Abstract
The protection of all environmental compartments (water, soil, air) is of great interest for the normal functioning of life on Earth. The environment is systematically polluted with different concentrations of physical, biological and chemical pollutants. For the purpose of environmental protection, numerous in situ and ex situ biological, chemical and physical remediation techniques have been developed. Most techniques have limitations, such as high cost, processing time or environmental feasibility. In general, biological techniques have proven to be the most environmentally friendly compared to chemical and physical techniques. Furthermore, remediation is an extremely complex procedure due to the complexity of the pollutant composition. Therefore, the implementation of individual physical, biological or chemical remediation techniques is often not sufficient for adequate remediation of the polluted environment. Accordingly, for more economical and efficient environmental remediation, it is recommended to use a combination of techniques that should meet the requirements of efficiency and treatment duration. Ultimately, this review provides a concise overview of the recent application of physical, biological and chemical remediation techniques to all compartments of the polluted environment. A critical review of existing knowledge on environmental remediation through a search of the relevant literature has helped to assess the basic challenges and limitations that arise in the issue of environmental remediation, as well as providing recommendations and guidelines for future research.
1. Introduction
The release of pollutants into the environment by numerous human activities and natural disasters causes different levels of pollution in the environment. Population growth, industrialization, rapid development in the agricultural sector, inadequate waste management and irresponsible release of pollutants into the environment contribute to the accumulation of inorganic and organic pollutants in water, soil and air [1]. Numerous chemical, physical and biological pollutants represent a toxicological threat to both the environment and human beings [2,3]. Nowadays, environmental protection issues are becoming more and more challenging, and they must be addressed in order to ensure safe and healthy conditions for life on Earth. Therefore, numerous remediation techniques have been developed to reduce the hazardous effects of a polluted environment. These techniques include biological, chemical and physical remediation [4,5,6,7,8]. Although various remediation techniques are available for remediation of the polluted environment, the choice of the appropriate technique is quite challenging and depends on a number of factors, such as the composition and concentration of pollutants in the polluted medium, operating costs, efficiency, feasibility, applicability and final impact on the environment. Hence, the purpose of this review is to provide an overview of the main types of environmental pollutants, as well as environmental remediation techniques that can be used, and summarize recent relevant research applied in the remediation of all environmental compartments. The main literature sources were the Web of Science Core Collection, Scopus, Science Direct, SciELO, Taylor & Francis, Wiley Springer and Google databases. The literature was searched using keywords related to environmental remediation and was mainly focused on the last five years. Most of the literature does not offer a combined critical report on the remediation of all environmental compartments but rather focuses on a single system. Therefore, this review aims to complete recent knowledge about the effectiveness, advantages and limitations of the application of remediation techniques to all environmental compartments with recommendations and guidelines for future research.
2. Categories of Environmental Pollutants and Their Impact on the Environment
The environment represents the natural habitat of organisms, including humans, and consists of three complex compartments, air, water and soil. It also represents a combination of biotic (living organisms) and abiotic (hydrosphere, lithosphere and atmosphere) components. Environmental pollution refers to the introduction of physical, chemical and biological contaminants in concentrations higher than those in the environment, thereby impairing the quality of the environment. This causes physical, chemical or biological changes in all environmental compartments. Ecosystem pollution has a number of consequences; it impairs the quality of the entire ecosystem and affects climate change and human health. Human activities have a negative impact on the environment by polluting drinking water, air and soil [3,9]. Specifically, rapid economic development and industrialization have led to an increase in living standards, and thus to significant environmental pollution as a consequence of non-compliance with increasingly strict legal regulations for the emission of pollutants into the environment. Pollutants can be of natural (volcanic eruption) or anthropogenic (industry, waste, wastewater, etc.) origin, biodegradable and non-biodegradable, primary and secondary. Biodegradable pollutants are decomposed under the action of living organisms (microorganisms), in contrast to non-biodegradable pollutants that are persistent in the environment. Primary pollutants are directly released into the environment from point or diffuse sources. Secondary pollutants are emitted as by-products of primary pollutants [3,9,10]. Pollutants are most often classified as chemical, physical and biological, as shown in Figure 1.
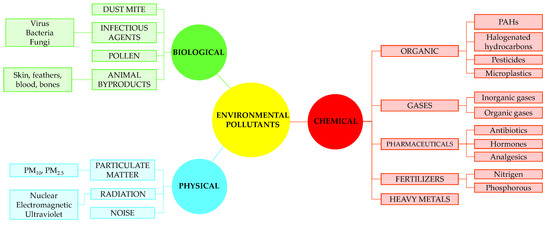
Figure 1.
Categories of environmental pollutants [11] (adapted with permission from Ref. [11], 2023, Muzammil Anjum).
The group of chemical pollutants includes inorganic and organic pollutants. Inorganic pollutants are highly toxic and non-biodegradable, and consist of radionuclides, heavy metals, metalloids and inorganic gases. The biogeochemical cycle and anthropogenic activities are the primary sources of inorganic contamination. The five priority heavy metals are arsenic, cadmium, chromium, lead and mercury. Heavy metal pollution is a serious problem because it degrades the quality of air, water and soil. The toxic, carcinogenic, mutagenic and teratogenic effects of heavy metals are the result of the tendency of bioaccumulation and biomagnification in living organisms [1,2,3,4,5]. Organic pollutants include volatile organic compounds (VOC), polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), aliphatic hydrocarbons, synthetic organic dyes, pesticides, pharmaceuticals, etc. Volatile organic compounds (VOCs) include toluene, benzene, ethylbenzene and xylene, while polycyclic aromatic hydrocarbons (PAHs) include benzopyrene, acenaphthylene, anthracene and fluoranthene. Pesticides are divided into herbicides, insecticides and fungicides, and most often contain compounds such as atrazine, epoxyconazole, endosulfan, dichlorodiphenyltrichloroethane (DDT), lindane, glyphosate, methoxychlor, tebuconazole and heavy metals. Organic pollutants have toxic, mutagenic and carcinogenic effects and are associated with the development of cancer in humans [1,3,11,12].
The group of physical pollutants includes particulate matter, radiation and noise. Particulate matter (PM) is formed in the atmosphere as a result of chemical reactions between different pollutants. PM is divided into PM10 (diameter 2.5 μm–10 μm) and PM2.5 (diameter < 2.5 μm), and can be of organic (PAHs, dioxins, benzene) or inorganic (carbon, chlorides, nitrates, sulfates, metals) origin. PM2.5 particles are considered a major health problem as they cause serious lung and cardiovascular diseases, as well as allergies in humans [9,11,13,14]. Radiation can be nuclear, electromagnetic and ultraviolet. Many household devices such as cell phone and Wi-Fi signals and microwave ovens are sources of radiation in close proximity to humans. Any unwanted sound that causes disturbances (damage or loss of hearing, headache, irregular heartbeat) is considered noise pollution, and it is most often a consequence of the operation of machines in industrial facilities [3,9,11].
Biological pollutants are toxic animals and microorganisms (bacteria, viruses, fungi), dust, allergens and pollen that pollute the environment, directly affecting human health by transmitting various diseases and causing allergic reactions and infections [9,11].
All of the listed pollutants are found in the air, water and soil, and chemical pollutants are of the greatest concern. For instance, the main sources of air pollution are industrial activities, traffic and natural disasters such as fires. The air is most often polluted by inorganic gases such as NOx, SOx, COx, Cl2, NH3, ozone and halogen gases, VOCs, heavy metal vapors and other greenhouse gases and particles. Primary air pollutants are those that are directly released into the environment from anthropogenic sources, fossil fuels combustion and traffic (SOx, NOx, VOCs). Secondary air pollutants are created in the atmosphere by chemical and photochemical reactions of primary pollutants and are often more harmful than primary ones. For example, ozone is a secondary pollutant. Ground-level ozone is formed from VOCs and NOx, while stratospheric ozone is formed from oxygen under a high-voltage electrical discharge. As a result of NOx and SOx in the atmosphere, acid rains occur, which have a negative effect on vegetation, monuments and soil [3,9,10,13,14]. The most common soil pollutants are fertilizers and pesticides since they are overused to meet the demand for food production and reduce plant diseases. In this way, persistent organic pollutants as well as the heavy metals contained in them are released into the soil, which leads to a significant impact on health and environmental conditions [1,3]. All of the listed inorganic and organic pollutants reach water systems as a result of direct discharge or under the influence of rain and soil washing.
A special group of pollutants, or emerging environmental pollutants, are of particular concern since they represent a major ecotoxicological problem for humans and other biotas, and include mainly microplastics, surfactants and pharmaceuticals. All the mentioned pollutants are actually derived from the basic chemical pollutants mentioned before.
Microplastic refers to plastic particles with a diameter of less than 5 mm, while nanoplastic refers to particles with a diameter of less than 1 μm [15]. It includes fragments, fibers, foam, pellets, and films, and is categorized as primary and secondary [16]. The primary source of microplastics includes particles of polyethylene, polypropylene, polystyrene, polyurethane, polyethylene terephthalate and polyvinyl chloride. It is purposefully produced and added to consumer products such as cosmetics, personal care products, pharmaceuticals, detergents, toothpaste, dyes, insecticides, fabric softeners, etc. Secondary microplastics are formed by the progressive fragmentation of macroplastics that are discarded and inadequately disposed of, most often by photooxidation under the influence of ultraviolet radiation or under the influence of physical, chemical and biological processes [15,16,17,18]. The resulting microplastics are widely distributed in the soil, water and air. Moreover, microplastics can adsorb various contaminants, which can be transferred to humans through the food chain. It enters the human body orally, by inhalation or through skin contact. Exposure to microplastics causes various toxic effects, such as oxidative stress, cytotoxicity, metabolic disorder, neurotoxicity, reproductive disorders, etc. [15,16,17,18,19,20,21].
Surfactants or surface-active substances are amphipathic compounds since they possess hydrophilic and hydrophobic groups. They are categorized as anionic, cationic, non-ionic, zwitterionic and gemini surfactants and biosurfactants [22,23]. Many consumer products, such as beauty products, soaps, detergents, personal care products, emulsifiers, pesticides, etc., contain surfactants. Globally, the most widely used surfactants in the world are benzalkonium chloride, linear alkylbenzene sulfonate, alkyl ethoxy sulfate, alkyl sulfate, alkylphenol ethoxylate, alkyl ethoxylates and ammonium-based quaternary structures [24,25]. Since they are used daily in households and industry, they are regularly discharged into waste water. However, due to the technological limitations of wastewater treatment and management, most of them end up in the environment. In addition, they are characterized by excellent adsorption capacity; therefore, they can be carriers of many more toxic contaminants in the environment. Surfactants easily penetrate the cell membrane, which reflects their ecotoxicity for living organisms. Finally, these chemicals are extremely toxic and are also categorized as endocrine disruptors [22,23,24,25].
Pharmaceuticals are human or veterinary medicinal products [25,26]. According to their biological activity and purpose, they are divided into antibiotics (treatment of bacterial infections), analgesics (they act to reduce pain), antineoplastics (used in cancer therapy), antidepressants, therapeutic hormones, non-steroidal anti-inflammatory drugs, etc. [20,27]. Pharmaceutical products are biologically active compounds. They can be excreted from the body in urine or feces as unchanged or as transformed secondary molecules, metabolites. Therefore, during normal consumption, they are excreted by human use and reach the environment through domestic waste water. Other pathways of reaching the environment are industrial wastewater and improper disposal of unused medicines [20,25,26,27]. Pharmaceutical products represent emerging pollutants and possible health risks for human health. Through the food chain and water consumption, they can affect the endocrine system [25,26,27].
Endocrine-disrupting chemicals are substances that disrupt the endocrine system, interfere with the action of hormones, resulting in disruption of reproductive, neurological and metabolic development, and even promote tumor growth [28,29,30,31]. In addition to pharmaceuticals and their metabolites found in the environment, endocrine disruptors also include other chemicals that are contained in various consumer products (personal care products, detergents, disinfectants, toothpaste, pesticides, plastics, etc.). They include a wide range of compounds such as polycyclic aromatic hydrocarbons, alkyl phenols, bisphenols, polychlorinated biphenyls, polybrominated biphenyls, dioxins, bisphenol A, phthalates, chlorpyrifos, dichlorodiphenyltrichloroethane, surfactants, heavy metals, parabens, etc. [20,28,29,30,31].
The mentioned pollutants are mobile and persistent in water, air, soil and sediments, even at low concentrations. Along with classic inorganic and organic pollutants, and emerging environmental pollutants, the risks and fate of produced nanomaterials are still being sought. Regulation of these compounds in the environment is a challenging task and requires an understanding the pollutant properties as well as their distribution [2,3].
Therefore, any emission of the mentioned pollutants into the environment, especially above the permissible values prescribed by law, represents a threat to the entire ecosystem. Since achieving a balance between industrialization and environmental protection is a challenge nowadays, great efforts are needed to limit future emissions of pollutants into the environment. Currently, already contaminated areas pose a risk for the spread of contamination. Therefore, in order to prevent the spread of pollution or complete remediation of polluted areas, various methods of environmental remediation have been developed.
3. Environmental Remediation Techniques
Remediation is a term generally used to refer to cleaning up or restoring a polluted environment. It represents taking measures to prevent the spread of pollution and further degradation of the environment to a level that enables future use, revitalization and recultivation. Therefore, the goal of remediation is to reduce the concentration of pollutants in the environment (air, water, soil) to an acceptable level or to remove them completely. Environmental remediation can be carried out in situ, i.e., directly at the site of pollution, or ex situ, i.e., outside the site of pollution, at the intended location for remediation. Due to the economy and simplicity of implementation, in situ remediation is more often applied [4,5,6,7,8]. Regardless of the environmental remediation implementation process, remediation techniques are divided into biological, chemical and physical as shown in Figure 2. Selection of the appropriate technique will depend on pollutant type, concentration, site conditions, process costs and time constraints.
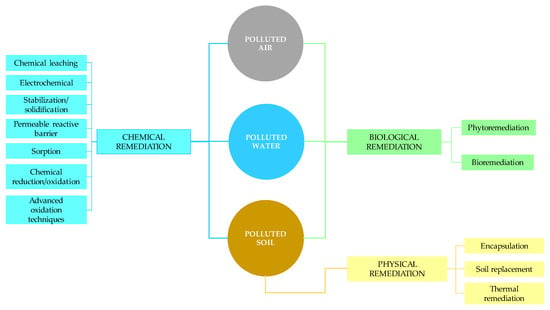
Figure 2.
Main remediation techniques for soil, water and air.
3.1. Biological Remediation
Biological remediation techniques are those that are initiated by biological organisms (plants, microorganisms, animals), substances and processes. Basically, it is divided into phytoremediation and bioremediation [6,7,32,33,34,35,36,37,38,39,40].
3.1.1. Phytoremediation
Phytoremediation is a biological technique that uses plants for partial or complete stabilization, fixation, decomposition, transfer or extraction of contaminants from soil, water and air. The principle of the technique is based on the ability of plants to concentrate and extract pollutants from the polluted medium in their parts (root, leaves, stem) [6,32,34,38]. Various types of plants can be used for phytoremediation, including trees, shrubs and aquatic plants. Plants for phytoremediation must be hyperaccumulators, i.e., they must possess the ability to accumulate pollutants above the level present in the soil. These plants should not show symptoms of any phytotoxicity [7,33,35,38]. Plants from the families Brassicaceae, Asteraceae, Violaceae, Fabaceae Caryophyllaceae, Euphorbiaceae, Lamiaceae, Flacourtaceae and Poaceae are mostly used for phytoremediation [7]. Phytoremediation can be applied to inorganic as well as organic pollutants. The effectiveness of phytoremediation depends on the type of plants, their ability to accumulate pollutants, their growth speed and weather conditions. Depending on the mechanism by which plants accumulate pollutants, phytoremediation is classified into phytovolatilization, phytostabilization, phytoextraction, rhizofiltration and phytodegradation [6,7,32,33,34,35,36,37,38,39,40].
Phytovolatilization
Phytovolatilization refers to the use of plants to accumulate pollutants inside the plant with their subsequent evaporation. At the same time, through metabolic activity, the plant transforms pollutants into less toxic volatile by-products and releases them into the atmosphere through transpiration. In order to improve the efficiency of phytovolatilization, genetically modified plants are generally used to increase the ability to accumulate and phytovolatilize pollutants. The application of phytovolatilization for remediation seems questionable because the pollutant is translocated into the atmosphere by evaporation, which is the main drawback of this technique [7,33,38].
Phytostabilization
Phytostabilization (Phytoimmobilization) is a technique that uses plant roots to reduce the bioavailability and mobility of pollutants from contaminated soil or sediment. The principle of the technique is based on the stabilization of pollutants by accumulation or complexation in the root or rhizosphere of the plant, the root zone. The advantages of this technique are its efficiency, relatively short implementation time and not requiring disposal of the plants. Since the concentration of pollutants in the soil does not decrease, this implies a limited use of the soil, which is also the main disadvantage of this technique [7,33,36,39].
Phytoextraction
Phytoextraction is based on the ability of plant roots to adsorb and concentrate heavy metals or organic pollutants from the soil into the above-ground parts of plants that can be harvested. Therefore, it is used most often for polluted soil, where the pollutant is transferred from the soil to the plant biomass. Unlike phytostabilization, phytoextraction actually removes pollutants from the soil. It is applicable for soils polluted with low to moderate levels of pollutants because most plants cannot survive in heavily polluted soil. Hence, the plants selected for this type of remediation must be hyper-accumulative. The advantages of phytoextraction are high efficiency, profitability, that it is not harmful to the environment and that there is no need to dispose of plants, no digging and no transportation [7,33,37,38].
Rhizofiltration
Rhizofiltration is a technique used for the remediation of heavy metals and organic pollutants from polluted waters through adsorption or deposition on plant roots. The criterion for plant selection is based on the expressed sorption properties of the plant’s roots, high development of root biomass and high tolerance to pollutants [35,38,40].
