Abstract
In this present work, a new kind of sulfurized hydrodesulfurization catalyst was synthesized via the hydrothermal treatment of MoS2, NiCO3·2Ni(OH)2·4H2O, and Al2O3 precursors, followed by annealing under a H2 atmosphere, which does not require a sulfurization process compared to traditional preparation methods. The influence of the annealing temperature and the type of Al2O3 precursor on the interactions between MoS2 and Al2O3 were studied using X-ray fluorescence spectroscopy, X-ray diffraction, N2 adsorption–desorption, Raman spectroscopy, X-ray photoelectron spectroscopy, and high-resolution transmission electron microscopy. The results indicated an increase in the number of stacked layers of the MoS2 catalyst, accompanied by a decrease in the degree of decoration of Ni atoms onto MoS2 nanoslabs, as a result of the strengthened MoS2–Al2O3 interaction. Subsequently, the efficiency of hydrodesulfurization (HDS) was evaluated using dibenzothiophene as a representative reactant, while establishing a correlation between the structure of the catalyst and its performance. The catalysts, using pseudo-boehmite as the precursor and calcined at 500 °C, synthesized by calcining pseudo-boehmite as the precursor for Al2O3 at a temperature of 500 °C and possessing suitable metal–support interactions, exhibited a reduced number of MoS2 stacking layers and lateral dimensions, along with an optimal decoration degree of Ni atoms, thereby resulting in the highest level of HDS activity.
1. Introduction
In recent times, the production of clean fuels has encountered significant obstacles attributed to the substandard quality of crude oil, alongside the prioritization of environmental preservation and the implementation of progressively rigorous environmental regulation [1,2]. HDS serves as the primary technique employed for the elimination of heteroatoms within petroleum fractions, thereby facilitating the production of refined fuels [3,4]. The research on HDS catalysts has garnered significant attention due to their pivotal role in HDS technology [5,6].
For more than eight decades, the refining industry has widely utilized alumina- or modified-alumina-supported MoS2 catalysts, typically enhanced by Co or Ni atoms, which are regarded as the most fundamental and significant catalysts for hydrotreating processes [7,8]. The existence of two distinct types of ‘Ni (Co)-Mo-S’ active phases, characterized by the presence of Ni or/and Co as promoting atoms situated at the edges and corners of MoS2 nano crystallites [9,10,11,12], has been widely acknowledged. Based on the concept of metal–support interactions (MSIs), the active phases can be categorized into two distinct types: type II and type I. The former exhibits complete sulfidation, which can be attributed to a weaker MSI [9,13]. Conversely, the latter displays lower stacking and incomplete sulfidation, which can be attributed to the presence of strong MSIs resulting from Mo-O-Al bridges. Consequently, the type II phase demonstrates significantly greater intrinsic activity, approximately twice as high as the alternative phase [14]. Indeed, MSIs have garnered considerable scholarly interest owing to their profound influence on the efficacy of catalysts [15,16,17,18].
The current focus of research on MSIs encompasses two key aspects: the adjustment of the support [17,19,20,21,22,23] and the modification of the preparation method [15,24,25,26,27]. Several studies have highlighted the direct influence of support characteristics on the strength of MSIs [28], consequently impacting catalyst performance. Researchers have primarily achieved support adjustment through alterations in the crystalline structure of Al2O3 [20,21], the incorporation of modifying elements [22,23,29], and the exploration of novel support materials [17]. The effect of various crystalline alumina types, namely γ-Al2O3, δ-Al2O3, and θ-Al2O3, on HDS activity was investigated by Gao et al. [20] and Wang et al. [21]. The findings indicated that the inclusion of optimal levels of MSIs in the catalysts supported by δ-Al2O3 led to a significant enhancement in HDS activity. However, γ-Al2O3 is widely used in the industry due to its large specific surface area and strength [30]. Consequently, researchers have begun to modify γ-Al2O3 by incorporating elements such as Mg, P, and B into the support [22,23,31]. For instance, Nadeina et al. [29] introduced B and P into the catalysts, which attenuated the MSI and consequently resulted in an improvement in HDS activity. Trejo et al. [22] and Mogica-Betancourt et al. [31] conducted studies to investigate the impact of incorporating MgO into the support material on the attenuation of MSIs, leading to the enhanced HDS activity of the catalysts. Furthermore, researchers have focused on identifying alternative support materials to replace alumina. For instance, Joshi et al. [17] employed the density functional theory (DFT) to theoretically examine the interactions between the active phase and the carrier in HDS catalysts. Their findings revealed a consistent correlation between the order of MSIs (SiO2 < carbon < Al2O3 < TiO2 < ZrO2 < Y2O3) and the relative abundance of type I phases. Furthermore, the researchers modified the MoS2 phase morphology by incorporating citric acid (CA), ethylene diamine tetraacetic acid (EDTA), and diethylenetriaminepentaacetic acid (DTPA) into the catalyst preparation process, thereby reducing the MSI and leading to a noteworthy improvement in the average stacked layer [24,25,32]. It is worth noting that the calcination temperature is a crucial factor in the catalyst preparation process, as supported by research demonstrating that higher calcination temperatures lead to an increase in the MSI of the catalyst [33,34,35]. This, in turn, influences the dispersion state, microstructure, and performance of the catalyst [15,36,37]. A study conducted by Abubakar et al. [38] specifically examined the impact of the calcination temperature on NiMo HDS catalysts and found that an optimal calcination temperature can result in a moderate MSI, thereby enhancing the dispersion and crystallinity of the active metal.
As previously stated, extensive research has been conducted on the influence of MSIs on the structure and performance of catalysts. In fact, the conventional industrial procedure for producing Mo-based hydroprocessing catalysts typically commences with the synthesis of oxide catalysts, such as CoO(NiO)-MoO3/Al2O3 [39] and CoO(NiO)-MoO3-P2O5/Al2O3 [40], which are subsequently converted into sulfide catalysts through the utilization of sulfur-containing compounds in the feed and H2 [41,42]. The existing body of research on MSIs has predominantly concentrated on investigating the oxidation state precursors of hydrogenation catalysts. Conversely, there is a scarcity within the literature of studies examining the impact of MSIs on the structure and performance of catalysts that directly interact with reactants and facilitate the sulfidation process. The utilization of H2-programmed elevated temperature reduction (H2-TPR) is a significant method for evaluating the MSIs of oxidized catalysts. However, in the case of sulfidized catalysts, H2 primarily serves the purpose of characterizing the sulfur composition in MoS2(WS2) rather than characterizing the MSI [43,44]. Its primary function is to determine the nature of sulfur in the catalysts and the level of ease or difficulty in creating sulfur vacancies [43,44]. However, recent investigations have explored the MSI of sulfided catalysts using CO/NO infrared (IR) adsorption [16,45,46]. These studies have revealed that a stronger MoS2–Al2O3 interactions results in a higher ratio of S-edge/Mo-edge in MoS2 [16], and a shift in the position of the Mo-edge peaks towards higher frequencies [45]. However, the sulfided HDS catalysts that were utilized in the investigations on the MSIs of sulfided catalysts were also acquired through the process of sulfiding the previously oxidized catalysts. Conversely, there is a lack of reported studies on the MSIs of sulfided HDS catalysts that were directly prepared.
In this study, supported sulfided catalysts were prepared through hydrothermal treatments of MoS2, NiCO3·2Ni(OH)2·4H2O, and Al2O3 precursors, followed by annealing in a H2 atmosphere. The impact of the annealing temperature and the type of Al2O3 precursor on the interactions between MoS2 and Al2O3 was investigated using various characterization methods. Furthermore, the HDS activity of the catalysts was evaluated using DBT as a model reactant. The findings indicate that an increase in MoS2–Al2O3 interactions leads to an increase in the number of MoS2 stacked layers in the catalysts, a decrease in auxiliary modification, and a decrease in catalyst HDS activity.
2. Experimental
2.1. Materials
No further purification was performed on all materials once they were received. Commercial bulk molybdenum disulfide (MoS2, crystalline powder, 99%) was purchased from Aladdin Industrial Corp. It used nickel carbonate (NiCO3·2Ni(OH)2·4H2O powder, 99.9%), pseudo-boehmite (Pb powder, 99%), and alumina (γ-Al2O3, 99.5%) supplied by Sinopec Changling Catalyst Company, Yueyang, China. Dibenzothiophene (DBT, 98%) and decanal were obtained from J&K Chemical, Shanghai, China, n-decane (99%) was procured from Tianjin Damao Chemical Reagent Factory, Tianjin, China, and aluminum isopropoxide (AIP, 98%) was supplied by Innochem, Kulaijaya, Malaysia.
