Diffusion Behavior of Carbon and Silicon in the Process of Preparing Silicon Steel Using Solid-State Decarburization
Abstract
:1. Introduction
2. Experimental Program
2.1. Modelling
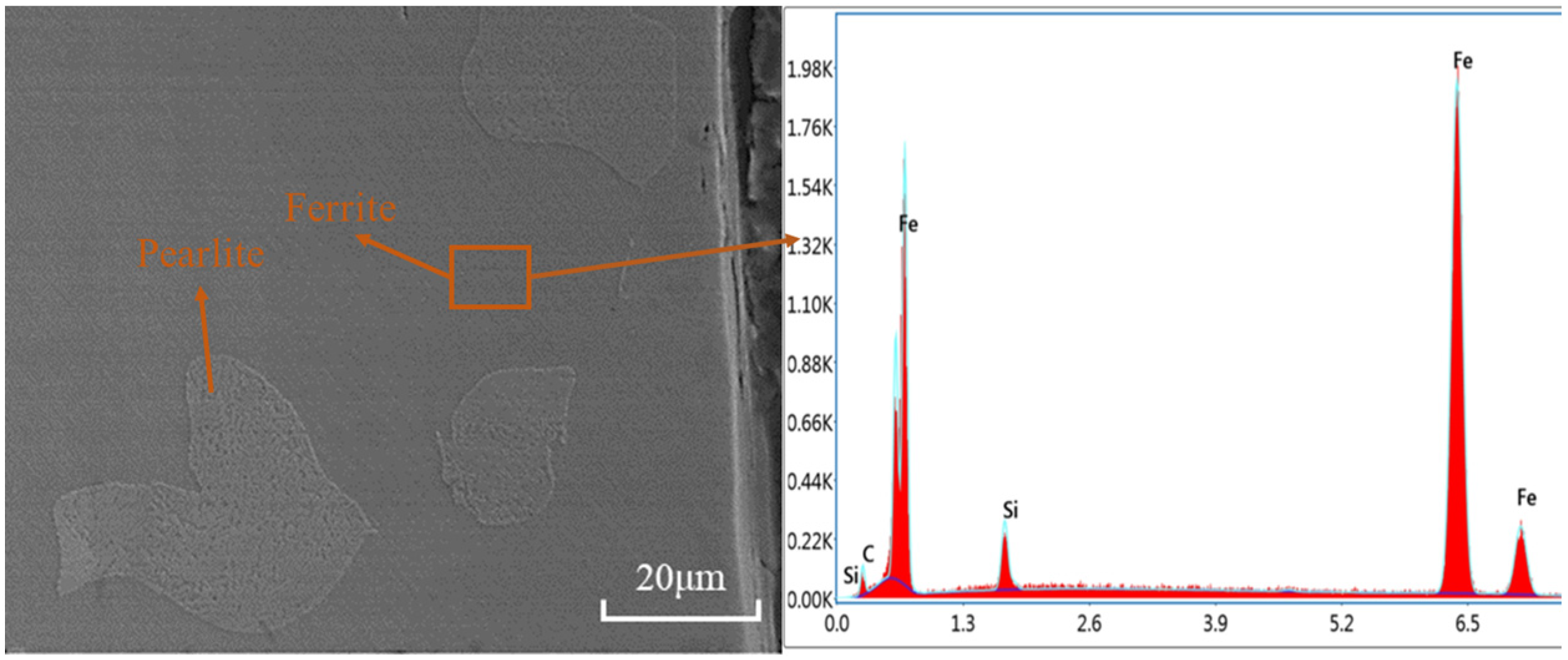
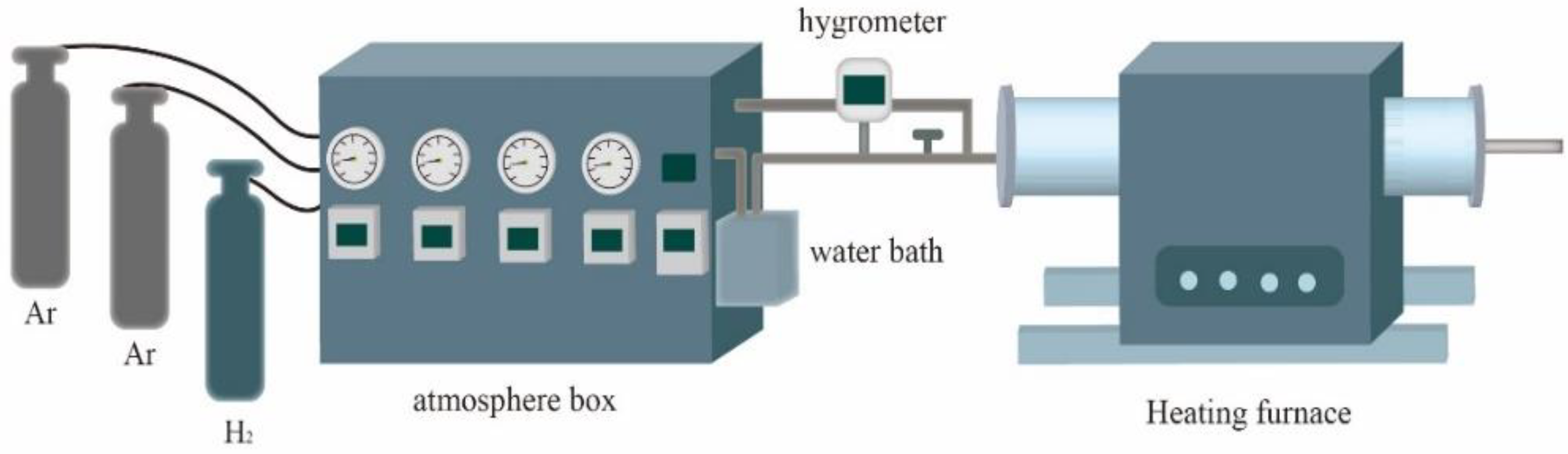
2.2. Experimental Materials and Methods
3. Results and Analysis
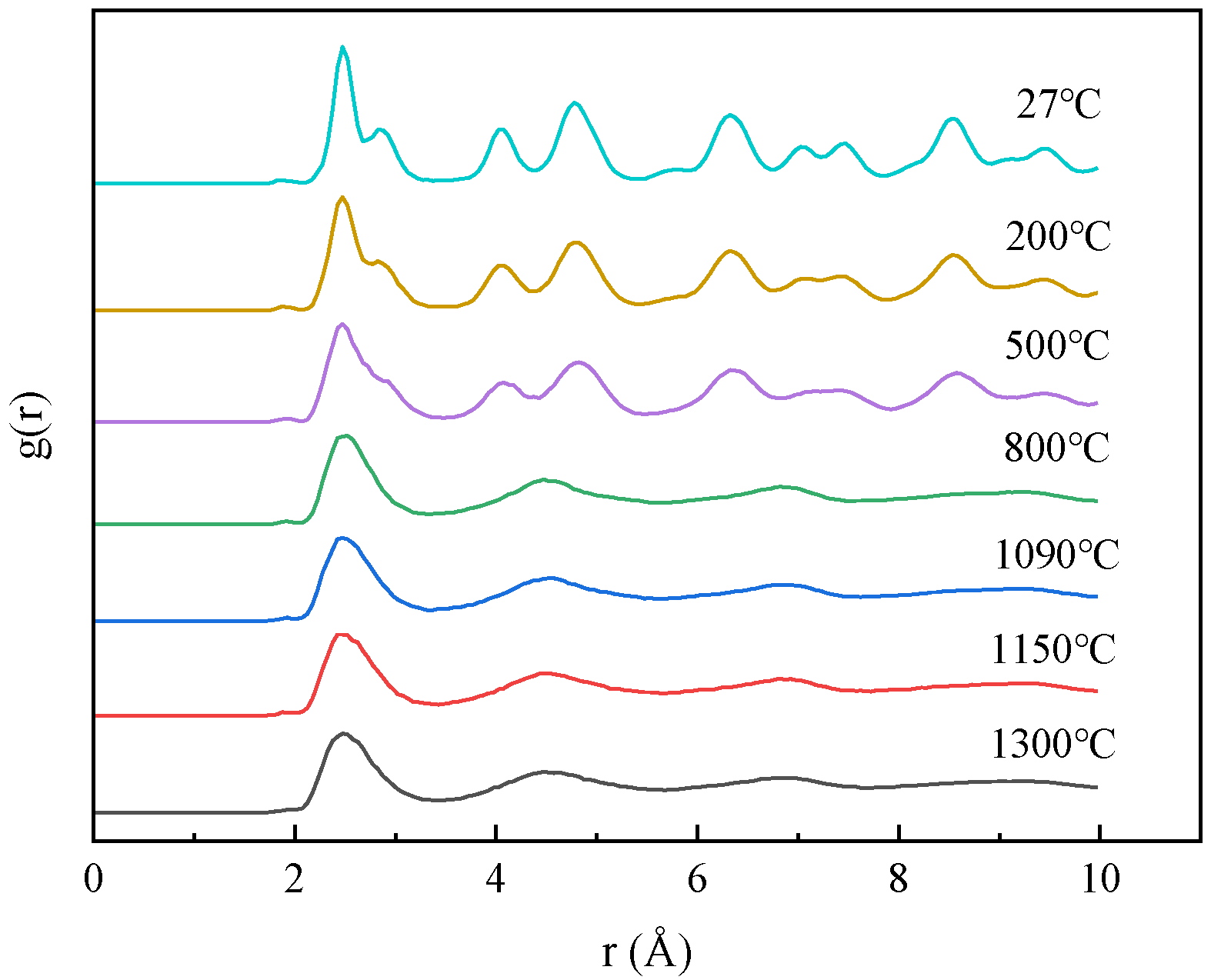
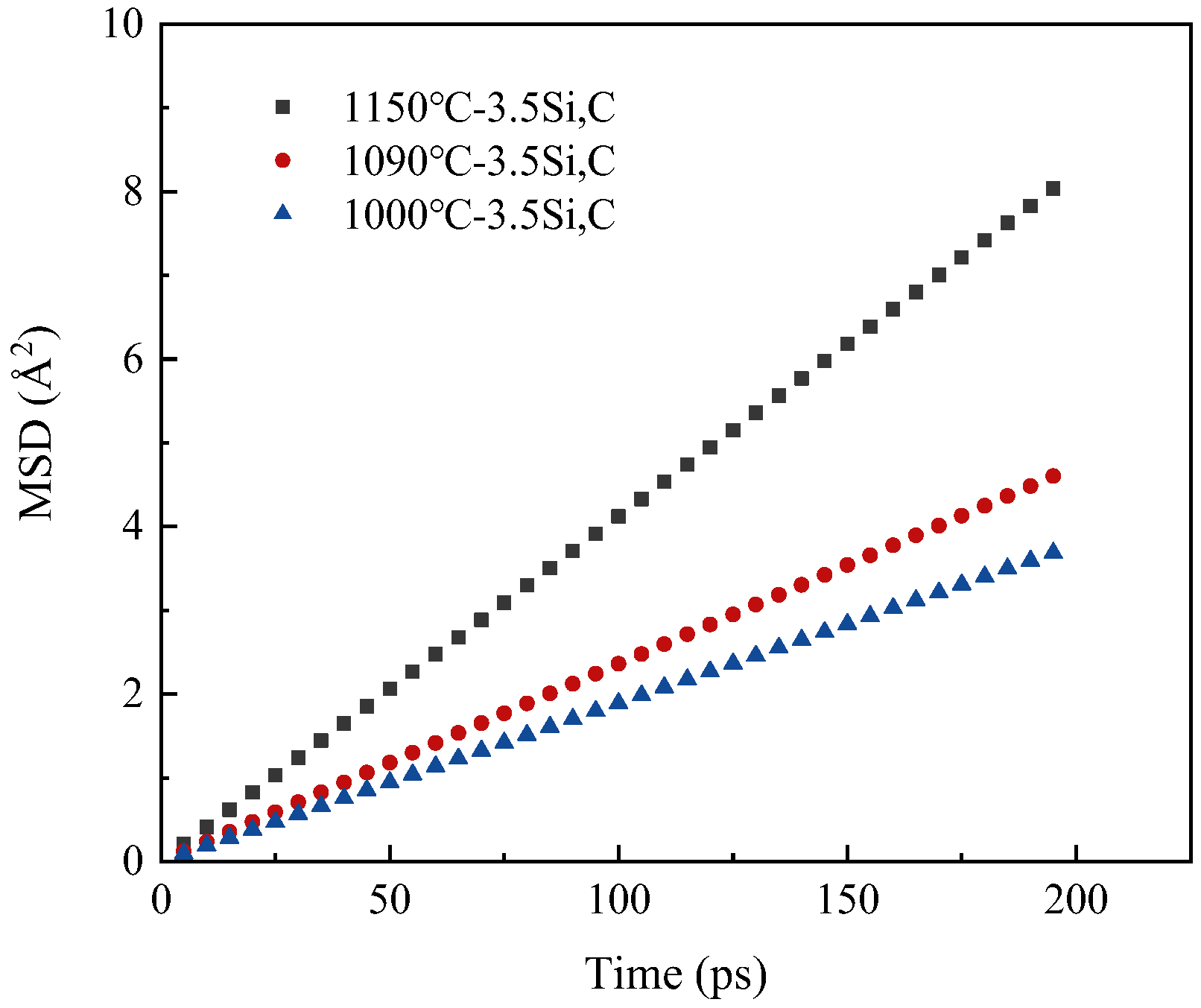
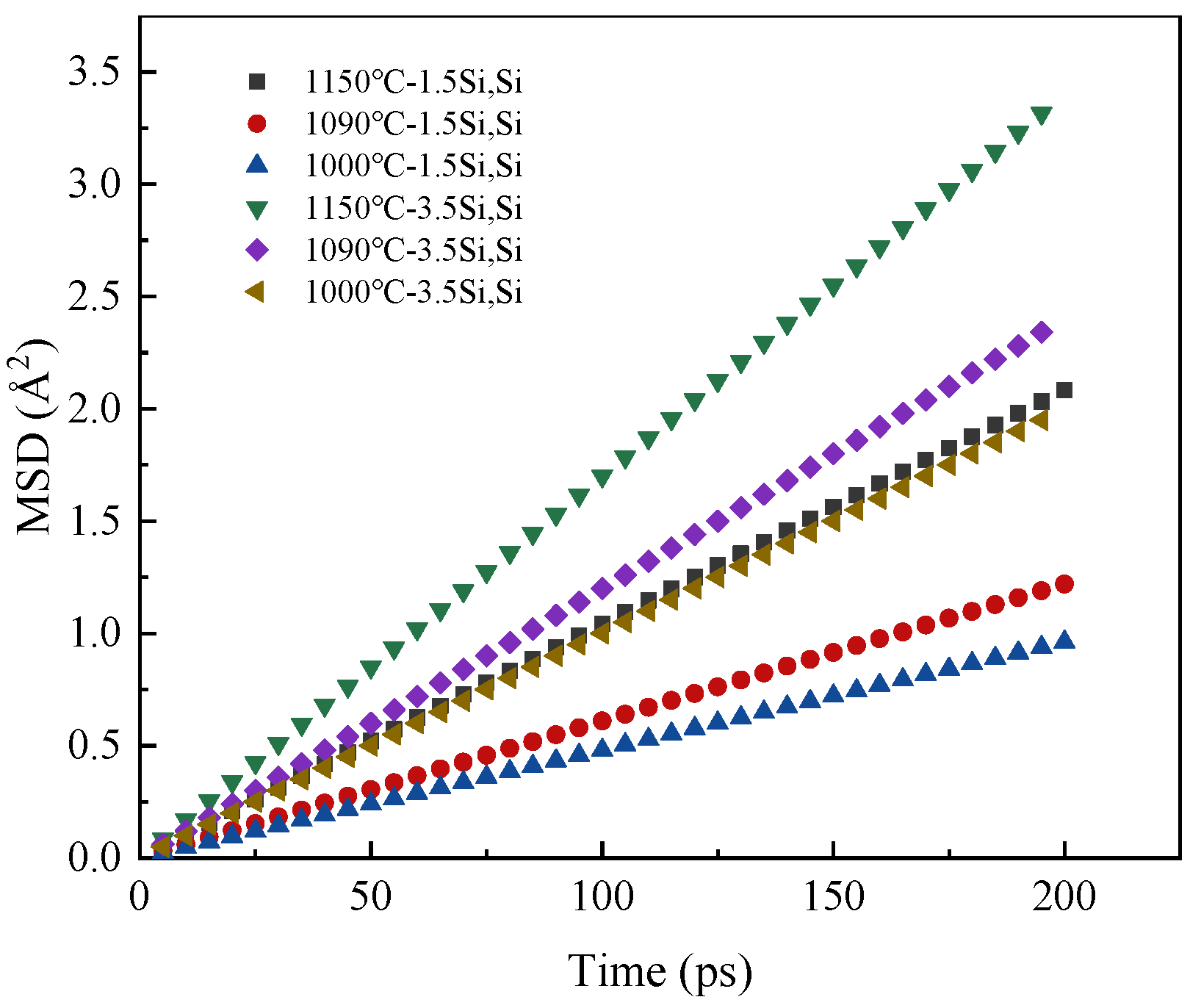
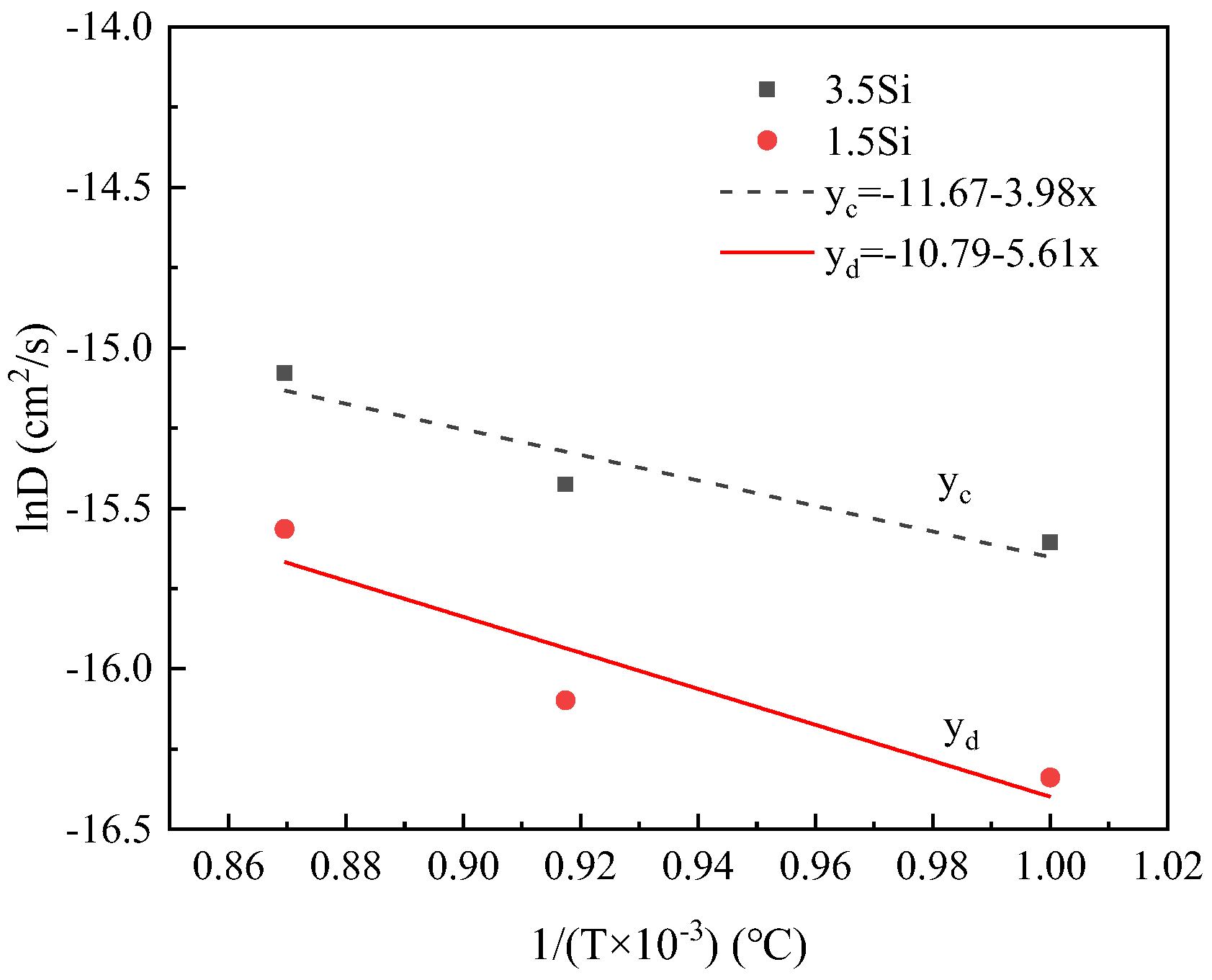
3.1. Effect of Temperature on Diffusion Behavior
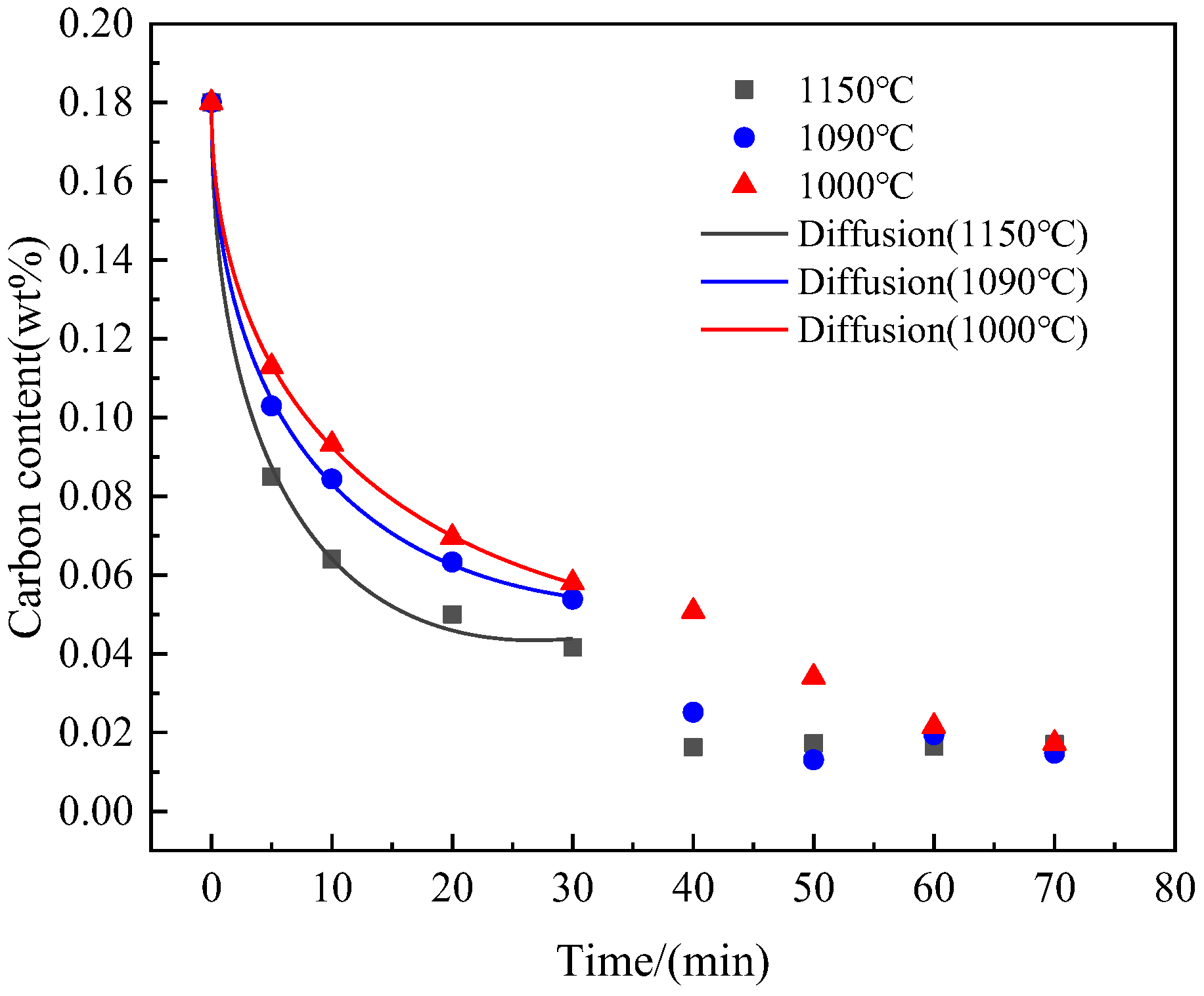
3.2. Verification of Simulation Results Combined with Decarburization Kinetics
- [%C]0—initial carbon content, wt%;
- k, k′ are the coefficients and their expressions are, respectively:
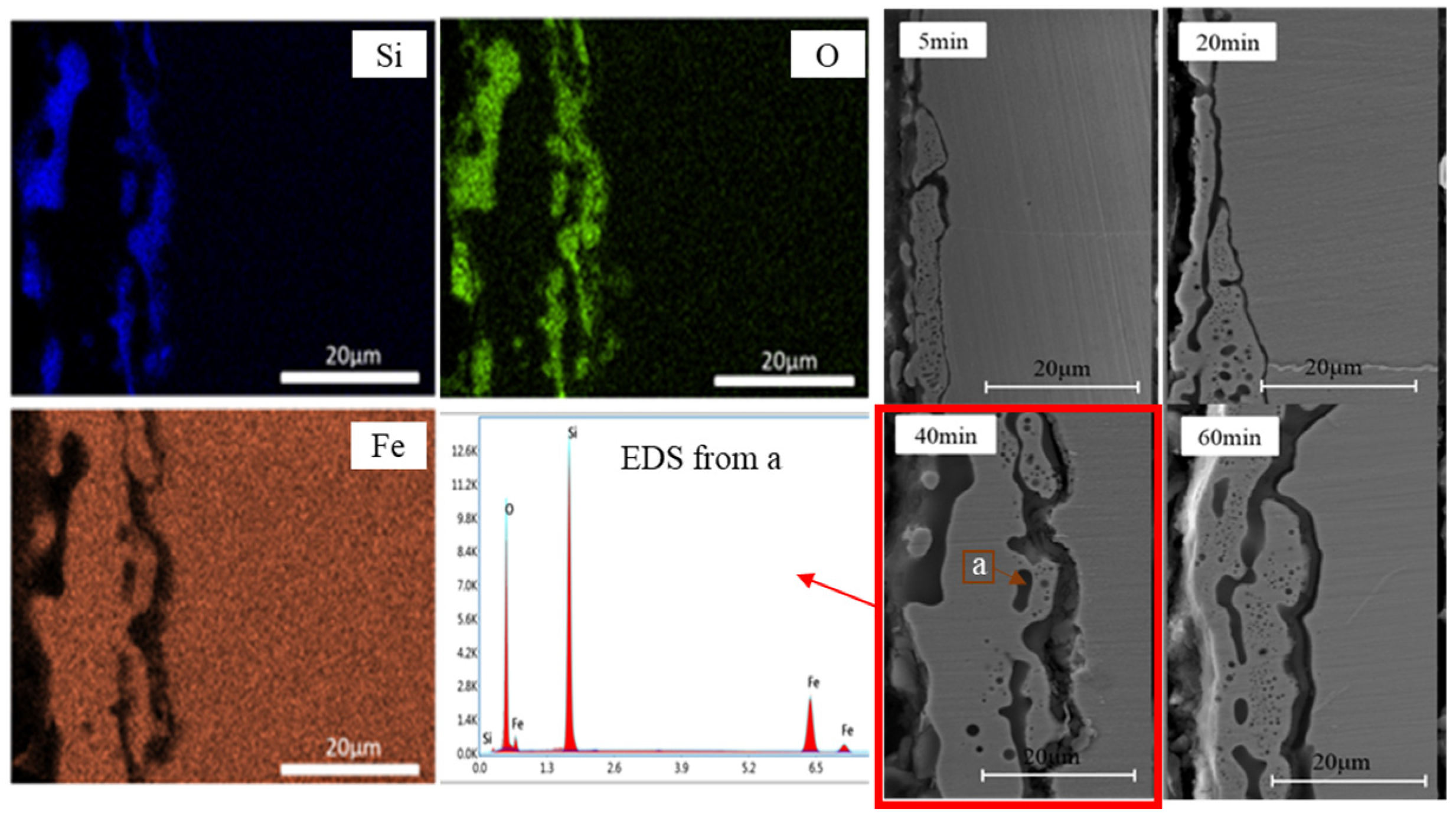
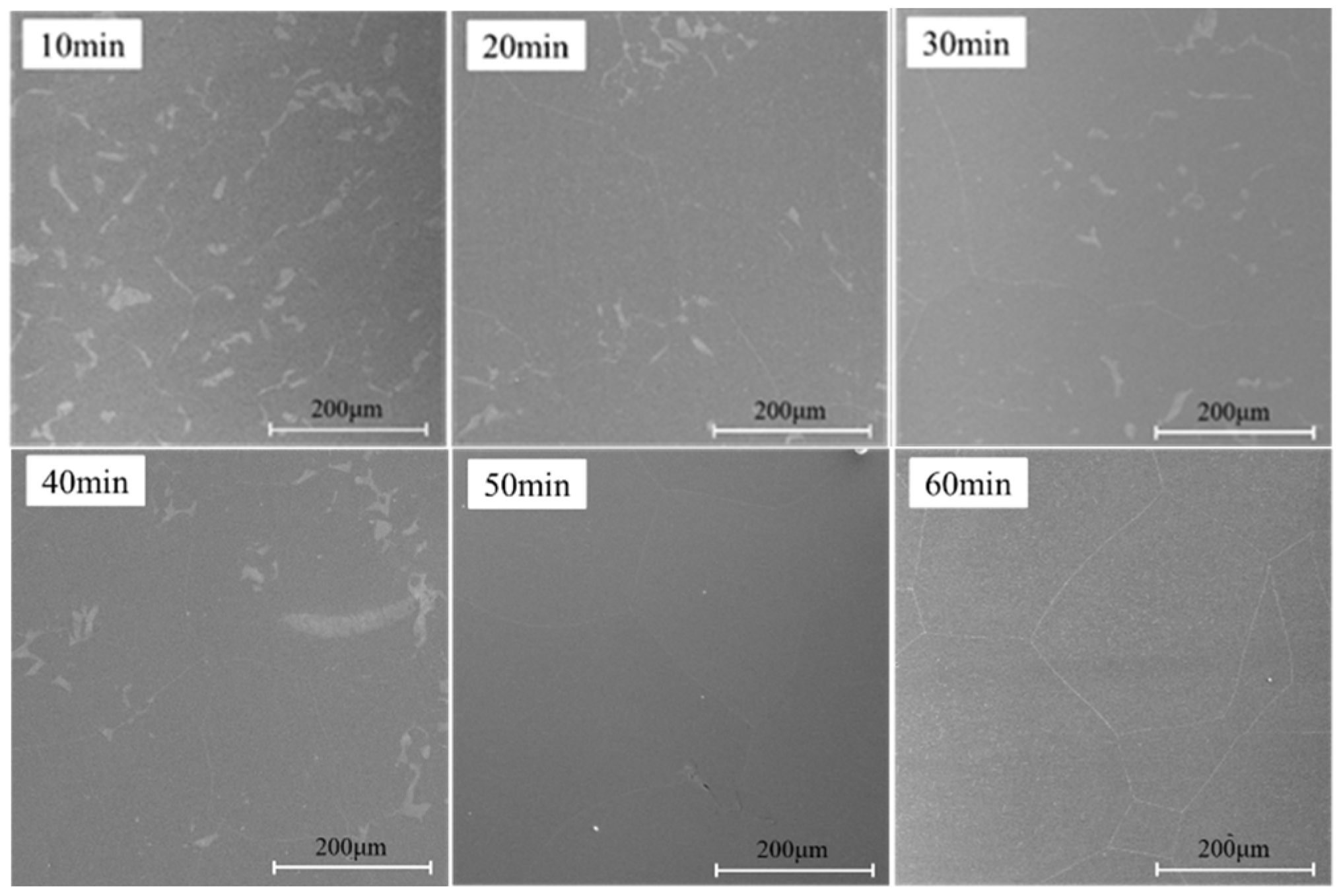
3.3. Effect of Carbon and Silicon Diffusion on Oxide Growth
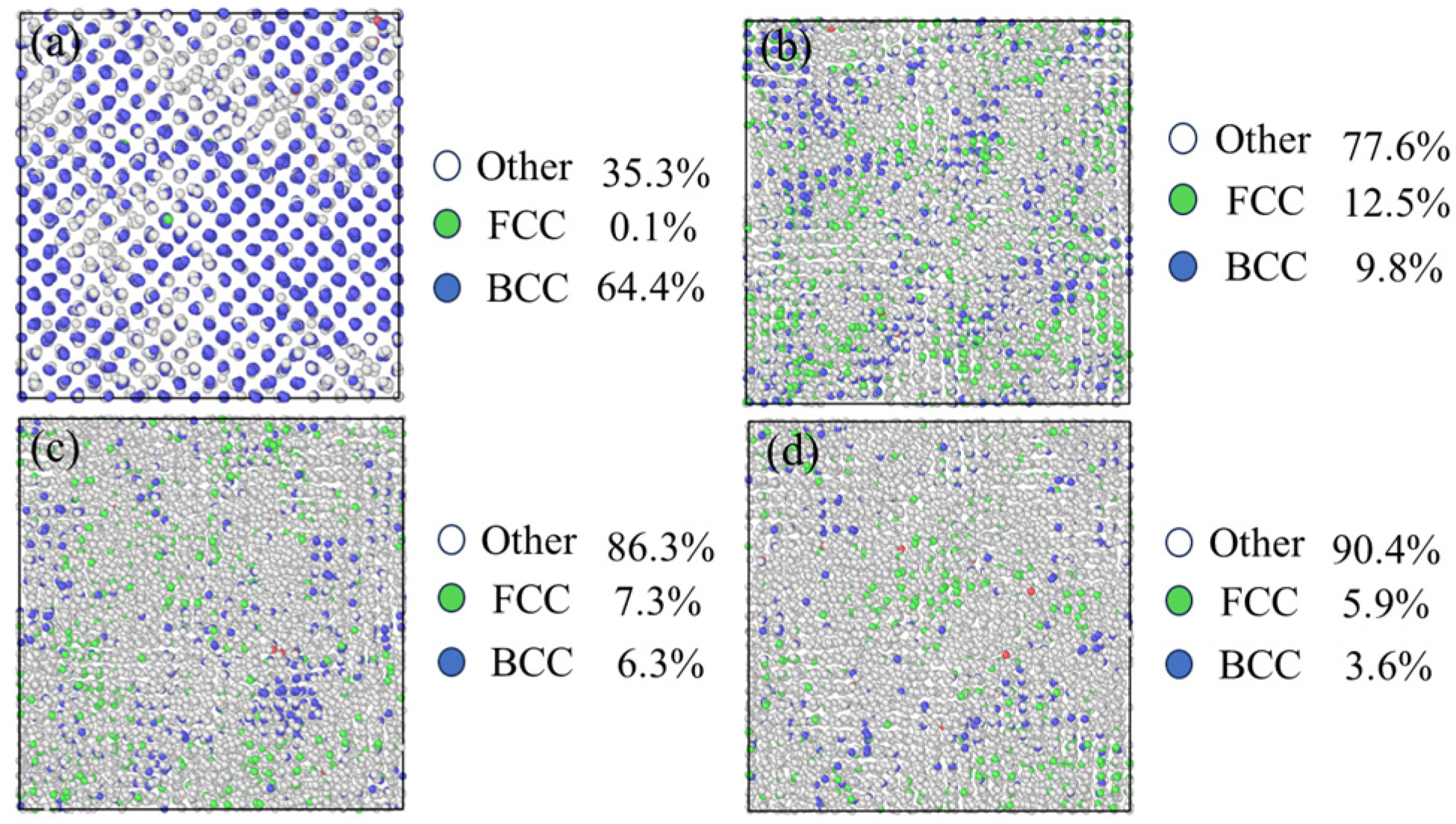
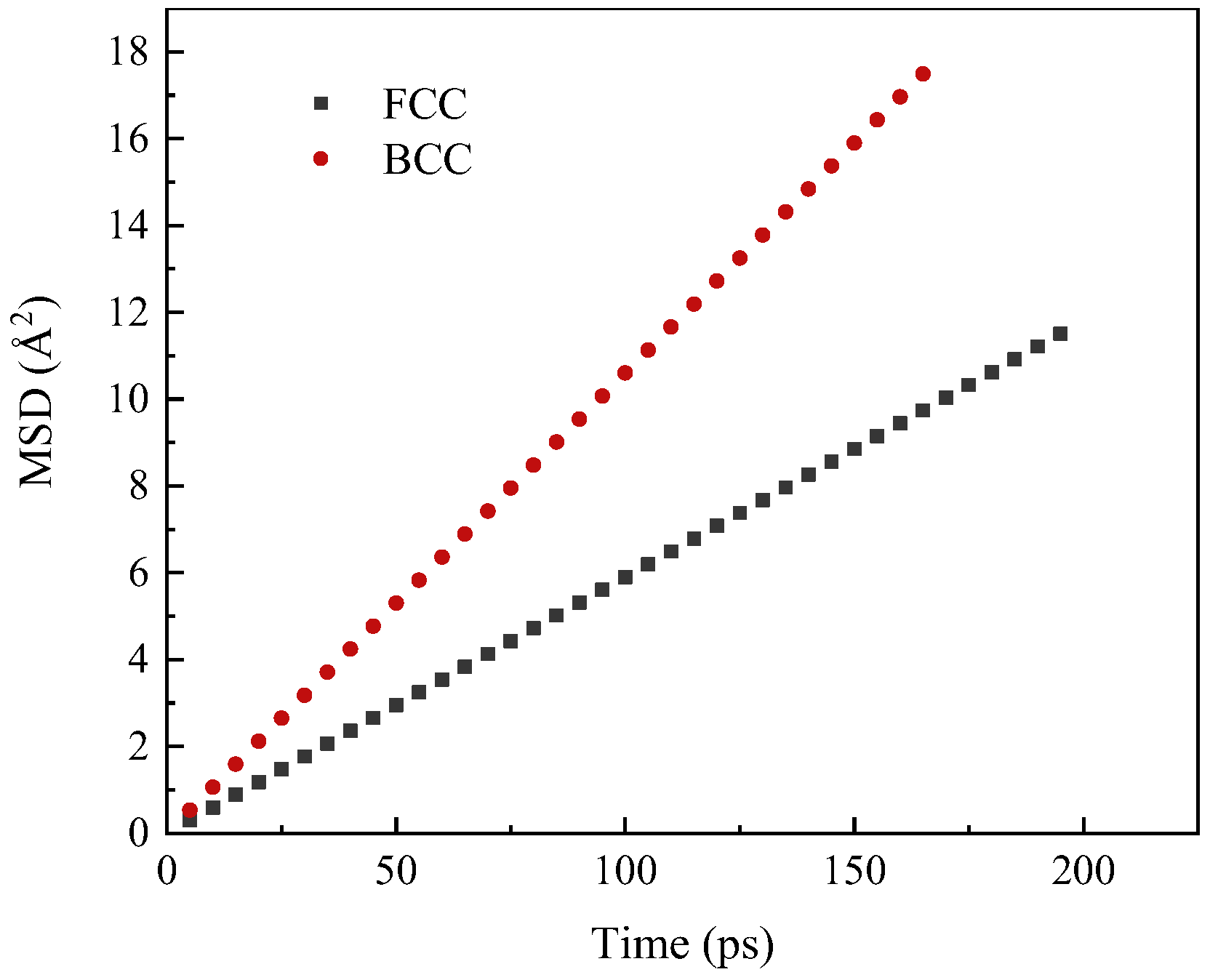
3.4. Effect of the Phase Transition on Diffusion Behavior
3.5. Effect of Silicon Content on Diffusion Behavior
4. Conclusions
- (1)
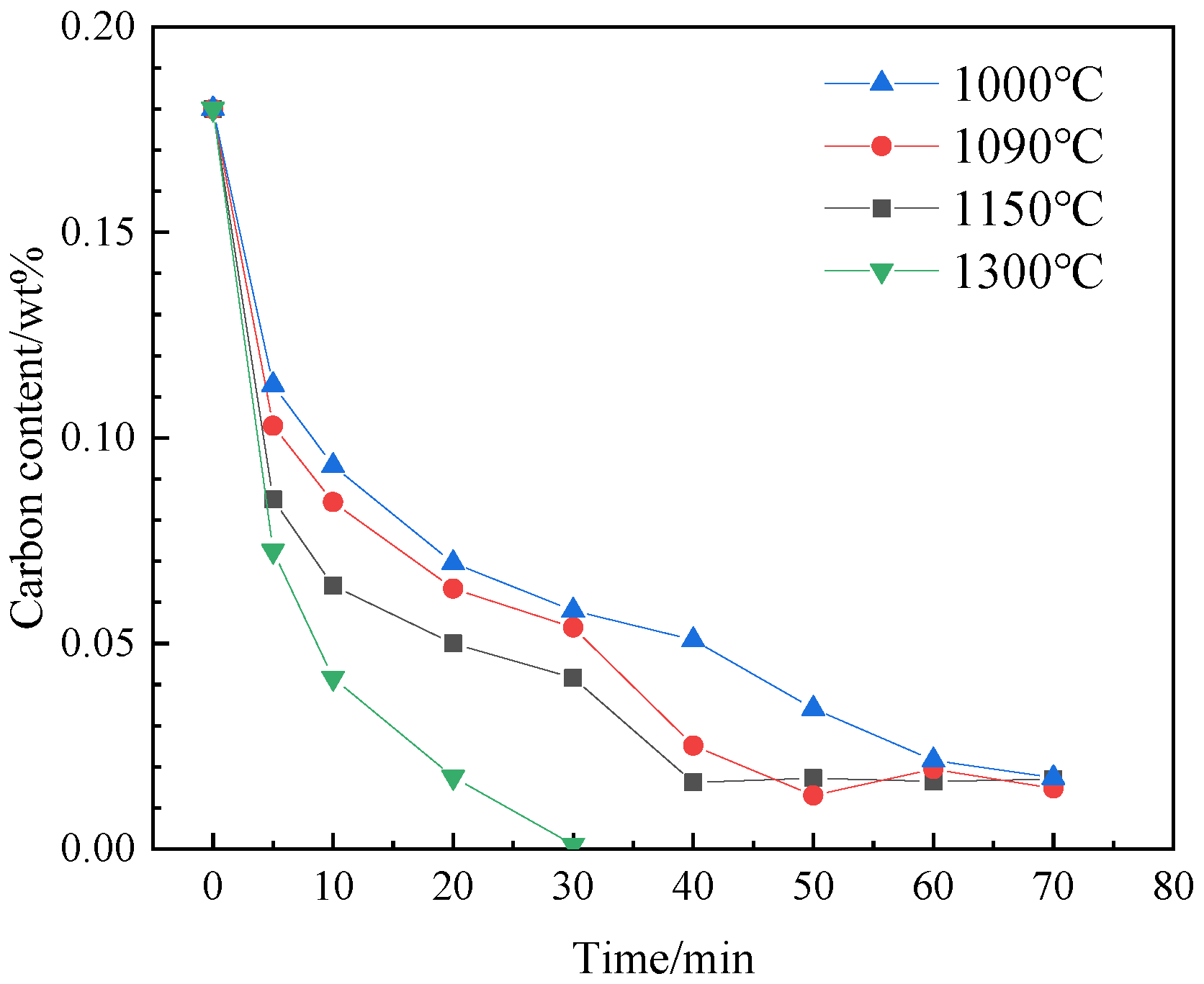
- The experimental results show that with the same decarburization time, the higher the temperature (1000–1300 °C), the faster the decarburization rate; Results from molecular dynamics simulations indicate that at temperatures below 800 °C, atoms maintain a tightly packed bcc structure, resulting in a low migration rate of carbon. When the temperature is higher than 800 °C, the atoms move violently and the crystal structure is destroyed. Some atoms tend to be