Phytodegradation
Phytodegradation (phytotransformation) is a technique used mainly for the degradation of organic pollutants using plant enzymes. If the decomposition takes place in the root zone with the action of microorganisms, then it is named rhizodegradation. Since there is an interaction between the plant and the microorganisms, it is not considered bioremediation [35,36].
Ultimately, the main advantage of all phytoremediation techniques is the possibility of in situ performance. In addition, phytoremediation is acceptable to the environment; therefore, it is often called green remediation. Furthermore, it is environmentally friendly, aesthetically pleasing, non-invasive, efficient, inexpensive, improves soil properties, applicable for large areas where there are no time constraints for performance and, finally, requires less manpower. However, phytoremediation also has disadvantages. It is very time-consuming, depends on climatic conditions, there can be resistance of plants to pollutants, it is not suitable for heavily polluted areas and it sometimes requires the harvesting of the above-ground parts of plants [6,32,40].
Meanwhile, this technique has seen significant progress using modern biotechnology. In fact, phytoremediation using plants transformed with genes, i.e., genetically modified plants, facilitate the entry and translocation of pollutants into specific plant organelles and tissues, enhancing the effectiveness of phytoremediation. Thus, using genetic engineering, specific genes can be transferred into plants to improve pollutant accumulation in roots, shoots or vacuoles. Indeed, genetic engineering makes it possible to design plants to remove specific pollutants. In this way, the rhizosphere of the plant can be adapted in order to increase the pollutant mobility to the roots of the plant, supporting accumulation in the roots or above-ground parts without the possibility of phytovolatilization. On the other hand, genetic manipulation enables increased pollutant accumulation, promotes growth and reduces plant oxidative stress. Accordingly, genetic engineering is considered promising for the wider application of phytoremediation [7,35,36].
3.1.2. Bioremediation
Bioremediation is a technique that removes, decomposes or immobilizes organic and inorganic pollutants from water or soil using microorganisms (bacteria, fungi and algae). The mechanism of bioremediation includes binding, immobilization, oxidation/reduction, extracellular complexation, intracellular accumulation and transformation of pollutants by microorganisms. Thus, the purpose of bioremediation is to convert contaminants into less toxic or non-toxic products that do not pose a threat to the environment. Bioremediation is mainly divided into three categories: bioaugmentation, biostimulation and animal remediation [33,34,37,41,42,43,44,45].
Bioaugmentation and Biostimulation
Bioaugmentation is the process of adding specific cultured exogenous microorganisms to contaminated soil or water for the purpose of biodegradation of targeted organic pollutants. Added microorganisms increase the rate of pollutant degradation, and for this reason bioaugmentation is used to accelerate pollutant degradation. In contrast, biostimulation implies the modification of the environment by adding nutrients (N, P, minerals), electron donors and acceptors to stimulate the activity of indigenous microorganisms in the soil for biodegradation [41,42,43,44,45].
Unlike organic pollutants, heavy metals are not decomposed by microorganisms, but accumulate them in the cellular structure. The main microbial processes of heavy metal removal are biosorption and bioleaching. Biosorption implies the immobilization of pollutants on the cellular structure of microorganisms through the processes of adsorption, ion exchange, complex formation, reduction and precipitation. The bioleaching process reduces the mobility of pollutants by using the ability of microorganisms to secrete metabolites such as enzymes that act on pollutants [37].
Bioremediation is an environmentally and economically acceptable, sustainable, non-invasive technique. It does not generate toxic by-products; therefore, it is applicable for in situ bioremediation of polluted soil and water, and the biggest drawback is the adaptation of microorganisms or their stimulation in the polluted area. Furthermore, it is relatively time consuming and dependent on environmental conditions for microbial metabolism [37,41,45]. The shortcomings of bioremediation can be overcome by genetic engineering, which allows the design of microorganisms with the desired characteristics for the removal of specific pollutants.
Specifically, genetically modified microorganisms are created by introducing proteins and genes into microorganisms through biotechnology or genetic engineering in order to improve their desired properties. Thus, through genetic manipulation, the method of metabolism of microorganisms is changed, which improves their catalytic potential. As a result of genetic changes, microbial enzymes involved in the process of pollutant degradation are induced; therefore, genetically modified microorganisms show improved bioremediation compared to classical ones. Bioremediation supported by genetically modified microorganisms is considered an economically profitable, simple, fast and ecologically safe technique. It depends on the survival of microorganisms in conditions of environmental stress. The main risk of genetic engineering is the introduction of non-native species into the environment and their impact on the biobalance [41,42,43].
Animal Remediation
It is well known, many types of macrofauna and mesofauna help the decomposition of organic substances in their own metabolism. Moreover, they enhance the metabolic activity of microbes in the soil. In addition, investigations have shown the possibility of using Styela plicata, Eisenia fetida and earthworms for heavy metal soil remediation. Therefore, using animals to remove pollutants from the environment is named animal remediation. This type of remediation is in the development phase since the abiotic conditions in the environment are often unfavorable for animals and their survival in contaminated soil [34,38].
3.2. Chemical Remediation
Chemical remediation is applied to contaminated soil, water systems and air using chemical agents and active substances that have the ability to decompose or remove pollutants. The main chemical remediation techniques include chemical leaching, electrochemical remediation, stabilization/solidification, permeable reactive barrier, sorption (adsorption and ion exchange), chemical reduction and oxidation, advanced oxidation techniques, photocatalysis and nanoremediation.
3.2.1. Chemical Leaching
Chemical leaching is a remediation technique that includes the use of chemical reagents for leaching and extraction of inorganic and organic pollutants from the polluted medium, most often soil and sediment. It can be performed in situ and ex situ. With the in situ technique, the leaching agent is directly introduced by injection or spraying into the contaminated soil, with the use of a system for digging up the soil, dosing the leaching agent, and a system for collecting the leachate and its treatment. In the case of ex situ leaching, the contaminated soil should be excavated and transported to the location intended for carrying out the procedure [33,46,47]. The mechanism of chemical leaching usually includes dissolution, extraction, precipitation, ion exchange, chelation, desorption and separation of pollutants. Inorganic acids (H2SO4, HCl, HNO3, H3PO4), organic acids (acetic, citric, malic, oxalic), chelating agents (ethylenediaminetetraacetic acid (EDTA), diethylenetriaminepentaacetic acid), humic and fulvic substances, surfactants and inorganic salt solutions (NaCl, Na2S2O3, KI) are used as leaching agents. Acidic leaching solutions reduce soil pH and thereby increase the solubility and mobility of metal ions. On the other hand, the use of acids has a negative impact on the soil since it destroys microorganisms and soil components (mineral components), which is not environmentally acceptable. For this reason, the use of organic acids and chelating reagents is justified. For example, a chelating agent like EDTA is expensive and poorly biodegradable. Hence, it is recommended to use more environmentally friendly cleaning agents such as citric and acetic acid [34,35,38,46,47]. Chemical leaching is an effective and fairly fast technique; therefore, it is considered one of the most cost-effective remediation techniques for highly contaminated soil and sediment. The main disadvantage of this technique is its ability to negatively affect soil fertility, microorganisms and soil mineral components. The use of leaching agents favors the creation of secondary pollution. This technique is quite challenging to perform in situ due to the direct impact on the soil and the surrounding area. As a consequence, in situ applicability is limited in contrast to ex situ application [7,35,37,46].
3.2.2. Electrochemical Remediation
Electroremediation works on the principle of electrokinesis, i.e., the movement of charged particles in a direct current electric field due to the formation of an electric potential gradient between two electrodes. The process includes four mechanisms: electromigration (movement of charged ions towards oppositely charged electrodes), electroosmosis (movement of the liquid phase), electrophoresis (movement of charged colloidal particles) and electrolysis (chemical reaction at the electrodes). Applying direct current to a contaminated medium (soil, sediment, water) causes the migration of charged species towards the electrodes. Thus, anions move towards the positively charged electrode (anode), and cations towards the negatively charged electrode (cathode) [7,35,36,38,48,49,50,51]. The goal of the procedure is to keep the pollutant in the vicinity of the electrodes with adequate treatment. For example, if pollutants are separated using a membrane, then this is physical separation, and the procedure is named electrokinetic remediation due to the movement of charged ions and particles in an electric field [38,50,51]. On the other hand, some metal species have poor conductivity, such as sulfides; therefore, it is necessary to use appropriate reagents (distilled water, inorganic and organic acids, chelates) that will promote their dissolution and mobility. In this way, an electrolytic environment is formed, which increases the mobility of pollutants, and thus the remediation efficiency [7]. However, the use of strong inorganic acids such as HCl, HNO3 is not recommended because they can damage the soil structure. Due to the need to add chemicals, the technique is named electrochemical remediation. Furthermore, during the electrochemical process, electrochemical reactions occur on the electrodes, from which the electrolysis of water is inevitable. Specifically, H+ ions are created at the anode and migrate towards the cathode, while OH− ions are created at the cathode and migrate towards the anode. Since H+ ions migrate twice as fast as OH− ions, this leads to acidification, which contributes to the dissolution of heavy metals, thus improving the efficiency of the process [38,49]. The effectiveness of this technique depends on the strength of the electric field, as well as the conductivity and mobility of the pollutant. This technique is not applicable for non-conductive pollutants and impermeable and dry soils. It is most often used for soil and sediment contaminated with conductive inorganic and organic pollutants, and less often for waste water. The advantages of this technique are the relatively short time of the procedure and its applicability for in situ and ex situ performance. In addition, it is easy to install and handle. Moreover, it is applicable at great depths and is environmentally friendly in the case of electrokinetic remediation [7,48]. The main limitation is the dependence on acidic pH, which sometimes requires the addition of chemicals and makes environmental acceptability questionable. Furthermore, it is not applicable for dry soils, there is a need to add electrolyte chemicals, it has a relatively low efficiency and it requires the consumption of electricity [35,37,48].
3.2.3. Stabilization/Solidification
Stabilization and solidification (S/S) are chemical remediation techniques that can be used together or separately to immobilize inorganic and organic contaminants, most often from soil and sediment. If they are used together, the first step is the addition of chemical reagents in order to reduce the mobility of the pollutant, or its stabilization. Chelating agents with functional groups containing nitrogen, oxygen, sulfur or phosphorus atoms are most often used and promote the immobilization of pollutants by precipitation, complexation or sorption. After that, solidifying agents are added to avoid future diffusion and subsequent leaching of pollutants into the environment (solidification) [33,36,37,52,53]. If used separately, then solidification represents the incorporation of the pollutant into the binder in order to immobilize the pollutant into a solid matrix. Inorganic (cement, asphalt, fly ash, clay, silicon oxide, lime, gypsum, zeolites, phosphates, minerals) and organic (microbes, animal manure, biomaterials, polymers, chitosan, alginate, agar) materials are used as binders [7,37]. As a separate technique, immobilization refers to reducing pollutant mobility and bioavailability in contaminated soil or sediment also by adding binding agents but without their solidification. For solidification, cement is preferred over other binders due to its wide availability and economic profitability, while asphalt is preferred for hydrocarbon solidification [7]. This technique can be performed in situ and ex situ. In situ S/S processes are usually preferred compared to ex situ because they require less labor and energy since ex situ implementation requires tools to dig and mix reagents with soil and transport. Furthermore, in situ performance is more suitable for shallow surfaces and may be limited for clay soils and large rocks. The main advantage of the in situ technique is the low cost, because it does not require excavation and disposal of soil. The main disadvantages of in situ application are the impracticability due to technical and geographical limitations of the location, and that the pollutants are permanently left at the location, which implies their possible redistribution in the future [7,36].
3.2.4. Permeable Reactive Barrier
Permeable reactive barrier (PRB) is an in situ technique that is most often applied for in situ groundwater remediation. The barrier is placed below the soil surface, perpendicular to the direction of the groundwater flow and filled with active material. Reactive material is a porous material that allows the flow of the contaminated plume through the PRB and has the ability to remove targeted contaminants through decomposition, immobilization, adsorption, transformation, and precipitation. Fillers for PRB must meet the characteristics of reactivity, stability, low cost, easy availability, hydraulic conductivity and compatibility with the environment. Various reactive materials are used as fillers for PRB, such as zero-valent iron, fly ash, Fe, lime, phosphate, zeolites, sand, activated carbon, etc. Depending on the type of reactive material, the removal of pollutants such as heavy metals, ammonium ions and organic pollutants takes place through physical and chemical processes. The advantages of this technique are its relatively easy implementation and the possibility of using different active materials. The disadvantages are that the efficiency is highly dependent on the water flow, and when the reactive material is saturated, it needs to be changed [54,55].
3.2.5. Sorption (Adsorption and Ion Exchange)
Adsorption and ion exchange are often related processes and occur simultaneously. For this reason, the universal term “sorption” is usually used, which implies the simultaneous occurrence of both processes. It is often the case that adsorption cannot be completely separated from ion exchange; therefore, the solid substance to which the pollutant sorbs is universally named a sorbent. Sorption mechanisms can be classified into three groups depending on the nature of the sorbate–sorbent bond. Physical sorption implies intermolecular interaction between sorbate and sorbent. This case of sorption refers exclusively to adsorption and some authors classify it as a physical method of remediation, which is correct. In contrast, chemical sorption or chemisorption involves the formation of a chemical bond between a sorbate and a sorbent and is defined as a chemical process. Electrostatic sorption or ion exchange involves Coulombic attractive forces that result in ionic interactions between the ions on the sorbent and those in its environment. Specifically, ion exchange is defined as the process of exchange of exchangeable ions from the solid phase (ion exchanger) with ions from the surrounding medium [38,48,55,56,57]. Sorbents used for adsorption and ion exchange can be divided into two groups, natural or synthetic and organic or inorganic. Natural sorbents include clays, natural zeolites, waste materials, biomass, etc. Synthetic sorbents usually include polymer materials that possess various acidic, basic or neutral active groups, and various synthesized materials such as synthetic zeolites, zero-valent iron, activated carbon, etc. [57]. Recently, various modifications of sorbents and synthesis of nanomaterials have been carried out in order to increase the efficiency of the sorption process. Sorption is a widely applicable remediation technique for removing inorganic (heavy metals, P, N) and organic (pesticides and various hydrocarbons) pollutants from polluted waters [38,48,55,56,57].
The implementation of the procedure is simple and flexible, and can be performed in situ or ex situ. The application of the ex situ procedure enables the successive utilization of the sorbent, its regeneration and utilization of the regenerate. In case the sorbent cannot be regenerated, it must be properly disposed of, which is a disadvantage of this technique. In general, the advantage of this technique lies in the existence of a large number of different available and inexpensive natural sorbents, as well as highly selective synthesized sorbents for the target groups of pollutants. Admittedly, sometimes the synthesis of targeted sorbents can make the implementation of the sorption process more expensive [38,48,56,57]. Ultimately, the Environmental Protection Agency (USEPA) declared the sorption process as one of the preferred wastewater treatment techniques [55].
3.2.6. Chemical Reduction and Oxidation
Chemical reduction/oxidation is one of the most commonly applied techniques for the remediation of polluted waters. It implies the use of reducing or oxidizing agents for the complete removal of pollutants or their conversion into a less toxic form. Reduction/oxidation reactions lead to the precipitation of compounds or the generation of free radicals that act on pollutants, whereby they are degraded or converted into a less toxic form [56,58,59]. Reduction is often used to convert toxic chromates into less toxic Cr3+, as well as organic compounds into less harmful products such as methane and ethane. Chemical oxidation is used to degrade organic contaminants such as PAHs, chlorinated hydrocarbons and inorganic contaminants such as cyanide [56,58]. Chemical extraction combined with oxidation can be very effective for removing organic and inorganic contaminants. The most commonly used combination of agents is a chelating agent (EDTA, citric acid) and hydrogen peroxide [38].
Reduction/oxidation techniques can be performed in situ and ex situ. Ex situ performance is preferable for inorganic pollutants, while in situ is preferable for organic pollutants, under the condition of complete oxidation to CO2 and H2O. The selection of the oxidant will depend on the type of pollutant and the characteristics of the polluted site. The most used oxidants are permanganate, ozone persulfate and hydrogen peroxide, and their advantages are availability, non-toxicity, good oxidizing power and relatively low price. Permanganate is used as a selective oxidant for the treatment of organic pollutants. Ozone is an oxidant capable of direct or indirect oxidation (creating hydroxyl radicals) of mostly organic pollutants (PAHs, pesticides, aliphatic hydrocarbons, etc.). Hydrogen peroxide (H2O2) is a strong oxidant, and its effectiveness increases in the presence of Fe2+ ions, where it is activated by the formation of free hydroxyl radicals that are effective for the decomposition of organic pollutants. The reaction of generating free hydroxyl radicals from the Fe2+/H2O2 system is also known as the Fenton reaction and is the basis for the development of advanced oxidation techniques (AOTs) [38,56,58,59].
3.2.7. Advanced Oxidation Techniques
Advanced oxidation techniques (AOTs) are a large group of physicochemical processes based on the generation of free hydroxyl radicals (HO•). The aforementioned Fenton oxidation involves the formation of hydroxyl radicals by the reaction of hydrogen peroxide (H2O2) with a liquid catalyst, Fe2+ (Fenton’s reagent, Fe2+/H2O2). The generated hydroxyl radical is considered one of the strongest oxidants (E° = 2.8 V) compared to classic oxidants such as ozone (E° = 2.10 V), persulfate (E° = 2.01 V), hydrogen peroxide (E° = 1.80 V) and permanganate (E° = 1.70 V) [57,58,59,60,61]. Fenton’s process depends on acidic pH (pH < 3) because the catalytic power of Fe2+ decreases in an alkaline medium due to its oxidation and precipitation in the form of Fe(OH)3. To overcome the drawbacks of the Fenton process, Fenton-like processes using Fe3+/H2O2 and Fe°/H2O2 systems have been investigated. However, they are less reactive since primarily hydroperoxyl radicals are generated (HO2•), which have a lower oxidizing power (E° = 1.65 V) [61,62].