2.2. Preparation of Sulfided Supported HDS Catalysts
2.2.1. Preparation of Catalysts and Supports with Different Annealing Temperatures
A series of sulfided supported HDS catalysts were synthesized through the hydrothermal treatment of commercially available MoS2, NiCO3·2Ni(OH)2·4H2O, and various Al2O3 precursors at different annealing temperatures under a H2 atmosphere. The specific preparation process involved the homogenous stirring of 1.50 g of commercial MoS2 powder, 0.96 g of NiCO3·2Ni(OH)2·4H2O powder, 10.1g of pseudo-boehmite powder, and 70 mL of deionized water at room temperature. The resulting mixture was then transferred to a 100mL rotary Teflon-lined stainless steel autoclave and subjected to hydrothermal treatment at 150 °C in a chamber furnace for a duration of 10 h. The resulting black solids were subsequently filtered, dried, and calcined under a H2 atmosphere. The catalysts were designated as Pb-500, Pb-550, and Pb-600, based on the respective H2 atmosphere annealing temperatures of 500, 550, and 600 °C. The NiO and MoO3 content of the three catalysts was analyzed using X-ray fluorescence spectroscopy (XRF, Rigaku ZSX-100e, Rigaku, Tokyo, Japan), and the obtained results are presented in Table 1. The data unequivocally demonstrate that all catalysts exhibit comparable metal loadings, approximately 6.3 wt% NiO and 14.6 wt% MoO3. Similarly, the pseudo-boehmite was subjected to the same treatment procedure, albeit excluding MoS2 and NiCO3·2Ni(OH)2·4H2O, leading to the formation of support materials referred to as Sup-500, Sup-550, and Sup-600.

Table 1.
XRF and N2 adsorption–desorption analysis results of the samples.
2.2.2. Catalyst Preparation from Different Alumina Precursors
HDS catalysts, specifically Al-500 and AIP-500, were synthesized using distinct Al2O3 precursors, namely γ-Al2O3 and aluminum isopropoxide (AIP), respectively, while maintaining an identical active metal content (15% MoO3). The synthesis procedure entailed subjecting the catalysts to hydrothermal treatment under uniform conditions, followed by annealing at 500 °C in an H2 atmosphere.
2.3. Characterizations of Sulfided NiMo Catalysts
Wide-angle X-ray diffraction (XRD) analysis was conducted using a power diffractometer (Bruker D5005 X’Pert) equipped with CuKa radiation (λ = 1.5406 Å). The recorded patterns spanned from 2θ = 5° to 2θ = 70°, employing a scanning speed of 2°·min−1.
The catalysts’ specific surface areas and pore structures were analyzed using a Micromeritics ASAP 2002 analyzer through N2 adsorption–desorption at liquid nitrogen temperature. Prior to measurement, the catalysts underwent degassing at 300 °C for 15 h under 105 Torr. The specific surface areas were determined using the Brunauer–Emmett–Teller (BET) model, while the pore size distributions were calculated using the Barret–Joyner–Halenda (BJH) method. The pore volumes were evaluated based on the N2 adsorption–desorption isotherms.
Raman experiments were conducted using a Raman spectrometer (LabRAM HR UV-NIR, Jobin Yvon, Newark, NJ, USA) that was equipped with a microscope. The He-Cd laser was utilized to excite the 325 nm line, while the 532 nm line was derived from a Nd-YAG laser. The laser power at the sample was carefully controlled to remain below 1 mW for 325 nm and below 5 mW for 532 nm.
High-resolution transmission electron microscopy (HRTEM) images of the sulfided bimetallic catalysts were acquired using a Philips Tecnai G2 F20 instrument, FEI Company, Hillsboro, OR, USA. The electron energy employed was 220 keV, with a Cs value of 0.5 mm. The instrument’s TEM mode provided a point-to-point resolution of 1.9 Å, and the specimen was tilted at ±40°. The images were captured using a TVIPS 1 k × 1 k CCD camera. In order to obtain an objective assessment of the lateral dimensions and stacking layers of the MoS2 samples, we conducted a random count of 300 MoS2 nanosheets from the HRTEM images for each MoS2 sample. Subsequently, we computed the lateral dimension and average number of stacking layers.
The X-ray photoelectron spectroscopy (XPS) experiments were conducted utilizing an ESCALab250 spectrometer manufactured by VG Scientific, Waltham, MA, USA. The quantification of the surface area of the elements was accomplished by employing the sensitivity factors provided by the VG program(Avantage 4.15, Mixed Gaussian–Lorentzian functions with an iterative least-squares computer program). All binding energies (BEs) were calibrated with respect to C1s, a type of adventitious carbon (284.80 eV). Furthermore, the Avantage software (version 5.967) can be employed to fit the XPS spectrum in order to discern the chemical states of the Ni species, namely Ni-Mo-S, Ni2+, NixSy, and Ni shake up.
2.4. Catalytic Performance of Sulfided NiMo Catalysts
The catalytic activities of the sulfided NiMo catalysts were assessed using a fixed microreactor with dimensions of a 500 mm length and a 10.0 mm diameter. The catalyst was finely ground to a size of 40–60 mesh and then mixed with 0.45 g of the catalyst and 1 g of quartz sand. This diluted sample was introduced into the constant temperature zone of the reactor, while the remaining space was filled with 40–60 mesh quartz sand. A model reactant consisting of a 1 wt% dibenzothio(DBT)/n-decane solution was selected for the HDS reactions of DBT. These reactions were carried out at temperatures of 260 °C, 280 °C, 300 °C, and 320 °C, a total pressure of 4 MPa, a weight hourly space velocity (WHSV) of 24.67 h−1, and a H2-to-feed volume ratio of 500.
The HDS of DBT was considered to be a pseudo-first-order reaction, taking into account the reaction conditions. The catalysts’ activity was determined using the provided equation [47].
where kHDS is the reaction rate constant of the DBT HDS (mol·g−1·s−1), F is the feeding rate of the reactant (mol·s−1), x is the total conversion of DBT, and m is the catalyst mass (gram).
The HDS of DBT yielded multiple products, as determined via a GC-MS analysis. Within the hydrogenation (HYD) pathway, tetrahydro-dibenzothiophene (THDBT) underwent further desulfurization to generate cyclohexylbenzene (CHB) and bicyclohexane (BCH), while direct desulfurization (DDS) resulted in the production of biliphenyl (BP). The selectivity calculations were performed using the following equations:
3. Results and Discussions
3.1. Effect of Annealing Temperatures on Catalyst Structure
3.1.1. XRD Analysis
The XRD spectra of the sulfided catalysts, synthesized with different annealing temperatures in a H2 atmosphere, are presented in Figure 1. In order to facilitate comparison, the XRD analysis was also conducted on γ-Al2O3 and pure MoS2, with their respective results also displayed in Figure 1. Based on the analysis of Figure 1, it is evident that the peaks observed at 37.7°, 45.8°, and 66.8° can be ascribed to γ-Al2O3. Additionally, the diffraction peaks at 14.4°, 32.7°, 33.5°, and 58.4° correspond to the crystal planes (002), (100), (101), and (110), respectively, in hexagonal 2H-MoS2 (JCPDS No. 37-1492). The intensities of the characteristic peaks of each crystal face of the sulfided catalysts, prepared at varying annealing temperatures in a H2 atmosphere, exhibited a discernible decrease when compared to commercial MoS2. Specifically, the (002), (100), and (110) characteristic peaks experienced a reduction in intensity. The (002) crystal face signifies the number of stacked layers of MoS2, while the (100) and (110) crystal faces represent the lateral size of MoS2 [1,48]. This suggests that the prepared catalysts underwent a reduction in both the stacked layers and lateral dimensions (which can also be reflected in the HRTEM, as shown below). In the hydrothermal environment, characterized by high temperature and pressure, the presence of a substantial volume of high-pressure gas induces the elongation and deformation of MoS2, leading to the partial disruption of the van der Waals forces between the MoS2 layers and the Mo-S bonds within these layers [49,50]. Simultaneously, the reaggregation of the exfoliated MoS2 particles was hindered, possibly attributed to the adsorption of Ni salts on the basal planes and edges of the MoS2 [51,52], along with the existence of anthropomorphic thin hydrotalcite that impedes the reaggregation process of MoS2. A direct relationship exists between the annealing temperature and the intensity of the characteristic peaks of MoS2 in an H2 atmosphere, indicating that the lateral size and number of stacked layers of MoS2 in the catalysts augment as the annealing temperature rises. In the catalysts that were synthesized through the conventional impregnation technique, the MSI exhibited an upward trend with the escalating calcination temperature, resulting in a decline in the number of stacked layers of MoS2 achieved through subsequent sulfidation as the calcination temperature increased [15,53]. The observed results differ from those obtained with catalysts prepared using conventional impregnation methods. This discrepancy can be attributed to the distinct preparation process employed for sulfidation catalysts, wherein commercial bulk MoS2 is directly utilized. Unlike the gradual nucleation and growth of the active metal in conventional impregnation, the direct preparation method allows for the stabilization of multiple layers of MoS2 on the Al2O3 surface. It can be inferred that the strength of the MoS2–Al2O3 interactions increases with higher annealing temperatures, leading to a more favorable stabilization of MoS2 layers.
