Comparison of the Effects of Jackfruit Seed Flour and Jackfruit Seed Starch in the Cookie Manufacturing Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Cookie Preparation
2.3. Analysis Method
2.3.1. Nutrition
2.3.2. Physical Properties
2.3.3. Color Attributes
2.3.4. Water Activity
2.3.5. Oil Holding Capacity and Water Holding Capacity
2.3.6. Hardness
2.3.7. Peroxide Value
2.3.8. Total Polyphenol Content (TPC)
2.3.9. DPPH Radical Scavenging Activity
2.3.10. Sensory Evaluation
2.3.11. Scanning Electron Microscopy (SEM)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Substitution Ratios of Jackfruit Seed Flour or Jackfruit Seed Starch on Cookie Quality
3.1.1. Physical Properties
3.1.2. Color Attributes
3.1.3. Physicochemical Properties
3.1.4. Sensory Evaluation
3.2. Comparison of Jackfruit Seed Starch Cookies and Jackfruit Seed Flour Cookies
3.2.1. Nutrition
3.2.2. Physicochemical Properties and Chemical Composition
3.2.3. Sensory Evaluation
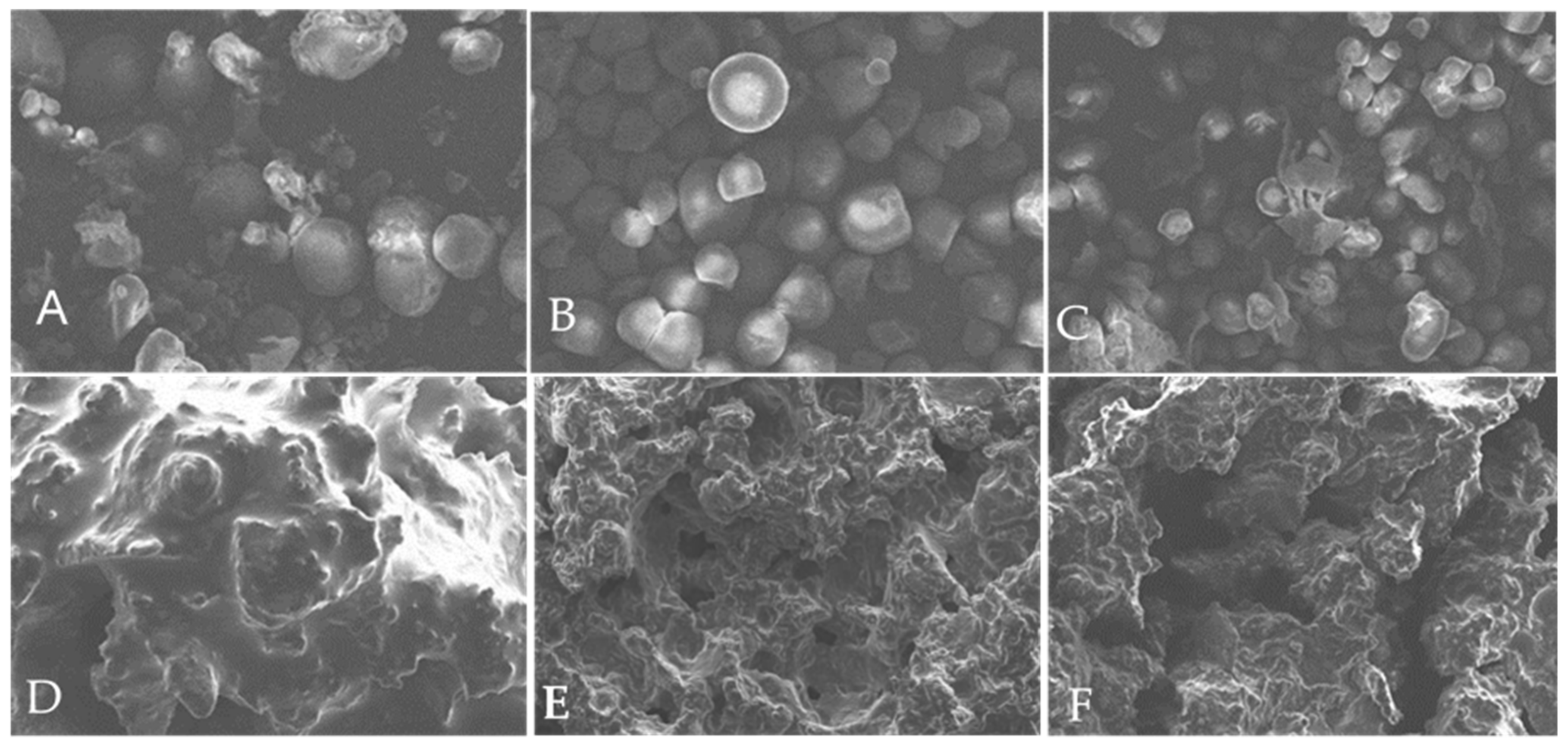
3.2.4. Morphological Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prakash, O.; Kumar, R.; Mishra, A.; Gupta, R. Review Article Artocarpus heterophyllus (Jackfruit): An Overview. Pharmacogn. Rev. 2009, 3, 353–358. [Google Scholar]
- Trejo Rodríguez, I.S.; Alcántara Quintana, L.E.; Algara Suarez, P.; Ruiz Cabrera, M.A.; Grajales Lagunes, A. Physicochemical Properties, Antioxidant Capacity, Prebiotic Activity and Anticancer Potential in Human Cells of Jackfruit (Artocarpus heterophyllus) Seed Flour. Molecules 2021, 26, 4854. [Google Scholar] [CrossRef] [PubMed]
- Aware, C.; Patil, R.; Gaikwad, S.; Yadav, S.; Bapat, V.; Jadhav, J. Evaluation of L-Dopa, Proximate Composition with In Vitro Anti-Inflammatory and Antioxidant Activity of Mucuna macrocarpa Beans: A Future Drug for Parkinson Treatment. Asian Pac. J. Trop. Biomed. 2017, 7, 1097–1106. [Google Scholar] [CrossRef]
- Baliga, M.S.; Shivashankara, A.R.; Haniadka, R.; Dsouza, J.; Bhat, H.P. Phytochemistry, Nutritional and Pharmacological Properties of Artocarpus heterophyllus Lam (Jackfruit): A Review. Food Res. Int. 2011, 44, 1800–1811. [Google Scholar] [CrossRef]
- Oluwamukomi, M.O.; Oluwalana, I.B.; Akinbowale, O.F. Physicochemical and Sensory Properties of Wheat-Cassava Composite Biscuit Enriched with Soy Flour. Afr. J. Food Sci. 2011, 5, 50–56. [Google Scholar]
- Montes, S.d.S.; Rodrigues, L.M.; Cardoso, R.d.C.V.; Camilloto, G.P.; Cruz, R.S. Tapioca and Rice Flour Cookies: Technological, Nutritional and Sensory Properties. Ciênc. Agrotecnol. 2015, 39, 514–522. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Wang, W.; Li, Y. Advanced Properties of Gluten-Free Cookies, Cakes, and Crackers: A Review. Trends Food Sci. Technol. 2020, 103, 200–213. [Google Scholar] [CrossRef]
