ND worldwide and the major cause of dementia [79]. AD is a progressive and chronic disabling pathology characterized by cognitive impairment, behavioral disturbances, and loss of memory and functional ability to perform daily tasks [80]. Its incidence increases proportionally with the aging of society, especially those over 75 years of age, and it is estimated that the current 45 million people affected will increase to triplicate the number of patients by 2050 [81]. The pathogenesis of AD is not fully understood, but neuronal loss and hippocampal and cortical atrophy are related to two main pathological features: the deposition of extracellular amyloid plaques and intracellular neurofibrillary tangles (NFT) [82]. The main component of amyloid plaques is the aberrant folded amyloid beta (Aβ) peptide with 42 amino acids [83], while hyperphosphorylated Tau protein (p-Tau) is the predominant element of NFT [84]. According to the amyloid cascade hypothesis, Aβ formation and subsequent aggregation in amyloid plaques is the initial step in the onset of AD and determines the following deposition of p-Tau in NFT. Cognitive impairment and progression of clinical symptoms correlate better with NFT accumulation than amyloid pathology [85]. Instead, progressive synaptic dysfunction and neuronal degeneration are attributed to neuroinflammation due to microglia activation [86].
Classical therapy for AD aims to improve progressive cognitive decline and impairment of functional abilities, such as memory. Pharmacological treatment includes acetylcholinesterase (AChE) inhibitors (donepezil, galantamine and rivastigmine) [87] and antagonists of N-metyl-D-Aspartate (NMDA) receptor (memantine) [88], in combination with non-pharmacological therapies to improve the performance of daily tasks [89,90]. However, current AD therapy is only symptomatic and manages the clinical manifestations of the disease for a short period of time. Available treatments do not correct the primary causes of the pathology and are unable to prevent neuronal loss and brain atrophy that lead to cognitive impairment. This is the main reason supporting the development of new strategies aimed at modifying the pathological course of the disease.
3.1.1. Dendrimers for AD Therapy
Dendrimers for Decreasing β-Amyloid Aggregates
In the amyloidogenic pathway, Aβ peptide is initially found as a monomer after the processing of the amyloid precursor protein (APP) by β-secretases, such as β-amyloid converting enzyme 1 (BACE1) and γ-secretase enzyme. The length of Aβ peptide depends on the cutting site of γ-secretase. The peptide composed of 42 amino acids is the major compound and the most toxic species due to its tendency to aggregate, although Aβ 40 is also found in amyloid deposits [91,92]. The progressive self-aggregation of Aβ peptide forms larger harmful species, such as oligomers, protofibrils, fibrils and the final insoluble amyloid plaques, which are the major hallmarks of the brains of AD patients [93]. Therefore, the reduction of toxic amyloid plaques is proposed as one of the most promising therapeutic strategies for slowing the progression of AD. To this end, several mechanisms involving DDs have been described, including direct interaction of DDs with Aβ oligomers, inhibition of Aβ aggregation and degradation of existing fibrils (Figure 2).
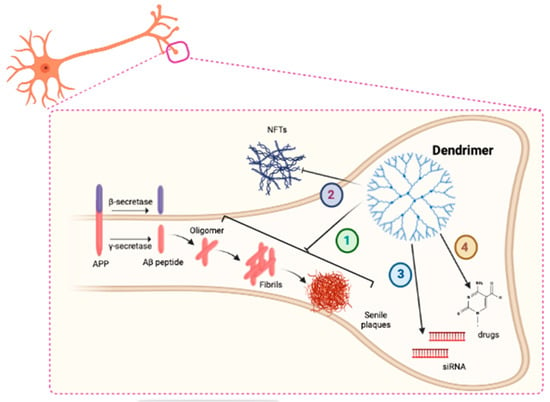
Figure 2.
Schematic representation of the main mechanism of action of DDs in AD (1). DDs could interact with Tau and reduce its aggregation into neurofibrillary tangles (NFTs) (2). DDs could act as delivery systems for siRNA molecules (3) and drugs (4) in neurons.
Several studies support the use of DDs as candidates for avoiding Aβ-induced toxicity. For example, Chafekar and colleagues described a G1 cysteine DD couple to the KLVFF peptide to form monomeric (K1) or tetrameric (K4) structures. Both complexes prevented Aβ 42 aggregation into amyloid fibrils, but the dendritic K4 form showed a better inhibitory profile than the K1 structure, and it was also able to disaggregate existing fibrils and reduce amyloid deposits, supporting its use for AD therapy [94].
Additionally, PAMAM DDs have also been employed as interesting anti-amyloidogenic tools. Three different generations of PAMAM DDs (G3, G4 and G5) avoided amyloid deposition of the 28 aa Aβ peptide (Aβ 1-28) and additionally promoted the disaggregation of existing fibrils in amorphous structures. The magnitude of the effect increased in a concentration-dependent manner and was also proportional to DD generation. The larger PAMAM DD generations were the most effective in disrupting the amyloid aggregation process [95]. In addition, the same group described the effect of G3 PAMAM DDs on the aggregation of Aβ 1-28 peptide. G3-PAMAM DD inhibited the formation of amyloid plaques due to a direct interaction between DD and the peptide. While low DD concentrations affected the nucleation process, high DD concentrations modified the elongation rate and the interaction of monomers with existing small aggregates [96]. Additionally, bio decorated PAMAM DDs have been explored as powerful tools to decrease the accumulation of senile plaques. G0 PAMAM DDs attached to helical β-peptide KLVFFAE or foldamers recognized Aβ oligomers avoiding their aggregation. Foldamer-DD complexes showed protective properties against Aβ toxicity in murine hippocampal slices by reducing long-term potentiation [97].
Although DDs have been demonstrated to be promising anti-amyloidogenic systems, cationic dendrimers are often cytotoxic because of the interaction with negative charges on cellular membranes [98]. One strategy for reducing the cellular toxicity of DDs is to decorate their surface with glycidic structures, such as maltose, to form glycodendrimers. This approach has been explored by several groups. For example, in a first study, Klementieva and colleagues showed that G4 and G5 maltose-PPI DDs reduced the aggregation of the amyloid peptide fragment of 40 aa (Aβ 40) in a generation-dependent manner. Moreover, they were biocompatible with PC12 and SH-SY5Y cell lines [99]. The G5 DDs avoided amyloid toxicity by forming amorphous structures, whereas the G4 conjugates decreased Aβ deposition without affecting preformed fibrils. A similar neuroprotective effect of PPI glycodendrimers on aberrant amyloid fibrillation has been observed in a second study. Different neutral and cationic G4 and G5 PPI DDs, coupled to maltose or maltotriose, protected SH-SY5Y cells against Aβ 42 toxicity, but only neutral PPI glycodendrimers reduced the toxicity of Aβ peptide obtained from human AD brain extracts. Moreover, both cationic and neutral G4 and G5 conjugates decreased Aβ levels in transgenic mouse models of AD, although they did not reverse memory impairment [51]. The protective effect of PPI DDs against Aβ 1-28 aggregation was also confirmed by modulation of amyloid fibrillation in a pH-dependent manner. At higher DD concentrations, the amyloidogenic process was totally inhibited, but at low DD concentrations, the amyloid aggregation process depended on pH. At acidic pH values, PPI dendrimers interacted more strongly with the peptide and interrupted the fibrillation process, reducing amyloid aggregates [100].
Among the potential molecules that can interfere with Aβ fibrillation, the effect of PLL DDs of the third (G3) and fifth generations (G5) was also characterized in the SH-SY5Y cell line. Only G3 DDs avoided amyloid plaque formation in solution and toxicity of Aβ 42 in neuroblastoma cells [101].
Oligomeric species of Aβ are considered the most neurotoxic species in the pathological course of AD. Phosphorous DDs have been proposed as powerful tools for controlling the fibrilization of intermediate oligomers and avoiding subsequent toxicity in AD. Specifically, G3 and G4 cationic phosphorus-containing DDs inhibited the aggregation cascade of Aβ 1-28 and reduced cytotoxicity in the N2a cell line [102,103].
A different useful option to prevent Aβ 1-28 toxicity is gallic acid-triethylene glycol (GATG) G3 morpholine-decorated DDs (mor-G3). Interestingly, although this kind of DDs accelerated the aggregation of Aβ 1-28 and increased the amount of fibrils, the complexation of mor-G3 DDs with fibrillary species showed a protective effect by reducing Aβ prefibrillary forms and cytotoxicity in B14 fibroblast [104].
Dendrimers for Decreasing Tau Aggregation
Tau is a neuronal protein that promotes the assembly of tubulin and contributes to the stability of microtubules. The deposition of Aβ peptide promotes the hyperphospho-rylation of Tau, which modifies its physiological function and determines its accumulation in NFTs [105].
DDs have been proposed as good candidates for reducing NFT deposition (Figure 2). For example, G3 and G4 phosphorus-containing DDs were able to interact with Tau and promote its assembly into amorphous structures, thereby reducing its aggregation in NFTs. Both DDs modified the length of fibrillary structures, and additionally, G3 derivatives also influenced the amount of fibrillar aggregates [102].