disordered, the migration rate of C is high, and the diffusion coefficient increases. The thin strip substrate undergoes a lattice transformation from face-centered cubic lattice austenite to body-centered cubic lattice ferrite. Notably, the diffusion coefficient of C in ferrite significantly exceeds that in austenite, giving rise to an observed enhancement in decarburization rate just before decarburization stagnation (30 min to 50 min);
- (2)
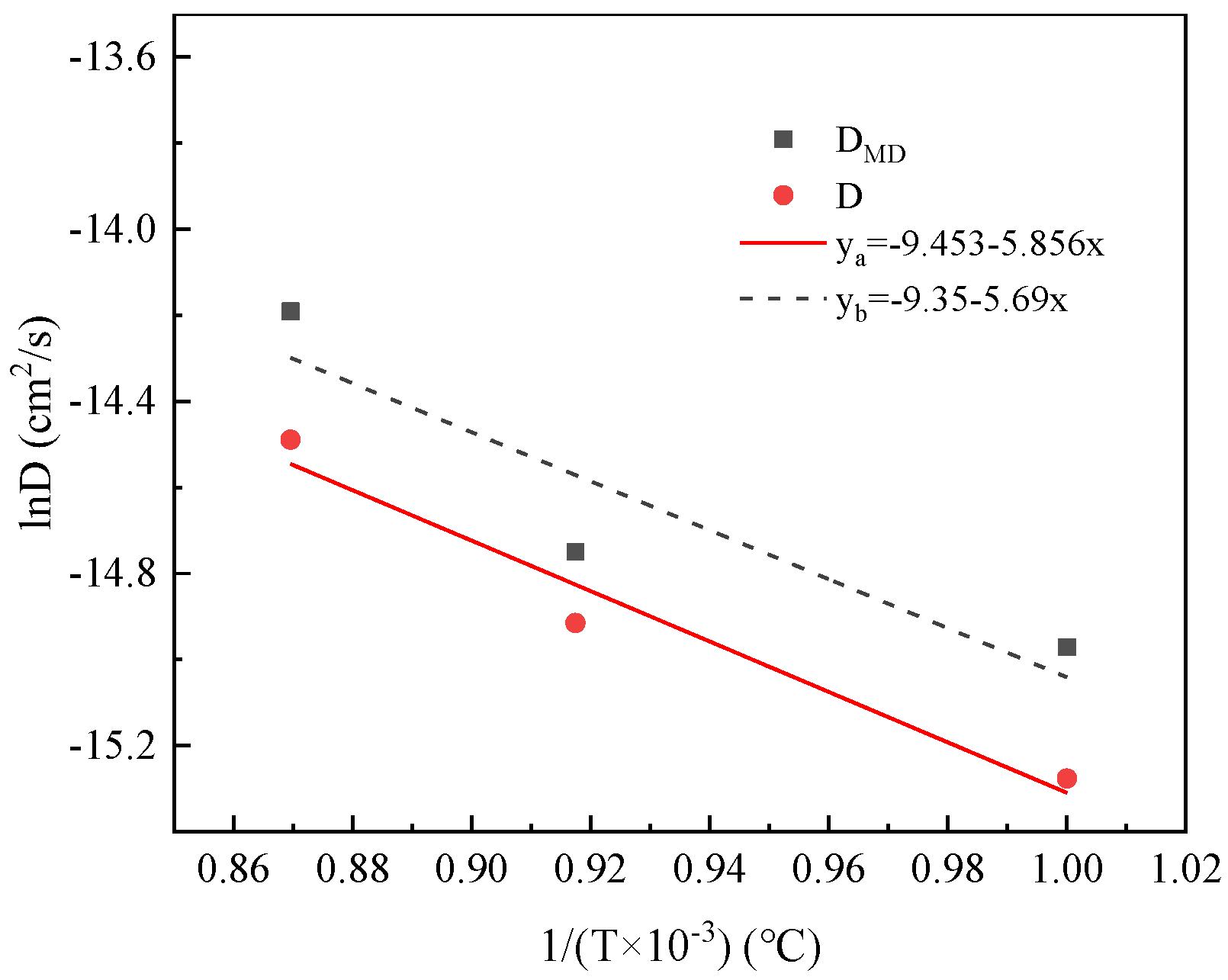
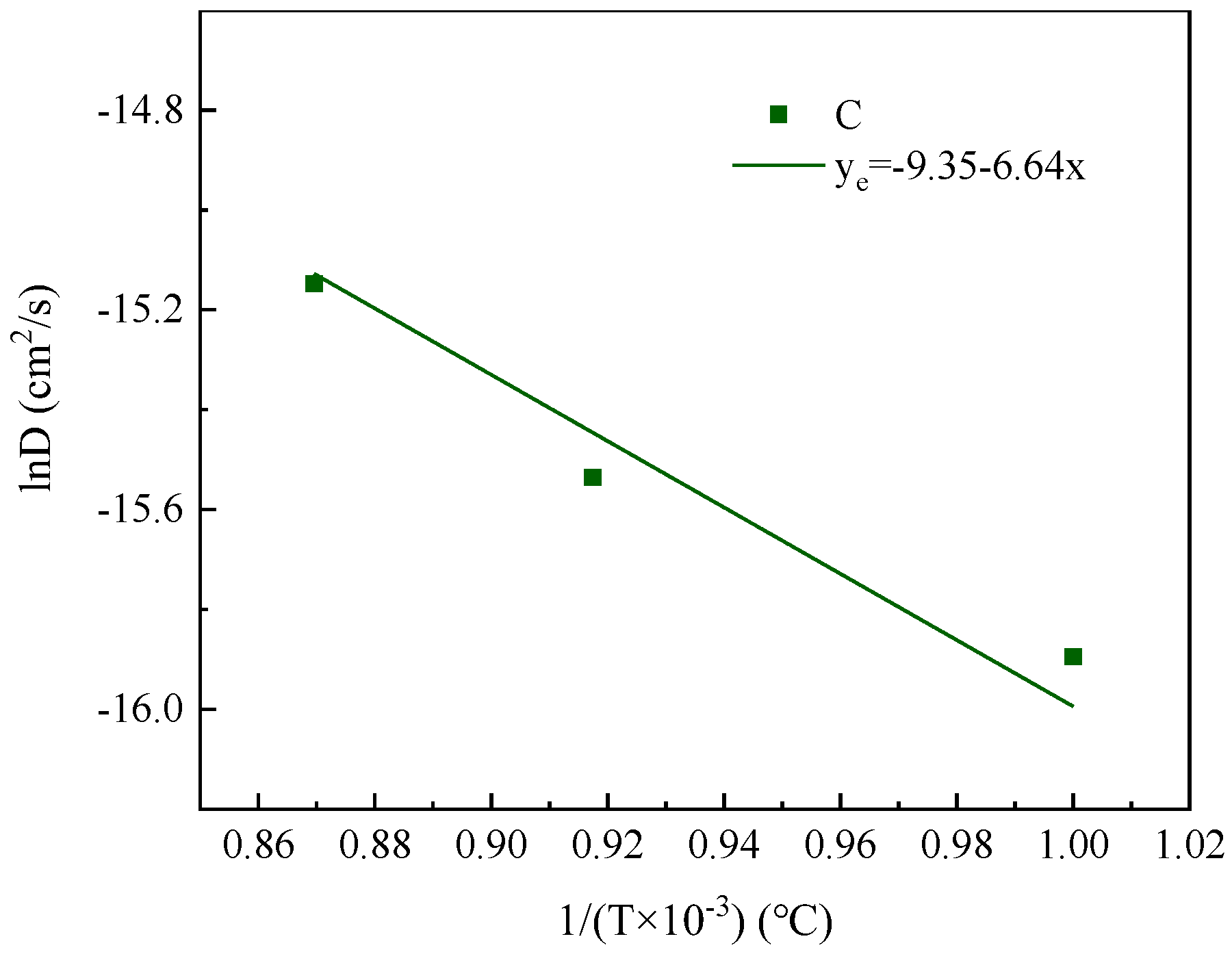
- Based on a decarburization model that considers carbon diffusion within the matrix as the rate-limiting step in the early stages of decarburization, the diffusion coefficient for carbon was determined. Subsequently, the diffusion activation energy for C in the Fe-3.5% Si-C alloy matrix was calculated as Q = 48.7 kJ·mol−1. Molecular dynamics simulations yielded a diffusion activation energy of 47.3 kJ·mol−1 for C in the Fe-3.5% Si-C alloy matrix, which closely aligns with experimental findings;
- (3)
- The diffusion coefficients of carbon were found to be D3.5Si, C = 7.84 × 10−9 exp(−48,686.7/RT) m2/s and D1.5Si, C = 8.69 × 10−10 exp(−55,204.9/RT) m2/s when the silicon content was 3.5 wt% and 1.5 wt%, respectively. In comparison to Si = 1.5 wt%, at the same decarburization time, Si = 3.5 wt% exhibited a higher decarburization rate during the initial 40 min. At a decarburization temperature of 1150 °C, the diffusion coefficients of C were determined to be 2.64 × 10−11 m2/s and 6.87 × 10−11 m2/s for Si contents of 1.5 wt% and 3.5 wt%, respectively. This suggests that an increase in silicon content leads to a reduction of the activation energy for the C diffusion and an increase in the carbon diffusion coefficient, thereby promoting the decarburization process;