- Lucini Mas, A.; Brigante, F.I.; Salvucci, E.; Ribotta, P.; Martinez, M.L.; Wunderlin, D.A.; Baroni, M.V. Novel Cookie Formulation with Defatted Sesame Flour: Evaluation of Its Technological and Sensory Properties. Changes in Phenolic Profile, Antioxidant Activity, and Gut Microbiota after Simulated Gastrointestinal Digestion. Food Chem. 2022, 389, 133122. [Google Scholar] [CrossRef]
- Hoang, N.H.; Do, H.H.; Dang, T.H.Y.; Ton, N.M.N.; Tran, T.T.T.; Le, V.V.M. Fiber-Enriched Biscuits Prepared with Enzyme-Treated Corncob Powder: Nutritional Composition, Physical Properties, and Sensory Acceptability. J. Food Process. Preserv. 2022, 46, e16784. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Zhang, W.; Zhang, H.; Guo, L.; Zheng, S.; Du, C. Effect of Black Soybean Flour Particle Size on the Nutritional, Texture and Physicochemical Characteristics of Cookies. LWT 2022, 164, 113649. [Google Scholar] [CrossRef]
- Carpentieri, S.; Larrea-Wachtendorff, D.; Donsì, F.; Ferrari, G. Functionalization of Pasta through the Incorporation of Bioactive Compounds from Agri-Food by-Products: Fundamentals, Opportunities, and Drawbacks. Trends Food Sci. Technol. 2022, 122, 49–65. [Google Scholar] [CrossRef]
- Eke-Ejiofor, J.; Beleyaq, E.A.; Onyenorah, N.I. The Effect of Processing Methods on the Functional and Compositional Properties of Jackfruit Seed Flour. Int. J. Nutr. Food Sci. 2014, 3, 166. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, A.; Dey, J.K.; Dey, U. Isolation of Starches from Non-Conventional Sources of North-Eastern Region of India. Pharma Innov. 2021, 10, 1117–1119. [Google Scholar]
- Shafi, M.; Baba, W.N.; Masoodi, F.A.; Bazaz, R. Wheat-Water Chestnut Flour Blends: Effect of Baking on Antioxidant Properties of Cookies. J. Food Sci. Technol. 2016, 53, 4278–4288. [Google Scholar] [CrossRef] [PubMed]
- Principle, A.; Apparatus, B. Official Methods of Analysis of AOAC INTERNATIONAL 18th Edition, 2005; AOAC International: Gaithersburg, MD, USA, 2005; pp. 4–5. [Google Scholar]
- Soares Araújo, R.R.; dos Santos Benfica, T.A.R.; Ferraz, V.P.; Moreira Santos, E. Nutritional Composition of Insects Gryllus assimilis and Zophobas morio: Potential Foods Harvested in Brazil. J. Food Compos. Anal. 2019, 76, 22–26. [Google Scholar] [CrossRef]
- Suriya, M.; Rajput, R.; Reddy, C.K.; Haripriya, S.; Bashir, M. Functional and Physicochemical Characteristics of Cookies Prepared from Amorphophallus paeoniifolius Flour. J. Food Sci. Technol. 2017, 54, 2156–2165. [Google Scholar] [CrossRef]