In order to overcome these drawbacks, there are numerous modifications of the classical Fenton process. For this purpose, external energy in the form of UV light, electricity and/or ultrasound is added to the Fenton process, resulting in photo-Fenton, electro-Fenton and sono-Fenton processes, as well as their combinations sono-photo-Fenton, photo-electro-Fenton and sono-electro-Fenton. Thus, free radicals are generated with the help of UV radiation, electrochemical oxidation, ultrasound and their combinations [38,56,57,58,60,61,62].
The generation of free radicals can also be achieved without Fenton’s reagent by the process of photolysis. Photolysis processes include ozone-based (O3/UV, O3/H2O2, and O3/H2O2/UV), hydrogen peroxide-based (H2O2/UV and the aforementioned Fenton-assisted processes) and photocatalytic (most commonly TiO2/UV and TiO2/H2O2/UV systems). Hydroxyl radicals are produced by ozonation, photolytic and photocatalytic splitting of hydrogen peroxide. All energy-assisted AOTs result in the generation of a higher concentration of hydroxyl radicals, causing a higher efficiency of the process [57,58,60,61].
The generated in situ HO• radical is a short-lived, extremely reactive oxidant that effectively mineralizes organic pollutants by various mechanisms such as dehydrogenation reactions, electrophilic addition to C=C double bonds, electron transfer, etc. Hydroxyl radicals are non-selective; they act on almost all types of organic pollutants, mineralizing them mostly completely to CO2 and water [57,58,62]. In addition, hydroxyl radicals do not generate secondary waste, they are not toxic, nor are they corrosive to equipment; therefore, AOTs are environmentally compatible. The main limitation of the wider use of AOTs is the high cost of reagents (ozone, hydrogen peroxide, Fe(II) salts) and energy consumption (generation of O3 or UV radiation). However, partial oxidation of organic pollutants can generate various by-products of questionable toxicity; therefore, ex situ use is limited [56,57,60,61]. These disadvantages can be overcome by using photocatalytic materials without the use of chemicals, for which a special group of AOTs, i.e., photocatalysis, has been developed.
3.2.8. Photocatalysis
Photocatalysis is the process of accelerating a photochemical reaction by the presence of a semiconductor that is activated by the absorption of UV radiation. The mechanism of photocatalysis is based on exposure of the photocatalytic material to UV radiation, whereby the metal absorbs energy and becomes excited, which causes the generation of superoxide radicals on the surface of the catalyst. The resulting radicals degrade pollutants, especially those of organic origin [58,62,63]. This technique is mainly used in water and air treatment, and is considered promising for remediation of organic pollutants [57,62,63]. Metal semiconductors, such as ZnO, WO3, CdS, NiO, GaP, TiO2 and others, are used as photocatalysts. The most suitable for in situ remediation is TiO2, since it has high photoactivity, low cost, chemical inertness, easy production and non-toxicity. The photoactivity of TiO2 can be improved by doping with transition or rare earth metals and by using photosensitive dyes. Composite materials of two or more types of photocatalytic materials are also used. As a rule, composite materials have proven to be more effective than single-component materials [57,58,62].
The application of photocatalysts is quite a challenge due to the fixation of the photocatalytic material on the support material. This is particularly important for air remediation applications to prevent the introduction of secondary pollutants (photocatalytic nanoparticles) into the purified air. The advantages of this technique are the high efficiency and non-selectivity of generated radicals towards pollutants. The disadvantages of this technique are the degradation of the photocatalytic material due to the surrounding conditions (aggressive media, air flow, mechanical abrasion, etc.) and compatibility with the environment. Furthermore, most photocatalytic materials are activated under the influence of the UV spectrum of light, which is often a limiting factor in their use. Specifically, the UV part of the solar spectrum makes up only 5% of the solar energy that reaches the Earth’s surface, which limits the application under the influence of solar energy [57,58,62,63].
3.2.9. Nanoremediation
Nanoremediation is a technique that uses natural or synthetic nanomaterials for the remediation of inorganic and organic pollutants present in water, air and soil through redox reactions, sorption, precipitation, complexation, coprecipitation, etc. [4,64,65,66]. Nanomaterials have particle sizes ranging from 1 to 100 nm [64]. Various types of natural nanomaterials are used, such as clay, metal oxides and organic substances, as well as synthetic ones based on TiO2, Fe, SiO2, carbon, graphene, nanotubes, polymers, micelles, nanomembranes, zeolites, zero-valent iron (nZVI), etc. [4]. Nanomaterials have many reaction sites on the surface, which makes them very reactive. They have good catalytic properties, a large specific surface area and a high degree of penetration into cracks; all of the above makes them promising for in situ and ex situ nanoremediation. In fact, nanoremediation is used in combination with biological and chemical techniques, that is, nanomaterials support the aforementioned techniques [4,64,65,66]. Although it is still in the development phase, this technique is promising for in situ nanoremediation because it does not require high costs for implementation and provides mostly complete degradation of pollutants. The high mobility of nanoparticles enables the application of this technique for in situ remediation of soil and sediment [36]. Furthermore, the issues that arise in nanoremediation are the potential toxicity of nanomaterials and their fate in the environment. Therefore, many questions remain open related to the effects and fate of nanomaterials, which require further research. However, these disadvantages could be compensated by the application of environmentally friendly nanomaterials in very small quantities, which would make the technique economically viable [36,37,38,64,65].
3.3. Physical Remediation
Physical remediation is most often applied to contaminated soil, and implies the removal of pollutants through physical mechanisms such as thermal remediation, vitrification, encapsulation and soil replacement.
3.3.1. Thermal Remediation
Thermal remediation is a technique by which a polluted medium, most often polluted soil and sediment, is subjected to heating under controlled conditions, with the aim of removing the contamination by evaporation. It is based on the volatility of pollutants and the heating of the polluted medium using steam, microwaves, infrared radiation, electricity, etc. [34,67,68]. Depending on the amount of heat used to vaporize the pollutant, it can be low temperature (90~320 °C) and high temperature (320~560 °C) [34,38,67]. The technique can be applied in situ or ex situ. The ex situ thermal remediation technique includes three steps, heating the polluted medium under controlled conditions during which the pollutant evaporates, subsequent condensation of the generated steam and treatment of the waste gas. During in situ thermal remediation, the pollutant in gaseous form evaporates into the atmosphere without collection, which is a disadvantage of in situ implementation. Since the pollutant is transferred to another location, ex situ performance is recommended. However, ex situ thermal remediation requires soil excavation and transportation, which significantly increases the cost of the process. This technique is applicable for the removal of organic (hydrocarbons, PAHs) and inorganic (heavy metals) pollutants [33,38,67].
The advantages of this technique are its short treatment time and high efficiency of contaminant removal, ability to treat hard-to-reach areas, applicability for highly contaminated areas, work safety and that it generally does not generate secondary pollution. However, the disadvantage is the need for a very high temperature to carry out the treatment and high capital costs for the treatment and gas emission control [37,38,67,68].
3.3.2. Vitrification
Vitrification implies high-temperature heat treatment of a contaminated medium, usually soil. By subjecting the contaminated medium to a very high temperature (1700–2000 °C), the medium melts, and upon cooling it solidifies and transforms into a vitrified inert mass in which the contaminants are immobilized [7,36,38,69,70,71,72]. Heavy metals and radionuclides are encapsulated in this glass matrix, while other organic contaminants are destroyed. Thus, the end product of vitrification is a vitrified glass-like product and usually has glass-like properties, characterized by low porosity and low leaching rate. The purpose of this technique is to immobilize contaminants, making them less toxic [33,70,71,72]. Vitrification can be performed in situ and ex situ. The in situ technique is preferred due to the relatively lower cost of implementation, since the ex situ implementation implies additional actions such as excavation, pretreatment, transportation of materials, which additionally increases the cost of implementation and applicability. Furthermore, vitrification is not applicable for dry soil since the source of thermal energy is an electric current that passes through moist and conductive soil by inserting electrodes into the contaminated area [7]. The advantages of this technique are its relatively simple application and high efficiency for cleaning large quantities of highly contaminated soil. The main limitations of vitrification are its applicability only for moist soils, and that it is very energy demanding. In addition, it is not applicable for soils with a high content of organic matter because it is extremely destructive and such soils lose their agricultural potential [7,38,72].
3.3.3. Encapsulation
Encapsulation is an environmental remediation technique that involves the separation and immobilization of contaminated soil, in order to prevent further spread of contaminants within a certain medium. This technique does not involve the removal of contaminants. The purpose is to ensure that the contaminant remains trapped within the closed area, in such a way that the level of environmental pollution does not worsen. The advantages of this technique are its simple application and economic profitability (low performance costs). The main disadvantages are that the encapsulation is not aesthetic and the contamination is not removed [7,48,53,69].
3.3.4. Soil Replacement
Soil replacement is the partial or complete replacement of contaminated soil with uncontaminated soil. In this way, the concentration of contaminants in the soil is reduced. The advantages of this technique are the effective isolation of pollutants and restoration of soil functionality [7,48]. Specifically, before implementing the replacement procedure, the contaminated area must be isolated from the environment with a physical barrier in order to prevent contamination of the surrounding areas and groundwater. Excavation, extraction of contaminated soil, transport and disposal of contaminated soil are then carried out, which makes this technique economically unprofitable. Since the replaced soil is treated as waste, the main challenge is therefore the necessity of processing the removed soil to avoid secondary contamination at another location. For the above reasons, this technique is applicable for heavily contaminated soils with a small surface area, since the treatment costs increase with the increase in the volume of the replaced soil [7,37,38,48].
4. Overview of Recent Knowledge on Environmental Remediation
4.1. Recent Knowledge on Biological Remediation of Soil, Water and Air
Biological remediation is applicable to all environmental compartments, soil, water and air. The results of investigation on phytoremediation of contaminated soil are shown in Table 1.

Table 1.
Results of investigation on phytoremediation of contaminated soil.
According to Landberg and Greger’s [73] investigation on a rural area of 2466 m2, in the village of Sunnersta in Sweden, the plant Salix viminalis was used to remediate soil contaminated with heavy metals (Cr, As, Cd, Zn, Cu, Pb, Ni), PAH and PCB compounds. After ten years of phytoremediation, all pollutants in the soil were significantly reduced, for heavy metals in the amount of 21–87% and for PAHs and PCBs from 25 to 73%. Most of the mentioned pollutants were removed in the highest percentage in the first five years. Remediation of oil-contaminated soil with an average concentration of 14,400 mg/kg was carried out by Panchenko et al. [74] in a field in the vicinity of an oil refinery in Volga, Russia, with the plants Melilotus officinalis, Agropyron cristatum, Medicago sativa L. and Lolium perenne L. The highest oil removal was achieved already after the first year of phytoremediation, by reducing their concentration to a value of 2500 mg/kg, and after 5 years to a value of 1100 mg/kg, i.e., 92.4%. The high phytoremediation efficiency was attributed to the rhizodegradation mechanism. Cheng et al. [75] collected petroleum-contaminated soil near an oil supply center in Taiwan and treated it with the plants Vetiveria zizanioides and Cymbopogon nardus itle. The initial amount of petroleum in the soil was 3000–8000 mg/kg. After 15 months of phytoremediation with Vetiveria zizanioides, petroleum concentrations were reduced by 89–90%, depending on the initial petroleum concentration. A similar result was obtained using the Cymbopogon nardus itle plant. A removal of 86% to 91% was achieved depending on the initial petroleum concentration in the soil. In addition to the achieved removal of petroleum hydrocarbons, the results showed that the plants had a positive effect on the stabilization of pH and electrical conductivity of the soil, as well as the number and diversity of microbiota in the rhizosphere of plants. Three studies were conducted by Chen et al. [76] (from September 2020 to January 2021, then from April to October 2021 and from July 2021 to July 2022) on three samples of cadmium-contaminated soil with concentrations of 0.7676 mg/kg, 1.3058 mg/kg and 1.0970 mg/kg. Phytoremediation of the soil with the plant Pennisetum hybridum resulted in the percentage of cadmium removal for three samples in amounts of 23.62%, 21.50% and 35.81%. It was observed that the root of Pennisetum hybridum has the highest phytoremediation potential.
Based on the aforementioned investigations, it is clearly observed that the application of phytoremediation in the remediation of soil contaminated with heavy metals and petroleum is a relatively highly effective, but also long-term procedure. The results of recent phytoremediation research (Table 1) indicate that the efficiency of phytoremediation depends on the type of pollutant, its concentration, the type of plant and the duration of remediation. Therefore, the application of phytoremediation will be desirable in places that do not have a direct negative impact on the human population and where the urgency of the remediation procedure is not required.
Investigations on phytoremediation of steel foundry wastewater contaminated with heavy metals (Al, As, Cd, Cr, Cu, Fe, Mn, Pb, Zn) using aquatic plants, Pistia stratiotes and Eichhornia crassipes was conducted by Aurangzeb et al. [77]. The results of the investigation are shown in Table 2. Depending on the initial heavy metal concentration, heavy metal removal in the range of 16–71% was achieved after treatment with Pistia stratiotes. On the other hand, using Eichhornia crassipes, the removal efficiency was from 48% to 83%. The results clearly showed the higher effectiveness of Eichhornia crassipes compared to Pistia stratiotes in removing the same initial concentrations of heavy metals. The mechanism of phytoremediation is attributed to the phytoextraction of heavy metals into plant tissues and roots.

Table 2.
Results of phytoremediation investigations of waters contaminated with heavy metals [77].
Phytoremediation tests of sediment contaminated with copper and lead are shown in Table 3.

Table 3.
Results of phytoremediation tests of contaminated sediment [78].
Song et al. [78] used a combination of plants, Vallisneria natans, Hydrilla verticillata and Myriophyllum spicatum, for phytoremediation of copper and lead polluted sediment of Le’an River near Dexing copper mine, located in Wannian, Shangrao City, Jiangxi Province. A copper removal efficiency of 26.1% was achieved when Vallisneria natans and Myriophyllum spicatum were planted together in the polluted sediment. With the simultaneous use of Hydrilla verticillata and Myriophyllum spicatum, a percentage of lead removal of 68.4% was achieved. The test indicated the possibility of reducing the concentrations of both copper and lead by in situ remediation, by planting the mentioned plants in the polluted sediment. It was concluded that Hydrilla verticillata has a positive effect on the number of microorganisms when planted together with Myriophyllum spicatum, which contributes to the removal of the examined heavy metals.
Summarizing the investigations of soil, water and sediment phytoremediation, all studies were mainly performed at the laboratory level and focused on the mechanism and efficiency of pollutant removal. Lifetime, growth rate and the fate of plants after use should be the goal of future research in order to provide the clearest possible guidelines for the use of phytoremediation in the field.
The well-known process of photosynthesis, the absorption of CO2 and release of oxygen, has been used as the potential of plants to remove pollutants from the air. Indoor air pollutants mainly include carbon monoxide, carbon dioxide, nitrogen oxides, VOCs, formaldehyde, benzene, particulates and others [79].
The assessment of the effectiveness of improving indoor air quality in studio apartments by phytoremediation with indoor plants are shown in Table 4.

Table 4.
Results of the assessment of the effectiveness of indoor air quality improvement in studio apartments by phytoremediation with indoor plants [80].
As specified in Table 4, the assessment of indoor air quality without and with the presence of indoor plants Sansevieria kirkii, Sansevieria trifasciata, Monstera deliciosa, Zamiifolia and Portulacaria afra in the period from December 2021 to January 2022 in four studio apartments with an area of 33 m2 was conducted by Sharma et al. [80]. The characteristics of the rooms were as follows: apartment 1 (with ventilation and plants), apartment 2 (without ventilation, with plants), apartment 3 (with ventilation and without plants) and apartment 4, which served as a control room (no ventilation, no plants). During two weeks, the measured data gave an insight into the indoor air quality. Significant differences were observed in the concentrations of pollutants in the apartments (Table 4). The measured values of PM2.5 and PM10 in the apartments showed higher concentrations than those measured outdoors. The main source of air pollution in apartments is attributed to activities such as baking. The average concentration of PM2.5 in apartment 4 (without plants, without ventilation) was 35.72 μg/m3, and 58.81 μg/m3 for PM10. The results showed that apartment 1 with plants and ventilation can significantly reduce the concentration of PM2.5 up to 64.61%, and PM10 up to 67.01%. The average concentration of VOCs in the apartment without plants and ventilation (apartment 4) reached a value of 800.41 μg/m3, while in the apartment with ventilation and plants (apartment 1) the highest reduction was observed, to a value of 56.35 μg/m3. The average concentration of formaldehyde in apartment 4, without plants and without ventilation, was 23.99 μg/m3. Apartment 1, with plants and ventilation, had a formaldehyde concentration of 6.02 μg/m3, similar to apartment 2, only with plants, which had a value of 5.98 μg/m3. The results revealed that average formaldehyde concentrations were significantly higher in apartments without indoor plants. Similar to other pollutants, CO2 concentrations were found to be higher in apartments without indoor plants and without ventilation (2616.36 × 103 μg/m3) than in apartments with plants and/or ventilation. Moreover, the lowest average CO2 concentration of 615.50 × 103 μg/m3 was recorded in apartment 1, with plants and ventilation. The results show that the average concentration of all air pollutants was significantly higher without plants and ventilation than with plants and ventilation. Even with ventilation and without plants (apartment 3), the average concentrations were higher than with ventilation and plants (apartment 1), indicating that ventilation cannot fully reduce pollution. The results indicate a simple and sustainable way of improving indoor air quality in apartments by phytoremediation with indoor plants.