- (4)
- Molecular dynamics simulations reveal that the diffusion coefficients of silicon were determined to be D3.5Si, Si = 8.54 × 10−10 exp(−33,089.7/RT) m2/s and D1.5Si, Si = 2.06 × 10−9 exp(−46,641.5/RT) m2/s when the Si content was 3.5 wt% and 1.5 wt%, respectively. At different temperatures, the diffusion coefficient of carbon was consistently higher than that of Si. Carbon diffuses to the surface before Si, consuming a significant amount of oxygen. When the Si content is 3.5 wt%, the diffusion coefficient of Si is relatively high, allowing it to diffuse to the near-surface region, where it combines with the remaining O to form a continuous banded SiO2 structure. In contrast, when the Si content is 1.5 wt%, the Si diffusion coefficient is significantly lower, causing the remaining O to diffuse within the matrix and oxidize upon encountering Si. In this case, SiO2 does not aggregate into banded structures but instead exhibits a highly-dispersed distribution.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, K.M. Production process and development of non-oriented electrical steel. China Metall. 2012, 22, 2430–2435. [Google Scholar]
- Zhang, Y.; Zhu, G.H.; Wu, Q. Development prospect of CSP production process for non-oriented electrical steel. Mod. Metall. 2013, 41, 1–4. [Google Scholar]
- Xiang, L.; Chen, S.L.; Pei, Y.H. Present situation and research progress of electrical steel produced by thin slab continuous casting and rolling in China. In Proceedings of the 11th China Iron and Steel Annual Conference, Beijing, China, 27 May 2017. [Google Scholar]
