Metal Oxide Nanoparticles’ Green Synthesis by Plants: Prospects in Phyto- and Bioremediation and Photocatalytic Degradation of Organic Pollutants
Abstract
1. Introduction
2. Green Synthesis Definition
3. Metal Oxide Nanoparticles
4. Factors Affecting the Synthesis of Inorganic Nanoparticles
4.1. pH
4.2. Temperature
4.3. Concentration of Extracts
4.4. Reaction Times
4.5. Stirring Speed
4.6. Choice of Species
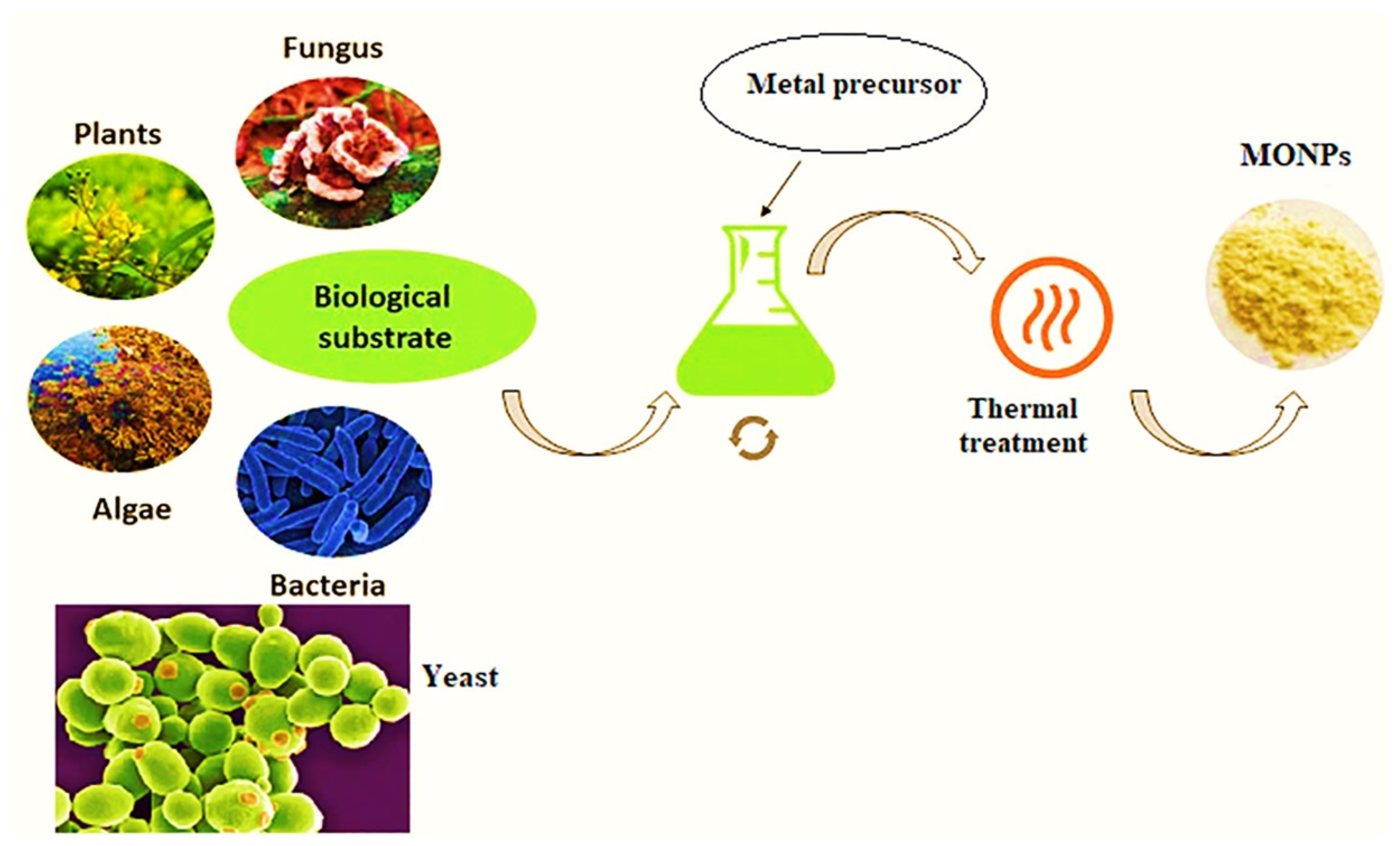
5. Various Techniques for Synthesizing MONPs
6. Biosynthesis of MONPs by Plants
7. Mechanism of Nanoparticle Synthesis by Plant Extract
- (a)
- The extracellular technique, which utilizes plant extract, is often preferred for nanoparticle synthesis due to its simplicity in handling, cost-effectiveness, and the absence of an additional step required to disrupt cells in order to recover internal nanoparticles for subsequent processing. In the extracellular method, metal salts are converted into metal nanoparticles using a suspension of plant extract. Plant extracts are abundant in various bioactive compounds, which serve as both reducing agents and capping agents during nanoparticle production. These compounds play a crucial role in reducing metal ions and stabilizing the resulting nanoparticles [89]. Metal nanoparticles are formed through the reduction of metal salts using bioactive substances released by plant cells in an extract medium. The process involves two stages: nucleation and crystal growth. During the nucleation stage, precursor metal salts (M+) are transformed into metal nanoparticles (M0) through a reaction facilitated by a component present in the plant extract. This reaction initiates the formation of small clusters of metal atoms that serve as the building blocks for the nanoparticles. In the crystal growth stage, the metal nanoparticles (M0) undergo complexation with other metal salts (M+), resulting in the formation of M0-M+ complexes. This complexation process enables the nanoparticles to grow in size. Additionally, the nanoparticles undergo adsorption, where they bind together to form nanocrystals, often in the form of metal nanoparticle dimers. Overall, the combination of nucleation and crystal growth processes using bioactive substances from plant extract allows for the production of metal nanoparticles [90].
- (b)
- In intracellular synthesis, metal salts are reduced within the cells through the assistance of various enzymes such as nitrate reductase and NADH- and NADPH-dependent enzymes. These enzymes are involved in essential metabolic processes like photosynthesis, nitrogen fixation, and respiration. Additionally, plant pigments such as chlorophyll also contribute to the intracellular reduction process [90]. Both living and dry biomasses of plants are capable of performing intracellular synthesis of nanoparticles. This process occurs as a defense mechanism of plant cells against stress conditions induced by metals. When exposed to metal ions, the metabolic machinery of plant cells is redirected to mitigate the toxic effects of metals. As a result, the cells utilize their inherent enzymatic systems and pigments to reduce metal salts and produce nanoparticles intracellularly [91]. The process of nanoparticle formation inside cells involves the binding of positively charged metal ions to the surface of the cell wall or to negatively charged protein or enzyme groups within the cytoplasm. Once the metal ions are trapped, they undergo reduction to form small nuclei, which serve as the starting point for nanoparticle synthesis. This process can lead to the production of nanoparticles with various morphologies. In the case of algal cells, there is an intracellular enzyme present that facilitates the internal conversion of metal salts into nanoparticles. This enzyme plays a crucial role in the reduction process, enabling the transformation of metal ions into nanoparticles within the cellular environment [91].