- Moritsuka, N.; Kawamura, K.; Tsujimoto, Y.; Rabenarivo, M.; Andriamananjara, A.; Rakotoson, T.; Razafimbelo, T. Comparison of Visual and Instrumental Measurements of Soil Color with Different Low-Cost Colorimeters. Soil Sci. Plant Nutr. 2019, 65, 605–615. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Li, D.; Guo, L.; Su, X.; Zhang, Y.; Wu, F.; Xu, X. Effect of Pigskin-Originated Gelatin on Properties of Wheat Flour Dough and Bread. Food Hydrocoll. 2019, 94, 183–190. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Sun, X.; Qin, W.; Wu, D.; Hu, B.; Raheem, D.; Yang, W.; Dong, H.; Vasanthan, T.; et al. High-Speed Shearing of Soybean Flour Suspension Disintegrates the Component Cell Layers and Modifies the Hydration Properties of Okara Fibers. LWT 2019, 116, 108505. [Google Scholar] [CrossRef]
- Bejaei, M.; Stanich, K.; Cliff, M.A. Modelling and classification of apple textural attributes using sensory, instrumental and compositional analyses. Foods 2021, 10, 384. [Google Scholar] [CrossRef]
- Duta, D.E.; Culetu, A.; Mohan, G. Sensory and Physicochemical Changes in Gluten-Free Oat Biscuits Stored under Different Packaging and Light Conditions. J. Food Sci. Technol. 2019, 56, 3823–3835. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Quah, E.P.L. Mridula and Camino. Food Chem. 2007, 103, 734–740. [Google Scholar] [CrossRef]
- Miśkiewicz, K.; Nebesny, E.; Rosicka-Kaczmarek, J.; Żyżelewicz, D.; Budryn, G. The Effects of Baking Conditions on Acrylamide Content in Shortcrust Cookies with Added Freeze-Dried Aqueous Rosemary Extract. J. Food Sci. Technol. 2018, 55, 4184–4196. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Roy, S.; Ali, M.J.; Hossain, M.R.; Sarker, M.S.H. Effect of Drying Temperature on Physiochemical Properties of Powder from Blanched and Unblanched Lemon Peel and Sensory Quality Evaluation of the Powder Fortified Biscuits. J. Food Eng. Technol. 2021, 10, 9–18. [Google Scholar] [CrossRef]
- Tư, H.D. Kỹ Thuật Phân Tích Cảm Quan Thực Phẩm; Khoa Học Kỹ Thuật: Hanoi, Vietnam, 2009; p. 145. [Google Scholar]
- Xu, D.; Zhou, X.; Lei, C.; Shang, Y.; Zhao, Y.; Wang, Z.; Zeng, F.; Liu, G. Development of Biscuits and Cookies Using Raw Dehydrated Potato Flour and Its Nutritional Quality and Volatile Aroma Compounds Evaluation. J. Food Process. Preserv. 2020, 44, e14528. [Google Scholar] [CrossRef]
- Baldino, N.; Gabriele, D.; Lupi, F.R.; De Cindio, B.; Cicerelli, L. Modeling of Baking Behavior of Semi-Sweet Short Dough Biscuits. Innov. Food Sci. Emerg. Technol. 2014, 25, 40–52. [Google Scholar] [CrossRef]
