Biogas Upgradation by CO2 Sequestration and Simultaneous Production of Acetic Acid by Novel Isolated Bacteria
Abstract
:1. Introduction
2. Materials and Methods
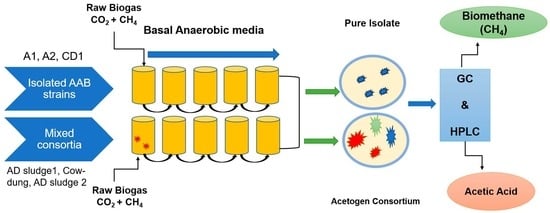
2.1. Bacterial Isolation and Culture Maintenance
2.2. Batch Experimental Conditions
2.3. Experimental Analysis
3. Result and Discussion
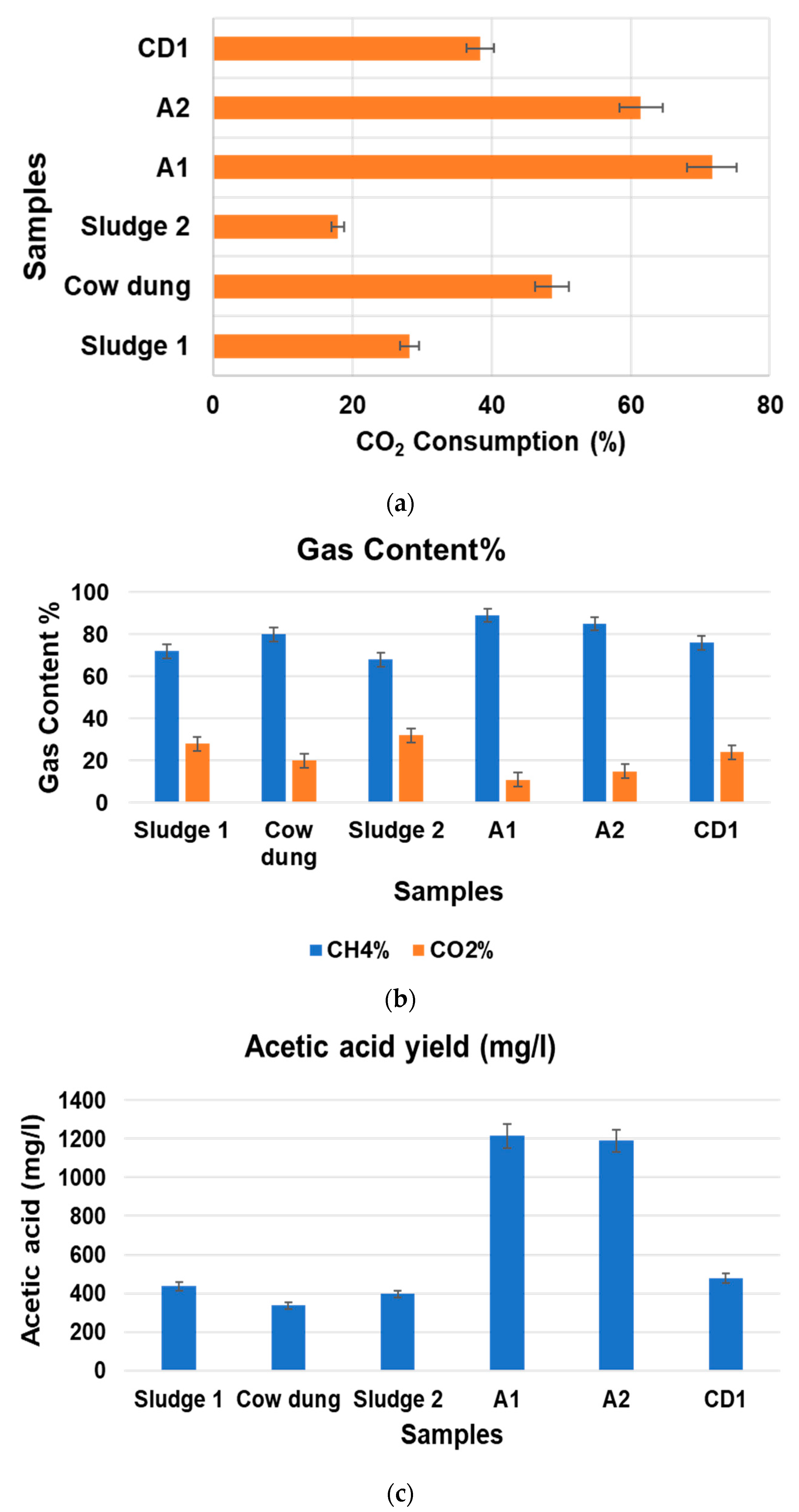
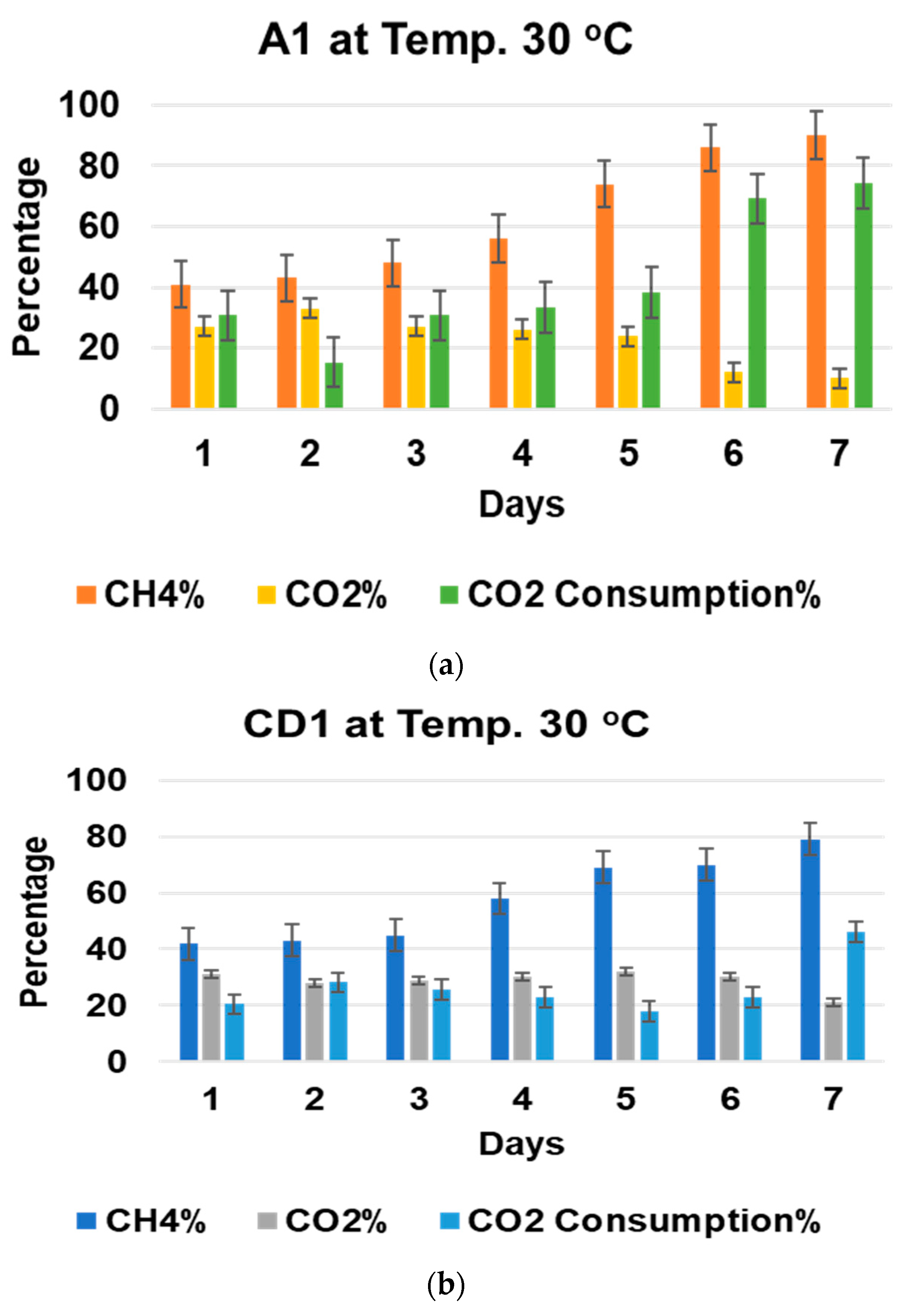
3.1. Comparision of Biogas Upgradation Using Mixed Microbial Consortia and Pure Isolates
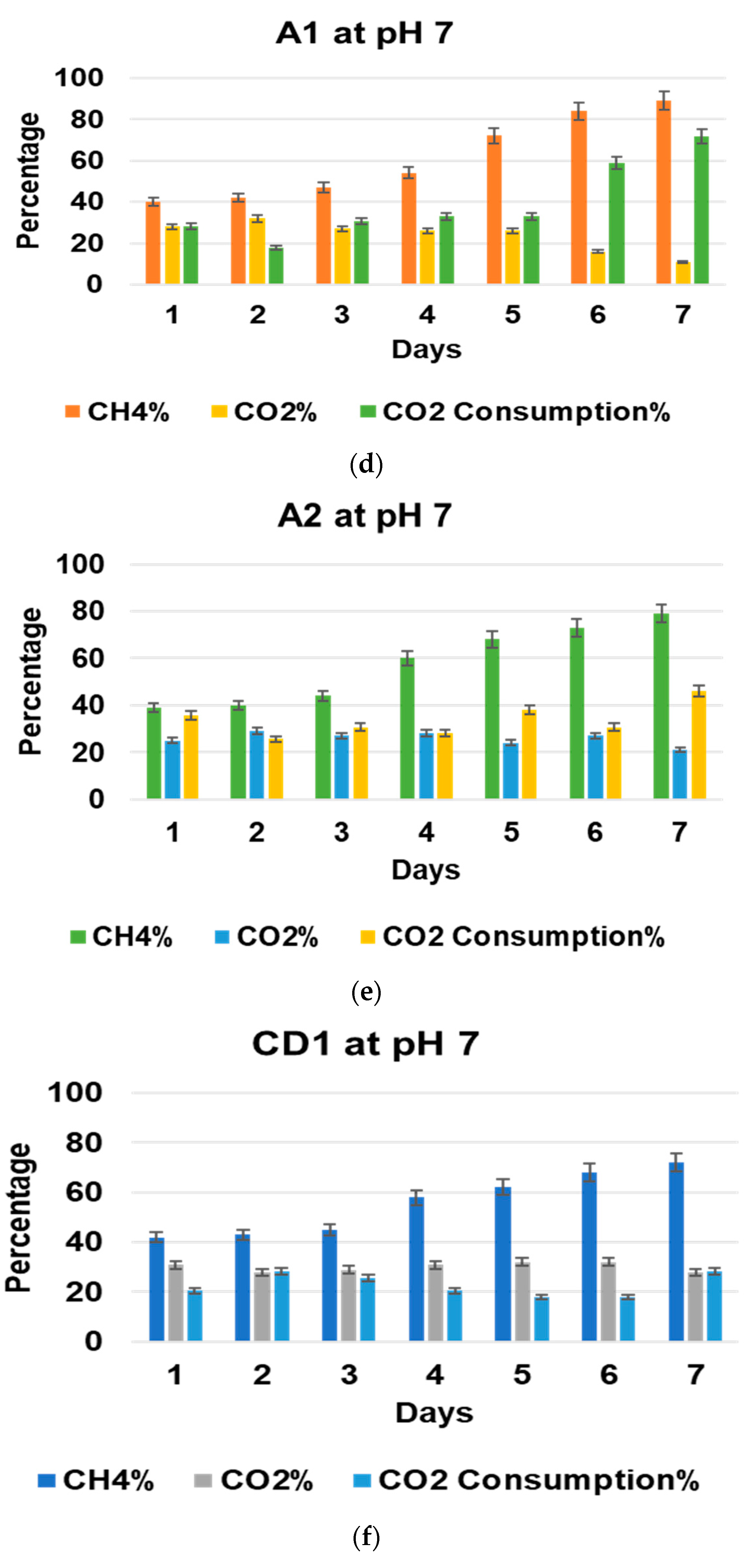
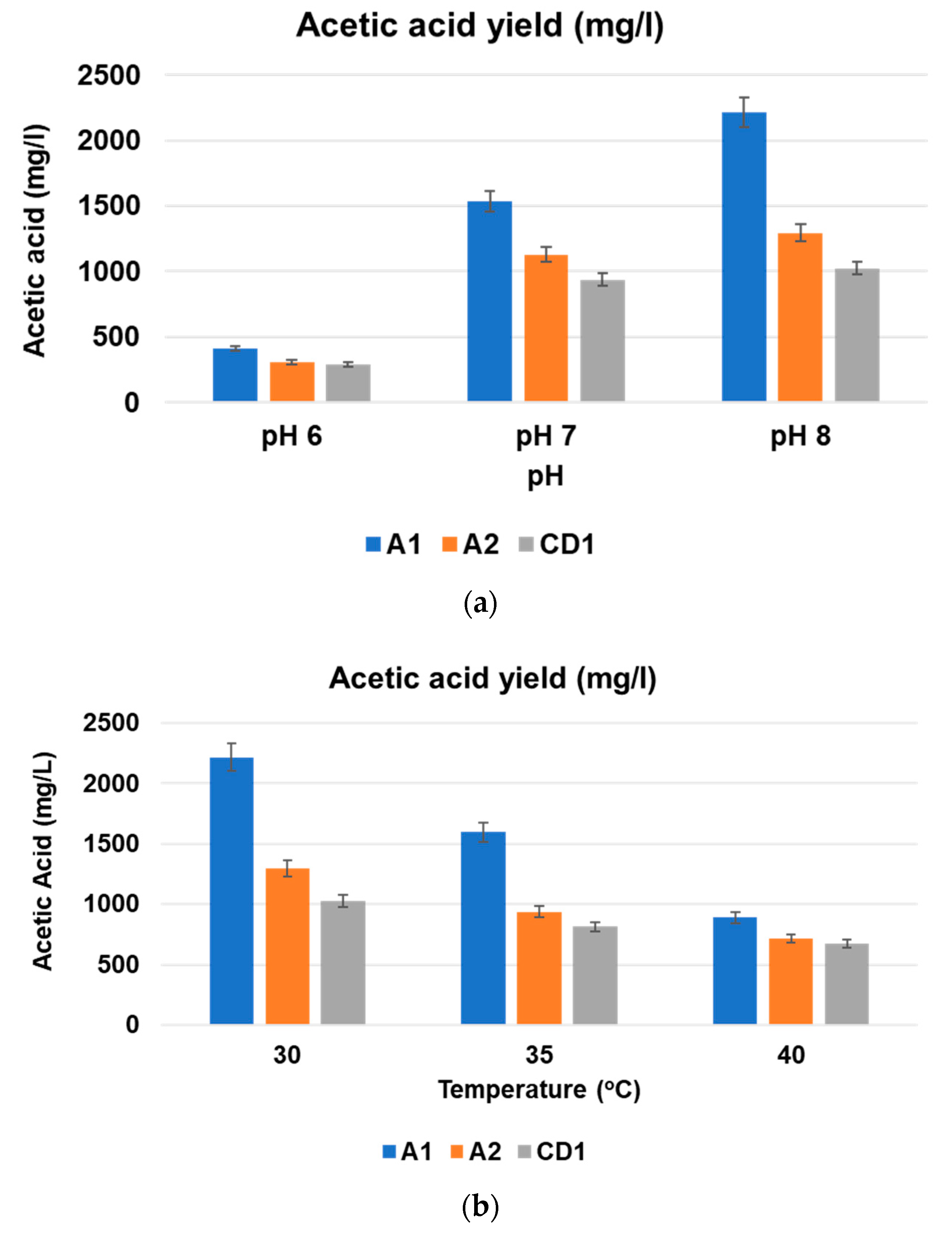
3.2. Effect of pH on Biogas Upgradation and Acetic Acid Production
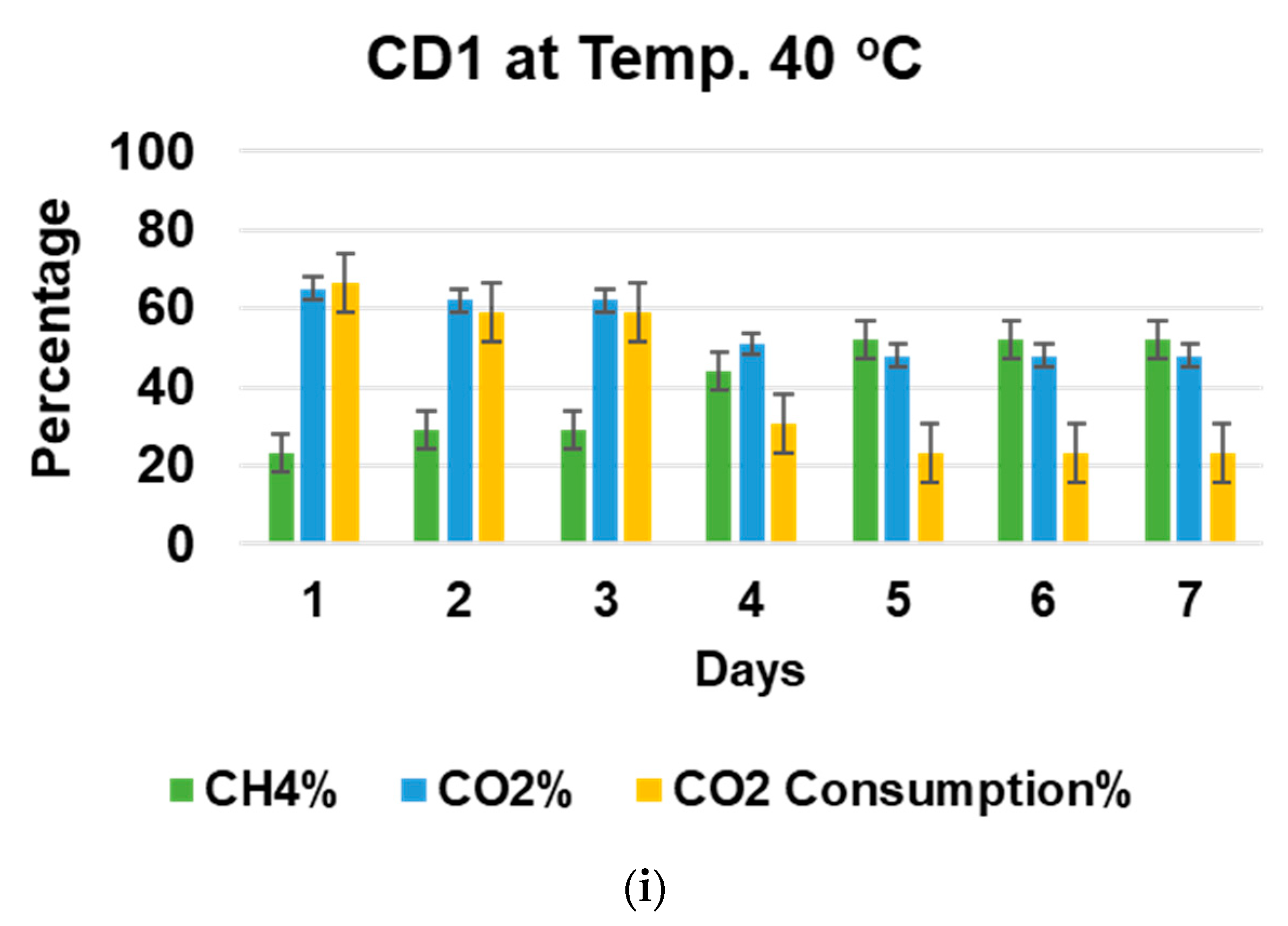
3.3. Effect of Incubation Temperature on Biogas Upgradation and Acetic Acid Production
3.4. Effect of pH and Temperature on CO2 Consumption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García, J.L.; Galán, B. Integrating Greenhouse Gas Capture and C1 Biotechnology: A Key Challenge for Circular Economy. Microb. Biotechnol. 2022, 15, 228–239. [Google Scholar] [CrossRef]