Dendrimers for AD Treatment with Different Mechanisms: Miscellaneous
In addition to their role as nanocarriers or anti-amyloidogenic systems, DDs are considered per se potential drugs to slow or delay the progression of AD. For example, G4 PPI DDs decorated with histidine and maltose (G4-PPI-HisMal) showed neuroprotective properties to address the cognitive decline associated with AD. The treatment of AD APP/PS1 transgenic mice with G4-PPI-HisMal increased the expression of synaptic markers and prevented memory impairment without affecting the amount of amyloid plaques [106]. The use of DDs to improve cognitive decline in AD has also been performed with smart DDs, such as ALZc3. ALZc3 is a Mg-coupling DD at its core with chelating properties and antioxidant activity, useful for improving spatial memory in a rat model of AD based on Aβ-peptide toxicity [107]. The neurodegenerative process of AD is also a consequence of neuroinflammation and the reduction of cholinergic activity. Apart from their role in the modulation of Tau aggregation, cationic phosphoDDs were proposed as interesting drugs for AD therapy due to the combination of several mechanisms, such as the reduction of oxidative stress and the inhibition of AChE activity, to enhance cholinergic transmission [103].
3.1.2. Dendrimers as Vehicles of Therapeutic Genes
Another potential strategy for AD is based on targeting Aβ production by inhibiting the enzymes responsible for its synthesis, such as BACE1 and γ-secretase. One approach to downregulating protein expression levels focused on the delivery of siRNA using DDs as vehicles (Figure 2). Zhang and colleagues developed an siRNA delivery system formed by dendrigraft poly-L-lysines (DGL) coupled with NL4 peptide and apolipoprotein A-1 to enhance cellular uptake. This biocompatible gene carrier allowed the silencing of the BACE1 protein in PC12 neuronal cells [108]. On the other hand, the inhibition of γ-secretase activity has also been performed using a different approach. G0 and G1 lysine dendrons were employed as vehicles for the γ-secretase modulator Flurbiprofen. Both types of complexes successfully increased the penetrance of the drug through an in vitro model of the BBB based on bEnd.3 endothelial cells and reduced the activity of γ-secretase in C6 glial cells, [109]. A multifunctional carrier consisting of PEGylated DGL has been studied for the treatment of AD. This vehicle was designed as a dual delivery system of a specific peptide to target NFTs (TLKIV peptide) and short hairpin RNA (shRNA) molecules to knock down the BACE1 enzyme. The carrier showed effectiveness in crossing the BBB in double APP/PS1 transgenic mice of AD and decreased the accumulation of NFT in the hippocampus and blocked the activity of BACE1, ameliorating memory decline [110].
3.1.3. Dendrimers as Vehicles of Drugs in AD
The successful treatment of AD requires the transport of drugs to the target tissue, the brain (Figure 2). DDs have been proposed as interesting drug delivery systems with the ability to cross biological barriers, including the BBB, and to increase the bioavailability of drugs in the brain. Current therapy for AD includes AChE inhibitors and modulators of glutamatergic excitotoxicity [111]. To support drug penetration across the BBB, different G3 PAMAM DDs conjugated with lactoferrin have been designed to vehicle the NMDA receptor antagonist memantine. The complex improved the pharmacokinetic properties of the drug, increased its bioavailability in the brain and ameliorated the memory decline in AD murine models [112]. The PAMAM-lactoferrin complex was also employed as an effective strategy to increase the brain bioavailability of rivastigmine, an AChE inhibitor. This system was more effective than rivastigmine alone in improving motor and spatial learning and slowing memory loss in in vivo models of the disease [113]. G4 and G4.5 PAMAM DDs have been reported to modify the bioavailability of carbamazepine. While the drug poorly dissolves in water, the complexes increased solubility properties and reduced cytotoxicity in human red blood cells and the N2a line, as well as the side effects in zebrafish [114].
Apart from improving drug pharmacokinetic properties, DDs have been demonstrated to be interesting tools for reducing the toxicity of classical treatments for AD. For example, tacrine treatment restores impaired cholinergic activity in AD patients but induces severe side effects, such as liver damage. G4 and G4.5 PAMAM DDs reduced tacrine-induced hepatotoxicity. The electrostatic interaction between DDs and tacrine reduced the cytotoxic effect of the drug on human red blood cells and the N2a cell line and the hepatotoxicity in zebrafish without altering its pharmacological activity [115].
3.1.4. Dendrimers for AD Diagnosis
The ability of dendrimers to interact with molecules has been exploited to detect key proteins involved in the pathological course of AD. For example, PAMAM DDs coupled to gold nanoparticles were employed to characterize Tau levels in the plasma and brain samples of AD patients by sandwich immunoassay and amperometry. To this end, Razzino and co-workers designed a 3D-Au-PAMAM covalently attached onto electrografted p-aminobenzoic acid, and the specific capture antibody was immobilized on the amino groups of the DD. This system resulted in high selectivity and a detection limit in the picomolar range [116].
The application of DD for the treatment and diagnosis of AD is summarized in Table 1.

Table 1.
DDs for AD therapy and diagnosis. Abbreviations: AD (Alzheimer’s disease), GATG (gallic acid-triethylene glycol), DD (dendrimer), PLL (poly-L—lysine), DGL (dendrigraft poly-L-lysines), NFT (neurofibrillary tangles), BACE1 (β-amyloid converting enzyme 1), PEG (polyethylenglicol), AChE (acetylcholinesterase).
3.2. Parkinson′s Disease
More than two hundred years ago, James Parkinson described PD as an involuntary shaking condition [117]. However, nowadays it is considered a complex and chronic neurodegenerative disorder that worsens with age and leads to intellectual and physical disability [118,119]. PD is the second most common pathological condition derived from neuronal degeneration and its prevalence is expected to increase in the following years with the aging of population, until 15 million people worldwide [120,121]. PD is clearly recognized by classical manifestations such as hands shaking, bradykinesia, resting tremor and postural instability. These symptoms arise in the late stages, when neuronal damage is not reversible, and worsen over time due to disease progression. In addition, patients also present non-motor manifestations, such as cognitive impairment, sleep disorders, depression and gastrointestinal or olfactory disturbances in the initial or prodromal phase [122]. Clinical symptoms are related to hypodopaminergic function due to the reduction of dopamine levels in the striatum derived from the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Moreover, abnormal cholinergic activity and widespread neuronal degeneration are also involved in both motor and non-motor symptoms. The second pathological hallmark of the disease is the identification of Lewy Bodies (LB) and Lewy Neurites (LN) as intraneuronal cytoplasmatic protein inclusions enriched in α-synuclein (α-syn) [123].
The current pharmacological treatment of PD is mainly based on dopaminergic therapy. The disease is basically managed with Levodopa, which, since the 1960s, has been the most effective treatment to restore dopaminergic activity, in combination with anticholinergic drugs [124]. However, these therapies are only symptomatic strategies and long-term ineffective because they do not modify the course of the disease. This challenge has been addressed by the development of DMT, which includes several approaches against relevant targets in PD pathology.
3.2.1. Dendrimers for PD Therapy
Dendrimers for Decreasing α-Synuclein Aggregates
Aggregation of proteins is a natural consequence of neuronal aging [125] but in some pathological situations such as PD, accumulation of toxic proteins accelerates and becomes a hallmark of the disease. Initially, protein aggregates play a protective neuronal strategy to isolate dangerous proteins, but the progressive accumulation of proteins, in particular α-syn, in cytosolic insoluble deposits is detrimental to neurons and triggers neurodegeneration [126,127]. Among the physiological functions of α-syn, its role in the control of intracellular vesicle trafficking and synaptic release has been highlighted [128]. The proper functionality of α-syn depends on its structure and correct folding. The protein acquires several conformations in equilibrium, from native monomers or tetramers to more complex structures, such as oligomers. α-syn is composed of three different domains: a hydrophobic central region, flanked by an N-terminal and a C-terminal domain. The central part of the protein is responsible for the acquisition of β-sheet conformation and oligomer formation [129]. Progressive accumulation of oligomers into α-syn fibrils within neurons constitutes the main element of the characteristic LB [130,131] (Figure 3). Aggregation of α-syn into fibrils and its subsequent cell-to-cell transmission represent a pathological gain of function mechanism for the protein, essential for the propagation of PD to surrounding cells [132]. Therefore, α-syn emerges as a key therapeutic target in PD, and decreasing α-syn deposition becomes a major approach to protect neurons from toxic aggregates, prevent neurodegeneration and delay the onset or progression of PD.
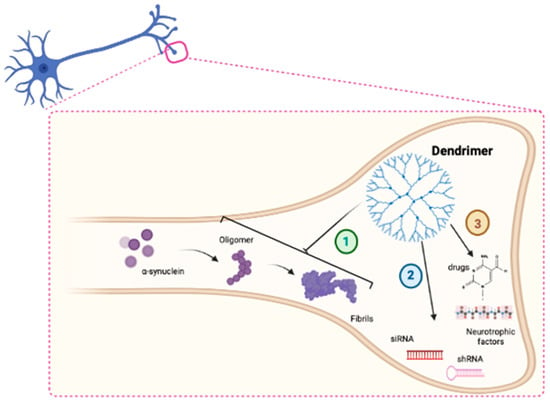
Figure 3.
Schematic representation of the main mechanism of action of DDs in PD (1). DDs could block α-syn fibrillation (2). DDs could act as delivery systems for siRNA molecules (3) and drugs in neurons.