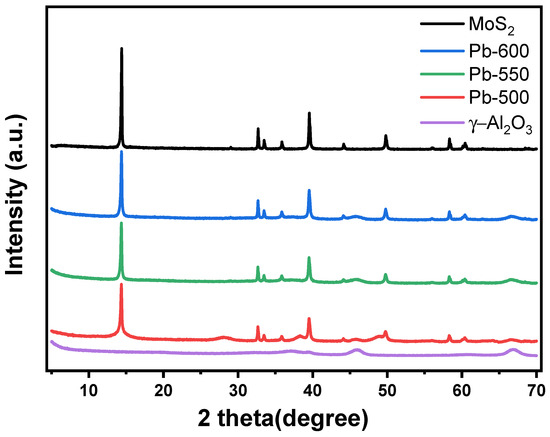
Figure 1.
XRD spectra of catalysts prepared at different annealing temperatures in a H2 atmosphere.
3.1.2. N2 Adsorption–Desorption
The N2 adsorption–desorption isotherms of the catalysts and supports are presented in Figure 2a. These isotherms exhibit a type IV behavior with characteristic H2-type hysteresis loops, indicating the presence of similar mesoporous structures. The pore size distribution plots, depicted in Figure 2b, demonstrate that the catalysts prepared at identical annealing temperatures exhibit a decrease in both the most probable pore size and the intensity of the pore distribution peaks, in comparison to the corresponding supports. The textural properties of the catalysts and supports, obtained through different annealing temperatures in a H2 atmosphere, are presented in Table 1. The data demonstrate that the catalysts exhibit a reduced specific surface area (Sg), pore volume (Vp), and most probable pore diameter (Dp) compared to the supports when prepared at the same annealing temperatures. This suggests that the MoS2 and Ni species are effectively dispersed on the surfaces of the γ-Al2O3 pores [54,55,56].
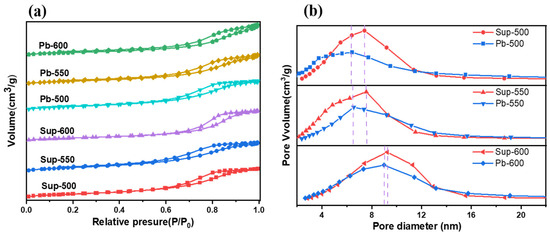
Figure 2.
N2 adsorption–desorption isotherms (a) and pore size distribution curves (b) of catalysts prepared at different annealing temperatures in a H2 atmosphere.
3.1.3. Raman Spectra
Figure 3 presents the Raman spectra of the catalysts synthesized under varying annealing temperatures in a H2 atmosphere. The spectra reveal the presence of two distinctive Raman activity modes of MoS2, specifically located at approximately 380 cm−1 and 404 cm−1. These modes correspond to the vibrational modes E12g and A1g, respectively [57,58]. E12g signifies the in-plane vibration of the two S atoms relative to the Mo atoms, while A1g represents the out-of-plane vibration of the S atoms in the opposite direction [59,60]. Based on the findings depicted in Figure 3, it is evident that the E12g vibration experiences a gradual redshift as the annealing temperature in the H2 atmosphere rises, while the A1g vibration undergoes a gradual blueshift. Consequently, the separation between the E12g and A1g vibrations increases. These findings imply a positive correlation between the quantity of MoS2 layers deposited on the γ-Al2O3 surface and the elevation in annealing temperature under a H2 atmosphere [61,62,63]. Given that the catalysts for sulfidation were prepared using commercial bulk MoS2, the MoS2 did not undergo nucleation and growth. Consequently, the stronger MoS2–Al2O3 interactions were advantageous for stabilizing multiple layers of MoS2 on the Al2O3 surface. It can be inferred that the MoS2–Al2O3 interactions intensified as the annealing temperature increased. These results align with the XRD data and previous research [61].
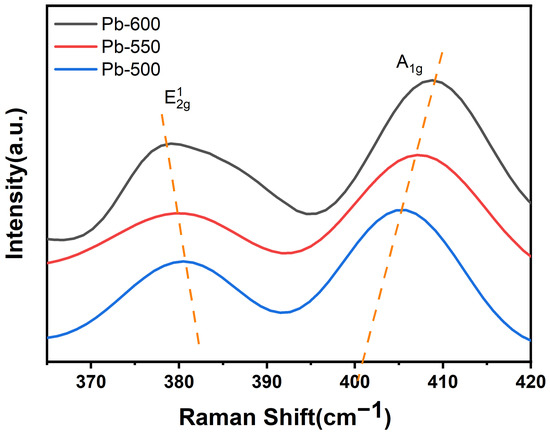
Figure 3.
Raman spectra of catalysts prepared at different annealing temperatures in a H2 atmosphere.
3.1.4. HRTEM Analysis
The morphology and microstructure of the catalysts obtained under varying annealing temperatures in a H2 atmosphere were examined using high-resolution transmission electron microscopy (HRTEM). Figure 4 illustrates that the observed stripes, exhibiting a lattice spacing of approximately 0.62 nm, correspond to the (002) plane of hexagonal MoS2. The commercially available bulk MoS2 samples consisted of regular flakes with lateral dimensions measuring a few hundred nanometers and over 30 stacked layers (Figure 4a). In contrast, the prepared catalyst samples exhibited a significant reduction in both their lateral size and stacked layers of MoS2, which aligns with the XRD findings. Furthermore, the catalysts exhibited a significant abundance of structural defects [64,65], as evidenced by the numerous discontinuous lattice streaks observed (Figure 4b–d). This occurrence, coupled with the reduction in the lateral size of MoS2, played a crucial role in enabling the catalysts to expose a greater number of active sites. Consequently, this enhancement in active site exposure contributed to the observed increase in HDS activity [1,66]. The statistical data pertaining to the lateral size of MoS2 and the average number of stacked layers in the HRTEM images are presented in Table 2. According to the data presented in Table 1, there is a correlation between the annealing temperature and the lateral size as well as the number of stacked layers of MoS2 in the catalysts. This observation aligns with the findings obtained from the XRD analysis.
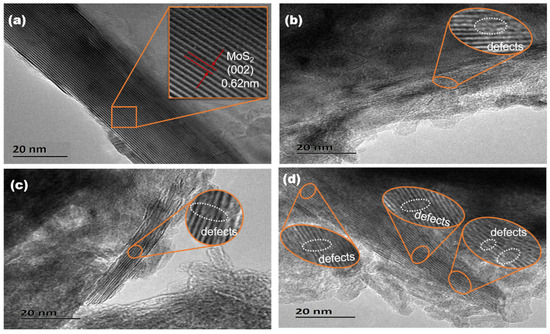
Figure 4.
HRTEM images of catalysts prepared at different annealing temperatures in a H2 atmosphere. (a) Commercial MoS2; (b) Pb-500; (c) Pb-550; (d) Pb-600.

Table 2.
HRTEM statistics of catalysts prepared at different annealing temperatures in a H2 atmosphere.
