1. Introduction
Electrolytic manganese slag is an acidic or weakly acidic industrial solid waste that contains a large number of ammonium salt compounds and heavy metal ions, such as Cu, Co, Ni, and Mn. The production of electrolytic manganese slag is subject to technical limitations, so domestic electrolytic manganese slag can only be effectively used in the form of piles, and piled electrolytic manganese slag has caused serious damage to the ecological environment. Resource utilization of electrolytic manganese slag: Electrolytic manganese slag contains a large amount of SiO2, Al2O3, Fe2O3, CaO, MgO, MnO and other main components, which shows that electrolytic manganese slag can be utilized for secondary use. The stacking of electrolytic manganese slag not only damages the ecological environment, but also represents a waste of resources destruction. At present, numerous scholars have performed considerable research on the harmlessness and resourcefulness of electrolytic manganese slag. However, given the complexity of the preparation process, the high cost of preparation, the harsh preparation environment, the different compositions of electrolytic manganese slag from different regions, and the inability to produce electrolytic manganese slag on a large scale industrially, there are very few reports on the actual application of electrolytic manganese slag. Currently, electrolytic manganese slag has caused serious impacts on the environment, so how to deal with electrolytic manganese slag quickly and effectively has become an urgent technical problem in China.
Geopolymers, as advanced inorganic polymer materials, exhibit intricate three-dimensional networks constructed from tetrahedral [AlO
4] and [SiO
4] structural units. Formed through controlled processes at moderate temperatures, they predominantly rely on rich sources of silicon-aluminum compounds, like clay, industrial byproducts, and slag [
1]. Noteworthy among these constituents are fly ash [
2], electrolytic manganese slag [
3], coal gangue [
4], and red mud [
5]. The pervasive availability of these silicon-aluminum-enriched industrial residues addresses the mounting challenge of waste accumulation, establishing geopolymer synthesis as an effective strategy. In contrast to conventional silicate-based cements, geopolymers present a multifaceted spectrum of enhanced attributes encompassing mechanical potency, resistance to corrosion, effective thermal insulation, and adept sequestration of heavy metal ions. These qualities render them propitious candidates for application spanning diverse domains, ranging from architectural engineering to waste management, aerospace technology, and defense systems [
6]. Kassym Yelemessov researched the prospects of the application of building structures made of polymer concrete composites on the basis of strength analysis [
7]. Basalt fiber polymer-modified reactive powder concrete (RPC) materials were prepared by Yafeng Gong [
8]. However, prevailing formulations of low-calcium fly ash-derived geopolymers confront issues, such as uncontrolled solidification times, hindering streamlined construction practices. Furthermore, their susceptibility to solidification shrinkage and fissuring underscores the need for performance optimization [
9].
Nano-materials, characterized by at least one dimension confined within the nanoscale range (1–100 nm) or constructed through self-assembly of units in this dimensional range, showcase unique physicochemical properties. This scale corresponds roughly to the compact arrangement of 10–1000 atoms. The distinctive nanoscale attributes, coupled with their reactivity reminiscent of volcanic ash, impart nano-materials with pronounced influences over the physicochemical attributes and microstructural configurations of geopolymers. A plethora of investigations underscores the propensity of nano-material integration to evoke discernible enhancements in macroscopic mechanical properties, microstructural refinement, and the overall durability of geopolymers [
10]. Noteworthy findings by Guo Xiaolu and colleagues posit that nano-material introduction, through chemical interplay, particle integration, and crystalline nucleation, augments the dissolution kinetics of silicon and aluminum species within alkaline matrices. This dynamic imparts elevated mechanical resilience [
11]. Preeminent nano-materials for geopolymer augmentation encompass nano-SiO
2 (NS), nano-TiO
2 (NT), nano-Al
2O
3 (NA), nano-clay (NC), carbon nanotubes (CNT), and graphene oxide (GO) [
12]. Distinguished by biocompatibility, non-toxicity, heightened whiteness, and chromatic purity, nano-sized CaCO
3 assumes dimensions markedly diminutive in scale, approximating one-tenth of regular light calcium particles. This imbues nano-sized CaCO
3 with distinct surface effects, size-dependent phenomena, quantum-scale attributes, and even macroscopic quantum tunneling effects, which are distinct from conventional calcium carbonate [
13]. FP Li [
14] found that the compressive strength of geopolymers with and without sodium dodecyl sulfate with 0.1% functionalized MWCNTs increased by 16.3% and 17.6%, respectively, and the modified geopolymers with 0.10% modified MWCNTs and 2.00% PVA fibers reached maximum compressive flexural strengths at 28 days of curing (38.43 MPa and 7.75 MPa). P Prochon [
15] found that compressive strengths of analyzed fly ash mortars with activators N5-S22 and N5-C10 (5 mol/dm (3) NaOH and 10% CaO) varied from 14.3 MPa to 5.9 MPa. KK Sun [
16] modified alkali-activated metakaolin mortar using nano-SiO
2 particles as an additive and found that nano-SiO
2 improved the compressive strength of geopolymer mortar and that the compressive strength could reach 24.9 MPa at 28 days.