8. Characterization of MONP Plant Synthesis
9. Adsorption Method for the Elimination of Various Pollutants
9.1. Application of Green MONPs Using Plant Extracts in Water Treatment
9.1.1. Removal of Heavy Metals
9.1.2. Fluoride Removal
9.2. Photocatalytic Activity of MONPs
9.2.1. Advantages of Nano-Photocatalysts
9.2.2. Factors Affecting the Photocatalytic Degradation
- (a)
- Amount of Catalyst
- (b)
- Amount of Pollutant
- (c)
- Influence of pH
- (d)
- Temperature, Time, and Morphology
9.2.3. General Reaction Mechanism of Photocatalysis
9.2.4. Application of Green MONPs’ Organic Pollutant Treatment by Photocatalytic Activity
9.2.5. Water Disinfection from Microbes
10. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BET | Surface area analysis |
| ICP-MS | Inductively coupled plasma-mass spectrometry |
| DLS | Dynamic light scattering |
| EDS | Energy-dispersive X-ray spectroscopy |
| EDX | Energy-dispersive X-ray |
| FTIR | Fourier transform infrared spectroscopy |
| NMR | Nuclear magnetic resonance spectroscopy |
| PL | Photoluminescence |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscope |
| TGA | Thermogravimetric analysis |
| XPSX-ray | Photoelectron spectroscopy |
| X-ray | X-ray diffraction technique |
References
- Abualnaja, K.M.; Alprol, A.E.; Abu-Saied, M.A.; Ashour, M.; Mansour, A.T. Removing of Anionic Dye from Aqueous Solutions by Adsorption Using of Multiwalled Carbon Nanotubes and Poly (Acrylonitrile-styrene) Impregnated with Activated Carbon. Sustainability 2021, 13, 7077. [Google Scholar] [CrossRef]
- Abualnaja, K.M.; Alprol, A.E.; Abu-Saied, M.A.; Mansour, A.T.; Ashour, M. Studying the Adsorptive Behavior of Poly(Acrylonaitrile-co-Styrene) and Carbon Nanotubes (Nanocomposites) Impregnated with Adsorbent Materials towards Methyl Orange Dye. Nanomaterials 2021, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Alprol, A.E.; Mansour, A.T.; El-Beltagi, H.S.; Ashour, M. Algal Extracts for Green Synthesis of Zinc Oxide Nanoparticles: Promising Approach for Algae Bioremediation. Materials 2023, 16, 2819. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, A.A.; Ayesu-Koranteng, E.; Shakantu, W. A Review of the Literature on the Environmental and Health Impact of Plastic Waste Pollutants in Sub-Saharan Africa. Pollutants 2022, 2, 531–545. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, J.; Yu, S.; Wang, P.; Sun, C. Tunable electronic properties of free-standing Fe-doped GaN nanowires as high-capacity anode of lithium-ion batteries. Arab. J. Chem. 2021, 14, 103161. [Google Scholar] [CrossRef]
- Mabrouk, M.M.; Ashour, M.; Labena, A.; Zaki, M.A.A.; Abdelhamid, A.F.; Gewaily, M.S.; Dawood, M.A.O.; Abualnaja, K.M.; Ayoub, H.F. Nanoparticles of Arthrospira platensis improves growth, antioxidative and immunological responses of Nile tilapia (Oreochromis niloticus) and its resistance to Aeromonas hydrophila. Aquacult. Res. 2022, 53, 125–135. [Google Scholar] [CrossRef]
- Sharawy, Z.Z.; Ashour, M.; Labena, A.; Alsaqufi, A.S.; Mansour, A.T.; Abbas, E.M. Effects of dietary Arthrospira platensis nanoparticles on growth performance, feed utilization, and growth-related gene expression of Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2022, 551, 737905. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Khedawy, M.; Abualnaja, K.M.; Shalaby, T.A.; Rayan, G.; Ramadan, K.M.; Ashour, M. Green Synthesis of Zinc Oxide Nanoparticles Using Red Seaweed for the Elimination of Organic Toxic Dye from an Aqueous Solution. Materials 2022, 15, 5169. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Alprol, A.E.; Mansour, A.T.; Abdelwahab, A.M.; Ashour, M. Advances in Green Synthesis of Metal Oxide Nanoparticles by Marine Algae for Wastewater Treatment by Adsorption and Photocatalysis Techniques. Catalysts 2023, 13, 888. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A.; Angulo, Y. Biofabrication of copper oxide nanoparticles using Andean blackberry (Rubus glaucus Benth.) fruit and leaf. J. Saudi Chem. Soc. 2017, 21, S475–S480. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. The Using of Nanoparticles of Microalgae in Remediation of Toxic Dye from Industrial Wastewater: Kinetic and Isotherm Studies. Materials 2022, 15, 3922. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.T.; Alprol, A.E.; Ashour, M.; Ramadan, K.M.; Alhajji, A.H.; Abualnaja, K.M. Do Red Seaweed Nanoparticles Enhance Bioremediation Capacity of Toxic Dyes from Aqueous Solution? Gels 2022, 8, 310. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Darwesh, O.; Shalapy, M.; Abo-Zeid, A.; Mahmoud, Y. Nano-bioremediation of municipal wastewater using myco-synthesized iron nanoparticles. Egypt. J. Chem. 2021, 64, 2499–2507. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of metal and metal oxide nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.; Khalil, N.; Ahmed, W.; Amin, N. Enhancement the bioremediation of crude oil by nanoparticle and biosurfactants. Egypt. J. Chem. 2017, 60, 835–848. [Google Scholar] [CrossRef][Green Version]
- Sundrarajan, M.; Bama, K.; Bhavani, M.; Jegatheeswaran, S.; Ambika, S.; Sangili, A.; Nithya, P.; Sumathi, R. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol. B Biol. 2017, 171, 117–124. [Google Scholar]
- Ruddaraju, L.K.; Pammi, S.; Pallela, P.V.K.; Padavala, V.S.; Kolapalli, V.R.M. Antibiotic potentiation and anti-cancer competence through bio-mediated ZnO nanoparticles. Mater. Sci. Eng. C 2019, 103, 109756. [Google Scholar] [CrossRef] [PubMed]