- Hong, L.K.; Ai, L.Q.; Cheng, R. Experimental study on Decarburization of Fe-C alloy strip by gas-solid reaction. Steel 2016, 51, 27–31. [Google Scholar]
- Mcdonald, C. Solid state steelmaking: Process technical and economic viability. Ironmak. Steelmak. 2012, 39, 487–489. [Google Scholar] [CrossRef]
- Park, J.O.; Long, T.V.; Sasaki, Y. Feasibility of solid-state steelmaking from cast iron-decarburization of rapidly solidified cast iron. Tetsu Hagane 2012, 98, 151–160. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, J.O.; Lee, J.S. Solid state steelmaking by decarburization of rapidly solidified high carbon iron sheet. Ironmak. Steelmak. 2012, 39, 530–534. [Google Scholar] [CrossRef]
- Liang, M.; Cheng, J.; Lin, W.M. Thermodynamic Study on Solid Phase Decarburization of High Carbon Ferromanganese Powder. Ferroalloy 2009, 40, 14–17. [Google Scholar]
- He, H.Q.; Wei, X.J.; Kang, G.Z. Study on solid state decarburization of high carbon ferromanganese in carbon dioxide atmosphere. Ferroalloy 2010, 41, 5–8. [Google Scholar]
- Li, Q. Gas Solid Decarburization of Fe—3.2% C Alloy Strip; North China University of Technology: Beijing, China, 2017. [Google Scholar]
- Li, Y.Q. Effect of Atmosphere Conditions on Gas Solid Reaction Decarburization of Iron Carbon Alloy Strip; North China University of Technology: Beijing, China, 2018. [Google Scholar]
- Cheng, R. Kinetic Analysis of Decarburization of Fe-C Alloy Strip by Gas-Solid Reaction; North China University of Technology: Beijing, China, 2018. [Google Scholar]