In order to broaden the types of mineral admixtures that can be applied to geopolymer modification and to further study the mechanism of action and engineering application of Ca2CO3 as a modifying material in the preparation of fly ash–manganese slag-based geopolymers, this study uses fly ash, manganese slag and nano-Ca2CO3 as raw materials to study the effect of the admixture of nano-Ca2CO3 on the mechanical properties of the fly ash–manganese slag geopolymer, and nano-Ca2CO3 was identified as the better modified material. In order to deeply explore the improvement effect of nano-Ca2CO3 on the mechanical properties of geopolymer, the effect of nano-Ca2CO3 doping on the mechanical properties of geopolymer was investigated. X-ray diffraction(XRD), scanning electron microscopy(SEM), attenuated total reflectance Fourier-transform infrared (ATR-FTIR) and other testing methods were used to characterize the geopolymer, and the leaching toxicity and radioactivity of the geopolymer were explored. The experimental results proved that the electrolytic manganese slag exhibits potential for used in re-plasticization and industrial preparation of building materials, which is a useful exploration of the reuse of electrolytic manganese slag and fly ash. This study can provide a technical reference for the resourceful utilization of waste.
2. Materials and Methods
2.1. Experimental Materials
Nanostructured calcium carbonate (CaCO
3) with an average particle size of 40 nm and a purity of 99% was synthesized by Beijing Boyu Gaoke New Material Technology Co, Ltd. (Beijing, China). Sodium silicate’s modulus is 2.0, and the sodium hydroxide employed is of analytical grade, possessing a purity exceeding 96.0%. The chemical composition of the coal fly ash (FA) is meticulously documented in
Table 1. Predominantly composed of silicon (Si) and aluminum (Al), the FA exhibits a Si:Al ratio of 2.08, rendering it suitable for employment in cementitious materials. A noteworthy feature is the calcium oxide (CaO) content of 3.31%, classifying it as a low-calcium fly ash. The chemical composition of the electrolytic manganese residue (EMR) is presented in
Table 2, prominently highlighting its silicon (Si) content.
2.2. Sample Preparation
In accordance with preliminary exploratory investigations, the compositional ratios of the geopolymeric blends of coal fly ash and electrolytic manganese residue (FA-EMR) with the incorporation of nanostructured calcium carbonate (CaCO
3) was established as delineated in
Table 3. Adhering to a consistent ratio of 70% coal fly ash and 30% electrolytic manganese residue, a total mass of 300 g of raw materials was meticulously quantified. Concurrently, 110 g of water was precisely measured. A predetermined quantity of nanostructured CaCO
3, sodium silicate, and sodium hydroxide was subsequently homogenized and agitated for 5 min. Subsequently, the amalgamated activator was judiciously introduced into the mixture of coal fly ash and electrolytic manganese residue and subjected to agitation at a speed of 1100 r/min for 10 min utilizing a clean slurry mixer. Following the attainment of homogeneity, the resultant geopolymeric mixture was cast into molds encompassing six layers, each possessing dimensions of 40 mm × 40 mm × 40 mm. Subsequently, these specimens were placed within a precisely controlled incubator maintained at a temperature of 70 ℃ for a curing period spanning 24 h. Upon completion of the curing interval, the specimens were demolded. The acquired specimens were subsequently exposed to an environmental chamber set at a temperature of 20 ± 1 ℃ and a relative humidity of 90%, with experimental evaluations undertaken subsequent to predetermined time intervals. In the course of this study, a total of 60 meticulously prepared specimens were utilized for comprehensive analysis.
2.3. Test Methods
The flexural strength tests for all specimens were conducted using a KZJ-5000 cement electric flexural testing machine, with a measurement precision of ±1%. The loading rate, implemented through a dual lever system, was set at 50 N/s. Similarly, the assessment of compressive strength was executed utilizing a YES-300 model cement automatic constant stress testing machine, with a loading rate fixed at 2.0 KN/s and a precision of ±1% in the test force measurement. The measurement scope of the testing apparatus ranged from 1% to 100% of the full-scale range. In each experimental set, the average of flexural and compressive strength values obtained from three test specimens was considered as the experimental outcome.
2.4. Weather Resistance Test
In order to expand the application range of fly ash–manganese residue geopolymers, it is necessary to investigate the effect of the surrounding environment on their performance, so weathering test research is essential. Weathering tests simulate changes in the performance of fly ash–manganese residue polymers in their natural environment. By subjecting them to a few days or one cycle inside a UV weathering chamber, it is possible to reproduce the changes that occur in the natural environment over months or even years.
The mass loss rate of the fly ash–manganese slag ground polymer test blocks after the weathering test can be calculated using Equation (1). The formula for calculating the mass loss rate of the test block is as follows:
where, ΔM is the mass loss rate of fly ash–manganese slag ground polymer specimens after the weathering test (%), M1 is the mass of the specimen before the weathering test (g), and M2 is the mass of the specimen after the weathering test (g).