- Madan, H.; Sharma, S.; Suresh, D.; Vidya, Y.; Nagabhushana, H.; Rajanaik, H.; Anantharaju, K.; Prashantha, S.; Maiya, P.S. Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: Their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Momeni, S.S.; Nasrollahzadeh, M.; Rustaiyan, A. Green synthesis of the Cu/ZnO nanoparticles mediated by Euphorbia prolifera leaf extract and investigation of their catalytic activity. J. Coll. Interf. Sci. 2016, 472, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Coll. Interf. Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. Dried Brown Seaweed’s Phytoremediation Potential for Methylene Blue Dye Removal from Aquatic Environments. Polymers 2022, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Alprol, A.E.; Khedawy, M.; Abualnaja, K.M.; Mansour, A.T. Equilibrium and Kinetic Modeling of Crystal Violet Dye Adsorption by a Marine Diatom, Skeletonema costatum. Materials 2022, 15, 6375. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.; Alprol, A.E.; Heneash, A.M.M.; Saleh, H.; Abualnaja, K.M.; Alhashmialameer, D.; Mansour, A.T. Ammonia Bioremediation from Aquaculture Wastewater Effluents Using Arthrospira platensis NIOF17/003: Impact of Biodiesel Residue and Potential of Ammonia-Loaded Biomass as Rotifer Feed. Materials 2021, 14, 5460. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Khan, S.A. Pharmaceutical pollution: A weakly regulated global environmental risk. Rev. Eur. Comp. Int. Environ. Law 2022, 31, 75–88. [Google Scholar] [CrossRef]
- Al-Qahtani, K.M. Cadmium removal from aqueous solution by green synthesis zero valent silver nanoparticles with Benjamina leaves extract. Egypt. J. Aquat. Res. 2017, 43, 269–274. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Fawzan, F.F. Adsorption study of heavy metal ions from aqueous solution by nanoparticle of wild herbs. Egypt. J. Aquat. Res. 2018, 44, 187–194. [Google Scholar] [CrossRef]
- Seabra, A.B.; Durán, N. Nanotoxicology of metal oxide nanoparticles. Metals 2015, 5, 934–975. [Google Scholar] [CrossRef]
- Vrček, I.V.; Žuntar, I.; Petlevski, R.; Pavičić, I.; Dutour Sikirić, M.; Ćurlin, M.; Goessler, W. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environ. Toxicol. 2016, 31, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R. Microbial Nanobionics; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Pal, G.; Rai, P.; Pandey, A. Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–26. [Google Scholar]
- Yu, S.; Liu, J.; Yin, Y.; Shen, M. Interactions between engineered nanoparticles and dissolved organic matter: A review on mechanisms and environmental effects. J. Environ. Sci. 2018, 63, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Singh, J.; Mishra, R.K.; Ojha, A.K. Electro-optical and magnetic properties of monodispersed colloidal Cu2O nanoparticles. J. Alloys Compd. 2013, 555, 123–130. [Google Scholar] [CrossRef]
- De Montferrand, C.; Hu, L.; Milosevic, I.; Russier, V.; Bonnin, D.; Motte, L.; Brioude, A.; Lalatonne, Y. Iron oxide nanoparticles with sizes, shapes and compositions resulting in different magnetization signatures as potential labels for multiparametric detection. Acta Biomater. 2013, 9, 6150–6157. [Google Scholar] [CrossRef]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Fatima Rana, N.; Menaa, F. Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef]
- Ahmad, P.; Tripathi, D.K.; Sharma, S.; Chauhan, D.K.; Dubey, N. Nanomaterials in Plants, Algae and Microorganisms; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Patra, J.K.; Baek, K.-H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomat. 2014, 2014, 417305. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Saratale, R.G.; Shinde, S.; Syed, A.; Ameen, F.; Ghodake, G. Green synthesis of silver nanoparticles using Laminaria japonica extract: Characterization and seedling growth assessment. J. Clean. Prod. 2018, 172, 2910–2918. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Swarnakar, S.; Ghosh, S.; Majumdar, S.; Banerjee, S. Disinfection of drinking water via algae mediated green synthesized copper oxide nanoparticles and its toxicity evaluation. J. Environ. Chem. Eng. 2019, 7, 102867. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, N.K.; Prasad, S.B.; Yadav, S.S.; Narayan, G.; Rai, A.K. The freshwater cyanobacterium Anabaena doliolum transformed with ApGSMT-DMT exhibited enhanced salt tolerance and protection to nitrogenase activity, but became halophilic. Microbiology 2013, 159, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thakur, R.; Duhan, J.S.; Chaudhury, A. Management of wilt disease of chickpea in vivo by silver nanoparticles biosynthesized by rhizospheric microflora of chickpea (Cicer arietinum). J. Chem. Technol. Biotechnol. 2018, 93, 3233–3243. [Google Scholar] [CrossRef]
- Salam, H.A.; Rajiv, P.; Kamaraj, M.; Jagadeeswaran, P.; Gunalan, S.; Sivaraj, R. Plants: Green route for nanoparticle synthesis. Int. Res. J. Biol. Sci. 2012, 1, 85–90. [Google Scholar]
- Cauerhff, A.; Castro, G.R. Bionanoparticles, a green nanochemistry approach. Electr. J. Biotechnol. 2013, 16, 11. [Google Scholar]
- Ruddaraju, L.K.; Pammi, S.V.N.; sankar Guntuku, G.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef]
- Yadav, M.; Kaur, P. A review on exploring phytosynthesis of silver and gold nanoparticles using genus Brassica. Int. J. Nanopart. 2018, 10, 165–177. [Google Scholar] [CrossRef]
- Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Pantilimon, C.; Drăgan, C.; Vidu, R.; Predescu, C.; Kuncser, V. Synthesis and characterization of dextran-coated iron oxide nanoparticles. R. Soc. Open Sci. 2018, 5, 171525. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.; Singh, A.; Singh, H.; Singh, S. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Chaudhury, A. Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimicrobial activity against opportunistic pathogens. Green Chem. Lett. Rev. 2016, 9, 33–38. [Google Scholar] [CrossRef]
- Ahmed, B.; Hashmi, A.; Khan, M.S.; Musarrat, J. ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv. Powder Technol. 2018, 29, 1601–1616. [Google Scholar] [CrossRef]
- Sharma, D.; Sabela, M.I.; Kanchi, S.; Mdluli, P.S.; Singh, G.; Stenström, T.A.; Bisetty, K. Biosynthesis of ZnO nanoparticles using Jacaranda mimosifolia flowers extract: Synergistic antibacterial activity and molecular simulated facet specific adsorption studies. J. Photochem. Photobiol. B Biol. 2016, 162, 199–207. [Google Scholar] [CrossRef]