- Ai, L.Q.; Hou, Y.B.; Hong, L.K. Kinetics of gas-solid decarburization of Fe-C alloy strip in H2/H2O atmosphere. J. Eng. Sci. 2021, 43, 816–824. [Google Scholar]
- Zhou, M.J. Decarburization Kinetics of Fe-C Alloy Strip by Gas-Solid Reaction in Ar-CO-CO2 Atmosphere; North China University of Technology: Beijing, China, 2022. [Google Scholar]
- Guo, Y.; Dai, F.Q.; Gao, Y. Study on decarburization process model of grain-oriented silicon steel. In Proceedings of the 2016 Seminar on Production Technology and Application of Domestic High Performance Electrical Steel, Dalian, China, 25 August 2016. [Google Scholar]
- Yang, S.Z.; Dai, F.Q.; Guo, Y. Numerical simulation analysis on decarburization process of grain-oriented silicon steel. J. Wuhan Univ. Sci. Technol. 2016, 39, 253–258. [Google Scholar]
- Hao, J.J.; Chen, J.; Han, P.D. Solid Phase Decarburization Kinetics of High-Carbon Ferrochrome Powders in the Microwave Field. Steel Res. Int. 2014, 85, 461–465. [Google Scholar] [CrossRef]
- Larssen, T.A.; Senk, D.; Tangstad, M. Reaction Rate Analysis of Manganese Ore Prereduction in CO-CO2 Atmosphere. Metall. Mater. Trans. B 2021, 52, 2087–2100. [Google Scholar] [CrossRef]
- Li, H.; Liang, J.L.; Li, Y.G. Study on the diffusion behavior of Si during annealing. Ind. Heat. 2014, 43, 30–32+41. [Google Scholar]
- Zhu, Y.B. Mathematical model of decarburization of steel during heating. Spec. Steel 1989, 3, 1–4. [Google Scholar]
- Zhang, C.; Xiang, L.; Dong, Y.L. Experimental study on decarburization annealing of grain-oriented silicon steel. J. Iron Steel Res. 2009, 21, 55–58. [Google Scholar]