- Saha, S.; Gupta, A.; Singh, S.R.K.; Bharti, N.; Singh, K.P.; Mahajan, V.; Gupta, H.S. Compositional and Varietal Influence of Finger Millet Flour on Rheological Properties of Dough and Quality of Biscuit. LWT 2011, 44, 616–621. [Google Scholar] [CrossRef]
- Aljobair, M.O. Physicochemical, Nutritional, and Sensory Quality and Storage Stability of Cookies: Effect of Clove Powder. Int. J. Food Prop. 2022, 25, 1009–1020. [Google Scholar] [CrossRef]
- Kamal, M.; Islam, M.; Aziz, M. Effect of Sweet Potato Flour of Two Local Varieties on Quality of Breads. J. Bangladesh Agric. Univ. 2014, 11, 301–306. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Muhammad, K. Functional Properties, Antioxidant Activities and Storage Stability of Cookies from Germinated Brown Rice and Rice-Potato Starch Composite Flour. Pertanika J. Trop. Agric. Sci. 2019, 42, 503–518. [Google Scholar]
- Giuberti, G.; Rocchetti, G.; Sigolo, S.; Fortunati, P.; Lucini, L.; Gallo, A. Exploitation of Alfalfa Seed (Medicago sativa L.) Flour into Gluten-Free Rice Cookies: Nutritional, Antioxidant and Quality Characteristics. Food Chem. 2018, 239, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Pittia, P.; Antonello, P. Chapter 2—Safety by Control of Water Activity: Drying, Smoking, and Salt or Sugar Addition. In Regulating Safety of Traditional and Ethnic Foods; Prakash, V., Martín-Belloso, O., Keener, L., Astley, S., Braun, S., McMahon, H., Lelieveld, H., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 7–28. ISBN 9780128006054. [Google Scholar]
- Galla, N.R.; Pamidighantam, P.R.; Karakala, B.; Gurusiddaiah, M.R.; Akula, S. Nutritional, Textural and Sensory Quality of Biscuits Supplemented with Spinach (Spinacia oleracea L.). Int. J. Gastron. Food Sci. 2017, 7, 20–26. [Google Scholar] [CrossRef]
- Varghese, C.; Wolodko, J.; Chen, L.; Doschak, M.; Srivastav, P.P.; Roopesh, M.S. Influence of Selected Product and Process Parameters on Microstructure, Rheological, and Textural Properties of 3D Printed Cookies. Foods 2020, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Červenka, L.; Brožková, I.; Vytřasová, J. Effects of the Principal Ingredients of Biscuits upon Water Activity. J. Food Nutr. Res. 2006, 45, 39–43. [Google Scholar]
- Xu, Y.C.; Leung, S.W.S.; Yeung, D.K.Y.; Hu, L.H.; Chen, G.H.; Che, C.M.; Man, R.Y.K. Structure-Activity Relationships of Flavonoids for Vascular Relaxation in Porcine Coronary Artery. Phytochemistry 2007, 68, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Drakos, A.; Kyriakakis, G.; Evageliou, V.; Protonotariou, S.; Mandala, I.; Ritzoulis, C. Influence of Jet Milling and Particle Size on the Composition, Physicochemical and Mechanical Properties of Barley and Rye Flours. Food