Table 5 shows the results of phytoremediation of polluted indoor air.

Table 5.
Results of phytoremediation of polluted indoor air.
Active botanical biofilter systems can be effective in removing indoor air pollutants. Ibrahim et al. [81] used a biofilter consisting of the Epipremnum aureum plant and mechanical ventilation with an air flow of 540 m3/h and achieved a removal efficiency of 54.5% for PM2.5, 65.4% for PM10 and 46.0% for VOCs (Table 5). Phytoremediation of benzene from indoor polluted air using two plants, Schefflera arboricola and Spathiphyllum wallisii, was investigated by Parsheh et al. [82] in a controlled environment using a plexiglass chamber. The average removal efficiency at different initial benzene concentrations (Table 5) was 91.0–97.0%. The toxic effect of benzene on the plants used was not determined at the tested concentrations. It can be concluded that this application is environmentally acceptable for the removal of benzene from polluted indoor air. According to a laboratory study conducted by Gong et al. [83], indoor benzene removal using Epipremnum aureum, Chlorophytum comosum, Hedera helix and Echinopsis tubiflora was 72% (Table 5). The results of the conducted investigations clearly indicate that phytoremediation of indoor polluted air is effective and can be used as a precautionary measure against possible unexpected pollutant emissions. Studies have shown that a plant’s potential for air phytoremediation is correlated with the plant’s transpiration rate and chlorophyll concentration. Accordingly, the specified parameters will facilitate the selection of plants for phytoremediation of air for future researchers.
Investigations have revealed that all parts of plants (leaves, roots, shoots) show effectiveness in reducing concentrations of pollutants in the air. It was observed that the efficiency of phytoremediation of pollutants from the air depends on the surface of the plant (density and size of the leaves) as well as the characteristics of the soil; more precisely, the microorganisms in the rhizosphere of the plant [84]. The mechanism for removing pollutants from the air is based on absorption in the leaves, followed by decomposition and transformation within the plant [85]. An investigation by Brilli et al. [86] confirmed the absorption of air pollutants through the leaves and their decomposition inside the plant. Moreover, through metabolic decomposition, pollutants become a source of carbon and energy for plants. Furthermore, Zhao et al. [87] determined the relationship of formaldehyde removal with microorganisms in the rhizosphere of the plant. On the other hand, removal of PM primarily takes place by accumulation on the leaf surface [88,89]. Therefore, plants with a larger leaf area are more efficient in removing particles. In the case of the latter, the rougher leaf of the plant proved to be more effective compared to the smooth leaves [90,91]. Finally, indoor air pollution is associated with a variety of health effects [92]. For example, VOCs cause respiratory and nervous effects [93], while PM are carriers of allergens and toxicants [94,95]. Accordingly, in addition to having an aesthetic effect indoors, indoor plants also represent an ecological approach to remediation and improvement of indoor air quality. Additional research is needed to expand the potential plant species in order to establish the mechanism of mitigation of toxicants, especially formaldehyde, benzene and VOCs.
The results of bioremediation of organic pollutants and heavy metals are presented in Table 6.

Table 6.
Results of bioremediation of organic pollutants and heavy metals.
For the biodegradation of pyrene at an initial concentration of 1000 mg/L, Marzuki et al. [96] used two types of bacteria, Bacillus licheniformis and Sphingobacterium. The result of the interaction of the bacterial suspension and pyrene during 30 days was the decomposition of pyrene by Bacillus licheniformis in an amount of 38.29%, as well as 39.00% by Sphingobacterium. Pyrene biodegradation products were simple organic compounds with alcohol and carboxylic acid groups for both types of bacteria. Testing of the bioremediation of anthracene and pyrene with the bacterial species Bacillus pumilus, Pseudomonas stutzeri and Acinetobacter calcoaceticus was carried out by Marzuki et al. [97]. During 25 days of interaction with pollutants, a decrease of 21.89% of anthracene and 7.71% of pyrene was observed. Acidic biodegradation products of two PAHs (alcohols, aldehydes, carboxylic acids and a small proportion of aromatic hydrocarbon components) inhibited the ability of bacteria to continue the biodegradation process. Furthermore, Marzuki et al. [98] investigated the bioremediation of waste contaminated with naphthalene, Cr(VI) and Cd using the bacteria Bacillus pumilus and Pseudomonas stutzeri. The initial concentration of Cr(VI) and Cd was 250 mg/L, and that of naphthalene was 1000 mg/L. Biodegradation efficiency using Bacillus pumilus bacteria for naphthalene was 7.16%, 56.30% for Cr(VI) and 61.23% for Cd. Biodegradation by the bacterium Pseudomonas stutzeri was 11.24% for naphthalene, 52.74% for Cr(VI) and 57.80% for Cd. An inhibitory effect of bacterial activity on the biodegradation of pyrene in the presence of heavy metals was observed. Gomaa [99] collected samples of calcareous soil in Egypt and used them to isolate bacteria that produce the urease enzyme. Bacteria Micrococcus sp. mixed with heavy metal salts in the concentration range of 0–10 mol/L showed a good ability to biosequestrate Cd and Pb over 2 days in the amount of 60.66% and 97.20%, respectively. The relatively satisfactory efficiency of bioremediation was attributed to the resistance of ureolytic bacteria to heavy metals.
Bioremediation is also used for the decomposition of endocrine disruptors such as pesticides, pharmaceuticals, bisphenols, phthalates, polychlorinated biphenyls, etc. Studies have shown the effective use of mushrooms for bioremediation of pesticides [100] and pharmaceuticals [101]. For example, Ding et al. [102] achieved 97.1% bioremediation efficiency of naproxen-contaminated water using Cymbella sp. In addition, studies have shown the possibility of microbiological activity for the degradation of organic compounds such as naphthalene [103], xylene [104] and PAHs [105]. Research by Jiang et al. [106] found that bacteria exposed to Cr6+ experience oxidative stress, thereby reducing their bioremediation efficiency. Therefore, bioremediation can be made more effective by applying genetic engineering, developing enzymes that act on the degradation of pollutants [107,108]. That is, microorganisms’ resistant to pollutants are the best choice for genetic manipulation [109,110]. Hence, contaminant-inhabited bacteria should be ideal candidates for genetic manipulation [111]. Genetically modified bacteria and fungi have been shown to be effective for the degradation of xenobiotics, pesticides and heavy metals [112,113]. Furthermore, bioremediation is often associated with phytoremediation. Specifically, rhizobacteria stimulate the growth of plants and contribute to the sorption of pollutants in the root zone. Therefore, the combination of microorganisms and plants acts synergistically on the remediation of pollutants [114]. Thus, Chen et al. [115] conducted a two-year study on soil contaminated with Zn, Cd and PAH compounds by phytoremediation using the plant Sedum alfredii and bioremediation using the bacteria Microbacterium sp. strain KL5 and Candida tropicalis strain C10. The experimental results presented in Table 7 indicate a more efficient removal of PAH compounds in the amount of 96.4% compared to heavy metals, 36.1% for Cd and 12.7% for Zn. Two-year bioremediation resulted in a decrease in the concentration of PAHs below the permitted concentrations for agricultural soil.

Table 7.
Results of soil phytoremediation and bioremediation [115].
The results presented in Table 6 and Table 7 indicate a mostly successful remediation of organic pollutants in relation to heavy metals. Studies have shown that bioremediation efficiency of up to 100% is achieved using a consortium of bacteria rather than a single bacterial species. In addition, the growth of bacterial cells continues even after the decomposition of organic pollutants. This indicates that bacterial cells can still carry out cell division and decomposition of hydrocarbon components for use as an energy source [116]. Furthermore, it is well known that microorganisms and plants reduce and/or remove pollutants from a polluted medium. However, according to recent advances in pollutant reduction, a combined system of microorganisms and plants has been shown to improve pollutant removal to an efficient level. In a pollutant-rich environment, the environment is depleted of nutrients. By introducing the appropriate plant and microorganism into such an environment, the plant interacts with the microorganism to survive under toxic conditions. This interaction leads to higher germination efficiency and enhanced root growth, resulting in enhanced pollutant degradation [117]. Therefore, the correct selection of plants and microorganisms can contribute to the effectiveness of bioremediation as well as to the improvement of soil properties. Although phytoremediation is a green technique, the slow growth of plants and the time required to achieve satisfactory efficiency are key challenges. It is recommended to use local plants and genetic engineering in order to overcome the mentioned shortcomings. The growth rate of plants can be improved by genetic engineering, the use of genetically modified plants or the addition of microorganisms to the soil.
4.2. Recent Knowledge on Chemical Remediation of Soil, Water and Air
In addition to phytoremediation and bioremediation, chemical remediation is one of the most applied and researched remediation techniques. This is supported by the fact that chemical remediation techniques are more numerous than others and applicable to all compartments of the environment (soil, water and air). The results of the chemical remediation investigations by leaching contaminated soil are shown in Table 8.

Table 8.
Results of contaminated soil leaching.
Artificially contaminated soil containing 700 mg Cu/kg, 530 mg Pb/kg, and 900 mg Zn/kg was used in a study by Park et al. [118]. A high-pressure soil washing device used tap water as a leaching agent. Under optimal experimental conditions, a removal of 37.7% for Cu, 36.6% for Pb and 45.1% for Zn was achieved. The remaining concentrations of heavy metals in the soil met the Korean Warning Standard and indicated the applicability of the chemical-free leaching procedure in case of urgent soil remediation. Zhang et al. [119] artificially polluted the soil with cadmium and phenanthrene, which was prepared by mixing sand and kaolinite clay in a ratio of 2:1. The soil remediation carried out by leaching with a 5 g/L rhamnolipid solution (an environmentally acceptable biosurfactant) at pH = 9 and 15 °C resulted in the removal of cadmium in the amount of 72.4% and phenanthrene in the amount of 84.8%. The biosurfactant contributed to the solubility of Cd and phenanthrene, which resulted in enhanced leaching and more effective remediation. Furthermore, Song and Nam [120] used a KCl solution to leach cesium-contaminated soil with a concentration of 1.47 mg/kg collected near a nuclear power plant in South Korea. The cesium removal efficiency was found to be 81.3% under the optimal leaching experimental conditions with 1 mol/L KCl solution at L/S = 20, pH = 2 for 2 h. It has been observed that the application of KCl does not eliminate nutrients from the soil, which justifies its use in contrast to conventional leaching agents. Hu et al. [121] investigated the two-stage remediation of zinc-contaminated soil with a concentration of 557.2 mg/kg by leaching with a 5 g/L citric acid solution and a 4 g/L chitosan (polysaccharide) solution. The efficiency of zinc removal from the soil by leaching was 63.9%. It was concluded that the main leaching mechanisms include dissolution, ion exchange and complexation of heavy metals as a result of the action of natural and degradable leaching agents. Copper, nickel and zinc contaminated soil from an industrial site in Dongguan, China, were leached with a solution obtained by mixing 0.05 mol/L EDTA and 0.20 mol/L citric acid, 0.05 mol/L EDTA with 0.20 mol/L oxalic acid and 0.05 mol/L EDTA with 0.20 mol/L tartaric acid in a study by Cheng et al. [122]. The removal percentage for three types of leaching solutions was 81.5%, 85.5% and 85.0% for Cu, 85.9%, 82.9% and 78.9% for Ni and 81.1%, 84.6% and 82.5% for Zn. The leaching conditions were pH 3.0, S/L = 1:10 and leaching time 6 h. The results revealed that EDTA has a stronger ability to chelate heavy metals, while acids lower the pH, which improves metal solubility. Remediation of contaminated soil by leaching is most often carried out ex situ and is applicable for smaller amounts of contaminated soil. The results of the investigations indicate a relatively high efficiency of pollutant removal by leaching with a relatively short time of procedure implementation. The use of environmentally friendly leaching agents is imperative for the ex situ application of this technique. The combination of chelating agents and acids seems promising because it acts synergistically on the effectiveness of the leaching process.
The results of the electrochemical soil remediation investigations are presented in Table 9.

Table 9.
Results of the electrochemical soil remediation investigations.
A pilot test and a test in environmental conditions were conducted by Cai et al. [123] on a cadmium-contaminated soil sample with an average concentration of 3.68 mg/kg. The sample was taken in Gaolian Village, Shaoguan City, Guangdong Province, China. After fourteen days of remediation, the efficiency of Cd removal from the soil in the pilot test was 87.0%, and 74.0% in environmental conditions. The result was attributed to the voltage gradient, which was five times higher in the pilot test than in environmental conditions. Also, the results showed that the efficiency of Cd removal from the soil was the highest in the upper soil layer of 0–10 cm. The removal of petroleum in the amount of 75.2% from petroleum-contaminated soil, using a graphite electrode at a voltage of 30 V and water as an electrolyte with the biosurfactant rhamnolipid in a period of 10 days, was carried out by Gidudu et al. [124]. Improved soil decontamination was the result of the application of high voltage and biosurfactant, which resulted in improved electroosmosis and electrophoresis of petroleum. In addition, the high voltage had no harmful effects on the bacteria. Alcántra et al. [125] achieved a removal efficiency of pyrene in the amount of 45.0% and fluoranthene in the amount of 57.0% using graphite electrodes and a 1% solution of the nonionic surfactant Tween 80 (polysorbate 80 produced from polyethoxylated sorbitan and oleic acid) and 0.1 mol/L of Na2SO4 solution, at 30 V and pH = 7 with an experiment length of 23 days. The test results confirmed that pH control was crucial for the efficiency of PAH remediation. In the work of Cong et al. [126], artificially contaminated soil with a 1000 mg/L chlorophenol solution was electrochemically remediated in a laboratory device using a graphite electrode. A voltage of 1200 V/m and a current of 10 mA were applied. After 140 min of the experiment, the removal efficiency of phenol was 72.0%, 80.2% for 2-chlorophenol, 81.6% for 2,4-dichlorophenol and 85.2% for 2,4,6-trichlorophenol. The obtained results indicated the feasibility of electrochemical remediation of soil contaminated with polychlorinated phenols. Taking into account the applicability of electrochemical remediation, it is observed that with the increase in the applied voltage, the remediation time is significantly reduced, with an increase in the efficiency of pollutant removal. However, shortening the remediation time significantly increases the costs of the procedure, which calls into question the cost-effectiveness of implementing electrochemical remediation, especially for larger amounts of contaminated soil.
Table 10 shows the results of electrochemical remediation of polluted water systems.

Table 10.
Results of electrochemical remediation of polluted water systems.
Abdulredha et al. [127] performed electrocoagulation of As(III) polluted water with a concentration of 300 mg/L, using a stainless steel electrode. At optimal operating conditions of electric current density of 6 mA/cm2, at pH = 9.0 and a duration of 30 min, the efficiency of As(III) removal was achieved in the amount of 81.0%. The outcome of the experiment pointed out that a higher current density contributes to shortening the working time and improves the removal of As(III). The need for pH control was also observed, with pH = 9 proving to be optimal. Furthermore, Babu et al. [128] achieved complete removal of As(III) from water with an initial As(III) concentration of 1 mg/L by applying a voltage of 5 V to Fe electrodes for 60 min of the experiment. Electrocoagulation with aeration significantly contributed to higher efficiency compared to electrocoagulation performed without aeration due to the formation of oxidizing agents such as ferryl ions in the process. Gooren et al. [129] investigated the removal of As(III) from a groundwater sample taken in Kocaeli province, Turkey, which was artificially contaminated with As(III). The electrochemical reactor consisted of a titanium cathode and an anode compartment (Al ball anode) which was aerated. Under optimal conditions of pH = 7.5, air flow of 6 L/min, current of 0.30 A and a time of 12 min, the achieved removal efficiency of As(III) was 99.2% for an initial concentration of 200 μg/L. As in the previous study, the results showed that electrocoagulation is dependent on the external oxidizing agent, the air flow rate. The remediation of the groundwater of the island of Tenerife (Spain) contaminated with fluorides with an initial concentration of 7.35 mg/L using aluminum electrodes at optimal conditions in a cell of 10 mA/cm2, pH = 7.8 and a time of 15 min was carried out by Betancor-Abreua et al. [130]. The results showed a fluoride removal efficiency of 85.9%. Removal of fluoride ions was achieved by electrocoagulation, i.e., absorption of fluoride ions on the formed Al(OH)3. López-Guzmán et al. [131] investigated the removal of fluoride and arsenic from water containing 5 mg/L fluoride and 80 μg/L arsenic. The optimal experimental conditions were pH = 5.0, treatment time of 15 min and a current of 4.5 mA/cm2. The achieved removal efficiency using the Fe-Al electrode was 85.7% for fluoride and 100.0% for arsenic. As with previous electrocoagulation studies, this study also revealed a pH dependence due to the formation of Fe and Al hydroxides that contribute to the removal of arsenic and fluoride. Comparing the results of electrochemical remediation of polluted soil and water, a higher efficiency of pollutant removal from water systems is observed, which is probably due to the facilitated electromigration of pollutants in the water medium. Moreover, the implementation time of electrochemical remediation is significantly longer for soil compared to water systems. This indicates a better applicability of electrochemical remediation for water systems.
Table 11 shows the results of the stabilization/solidification study of contaminated soil.

Table 11.
Results of the stabilization/solidification study of contaminated soil.