- Al-Quraan, A.; Darwish, H.; Malkawi, A.M. Renewable Energy Role in Climate Stabilization and Water Consumption Minimization in Jordan. Processes 2023, 11, 2369. [Google Scholar] [CrossRef]
- Al-Quraan, A.; Al-Mahmodi, M.; Al-Asemi, T.; Bafleh, A.; Bdour, M.; Muhsen, H.; Malkawi, A. A New Configuration of Roof Photovoltaic System for Limited Area Applications—A Case Study in KSA. Buildings 2022, 12, 92. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, M.; Bolan, N.S.; Kapley, A.; Kumar, R.; Singh, L. Multidimensional Approaches of Biogas Production and Up-Gradation: Opportunities and Challenges. Bioresour. Technol. 2021, 338, 125514. [Google Scholar] [CrossRef]
- Yong, G.T.X.; Chan, Y.J.; Lau, P.L.; Ethiraj, B.; Ghfar, A.A.; Mohammed, A.A.A.; Shahid, M.K.; Lim, J.W. Optimization of the Performances of Palm Oil Mill Effluent (POME)-Based Biogas Plants Using Comparative Analysis and Response Surface Methodology. Processes 2023, 11, 1603. [Google Scholar] [CrossRef]
- Paolini, V.; Petracchini, F.; Segreto, M.; Tomassetti, L.; Naja, N.; Cecinato, A. Environmental Impact of Biogas: A Short Review of Current Knowledge. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2018, 53, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A.; Khan, M.R.; Pirozzi, D.; Ab Wahid, Z. Financial Sustainability of Biogas Technology: Barriers, Opportunities, and Solutions. Energy Sources Part B Econ. Plan. Policy 2016, 11, 841–848. [Google Scholar] [CrossRef]
- Park, Y.; Khim, J.; Kim, J.D. Application of a Full-Scale Horizontal Anaerobic Digester for the Co-Digestion of Pig Manure, Food Waste, Excretion, and Thickened Sewage Sludge. Processes 2023, 11, 1294. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Pohořelý, M.; Meers, E.; Skoblia, S.; Moško, J.; Jeremiáš, M. Potential of Coupling Anaerobic Digestion with Thermochemical Technologies for Waste Valorization. Fuel 2021, 294, 120533. [Google Scholar] [CrossRef]