Chem. 2017, 215, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Tra, T.T.T.; Phúc, L.N.; Yn, V.T.N.; Sang, L.T.; Thu, N.T.A.; Nguyet, T.N.M.; Mn, L.V.V. Use of Wheat Flour and Spent Coffee Grounds in the Production of Cookies with High Fiber and Antioxidant Content: Effects of Spent Coffee Grounds Ratio on the Product Quality. IOP Conf. Ser. Earth Environ. Sci. 2021, 947, 012044. [Google Scholar] [CrossRef]
- Eleazu, C.; Eleazu, K.; Aniedu, C.; Amajor, J.; Ikpeama, A.; Ebenzer, I. Effect of Partial Replacement of Wheat Flour with High Quality Cassava Flour on the Chemical Composition, Antioxidant Activity, Sensory Quality, and Microbial Quality of Bread. Prev. Nutr. Food Sci. 2014, 19, 115–123. [Google Scholar] [CrossRef]
- Sato, E.; Kohno, M.; Hamano, H.; Niwano, Y. Increased Anti-Oxidative Potency of Garlic by Spontaneous Short-Term Fermentation. Plant Foods Hum. Nutr. 2006, 61, 157–160. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S. The Significance of Protein in Food Intake and Body Weight Regulation. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 635–638. [Google Scholar] [CrossRef]
- Hasan, S.M.K.; Hossain, M.A.; Hossain, M.J.; Roy, J.; Sarker, M.S.H. Preparation of Biscuit from Jackfruit (Artocarpus heterophyllus) Seed Flour. Agriculturists 2010, 8, 10–18. [Google Scholar]
- Kulthe, A.A.; Pawar, V.D.; Kotecha, P.M.; Chavan, U.D.; Bansode, V.V. Development of High Protein and Low Calorie Cookies. J. Food Sci. Technol. 2014, 51, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A. Development, Nutrient Composition and Sensory Properties of Biscuits Produced from Composite Flour of Wheat and African Yam Bean. Br. J. Appl. Sci. Technol. 2014, 4, 1925–1933. [Google Scholar] [CrossRef]
- Schopf, M.; Scherf, K.A. Water Absorption Capacity Determines the Functionality of Vital Gluten Related to Specific Bread Volume. Foods 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Bello, F.A. Physicochemical and Sensory Properties of Cookies Produced from Wheat, Unripe Plantain and Germinated Fluted Pumpkin Seed Composite Flour. Food Sci. Qual. Manag. 2020, 96, 36–43. [Google Scholar]
- Cummings, J.H.; Stephen, A.M. Carbohydrate Terminology and Classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Oliva, N.; Cini, E. A Systematic Review of Gluten-Free Dough and Bread: Dough Rheology, Bread Characteristics, and Improvement Strategies. Appl. Sci. 2020, 10, 6559. [Google Scholar] [CrossRef]
- Mancebo, C.M.; Rodriguez, P.; Gómez, M. Assessing Rice Flour-Starch-Protein Mixtures to Produce Gluten Free Sugar-Snap Cookies. LWT 2016, 67, 127–132. [Google Scholar] [CrossRef]