DDs have emerged as effective tools to block α-syn fibrillation (Figure 3). Two independent studies have shown the anti-aggregating properties of PAMAM DDs in PD. In the first of these, Rekas and colleagues demonstrated that G3 to G5 PAMAM DDs inhibited α-syn fibrillation and disaggregated preformed fibrils. The dual effect is dependent on the concentration and generation number [133]. In a second study, a further characterization of PAMAM DDs’s structure suggested that only G4 DDs, with terminal amino groups, were able to inhibit α-syn fibrils formation, unlike G3.5 DDs, functionalized with carboxyl groups on the surface [134].
On the other hand, a different DD system, based on phosphorous addition, has been proposed as a promising candidate to reduce α-syn aggregation. Milowska and colleagues showed that G3 and G4 phosphorous DDs blocked α-syn fibrillation until a 0.5 µM concentration [135]. These authors also demonstrated that phosphorous DDs with antiviral properties, termed viologen-phosphorous DDs, are suitable compounds for controlling α-syn aggregation and toxic fibril formation [136].
Alternatively, CBS DDs have been described as effective therapeutic agents in a rotenone-induced mouse model of PD. DDs exerted a dual neuroprotective mechanism due to both their ability to encapsulate and complex rotenone and to prevent fibrillation of α-syn [137]. Interestingly, CBS DDS prevented rotenone damage more efficiently than PAMAM, phosphorous and viologen-phosphorous DDs [138]. The effectiveness of CBS DDS in modulating the aberrant accumulation of α-syn has also been explored in induced pluripotent stem cell (iPSC) lines obtained from PD patients carrying the LRRK2 G2019S mutation. These results highlight the potential of CBS DDs for PD therapy [139].
3.2.2. Dendrimers as Vehicles of Therapeutic Genes
It is widely accepted that PD has a multifactorial origin. PD is the result of a complex relationship between individual genetic susceptibility and environmental factors that increase the risk of developing the disease [140]. Although most PD cases are sporadic forms, a small proportion of PD patients receive a pathological genetic variant through a familial hereditary pattern. Among the main genetic causes linked to PD it has been described mutations in several genes such as SNCA, LRRK2, PINK1, DJ-1, VPS35 and Parkin [141]. Loss-of-function studies have been designed to reduce protein expression levels derived from key genes to minimal amounts, mimicking the effect of protein silencing. In this context, DDs have emerged as potential carriers for introducing genetic materials, such as nucleic acids, into the cells (Figure 3). In physiological conditions, positive charges on the surface of DDs (generally amine groups) can react with negative charges of nucleic acids to form dendriplexes, which are internalized through different cellular mechanisms [142]. Among the genetic causes related to PD, the discovery of mutations in the gene encoding α-syn (SNCA) was the first characterized [143]. In particular, missense point mutations and duplications or triplications of the SNCA gene are linked to autosomal dominant forms of PD [144]. One possible explanation for the toxicity is the increase in protein levels. A potential therapeutic option for managing α-syn load is the reduction of protein synthesis, although downregulation of α-syn has typically been accomplished by viral carriers rather than by dendrimeric structures [145,146,147].
Loss-of-function studies can also be designed to modulate cellular pathways that are modified during the pathological course of the disease. Restoring the activity of specific targets through RNA technology is an alternative to ameliorate the symptoms. For example, a delivery system based on a DGL vector, coupled with rabies virus glycoprotein peptide and complexed with caspase-3 shRNA, successfully improved motor performance in rotenone-treated rats as a murine model of PD. This strategy reduced caspase-3 protein expression and dopaminergic neuronal loss [148].
3.2.3. Dendrimers as Vehicles of Drugs or Trophic Factors
Among the main reasons supporting the use of DDs as vehicles for therapeutic compounds are the improvement of pharmacokinetic properties and the safety profile [149]. A DD system based on G4.5 of carboxyl decorated PAMAM DDS was successfully used to complex the drug carbamazepine. This formulation improved specific drug properties, such as solubility and safety profile, due to the reduction of the dose required and consequent toxicity in human red blood cells, the N2a cell line and zebrafish [114].
However, the main challenge in the treatment of NDs, including PD, is the transport of drugs into the brain. Drugs are combined with DDs to improve pharmacodynamic properties and enhance the access of the active compounds to specific biological targets (Figure 3). For example, the gene encoding for the trophic factor human glial-derived neurotrophic factor (hGDNF) was loaded into an angiopep-coupled DGL system to increase BBB penetration. Angiopep binds to low-density lipoprotein receptor-related protein (LRP), which is expressed at a high level in the BBB. The system showed increased gene expression in rat brains and neuroprotective effects in the rotenone-induced PD model, with recovery of the parkinsonian phenotype, motor activity and amelioration of dopaminergic loss [150]. In another study, the neuroprotection of hGDNF encapsulated in lactoferrin-decorated PAMAM DDs was confirmed. After treatment with the complex, GDNF overexpression increased neurotransmitter levels, prevented neuronal loss and improved motor activity performance in a rotenone-induced rat model [151]. On the other hand, specific antagonists of adenosine receptors (AR), such as theophylline or caffeine, have shown therapeutic potential for the treatment of PD [152]. A study by Kecskes and colleagues described that G4 PEGylated PAMAM DDs conjugated to xanthines enhanced receptor affinity and increased the interaction with binding sites [153].
The application of DD for the treatment and diagnosis of PD is summarized in Table 2.

Table 2.
DDs for PD therapy and diagnosis. Abbreviations: CBS (carbosilane) DDs (dendrimers), α-syn (α-synuclein), DGL (dendrigraft poly-L-lysines), hGDNF (human glial-derived neurotrophic factor), AR (adenosine receptor), mHippoE-18 (embryonic mouse hippocampal cell line), GATG (gallic acid-triethylene glycol) PLL (poly-L-lysine), DGL (dendrigraft poly-L-lysines), NFT (neurofibrillary tangles), BACE1 (β-amyloid converting enzyme 1), PEG (polyethylenglicol), AChE (acetylcholinesterase).
3.3. Multiple Sclerosis
MS was defined as a clinicopathological entity more than 150 years ago by French neurologist Jean-Martin Charcot, who coined the concept of “sclerosis in plaques” [154]. Currently, it is described as an autoimmune inflammatory disorder of the central nervous system (CNS). It is characterized by the presence of inflammation and lymphocyte infiltration, leading to damage to the integrity of axons and myelin sheaths and causing neurological disabilities over time. Symptoms and impairments include visual problems, numbness, weakness and tingling pain [155]. MS is one of the most common causes of neurological disabilities worldwide and mainly affects young people in their 20s–40s at disease onset. An estimated 2.3 million people worldwide suffer from MS, with a prevalence of 50–300 per 100,000 population [156].
The underlying etiology of MS is still not known, but CNS inflammation and demyelinated nerve cells are the main hallmarks of the pathology. Initially, disruption of blood vessels in the BBB allows autoreactive T cells against myelinic components to migrate across the BBB and trigger the further recruitment of immune cells from the peripheral blood [157]. T lymphocytes attack myelin in the brain and spinal cord, causing degeneration of oligodendrocytes and gradually leading to neuronal demyelination and the clustering of inflammatory cells around demyelinated plaques [158]. The mutual interaction of microglia, astrocytes and T-cells contributes to chronic demyelination and neuronal loss over time [159].
DMT is the standard medication used to treat MS. There are currently more than 15 FDA-approved DMT that modify, modulate or suppress the immune system, limit neuroinflammation in the CNS, and prevent the relapse and the formation of new lesions. However, DMT only improves disability progression for 2–3 years after the beginning of the treatment, none target cognitive impairment, and produce significant side-effects [160]. Therefore, new therapies that reverse disease progression and reduce adverse events are needed.
3.3.1. Dendrimers for MS Therapy
Dendrimers for Decreasing Neuroinflammation
Neuroinflammation plays a key role in the pathogenesis of MS. The inherent anti-inflammatory activity of certain DDs has been known since the first decade of the 21st century.
In 2008, nonlinear peptide DDs synthesized as octamers of an encephalitogenic proteolipid attached to a central lysine core reduced the course of experimental allergic encephalomyelitis (EAE) in mice by altering antigen-specific cell trafficking and inducing the retention of peptide-specific T cells in the spleen [161]. Shortly later, Chauhan and co-workers unexpectedly found that PAMAM DDs ended by carboxylate, amine or hydroxyl groups exhibited anti-inflammatory properties in vivo. This anti-inflammatory activity was higher for DDs possessing amine or hydroxyl surface groups and lower for carboxylate-ended DDs [162].
Furthermore, anionic phosphorus-based DDs capped by amino-bisphosphonate groups, and especially DDs azabisphosphonate (ABP), activated anti-inflammatory and immunosuppressive human monocytes, reducing the inflammatory response. The effect of phosphorous dendrimers was demonstrated in vivo in different models of peripheral inflammation in mice [163] and were then subject to a patent describing their use as anti-inflammatory agents for the treatment of autoimmune diseases [164]. More recently, anionic ABP DDs have been shown to reduce neuroinflammation by promoting IL-10 producing CD4+T lymphocytes and inhibit the progression of EAE, producing results similar to those of approved drugs [165]. In contrast, the anti-inflammatory properties of neutral phosphorous DDs have been poorly explored. In 2013, Blattes and co-workers reported that mannosylated G3 and G4 poly(phosphorhydrazone) (PPH) DDs inhibited neutrophil influx to the site of inflammation and reduced the release of pro-inflammatory cytokines, such as TNFα and IL-12, by binding to the C-type lectin receptor DC-SIGN [166]. More recently, Posadas and co-workers demonstrated the anti-inflammatory activity of neutral PPH dendrimers (G and G4) in vitro and in vivo by inhibiting the NFkB activation pathway [29]. Therefore, phosphorous DDs display powerful anti-inflammatory properties that could be exploited in the treatment of autoimmune diseases, such as MS.