Anand-Reddy et al. [132] mixed artificial lead and zinc contaminated soil with limestone-calcined clay cement (LC3) and solidified it over a period of 28 days. The toxicity of the material was then tested according to the standard toxicity characteristic leaching protocol (TCLP). The results indicated a decrease in the concentration of Zn and Pb by increasing the solidification time. The decrease in eluted concentrations was attributed to increased pH values and the formation of metal hydroxides in the presence of free available Ca(OH)2 and Ca2+ ions in the binding material. Moreover, when limestone is added to calcined clay, it reacts to form carbo-aluminates which tend to reduce the mobility of heavy metals by forming insoluble metal hydroxides, thereby increasing the effectiveness of immobilization and reducing the leaching of zinc and lead. It was concluded that stabilization with LC3 promotes the immobilization of Zn and Pb from contaminated soil, which further reduces the possibility of their leaching. In addition to the above, there is also an increase in strength in the treated samples, which is a consequence of the hydration reaction. The stabilization/solidification efficiency was 88.0% for Zn and 99.0% for Pb, after 28 days of solidification. Hu et al. [133] used a tailings-based geopolymer to immobilize Ba and Pb. The compressive strength of the prepared tailings-based geopolymer after 7 days of solidification reached a value of 35 MPa. The maximum concentration of Pb and Ba in the leachate did not exceed the values of 0.1 mg Pb/L and 0.4 mg Ba/L. The results of leaching showed that the prepared tailings-based geopolymer was able to effectively immobilize heavy metal cations by >95.0%. Treatment of copper-contaminated sediment samples collected in the Sembrong River, Malaysia by stabilization/solidification using Portland composite cement as the main binder with the addition of rice husk ash was carried out by Aliyu et al. [134]. A high percentage of Cu stabilization was observed after 28 days leaching according to the TCLP procedure, in the amount of 97.8%. The results showed that the partial replacement of cement with rice husk ash in the binder system increased the strength and reduced the leaching ability of Cu from the polluted sediment. Soil containing 170.4 mg As/kg was sampled by Li et al. [135] in the Great Bay area of South China and treated by solidification/stabilization using a mixture of cement and blast slag. The results showed that with the 10% binder application, more than 80% of As was effectively stabilized at pH = 5.5–6.5 during the 28 days of the experiment. The stabilization/solidification technique is very important for the stabilization or disposal of hazardous waste, especially that obtained after ex situ remediation using different sorbents. Studies have shown that the addition of sorbents saturated with heavy metals to cement increases its strength, which justifies the process of their stabilization/solidification.
Table 12 shows the results of investigations on the remediation of polluted water systems using a permeable reactive barrier.

Table 12.
Results of investigations on the remediation of polluted water systems using a permeable reactive barrier.
Lee et al. [136] investigated the possibility of zeolitic rocks as a filler for a permeable reactive barrier (PRB) for the purpose of remediation of zinc-contaminated groundwater. Zinc concentrations in the effluent decreased until equilibrium was established, from 434 to 5 mg/L, whereby the zinc removal efficiency in the amount of 99.0% was achieved as a result of sorption on zeolite. Jun et al. [137] used a laboratory reactor to simulate PRB for the remediation of heavy metal landfill leachate-contaminated groundwater with initial concentrations of 82.8 mg Zn/L, 13.8 mg Mn/L, 555.9 mg Ca/L, 186.4 mg Mg/L, 0, 08 mg Cd/L, 0.2 mg Cr/L, 1.2 mg Sr/L and 16.2 mg Al/L. Zero-valent iron (ZVI) and zeolite were used as reactive media. The removal efficiency was 97.2% for Zn, 99.6% for Mn, 81.7% for Ca, 95.9% for Mg, 95.2% for Cd, 70.7% for Cr, 90.5% for Sr and 58.7% for Al. Historical storage of ore containing sulfide minerals at an industrial site in British Columbia, Canada led to soil and groundwater contamination. Significant amounts of heavy metals, including Cu, Cd, Co, Ni and Zn, have been released into groundwater by oxidation of sulfide minerals. Ludwig et al. [138] placed a pilot-scale PRB in the path of the dissolved heavy-metal plume; the reactive mixture used in the barrier consisted of 15% leaf compost, 84% pea gravel, 1% limestone by volume and sulfate-reducing bacteria. After 21 months, there was a significant removal of heavy metals. Heavy metal concentrations decreased as follows: from 3.63 mg/L to 0.0105 mg/L for Cu, from 0.0153 mg/L to 0.0002 mg/L for Cd, from 0.0053 mg/L to 0.011 mg/L for Co, from 0.131 mg/L to 0.033 mg/L for Ni and from 2.41 mg/L to 0.136 mg/L for Zn. Expressed as removal efficiency for Cu, Cd, Co, Ni and Zn, this was 99.7%, 98.7%, 79.2%, 74.8% and 94.4%, respectively. Guo et al. [139] conducted laboratory testing of nitrate removal from synthetic and real wastewater using PRB filled with modified raw wheat straw and with the addition of denitrifying bacteria in an amount up to 35%. During the 370 days of the experiment, a nitrate removal efficiency of 90.0% was achieved in laboratory conditions and 60.0% in the field for initial nitrate concentrations of 27.80–59.86 mg/L. The results showed that nitrate removal is associated with simultaneous chemical reduction and biodenitrification, which becomes dominant in the final phase of the process. The results of the investigations show that the application of PRB can be very effective for the removal of pollutants from water systems. Although the duration of the procedure is relatively long, the extremely high removal efficiency compensates for the time of the procedure. The wide range of PRB materials makes this technique applicable to all systems contaminated with different types of pollutants. For example, one of the reactive media used for water remediation using PRB is natural zeolite, due to its high cation exchange capacity. Ultimately, the application of PRB can be significant in preventing the spread of potential contamination by placement in areas of high vulnerability of water systems.
Table 13 shows the results of remediation of polluted air by photocatalysis.

Table 13.
Results of remediation of polluted air by photocatalysis.
Using multicomponent oxide thin films of ZnO/Zn2TiO4 and TiO2 as photocatalysts on a glass substrate, Hernández-García et al. [140] successfully carried out the degradation of benzene in the gaseous state. The initial benzene concentration was 0.11 ppm. The process took place in a batch type reactor, at room temperature. The results show the degradation of benzene in the amount of 95% for a period of 4 h using the ZnO/Zn2TiO4 photocatalyst. Furthermore, by applying thin layers of TiO2, a degradation of only 70% was achieved under the same measurement conditions. The synergistic effect of the photocatalyst proved to be better. Yamada et al. [141] evaluated the photocatalytic properties of a TiO2 coating, produced by spraying on a steel substrate, by removing NOx. The NOx removal of 87% indicated that the TiO2 coating had good photocatalytic properties. Hernández-García et al. [142] investigated the photodegradation of gaseous benzene with an initial concentration of 110 ppm without and with photocatalysts (CdO, TiO2, CdO/CdTiO3) in a batch reactor at room temperature with UV irradiation. The efficiency of benzene removal was 25%without a photocatalyst, 40% with CdO and 70% with TiO2 for a UV irradiation time of 4 h. Mehrizadeh et al. [143] studied the application of ZnFe2O4 nanoparticles for the removal of toluene from the gaseous phase by a photocatalytic process under UV and visible irradiation in a photoreactor. The prepared nanoparticles were able to remove 60% of toluene. Abidia et al. [144] proved that the photocatalytic test carried out in a reactor with visible light and CuxO/TiO2 on polyester cloth affects the removal of polluted air with chloroform. Chloroform concentration decreased up to 71% within 15 h of irradiation. Optimal removal was achieved at a catalyst deposition current of 80 A and a catalyst deposition time of 20 s. Removal of chloroform in the amount of 71% under the action of CuxO/TiO2 on polyester cloth was achieved in 35 h, which shows a good catalytic ability to remove chloroform. Photocatalysis appears to be a promising technique for the remediation of polluted air, as well as wastewater primarily polluted with biologically non-degradable organic compounds, since the application of irradiation to the photocatalytic material produces free radicals that in a short time indiscriminately and highly efficiently degrade pollutants.
Emerging environmental pollutants such as endocrine disruptors have attracted the special attention of scientists due to their extremely negative impact on human health and the environment. This group of substances includes pharmaceuticals, pesticides, bisphenol A, etc. The most commonly applied techniques for their remediation are advanced oxidation techniques including photocatalysis and nanoremediation.
Advanced oxidation techniques (AOTs) have been widely applied to remove endocrine disruptors from polluted waters. Several studies have applied ozone to degrade pharmaceuticals [145,146,147]. Photodegradation by UV radiation has also been applied to various types of pharmaceuticals. Thus, Timm et al. [148] achieved ≈100% removal of amoxicillin and ampicillin, 95% of penicillin and 90% of piperacillin. Another study conducted by Ribeiro et al. [149] observed 96% photodegradation of ceftiofur and 92% of cefapirin. Almost 100% removal of enoxacin was observed by Lastre-Acosta et al. [150]. All investigations were carried out by photodegradation without the use of chemicals. Although the treatment is relatively short, the application of photolysis is not often used, unlike the combination of UV radiation with other oxidants. Yao et al. [151] showed that the O3/UV combination enables 90% degradation efficiency of chloramphenicol from groundwater, while Wardenier et al. [152] applied the same system for the degradation of bisphenol A and achieved a degradation efficiency of >80%. The aforementioned investigations suggest that the combination of ozone and UV light increases the efficiency of degradation due to the generation of higher amounts of hydroxyl radicals. A similar finding was observed in the study by Gomes et al. [153] who applied the O3/H2O2 system for sulfamethoxazole degradation and achieved almost 100% degradation in 45 min of treatment. In the O3/ H2O2 system, H2O2 accelerates the decomposition of ozone and the generation of radicals. The H2O2/UV combination has been used to degrade cephalexin [154,155], ofloxacin [154,156], norfloxacin [154], roxithromycin [157] and parabens [158]. Collectively, all studies achieved >99% degradation efficiency in a short period of 3 to 45 min. In relation to the aforementioned double AOT combinations, the O3/H2O2/UV system proved to be even more effective in the degradation of pharmaceuticals [159,160].
On the other hand, several studies have been conducted using Fenton’s reagent (Fe2+/H2O2) for the degradation of various pharmaceuticals such as amoxicillin [161] and ciprofloxacin [162,163,164]. The degradation efficiency was observed in the range of 70–83%. A catalytically assisted heterogeneous Fenton-like process has been investigated for the removal of ciprofloxacin [165] and tetracycline [166,167]. The use of heterogeneous catalysts improved the degradation efficiency compared to the Fenton process. However, the efficiency of the photo-Fenton process (Fe2+/H2O2/UV) on the degradation of amoxicillin [168], ampicillin [169] and trimethoprim [170] further increased with a decrease in treatment time (6–20 min). The use of UV radiation in the photo-Fenton process accelerates the generation of hydroxyl radicals, alongside a lower consumption of H2O2, which contributes to the economy of this process. In summary, based on treatment efficiency and duration, AOTs are superior compared to conventional water treatment techniques. This is one of the fundamental reasons for the increase in research on emerging environmental pollutants using these techniques.
Within the framework of advanced techniques, nanoremediation represents a recent innovative technique for the removal of emerging environmental pollutants from water and soil [171,172]. Numerous types of nanomaterials have been investigated for the removal of heavy metals [173,174,175,176,177,178] and organic substances [179,180,181,182]. Nanoremediation has also been used to remove endocrine disruptors such as herbicides [183,184], naphthalene [185], DDT [186], bisphenol A [187,188] and pharmaceuticals [189,190]. Studies have reported a high application of photocatalytic activation of nanomaterials due to the generation of superoxide radicals that intensify the degradation process. Various nanomaterials, nanocomposites and bionanomaterials were used in the aforementioned studies. However, investigations were not focused on assessing the toxic effect of nanomaterials on humans and the environment. For example, iron oxide nanoparticles have been observed to exhibit a mutagenic effect [191]. Therefore, future investigations should focus more on the fate and effects of these materials on the ecosystem.
A special group of pollutants consists of microplastics, and extensive research into physical, chemical and biological techniques of environmental remediation is being carried out [19,192]. Since biological methods of remediation are low-efficiency and still in the development phase, more attention will be paid to chemical methods. Chemical microplastic remediation techniques such as adsorption [193], electrocoagulation [194,195,196,197], AOTs [198] and photocatalysis [199,200] have been investigated. Electrocoagulation leads to the formation of microcoagulants and loss of stability of suspended microplastic particles due to coagulation. It is more effective than classic coagulation, and the operation time and the consumption of chemicals are reduced. Investigations have shown that photocatalysis is very effective in the degradation of microplastics, and the future of ecological application is promised by biophotocatalytic materials. Along with the development of techniques for remediation of the environment polluted by microplastics, the main strategy is to introduce restrictions on its use in production.
4.3. Recent Knowledge on Physical Remediation of Contaminated Soil and Sediment
As already mentioned, physical remediation is applicable exclusively for contaminated soil and sediment. Techniques such as separation, capping/encapsulation and soil mixing do not actually represent remediation because their purpose is to prevent the spread of contamination. In contrast to the mentioned techniques, thermal treatment represents remediation; therefore, Table 14 shows the results of thermal remediation investigations of contaminated soil and sediment.

Table 14.
Results of thermal remediation investigations of contaminated soil and sediment.
Removal of PAH compounds (phenanthrene, pyrene, benzopyrene) from artificially polluted soil by thermal remediation, by heating the soil in a quartz tube, was carried out by Liu et al. [201]. The concentration of PAH compounds in the contaminated soil was 1.2 mg/kg. The results showed that more benzopyrene was retained in the soil due to its higher thermal stability. Furthermore, a slight decrease of PAH compounds was observed at temperatures up to 100 °C. When the temperature was set to 200 °C, the content of benzopyrene decreased by 23%, while the content of phenanthrene and pyrene decreased by only 1%. Furthermore, when the temperature was set to 400 °C, the residual amount of all three PAH compounds in the soil was 0.5–1%, because the boiling point of PAHs is approximately around 400 °C, and there was no significant difference even at 800 °C. However, PAH compounds were removed in the following order: phenanthrene, pyrene and benzopyrene, depending on the molecular structure, boiling point and number of rings. Heat treatment at 400 °C caused significant changes in the composition of organic matter, because pyrolysis occurred, and thus the reduction of organic matter. This was the reason for the change in the physical properties and bioavailability of organic matter in the treated soil by thermal remediation. Sörengård et al. [202] carried out thermal remediation of artificially polluted soil with perfluoroalkyl and polyfluoroalkyl substances (PFAS) with initial concentrations of 4 mg/kg and 0.025 mg/kg. The soil was subjected to thermal remediation at temperatures from 150 °C to 550 °C for 75 min. The concentrations of perfluoroalkyl and polyfluoroalkyl decreased by 43% and 79%, respectively, at a temperature of 350 °C. More than 99% of PFAS were removed at temperatures of 450 °C and 550 °C. In a laboratory-experimental study, Bulmău et al. [203] conducted thermal remediation of sampled soil from a highly polluted site as a result of anthropogenic activities associated with petroleum refining. The initial concentrations of benzoanthracene, benzopyrene, pyrene and total PAHs were 0.257 mg/kg, 0.050 mg/kg, 0.089 mg/kg and 0.989 mg/kg, respectively. After 30 min of thermal remediation at 650 °C, the decontaminated soil had a concentration of benzoanthracene in the amount of 0.020 mg/kg, benzopyrene an amount of 0.002 mg/kg, pyrene an amount of 0.014 mg/kg and total PAHs an amount of 0.201 mg/kg; expressed as a percentage, the values were 92.9%, 96%, 84.3% and 79.7%, respectively. Maa et al. [204] collected soil samples from agricultural land near a mercury mining area in Tongren, Guizhou Province, China. The heat treatment was carried out in a laboratory rotary furnace with a mercury vapor treatment system. The mercury removal was greatly improved by the addition of citric acid. The concentration of mercury in the soil was reduced from 134 mg/kg to 1.1 mg/kg when the soil was treated at 400 °C for 60 min. Hydrocarbon-contaminated marine sediment of Augusta Bay, Italy, with an initial concentration of 1370 mg/kg was thermally treated by Falciglia et al. [205]. An electric furnace with a gas outlet connected to a VOC capture system made of granular activated carbon was used. The results revealed that temperatures ranging from 200 °C to 280 °C resulted in an overall hydrocarbon removal efficiency of 75% to 85% within 10 min. The maximum removal efficiency was 89% at 200 °C for 30 min. Thermal remediation is suitable for soil contaminated with substances whose heat treatment does not produce toxic gaseous compounds dangerous to the environment. This type of processing can be performed in situ. However, in case of formation of toxic gaseous products, it is necessary to carry out remediation in an ex situ mode with convenient collection of toxic gaseous products, which increases the cost of performing the procedure [67].
5. Overview of the Applicability and Selection of Appropriate Technique for the Remediation of the Polluted Environment
Various techniques for the remediation of polluted environments have been investigated with the aim of mitigating the harmful effects and consequences for the ecosystem. It is well known that remediation is a challenging process due to the complexity of the performance and financial expenses. Therefore, the evaluation of efficiency in terms of energy requirements, flexibility in processing different pollutants and the level of generation of waste as a by-product at the end of the treatment process is very critical in the development and application of any treatment technique.
Most techniques are applicable in situ and ex situ, such as phytoremediation, bioremediation, electrochemical remediation, solidification/stabilization, use of permeable reactive barriers, AOTs, photocatalysis, nanoremediation and physical soil treatment techniques. However, chemical soil leaching is carried out ex situ, which makes the implementation even more expensive. In general, in situ remediation is more cost competitive and preferable than ex situ performance.
Physical remediation techniques are relatively highly effective. They are applicable for small areas of highly polluted soil and are often destructive and economically very demanding. Specifically, these techniques require a large amount of manpower and material resources. For example, soil excavation is considered the most expensive remediation approach. In addition to the above requirements, physical remediation techniques are considered the most effective as they require minimal implementation time compared to other techniques.
Chemical remediation techniques are applicable to all polluted environmental compartments. They are mostly fast, simple, easy to use and relatively economical, and their effectiveness depends on the type of pollutant. However, they are often not environmentally acceptable due to the application and generation of additional pollutants in the environment. Compared to physical remediation, they are considered more economical due to the relatively small doses of chemical agents used. For example, the stabilization and retention of metals in soil is suitable for application in large areas, with the condition of metal stability.