- Brennan, C.S.; Samyue, E. Evaluation of Starch Degradation and Textural Characteristics of Dietary Fiber Enriched Biscuits. Int. J. Food Prop. 2004, 7, 647–657. [Google Scholar] [CrossRef]
- Agrahar-Murugkar, D.; Gulati, P.; Kotwaliwale, N.; Gupta, C. Evaluation of Nutritional, Textural and Particle Size Characteristics of Dough and Biscuits Made from Composite Flours Containing Sprouted and Malted Ingredients. J. Food Sci. Technol. 2015, 52, 5129–5137. [Google Scholar] [CrossRef]
- Ojha, P.; Pathak, G.; Maharjan, S.; Manandhar, U.; Maharjan, S.; Karki, R. Quality and textural properties evaluation of gluten-free biscuit developed from maize, rice, buckwheat, and soybean. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2022, 23, 295–305. [Google Scholar]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Bhat, V.; Mutha, A.; Dsouza, M.R. Pharmacognostic and Physiochemical Studies of Artocarpus heterophyllus Seeds. Int. J. ChemTech Res. 2017, 10, 525–536. [Google Scholar]
- Sakac, M.; Torbica, A.; Sedej, I.; Hadnadev, M. Influence of Breadmaking on Antioxidant Capacity of Gluten Free Breads Based on Rice and Buckwheat Flours. Food Res. Int. 2011, 44, 2806–2813. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis; Springer: New York, NY, USA, 2018; ISBN 9781493966745. [Google Scholar]
- Azeez, S.; Lasekan, O.; Jinap, S.; Sulaiman, R. Effect of Roasting Conditions on the Browning Intensity and Structural Changes in Jackfruit (Artocarpus hetrophyllus) Seeds. J. Food Sci. Technol. 2015, 52, 8050–8058. [Google Scholar] [CrossRef] [PubMed]
- Baer, C.; Jaeschke, T.; Mertmann, P.; Pohl, N.; Musch, T. A MmWave Measuring Procedure for Mass Flow Monitoring of Pneumatic Conveyed Bulk Materials. IEEE Sens. J. 2014, 14, 3201–3209. [Google Scholar] [CrossRef]
- Dachana, K.B.; Rajiv, J.; Indrani, D.; Prakash, J. Effect of Dried Moringa (Moringa oleifera Lam) Leaves on Rheological, Microstructural, Nutritional, Textural and Organoleptic Characteristics of Cookies. J. Food Qual. 2010, 33, 660–677. [Google Scholar] [CrossRef]

| Cookie Samples | Diameter (cm) | Thickness (mm) | Spread Ratio |
|---|---|---|---|
| CC | 5.97 a ± 0.06 | 3.97 a ± 0.06 | 1.50 a ± 0.03 |
| JSSC | |||
| 10% | 6.03 bc ± 0.06 | 3.93 a ± 0.06 | 1.52 bc ± 0.15 |
| 20% | 6.07 abc ± 0.06 | 3.93 a ± 0.06 | 1.54 bc ± 0.15 |
| 30% | 6.13 ab ± 0.15 | 3.90 a ± 0.10 | 1.57 ab ± 0.16 |
| 40% | 6.17 a ± 0.12 | 3.87 a ± 0.12 | 1.57 a ± 0.16 |
| JSFC | |||
| 10% | 5.83 b ± 0.06 | 3.93 a ± 0.06 | 1.47 ab ± 0.03 |
| 20% | 5.63 c ± 0.06 | 3.93 a ± 0.06 | 1.43 abc ± 0.07 |
| 30% | 5.47 d ± 0.06 | 3.90 a ± 0.10 | 1.40 bc ± 0.05 |
| 40% | 5.30 e ± 0.10 | 3.87 a ± 0.12 | 1.37 c ± 0.05 |