Dendrimers as Vehicles of Drugs
Disruption of the BBB and the presence of neuroinflammation characterized by excessive activation of T-cells and microglia represent the main hallmarks of MS. Interestingly, G4 hydroxyl-terminated PAMAM DDs show a remarkable ability to selectively deliver loaded drugs to activated brain microglia. Although hydroxyl DDs cannot cross the intact BBB, the impaired BBB in MS allows them to penetrate into the brain and be taken up by activated glial cells supporting these structures to selective drug delivery to the CNS [167]. In this regard, hydroxyl-G6 PAMAM DD conjugated with minocycline showed higher efficiency in crossing the BBB and reducing CNS inflammation than the free drug [168]. Similarly, conjugation of 2-(phosphonomethyl)-pentanedioic acid (2-PMPA), a selective inhibitor of the enzyme glutamate carboxypeptidase II, to the surface of G4 hydroxyl-terminated PAMAM DDs resulted in potent brain penetrant and selective uptake in microglia that improved cognitive function [169].
Dendritic polyglycerol derivatives (dPG) are another type of DD with a similar safety to linear PEG or dextran, useful for systemic drug delivery [170]. Recently, dPG microspheres loaded with dimethilfumarate (DMF), a DMT approved by the FDA, and curcumin (CUR), a naturally occurring polyphenol with therapeutic potential in MS, have shown a good long-term release of both drugs with low in vitro toxicity, although future biocompatibility studies in more complex biological models are required [171].
Finally, a peptide DD with a precise amino acid sequence similar to glatiramer acetate (GA), one of the most successful first-line treatments for MS, has recently been obtained. This DD has been shown to steer monocytes toward an anti-inflammatory state, providing a suitable point for developing new immunomodulatory DDs [172].
3.3.2. Dendrimers for MS Diagnosis
The ability of DDs to interact with molecules has been exploited to detect two MS biomarkers, myelin basic protein (MBP) and Tau protein, in CSF and serum. A 1G tri-methylolpropane tris[poly(propyleneglycol) was coupled to graphene oxide and the specific antibodies that recognize the target proteins to obtain a nanoimmunosensor quite similar to the commercially available ELISA kit [173].
Furthermore, DDs could represent a new platform for the detection of lesions in the brain since they are systems capable of crossing the BBB and improving the delivery of contrast agents. In addition, metallic nanoparticles such as iron, gold or silver coated on the surface of DDs raise the possibility of their application as contrast agents. These systems, already tested in oncological diseases, could be extended to MS diagnostics [174].
The application of DD for the treatment and diagnosis of MS is summarized in Table 3.

Table 3.
DDs for MS therapy and diagnosis: Abbreviations: MAPs (Multiple Ag peptides), GPBP (guinea pig myelin basic protein), ABP (azabisphosphonate), PPH (poly(phosphorhydrazone), BBB (Blood–brain barrier), 2-PMPA (2-(phosphonomethyl)-pentanedioic acid), dPG (dendritic polyglycerol, DMF (dimethilfumarate), GA (glatiramer acetate), Ab (antibody), MBP (myelin basic protein)), CSF (cerebrospinal fluid).
3.4. Amyotrophic Lateral Sclerosis (ALS)
First described by Charles Bell in 1824, it was the neurologist Jean-Martin Charcot, who began using the term ALS in 1874 [175]. ALS is a progressive ND affecting motor neurons in the brain and spinal cord. Unlike MS, symptoms begin with muscle weakness and progress to affect speech, swallowing and finally breathing. Similar to other NDs, misdiagnosis determines disease prognosis and a patient’s quality of life [155].
The annual incidence of ALS is around 1-2.6 new cases per 100,000 persons. This disease is characterized by rapid progression with average survival 3–4 years from onset, whereas the average age of onset nowadays is 59–60 years [176]. Although the etiology remains unknown, a subset of cases (~10%) appears to be inherited. Approximately 1–2% of cases are related to mutations in the gene encoding superoxide dismutase-1 (SOD1), in which more than 130 mutations have been identified. Mutant SOD1 displays an enhanced propensity to misfold and aggregate, contributing to spinal motor neuron toxicity [177].
Functionality loss, haploinsufficiency, RNA toxicity and proteotoxicity seem to be the main pathological mechanisms in ALS. In addition, elevated levels of glutamate from the catabolism of the neuropeptide N-acetyl-aspartyl-glutamate (NAAG) by glutamate carboxypeptidase II (GCPII) hyperactivate NMDA receptors, leading to excitotoxic death [178,179].
Currently, the specific drugs approved by the FDA for the treatment of ALS are Riluzol, a glutamine agonist, and Edaravone, a free radical scavenger. Neither of these prolongs patient survival by more than 2–3 months, highlighting the need for new therapeutic strategies in ALS [180,181].
DDs for ALS Therapy
Although ALS is a devasting ND with no cure to date, the use of nanomedicine remains poorly explored [182]. Regarding DD application in ALS, recently, an hydroxylated G4 PAMAM DD covalently attached to 2-(Phosphonomethyl)pentanedioic acid, a potent GCPII inhibitor, resulted in potent inhibition of this enzymatic activity in activated macrophages infiltrating the muscle and significantly delayed neuromuscular junction denervation and loss of muscle function in a SODG93A mice model of ALS [183].
4. Conclusions
Current therapies available for NDs, such as PD, AD and MS, are able to reduce clinical manifestations and improve the quality of life of patients. The big challenge in the treatment of these pathologies is the development of strategies that prevent neurodegeneration and delay the onset or progression of these diseases. DDs have gained attention in recent decades due to their potential as drug vehicles usually employed for the management of NDs. In addition to being useful delivery systems for improving the pharmacokinetic properties of drugs and reducing their toxicity, DDs have been shown to exert anti-aggregation properties to reduce protein accumulation or promote protein disaggregation, and certain structures display inherent anti-inflammatory properties. DDs are able to interact with key therapeutic targets in AD, PD and MS to decrease aberrant protein deposits and protect neurons from toxic aggregates and to reduce neuroinflammation in NDs. However, DDs are not only useful agents for the therapy of NDs; they have thus far proven to be interesting diagnostic tools for AD. However, although the potential of DDs for the therapy of NDs has been highlighted, further efforts are needed to characterize the behavior of DDs in humans before planning the development of future clinical trials.
Author Contributions
Conceptualization, I.P.; writing—original draft preparation, I.P. and M.D.P.-C.; writing—review and editing, I.P. and M.D.P.-C.; funding acquisition, I.P. and M.D.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Junta de Comunidades de Castilla-La Mancha (grants number SBPLY/19/180501/000060 to M.D.P.-C. and SBPLY/19/180501/000067 to I.P.; Diputación Provincial de Albacete-Universidad de Castilla-La Mancha (grant number DIPUAB-2021-5) to M.D.P.-C.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. No new data were created or analyzed in this study. Data sharing is not applicable to this article.