- Agren, J. Computer simulations of the austenite/ferrite diffusional transformations in low alloyed steels. Acta Metall. 1982, 30, 841–851. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Ai, L.Q.; Hong, L.K. Experimental study on Decarburization of Fe-C-Si alloy in solid state. J. Mater. Metall. 2023, 22, 45–51+66. [Google Scholar]
- Liu, Y.B.; Zhang, W.; Tong, Q. Effects of Si and Cr on complete decarburization behavior of high carbon steels in atmosphere of 2 vol.% O2. J. Iron Steel Res. 2016, 23, 1316–1322. [Google Scholar] [CrossRef]
- Li, N. Molecular Dynamics Simulation of Tensile Behavior of Ti2Cu Inter Metallics; Changchun University of Technology: Changchun, China, 2022. [Google Scholar]
- Wang, W.T.; Wang, H.; Lu, X.G. Molecular dynamics simulation of tracer diffusion coefficient of liquid Ni Al Binary Alloy. Shanghai Met. 2021, 43, 108–111. [Google Scholar]
- Tapasa, K.; Barashev, A.V.; Bacon, D.J. Computer simulation of carbon diffusion and vacancy–carbon interaction in α-iron. Acta Mater. 2007, 55, 1–11. [Google Scholar] [CrossRef]
- Ye, Y.J.; Zhang, L.T.; Cheng, L.F.; Xu, Y.D. Molecular dynamics simulation of diffusion behavior of amorphous Si-B-C system. J. Inorg. Mater. 2006, 21, 843–847. [Google Scholar]
- Sun, Y.C. Molecular Dynamics Simulation of Oxygen Isotope Diffusion in Molten SiO2; Nanjing University: Nanjing, China, 2012. [Google Scholar]
- Shi, X.T.; Gao, X.G.; Zhong, Y. Molecular dynamics simulation of oxygen diffusion in molten SiO2. J. Nanjing Univ. Aeronaut. Astronaut. 2021, 53, 821–826. [Google Scholar]