| Cookie Samples | L* | a* | b* | ΔE |
|---|---|---|---|---|
| CC | 71.25 a ± 1.43 | 5.58 c ± 0.18 | 28.49 a ± 1.03 | - |
| JSSC | ||||
| 10% | 70.01 a ± 0.90 | 7.28 b ± 0.69 | 28.35 a ± 0.83 | 2.75 b ± 1.07 |
| 20% | 69.77 a ± 0.90 | 7.73 ab ± 0.48 | 27.33 ab ± 0.45 | 3.35 b ± 1.06 |
| 30% | 69.77 a ± 1.35 | 7.90 ab ± 0.45 | 27.30 ab ± 0.55 | 3.84 ab ± 1.02 |
| 40% | 66.83 b ± 1.06 | 8.25 a ± 0.27 | 26.73 b ± 0.69 | 5.63 a ± 0.85 |
| JSFC | ||||
| 10% | 57.79 b ± 1.57 | 12.28 b ± 0.23 | 20.33 a ± 1.14 | 17.21 c ± 0.78 |
| 20% | 49.73 c ± 1.09 | 12.38 ab ± 0.31 | 15.40 ab ± 0.42 | 26.10 b ± 2.28 |
| 30% | 49.68 c ± 0.47 | 12.73 ab ± 0.12 | 15.27 ab ± 0.17 | 26.30 b ± 1.83 |
| 40% | 45.56 d ± 1.09 | 12.75 a ± 0.23 | 12.35 b ± 0.42 | 31.22 a ± 1.45 |
| Cookie Samples | Moisture Content (%) | Water Activity | TPC (mgGAE/100 g DM) | DPPH (mgAAE/100 g DM) |
|---|---|---|---|---|
| CC | 2.35 a ± 0.09 | 0.33 a ± 0.02 | 34.42 d ± 0.47 | 11.70 d ± 0.14 |
| JSSC | ||||
| 10% | 2.06 b ± 0.06 | 0.35 a ± 0.01 | 34.13 ab ± 0.12 | 11.64 a ± 0.29 |
| 20% | 2.71 a ± 0.26 | 0.36 a ± 0.01 | 33.73 b ± 0.32 | 10.83 b ± 0.14 |
| 30% | 2.80 a ± 0.61 | 0.35 a ± 0.01 | 32.94 c ± 0.15 | 10.57 bc ± 0.05 |
| 40% | 2.65 ab ± 0.35 | 0.36 a ± 0.02 | 29.60 d ± 0.12 | 10.35 c ± 0.02 |
| JSFC | ||||
| 10% | 2.32 a ± 0.14 | 0.33 a ± 0.01 | 34.61 d ± 0.49 | 11.87 cd ± 0.28 |
| 20% | 2.46 a ± 0.19 | 0.33 a ± 0.02 | 56.58 c ± 1.54 | 12.16 c ± 0.08 |
| 30% | 2.35 a ± 0.07 | 0.34 a ± 0.02 | 81.40 b ± 1.38 | 12.58 b ± 0.05 |
| 40% | 2.38 a ± 0.19 | 0.34 a ± 0.01 | 102.04 a ± 1.56 | 13.20 a ± 0.22 |
| Cookie Samples | Texture | Color | Odor | Taste | Overall Acceptability |
|---|---|---|---|---|---|
| CC | 4.20 a ± 0.80 | 4.07 a ± 0.91 | 3.63 ab ± 0.72 | 3.70 a ± 0.70 | 3.90 a ± 0.28 |
| JSSC | |||||
| 10% | 4.00 a ± 0.95 | 3.8 ab ± 0.96 | 3.43 a ± 0.73 | 3.43 a ± 1.07 | 3.67 a ± 0.93 |
| 20% | 3.93 ab ± 0.91 | 3.6 ab ± 1.10 | 3.33 a ± 0.84 | 3.43 a ± 0.63 | 3.57 a ± 0.19 |
| 30% | 3.93 ab ± 0.78 | 3.33 b ± 1.09 | 3.33 a ± 0.96 | 3.47 a ± 1.10 | 3.52 a ± 0.15 |
| 40% | 3.50 b ± 0.97 | 2.53 c ± 1.13 | 3.23 a ± 0.77 | 2.70 b ± 0.89 | 2.99 b ± 0.14 |
| JSFC | |||||
| 10% | 3.67 b ± 1.15 | 3.83 a ± 0.95 | 3.47 b ± 0.73 | 3.27 ab ± 1.11 | 3.56 ab ± 0.25 |
| 20% | 3.60 b ± 0.89 | 3.23 b ± 1.14 | 3.93 a ± 0.98 | 3.13 b ± 1.38 | 3.48 ab ± 0.16 |
| 30% | 3.50 b ± 0.68 | 3.20 b ± 1.02 | 3.40 b ± 0.86 | 3.57 ab ± 0.77 | 3.42 b ± 0.37 |
| 40% | 2.90 c ± 0.88 | 2.07 c ± 0.87 | 2.40 ± 0.77 | 2.33 c ± 1.15 | 2.43 c ± 0.35 |
| Cookie Samples | CC | JSSC | JSFC |
|---|---|---|---|
| Protein content (% DM) | 8.92 a ± 0.10 | 7.69 b ± 0.20 | 8.74 a ± 0.16 |