The rate of loss of the compressive strength of the fly ash–manganese slag ground polymer specimen after the weathering test can be calculated using Equation (2). The formula for calculating the rate of loss of compressive strength of test pieces is as follows:
where ΔF is the loss of the compressive strength of the fly ash–manganese slag ground polymer specimens after the weathering test (%); F1 is the compressive strength of the specimen before the weathering test (MPa); and F2 is the compressive strength of the specimen after the weathering test (MPa).
2.5. Radioactivity and Leaching Toxicity Testing
This test uses the CIT-3000F building material radioactivity detector to test the radioactivity of the fly ash–manganese slag polymer. The specific test method is as follows: the fly ash–manganese slag polymer test piece to be tested is crushed, then ground, and sieved through the 80 mesh. Then, 500 g (accuracy value 0.1 g) of fly ash–manganese slag polymer powder is weighed and placed into the standard sample box. Measurements were performed after 24 h of resting. According to HJ557-2010 solid waste leaching toxicity leaching method, the horizontal oscillation method, and GB5085.3-2007 hazardous waste identification standards, the identification of manganese slag samples and the geopolymer leaching toxicity of Mn, Cr, Cr (VI), Hg, and Pb ions were assessed.
2.6. Microstructural Characterization
The mineral composition of the geopolymer composite derived from the combination of fly ash and manganese slag was analyzed utilizing the Smart Lab SE multifunctional X-ray diffractometer. The scanning was executed over a 2θ range spanning from 10°to 70°. The microscopic morphology of the resulting fly ash–manganese slag geopolymer was observed employing the Flex1000 scanning electron microscope. Furthermore, the characterization of the fly ash–manganese slag geopolymer was conducted using Fourier-transform infrared spectroscopy (ATR-FTIR).
3. Results
3.1. Compressive Strength Analysis
Figure 1 depicts the compressive strength evolution of distinct specimens of the fly ash–manganese slag geopolymer measured at 7 and 28 days with varying nano-CaCO
3 concentrations. As shown in
Figure 1, an escalating nano-CaCO
3 content corresponds to an augmented initial strength across the specimens. This phenomenon emanates from the nano-scaled dimensions of the nano-CaCO
3 particles, enabling their ingress into the microfine porosity of the system. This confers a densifying effect that fosters heightened compressive strength [
17]. Additionally, the integration of nano-CaCO
3 imparts an elevated concentration of monomeric calcium, which, in turn, catalyzes the assembly of monomeric entities that expedite the geopolymeric polymerization process. This, in effect, accelerates the kinetics of geopolymer formation, engendering a proliferation of intricate three-dimensional gel networks that decisively contribute to the enhanced mechanical attributes [
18].
An increase in the pH of the reaction system accentuates the dissolution kinetics of both fly ash and manganese slag, thereby expediting the liberation of active SiO
2 and AlO
3 moieties. This, subsequently, stimulates the formation of hydration products and geopolymeric constructs, consequently endowing an elevated compressive strength upon the geopolymer matrix [
19]. The insights gleaned from
Figure 1 illuminate that an amplification in the activator dosage results in a collective amplification of the compressive strength of the fly ash–manganese slag geopolymer. The pinnacle compressive strength performance is realized when the nano-CaCO
3 concentration is set at 3.5%, the silica sodium content is maintained at 10%, and the NaOH quantum is established at 12%. However, it is noteworthy that an excessive NaOH quotient may induce an overly elevated pH environment during the geopolymeric reaction sequence. This prompts the rapid dissolution and subsequent accumulation of silicate and aluminate ions, thereby facilitating escalated reaction kinetics and concurrent heightened hydration heat release that accelerates the reaction sequence. Consequently, this may elevate internal temperatures during rapid condensation, thereby yielding temperature-induced stresses and microscopic fissures that ultimately culminate in a diminution of the geopolymer’s compressive strength [
20].
Although silica sodium is adept at augmenting the active SiO
2 load within the geopolymer structure, an excessive dosing could accelerate reaction kinetics, thereby fostering the genesis of larger voids within the evolving geopolymer matrix. This, in turn, may lead to compromised mechanical resilience within the specimens [
21]. The judicious incorporation of nano-CaCO
3 noticeably elevates the compressive strength of the specimens. Nevertheless, it is prudent to exercise caution in preventing an undue surplus of nano-CaCO
3 within the geopolymer composition, as this might hinder optimal dispersion within the matrix. Consequently, such agglomeration could curtail the participation of certain nano-CaCO
3 particles in the reaction sequence, ultimately contributing to a reduction in the compressive strength of the fly ash–manganese slag geopolymer [
22]. The outcomes substantiate that the zenith of compressive strength attainment in the fly ash–manganese slag geopolymer is achieved through a judicious combination of 3.5% nano-CaCO
3 content, 10% silica sodium content, and a 12% NaOH composition, and the 28-day strength reached 25.6 MPa. Compared to other fly ash geopolymers, the compressive strength of the geopolymer prepared in this research is significantly improved.