3.1.5. XPS Analysis
Figure 5a–f present the Mo3d and Ni2p spectra, along with their corresponding deconvoluted curves, of Pb-500, Pb-550, and Pb-600, thereby offering significant elucidation on the chemical composition of Mo species and Ni species. The binding energies of the Mo3d5/2 and Mo3d3/2 peaks for Mo4+ (MoS2) are observed at approximately 229.0 and 232.1 eV, respectively, while those for Mo6+ (MoO3) are observed at around 232.5 and 235.7 eV [2,21]. Additionally, the binding energy of the S2s peak is observed at approximately 226.2 eV [2,21]. The binding energy of the Ni2p peak for the Ni-Mo-S active phase is observed at approximately 855.1 eV, whereas for the Ni sulfides (NiSx), it is observed at approximately 853.2 eV [67]. Additionally, the binding energy for oxidic Ni2+ species (such as NiOx, including NiAl2O4 and NiO) is observed at approximately 856.7 eV [67]. Furthermore, the peak observed at approximately 862.5 eV in the Ni2p spectra is attributed to the shake up of the oxidic Ni2+ species [67]. It has been clearly observed that the spectra of the Mo3d in the catalyst series exhibit minimal variation, with peaks corresponding to both MoS2 and MoO3 species. The fitting curves of all three catalysts display nearly identical dominant characteristic peaks of MoS2, along with weaker characteristic peaks of the MoO3 species. However, notable differences can be observed in the Ni2p spectra of the catalysts prepared under different conditions, particularly when the annealing temperature reaches 600 °C. As the annealing temperature rises, the area of the Ni-Mo-S peaks exhibits a gradual decline, whereas the peak area of the characteristic peak of the oxidized Ni2+ species experiences a slow increase. At a annealing temperature of 600 °C, the Ni-Mo-S peak becomes nearly imperceptible, potentially due to the intensified metal–support interactions resulting from the elevated annealing temperature, thereby inducing a modification in the active metal state [46,68].
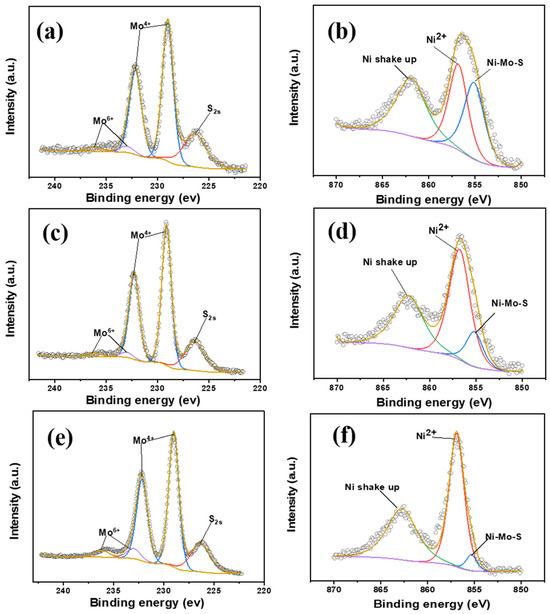
Figure 5.
Fitted curves of Mo3d and Ni2p3/2 orbitals for catalysts prepared at different annealing temperatures in a H2 atmosphere: Pb-500 (a,b); Pb-550 (c,d); Pb-600 (e,f).
The distribution of active phases was examined by conducting a quantitative analysis on the Mo and Ni species, as presented in Table 3. Specifically, the sulfidation degree of the molybdenum species (Mosul) was determined by calculating the ratio of Mo4+ (MoS2) to the combined sum of Mo4+ (MoS2), Mo5+ (MoOxSy), and Mo6+ (MoO3). This can be expressed as Mosul = Mo4+/(Mo4+ + Mo5+ + Mo6+) [2,54]. Similarly, the decoration degree of Ni species on the edges of the MoS2 nanoslabs, denoted as NiNiMoS/NiNitotal, was determined based on the atom ratio of Ni2+ in the Ni-Mo-S phase (NiNiMoS) to the combined sum of NiNiMoS, Ni2+ in the NiSx phase (NiNiSx), and oxidic Ni2+ (NiNiOx). Thus, NiNiMoS/NiNitotal = NiNiMoS/(NiNiMoS + NiNiSx + NiNiOx) [2,15]. The observation reveals that the Mosul values of the three catalysts exhibit minimal variation as the annealing temperature increases in a H2 atmosphere. By considering the findings from the XRD and Raman analyses, it can be inferred that the annealing temperature solely influences the number of MoS2 layers stacked on the γ-Al2O3 surface by modifying the interactions between MoS2 and Al2O3, while having a negligible impact on the sulfidation degree of Mo species. Contrarily, according to the data presented in Table 3, it is evident that the level of adornment exhibited by Ni species experiences a notable decline as the annealing temperature rises. Specifically, the NiNiMoS/NiNitotal ratio follows the descending sequence: Pb-500 (31.6%) > Pb-550 (10.9%) > Pb-600 (2.58%). The observed phenomenon can be attributed to the augmentation of the lateral dimensions of MoS2 on the Al2O3 surface as the annealing temperature rises in the H2 atmosphere. This leads to a reduction in the quantity of edge sites in MoS2 that can accommodate Ni species, thereby hindering the formation of the Ni-Mo-S active phase. The observed increase in Mo/Al with the increasing annealing temperature, as presented in Table 3, can be attributed to the rise in the number of stacked layers of MoS2 on the Al2O3 surface and the increase in Mo species detected via XPS. Conversely, the decrease in Ni/Al with the increasing annealing temperature can be attributed to the reduction in Ni species involved in the interactions with MoS2 to form Ni-Mo-S. Additionally, the interactions between Ni species and the support is enhanced with the annealing temperature increasing, potentially resulting in the migration of Ni from the Al2O3 surface into the Al2O3 crystals to form NiAlO4 [15].

Table 3.
XPS analysis results of the samples.
3.2. Effect of Alumina Precursors on Catalyst Structure
3.2.1. XRD Analysis
The catalyst structure changes resulting from metal–support interactions at various annealing temperatures in a H2 atmosphere were examined, with a focus on the proposed pseudo-boehmite as the support precursor. Additionally, the impact of different Al2O3 precursors on the catalyst structure was investigated under identical annealing conditions. The XRD spectra of sulfided HDS catalysts, prepared using various Al2O3 precursors, are presented in Figure 6. This observation clearly indicates that all catalysts exhibit similar characteristic peaks, specifically at 2θ = 14.4°, 32.7°, 33.5°, 35.8°, 39.6°, 44.1°, 49.8°, 58.4°, and 60.4°, which can be attributed to MoS2. Furthermore, the intensities of these MoS2 characteristic peaks in the catalysts prepared using different precursors show a slight increase in the following sequence: Pb-500 < Al-500 < AIP-500. Given that the catalysts for sulfidation were prepared using commercial bulk MoS2, the MoS2 did not undergo nucleation and growth. Consequently, the stronger MoS2–Al2O3 interactions were advantageous for stabilizing multiple layers of MoS2 on the Al2O3 surface. It can be inferred that the MoS2–Al2O3 interactions is enhanced in the order of Pb-500 < Al-500 < AIP-500. Besides that, it is worth noting that Pb-500 displayed weak characteristic peaks of pseudo-boehmite at 27.9°, in addition to the characteristic peaks of γ-Al2O3 at 37.7°, 45.8°, and 66.8°, which probably originated from the insufficient annealing of the proposed pseudo-boehmite.
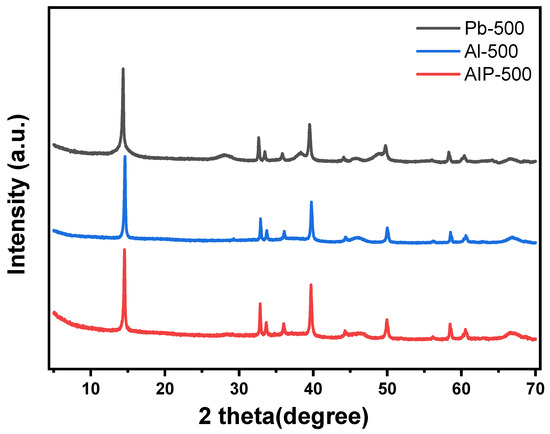
Figure 6.
XRD spectra of catalysts prepared with different Al2O3 precursors.