Physical remediation techniques such as thermal remediation and vitrification are generally not applicable on agricultural land due to their destructive nature. On the other hand, certain chemical techniques such as sorption and biological techniques can be applied to large agricultural areas due to their non-destructive nature. Thus, the remediation of agricultural soil requires careful application of thermal desorption, since too high a temperature can cause thermal decomposition of organic substances in the soil and make the soil unsuitable for agricultural purposes.
Nowadays, sorption is one of the most studied techniques due to the simplicity of performance, efficiency and economic profitability using natural sorbents. In addition, waste materials (by-products) of an industrial process are often used for sorption purposes, which realizes the principle of circular economy. Since sorption is sometimes a reversible process, it additionally contributes to the multipurpose use of materials for remediation purposes. Investigations are systematically carried out to find new materials such as nanomaterials, biochar and other natural modified materials that would meet the requirements of high efficiency and optimal cost. Therefore, environmentally friendly sorbents can be used as fillers for PRB and in situ immobilization of pollutants. Techniques using new materials such as sorption on modified sorbents and photocatalysis have shown significant efficiency in wastewater treatment.
The removal of organic pollutants is very challenging, especially with the use of biological techniques. AOTs have proven to be superior for the removal of emerging environmental pollutants compared to classical chemical techniques, especially biological ones. However, their application is quite limited due to high operating costs. To reduce costs, AOTs are coupled with photocatalysis. For example, energy costs are reduced by using photocatalysts that can be activated by a wider part of the light spectrum.
Compared to physical and chemical remediation techniques, biological remediation is a relatively more economical but environmentally friendly in situ non-destructive safe “green” remediation technique. It shows advantages in terms of field applicability, cost and environmental performance safety. The main limitations of biological remediation are the long implementation period and its applicability for slightly to moderately contaminated soils.
More recent research is being conducted with the aim of finding indigenous plants that have the ability to remove pollutants. In addition, the development of bioengineering leads to the improvement of the applicability of biological remediation using modified plant species and specific microorganisms resistant to the toxicity of pollutants. This could contribute to compensating for the shortcomings of biological remediation by shortening the remediation time.
Ultimately, all techniques have advantages and limitations. It is necessary to take into account all the factors that affect the feasibility of the remediation process when choosing the best technique. Finally, the cost analysis is the basis for making a decision on the remediation of the polluted media.
6. Future Directions and Conclusions
This review compares the effectiveness of different remediation techniques applied to the polluted environment. Their application is an extremely complex and demanding task. Specifically, the impact of environmental pollution with different types of pollutants directly affects the living world as a result of the destruction of natural resources. Therefore, in most countries nowadays, the level of awareness of the human population about environmental protection is increasing as a result of increasing information and the efforts of scientists and associations whose goal is to preserve the environment as a whole. In addition, stricter legal regulations are being introduced for the emission of pollutants in order to reduce their emission into the environment. Remediation of the polluted environment is becoming an increasing challenge due to the complexity of the pollutants’ composition in the environment. The various applied remediation techniques, physical, biological and chemical, have their advantages and limitations in terms of efficiency, feasibility, flexibility and energy requirements. Sometimes low processing efficiency and high operating costs require finding an alternative technique that will meet the requirements of efficiency and remediation time.
However, the use of individual techniques often does not meet the requirements of high efficiency in a short period of time, and a combination of two or more techniques consecutively or simultaneously is recommended. A solution could be found by using a hybrid system that combines two or more physical, biological or chemical treatment processes. This method of processing should be more efficient, especially for removing the complex composition of different types of pollutants. For example, physical containment of pollutants combined with biological remediation appears to be a good approach to environmental remediation. Furthermore, phytoremediation can be effectively used in combination with bioremediation. Thus, bioremediation can effectively reduce the bioavailability of organic and inorganic pollutants in the polluted medium. Then, phytoremediation can be used to remove lower residual concentrations of pollutants. The application of genetic engineering is promising in the commercialization of phytoremediation and bioremediation. Specifically, the use of genetically modified plants and microorganisms in accordance with the requirements of the contaminated medium can be a very effective tool for more efficient remediation. Although genetic engineering contributes to the development of bioremediation, there are gaps in knowledge about the fate and impact of genetically modified microorganisms in the environment. Accordingly, additional efforts are needed to reveal their potential negative effects on human health and the balance of the biological ecosystem. Also, the application of nanoremediation requires systematic research on the fate and potential toxicity of nanomaterials on the environment. In the future, the integration of nanoremediation with other techniques should be an imperative for sustainable green remediation. Synthesis of bionanomaterials should be an economical and environmentally friendly solution for sustainable in situ application in the field.
Therefore, the strategy for the development of future hybrid remediation techniques is to research green, environmentally friendly in situ techniques based on high efficiency and rapid remediation. However, most published investigations have been conducted at the laboratory level. Therefore, there is a need for further systematic research on this issue on real samples and in the field in order to understand the suitability, sustainability and applicability of these techniques for practical purposes. It would also be desirable to examine the synergistic effect of combined remediation techniques.
The proposed hybrid method of remediation could lead to the development of innovative techniques. Therefore, the following recommendations and future guidelines for improving environmental remediation are proposed: (I) simultaneous or consecutive combination of different remediation techniques, (II) confirmation of the applicability of hybrid remediation techniques on real samples and in the field, (III) synthesis of new “green” materials without affecting the environment and (IV) in addition to environmental requirements, consider the applicability costs of hybrid remediation techniques.
Author Contributions
Conceptualization, investigation, methodology, formal analysis, writing—original draft preparation, writing—review and editing, M.U. and A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the bilateral Croatian–Slovenian project “Natural modified sorbents as materials for remediation of mercury contaminated environment” (2020–2023), founded by the Croatian Ministry of Science and Education and the Slovenian Research Agency (ARRS).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhavya, G.; Belorkar, S.A.; Mythili, R.; Geetha, N.; Shetty, H.S.; Udikeri, S.S.; Jogaiah, S. Remediation of emerging environmental pollutants: A review based on advances in the uses of eco-friendly biofabricated nanomaterials. Chemosphere 2021, 275, 129975. [Google Scholar] [CrossRef]
- Aryal, N.; Wood, J.; Rijal, I.; Deng, D.; Jha, M.K.; Ofori-Boadu, A. Fate of environmental pollutants: A review. Water Environ. Res. 2020, 92, 1587–1594. [Google Scholar] [CrossRef]
- Özkara, A.; Akyıl, D. Environmental Pollution and Pollutants on the Ecosystem: A Review. Türk Bilimsel Derlemeler Dergisi 2018, 11, 11–17. Available online: https://derleme.gen.tr/index.php/derleme/article/view/253 (accessed on 5 November 2023).
- Hussain, A.; Rehman, F.; Rafeeq, H.; Waqas, M.; Asghar, A.; Afsheen, N.; Rahdar, A.; Bilal, M.; Iqbal, H.M.N. In-situ, Ex-situ, and nano-remediation strategies to treat polluted soil, water, and air—A review. Chemosphere 2022, 289, 133252. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Kamyab, H.; Rajasimman, M.; Rajamohan, N.; Ngo, G.H.; Xia, C. Physico-chemical and biological remediation techniques for the elimination of endocrine-disrupting hazardous chemicals. Environ. Res. 2023, 232, 116363. [Google Scholar] [CrossRef]
- Wei, Z.; Le, Q.V.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.Á.; Segura, A. Past, present and future trends in the remediation of heavy-metal contaminated soil—Remediation techniques applied in real soil-contamination events. Heliyon 2023, 9, e16692. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Sheoran, K.; Siwal, S.S.; Kapoor, D.; Singh, N.; Saini, A.K.; Alsanie, W.F.; Thakur, V.K. Air Pollutants Removal Using Biofiltration Technique: A Challenge at the Frontiers of Sustainable Environment. ACS Eng. Au. 2022, 2, 378–396. [Google Scholar] [CrossRef]
- Qadeer, S.; Anjum, M.; Khalid, A. Emerging Environmental Pollutants: Issues and Challenges. In Environmental Contamination and Remediation; Anwar, Y., Rehman Hakeem, K., Alharby, H.F., Alghamdi, K.M., Eds.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2018; pp. 1–40. [Google Scholar]
- Doyle, S.; Meade, E.; Fowley, C.; Garvey, M. A Comprehensive Review of Current Environmental Pollutants of Pharmaceutical, Agricultural and Industrial Origin. Eur. Exp. Biol. 2020, 10, 2. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Quarmby, S.; Santos, G.; Mathias, M. Air Quality Strategies and Technologies: A Rapid Review of the International Evidence. Sustainability 2019, 11, 2757. [Google Scholar] [CrossRef]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.S.; Wu, Y.S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef]
- Kim, Y.N.; Yoon, J.H.; Kim, K.H. Microplastic contamination in soil environment—A review. Soil. Sci. Ann. 2020, 71, 300–308. [Google Scholar] [CrossRef]
- Anik, A.H.; Hossain, S.; Alam, M.; Sultan, M.B.; Tanvir Hasnine, M.D.; Rahman, M.M. Microplastics pollution: A comprehensive review on the sources, fates, effects, and potential remediation. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100530. [Google Scholar] [CrossRef]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef]
- Menéndez-Pedriza, A.; Jaumot, J. Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects. Toxics 2020, 8, 40. [Google Scholar] [CrossRef]
- Saxena, N.; Merajul Islam, M.; Baliyan, S.; Sharma, D.A. Comprehensive Review on Removal of Environmental Pollution using Surfactant Based Remediation Process. RSC Sustain. 2023. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Arora, J.; Ranjan, A.; Chauhan, A.; Biswas, R.; Rajput, V.D.; Sushkova, S.; Mandzhieva, S.; Minkina, T.; Jindal, T. Surfactant pollution, an emerging threat to ecosystem: Approaches for effective bacterial degradation. J. Appl. Microbiol. 2022, 133, 1229–1244. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Bilal, M.; Hussain, T.; Sher, F.; Rizwan, K. Surfactants-based remediation as an effective approach for removal of environmental pollutants-A review. J. Mol. Liq. 2020, 318, 113960. [Google Scholar] [CrossRef]
- Miettinen, M.; Khan, S.A. Pharmaceutical pollution: A weakly regulated global environmental risk. Rev. Eur. Comp. Int. Environ. Law 2022, 31, 75–88. [Google Scholar] [CrossRef]
- Kümmerer, K. Pharmaceuticals in the Environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Ahn, C.; Jeung, E.B. Endocrine-Disrupting Chemicals and Disease Endpoints. Int. J. Mol. Sci. 2023, 24, 5342. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Stiefel, C.; Stintzing, F. Endocrine-active and endocrine-disrupting compounds in food—Occurrence, formation and relevance. NFS 2023, 31, 57–92. [Google Scholar] [CrossRef]
- Razzaq, R. Phytoremediation: An Environmental Friendly Technique—A Review. J. Environ. Anal. Chem. 2017, 4, 195. [Google Scholar] [CrossRef]
- Teng, D.; Mao, K.; Ali, W.; Xu, G.; Huang, G.; Niazi, N.K.; Fenga, X.; Zhang, H. Describing the toxicity and sources and the remediation technologies for mercury-contaminated soil. RSC Adv. 2020, 10, 23221–23232. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Ifon, B.E.; Togbé, A.; Tometin, L.A.S.; Suanon, F.; Yessoufou, A. Metal-Contaminated Soil Remediation. In Metals in Soil—Contamination and Remediation; Begum, Z.A., Rahman, I.M.M., Hasegawa, H., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- He, F.; Gao, J.; Pierce, E.; Strong, P.J.; Wang, H.; Liang, L. In situ remediation technologies for mercury-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 8124–8147. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F.; Shanthakumar, S. Remediation of Metal/Metalloid-Polluted Soils: A Short Review. Appl. Sci. 2021, 11, 4134. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef]
- Ankit; Bauddh, K.; Korstad, J. Phycoremediation: Use of Algae to Sequester Heavy Metals. Hydrobiology 2022, 1, 288–303. [Google Scholar] [CrossRef]
- Lee, B.X.Y.; Hadibarata, T.; Yuniarto, A. Phytoremediation Mechanisms in Air Pollution Control: A Review. Water Air Soil. Pollut. 2020, 231, 437. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Amin, L.; Sidik, N.M. Genetically engineered organisms for bioremediation of pollutants in contaminated sites. Chin. Sci. Bull. 2014, 59, 703–714. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Ramesh, B.; Srinivasan, S. Removal of toxic heavy metals using genetically engineered microbes: Molecular tools, risk assessment and management strategies. Chemosphere 2022, 298, 134341. [Google Scholar] [CrossRef]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M.N. Genetically engineered microorganisms for environmental remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef]
- Sharma, P.; Bano, A.; Singh, S.P.; Dubey, N.K.; Chandra, R.; Iqbal, H.M.N. Recent advancements in microbial-assisted remediation strategies for toxic contaminants. CLCE 2022, 2, 100020. [Google Scholar] [CrossRef]
- Sharma, I. Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In Trace Metals in the Environment—New Approaches and Recent Advances; Murillo-Tovar, M.A., Saldarriaga-Noreña, H., Saeid, A., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Chu, C.Y.; Ko, T.H. Evaluation of Acid Leaching on the Removal of Heavy Metals and Soil Fertility in Contaminated Soil. J. Chem. 2018, 2018, 5036581. [Google Scholar] [CrossRef]
- Saint-Fort, R. Surfactants and Their Applications for Remediation of Hydrophobic Organic Contaminants in Soils. In Surfactants and Detegents; Dutta, A.K., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- Bazant, M.Z. Electrokinetics meets electrohydrodynamics. J. Fluid. Mech. 2015, 782, 1–4. [Google Scholar] [CrossRef]
- Lukman, S.; Essa, M.H.; Mu’azu, N.D.; Bukhari, A. Coupled Electrokinetics-Adsorption Technique for Simultaneous Removal of Heavy Metals and Organics from Saline-Sodic Soil. Sci. World J. 2013, 2013, 346910. [Google Scholar] [CrossRef]
- Shenbagavalli, S.; Mahimairaja, S. Electro kinetic remediation of contaminated habitats. Afr. J. Environ. Sci. Technol. 2010, 4, 13. Available online: https://www.ajol.info/index.php/ajest/article/view/71410 (accessed on 5 November 2023).