| Fat content (% DM) | 22.33 a ± 0.20 | 22.37 a ± 0.25 | 21.75 b ± 0.22 |
| Ash content (% DM) | 0.94 b ± 0.04 | 0.89 b ± 0.03 | 1.67 a ± 0.12 |
| Crude fiber (% DM) | 2.13 b ± 0.12 | 2.00 b ± 0.20 | 4.67 a± 0.50 |
| Carbohydrate content (% DM) | 68.37 b ± 0.01 | 69.07 a ± 0.15 | 67.20 c ± 0.07 |
| Cookie Samples | CC | JSSC | JSFC |
|---|---|---|---|
| Moisture content (% DM) | 2.35 a ± 0.09 | 2.80 a ± 0.61 | 2.35 a ± 0.07 |
| Water activity | 0.33 a ± 0.02 | 0.35 a ± 0.01 | 0.34 a ± 0.02 |
| Water holding capacity (g/g) | 1.64 a ± 0.05 | 1.53 ab ± 0.04 | 1.45 b ± 0.07 |
| Oil holding capacity (g/g) | 1.76 a ± 0.04 | 1.36 b ± 0.05 | 1.72 a ± 0.04 |
| L* | 71.25 a ± 1.43 | 69.77 a ± 1.35 | 49.68 b ± 0.47 |
| a* | 5.58 c ± 0.18 | 7.90 b ± 0.45 | 12.73 a ± 0.12 |
| b* | 28.49 a ± 1.03 | 27.30 a ± 0.55 | 15.27 b ± 0.17 |
| PV (meqO2/kg) | 0.77 a ± 0.21 | 0.80 a ± 0.28 | 0.73 a ± 0.14 |
| Hardness (N) | 3.27 c ± 0.52 | 3.72 b ± 0.37 | 5.80 a ± 0.36 |
| TPC (mgGAE/100 g DM) | 34.42 b ± 0.47 | 32.94 b ± 0.15 | 81.40 a ± 1.38 |
| DPPH (mgAAE/100 g DM) | 11.70 b ± 0.14 | 10.57 c ± 0.05 | 12.58 a ± 0.05 |
| Cookie Samples | CC | JSSC | JSFC |
|---|---|---|---|
| Texture | 4.20 a ± 0.80 | 3.93 a ± 0.78 | 3.50 b ± 0.68 |
| Color | 4.07 a ± 0.91 | 3.33 b ± 1.09 | 3.20 b ± 1.02 |
| Odor | 3.63 a ± 0.72 | 3.33 a ± 0.96 | 3.40 a ± 0.86 |
| Taste | 3.70 a ± 0.70 | 3.47 a ± 1.10 | 3.57 a ± 0.77 |
| Overall acceptability | 3.90 a ± 0.28 | 3.52 ab ± 0.15 | 3.42 b ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van, C.K.; Nguyen, T.H.; Nguyen, T.T.N.H.; Nguyen, P.T.N.; Tran, T.T.; Hoang, Q.B. Comparison of the Effects of Jackfruit Seed Flour and Jackfruit Seed Starch in the Cookie Manufacturing Process. Processes 2023, 11, 3194. https://doi.org/10.3390/pr11113194
Van CK, Nguyen TH, Nguyen TTNH, Nguyen PTN, Tran TT, Hoang QB. Comparison of the Effects of Jackfruit Seed Flour and Jackfruit Seed Starch in the Cookie Manufacturing Process. Processes. 2023; 11(11):3194. https://doi.org/10.3390/pr11113194
Chicago/Turabian StyleVan, Chi Khang, Thi Han Nguyen, Trinh Thi Nhu Hang Nguyen, Phu Thuong Nhan Nguyen, Thi Tuu Tran, and Quang Binh Hoang. 2023. "Comparison of the Effects of Jackfruit Seed Flour and Jackfruit Seed Starch in the Cookie Manufacturing Process" Processes 11, no. 11: 3194. https://doi.org/10.3390/pr11113194
APA StyleVan, C. K., Nguyen, T. H., Nguyen, T. T. N. H., Nguyen, P. T. N., Tran, T. T., & Hoang, Q. B. (2023). Comparison of the Effects of Jackfruit Seed Flour and Jackfruit Seed Starch in the Cookie Manufacturing Process. Processes, 11(11), 3194. https://doi.org/10.3390/pr11113194