- Wang, G.L.; Yu, A.B.; Yuan, J.D. Molecular dynamics simulation of diamond/Ti hot press diffusion process. Surf. Technol. 2022, 51, 435–442. [Google Scholar]
- Timmerscheidt, A.T.; Appen, V.J.; Dronskowski, R. A molecular-dynamics study on carbon diffusion in face-centered cubic iron. Comput. Mater. Sci. 2014, 91, 235–239. [Google Scholar] [CrossRef]
- Aslam, I.; Baskes, M.I.; Dickel, D.E. Thermodynamic and kinetic behavior of low-alloy steels: An atomic level study using an Fe-Mn-Si-C modified embedded atom method (MEAM) potential. Materialia 2019, 8, 100473. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildsley, D.J. Computer Simulation of Liquid; Oxford University Press: New York, NY, USA, 1987; Volume 28. [Google Scholar]
- Honeycutt, D.J.; Andersen, H.C. Molecular dynamics study of melting and freezing of small Lennard–Jones clusters. J. Chem. Phys. 1987, 91, 4950–4963. [Google Scholar] [CrossRef]
- Wang, S.G.; Liu, C.X.; Jian, Z.Y. Molecular dynamics simulation of diffusion coefficient of Al Cu alloy. J. Xi’an Technol. Univ. 2018, 38, 559–564. [Google Scholar]
- Ye, Q.; Zhao, S.J. Molecular dynamics simulation of continuous cooling transformation in Fe based alloys. Shanghai Met. 2012, 34, 9–12. [Google Scholar]
- Hou, Y.B. Decarburization of Fe-C Alloy by Staged Heating in CO-CO2 Atmosphere; North China University of Technology: Beijing, China, 2021. [Google Scholar]
- Zhou, Y.Q.; Ai, L.Q.; Hong, L.K. Evolution of oxide layer in solid decarburization process of Fe-C-Si alloy[J/OL]. Steel 2023. [Google Scholar]
- Luo, H.W.; Xiang, R.; Chen, L.F. Modeling decarburization kinetics of grain-oriented silicon steel. Chin. Sci. Bull. 2014, 59, 1778–1783. [Google Scholar] [CrossRef]