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease—Repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.B.; Barker, R.A. Recent developments in the treatment of Parkinson’s Disease. F1000Research 2020, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- McFarthing, K.; Buff, S.; Rafaloff, G.; Dominey, T.; Wyse, R.K.; Stott, S.R.W. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. J. Park. Dis. 2020, 10, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Vaz, M.; Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef]
- Yu, T.-W.; Lane, H.-Y.; Lin, C.-H. Novel Therapeutic Approaches for Alzheimer’s Disease: An Updated Review. Int. J. Mol. Sci. 2021, 22, 8208. [Google Scholar] [CrossRef]
- Athar, T.; Al Balushi, K.; Khan, S.A. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol. Biol. Rep. 2021, 48, 5629–5645. [Google Scholar] [CrossRef]
- Schneider, S.A.; Alcalay, R.N. Neuropathology of genetic synucleinopathies with parkinsonism: Review of the literature: Neuropathology of Genetic Parkinson‘s Disease. Mov. Disord. 2017, 32, 1504–1523. [Google Scholar] [CrossRef]
- Ghobadinezhad, F.; Ebrahimi, N.; Mozaffari, F.; Moradi, N.; Beiranvand, S.; Pournazari, M.; Rezaei-Tazangi, F.; Khorram, R.; Afshinpour, M.; Robino, R.A.; et al. The emerging role of regulatory cell-based therapy in autoimmune disease. Front. Immunol. 2022, 13, 1075813. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Gallego-Yerga, L.; Posadas, I.; de la Torre, C.; Ruiz-Almansa, J.; Sansone, F.; Ortiz Mellet, C.; Casnati, A.; García Fernández, J.M.; Ceña, V. Docetaxel-Loaded Nanoparticles Assembled from β-Cyclodextrin/Calixarene Giant Surfactants: Physicochemical Properties and Cytotoxic Effect in Prostate Cancer and Glioblastoma Cells. Front. Pharmacol. 2017, 8, 249. [Google Scholar] [CrossRef]
- Bölükbas, D.A.; Datz, S.; Meyer-Schwickerath, C.; Morrone, C.; Doryab, A.; Gößl, D.; Vreka, M.; Yang, L.; Argyo, C.; Rijt, S.H.; et al. Organ-Restricted Vascular Delivery of Nanoparticles for Lung Cancer Therapy. Adv. Ther. 2020, 3, 2000017. [Google Scholar] [CrossRef]
- Ou, H.; Cheng, T.; Zhang, Y.; Liu, J.; Ding, Y.; Zhen, J.; Shen, W.; Xu, Y.; Yang, W.; Niu, P.; et al. Surface-adaptive zwitterionic nanoparticles for prolonged blood circulation time and enhanced cellular uptake in tumor cells. Acta Biomater. 2018, 65, 339–348. [Google Scholar] [CrossRef]
- Ai, X.; Wang, S.; Duan, Y.; Zhang, Q.; Chen, M.S.; Gao, W.; Zhang, L. Emerging Approaches to Functionalizing Cell Membrane-Coated Nanoparticles. Biochemistry 2021, 60, 941–955. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Dhommati, R.; Jain, A.; Challa, V.G.S.; Shaheen, M.; Khan, W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur. J. Pharm. Sci. 2016, 92, 224–234. [Google Scholar] [CrossRef]
- Akbari, V.; Rezazadeh, M.; Hanaie, N.S.; Hasanzadeh, F. Preparation and in vitro characterization of histidine trimethyl chitosan conjugated nanocomplex incorporated into injectable thermosensitive hydrogels for localized gene delivery. Biotechnol. Appl. Biochem. 2022, 69, 1047–1057. [Google Scholar] [CrossRef]
- Mashel, T.V.; Tarakanchikova, Y.V.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S.; Lepik, K.V.; Fehse, B. Overcoming the delivery problem for therapeutic genome editing: Current status and perspective of non-viral methods. Biomaterials 2020, 258, 120282. [Google Scholar] [CrossRef]
- Kuhn, J.; Papanastasiou, G.; Tai, C.-W.; Moran, C.M.; Jansen, M.A.; Tavares, A.A.; Lennen, R.J.; Corral, C.A.; Wang, C.; Thomson, A.J.; et al. Tri-modal imaging of gold-dotted magnetic nanoparticles for magnetic resonance imaging, computed tomography and intravascular ultrasound: An in vitro study. Nanomedicine 2020, 15, 2433–2445. [Google Scholar] [CrossRef]
- Yang, L.; Kim, T.; Cho, H.; Luo, J.; Lee, J.; Chueng, S.D.; Hou, Y.; Yin, P.T.; Han, J.; Kim, J.H.; et al. Hybrid Graphene-Gold Nanoparticle-Based Nucleic Acid Conjugates for Cancer-Specific Multimodal Imaging and Combined Therapeutics. Adv. Funct. Mater. 2021, 31, 2006918. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Mu, M.; Yang, B.; Chen, X.; Lee, W.Y.W.; Ke, Y.; Yung, W.H.; Tang, B.Z.; Bian, L. Multifunctional Nanoprobe for the Delivery of Therapeutic siRNA and Real-Time Molecular Imaging of Parkinson’s Disease Biomarkers. ACS Appl. Mater. Interfaces 2021, 13, 11609–11620. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Prato, M. Nanomaterials for (Nano)medicine. ACS Med. Chem. Lett. 2013, 4, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood Dar, A.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. BioMed. Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Cai, R.; Chen, C. The Crown and the Scepter: Roles of the Protein Corona in Nanomedicine. Adv. Mater. 2019, 31, 1805740. [Google Scholar] [CrossRef]
- Ye, H.; Shen, Z.; Yu, L.; Wei, M.; Li, Y. Manipulating nanoparticle transport within blood flow through external forces: An exemplar of mechanics in nanomedicine. Proc. R. Soc. Math. Phys. Eng. Sci. 2018, 474, 20170845. [Google Scholar] [CrossRef]
- de la Torre, C.; Ceña, V. The Delivery Challenge in Neurodegenerative Disorders: The Nanoparticles Role in Alzheimer’s Disease Therapeutics and Diagnostics. Pharmaceutics 2018, 10, 190. [Google Scholar] [CrossRef]
- Posadas, I.; Romero-Castillo, L.; El Brahmi, N.; Manzanares, D.; Mignani, S.; Majoral, J.-P.; Ceña, V. Neutral high-generation phosphorus dendrimers inhibit macrophage-mediated inflammatory response in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2017, 114, E7660–E7669. [Google Scholar] [CrossRef]
- Mohindra, P.; Desai, T.A. Micro- and nanoscale biophysical cues for cardiovascular disease therapy. Nanomed. Nanotechnol. Biol. Med. 2021, 34, 102365. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L. Nanomaterials arising amid antibiotic resistance. Nat. Rev. Microbiol. 2021, 19, 5–6. [Google Scholar] [CrossRef]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef]
- Tavakol, S.; Zahmatkeshan, M.; Mohammadinejad, R.; Mehrzadi, S.; Joghataei, M.T.; Alavijeh, M.S.; Seifalian, A. The role of nanotechnology in current COVID-19 outbreak. Heliyon 2021, 7, e06841. [Google Scholar] [CrossRef]
- Játiva, P.; Ceña, V. Use of nanoparticles for glioblastoma treatment: A new approach. Nanomedicine 2017, 12, 2533–2554. [Google Scholar] [CrossRef]
- FDA. FDA Approves Liposome-Encapsulated Combination of Daunorubicin-Cytarabine for Adults with Some Types of Poor Prognosis AML. 2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-liposome-encapsulated-combination-daunorubicin-cytarabine-adults-some-types-poor (accessed on 2 December 2022).
- FDA. FDA Approves First-of-Its Kind Targeted RNA-Based Therapy to Treat a Rare Disease. 2018. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-targeted-rna-based-therapy-treat-rare-disease (accessed on 2 December 2022).
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Tomalia, D.A. The dendritic state. Mater. Today 2005, 8, 34–46. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Which Dendrimer to Attain the Desired Properties? Focus on Phosphorhydrazone Dendrimers. Molecules 2018, 23, 622. [Google Scholar] [CrossRef]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Igne Ferreira, E.; Seoud, O.E.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef]
- Gupta, V.; Nayak, S. Dendrimers: A Review on Synthetic Approaches. J. Appl. Pharm. Sci. 2015, 5, 117–122. [Google Scholar] [CrossRef]
- Konopka, M.; Janaszewska, A.; Klajnert-Maculewicz, B. Intrinsic Fluorescence of PAMAM Dendrimers—Quenching Studies. Polymers 2018, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Vögtle, F.; Richardt, G.; Werner, N. Dendrimer Chemistry; Wiley-VCH: Weinheim, Germany, 2009; ISBN 978-3-527-32066-0. [Google Scholar]
- Pérez-Martínez, F.C.; Guerra, J.; Posadas, I.; Ceña, V. Barriers to Non-Viral Vector-Mediated Gene Delivery in the Nervous System. Pharm. Res. 2011, 28, 1843–1858. [Google Scholar] [CrossRef]
- Somani, S.; Laskar, P.; Altwaijry, N.; Kewcharoenvong, P.; Irving, C.; Robb, G.; Pickard, B.S.; Dufès, C. PEGylation of polypropylenimine dendrimers: Effects on cytotoxicity, DNA condensation, gene delivery and expression in cancer cells. Sci. Rep. 2018, 8, 9410. [Google Scholar] [CrossRef]