- Al-Ruqeishi, M.S.; Mohiuddin, T.; Al-Saadi, L.K. Green synthesis of iron oxide nanorods from deciduous Omani mango tree leaves for heavy oil viscosity treatment. Arab. J. Chem. 2019, 12, 4084–4090. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green approach for fabrication and applications of zinc oxide nanoparticles. Bioinorg. Chem. Applic. 2014, 2014, 523869. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, S.; Buledi, J.A.; Mal, D.; Solangi, A.R.; Balouch, A.; Hyder, A. Importance and analytical perspective of green synthetic strategies of copper, zinc, and titanium oxide nanoparticles and their applications in pathogens and environmental remediation. Curr. Anal. Chem. 2021, 17, 1169–1181. [Google Scholar] [CrossRef]
- Sharma, J.K.; Srivastava, P.; Ameen, S.; Akhtar, M.S.; Singh, G.; Yadava, S. Azadirachta indica plant-assisted green synthesis of Mn3O4 nanoparticles: Excellent thermal catalytic performance and chemical sensing behavior. J. Coll. Interf. Sci. 2016, 472, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar]
- Surendra, T.; Roopan, S.M. Photocatalytic and antibacterial properties of phytosynthesized CeO2 NPs using Moringa oleifera peel extract. J. Photochem. Photobiol. B Biol. 2016, 161, 122–128. [Google Scholar] [CrossRef]
- Moon, S.A.; Salunke, B.K.; Alkotaini, B.; Sathiyamoorthi, E.; Kim, B.S. Biological synthesis of manganese dioxide nanoparticles by Kalopanax pictus plant extract. IET Nanobiotechnol. 2015, 9, 220–225. [Google Scholar] [CrossRef]
- Vidya, C.; Prabha, M.C.; Raj, M.A. Green mediated synthesis of zinc oxide nanoparticles for the photocatalytic degradation of Rose Bengal dye. Environ. Nanotechnol. Monit. Manag. 2016, 6, 134–138. [Google Scholar]
- Stan, M.; Popa, A.; Toloman, D.; Dehelean, A.; Lung, I.; Katona, G. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater. Sci. Semicond. Process. 2015, 39, 23–29. [Google Scholar] [CrossRef]
- Suresh, D.; Shobharani, R.; Nethravathi, P.; Kumar, M.P.; Nagabhushana, H.; Sharma, S. Artocarpus gomezianus aided green synthesis of ZnO nanoparticles: Luminescence, photocatalytic and antioxidant properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 128–134. [Google Scholar] [CrossRef]
- Kumar, P.V.; Pammi, S.; Shameem, U. A Green approach for the synthesis of iron oxide nanoparticles by using roots of A. racemosus and its deg-radation of dye methyl orange. Int. J. Pharm. Drug Anal. 2018, 6, 22–28. [Google Scholar]
- Ardakani, L.S.; Alimardani, V.; Tamaddon, A.M.; Amani, A.M.; Taghizadeh, S. Green synthesis of iron-based nanoparticles using Chlorophytum comosum leaf extract: Methyl orange dye degradation and antimicrobial properties. Heliyon 2021, 7, 1. [Google Scholar]
- Karpagavinayagam, P.; Vedhi, C. Green synthesis of iron oxide nanoparticles using Avicennia marina flower extract. Vacuum 2019, 160, 286–292. [Google Scholar] [CrossRef]
- Lohrasbi, S.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Ghasemi, Y.; Amani, A.M.; Taghizadeh, S. Green synthesis of iron nanoparticles using Plantago major leaf extract and their application as a catalyst for the decolorization of azo dye. BioNanoScience 2019, 9, 317–322. [Google Scholar] [CrossRef]
- Rostamizadeh, E.; Iranbakhsh, A.; Majd, A. Green synthesis of Fe2O3 nanoparticles using fruit extract of Cornus mas L. and its growth-promoting roles in barley. J. Nanostruct. Chem. 2020, 10, 125–130. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Jamzad, M.; Kamari Bidkorpeh, M. Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis L. leaves and evaluation of the antimicrobial activity. J. Nanostructure Chem. 2020, 10, 193–201. [Google Scholar] [CrossRef]
- Belaiche, Y.; Khelef, A.; Laouini, S.E.; Bouafia, A.; Tedjani, M.L.; Barhoum, A. Green synthesis and characterization of silver/silver oxide nanoparticles using aqueous leaves extract of Artemisia herba-alba as reducing and capping agents. Rev. Romana Mater. 2021, 51, 342–352. [Google Scholar]
- Roopan, S.M.; Bharathi, A.; Prabhakarn, A.; Rahuman, A.A.; Velayutham, K.; Rajakumar, G.; Padmaja, R.; Lekshmi, M.; Madhumitha, G. Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 98, 86–90. [Google Scholar] [CrossRef]
- Fu, L.; Fu, Z. Plectranthus amboinicus leaf extract–assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram. Int. 2015, 41, 2492–2496. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Rao, Y.S.; Balaji, T.; Prathima, B.; Jyothi, N. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater. Lett. 2013, 100, 241–244. [Google Scholar] [CrossRef]
- Vidhu, V.; Philip, D. Biogenic synthesis of SnO2 nanoparticles: Evaluation of antibacterial and antioxidant activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 372–379. [Google Scholar] [CrossRef]
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Synthesis, characterization and photocatalytic activity of ZnO nanoparticles prepared by biological method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 143, 304–308. [Google Scholar] [CrossRef]
- Sankar, R.; Manikandan, P.; Malarvizhi, V.; Fathima, T.; Shivashangari, K.S.; Ravikumar, V. Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 746–750. [Google Scholar] [CrossRef]
- Diallo, A.; Manikandan, E.; Rajendran, V.; Maaza, M. Physical & enhanced photocatalytic properties of green synthesized SnO2 nanoparticles via Aspalathus linearis. J. Alloys Compd. 2016, 681, 561–570. [Google Scholar]
- Suresh, D.; Nethravathi, P.; Rajanaika, H.; Nagabhushana, H.; Sharma, S. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Proces. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Thovhogi, N.; Park, E.; Manikandan, E.; Maaza, M.; Gurib-Fakim, A. Physical properties of CdO nanoparticles synthesized by green chemistry via Hibiscus Sabdariffa flower extract. J. Alloys Compd. 2016, 655, 314–320. [Google Scholar] [CrossRef]
- Ijaz, F.; Shahid, S.; Khan, S.A.; Ahmad, W.; Zaman, S. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activitie. Trop. J. Pharm. Res. 2017, 16, 743–753. [Google Scholar] [CrossRef]
- Malleshappa, J.; Nagabhushana, H.; Sharma, S.; Vidya, Y.; Anantharaju, K.; Prashantha, S.; Prasad, B.D.; Naika, H.R.; Lingaraju, K.; Surendra, B. Leucas aspera mediated multifunctional CeO2 nanoparticles: Structural, photoluminescent, photocatalytic and antibacterial properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 452–462. [Google Scholar] [CrossRef]