3.2. Weatherability Test Results and Analysis
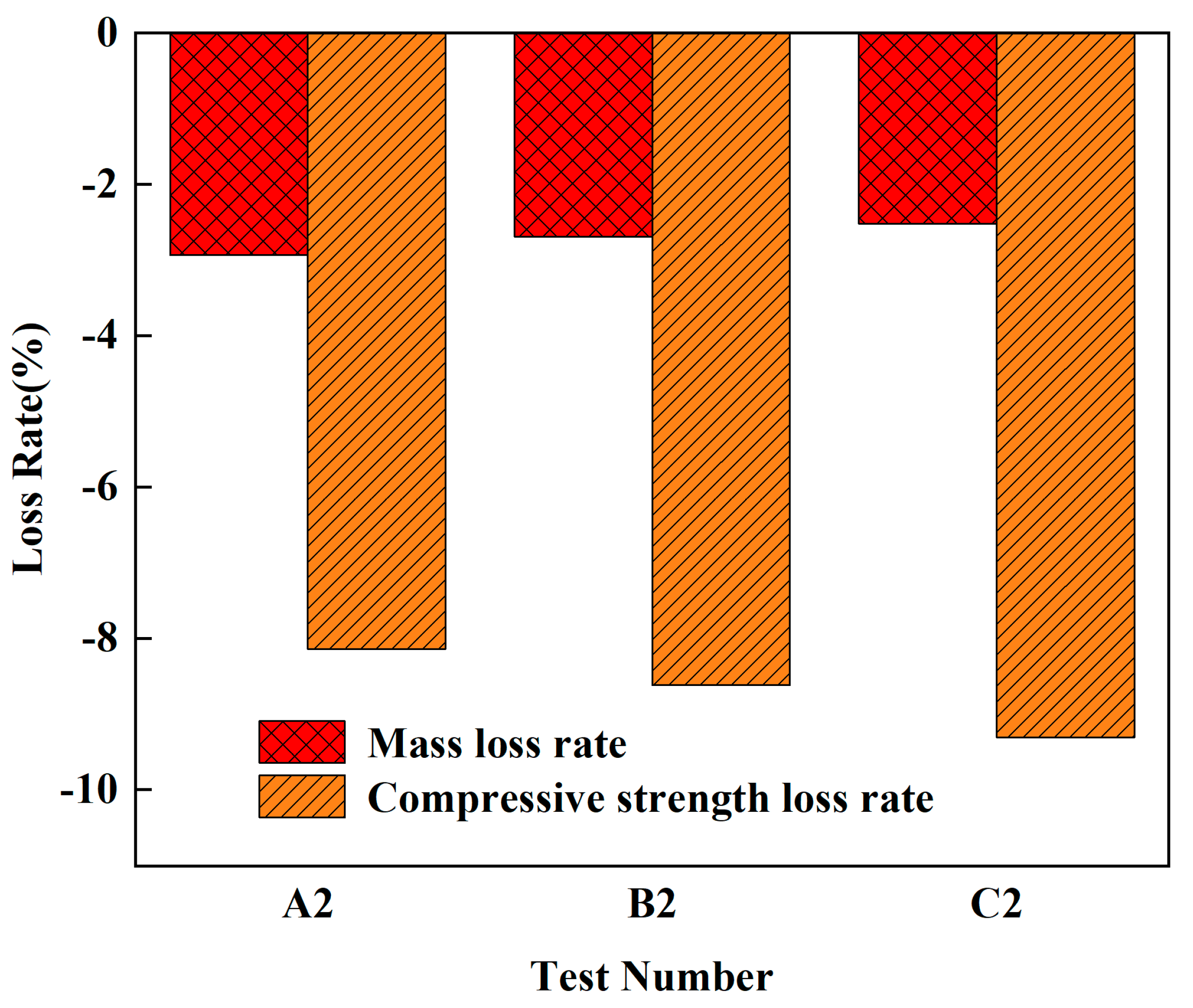
As can be seen in
Figure 2, the mass of the fly ash–manganese slag ground polymer test blocks varied in a small range, but the strength varied more. The increase in mass is due to the closed micropores on the surface of the test block during the weathering spraying process, which absorbed more water, and the geopolymer also absorbed part of the water during the later polymerization process. The mechanical properties of A2, B2, and C2 were improved because the fly ash–manganese residue geopolymer test block absorbed more water, which promoted the secondary polymerization inside the test block, and the overall structure was more and more dense.
Group B2 geopolymer mass increased by 2.27%, and compressive strength increased by 8.31%. According to the artificial climate accelerated aging test method for building materials stipulated in GB/T 16259-2008, fly ash–manganese slag geopolymer can effectively improve the climate resistance of geopolymer within a certain range.
In summary, fly ash–manganese slag geopolymer in a certain range can effectively improve the weather resistance of the geopolymer. Even when used in outdoor environments and subject to long-term exposure to sunlight, rain, condensation, temperature and other environmental changes, the appearance of the fly ash-manganese slag geopolymer test piece is not dramatically altered. In addition, these conditions will not affect its safety and reliability.