- Klementieva, O.; Aso, E.; Filippini, D.; Benseny-Cases, N.; Carmona, M.; Juvés, S.; Appelhans, D.; Cladera, J.; Ferrer, I. Effect of Poly(propylene imine) Glycodendrimers on β-Amyloid Aggregation in Vitro and in APP/PS1 Transgenic Mice, as a Model of Brain Amyloid Deposition and Alzheimer’s Disease. Biomacromolecules 2013, 14, 3570–3580. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Barani, L.; Kasaeian, A. Biomimetics in drug delivery systems: A critical review. J. Control Release 2017, 253, 97–109. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-0-521-51580-1. [Google Scholar]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Maleki, B.; Sheikh, S. Nano polypropylenimine dendrimer (DAB-PPI-G 1): As a novel nano basic-polymer catalyst for one-pot synthesis of 2-amino-2-chromene derivatives. RSC Adv. 2015, 5, 42997–43005. [Google Scholar] [CrossRef]
- Golshan, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Mohammadi, M. Synthesis of poly(propylene imine) dendrimers via homogeneous reduction process using lithium aluminium hydride: Bioconjugation with folic acid and doxorubicin release kinetics. Appl. Organomet. Chem. 2017, 31, e3789. [Google Scholar] [CrossRef]
- Idris, A.O.; Mamba, B.; Feleni, U. Poly (propylene imine) dendrimer: A potential nanomaterial for electrochemical application. Mater. Chem. Phys. 2020, 244, 122641. [Google Scholar] [CrossRef]
- Chen, S.; Huang, S.; Li, Y.; Zhou, C. Recent Advances in Epsilon-Poly-L-Lysine and L-Lysine-Based Dendrimer Synthesis, Modification, and Biomedical Applications. Front. Chem. 2021, 9, 659304. [Google Scholar] [CrossRef]
- Hatano, K.; Matsuoka, K.; Terunuma, D. Carbosilane glycodendrimers. Chem. Soc. Rev. 2013, 42, 4574–4598. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.-O.; Majoral, J.-P. Biological properties of phosphorus dendrimers. New J. Chem. 2010, 34, 1512. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.-M.; Lahana, R.; Majoral, J.-P. A General Synthetic Strategy for Neutral Phosphorus-Containing Dendrimers. A J. Ger. Chem. Soc. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.-S.; Akter, W.; Gautam, R.K.; Uddin, S.; Rahman, S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. BioMed Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef]
- Hsu, H.; Bugno, J.; Lee, S.; Hong, S. Dendrimer-based nanocarriers: A versatile platform for drug delivery. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1409. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Tarach, P.; Janaszewska, A. Recent Advances in Preclinical Research Using PAMAM Dendrimers for Cancer Gene Therapy. Int. J. Mol. Sci. 2021, 22, 2912. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M. Recent Advances in Preclinical Studies and Potential Applications of Dendrimers as Drug Carriers in the Central Nervous System. Curr. Pharm. Des. 2017, 23, 3105–3119. [Google Scholar] [CrossRef] [PubMed]
- Chaniotakis, N.; Thermos, K.; Kalomiraki, M. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2015, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Tripathi, R.; Chen, L.; Caminade, A.-M.; Shi, X.; Majoral, J.-P. New Ways to Treat Tuberculosis Using Dendrimers as Nanocarriers. Pharmaceutics 2018, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Parveen, F.; Torchilin, V. Developments in Treatment Methodologies Using Dendrimers for Infectious Diseases. Molecules 2021, 26, 3304. [Google Scholar] [CrossRef]
- Mlynarczyk, D.T.; Dlugaszewska, J.; Kaluzna-Mlynarczyk, A.; Goslinski, T. Dendrimers against fungi—A state of the art review. J. Control Release 2021, 330, 599–617. [Google Scholar] [CrossRef]
- Kheiriabad, S.; Ghaffari, M.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM Dendrimers as a Delivery System for Small Interfering RNA. In RNA Interference and CRISPR Technologies; Sioud, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2115, pp. 91–106. ISBN 978-1-07-160289-8. [Google Scholar]
- Laurini, E.; Aulic, S.; Marson, D.; Fermeglia, M.; Pricl, S. Cationic Dendrimers for siRNA Delivery: An Overview of Methods for In Vitro/In Vivo Characterization. In Design and Delivery of SiRNA Therapeutics; Ditzel, H.J., Tuttolomondo, M., Kauppinen, S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2282, pp. 209–244. ISBN 978-1-07-161297-2. [Google Scholar]
- Perez-Martinez, F.C.; Ocana, A.V.; Perez-Carrion, M.D.; Cena, V. Dendrimers As Vectors for Genetic Material Delivery to the Nervous System. Curr. Med. Chem. 2012, 19, 5101–5108. [Google Scholar] [CrossRef]
- Tambe, V.; Thakkar, S.; Raval, N.; Sharma, D.; Kalia, K.; Tekade, R.K. Surface Engineered Dendrimers in siRNA Delivery and Gene Silencing. Curr. Pharm. Des. 2017, 23, 2952–2975. [Google Scholar] [CrossRef]
- Bober, Z.; Bartusik-Aebisher, D.; Aebisher, D. Application of Dendrimers in Anticancer Diagnostics and Therapy. Molecules 2022, 27, 3237. [Google Scholar] [CrossRef]
- Caminade, A.-M. Phosphorus Dendrimers as Nanotools against Cancers. Molecules 2020, 25, 3333. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Ceña, V.; Shcharbin, D.; Bryszewska, M.; Majoral, J.-P. In vivo therapeutic applications of phosphorus dendrimers: State of the art. Drug Discov. Today 2021, 26, 677–689. [Google Scholar] [CrossRef]
- Caminade, A.-M. Phosphorus dendrimers for nanomedicine. Chem. Commun. 2017, 53, 9830–9838. [Google Scholar] [CrossRef]
- Stelzmann, R.A.; Norman Schnitzlein, H.; Reed Murtagh, F. An english translation of alzheimer’s 1907 paper, “uber eine eigenartige erkankung der hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primer 2021, 7, 33. [Google Scholar] [CrossRef]
- Lynch, C. World Alzheimer Report 2019: Attitudes to dementia, a global survey: Public health: Engaging people in ADRD research. Alzheimers Dement. 2020, 16, e038255. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Tolar, M.; Hey, J.; Power, A.; Abushakra, S. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer’s Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int. J. Mol. Sci. 2021, 22, 6355. [Google Scholar] [CrossRef]
- Bejanin, A.; Schonhaut, D.R.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 2017, 140, 3286–3300. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Femminella, G.D.; Ninan, S.; Atkinson, R.; Fan, Z.; Brooks, D.J.; Edison, P. Does Microglial Activation Influence Hippocampal Volume and Neuronal Function in Alzheimer’s Disease and Parkinson’s Disease Dementia? J. Alzheimers Dis. 2016, 51, 1275–1289. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Kabir, T.; Sufian, M.A.; Uddin, S.; Begum, M.M.; Akhter, S.; Islam, A.; Mathew, B.; Islam, S.; Amran, S.; Ashraf, G. NMDA Receptor Antagonists: Repositioning of Memantine as a Multitargeting Agent for Alzheimer’s Therapy. Curr. Pharm. Des. 2019, 25, 3506–3518. [Google Scholar] [CrossRef] [PubMed]
- Fish, P.V.; Steadman, D.; Bayle, E.D.; Whiting, P. New approaches for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2019, 29, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Makin, S. The amyloid hypothesis on trial. Nature 2018, 559, S4–S7. [Google Scholar] [CrossRef]
- Chafekar, S.M.; Malda, H.; Merkx, M.; Meijer, E.W.; Viertl, D.; Lashuel, H.A.; Baas, F.; Scheper, W. Branched KLVFF Tetramers Strongly Potentiate Inhibition of β-Amyloid Aggregation. ChemBioChem 2007, 8, 1857–1864. [Google Scholar] [CrossRef]
- Klajnert, B.; Cortijo-Arellano, M.; Cladera, J.; Bryszewska, M. Influence of dendrimer’s structure on its activity against amyloid fibril formation. Biochem. Biophys. Res. Commun. 2006, 345, 21–28. [Google Scholar] [CrossRef]
- Klajnert, B.; Cortijo-Arellano, M.; Bryszewska, M.; Cladera, J. Influence of heparin and dendrimers on the aggregation of two amyloid peptides related to Alzheimer’s and prion diseases. Biochem. Biophys. Res. Commun. 2006, 339, 577–582. [Google Scholar] [CrossRef]
- Fülöp, L.; Mándity, I.M.; Juhász, G.; Szegedi, V.; Hetényi, A.; Wéber, E.; Bozsó, Z.; Simon, D.; Benkő, M.; Király, Z.; et al. A Foldamer-Dendrimer Conjugate Neutralizes Synaptotoxic β-Amyloid Oligomers. PLoS ONE 2012, 7, e39485. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Klementieva, O.; Benseny-Cases, N.; Gella, A.; Appelhans, D.; Voit, B.; Cladera, J. Dense Shell Glycodendrimers as Potential Nontoxic Anti-amyloidogenic Agents in Alzheimer’s Disease. Amyloid–Dendrimer Aggregates Morphology and Cell Toxicity. Biomacromolecules 2011, 12, 3903–3909. [Google Scholar] [CrossRef]
- Klajnert, B.; Cladera, J.; Bryszewska, M. Molecular Interactions of Dendrimers with Amyloid Peptides: pH Dependence. Biomacromolecules 2006, 7, 2186–2191. [Google Scholar] [CrossRef]
- Neelov, I.M.; Janaszewska, A.; Klajnert, B.; Bryszewska, M.; Makova, N.Z.; Hicks, D.; Pearson, H.A.; Vlasov, G.P.; Ilyash, M.Y.; Vasilev, D.S.; et al. Molecular Properties of Lysine Dendrimers and their Interactions with Aβ-Peptides and Neuronal Cells. Curr. Med. Chem. 2012, 20, 134–143. [Google Scholar] [CrossRef]