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Biosynthesis and photocatalytic properties of SnO2 nanoparticles prepared using aqueous extract of cauliflower. J. Clust. Sci. 2017, 28, 1883–1896. [Google Scholar] [CrossRef]
- Kumar, P.N.; Sakthivel, K.; Balasubramanian, V. Microwave assisted biosynthesis of rice shaped ZnO nanoparticles using Amorphophallus konjac tuber extract and its application in dye sensitized solar cells. Mater. Sci. Pol. 2017, 35, 111–119. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef]
- Ramanan, V.; Thiyagarajan, S.K.; Raji, K.; Suresh, R.; Sekar, R.; Ramamurthy, P. Outright green synthesis of fluorescent carbon dots from eutrophic algal blooms for in vitro imaging. ACS Sustain. Chem. Eng. 2016, 4, 4724–4731. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, G.-D. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 487–495. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; El-Desoky, H.S.; El-Moselhy, K.M.; Amer, A.; Abou El-Naga, E.H.; Mohamedein, L.I.; Al-Prol, A.E. Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. Egypt. J. Aquat. Res. 2014, 40, 235–242. [Google Scholar] [CrossRef]
- Yasir, M.; Šopík, T.; Ali, H.; Kimmer, D.; Sedlařík, V. Green synthesis of titanium and zinc oxide nanoparticles for simultaneous photocatalytic removal of estrogens in wastewater. In Proceedings of the NANOCON 2021—International Conference on Nanomaterials, Brno, Czech Republic, 20–22 October 2021. [Google Scholar]
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. 2016, 23, 13754–13788. [Google Scholar] [CrossRef]
- Srivastava, V.; Gusain, D.; Sharma, Y.C. Synthesis, characterization and application of zinc oxide nanoparticles (n-ZnO). Ceram. Int. 2013, 39, 9803–9808. [Google Scholar] [CrossRef]
- Ehrampoush, M.H.; Miria, M.; Salmani, M.H.; Mahvi, A.H. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract. J. Environ. Health Sci. Eng. 2015, 13, 84. [Google Scholar] [CrossRef]
- Fazlzadeh, M.; Khosravi, R.; Zarei, A. Green synthesis of zinc oxide nanoparticles using Peganum harmala seed extract, and loaded on Peganum harmala seed powdered activated carbon as new adsorbent for removal of Cr (VI) from aqueous solution. Ecol. Eng. 2017, 103, 180–190. [Google Scholar] [CrossRef]
- Mukherjee, D.; Ghosh, S.; Majumdar, S.; Annapurna, K. Green synthesis of α-Fe2O3 nanoparticles for arsenic (V) remediation with a novel aspect for sludge management. J. Environ. Chem. Eng. 2016, 4, 639–650. [Google Scholar] [CrossRef]
- Somu, P.; Paul, S. Casein based biogenic-synthesized zinc oxide nanoparticles simultaneously decontaminate heavy metals, dyes, and pathogenic microbes: A rational strategy for wastewater treatment. J. Chem. Technol. Biotechnol. 2018, 93, 2962–2976. [Google Scholar] [CrossRef]
- Azizi, S.; Mahdavi Shahri, M.; Mohamad, R. Green synthesis of zinc oxide nanoparticles for enhanced adsorption of lead ions from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Yoon, M. Rapid removal of cadmium ions using green-synthesized Fe3O4 nanoparticles capped with diethyl-4-(4 amino-5-mercapto-4 H-1, 2, 4-triazol-3-yl) phenyl phosphonate. RSC Adv. 2015, 5, 65444–65453. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G. New frontiers in the biosynthesis of metal oxide nanoparticles and their environmental applications: An overview. SN Appl. Sci. 2019, 1, 928. [Google Scholar] [CrossRef]
- Dubey, S.; Sharma, Y.C. Calotropis procera mediated one pot green synthesis of Cupric oxide nanoparticles (CuO-NPs) for adsorptive removal of Cr (VI) from aqueous solutions. Appl. Organomet. Chem. 2017, 31, e3849. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, V.; Kim, K.-H.; Rawat, M. Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes. Environ. Res. 2019, 177, 108569. [Google Scholar] [CrossRef]
- Khani, R.; Roostaei, B.; Bagherzade, G.; Moudi, M. Green synthesis of copper nanoparticles by fruit extract of Ziziphus spina-christi (L.) Willd.: Application for adsorption of triphenylmethane dye and antibacterial assay. J. Mol. Liq. 2018, 255, 541–549. [Google Scholar] [CrossRef]
- Sathyavathi, S.; Manjula, A.; Rajendhran, J.; Gunasekaran, P. Extracellular synthesis and characterization of nickel oxide nanoparticles from Microbacterium sp. MRS-1 towards bioremediation of nickel electroplating industrial effluent. Bioresour. Technol. 2014, 165, 270–273. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Al-Qahtani, K.M.; Alflaij, S.O.; Al-Qahtani, S.F.; Alsamhan, F.A. Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci. Rep. 2021, 11, 12547. [Google Scholar] [CrossRef]
- Vidovix, T.B.; Quesada, H.B.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Adsorption of Safranin-O dye by copper oxide nanoparticles synthesized from Punica granatum leaf extract. Environ. Technol. 2022, 43, 3047–3063. [Google Scholar] [CrossRef]
- Debnath, P.; Mondal, N.K. Effective removal of congo red dye from aqueous solution using biosynthesized zinc oxide nanoparticles. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100320. [Google Scholar] [CrossRef]
- Nayak, A.; Sahoo, J.K.; Sahoo, S.K.; Sahu, D. Removal of congo red dye from aqueous solution using zinc oxide nanoparticles synthesised from Ocimum sanctum (Tulsi leaf): A green approach. Int. J. Environ. Anal. Chem. 2022, 102, 7889–7910. [Google Scholar] [CrossRef]
- Mittal, H.; Morajkar, P.P.; Al Alili, A.; Alhassan, S.M. In-situ synthesis of ZnO nanoparticles using gum arabic based hydrogels as a self-template for effective malachite green dye adsorption. J. Polym. Environ. 2020, 28, 1637–1653. [Google Scholar] [CrossRef]
- Suriyaraj, S.; Selvakumar, R. Advances in nanomaterial based approaches for enhanced fluoride and nitrate removal from contaminated water. RSC Adv. 2016, 6, 10565–10583. [Google Scholar] [CrossRef]
- Zeng, Y.; Xue, Y.; Liang, S.; Zhang, J. Removal of fluoride from aqueous solution by TiO2 and TiO2–SiO2 nanocomposite. Chem. Speciat. Bioavailab. 2017, 29, 25–32. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Zhao, N.; Yang, W.; You, X. Sulfate-doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water. Water Res. 2013, 47, 4040–4049. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.; Shimabuku, Q.L.; Fernandes Silva, M.; Bergamasco, R. Iron-oxide nanoparticles by the green synthesis method using Moringa oleifera leaf extract for fluoride removal. Environ. Technol. 2018, 39, 2926–2936. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Khan, S. Defluoridation technology for drinking water and tea by green synthesized Fe3O4/Al2O3 nanoparticles coated polyurethane foams for rural communities. Sci. Rep. 2017, 7, 8070. [Google Scholar] [CrossRef] [PubMed]