3.3. Radioactivity Analysis
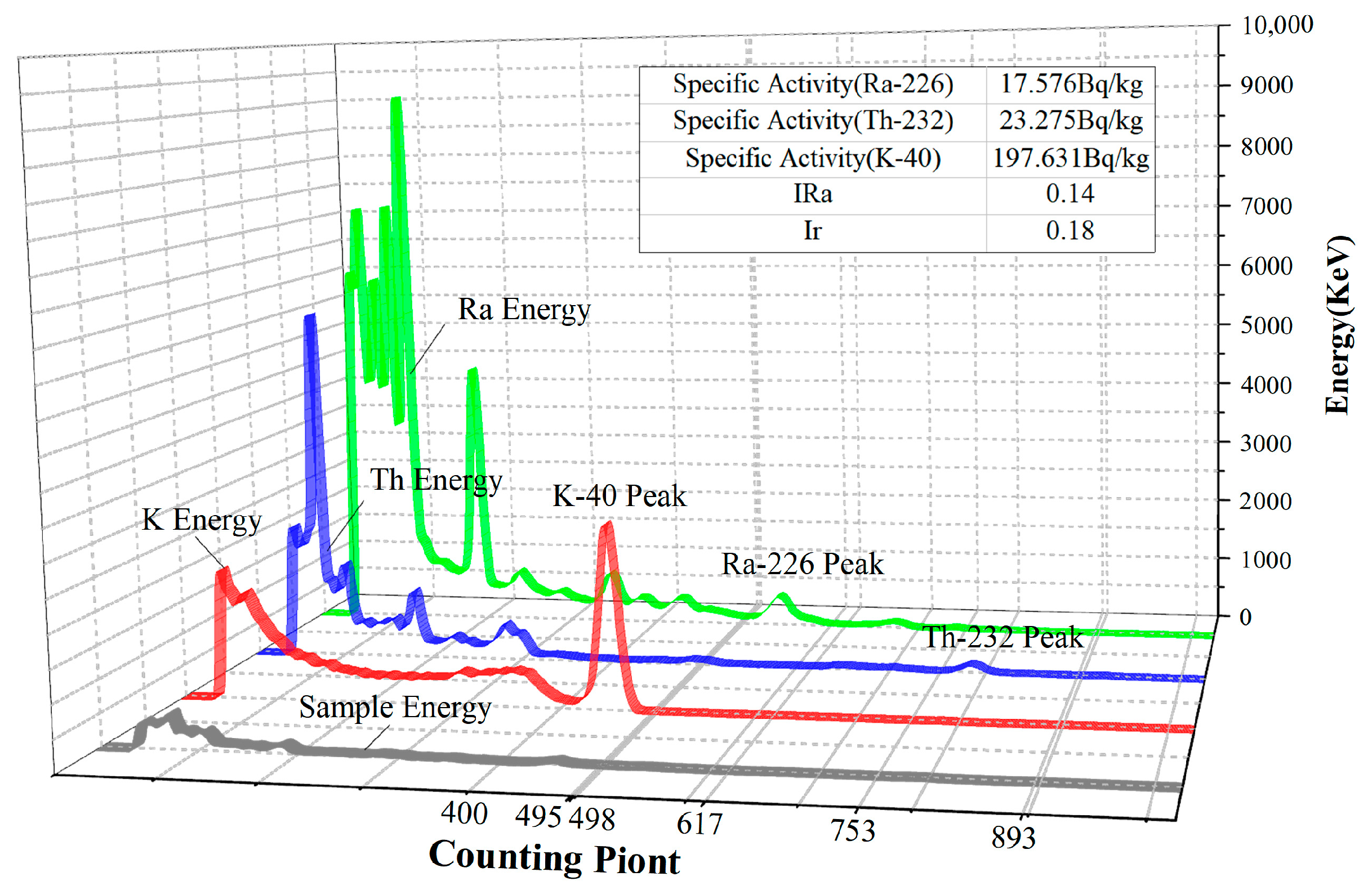
Group B2 fly ash–manganese slag geopolymer 28-day test blocks were tested for radioactivity according to the above test method, and the test detection energy spectrum is shown in
Figure 3.
According to the latest national standard GB6556-2010, the specific activity of natural radionuclides Ra-226, Th-232, and K-40 for class A finishing materials must simultaneously meet IRa ≤ 1.0 and Ir ≤ 1.3, and the scope of use of class A materials is not limited. For Class B finishing materials, the specific activity of natural radionuclides must simultaneously meet IRa ≤ 1.3 and Ir ≤ 1.9. Class B building materials can be used for exterior wall decoration of civil buildings or other buildings. Materials that do not meet the requirements of Class A and B finishing materials but meet the requirements of Ir ≤ 2.8 are Class C finishing materials, which can only be used for exterior wall decoration of buildings or other exterior wall projects.
The fly ash–manganese slag geopolymer studied in this project is mainly used for the exterior finishes of civil buildings or other buildings, as well as outdoor and road occasions. Therefore, it is sufficient for the prepared geopolymer to meet the requirements of Class B decorative decoration materials in terms of its radioactivity index. According to the results of the radioactivity test, the IRa and Ir values of the mortar after 28 days of maintenance can be compared with the standard, and the geopolymer radioactive indexes meet the standard of class A building materials, which is not harmful to human beings. Thus, this material can be applied in any building decoration field.