- Wasiak, T.; Ionov, M.; Nieznanski, K.; Nieznanska, H.; Klementieva, O.; Granell, M.; Cladera, J.; Majoral, J.-P.; Caminade, A.M.; Klajnert, B. Phosphorus Dendrimers Affect Alzheimer’s (Aβ 1–28) Peptide and MAP-Tau Protein Aggregation. Mol. Pharm. 2012, 9, 458–469. [Google Scholar] [CrossRef]
- Wasiak, T.; Marcinkowska, M.; Pieszynski, I.; Zablocka, M.; Caminade, A.-M.; Majoral, J.-P.; Klajnert-Maculewicz, B. Cationic phosphorus dendrimers and therapy for Alzheimer’s disease. New J. Chem. 2015, 39, 4852–4859. [Google Scholar] [CrossRef]
- Klajnert, B.; Wasiak, T.; Ionov, M.; Fernandez-Villamarin, M.; Sousa-Herves, A.; Correa, J.; Riguera, R.; Fernandez-Megia, E. Dendrimers reduce toxicity of Aβ 1-28 peptide during aggregation and accelerate fibril formation. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1372–1378. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-Beta: A Crucial Factor in Alzheimer’s Disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef]
- Al-azzawi, S.; Masheta, D.; Guildford, A.; Phillips, G.; Santin, M. Dendrimeric Poly(Epsilon-Lysine) Delivery Systems for the Enhanced Permeability of Flurbiprofen across the Blood-Brain Barrier in Alzheimer’s Disease. Int. J. Mol. Sci. 2018, 19, 3224. [Google Scholar] [CrossRef]
- Liu, Y.; An, S.; Li, J.; Kuang, Y.; He, X.; Guo, Y.; Ma, H.; Zhang, Y.; Ji, B.; Jiang, C. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials 2016, 80, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Aso, E.; Martinsson, I.; Appelhans, D.; Effenberg, C.; Benseny-Cases, N.; Cladera, J.; Gouras, G.; Ferrer, I.; Klementieva, O. Poly(propylene imine) dendrimers with histidine-maltose shell as novel type of nanoparticles for synapse and memory protection. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Sales, R.; Ashiri, M.; Hafizi, M.; Kalanaky, S.; Maghsoudi, A.H.; Fakharzadeh, S.; Maghsoudi, N.; Nazaran, M.H. Neuroprotective Effect of New Nanochelating-Based Nano Complex, ALZc3, Against Aβ (1–42)-Induced Toxicity in Rat: A Comparison with Memantine. Pharm. Res. 2020, 37, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gu, Z.; Shen, L.; Liu, X.; Lin, H. A Dual Targeting Drug Delivery System for Penetrating Blood-Brain Barrier and Selectively Delivering siRNA to Neurons for Alzheimer’s Disease Treatment. Curr. Pharm. Biotechnol. 2018, 18, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kettle, C.; Morton, D.W. A molecular approach in drug development for Alzheimer’s disease. Biomed. Pharmacother. 2018, 106, 553–565. [Google Scholar] [CrossRef]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin Coupled Lower Generation PAMAM Dendrimers for Brain Targeted Delivery of Memantine in Aluminum-Chloride-Induced Alzheimer’s Disease in Mice. Bioconjug. Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef]
- Gothwal, A.; Singh, H.; Jain, S.K.; Dutta, A.; Borah, A.; Gupta, U. Behavioral and Biochemical Implications of Dendrimeric Rivastigmine in Memory-Deficit and Alzheimer’s Induced Rodents. ACS Chem. Neurosci. 2019, 10, 3789–3795. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Temprana, C.F.; Alonso, S.D.V.; Prieto, M.J. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: A biophysical and nanotoxicological characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Alonso, S.D.V.; Prieto, M.J. Combined Therapy for Alzheimer’s Disease: Tacrine and PAMAM Dendrimers Co-Administration Reduces the Side Effects of the Drug without Modifying its Activity. AAPS PharmSciTech 2020, 21, 110. [Google Scholar] [CrossRef]
- Razzino, C.A.; Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; Campuzano, S.; et al. An electrochemical immunosensor using gold nanoparticles-PAMAM-nanostructured screen-printed carbon electrodes for tau protein determination in plasma and brain tissues from Alzheimer patients. Biosens. Bioelectron. 2020, 163, 112238. [Google Scholar] [CrossRef]
- Parkinson, J. An Essay on the Shaking Palsy. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 223–236. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy: The Shaking Palsy: Past, Present and Future. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Rocca, W.A. The burden of Parkinson’s disease: A worldwide perspective. Lancet Neurol. 2018, 17, 928–929. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy Body in Parkinson’s Disease and Related Neurodegenerative Disorders. Mol. Neurobiol. 2013, 47, 495–508. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548. [Google Scholar] [CrossRef]
- Thal, D.R. Neurodegeneration in Normal Brain Aging and Disease. Sci. Aging Knowl. Environ. 2004, 2004, pe26. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. What is the role of protein aggregation in neurodegeneration? Nat. Rev. Mol. Cell Biol. 2005, 6, 891–898. [Google Scholar] [CrossRef]
- Chartier, S.; Duyckaerts, C. Is Lewy pathology in the human nervous system chiefly an indicator of neuronal protection or of toxicity? Cell Tissue Res. 2018, 373, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Bendor, J.T.; Logan, T.P.; Edwards, R.H. The Function of α-Synuclein. Neuron 2013, 79, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Bisi, N.; Feni, L.; Peqini, K.; Pérez-Peña, H.; Ongeri, S.; Pieraccini, S.; Pellegrino, S. α-Synuclein: An All-Inclusive Trip Around its Structure, Influencing Factors and Applied Techniques. Front. Chem. 2021, 9, 666585. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Duda, J.E.; Lee, V.M.-Y.; Trojanowski, J.Q. Neuropathology of synuclein aggregates: New insights into mechanisms of neurodegenerative diseases. J. Neurosci. Res. 2000, 61, 121–127. [Google Scholar] [CrossRef]
- Brundin, P.; Melki, R. Prying into the Prion Hypothesis for Parkinson’s Disease. J. Neurosci. 2017, 37, 9808–9818. [Google Scholar] [CrossRef]
- Rekas, A.; Lo, V.; Gadd, G.E.; Cappai, R.; Yun, S.I. PAMAM Dendrimers as Potential Agents against Fibrillation of α -Synuclein, a Parkinson’s Disease-Related Protein. Macromol. Biosci. 2009, 9, 230–238. [Google Scholar] [CrossRef]
- Milowska, K.; Malachowska, M.; Gabryelak, T. PAMAM G4 dendrimers affect the aggregation of α-synuclein. Int. J. Biol. Macromol. 2011, 48, 742–746. [Google Scholar] [CrossRef]
- Milowska, K.; Gabryelak, T.; Bryszewska, M.; Caminade, A.-M.; Majoral, J.-P. Phosphorus-containing dendrimers against α-synuclein fibril formation. Int. J. Biol. Macromol. 2012, 50, 1138–1143. [Google Scholar] [CrossRef]
- Milowska, K.; Grochowina, J.; Katir, N.; El Kadib, A.; Majoral, J.-P.; Bryszewska, M.; Gabryelak, T. Viologen-Phosphorus Dendrimers Inhibit α-Synuclein Fibrillation. Mol. Pharm. 2013, 10, 1131–1137. [Google Scholar] [CrossRef]
- Milowska, K.; Szwed, A.; Mutrynowska, M.; Gomez-Ramirez, R.; de la Mata, F.J.; Gabryelak, T.; Bryszewska, M. Carbosilane dendrimers inhibit α-synuclein fibrillation and prevent cells from rotenone-induced damage. Int. J. Pharm. 2015, 484, 268–275. [Google Scholar] [CrossRef]
- Milowska, K.; Szwed, A.; Zablocka, M.; Caminade, A.-M.; Majoral, J.-P.; Mignani, S.; Gabryelak, T.; Bryszewska, M. In vitro PAMAM, phosphorus and viologen-phosphorus dendrimers prevent rotenone-induced cell damage. Int. J. Pharm. 2014, 474, 42–49. [Google Scholar] [CrossRef]
- Ferrer-Lorente, R.; Lozano-Cruz, T.; Fernández-Carasa, I.; Miłowska, K.; de la Mata, F.J.; Bryszewska, M.; Consiglio, A.; Ortega, P.; Gómez, R.; Raya, A. Cationic Carbosilane Dendrimers Prevent Abnormal α-Synuclein Accumulation in Parkinson’s Disease Patient-Specific Dopamine Neurons. Biomacromolecules 2021, 22, 4582–4591. [Google Scholar] [CrossRef]
- Tran, J.; Anastacio, H.; Bardy, C. Genetic predispositions of Parkinson’s disease revealed in patient-derived brain cells. Npj Park. Dis. 2020, 6, 8. [Google Scholar] [CrossRef]
- Bandres-Ciga, S.; Diez-Fairen, M.; Kim, J.J.; Singleton, A.B. Genetics of Parkinson’s disease: An introspection of its journey towards precision medicine. Neurobiol. Dis. 2020, 137, 104782. [Google Scholar] [CrossRef]
- Braun, C.S.; Vetro, J.A.; Tomalia, D.A.; Koe, G.S.; Koe, J.G.; Russell Middaugh, C. Structure/Function Relationships of Polyamidoamine/DNA Dendrimers as Gene Delivery Vehicles. J. Pharm. Sci. 2005, 94, 423–436. [Google Scholar] [CrossRef]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Henderson, M.X.; Trojanowski, J.Q.; Lee, V.M.-Y. α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci. Lett. 2019, 709, 134316. [Google Scholar] [CrossRef]