3.2.2. Raman Spectra
The Raman spectra of the catalysts, prepared using various Al2O3 precursors, are presented in Figure 7. The vibrational modes of MoS2, namely E12g and A1g, are observed at approximately 380 cm−1 and 404 cm−1, respectively [57,58]. The peaks corresponding to these vibrational modes exhibit slight shifts in the three catalysts. Specifically, the E12g mode experiences a shift towards higher frequencies, while the A1g mode undergoes a shift towards lower frequencies. These shifts occur in the order of Pb-500 > Al-500 > AIP-500, suggesting that the number of stacked layers of MoS2 in the catalysts, prepared using different Al2O3 precursors, follows a descending order of AIP-500 > Al-500 > Pb-500 [61,62,63]. The quantity of MoS2 layers stacked on the γ-Al2O3 surface exhibits a strong correlation with the MoS2–Al2O3 interactions. Enhanced MoS2–Al2O3 interactions promote the stabilization of a greater number of stacked MoS2 layers on the γ-Al2O3 surface. This observation aligns with the findings obtained from catalysts prepared under various annealing temperatures in a H2 atmosphere.
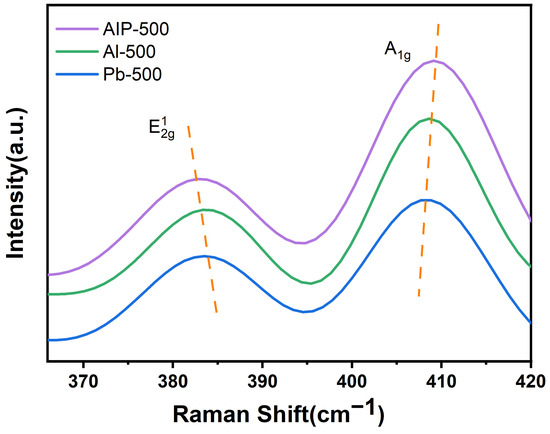
Figure 7.
Raman spectra of catalysts prepared with different Al2O3 precursors.
3.2.3. XPS Analysis
XPS was employed to conduct a comprehensive investigation into the chemical state of Mo and Ni species present on the surface of sulfided catalysts prepared using various Al2O3 precursors. The fitting spectra of Mo3d and Ni2p3/2 for the three catalysts are depicted in Figure 8. In the Mo3d5/2 spectra, all three catalysts exhibited distinctive peaks corresponding to Mo4+ and Mo6+ at binding energies of approximately 229.0 eV and 232.5 eV, respectively [2,21], and the shapes and areas of these peaks exhibited minimal variation. In the Ni2p3/2 spectra, the catalysts synthesized with different Al2O3 precursors displayed peaks attributed to Ni-Mo-S, Ni2+ and Ni shake up, located at approximately 855.1 eV, 856.7 eV, and 862.5 eV, respectively [67]. Interestingly, the order of peak areas of the Ni-Mo-S active phases in the three catalysts decrease as follows: Pb-500 > Al-500 > AIP-500. The peak area occupancies of the various Mo and Ni species are provided in Table 4. It is evident from Table 4 that the different support precursors have a minimal impact on Mosul, while they significantly influence NiNiMoS/NiNitotal. Specifically, NiNiMoS/NiNitotal decreases in the order of Pb-500 > Al-500 > AIP-500, which is contrary to the number of stacked layers of MoS2 on the γ-Al2O3 surface. The observed variation in the Ni-Mo-S results for the catalysts synthesized at various annealing temperatures in a hydrogen atmosphere suggests that this phenomenon could be attributed to the influence of the MoS2–Al2O3 interactions on the state of the nickel species.
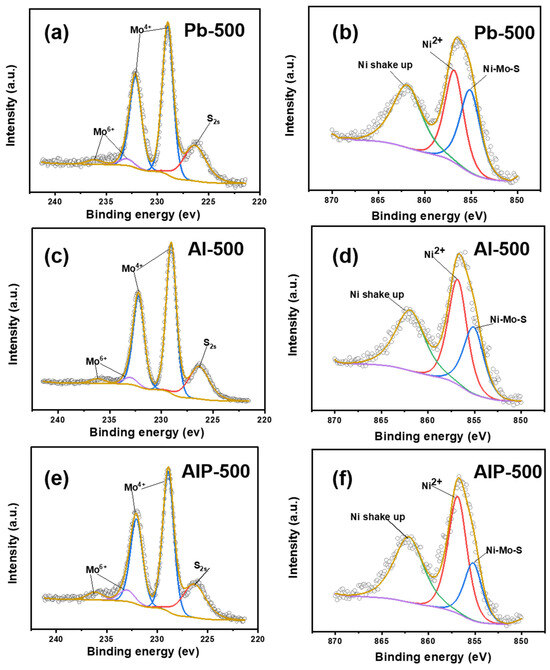
Figure 8.
Fitted curves of Mo3d and Ni2p3/2 orbitals for catalysts prepared at different support precursors: Pb-500 (a,b); Al-500 (c,d); AIP-500 (e,f).

Table 4.
Results of product distribution of DBT from different catalysts at comparable conversions.
3.3. Activity Evaluation Results
The HDS catalytic activities of the catalysts were assessed by using DBT as a model reactant. Figure 9 illustrates the correlation between the reactivity (kHDS) of DBT across various catalysts and the reaction temperature. The results indicate that the kHDS exhibits an upward trend as the reaction temperature rises. Furthermore, the catalyst’s activity diminishes in the following sequence at identical reaction temperatures: Pb-500 > Al-500 > AIP-500 > Pb-550 > Pb-600. On one hand, the increase in annealing temperature leads to an increase in the lateral size of MoS2, resulting in a reduction in the edge sites available for accommodating Ni species and forming the Ni-Mo-S active phase. Consequently, the catalysts exhibit a decrease in activity with the increasing annealing temperature. On the other hand, the enhancement of MSIs in catalysts prepared using different Al2O3 precursors may hinder the formation of the Ni-Mo-S active phase. As a result, the order of catalytic activity for different precursors is opposite to that of the MSI, with Pb-500 exhibiting the highest activity, followed by Al-500 and AIP-500.
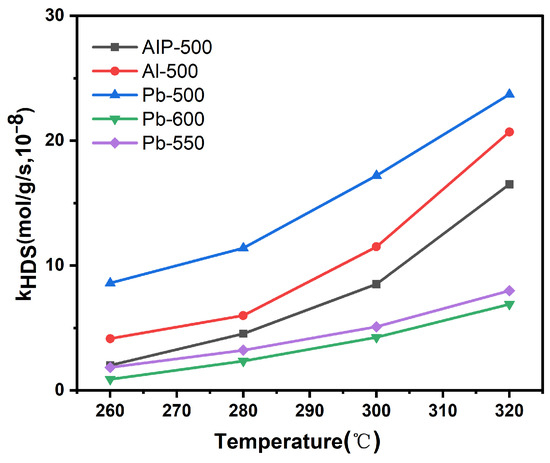
Figure 9.
Temperature dependence of DBT conversion on different catalyst samples.
As depicted in Figure 10, the HDS reaction of DBT on the catalyst occurs primarily through two distinct pathways: hydrogenation (HYD) and direct desulfurization (DDS) [69,70]. In the DDS pathway, DBT undergoes the reaction by firstly adsorbing on the S vacancy of the catalyst in the σ mode, and then occurring C-S bond breakage to generate biphenyl (BP) [69,71]. Conversely, in the HYD pathway, DBT is adsorbed onto the catalyst in the π-mode via the aromatic system and is initially hydrogenated to generate the intermediate tetrahydro-dibenzothiophene (THDBT) [69,71]. Subsequently, THDBT undergoes desulfurization, leading to the formation of cyclohexylbenzene (CHB), which is further hydrogenated to yield bicyclohexane (BCH) [69,71]. The selectivity of various catalysts for the HDS of DBT at a reaction temperature of 280 °C is presented in Table 4. The results indicate that the DBT reaction pathway of the catalysts prepared is primarily governed by the DDS pathway. Furthermore, the table reveals that the ratio of SHYD/SDDS decreases in the following sequence: Pb-500 > Pb-550 > Pb-600. This suggests that an increase in the number of MoS2 layers predominantly impairs the HYD selectivity and consequently reduces the HDS activity. Additionally, the HYD selectivity of the catalysts, prepared using different Al2O3 precursors, also diminishes with an increase in the number of MoS2 stacking layers.