- Yang, S.Z. Study on Decarburization of Grain-Oriented Silicon Steel; Wuhan University of Science and Technology: Wuhan, China, 2016. [Google Scholar]







| Si | C | Fe | S | P | O |
|---|---|---|---|---|---|
| 3.5 | 0.18 | 96.32 | <0.02 | <0.027 | <0.066 |
| 1.5 | 0.18 | 98.32 | <0.02 | <0.027 | <0.066 |
| Temperature (°C) | Expression | Adj. R-Square |
|---|---|---|
| 1000 | [%C]t = 0.18 − 0.03499t1/2 + 000232t | 0.99989 |
| 1090 | [%C]t = 0.18 − 0.0414t1/2 + 000333t | 0.99929 |
| 1150 | [%C]t = 0.18 − 0.05279t1/2 + 00051t | 0.99705 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Ai, L.; Hong, L.; Zhou, Y.; Zhu, G.; Sun, C. Diffusion Behavior of Carbon and Silicon in the Process of Preparing Silicon Steel Using Solid-State Decarburization. Processes 2023, 11, 3176. https://doi.org/10.3390/pr11113176
Wen L, Ai L, Hong L, Zhou Y, Zhu G, Sun C. Diffusion Behavior of Carbon and Silicon in the Process of Preparing Silicon Steel Using Solid-State Decarburization. Processes. 2023; 11(11):3176. https://doi.org/10.3390/pr11113176
Chicago/Turabian StyleWen, Li, Liqun Ai, Lukuo Hong, Yuqing Zhou, Guangpeng Zhu, and Caijiao Sun. 2023. "Diffusion Behavior of Carbon and Silicon in the Process of Preparing Silicon Steel Using Solid-State Decarburization" Processes 11, no. 11: 3176. https://doi.org/10.3390/pr11113176
APA StyleWen, L., Ai, L., Hong, L., Zhou, Y., Zhu, G., & Sun, C. (2023). Diffusion Behavior of Carbon and Silicon in the Process of Preparing Silicon Steel Using Solid-State Decarburization. Processes, 11(11), 3176. https://doi.org/10.3390/pr11113176