- Zandsalimi, Y.; Taymori, P.; Darvishi Cheshmeh Soltani, R.; Rezaee, R.; Abdullahi, N.; Safari, M. Photocatalytic removal of Acid Red 88 dye using zinc oxide nanoparticles fixed on glass plates. J. Adv. Environ. Health Res. 2015, 3, 102–110. [Google Scholar]
- Yasir, M.; Ali, H.; Masar, M.; Ngwabebhoh, F.A.; Zubair, M.; Sopik, T.; Machovsky, M.; Kuritka, I.; Sedlarik, V. Design and fabrication of TiO2/Nd polyurethane nanofibers based photoreactor: A continuous flow kinetics study for Estriol degradation and mechanism. J. Water Process Eng. 2023, 56, 104271. [Google Scholar] [CrossRef]
- Wei, Y.; Hao, J.-G.; Zhang, J.-L.; Huang, W.-Y.; Ouyang, S.-b.; Yang, K.; Lu, K.-Q. Integrating Co (OH) 2 nanosheet arrays on graphene for efficient noble-metal-free EY-sensitized photocatalytic H2 evolution. Dalton Trans. 2023, 52, 13923–13929. [Google Scholar] [CrossRef]
- Chen, H.-Q.; Hao, J.-G.; Wei, Y.; Huang, W.-Y.; Zhang, J.-L.; Deng, T.; Yang, K.; Lu, K.-Q. Recent Developments and Perspectives of Cobalt Sulfide-Based Composite Materials in Photocatalysis. Catalysts 2023, 13, 544. [Google Scholar] [CrossRef]
- Subramanian, H.; Krishnan, M.; Mahalingam, A. Photocatalytic dye degradation and photoexcited anti-microbial activities of green zinc oxide nanoparticles synthesized via Sargassum muticum extracts. RSC Adv. 2022, 12, 985–997. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Pandey, S.; Ray, S.S. Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Singh, N.; Chakraborty, R.; Gupta, R.K. Mutton bone derived hydroxyapatite supported TiO2 nanoparticles for sustainable photocatalytic applications. J. Environ. Chem. Eng. 2018, 6, 459–467. [Google Scholar] [CrossRef]
- Buazar, F.; Alipouryan, S.; Kroushawi, F.; Hossieni, S. Photodegradation of odorous 2-mercaptobenzoxazole through zinc oxide/hydroxyapatite nanocomposite. Appl. Nanosci. 2015, 5, 719–729. [Google Scholar] [CrossRef]
- Rezaee, A.; Rangkooy, H.; Khavanin, A.; Jafari, A.J. High photocatalytic decomposition of the air pollutant formaldehyde using nano-ZnO on bone char. Environ. Chem. Lett. 2014, 12, 353–357. [Google Scholar] [CrossRef]
- Hassan, S.S.; El Azab, W.I.; Ali, H.R.; Mansour, M.S. Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045012. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Chandrasekaran, S.; Salla, S.; Elakkiya, V.; Senthil, R.; Nithyadharseni, P.; Maiyalagan, T.; Micheal, K.; Ayeshamariam, A.; Arasu, M.V. Recent developments of metal oxide based heterostructures for photocatalytic applications towards environmental remediation. J. Solid State Chem. 2018, 267, 35–52. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 78–84. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, L.; Han, F.; Wang, A.; Cai, W.; Yu, J.; Yang, J.; Peng, F. Green biosynthesis and characterization of zinc oxide nanoparticles using Corymbia citriodora leaf extract and their photocatalytic activity. Green Chem. Lett. Rev. 2015, 8, 59–63. [Google Scholar] [CrossRef]
- Fulekar, J.; Dutta, D.P.; Pathak, B.; Fulekar, M.H. Novel microbial and root mediated green synthesis of TiO2 nanoparticles and its application in wastewater remediation. J. Chem. Technol. Biotechnol. 2018, 93, 736–743. [Google Scholar] [CrossRef]
- Muthukumar, H.; Matheswaran, M. Amaranthus spinosus leaf extract mediated FeO nanoparticles: Physicochemical traits, photocatalytic and antioxidant activity. ACS Sustain. Chem. Eng. 2015, 3, 3149–3156. [Google Scholar] [CrossRef]
- Rather, M.Y.; Sundarapandian, S. Magnetic iron oxide nanorod synthesis by Wedelia urticifolia (Blume) DC. leaf extract for methylene blue dye degradation. Appl. Nanosci. 2020, 10, 2219–2227. [Google Scholar] [CrossRef]
- Prasad, C.; Yuvaraja, G.; Venkateswarlu, P. Biogenic synthesis of Fe3O4 magnetic nanoparticles using Pisum sativum peels extract and its effect on magnetic and Methyl orange dye degradation studies. J. Magn. Magn. Mater. 2017, 424, 376–381. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Galeas, S.; Sharma, V.; Guerrero, V.H.; Debut, A.; Cumbal, L. Characterization and application of biosynthesized iron oxide nanoparticles using Citrus paradisi peel: A sustainable approach. Inorg. Chem. Commun. 2020, 119, 108116. [Google Scholar] [CrossRef]
- Nethravathi, P.; Kumar, M.P.; Suresh, D.; Lingaraju, K.; Rajanaika, H.; Nagabhushana, H.; Sharma, S. Tinospora cordifolia mediated facile green synthesis of cupric oxide nanoparticles and their photocatalytic, antioxidant and antibacterial properties. Mater. Sci. Semicond. Proces. 2015, 33, 81–88. [Google Scholar]
- Sreekanth, T.; Shim, J.-J.; Lee, Y.R. Degradation of organic pollutants by bio-inspired rectangular and hexagonal titanium dioxide nanostructures. J. Photochem. Photobiol. B Biol. 2017, 169, 90–95. [Google Scholar] [CrossRef]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Kennedy, L.J.; Ramalingam, R.J.; Al-Lohedan, H.A. Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochem. Photobiol. B Biol. 2018, 180, 39–50. [Google Scholar] [CrossRef]
- Reddy Yadav, L.; Manjunath, K.; Archana, B.; Madhu, C.; Raja Naika, H.; Nagabhushana, H.; Kavitha, C.; Nagaraju, G. Fruit juice extract mediated synthesis of CeO2 nanoparticles for antibacterial and photocatalytic activities. Eur. Phys. J. Plus 2016, 131, 154. [Google Scholar] [CrossRef]
- Aminuzzaman, M.; Ying, L.P.; Goh, W.-S.; Watanabe, A. Green synthesis of zinc oxide nanoparticles using aqueous extract of Garcinia mangostana fruit pericarp and their photocatalytic activity. Bull. Mater. Sci. 2018, 41, 50. [Google Scholar] [CrossRef]
- Li, S.-W.; Gao, R.-M.; Zhao, J.-s. Deep oxidative desulfurization of fuel catalyzed by modified heteropolyacid: The comparison performance of three kinds of ionic liquids. ACS Sustain. Chem. Eng. 2018, 6, 15858–15866. [Google Scholar] [CrossRef]