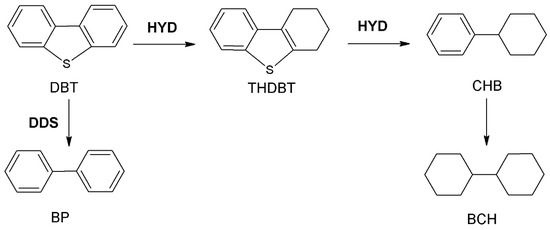
Figure 10.
HDS reaction pathways of DBT.
4. Conclusions
Five sulfided, supported HDS catalysts were prepared by employing MoS2, nickel salts, and different alumina precursors, first through a hydrothermal treatment, followed by annealing at different temperatures in a H2 atmosphere. The characterization and evaluation of the HDS activity revealed that the catalysts prepared via hydrothermal treatment and annealing in a H2 atmosphere exhibited a reduction in both the stacking layer and the lateral dimensions of MoS2 compared to its initial state, while also introducing a certain degree of structural defects. The rise in metal–support interactions as the annealing temperature increases in a H2 atmosphere results in an augmentation of the stacked layers and lateral dimensions of MoS2 on the surfaces of Al2O3. Consequently, the reduction in edge sites utilized by MoS2 for accommodating Ni species hampers the formation of Ni-Mo-S. This decrease in the proportion of active Ni-Mo-S phases formed leads to a decline in the catalytic activity for HDS as the annealing temperature of the catalyst increases. Furthermore, the stacking thickness of the MoS2 catalyst on the surfaces of Al2O3, synthesized from various alumina precursors and subjected to identical annealing conditions, exhibits an ascending trend in the sequence of Pb-500 < Al-500 < AIP-500. Consequently, it can be inferred that the metal–support interactions strength of distinct Al2O3 precursors also follows this order, as deduced from the impact of the annealing temperature on the number of MoS2 layers stacked. This phenomenon subsequently leads to a converse pattern in the augmentation of the share of the Ni-Mo-S active phase and the HDS activity.
This study directly examines the impact of the annealing temperature and alumina precursor on the metal–support interactions in sulfided HDS catalysts. The catalysts prepared through annealing at 500 °C, utilizing a pseudo-boehmite precursor, exhibit superior HDS performance. The utilization of commercial MoS2 for the direct synthesis of sulfided HDS catalysts in this study circumvents various challenges associated with the conventional sulfidation process of hydrotreating catalysts, including environmental, economic, and safety concerns.
Author Contributions
Formal analysis, H.Y.; Resources, Q.Y.; Writing—original draft, C.Y.; Writing—review & editing, Q.D. and A.H. All authors have read and agreed to the published version of the manuscript.
Funding
It is our pleasure to acknowledge that the support for this work provided by the Key Technologies Research and Development Program (Grant No. 2021YFA1501204) and the China Petrochemical Corporation (Sinopec Group 121043-2).
Data Availability Statement
The experimental design, analysis, and results, along with the data analysis and processing procedures, have been comprehensively delineated. However, the raw data cannot be disclosed due to laboratory policies or confidentiality agreements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, D.; Li, J.; Ma, H.; Yang, C.; Pan, Z.; Qu, W.; Tian, Z. Layer-structure adjustable MoS2 catalysts for the slurry-phase hydrogenation of polycyclic aromatic hydrocarbons. J. Energy Chem. 2021, 63, 294–304. [Google Scholar] [CrossRef]
- Gao, Y.; Han, W.; Long, X.; Nie, H.; Li, D. Preparation of hydrodesulfurization catalysts using MoS3 nanoparticles as a precursor. Appl. Catal. B Environ. 2018, 224, 330–340. [Google Scholar] [CrossRef]
- Bodin, A.; Christoffersen, A.N.; Elkjær, C.F.; Brorson, M.; Kibsgaard, J.; Helveg, S.; Chorkendorff, I. Engineering Ni-Mo-S Nanoparticles for Hydrodesulfurization. Nano Lett. 2018, 18, 3454–3460. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Kawanishi, K.; Tsujino, T.; Al-Otaibi, A.M.; Uemichi, Y. Catalytic Activities of Noble Metal Phosphides for Hydrogenation and Hydrodesulfurization Reactions. Catalysts 2018, 8, 160. [Google Scholar] [CrossRef]
- Alsalme, A.; Alzaqri, N.; Alsaleh, A.; Siddiqui, M.R.H.; Alotaibi, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Efficient Ni–Mo hydrodesulfurization catalyst prepared through Keggin polyoxometalate. Appl. Catal. B Environ. 2016, 182, 102–108. [Google Scholar] [CrossRef]
- Duan, A.; Li, T.; Zhao, Z.; Liu, B.; Zhou, X.; Jiang, G.; Liu, J.; Wei, Y.; Pan, H. Synthesis of hierarchically porous L-KIT-6 silica–alumina material and the super catalytic performances for hydrodesulfurization of benzothiophene. Appl. Catal. B Environ. 2015, 165, 763–773. [Google Scholar] [CrossRef]
- Zepeda, T.A.; de León, J.N.D.; Alonso, G.; Infantes-Molina, A.; Galindo-Ortega, Y.; Huirache-Acuña, R.; Fuentes, S. Hydrodesulfurization activity of Ni-containing unsupported Ga(x)WS2 catalysts. Catal. Commun. 2019, 130, 105760. [Google Scholar] [CrossRef]
- Yue, L.; Li, G.; Zhang, F.; Chen, L.; Li, X.; Huang, X. Size-dependent activity of unsupported Co–Mo sulfide catalysts for the hydrodesulfurization of dibenzothiophene. Appl. Catal. A Gen. 2016, 512, 85–92. [Google Scholar] [CrossRef]
- Berhault, G.; Perez De la Rosa, M.; Mehta, A.; Yácaman, M.J.; Chianelli, R.R. The single-layered morphology of supported MoS2-based catalysts—The role of the cobalt promoter and its effects in the hydrodesulfurization of dibenzothiophene. Appl. Catal. A Gen. 2008, 345, 80–88. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Helveg, S.; Lægsgaard, E.; Stensgaard, I.; Clausen, B.; Topsøe, H.; Besenbacher, F. Atomic-Scale Structure of Co–Mo–S Nanoclusters in Hydrotreating Catalysts. J. Catal. 2001, 197, 1–5. [Google Scholar] [CrossRef]
- Besenbacher, F.; Brorson, M.; Clausen, B.; Helveg, S.; Hinnemann, B.; Kibsgaard, J.; Lauritsen, J.; Moses, P.; Nørskov, J.; Topsøe, H. Recent STM, DFT and HAADF-STEM studies of sulfide-based hydrotreating catalysts: Insight into mechanistic, structural and particle size effects. Catal. Today 2008, 130, 86–96. [Google Scholar] [CrossRef]
- Topsøe, H.; Clausen, B.S.; Topsøe, N.Y.; Pedersen, E. Recent Basic Research in Hydrodesulfurization Catalysis. Ind. Eng. Chem. Fundam. 1986, 25, 25–36. [Google Scholar] [CrossRef]
- Romero-Galarza, A.; Gutiérrez-Alejandre, A.; Ramírez, J. Analysis of the promotion of CoMoP/Al2O3 HDS catalysts prepared from a reduced H–P–Mo heteropolyacid Co salt. J. Catal. 2011, 280, 230–238. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ochiai, K.; Kawano, M.; Kubota, T. Evaluation of the maximum potential activity of Co–Mo/Al2O3 catalysts for hydrodesulfurization. J. Catal. 2004, 222, 143–151. [Google Scholar] [CrossRef]
- Liu, Z.; Han, W.; Hu, D.; Sun, S.; Hu, A.; Wang, Z.; Jia, Y.; Zhao, X.; Yang, Q. Effects of Ni–Al2O3 interaction on NiMo/Al2O3 hydrodesulfurization catalysts. J. Catal. 2020, 387, 62–72. [Google Scholar] [CrossRef]
- Dominguez Garcia, E.; Chen, J.; Oliviero, E.; Oliviero, L.; Maugé, F. New insight into the support effect on HDS catalysts: Evidence for the role of Mo-support interaction on the MoS2 slab morphology. Appl. Catal. B Environ. 2020, 260, 117975. [Google Scholar] [CrossRef]