Table 4 shows the results of heavy metal toxicity leaching from the original sample of EMR and the ground polymer of fly ash–manganese slag of group B2. Cr, Cr(VI), Hg, and Pb were not detected in the original sample and the ground polymer, so the EMR did not reach the limit of hazardous characteristic indexes and was not hazardous waste. However, the Mn content was too high, making the material hazardous to a certain extent.
The geopolymer curing effect mainly involves the fixation of heavy metal ions by the gelling material through physical encapsulation and adsorption, while the stabilization effect refers to the entry of heavy metal ions into the skeletal structure of the matrix through chemical bonding [
23], i.e., the curing/stabilizing process is a physicochemical reaction between silica-aluminum materials, alkaline exciters and heavy metals, which can provide better curing/stabilizing effect. The Mn curing rate of 96.77% can be obtained as shown in
Table 4, and Mn ion curing was achieved.
3.4. XRD Analysis
Figure 4 depicts the X-ray diffraction (XRD) patterns of the fly ash–manganese slag geopolymer specimens with diverse dosages of nano-CaCO
3 incorporated and subjected to a curing duration of 28 days. Notably, the XRD profiles reveal an amorphous disposition within the angular range of 15° to 30° for all three specimen cohorts. Remarkably, within the 20° to 40° span, discernible attenuation of higher-intensity diffraction peaks is observed compared to the constituent materials, fly ash, and manganese slag. This absence signifies the disruption of the glassy phase within the fly ash–manganese slag composite, which is attributed to the effects of the activation process [
24]. It is noteworthy, however, that the reaction products across all experimental groups exhibit a noteworthy degree of consistency, dominated by amorphous phases alongside quartz, mullite, and minor zeolitic phases. A salient observation from
Figure 4 is the conspicuous reduction in the diffraction intensity of the quartz peak in the experimental groups relative to the unmodified fly ash and manganese slag. This reduction underscores the occurrence of geopolymerization consequent to the introduction of nano-CaCO
3. The principal hydration product of geopolymerization, namely, the amorphous aluminosilicate hydrate (N-A-S-H), is characterized by its diminished diffraction peak, which is attributed to its inherently amorphous nature [
25]. Furthermore, the XRD analysis unveils distinctive peaks corresponding to Ca
3SiO
5 and NaAlSi
3O
8 in the fly ash–manganese slag geopolymer specimens enriched with nano-CaCO