- Bishnoi, S.; Kumar, A.; Selvaraj, R. Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater. Res. Bull. 2018, 97, 121–127. [Google Scholar] [CrossRef]
- Alshehri, A.; Malik, M.A.; Khan, Z.; Al-Thabaiti, S.A.; Hasan, N. Biofabrication of Fe nanoparticles in aqueous extract of Hibiscus sabdariffa with enhanced photocatalytic activities. RSC Adv. 2017, 7, 25149–25159. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; LewisOscar, F.; Pugazhendhi, A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J. Photochem. Photobiol. B Biol. 2019, 192, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Maharana, M.; Sen, S. Peltophorum pterocarpum leaf extract mediated green synthesis of novel iron oxide particles for application in photocatalytic and catalytic removal of organic pollutants. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–14. [Google Scholar]
- Bhattacharjee, A.; Ahmaruzzaman, M. Photocatalytic-degradation and reduction of organic compounds using SnO2 quantum dots (via a green route) under direct sunlight. RSC Adv. 2015, 5, 66122–66133. [Google Scholar] [CrossRef]
- Thandapani, K.; Kathiravan, M.; Namasivayam, E.; Padiksan, I.A.; Natesan, G.; Tiwari, M.; Giovanni, B.; Perumal, V. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ. Sci. Pollut. Res. 2018, 25, 10328–10339. [Google Scholar] [CrossRef]
- Reddy Yadav, L.; Lingaraju, K.; Daruka Prasad, B.; Kavitha, C.; Banuprakash, G.; Nagaraju, G. Synthesis of CeO2 nanoparticles: Photocatalytic and antibacterial activities. Eur. Phys. J. Plus 2017, 132, 239. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Manjunath, K.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Rauvolfia serpentina-mediated green synthesis of CuO nanoparticles and its multidisciplinary studies. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 1134–1140. [Google Scholar] [CrossRef]
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Prasad, A.R.; Ammal, P.R.; Joseph, A. Effective photocatalytic removal of different dye stuffs using green synthesized zinc oxide nanogranules. Mater. Res. Bull. 2018, 102, 116–121. [Google Scholar] [CrossRef]
- Nava, O.; Soto-Robles, C.; Gómez-Gutiérrez, C.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Olivas, A.; Luque, P. Fruit peel extract mediated green synthesis of zinc oxide nanoparticles. J. Mol. Struct. 2017, 1147, 1–6. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green synthesis of zinc oxide nanoparticles using pomegranate fruit peel and solid coffee grounds vs. chemical method of synthesis, with their biocompatibility and antibacterial properties investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. One pot synthesis and characterization of gold nanocatalyst using Sacha inchi (Plukenetia volubilis) oil: Green approach. J. Photochem. Photobiol. B Biol. 2016, 158, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Ullah, Z.; Fazal, A.; Irfan, M. Use of vegetable waste extracts for controlling microstructure of CuO nanoparticles: Green synthesis, characterization, and photocatalytic applications. J. Chem. 2017, 2017, 2721798. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Mathimani, T.; Santhanam, N.; Phuong, T.N.; Brindhadevi, K.; Pugazhendhi, A. Green synthesis of cobalt-oxide nanoparticle using jumbo Muscadine (Vitis rotundifolia): Characterization and photo-catalytic activity of acid Blue-74. J. Photochem. Photobiol. B Biol. 2020, 211, 112011. [Google Scholar] [CrossRef] [PubMed]
- El-Aassar, A.H.; Said, M.; Abdel-Gawad, A.; Shawky, H. Using silver nanoparticles coated on activated carbon granules in columns for microbiological pollutants water disinfection in Abu Rawash area, Great Cairo, Egypt. Aust. J. Basic Appl. Sci. 2013, 7, 422–432. [Google Scholar]
- Mostafaii, G.; Chimehi, E.; Gilasi, H.; Iranshahi, L. Investigation of zinc oxide nanoparticles effects on removal of total coliform bacteria in activated sludge process effluent of municipal wastewater. J. Environ. Sci. Technol. 2017, 10, 49–55. [Google Scholar] [CrossRef][Green Version]
- Sharmila, G.; Muthukumaran, C.; Sandiya, K.; Santhiya, S.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Thirumarimurugan, M. Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J. Nanostruct. Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Kumar, S.; Dilbaghi, N. Interaction of ZnO nanoparticles with food borne pathogens Escherichia coli DH5α and Staphylococcus aureus 5021 & their bactericidal efficacy. AIP Conf. Proc. 2011, 1393, 153–154. [Google Scholar]
- Sankar, R.; Prasath, B.B.; Nandakumar, R.; Santhanam, P.; Shivashangari, K.S.; Ravikumar, V. Growth inhibition of bloom forming cyanobacterium Microcystis aeruginosa by green route fabricated copper oxide nanoparticles. Environ. Sci. Pollut. Res. 2014, 21, 14232–14240. [Google Scholar] [CrossRef]


| Species | Plant Portion | MONPs | Dimensions (nm) | Form | Refs. |
|---|---|---|---|---|---|
| Cynodon dactylon | Leaf | TiO2 | 13–34 | Hexagonal | [59] |
| Azadirachta indica | Leaf | Mn3O4 | 18.2 | Spherical | [60] |
| Leaf | ZnO | 9.6–25.5 | Hexagonal wurtzite | [61] | |
| Moringa oleifera | Leaf | ZnO | 12.27–30.51 | - | [62] |
| Peel | CeO2 | 45 | Spherical | [63] | |
| Kalopanax pictus | Leaf | MnO2 | 19.2 | Spherical | [64] |
| Artocarpus hetero- | Leaf | ZnO | 15–25 | Hexagonal wurtzite | [65] |
| Allium sativum | Bulb | ZnO | 14–70 | Hexagonal wurtzite | [66] |
| Petroselinum crispum | Leaf | ZnO | 14–70 | Hexagonal wurtzite | [66] |
| Artocarpus gomezi- anus | Fruits | ZnO | 11.53 | Spherical, hexagonal wurtzite | [67] |
| Asparagus racemosus | Root | Fe2O4 | 30–40 | Spherical | [68] |
| Chlorophytum comosum | Leaf | Fe3O4 | 100 | - | [69] |
| Punica granatum | Seed | Fe3O4 | 25–55 | Semi-spherical | [70] |
| Avicennia marine | Flower | Fe3O4 | 30–100 | Honeycomb | [71] |
| Cornelian cherry | Fruit | Fe3O4 | 29–37 | Spherical | [72] |
| Borassusflabellifer | Seed | Fe3O4 | 10–40 | Hexagonal | [73] |
| Laurus nobilis | Leaf | Fe3O4 | 8.03 ± 8.99 | Spherical and hexagonal | [74] |
| Punica granatum L. | Leaf | Fe3O4 | 21–23 nm | - | [75] |