- Joshi, Y.; Ghosh, P.; Daage, M.; Delgass, W. Support effects in HDS catalysts: DFT analysis of thiolysis and hydrolysis energies of metal–support linkages. J. Catal. 2008, 257, 71–80. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Yang, W.; Hussain, M. Recent developments in alumina supported hydrodesulfurization catalysts for the production of sulfur-free refinery products: A technical review. Catal. Rev. 2020, 64, 1–86. [Google Scholar] [CrossRef]
- Bara, C.; Plais, L.; Larmier, K.; Devers, E.; Digne, M.; Lamic-Humblot, A.-F.; Pirngruber, G.D.; Carrier, X. Aqueous-Phase Preparation of Model HDS Catalysts on Planar Alumina Substrates: Support Effect on Mo Adsorption and Sulfidation. J. Am. Chem. Soc. 2015, 137, 15915–15928. [Google Scholar] [CrossRef]
- Cao, J.; Xia, J.; Zhang, Y.; Liu, X.; Bai, L.; Xu, J.; Yang, C.-A.; Zheng, S.; Yang, T.; Tang, K.; et al. Influence of the alumina crystal phase on the performance of CoMo/Al2O3 catalysts for the selective hydrodesulfurization of fluid catalytic cracking naphtha. Fuel 2021, 289, 119843. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Zheng, P.; Chen, Z.; Duan, A.; Xu, C.; Jiao, J.; Zhang, H.; Cao, Z.; Ge, B. Synthesis of NiMo catalysts supported on mesoporous Al2O3 with different crystal forms and superior catalytic performance for the hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene. J. Catal. 2016, 344, 680–691. [Google Scholar] [CrossRef]
- Trejo, F.; Rana, M.; Ancheyta, J. CoMo/MgO–Al2O3 supported catalysts: An alternative approach to prepare HDS catalysts. Catal. Today 2008, 130, 327–336. [Google Scholar] [CrossRef]
- Zepeda, T.A.; Pawelec, B.; Obeso-Estrella, R.; Díaz de León, J.N.; Fuentes, S.; Alonso-Núñez, G.; Fierro, J.L.G. Competitive HDS and HDN reactions over NiMoS/HMS-Al catalysts: Diminishing of the inhibition of HDS reaction by support modification with P. Appl. Catal. B Environ. 2016, 180, 569–579. [Google Scholar] [CrossRef]
- Al-Dalama, K.; Stanislaus, A. A comparative study of the influence of chelating agents on the hydrodesulfurization (HDS) activity of alumina and silica-alumina-supported CoMo catalysts. Energy Fuels 2006, 20, 1777–1783. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Inaba, M.; Tsunoda, T.; Hamakawa, S.; Suzuki, K.; Hayakawa, T. Dry reforming of methane over supported noble metals: A novel approach to preparing catalysts. Catal. Commun. 2003, 4, 493–498. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Liu, D.; Liu, B.; Lu, Y.; Chai, Y.; Liu, C. Study of the Promotion Effect of Citric Acid on the Active NiMoS Phase in NiMo/Al2O3 Catalysts. Ind. Eng. Chem. Res. 2019, 58, 17195–17206. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhao, Z.; Chen, Z.-T.; Li, J.-M.; Duan, A.-J.; Xu, C.-M.; Gao, D.-W.; Cao, Z.-K.; Zheng, P.; Fan, J.-Y. Effect of synthesis temperature on structure-activity-relationship over NiMo/γ-Al2O3 catalysts for the hydrodesulfurization of DBT and 4,6-DMDBT. Fuel Process. Technol. 2017, 161, 52–61. [Google Scholar] [CrossRef]
- Dhar, G.M.; Srinivas, B.; Rana, M.; Kumar, M.; Maity, S. Mixed oxide supported hydrodesulfurization catalysts—A review. Catal. Today 2003, 86, 45–60. [Google Scholar] [CrossRef]
- Nadeina, K.A.; Kazakov, M.O.; Danilova, I.G.; Kovalskaya, A.A.; Stolyarova, E.A.; Dik, P.P.; Gerasimov, E.Y.; Prosvirin, I.P.; Chesalov, Y.A.; Klimov, O.V.; et al. The influence of B and P in the impregnating solution on the properties of NiMo/γ-δ-Al2O3 catalysts for VGO hydrotreating. Catal. Today 2019, 329, 2–12. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Yan, Z. Facile route to prepare bimodal mesoporous γ-Al2O3 as support for highly active CoMo-based hydrodesulfurization catalyst. Appl. Catal. B Environ. 2012, 121–122, 50–56. [Google Scholar] [CrossRef]
- Mogica-Betancourt, J.C.; López-Benítez, A.; Montiel-López, J.R.; Massin, L.; Aouine, M.; Vrinat, M.; Berhault, G.; Guevara-Lara, A. Interaction effects of nickel polyoxotungstate with the Al2O3–MgO support for application in dibenzothiophene hydrodesulfurization. J. Catal. 2014, 313, 9–23. [Google Scholar] [CrossRef]
- Kishan, G.; Van Veen, J.; Niemantsverdriet, J. Realistic surface science models of hydrodesulfurization catalysts on planar thin-film supports: The role of chelating agents in the preparation of CoW/SiO2 catalysts. Top. Catal. 2004, 29, 103–110. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Lu, J.; Lu, H.; He, S.; Song, D.; Luo, Y.; Liu, J. Study of the Metal–Support Interaction and Electronic Effect Induced by Calcination Temperature Regulation and Their Effect on the Catalytic Performance of Glycerol Steam Reforming for Hydrogen Production. Nanomaterials 2021, 11, 3149. [Google Scholar] [CrossRef]
- Wang, B.; Ding, G.; Shang, Y.; Lv, J.; Wang, H.; Wang, E.; Li, Z.; Ma, X.; Qin, S.; Sun, Q. Effects of MoO3 loading and calcination temperature on the activity of the sulphur-resistant methanation catalyst MoO3/γ-Al2O3. Appl. Catal. A Gen. 2012, 431–432, 144–150. [Google Scholar] [CrossRef]
- He, L.; Ren, Y.; Yue, B.; Tsang, S.C.E.; He, H. Tuning Metal–Support Interactions on Ni/Al2O3 Catalysts to Improve Catalytic Activity and Stability for Dry Reforming of Methane. Processes 2021, 9, 706. [Google Scholar] [CrossRef]
- Liu, H.; Yin, C.; Liu, B.; Li, X.; Li, Y.; Chai, Y.; Liu, C. Effect of calcination temperature of unsupported NiMo catalysts on the hydrodesulfurization of dibenzothiophene. Energy Fuels 2014, 28, 2429–2436. [Google Scholar] [CrossRef]
- Wang, H.; Fan, Q.; Yang, Z.; Tang, S.; Chen, J.; Wu, Y. A novel pre-sulfided hydrotreating catalyst derived from thiomolybdate intercalated NiAl LDHs. Mol. Catal. 2019, 468, 1–8. [Google Scholar] [CrossRef]
- Abubakar, U.C.; Alhooshani, K.R.; Adamu, S.; Al Thagfi, J.; Saleh, T.A. The effect of calcination temperature on the activity of hydrodesulfurization catalysts supported on mesoporous activated carbon. J. Clean. Prod. 2019, 211, 1567–1575. [Google Scholar] [CrossRef]
- Fujikawa, T. Development of new CoMo HDS catalyst for ultra-low sulfur diesel fuel production. J. Jpn. Pet. Inst. 2007, 50, 249–261. [Google Scholar] [CrossRef]
- Bergwerff, J.A.; Visser, T.; Leliveld, G.; Rossenaar, B.D.; de Jong, K.P.; Weckhuysen, B.M. Envisaging the physicochemical processes during the preparation of supported catalysts: Raman microscopy on the impregnation of Mo onto Al2O3 extrudates. J. Am. Chem. Soc. 2004, 126, 14548–14556. [Google Scholar] [CrossRef]
- Woolfolk, L.G.; Geantet, C.; Massin, L.; Laurenti, D.; Reyes, J.A.D.L. Solvent effect over the promoter addition for a supported NiWS hydrotreating catalyst. Appl. Catal. B Environ. 2017, 201, 331–338. [Google Scholar] [CrossRef]