- Yan, L.; Zhao, W. Application Research on Soil and Water Environmental Pollution Remediation Technology. Earth Environ. Sci. 2019, 384, 012212. [Google Scholar] [CrossRef]
- Camenzuli, D.; Gore, D. Immobilization and Encapsulation of Contaminants Using Silica Treatments: A Review. Remediation 2013, 24, 49–67. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Sulaymon, A.H.; Khaliefa, Q.M. A review of permeable reactive barrier as passive sustainable technology for groundwater remediation. Int. J. Environ. Sci. Technol. 2018, 15, 1123–1138. [Google Scholar] [CrossRef]
- Al-Hashimi, O.; Hashim, K.; Loffill, E.; Marolt Čebašek, T.; Nakouti, I.; Faisal, A.A.H.; Al-Ansari, N. A Comprehensive Review for Groundwater Contamination and Remediation: Occurrence, Migration and Adsorption Modelling. Molecules 2021, 26, 5913. [Google Scholar] [CrossRef]
- Jovanović, T.; Petrović, M.; Kostić, M.; Bojić, D.; Bojić, A. Chemical remediation technologies. FU Phys. Chem. Technol. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Copty, N.K.; Ali, G.A.M.; Bashir, M.J.K.; Abu Amr, S.S.; Abushammala, M.F.M.; Nassani, D.E.; Al Maskari, T. An overview of chemical oxidation-based remediation technologies for non-aqueous phase liquids removal from soil. Global Nest J. 2022, 24, 74–86. [Google Scholar] [CrossRef]
- Yoo, J.C.; Lee, C.; Lee, J.S.; Baek, K. Simultaneous application of chemical oxidation and extraction processes is effective at remediating soil Co-contaminated with petroleum and heavy metals. J. Environ. Manag. 2017, 186, 314–319. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552. [Google Scholar] [CrossRef]
- Litter, M.I.; Quici, N. Photochemical Advanced Oxidation Processes for Water and Wastewater Treatment. Recent. Pat. Eng. 2010, 4, 217–241. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Heyst, B.V. An overview of photocatalyst immobilization methods for air pollution remediation. Chem. Eng. J. 2019, 391, 123490. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Ali, G.A.M.; Bashir, M.J.K.; Copty, N.K.; Amr, S.S.A.; Abushammala, M.F.M.; Al Maskari, T. Recent Advances of Nanoremediation Technologies for Soil and Groundwater Remediation: A Review. Water 2021, 13, 2186. [Google Scholar] [CrossRef]
- Kumar, L.; Ragunathan, V.; Chugh, M.; Bharadvaja, N. Nanomaterials for remediation of contaminants: A review. Environ. Chem. Lett. 2021, 19, 3139–3163. [Google Scholar] [CrossRef]
- Roy, A.; Sharma, A.; Yadav, S.; Jule, L.T.; Krishnaraj, R. Nanomaterials for Remediation of Environmental Pollutants. Bioinorg. Chem. Appl. 2021, 2021, 1764647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dong, F.; Feng, Y.; Li, Y.; Dong, Y. Thermal desorption for remediation of contaminated soil: A review. Chemosphere 2019, 221, 841–855. [Google Scholar] [CrossRef]
- Saeedi, M.; Li, L.Y.; Grace, J.R. Effect of Co-existing Heavy Metals and Natural Organic Matter on Sorption/Desorption of Polycyclic Aromatic Hydrocarbons in Soil: A Review. J. Pollut. 2020, 6, 1–24. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Dellisanti, F.; Rossi, P.L.; Valdre, G. Infield remediation of tons of heavy metal-rich waste by Joule heating vitrification. Int. J. Miner. Process. 2009, 93, 239–245. [Google Scholar] [CrossRef]
- McCloy, J.S.; Schuller, S. Vitrification of wastes: From unwanted to controlled crystallization, a review. C. R. Géosci. 2022, 354, 121–160. [Google Scholar] [CrossRef]
- Trifunović, V. Vitrification as a method of soil remediation. Zastita Materijala 2021, 62, 166–179. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, M. Phytoremediation Using Willow in Industrial Contaminated Soil. Sustainability 2022, 14, 8449. [Google Scholar] [CrossRef]
- Panchenko, L.; Muratova, A.; Dubrovskaya, E.; Golubev, S.; Turkovskaya, O. Natural and Technical Phytoremediation of Oil-Contaminated Soil. Life 2023, 13, 177. [Google Scholar] [CrossRef]
- Cheng, P.C.; Lin, Y.C.; Lin, M.S.; Lin, S.L.; Hsiao, Y.H.; Huang, C.Y.; Tu, P.C.; Cheng, S.F. Phytoremediation Efficiency of Weathered Petroleum-Contaminated Soils by Vetiveria zizanioides and Cymbopogon nardus itle. Eng. Proc. 2023, 38, 63. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Z.; Hu, K.; Wu, Q.T. Phytoextraction and Migration Patterns of Cadmium in Contaminated Soils by Pennisetum hybridum. Plants 2023, 12, 2321. [Google Scholar] [CrossRef]
- Aurangzeb, N.; Nisa, S.; Bibi, Y.; Javed, F.; Hussain, F. Phytoremediation potential of aquatic herbs from steel foundry effluent. Braz. J. Chem. Eng. 2014, 31, 881–886. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Zhang, J.; Wan, Y. Phytoremediation Competence of Composite Heavy-Metal-Contaminated Sediments by Intercropping Myriophyllum spicatum L. with Two Species of Plants. Int. J. Environ. Res. Public Health 2023, 20, 3185. [Google Scholar] [CrossRef] [PubMed]
- Davardoost, F.; Kahforoushan, D. Modeling the Dispersion of Volatile Organic Compounds in Indoor Environment. Chem. Eng. Technol. 2019, 42, 549–559. [Google Scholar] [CrossRef]
- Sharma, S.; Bakht, A.; Jahanzaib, M.; Lee, H.; Park, D. Evaluation of the Effectiveness of Common Indoor Plants in Improving the Indoor Air Quality of Studio Apartments. Atmosphere 2022, 13, 1863. [Google Scholar] [CrossRef]
- Ibrahim, I.Z.; Chong, W.T.; Yusoff, S.; Wang, C.T.; Xiang, X.; Muzammil, W.K. Evaluation of common indoor air pollutant reduction by a botanical indoor air biofilter system. Indoor Built Environ. 2021, 30, 7–21. [Google Scholar] [CrossRef]
- Parseha, I.; Teirib, H.; Hajizadehc, Y.; Ebrahimpour, K. Phytoremediation of benzene vapors from indoor air by Schefflera arboricola and Spathiphyllum wallisii plants. Atmos. Pollut. Res. 2018, 9, 1083–1087. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, T.; Wang, P.; Lin, Y.; Zheng, R.; Zhao, Y.; Xu, B. Fundamentals of Ornamental Plants in Removing Benzene in Indoor Air. Atmosphere 2019, 10, 221. [Google Scholar] [CrossRef]
- Khaksar, G.; Treesubsuntorn, C.; Thiravetyan, P. Effect of endophytic Bacillus cereus ERBP inoculation into nonnative host: Potentials and challenges for airborne formaldehyde removal. Plant Physiol. Biochem. 2016, 107, 326–336. [Google Scholar] [CrossRef]
- Zhang, W.K.; Wang, B.; Niu, X. Adsorption Capacity of the Air Particulate Matter in Urban Landscape Plants in Different Polluted Regions of Beijing. Huan Jing Ke Xue 2015, 36, 2381–2388. Available online: https://pubmed.ncbi.nlm.nih.gov/26489302/ (accessed on 5 November 2023). [PubMed]
- Brilli, F.; Fares, S.; Ghirardo, A.; de Visser, P.; Calatayud, V.; Munoz, A.; Annesi-Maesano, I.; Sebastiani, F.; Alivernini, A.; Varriale, V.; et al. Plants for Sustainable Improvement of Indoor Air Quality. Trends Plant Sci. 2018, 23, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Y.; Zhao, Y.Y.; Liang, H.X.; Su, Y.H. Formaldehyde removal in the air by six plant systems with or without rhizosphere microorganisms. Int. J. Phytoremediat. 2019, 21, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.S.; Xu, W.; Mo, L.; Heal, M.R.; Xu, X.W.; Yu, X.X. Quantifying particulate matter accumulated on leaves by 17 species of urban trees in Beijing, China. Environ. Sci. Pollut. Res. 2018, 25, 12545–12556. [Google Scholar] [CrossRef] [PubMed]
- Pettit, T.; Irga, P.J.; Torpy, F.R. The in situ pilot-scale phytoremediation of airborne VOCs and particulate matter with an active green wall. Air Qual. Atmos. Health 2018, 12, 33–44. [Google Scholar] [CrossRef]
- Lu, S.; Yang, X.; Li, S.; Chen, B.; Jiang, Y.; Wang, D.; Xu, L. Effects of plant leaf surface and different pollution levels on PM2.5 adsorption capacity. Urban For. Urban Green. 2018, 34, 64–70. [Google Scholar] [CrossRef]
- Jeong, N.R.; Kim, K.J.; Yoon, J.H.; Han, S.W.; You, S. Evaluation on the potential of 18 species of indoor plants to reduce particulate matter. J. People Plants Environ. 2020, 23, 637–646. [Google Scholar] [CrossRef]
- Ravindra, K.; Kaur-Sidhu, M.; Mor, S. Air pollution in rural households due to solid biomass fuel use and its health impacts. In Indoor Environmental Quality; Sharma, A., Goyal, R., Mittal, R., Eds.; Lecture Notes in Civil Engineering; Springer: Singapore, 2020; Volume 60, pp. 27–33. [Google Scholar] [CrossRef]
- Mozaffar, A.; Zhang, Y.L. Atmospheric volatile organic compounds (VOCs) in China: A review. Curr. Pollut. Rep. 2020, 6, 250–263. [Google Scholar] [CrossRef]
- Ravindra, K. Emission of black carbon from rural households kitchens and assessment of lifetime excess cancer risk in villages of North India. Environ. Int. 2019, 122, 201–212. [Google Scholar] [CrossRef]
- Ravindra, K.; Goyal, A.; Kumar, S.; Aggarwal, A.; Mor, S. Pollen Calendar to depict seasonal periodicities of airborne pollen species in a city situated in Indo-Gangetic plain, India. Atmos. Environ. 2021, 262, 118649. [Google Scholar] [CrossRef]
- Marzuki, I.; Nisaa, K.; Asaf, R.; Athirah, A.; Paena, M.; Susianingsih, E.; Nurhidayah, N.; Kadriah, I.A.K.; Kamaruddin, K.; Sahabuddin, S.; et al. Comparison of Pyrene Biodegradation Using Two Types of Marine Bacterial Isolates. Sustainability 2022, 14, 9890. [Google Scholar] [CrossRef]
- Marzuki, I.; Asaf, R.; Paena, M.; Athirah, A.; Nisaa, K.; Ahmad, R.; Kamaruddin, M. Anthracene and Pyrene Biodegradation Performance of Marine Sponge Symbiont Bacteria Consortium. Molecules 2021, 26, 6851. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, I.; Daris, L.; Nisaa, K.; Emelda, A. The power of biodegradation and bio-adsorption of bacteria symbiont sponges sea on waste contaminated of polycyclic aromatic hydrocarbons and heavy metals. Earth Environ. Sci. 2020, 584, 012013. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Biosequestration of heavy metals by microbially induced calcite precipitation of ureolytic bacteria. Rom. Biotechnol. Lett. 2019, 24, 147–153. [Google Scholar] [CrossRef]
- Soares, P.R.S.; Birolli, W.G.; Ferreira, I.M.; Porto, A.L.M. Biodegradation pathway of the organophosphate pesticides chlorpyrifos, methyl parathion and profenofos by the marine-derived fungus Aspergillus sydowii CBMAI 935 and its poten-tial for methylation reactions of phenolic compounds. Mar. Pollut. Bull. 2021, 166, 112185. [Google Scholar] [CrossRef]
- Holanda, F.H.; Birolli, W.G.; Morais, E.D.S.; Sena, I.S.; Ferreira, A.M.; Faustino, S.M.M.; Solon, L.G.D.S.; Porto, A.L.; Ferreira, I.M. Study of biodegradation of chloramphenicol by endophytic fungi isolated from Bertholletia excelsa (Brazil nuts). Biocatal. Agric. Biotechnol. 2019, 20, 101200. [Google Scholar] [CrossRef]
- Ding, T.; Lin, K.; Yang, B.; Yang, M.; Li, J.; Li, W.; Gan, J. Biodegradation of naproxen by freshwater algae Cymbella sp. and Scenedesmus quadricauda and the comparative toxicity. Bioresour. Technol. 2017, 238, 164–173. [Google Scholar] [CrossRef]
- Mohapatra, B.; Phale, P.S. Microbial degradation of naphthalene and substituted naphthalenes: Metabolic diversity and genomic insight for bioremediation. Front. Bioeng. Biotechnol. 2021, 9, 602445. [Google Scholar] [CrossRef]
- Miri, S.; Rasooli, A.; Brar, S.K.; Rouissi, T.; Martel, R. Biodegradation of p-xylene—A comparison of three psychrophilic Pseudomonas strains through the lens of gene expression. Environ. Sci. Pollut. Res. 2022, 29, 21465–21479. [Google Scholar] [CrossRef]
- Sangkharak, K.; Choonut, A.; Rakkan, T.; Prasertsan, P. The degradation of phenanthrene, pyrene, and fluoranthene and its conversion into medium-chain-length polyhydroxyalkanoate by novel polycyclic aromatic hydrocarbon-degrading bacteria. Curr. Microbiol. 2020, 77, 897–909. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, T.; Peng, O.; Chen, A.; Tie, B.; Shao, J. Responses of microbial community and soil enzyme to heavy metal passivators in cadmium contaminated paddy soils: An in situ field experiment. Int. Biodeterior. Biodegrad. 2021, 164, 105292. [Google Scholar] [CrossRef]
- Mehta, K.; Shukla, A.; Saraf, M. Articulating the exuberant intricacies of bacterial exopolysaccharides to purge environmental pollutants. Heliyon 2021, 7, e08446. [Google Scholar] [CrossRef]
- Gallo, G.; Puopolo, R.; Carbonaro, M.; Maresca, E.; Fiorentino, G. Extremophiles, a nifty tool to face environmental pollution: From exploitation of metabolism to genome engineering. Int. J. Environ. Res. Publ. Health 2021, 18, 5228. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Prakash, D. Novel bioremediation methods. In Waste Management: Concepts, Methodologies, Tools, and Applications; IGI Global: New York, NY, USA, 2020; pp. 1627–1643. [Google Scholar]
- Jaiswal, S.; Shukla, P. Alternative strategies for microbial remediation of pollutants via synthetic biology. Front. Microbiol. 2020, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Shakhreliya, S.; Maurya, R.; Pandey, V.C.; Gohil, N.; Bhattacharjee, G.; Alzahrani, K.J. CRISPR-assisted strategies for futuristic phytoremediation. In Assisted Phytoremediation; Pandey, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–220. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Pant, G.; Garlapati, D.; Agrawal, U.; Prasuna, R.G.; Mathimani, T.; Pugazhendhi, A. Biological approaches practised using genetically engineered microbes for a sustainable environment: A review. J. Hazard. Mater. 2021, 405, 124631. [Google Scholar] [CrossRef]
- Weyens, N.; Thijs, S.; Popek, R.; Witters, N.; Przybysz, A.; Espenshade, J.; Gawronska, H.; Vangronsveld, J.; Gawronski, S.W. The role of plant-microbe interactions and their exploitation for phytoremediation of air pollutants. Int. J. Mol. Sci. 2015, 16, 25576–25604. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tan, M.; Ma, J.; Zhang, S.; Li, G.; Qua, J. Efficient remediation of PAH-metal co-contaminated soil using microbial-plant combination: A greenhouse study. J. Hazard. Mater. 2016, 302, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Marzuki, I.; Rosmiati, R.; Mustafa, A.; Sahabuddin, S.; Tarunamulia, T.; Susianingsih, E.; Hendrajat, E.A.; Sahrijanna, A.; Muslimin, M.; Ratnawati, E.; et al. Potential Utilization of Bacterial Consortium of Symbionts Marine Sponges in Removing Polyaromatic Hydrocarbons and Heavy Metals, Review. Biology 2023, 12, 86. [Google Scholar] [CrossRef]
- Supreeth, M. Enhanced remediation of pollutants by microorganisms–plant combination. Int. J. Environ. Sci. Technol. 2022, 19, 4587–4598. [Google Scholar] [CrossRef]
- Park, S.H.; Koutsospyros, A.; Moon, D.H. Optimization of a High-Pressure Soil Washing System for Emergency Recovery of Heavy Metal-Contaminated Soil. Agriculture 2022, 12, 2054. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Wang, S.; Zhao, S. Improved remediation of co-contaminated soils by heavy metals and PAHs with biosurfactant-enhanced soil washing. Sci. Rep. 2022, 12, 3801. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Nam, K. Development of a potassium-based soil washing solution using response surface methodology for efficient removal of cesium contamination in soil. Chemosphere 2023, 332, 138854. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282, 131092. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lin, Q.; Wang, Y.; Luo, H.; Huang, Z.; Fu, H.; Chen, H.; Xiao, R. The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids. Arab. J. Chem. 2020, 13, 5160–5170. [Google Scholar] [CrossRef]
- Cai, Z.; Sun, Y.; Deng, Y.; Zheng, X.; Sun, S.; Romantschuk, M.; Sinkkonen, A. In situ electrokinetic (EK) remediation of the total and plant available cadmium (Cd) in paddy agricultural soil using low voltage gradients at pilot and full scales. Sci. Total Environ. 2021, 785, 147277. [Google Scholar] [CrossRef] [PubMed]
- Gidudu, B.; Nkhalambayausi Chirwa, E.M. The combined application of a high voltage, low electrode spacing, and biosurfactants enhances the bio-electrokinetic remediation of petroleum contaminated soil. J. Clean. Prod. 2020, 276, 122745. [Google Scholar] [CrossRef]
- Alcántara, M.T.; Gómez, J.; Pazos, M.; Sanromán, M.A. Electrokinetic remediation of PAH mixtures from kaolin. J. Hazard. Mater. 2010, 179, 1156–1160. [Google Scholar] [CrossRef]
- Cong, Y.; Ye, Q.; Wu, Z. Electrokinetic behaviour of chlorinated phenols in soil and their electrochemical degradation. Process Saf. Environ. Prot. 2005, 83, 178–183. [Google Scholar] [CrossRef]
- Abdulredha, M.; Ismael, H.I.; Khalaf, Z.D.; Abood, E.S. Adopting electrocoagulation technology for removing arsenic from contaminated water. Earth Environ. Sci. 2022, 1088, 012020. [Google Scholar] [CrossRef]
- Syam Babu, D.; Nidheesh, P.V.; Suresh Kumar, M. Arsenite removal from aqueous solution by aerated iron electrocoagulation process. Sep. Sci. Technol. 2019, 56, 184–193. [Google Scholar] [CrossRef]
- Goren, A.Y.; Kobya, M.; Oncel, M.S. Arsenite removal from groundwater by aerated electrocoagulation reactor with Al ball electrodes: Human health risk assessment. Chemosphere 2020, 251, 126363. [Google Scholar] [CrossRef] [PubMed]
- Betancor-Abreua, A.; Menaa, V.F.; Gonzáleza, S.; Delgadob, S.; Soutoa, R.M.; Santana, J.J. Design and optimization of an electrocoagulation reactor for fluoride remediation in underground water sources for human consumption. J. Water Process. Eng. 2019, 31, 100865. [Google Scholar] [CrossRef]