| Annona squamosal | Peel | TiO2 | 23 ± 2 | Spherical | [76] |
| Plectranthus amboinicus | Leaf | ZnO | 50–180 | Rod shape | [77] |
| Plantain peel | Peel | Fe3O4 | <50 | Spherical | [78] |
| Saraca indica | Flower | SnO2 | 2–4 | Circular | [79] |
| Phyllanthus niruri | Leaf | ZnO | 25.61 | Hexagonal wurtzite | [80] |
| Carica papaya | Leaf | CuO | 140 | Rod shape | [81] |
| Aspalathus linearis | Leaf | SnO2 | 8.23 | - | [82] |
| Cassia fistula | Leaf | ZnO | 5–15 | Hexagonal wurtzite | [83] |
| Hibiscus Sabdariffa | Flower | CdO | 113 | Cuboid shape | [84] |
| Abutilon indicum | Leaf | CuO | 16.78 | Hexagonal wurtzite | [85] |
| Leucas aspera | Leaf | CeO2 | 4–13 | Uniform microspheres | [86] |
| Brassica oleracea | Leaf | SnO2 | 3.62–6.34 | Quasi-spherical | [87] |
| Amorphophallus | Root | ZnO | 19.6 | Rice | [88] |
| No. | Studied Properties (Information) | Characterization Techniques |
|---|---|---|
| 1 | Elemental–chemical composition | NMR, SEM-EDX, ICP-MS, XRD, XPS, |
| 2 | Shape | TEM, AFM |
| 3 | Chemical state–oxidation state | XPS |
| 4 | Size | TEM, XRD, DLS, SEM, ICP-MS, UV-Vis |
| 5 | Crystal structure | XRD |
| 6 | Size distribution | DLS, ICP-MS, SEM |
| 7 | Growth kinetics | NMR, TEM |
| 8 | Surface composition, arrangement, density, ligand binding | NMR, FTIR, TGA, XPS |
| 9 | Surface area | NMR, BET, Zeta potential |
| 10 | Agglomeration state | TEM, UV-Vis, Zeta potential, SEM, DLS |
| 11 | 3D visualization | AFM, SEM |
| 12 | Dispersion of MONPs in matrices | AFM, TEM, SEM |
| 13 | Detection of NPs | TEM, SEM |
| 14 | Optical properties | UV-Vis, PL |
| Plant Extract | Green MONPs | Pollutant Elimination | Initial Conc. (mg L−1) | Contact Time (min) | pH | Amount NPs (g L−1) | Removal Efficiency (%) | Sorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Peganum harmala | ZnO-NPs | Cr(VI) | 1000 | 30 | 2 | 2 | 97.6 | 74.7 | [98] |
| Aloe vera | α-Fe2O3-NPs | As(V) | 2–30 | - | 6–8 | 0.5 | - | 38.4 | [99] |
| Casein | ZnO-NPs | Cd(II) | 500 | 30 | 7 | 2 | 85.6 | 156.7 | [100] |
| Tangerine peel | Fe2O3-NPs | Cd(II) | 15 | 90 | 4 | 0.4 | 88.7 | - | [97] |
| Zingiber zerumbet | ZnO-NPs | Pb(II) | 25 | 60 | 5 | 0.1 | 93 | 19.6 | [101] |
| Ananas comosus | Fe3O4-NPs | Cd(II) | 60 | 10 | 6 | 0.1 | 96.1 | 49.1 | [102] |
| Emblicao fficinallis | ZnO-NPs | As(III) | 0.002 | 60 | 5 | 2.5 | 96.7 | [103] | |
| Calotropis procera | CuO-NPs | Cr(VI) | 5 | 120 | 2 | 18.8 | 99.9 | [104] | |
| Jatropha curcas | TiO2-NPs | Cr(VI) | - | - | 8.2 | - | 76.5 | - | [105] |
| Psidium guajava | CuO-NPs | Nile blue | 93 | [106] | |||||
| Ziziphus spina-christi | CuO NPs | CV dye | 7.5 min | 95 | [107] | ||||
| Microbac-terium sp | NiO | Ni(II) | 120 | 7 | 2.172 | 95 | [108] | ||
| Mint leaf extract | CuO | Pb(II) Ni(II) Cd(II) | 20 | 60 min | 6 | 0.5 | 88.8 54.9 15.6 | [109] | |
| Punica granatum leaf extract | CuO | SO dye | - | 180 min | 6.1 | 1 | 189.5 | [110] |
| Advantages | Disadvantages |
|---|---|
| Lower toxicity | Long duration time |
| Excellent photoactive properties | |
| Availability | |
| High chemical stability | Limited applications |
| Low cost | |
| Eco-friendliness |
| Plants | Photocatalyst | Types of Dyes | Degradation (%) | Refs. |
|---|---|---|---|---|
| Banana crust | CuONPs | Congo Red (CR) | 88 | [143] |
| Amaranthus spinosus, leaf | FeONPs | Methyl Orange (MO) | 75 | [144] |
| FeONPs | Methylene Blue (MB) | 69 | ||
| Cynometra ramiflora | FeONPs | MB | High | [145] |
| Asparagus racemosus | FeONPs | MO | Good | [68] |
| Hibiscus sabdariffa, flower | FeONPs | Congo Red | 96.1% | [146] |
| Ruellia tuberose, leaf | FeONPs | Crystal Violet | 80% | [147] |
| Peltophorum Pterocarpum, leaf | Fe3O4 | Rhodamine | High | [148] |
| Sugar cane, juice | SnO2NPs | Rose Bengal | 99.3 | [149] |
| Sugar cane, juice | SnO2NPs | MB | 96.8 | |
| Rhizoma Coptidis | TiO2NPs | MB | 71 | [140] |
| Parthenium hysterophorus | TiO2NPs | MB | 92.5 | [150] |
| TiO2NPs | MO | 81.5 | ||
| TiO2NPs | Crystal Violet (CV) | 79.7 | ||
| TiO2NPs | Alizarin Red | 77.3 | ||
| Starch | TiO2NPs | MB | 100 | 196 |
| Aspalathus linearis | NiONPs | MB | Good | [151] |
| Eucalyptus globulus Eucalyptus globulus | ZnONPs | MB | 98.3 | [152] |
| ZnONPs | MO | 96.66 | ||
| Lagerstroemia speciosa | ZnONPs | MO | 93.5 | [153] |
| Cassia fistula, leaf | ZnONPs | MB | 98.71 | [83] |
| Artocarpus hetero phyllus, leaf | ZnONPs | Rose Bengal | 85 | [65] |
| Lemon juice | ZnONPs | MB | 91.17 | [154] |
| ZnONPs | CR | 90 | ||
| ZnONPs | Rhodamine B | 98 | ||
| Citrus aurantifolia (lemon), Citrus paradisi | ZnONPs | MB | 97 | [155] |
| Punica granatum, peel | ZnO | Antibacterial property | Good | [156] |
| Eucalyptus globulus | MB | 98.3 | [157] | |
| Moringa oleifera, peel | CeO2 | CV | 97.5 | [63] |
| Cauliflower | SnO2 | MB | 91.89 | [87] |
| Cauliflower waste, potato peel | CuO | MB | 87.37–96 | [158] |
| Vitis rotundifolia, fruit | CoO | Acid blue | High | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashour, M.; Mansour, A.T.; Abdelwahab, A.M.; Alprol, A.E. Metal Oxide Nanoparticles’ Green Synthesis by Plants: Prospects in Phyto- and Bioremediation and Photocatalytic Degradation of Organic Pollutants. Processes 2023, 11, 3356. https://doi.org/10.3390/pr11123356
Ashour M, Mansour AT, Abdelwahab AM, Alprol AE. Metal Oxide Nanoparticles’ Green Synthesis by Plants: Prospects in Phyto- and Bioremediation and Photocatalytic Degradation of Organic Pollutants. Processes. 2023; 11(12):3356. https://doi.org/10.3390/pr11123356
Chicago/Turabian StyleAshour, Mohamed, Abdallah Tageldein Mansour, Abdelwahab M. Abdelwahab, and Ahmed E. Alprol. 2023. "Metal Oxide Nanoparticles’ Green Synthesis by Plants: Prospects in Phyto- and Bioremediation and Photocatalytic Degradation of Organic Pollutants" Processes 11, no. 12: 3356. https://doi.org/10.3390/pr11123356
APA StyleAshour, M., Mansour, A. T., Abdelwahab, A. M., & Alprol, A. E. (2023). Metal Oxide Nanoparticles’ Green Synthesis by Plants: Prospects in Phyto- and Bioremediation and Photocatalytic Degradation of Organic Pollutants. Processes, 11(12), 3356. https://doi.org/10.3390/pr11123356