- Okamoto, Y.; Hioka, K.; Arakawa, K.; Fujikawa, T.; Ebihara, T.; Kubota, T. Effect of sulfidation atmosphere on the hydrodesulfurization activity of SiO2-supported Co–Mo sulfide catalysts: Local structure and intrinsic activity of the active sites. J. Catal. 2009, 268, 49–59. [Google Scholar] [CrossRef]
- Puello-Polo, E.; Gutiérrez-Alejandre, A.; González, G.; Brito, J.L. Relationship Between Sulfidation and HDS Catalytic Activity of Activated Carbon Supported Mo, Fe–Mo, Co–Mo and Ni–Mo Carbides. Catal. Lett. 2010, 135, 212–218. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Liu, T.; Jiang, Z.; Li, C. Optimizing both the CoMo/Al2O3 catalyst and the technology for selectivity enhancement in the hydrodesulfurization of FCC gasoline. Appl. Catal. A Gen. 2019, 575, 187–197. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Singh, S.; Schachtl, E.; Kim, J.; Kondratieva, E.; Hein, J.; Lercher, J.A. Effects of the Support on the Performance and Promotion of (Ni)MoS2 Catalysts for Simultaneous Hydrodenitrogenation and Hydrodesulfurization. ACS Catal. 2014, 4, 1487–1499. [Google Scholar] [CrossRef]
- Chen, J.; Labruyere, V.; Maugé, F.; Quoineaud, A.-A.; Hugon, A.; Oliviero, L. IR Spectroscopic Evidence for MoS2 Morphology Change with Sulfidation Temperature on MoS2/Al2O3 Catalyst. J. Phys. Chem. C 2014, 118, 30039–30044. [Google Scholar] [CrossRef]
- Gao, D.; Duan, A.; Zhang, X.; Zhao, Z.; Hong, E.; Li, J.; Wang, H. Synthesis of NiMo catalysts supported on mesoporous Al-SBA-15 with different morphologies and their catalytic performance of DBT HDS. Appl. Catal. B Environ. 2015, 165, 269–284. [Google Scholar] [CrossRef]
- Ye, L.; Xu, H.; Zhang, D.; Chen, S. Synthesis of bilayer MoS2 nanosheets by a facile hydrothermal method and their methyl orange adsorption capacity. Mater. Res. Bull. 2014, 55, 221–228. [Google Scholar] [CrossRef]
- Liu, Y.D.; Ren, L.; Qi, X.; Yang, L.W.; Hao, G.L.; Li, J.; Wei, X.L.; Zhong, J.X. Preparation, characterization and photoelectrochemical property of ultrathin MoS2 nanosheets via hydrothermal intercalation and exfoliation route. J. Alloys Compd. 2013, 571, 37–42. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Mei, L.; Ma, D.; Liao, Y.; Zu, Y.; Xu, P.; Yin, W.; Gu, Z. A two-step gas/liquid strategy for the production of N-doped defect-rich transition metal dichalcogenide nanosheets and their antibacterial applications. Nanoscale 2020, 12, 8415–8424. [Google Scholar] [CrossRef]
- Rajendhran, N.; Palanisamy, S.; Periyasamy, P.; Venkatachalam, R. Enhancing of the tribological characteristics of the lubricant oils using Ni-promoted MoS2 nanosheets as nano-additives. Tribol. Int. 2018, 118, 314–328. [Google Scholar] [CrossRef]
- Backes, C.; Berner, N.C.; Chen, X.; Lafargue, P.; LaPlace, P.; Freeley, M.; Duesberg, G.S.; Coleman, J.N.; McDonald, A.R. Functionalization of liquid-exfoliated two-dimensional 2H-MoS2. Angew. Chem. Int. Ed. 2015, 54, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.A.; Alhooshani, K.; Ali, S.A. Single-pot synthesis of Ti-SBA-15-NiMo hydrodesulfurization catalysts: Role of calcination temperature on dispersion and activity. Appl. Catal. B Environ. 2017, 203, 428–441. [Google Scholar] [CrossRef]
- Han, W.; Yuan, P.; Fan, Y.; Shi, G.; Liu, H.; Bai, D.; Bao, X. Preparation of supported hydrodesulfurization catalysts with enhanced performance using Mo-based inorganic–organic hybrid nanocrystals as a superior precursor. J. Mater. Chem. 2012, 22, 25340. [Google Scholar] [CrossRef]
- Han, W.; Nie, H.; Long, X.; Li, M.; Yang, Q.; Li, D. A study on the origin of the active sites of HDN catalysts using alumina-supported MoS3 nanoparticles as a precursor. Catal. Sci. Technol. 2016, 6, 3497–3509. [Google Scholar] [CrossRef]
- Fan, Y.; Bao, X.; Wang, H.; Chen, C.; Shi, G. A surfactant-assisted hydrothermal deposition method for preparing highly dispersed W/γ-Al2O3 hydrodenitrogenation catalyst. J. Catal. 2007, 245, 477–481. [Google Scholar] [CrossRef]
- Gao, M.-R.; Chan, M.K.Y.; Sun, Y. Edge-terminated molybdenum disulfide with a 9.4-Å interlayer spacing for electrochemical hydrogen production. Nat. Commun. 2015, 6, 7493. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Gołasa, K.; Grzeszczyk, M.; Bożek, R.; Leszczyński, P.; Wysmołek, A.; Potemski, M.; Babiński, A. Resonant Raman scattering in MoS2—From bulk to monolayer. Solid State Commun. 2014, 197, 53–56. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, C.; Zhou, P.; Liu, Z.; Xu, Y.; Wang, D.; Fang, B. Enhanced hydrogen evolution catalysis from osmotically swollen ammoniated MoS2. J. Mater. Chem. A 2015, 3, 13050–13056. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Xiao, B.; Pradhan, A.K. Energy band alignment of high-k oxide heterostructures at MoS2/Al2O3 and MoS2/ZrO2 interfaces. J. Appl. Phys. 2016, 120, 125305. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single-and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Chai, J.; Lu, X.; Wong, L.M.; Wong, T.I.; Pan, J.; Xiong, Q.; Chi, D.; Wang, S. Growth of wafer-scale MoS2 monolayer by magnetron sputtering. Nanoscale 2015, 7, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Zhao, X.; Cui, K.; Shen, Q.; Li, P.; Qu, X.; Jiao, L. Molecular Engineering on MoS2 Enables Large Interlayers and Unlocked Basal Planes for High-Performance Aqueous Zn-Ion Storage. Angew. Chem. Int. Ed. 2021, 60, 20286–20293. [Google Scholar] [CrossRef]
- Lai, W.; Chen, Z.; Zhu, J.; Yang, L.; Zheng, J.; Yi, X.; Fang, W. A NiMoS flower-like structure with self-assembled nanosheets as high-performance hydrodesulfurization catalysts. Nanoscale 2016, 8, 3823–3833. [Google Scholar] [CrossRef]
- Wang, S.; An, C.; Yuan, J. Synthetic Fabrication of Nanoscale MoS2-Based Transition Metal Sulfides. Materials 2010, 3, 401–433. [Google Scholar] [CrossRef]
- Qiu, L.; Xu, G. Peak overlaps and corresponding solutions in the X-ray photoelectron spectroscopic study of hydrodesulfurization catalysts. Appl. Surf. Sci. 2010, 256, 3413–3417. [Google Scholar] [CrossRef]
- Chen, J.; Oliviero, L.; Portier, X.; Maugé, F. On the morphology of MoS2slabs on MoS2/Al2O3 catalysts: The influence of Mo loading. RSC Adv. 2015, 5, 81038–81044. [Google Scholar] [CrossRef]
- Yi, Y.; Jin, X.; Wang, L.; Zhang, Q.; Xiong, G.; Liang, C. Preparation of unsupported Ni–Mo–S catalysts for hydrodesulfurization of dibenzothiophene by thermal decomposition of tetramethylammonium thiomolybdates. Catal. Today 2011, 175, 460–466. [Google Scholar] [CrossRef]
- Lumbreras, J.A.; Huirache-Acuña, R.; Rivera-Muñoz, E.M.; Berhault, G.; Alonso-Núñez, G. Unsupported Ni/Mo(W)S2 Catalysts from Hexamethylenediammonium Thiometallates Precursors: In Situ Activation During the HDS of DBT. Catal. Lett. 2009, 134, 138–146. [Google Scholar] [CrossRef]
- Yoosuk, B.; Kim, J.H.; Song, C.; Ngamcharussrivichai, C.; Prasassarakich, P. Highly active MoS2, CoMoS2 and NiMoS2 unsupported catalysts prepared by hydrothermal synthesis for hydrodesulfurization of 4,6-dimethyldibenzothiophene. Catal. Today 2008, 130, 14–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).