- Khodr, C.E.; Becerra, A.; Han, Y.; Bohn, M.C. Targeting alpha-synuclein with a microRNA-embedded silencing vector in the rat substantia nigra: Positive and negative effects. Brain Res. 2014, 1550, 47–60. [Google Scholar] [CrossRef]
- Khodr, C.E.; Sapru, M.K.; Pedapati, J.; Han, Y.; West, N.C.; Kells, A.P.; Bankiewicz, K.S.; Bohn, M.C. An alpha-synuclein AAV gene silencing vector ameliorates a behavioral deficit in a rat model of Parkinson’s disease, but displays toxicity in dopamine neurons. Brain Res. 2011, 1395, 94–107. [Google Scholar] [CrossRef]
- Gorbatyuk, O.S.; Li, S.; Nash, K.; Gorbatyuk, M.; Lewin, A.S.; Sullivan, L.F.; Mandel, R.J.; Chen, W.; Meyers, C.; Manfredsson, F.P.; et al. In Vivo RNAi-Mediated α-Synuclein Silencing Induces Nigrostriatal Degeneration. Mol. Ther. 2010, 18, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, Y.; An, S.; Kuang, Y.; He, X.; Ma, H.; Li, J.; Lv, J.; Zhang, N.; Jiang, C. Targeting Caspase-3 as Dual Therapeutic Benefits by RNAi Facilitating Brain-Targeted Nanoparticles in a Rat Model of Parkinson’s Disease. PLoS ONE 2013, 8, e62905. [Google Scholar] [CrossRef]
- Langer, R. New Methods of Drug Delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ma, H.; Guo, Y.; Liu, S.; Kuang, Y.; Shao, K.; Li, J.; Liu, Y.; Han, L.; Huang, S.; et al. Angiopep-Conjugated Nanoparticles for Targeted Long-Term Gene Therapy of Parkinson’s Disease. Pharm. Res. 2013, 30, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Liu, Y.; Wu, D.; Feng, L.; Jiang, C.; Pei, Y. Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J. Neurol. Sci. 2010, 290, 123–130. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.-G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef]
- Kecskés, A.; Tosh, D.K.; Wei, Q.; Gao, Z.-G.; Jacobson, K.A. GPCR Ligand Dendrimer (GLiDe) Conjugates: Adenosine Receptor Interactions of a Series of Multivalent Xanthine Antagonists. Bioconjug. Chem. 2011, 22, 1115–1127. [Google Scholar] [CrossRef]
- Charcot, J.M. Histologie de la Sclérose en Plaques; HACHETTE LIVRE-BNF: Paris, France, 1868. [Google Scholar]
- Deeb, O.; Nabulsi, M. Exploring Multiple Sclerosis (MS) and Amyotrophic Lateral Scler osis (ALS) as Neurodegenerative Diseases and their Treatments: A Review Study. Curr. Top. Med. Chem. 2020, 20, 2391–2403. [Google Scholar] [CrossRef]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef]
- Steinman, L. Immunology of Relapse and Remission in Multiple Sclerosis. Annu. Rev. Immunol. 2014, 32, 257–281. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Kumar, B. A review on nanotechnology based innovations in diagnosis and treatment of multiple sclerosis. J. Cell Immunother. 2018, 4, 56–64. [Google Scholar] [CrossRef]
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J.; the MSCOI Study Group; the European Multiple Sclerosis Platform. New insights into the burden and costs of multiple sclerosis in Europe. Mult. Scler. J. 2017, 23, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, K.W.; Wagner, C.R.; Whitham, R.H.; Hinrichs, D.J. Synthetic Peptide Dendrimers Block the Development and Expression of Experimental Allergic Encephalomyelitis. J. Immunol. 2008, 181, 3301–3309. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Diwan, P.V.; Jain, N.K.; Tomalia, D.A. Unexpected In Vivo Anti-Inflammatory Activity Observed for Simple, Surface Functionalized Poly(amidoamine) Dendrimers. Biomacromolecules 2009, 10, 1195–1202. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.-O.; Caminade, A.-M.; Majoral, J.-P.; Eisenberg, R.A.; Fournié, J.-J.; Cantagrel, A.; et al. A Phosphorus-Based Dendrimer Targets Inflammation and Osteoclastogenesis in Experimental Arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef]
- Poupot, M.; Poupot, R.; Fournie, J.J.; Portevin, D.; Fruchon, S.; Davignon, J.L.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Rolland, O. Phosphorylated Dendrimers as Antiinflammatory Drug (WO 2010/013086); European patent Office: Munich, Germany, 2010. [Google Scholar]
- Hayder, M.; Varilh, M.; Turrin, C.-O.; Saoudi, A.; Caminade, A.-M.; Poupot, R.; Liblau, R.S. Phosphorus-Based Dendrimer ABP Treats Neuroinflammation by Promoting IL-10-Producing CD4+ T Cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef]
- Blattes, E.; Vercellone, A.; Eutamène, H.; Turrin, C.-O.; Théodorou, V.; Majoral, J.-P.; Caminade, A.-M.; Prandi, J.; Nigou, J.; Puzo, G. Mannodendrimers prevent acute lung inflammation by inhibiting neutrophil recruitment. Proc. Natl. Acad. Sci. USA 2013, 110, 8795–8800. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, R.; Zhang, Z.; Liaw, K.; Kambhampati, S.P.; Porterfield, J.E.; Lin, K.C.; DeRidder, L.B.; Kannan, S.; Kannan, R.M. Dense hydroxyl polyethylene glycol dendrimer targets activated glia in multiple CNS disorders. Sci. Adv. 2020, 6, eaay8514. [Google Scholar] [CrossRef]
- Sharma, R.; Kim, S.-Y.; Sharma, A.; Zhang, Z.; Kambhampati, S.P.; Kannan, S.; Kannan, R.M. Activated Microglia Targeting Dendrimer–Minocycline Conjugate as Therapeutics for Neuroinflammation. Bioconjugate Chem. 2017, 28, 2874–2886. [Google Scholar] [CrossRef]
- Hollinger, K.R.; Sharma, A.; Tallon, C.; Lovell, L.; Thomas, A.G.; Zhu, X.; Wiseman, R.; Wu, Y.; Kambhampati, S.P.; Liaw, K.; et al. Dendrimer-2PMPA selectively blocks upregulated microglial GCPII activity and improves cognition in a mouse model of multiple sclerosis. Nanotheranostics 2022, 6, 126–142. [Google Scholar] [CrossRef]
- Khandare, J.; Mohr, A.; Calderón, M.; Welker, P.; Licha, K.; Haag, R. Structure-biocompatibility relationship of dendritic polyglycerol derivatives. Biomaterials 2010, 31, 4268–4277. [Google Scholar] [CrossRef]
- Da Silva, P.V.; De Queiroz, A.A.A. Long term multiple sclerosis drug delivery using dendritic polyglycerol flower-like microspheres. J. Biomater. Sci. Polym. Ed. 2019, 31, 188–206. [Google Scholar] [CrossRef]
- Erzina, D.; Capecchi, A.; Javor, S.; Reymond, J. An Immunomodulatory Peptide Dendrimer Inspired from Glatiramer Acetate. Angew. Chem. Int. Ed. 2021, 60, 26403–26408. [Google Scholar] [CrossRef]
- Derkus, B.; Bozkurt, P.A.; Tulu, M.; Emregul, K.C.; Yucesan, C.; Emregul, E. Simultaneous quantification of Myelin Basic Protein and Tau proteins in cerebrospinal fluid and serum of Multiple Sclerosis patients using nanoimmunosensor. Biosens. Bioelectron. 2017, 89, 781–788. [Google Scholar] [CrossRef]
- Wang, S.H.; Shi, X.; Van Antwerp, M.; Cao, Z.; Swanson, S.D.; Bi, X.; Baker, J.R. Dendrimer-Functionalized Iron Oxide Nanoparticles for Specific Targeting and Imaging of Cancer Cells. Adv. Funct. Mater. 2007, 17, 3043–3050. [Google Scholar] [CrossRef]
- Rowland, L.P. How Amyotrophic Lateral Sclerosis Got Its Name. Arch. Neurol. 2001, 58, 512–515. [Google Scholar] [CrossRef]
- Pichla, M.; Bartosz, G.; Sadowska-Bartosz, I. The Antiaggregative and Antiamyloidogenic Properties of Nanoparticles: A Promising Tool for the Treatment and Diagnostics of Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 3534570. [Google Scholar] [CrossRef]
- Bruijn, L.I.; Miller, T.M.; Cleveland, D.W. Unraveling the mechanisms involved in motor neuron degeneration in als. Annu. Rev. Neurosci. 2004, 27, 723–749. [Google Scholar] [CrossRef]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Unnikrishnan, M.K.; Uddin, S.; Mathew, G.E.; Pratap, R.; Marathakam, A.; Mathew, B. Revisiting the blood-brain barrier: A hard nut to crack in the transportation of drug molecules. Brain Res. Bull. 2020, 160, 121–140. [Google Scholar] [CrossRef]
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliffe, M.; Smith, A. Riluzole: Real-world evidence supports significant extension of median survival times in patients with amyotrophic lateral sclerosis. Degener. Neurol. Neuromuscul. Dis. 2017, ume 7, 61–70. [Google Scholar] [CrossRef]
- Oskarsson, B.; Gendron, T.F.; Staff, N.P. Amyotrophic Lateral Sclerosis: An Update for 2018. Mayo Clin. Proc. 2018, 93, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.S.; Sarmadi, V.H.; Larijani, G.; Garahgheshlagh, S.N.; Ramezani, S.; Moghadamifar, S.; Mohebi, S.L.; Milan, P.B.; Haramshahi, S.M.A.; Ahmadirad, N.; et al. Regenerative medicine improve neurodegenerative diseases. Cell Tissue Bank. 2022, 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tallon, C.; Sharma, A.; Zhang, Z.; Thomas, A.G.; Ng, J.; Zhu, X.; Donoghue, A.; Schulte, M.; Joe, T.R.; Kambhampati, S.P.; et al. Dendrimer-2PMPA Delays Muscle Function Loss and Denervation in a Murine Model of Amyotrophic Lateral Sclerosis. Neurotherapeutics 2022, 19, 274–288. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).