3. These discernible manifestations complement the prevailing peaks attributed to minerals such as quartz and mullite [
26].
3.5. ATR-FTIR Analysis
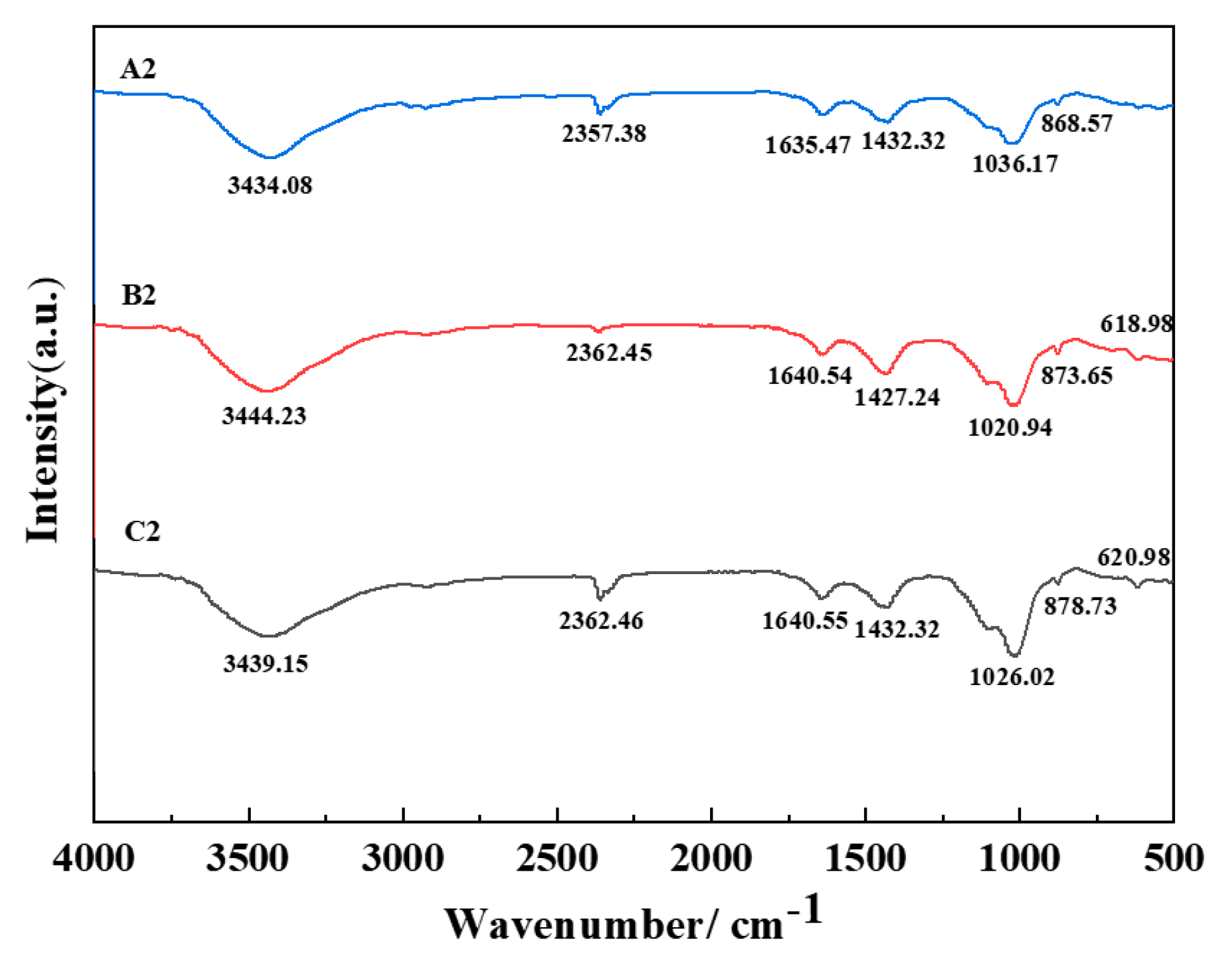
Figure 5 depicts the infrared spectra ranging from 4000 cm
−1 to 500 cm
−1 of geopolymer specimens derived from the amalgamation of fly ash and manganese slag and augmented with distinct quantities of nano-CaCO
3 following a curing period of 28 days. The pronounced band centered around 3440 cm
−1 corresponds to the stretching vibration of H
2O molecules, while the broader peak proximal to 1640 cm
−1 signifies the OH- bending vibration of water, which is indicative of the water content within the geopolymeric matrix. The extended feature at 2360 cm
−1 emanates from the carbonization of O-C-O bonds [
27]. The asymmetrical stretching vibration peak observable at 1430 cm
−1, which originates from CO
32- ions, signifies the interaction between atmospheric CO
2 and alkali metal cations diffusing within the geopolymeric structure. The encompassing spectral region around 1030 cm
−1, which represents the Si-O-T asymmetric vibrations intrinsic to the amorphous N-A-S-H gel, serves as a versatile tool for discerning fluctuations in products arising from aluminosilicate gel reactions [
28]. The spectral absorption in proximity to 870 cm
−1 corresponds to the Si-O-T asymmetric stretching vibrations inherent in the C-S-H gel. The frequency of the asymmetric bending vibrations of Si-O bonds materializes around 620 cm
−1 [
29].
The comprehensive examination offered in
Figure 5 unveils a notable shift in the infrared absorption peak proximal to 1020 cm
−1, indicative of an intricate interplay among active species within fly ash, manganese slag, and the applied activating agent post-integration. Notably, the most conspicuous shift is discerned in the B2 specimen. This phenomenon is attributed to the introduction of Al
3+ ions, supplanting Si
4+ ions, thus engendering heightened aluminum content within the aluminosilicate network. Consequently, Si-O-T bonds experience disruption, ultimately facilitating the depolymerization of the geopolymeric matrix and elevating its polymerization degree [
30].
It can be suggested that the possible reaction between sodium silicate and the geopolymer is due to the hydrolysis of sodium silicate reacting with the reactive -OH groups in the geopolymer. Thus, Si-O-C bonds are introduced in the silica-aluminate network structure, which complicates the network structure, and, consequently, the compressive strength of the manganese slag–fly ash geopolymer is greatly improved [
31].