- López-Guzmán, M.; Alarcón-Herrera, M.T.; Irigoyen-Campuzano, J.R.; Torres-Castañón, L.A.; Reynoso-Cuevas, L. Simultaneous removal of fluoride and arsenic from well water by electrocoagulation. Sci. Total Environ. 2019, 678, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Anand Reddy, V.; Solanki, C.H.; Kumar, S.; Reddy, K.R.; Du, Y.J. Stabilization/Solidification of Zinc- and Lead-Contaminated Soil Using Limestone Calcined Clay Cement (LC3): An Environmentally Friendly Alternative. Sustainability 2020, 12, 3725. [Google Scholar] [CrossRef]
- Hu, S.; Zhong, L.; Yang, X.; Bai, H.; Ren, B.; Zhao, Y.; Zhang, W.; Ju, X.; Wen, H.; Mao, S.; et al. Synthesis of rare earth tailing-based geopolymer for efficiently immobilizing heavy metals. Constr. Build. Mater. 2020, 254, 119273. [Google Scholar] [CrossRef]
- Aliyu, M.K.; Abd Karim, A.T.; Chan, C.M.; Oyekanmi, A.A.; Hossain, K.; Ismail, N. Mobility of copper and its micro-structure characteristics in contaminated river sediment through stabilization by using cement and rice husk ash. Water Environ. J. 2020, 34, 229–238. [Google Scholar] [CrossRef]
- Li, J.S.; Chen, L.; Zhan, B.; Wang, L.; Poon, C.S.; Tsang, D.C.W. Sustainable stabilization/solidification of arsenic-containing soil by blast slag and cement blends. Chemosphere 2021, 271, 129868. [Google Scholar] [CrossRef]
- Lee, S.H.; Jo, H.Y.; Yun, S.T.; Lee, Y.J. Evaluation of factors affecting performance of a zeolitic rock barrier to remove zinc from water. J. Hazard. Mater. 2010, 175, 224–234. [Google Scholar] [CrossRef]
- Jun, D.; Yongsheng, Z.; Weihong, Z.; Mei, H. Laboratory study on sequenced permeable reactive barrier remediation for landfill leachate-contaminated groundwater. J. Hazard. Mater. 2009, 161, 224–230. [Google Scholar] [CrossRef]
- Ludwig, R.D.; McGregor, R.G.; Blowes, D.W.; Benner, S.G.; Mountjoy, K. A permeable reactive barrier for treatment of heavy metals. Ground Water 2002, 40, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Qi, L.; Bai, Y.; Yin, L.; Li, L.; Zhang, W. Geochemical stability of zero-valent iron modified raw wheat straw innovatively applicated to in situ permeable reactive barrier: N2 selectivity and long-term denitrification. Ecotoxicol. Environ. Saf. 2021, 224, 112649. [Google Scholar] [CrossRef]
- Hernández-García, F.A.; Torres-Delgado, G.; Castanedo-Pérez, R.; Zelaya-Ángel, O. Gaseous benzene degradation by photocatalysis using ZnO+Zn2TiO4 thin films obtained by sol-gel process. Environ. Sci. Pollut. Res. 2016, 23, 13191–13199. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kandori, Y.; Sato, K.; Fukumoto, M. Fabrication of Titanium Dioxide Photocatalyst Coatings by Cold Spray. J. Solid Mech. Mater. Eng. 2009, 3, 210–216. [Google Scholar] [CrossRef]
- Hernández-García, F.A.; Torres-Delgado, G.; Castanedo-Pérez, R.; Zelaya-Ángel, O. Photodegradation of gaseous C6H6 using CdO + CdTiO3 and TiO2 thin films obtained by sol–gel technique. J. Photochem. Photobiol. A Chem. 2015, 310, 52–59. [Google Scholar] [CrossRef]
- Mehrizadeh, H.; Niaei, A.; Tseng, H.H.; Salari, D.; Khataee, A. Synthesis of ZnFe2O4 nanoparticles for photocatalytic removal of toluene from gas phase in the annular reactor. J. Photochem. Photobiol. A Chem. 2017, 332, 188–195. [Google Scholar] [CrossRef]
- Abidia, M.; Assadia, A.A.; Bouzazaa, A.; Hajjaji, A.; Bessais, B.; Rtimic, S. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuxO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Alsager, O.A.; Alnajrani, M.N.; Abuelizz, H.A.; Aldaghmani, I.A. Removal of antibiotics from water and waste milk by ozonation: Kinetics, byproducts, and antimicrobial activity. Ecotoxicol. Environ. Saf. 2018, 158, 114–122. [Google Scholar] [CrossRef]
- Östman, M.; Björlenius, B.; Fick, J.; Tysklind, M. Effect of full-scale ozonation and pilot-scale granular activated carbon on the removal of biocides, antimycotics and antibiotics in a sewage treatment plant. Sci. Total Environ. 2019, 649, 1117–1123. [Google Scholar] [CrossRef]
- Wang, H.; Mustafa, M.; Yu, G.; Östman, M.; Cheng, Y.; Wang, Y.; Tysklind, M. Oxidation of emerging biocides and antibiotics in wastewater by ozonation and the electro-peroxone process. Chemosphere 2019, 235, 575–585. [Google Scholar] [CrossRef]
- Timm, A.; Borowska, E.; Majewsky, M.; Merel, S.; Zwiener, C.; Bräse, S.; Horn, H. Photolysis of four β-lactam antibiotics under simulated environmental conditions: Degradation, transformation products and antibacterial activity. Sci. Total Environ. 2019, 651, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Lutze, H.V.; Schmidt, T.C. Base-catalyzed hydrolysis and speciation-dependent photolysis of two cephalosporin antibiotics, ceftiofur and cefapirin. Water Res. 2018, 134, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lastre-Acosta, A.M.; Barberato, B.; Parizi, M.P.S.; Teixeira, A.C.S.C. Direct and indirect photolysis of the antibiotic enoxacin: Kinetics of oxidation by reactive photo-induced species and simulations. Environ. Sci. Pollut. Res. 2019, 26, 4337–4347. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Ur Rehman, S.W.; Wang, H.; Yang, H.; Yu, G.; Wang, Y. Pilot-scale evaluation of micropollutant abatements by conventional ozonation, UV/O3, and an electro-peroxone process. Water Res. 2018, 138, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Wardenier, N.; Liu, Z.; Nikiforov, A.; Van Hulle, S.W.H.; Leys, C. Micropollutant elimination by O3, UV and plasma-based AOPs: An evaluation of treatment and energy costs. Chemosphere 2019, 234, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.S.; Gando-Ferreira, L.M.; Quinta-Ferreira, R.M.; Martins, R.C. Removal of sulfamethoxazole and diclofenac from water: Strategies involving O3 and H2O2. Environ. Technol. 2018, 39, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cho, D.W.; Graham, N.J.D.; Hou, D.; Yip, A.C.K.; Khan, E.; Song, H.; Li, Y.; Tsang, D.C.W. Degradation of antibiotics by modified vacuum-UV based processes: Mechanistic consequences of H2O2 and K2S2O8 in the presence of halide ions. Sci. Total Environ. 2019, 664, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, H.; Teymori, M.; Aghapour, A.A.; Jafari, S.J.; Taghipour, S.; Bargeshadi, R. Photodegradation of ceftriaxone in aqueous solution by using UVC and UVC/H2O2 oxidation processes. Appl. Water Sci. 2019, 9, 81. [Google Scholar] [CrossRef]
- Urbano, V.R.; Peres, M.S.; Maniero, M.G.; Guimarães, J.R. Abatement and toxicity reduction of antimicrobials by UV/H2O2 process. J. Environ. Manag. 2017, 193, 439–447. [Google Scholar] [CrossRef]
- Li, W.; Xu, X.; Lyu, B.; Tang, Y.; Zhang, Y.; Chen, F.; Korshin, G. Degradation of typical macrolide antibiotic roxithromycin by hydroxyl radical: Kinetics, products, and toxicity assessment. Environ. Sci. Pollut. Res. 2019, 26, 14570–14582. [Google Scholar] [CrossRef]
- Álvarez, M.A.; Ruidíaz-Martínez, M.; Cruz-Quesada, G.; López-Ramón, M.V.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Mota, A.J. Removal of parabens from water by UV-driven advanced oxidation processes. Chem. Eng. J. 2020, 379, 122334. [Google Scholar] [CrossRef]
- Luu, H.T.; Minh, D.N.; Lee, K. Effects of advanced oxidation of penicillin on biotoxicity, biodegradability and subsequent biological treatment. Appl. Chem. Eng. 2018, 29, 690–695. [Google Scholar] [CrossRef]
- Moradi, M.; Moussavi, G. Investigation of chemical-less UVC/VUV process for advanced oxidation of sulfamethoxazole in aqueous solutions: Evaluation of operational variables and degradation mechanism. Sep. Purif. Technol. 2018, 190, 90–99. [Google Scholar] [CrossRef]
- Türkay, G.K.; Kumbur, H. Investigation of amoxicillin removal from aqueous solution by Fenton and photocatalytic oxidation processes. Kuwait J. Sci. 2019, 46, 85–93. [Google Scholar]
- Salari, M.; Rakhshandehroo, G.R.; Nikoo, M.R. Degradation of ciprofloxacin antibiotic by Homogeneous Fenton oxidation: Hybrid AHP-PROMETHEE method, optimization, biodegradability improvement and identification of oxidized by-products. Chemosphere 2018, 206, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, G.R.; Salari, M.; Nikoo, M.R. Optimization of degradation of ciprofloxacin antibiotic and assessment of degradation products using full factorial experimental design by Fenton homogenous process. Glob. Nest J. 2018, 20, 324–332. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, A. Degradation of ciprofloxacin using Fenton’s oxidation: Effect of operating parameters, identification of oxidized by-products and toxicity assessment. Chemosphere 2018, 193, 1181–1188. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Yu, G.; Xie, S.; Li, C.; Lai, D.; Li, Z.; You, F.; Wang, Y. The synthesis of heterogeneous Fenton-like catalyst using sewage sludge biochar and its application for ciprofloxacin degradation. Sci. Total Environ. 2019, 654, 1284–1292. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, Q.; Kong, Y.; Shen, C.; Hao, H.; Dionysiou, D.D.; Shi, B. Enhanced antibiotic removal through a dual-reaction-center Fenton-like process in 3D graphene based hydrogels. Environ. Sci. Nano 2019, 6, 388–398. [Google Scholar] [CrossRef]
- Hinojosa Guerra, M.M.; Oller Alberola, I.; Malato Rodriguez, S.; Agüera López, A.; Acevedo Merino, A.; Quiroga Alonso, J.M. Oxidation mechanisms of amoxicillin and paracetamol in the photo-Fenton solar process. Water Res. 2019, 156, 232–240. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Raj, S.; Prakash, H.; Fatta-Kassinos, D. Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated wastewater effluents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- Wang, Q.; Pang, W.; Mao, Y.; Sun, Q.; Zhang, P.; Ke, Q.; Yu, H.; Dai, C.; Zhao, M. Study of the degradation of trimethoprim using photo-Fenton oxidation technology. Water 2019, 11, 207. [Google Scholar] [CrossRef]
- Ganie, A.S.; Bano, S.; Khan, N.; Sultana, S.; Rehman, Z.; Rahman, M.M.; Sabir, S.; Coulon, F.; Khan, M.Z. Nanoremediation technologies for sustainable remediation of contaminated environments: Recent advances and challenges. Chemosphere 2021, 275, 130065. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Sarkar, B.; Khan, E.; Alessi, D.S.; Biswas, J.K.; Manjaiah, K.M.; Eguchi, M.; Wu, K.C.W.; Yamauchi, Y.; Ok, Y.S. Nanomaterials for sustainable remediation of chemical contaminants in water and soil. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2611–2660. [Google Scholar] [CrossRef]
- Guerra, F.; Attia, M.; Whitehead, D.; Alexis, F. Nanotechnology for environmental remediation: Materials and applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wang, G.; Peng, C.; Tan, J.; Wan, J.; Sun, P.; Li, Q.; Ji, X.; Zhang, Q.; Wu, Y.; et al. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review. J. Hazard. Mater. 2022, 426, 127993. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Rodríguez-Valdés, E.; Alonso, J.; Baragaño, D.; Gallego, J.R.; Lobo, M.C. Nanoremediation and long-term monitoring of brownfield soil highly polluted with as and Hg. Sci. Total Environ. 2019, 675, 165–175. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, H.; Kumar, A. Remediation of Arsenic by Metal/Metal Oxide Based Nanocomposites/Nanohybrids: Contamination Scenario in Groundwater, Practical Challenges, and Future Perspectives. Sep. Purif. Rev. 2020, 50, 283–314. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Álvarez, M.A.; Alonso, J.; Lobo, M.C. Effectiveness of nanoscale zero-valent iron for the immobilization of Cu and/or Ni in water and soil samples. Sci. Rep. 2020, 10, 15927. [Google Scholar] [CrossRef]
- Mpouras, T.; Polydera, A.; Dermatas, D.; Verdone, N.; Vilardi, G. Multi wall carbon nanotubes application for treatment of Cr(VI)-contaminated groundwater; Modeling of batch & column experiments. Chemosphere 2021, 269, 128749. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, H.; Tian, R.; Li, R.; Xie, Q. Remediation of Trichloroethylene-Contaminated Groundwater by Sulfide-Modified Nanoscale Zero-Valent Iron Supported on Biochar: Investigation of Critical Factors. Water Air Soil. Pollut. 2020, 231, 432. [Google Scholar] [CrossRef]
- Murgueitio, E.; Cumbal, L.; Abril, M.; Izquierdo, A.; Debut, A.; Tinoco, O. Green synthesis of iron nanoparticles: Application on the removal of petroleum oil from contaminated water and soils. J. Nanotechnol. 2018, 2018, 4184769. [Google Scholar] [CrossRef]
- Lin, K.S.; Mdlovu, N.V.; Chen, C.Y.; Chiang, C.L.; Dehvari, K. Degradation of TCE, PCE, and 1,2–DCE DNAPLs in contaminated groundwater using polyethylenimine-modified zero-valent iron nanoparticles. J. Clean. Prod. 2018, 175, 456–466. [Google Scholar] [CrossRef]
- Tian, H.; Liang, Y.; Yang, D.; Sun, Y. Characteristics of PVP–stabilised NZVI and application to dechlorination of soil–sorbed TCE with ionic surfactant. Chemosphere 2020, 239, 124807. [Google Scholar] [CrossRef]
- Kamarudin, N.S.; Jusoh, R.; Jalil, A.A.; Setiabudi, H.D.; Sukor, N.F. Synthesis of silver nanoparticles in green binary solvent for degradation of 2,4-D herbicide: Optimization and kinetic studies. Chem. Eng. Res. Des. 2020, 159, 300–314. [Google Scholar] [CrossRef]
- Kamarudin, N.S.; Jusoh, R.; Setiabudi, H.D.; Jusoh, N.W.C.; Jaafar, N.F.; Sukor, N.F. Cymbopogon nardus mediated synthesis of ag nanoparticles for the photocatalytic degradation of 2,4-dicholorophenoxyacetic acid. Bull. Chem. React. Eng. Catal. 2019, 14, 173–181. [Google Scholar] [CrossRef]
- Abbas, S.; Nasreen, S.; Haroon, A.; Ashraf, M.A. Synthesis of silver and copper nanoparticles from plants and application as adsorbents for naphthalene decontamination. Saudi J. Biol. Sci. 2020, 27, 1016–1023. [Google Scholar] [CrossRef]
- Blundell, S.P.; Owens, G. Evaluation of enhancement techniques for the dechlorination of DDT by nanoscale zero-valent iron. Chemosphere 2021, 264, 128324. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, X.; Mu, M.; Jiang, Y.; Li, X.; Zhang, H.; Ouyang, T. Anatase TiO2@MIL-101(Cr) nanocomposite for photocatalytic degradation of bisphenol A. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124745. [Google Scholar] [CrossRef]
- Huang, Y.; Su, M.; Zhou, Y.; Chen, D.; Xu, Z.; Zhang, H.; Liao, C. LiCl–CN nanotubes ceramic films with highly efficient visible light—Driven photocatalytic active for bisphenol A degradation and efficient regeneration process. Ceram. Int. 2020, 46, 26492–26501. [Google Scholar] [CrossRef]
- Rajendran, K.; Sen, S. Adsorptive removal of carbamazepine using biosynthesized hematite nanoparticles. Environ. Nanotechnol. Monit. Manage. 2018, 9, 122–127. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.; Farag, R.S.; Abdel-Gawad, S.A. Carbamazepine removal from aqueous solution by green synthesis zero-valent iron/Cu nanoparticles with Ficus benjamina leaves’ extract. Int. J. Environ. Res. 2019, 13, 843–852. [Google Scholar] [CrossRef]
- Dissanayake, N.M.; Current, K.M.; Obare, S.O. Mutagenic Effects of Iron Oxide Nanoparticles on Biological Cells. Int. J. Mol. Sci. 2015, 16, 23482–23516. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Xie, Y.; Wang, J. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhu, Y.; Zeng, G.; Zhang, Y.; Yang, Y.; Wen, X.; Chen, M.; Yi, H. Removal of microplastics via drinking water treatment: Current knowledge and future directions. Chemosphere 2020, 251, 126612. [Google Scholar] [CrossRef]
- Kiendrebeogo, K.; Karimi Estahbanati, M.R.; Khosravanipour Mostafazadeh, A.; Drogui, P.; Tyagi, R.D. Treatment of microplastics in water by anodic oxidation: A case study for polystyrene. Environ. Pollut. 2021, 269, 116168. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; Vieira de Melo, J.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of microbeads from wastewater using electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Sin, J.C.; Zeng, H.; Lin, H.; Li, H.; Chai, Y.Y.; Choong, M.K.; Mohamed, A.R. Green synthesis of Fe-ZnO nanoparticles with improved sunlight photocatalytic performance for polyethylene film deterioration and bacterial inactivation. Mater. Sci. Semicond. Process. 2021, 123, 105574. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for micro-plastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Liu, C.; Shi, H.; Wang, C.; Fei, Y.; Han, Z. Thermal Remediation of Soil Contaminated with Polycyclic Aromatic Hydrocarbons: Pollutant Removal Process and Influence on Soil Functionality. Toxics 2022, 10, 474. [Google Scholar] [CrossRef]
- Sörengård, M.; Lindh, A.S.; Ahrens, L. Thermal desorption as a high removal remediation technique for soils contaminated with per- and polyfluoroalkyl substances (PFASs). PLoS ONE 2020, 15, e0234476. [Google Scholar] [CrossRef]
- Bulmău, C.; Mărculescu, C.; Lu, S.; Qi, Z. Analysis of thermal processing applied to contaminated soil for organic pollutants removal. J. Geochem. Explor. 2014, 147, 298–305. [Google Scholar] [CrossRef]
- Maa, F.; Peng, C.; Houc, D.; Wua, B.; Zhanga, Q.; Li, F.; Gua, Q. Citric acid facilitated thermal treatment: An innovative method for the remediation of mercury contaminated soil. J. Hazard. Mater. 2015, 300, 546–552. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Lumia, L.; Giustra, M.G.; Gagliano, E.; Roccaro, P.; Vagliasindi, F.G.A.; Di Bella, G. Remediation of petrol hydrocarbon-contaminated marine sediments by thermal desorption. Chemosphere 2020, 260, 127576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).