3.6. Structural and Morphological Analysis
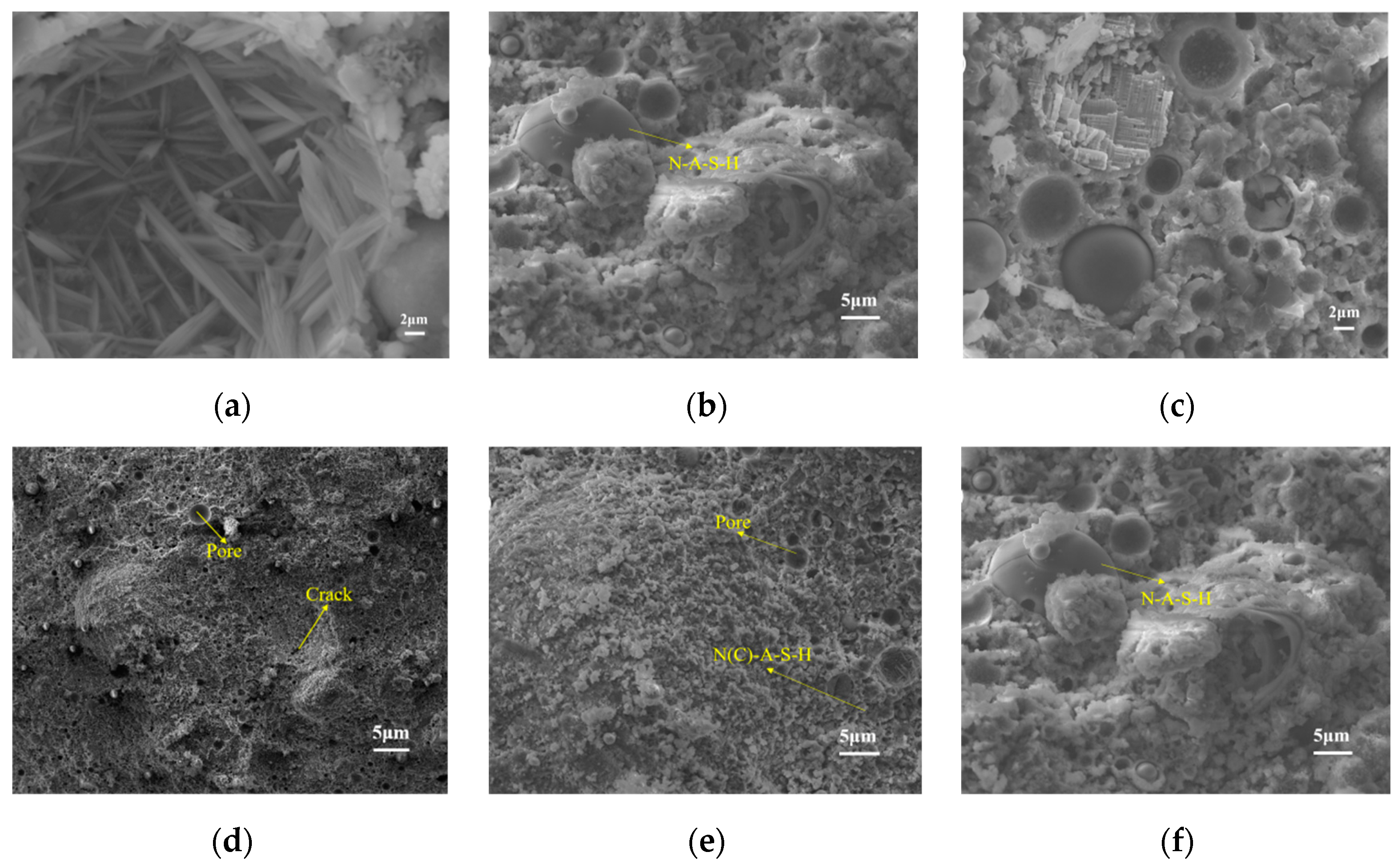
Figure 6 depicts the scanning electron microscopy (SEM) micrographs of the fly ash–manganese slag geopolymer samples with a 3.5% inclusion of nano-CaCO
3, examined after 7 and 28 days of curing. Specifically, panels a, b, and c pertain to the 7-day curing period, revealing a discernible structural disposition characterized by a porous and fractured architecture. Some insufficiently hydrated fly ash particles adopt a spherical morphology with smooth surfaces. A substantial proportion of these surfaces bear the deposition of fibrillar hydrolysis products and granular gel precipitates, while non-hydrated fly ash particles are embedded within the gel matrix [
32] and formed a three-dimensional mesh. Concurrently, a prominent presence of angular crystalline entities, possibly corresponding to granular SiO
2 crystals and Ca(OH)
2, emerges in proximity to the fly ash, as evidenced in panel a [
33].
Upon the interaction between nano-CaCO
3 and the alkali-activated solution, an abundance of needle-like crystals proliferating on the surface of the hydrating gel are noted in the 7-day micrograph in panel a. This phenomenon could be attributed to the minute dimensions of the nano-CaCO
3 particles, facilitating their role as nucleation sites for hydration products. This, in turn, expedites the hydration kinetics of the fly ash–manganese slag geopolymer and underscores the propensity of nano-CaCO
3 to foster crystal refinement [
34]. Panels d and e collectively unveil an intriguing coexistence of amorphous gel and other discernible phases, indicative of higher nano-CaCO
3 content. Notably, instances of pore filling become evident, inducing a marked reduction in both porosity and fissures. This transformative architectural shift reflects a more densely compacted structure and heightened compressive strength, thereby delineating the intricate interplay between the alkali-activated solution and nano-CaCO
3, consequently amplifying the geopolymer’s compressive resilience.
Micrograph c captures the occurrence of internal voids within the geopolymer matrix as a consequence of excessive nano-CaCO
3 content. This phenomenon leads to the expansion of cracks, potentially culminating in crack propagation, driven by the augmented specific surface area of nano-CaCO
3. The exacerbation of this phenomenon due to inadequate dispersion characteristics of nano-CaCO
3 contributes to the attenuation of the compressive strength of the fly ash-manganese slag geopolymer upon excessive incorporation [
35]. Micrographs e and f distinctly showcase an assorted array of aluminosilicate structures within the geopolymer matrix, encompassing noteworthy constituents, such as the N-A-S-H and C-A-S-H phases. Collectively, these diverse phases significantly enhance the fly ash–manganese slag geopolymer’s compressive strength.