- Kougias, P.G.; Treu, L.; Benavente, D.P.; Boe, K.; Campanaro, S.; Angelidaki, I. Ex-Situ Biogas Upgrading and Enhancement in Different Reactor Systems. Bioresour. Technol. 2017, 225, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of Appropriate Biogas Upgrading Technology—A Review of Biogas Cleaning, Upgrading and Utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Soto, C.; Palacio, L.; Muñoz, R.; Prádanos, P.; Hernandez, A. Recent Advances in Membrane-Based Biogas and Biohydrogen Upgrading. Processes 2022, 10, 1918. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Hollow Fiber Membrane Based H2 Diffusion for Efficient in Situ Biogas Upgrading in an Anaerobic Reactor. Appl. Microbiol. Biotechnol. 2013, 97, 3739–3744. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Chiu, S.Y.; Huang, T.T.; Dai, L.; Hsu, L.K.; Lin, C.S. Ability of a Mutant Strain of the Microalga Chlorella sp. to Capture Carbon Dioxide for Biogas Upgrading. Appl. Energy 2012, 93, 176–183. [Google Scholar] [CrossRef]
- Xu, H.; Wang, K.; Holmes, D.E. Bioelectrochemical Removal of Carbon Dioxide (CO2): An Innovative Method for Biogas Upgrading. Bioresour. Technol. 2014, 173, 392–398. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Angelidaki, I. In-Situ Biogas Upgrading in Thermophilic Granular UASB Reactor: Key Factors Affecting the Hydrogen Mass Transfer Rate. Bioresour. Technol. 2016, 221, 485–491. [Google Scholar] [CrossRef]
- Vinardell, S.; Astals, S.; Peces, M.; Cardete, M.A.; Fernández, I.; Mata-Alvarez, J.; Dosta, J. Advances in Anaerobic Membrane Bioreactor Technology for Municipal Wastewater Treatment: A 2020 Updated Review. Renew. Sustain. Energy Rev. 2020, 130, 109936. [Google Scholar] [CrossRef]
- Groher, A.; Weuster-Botz, D. Comparative Reaction Engineering Analysis of Different Acetogenic Bacteria for Gas Fermentation. J. Biotechnol. 2016, 228, 82–94. [Google Scholar] [CrossRef]
- Vandecasteele, J. Experimental and Modelling Study of Pure-Culture Syngas Fermentation for Biofuels Production. Master’s Thesis, Ghent University, Ghent, Belgium, 2016. [Google Scholar]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Kovalev, A.A.; Zhuravleva, E.A.; Pareek, N.; Vivekanand, V. Enhanced Production of Acetic Acid through Bioprocess Optimization Employing Response Surface Methodology and Artificial Neural Network. Bioresour. Technol. 2023, 376, 128930. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the Biomethane Potential (BMP) of Solid Organic Wastes and Energy Crops: A Proposed Protocol for Batch Assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- Hniman, A.; Prasertsan, P.; O-Thong, S. Community Analysis of Thermophilic Hydrogen-Producing Consortia Enriched from Thailand Hot Spring with Mixed Xylose and Glucose. Int. J. Hydrogen Energy 2011, 36, 14217–14226. [Google Scholar] [CrossRef]
- Ye, R.; Jin, Q.; Bohannan, B.; Keller, J.K.; Bridgham, S.D. Homoacetogenesis: A Potentially Underappreciated Carbon Pathway in Peatlands. Soil Biol. Biochem. 2014, 68, 385–391. [Google Scholar] [CrossRef]
- Müller, V. New Horizons in Acetogenic Conversion of One-Carbon Substrates and Biological Hydrogen Storage. Trends Biotechnol. 2019, 37, 1344–1354. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas Fermentation Process Development for Production of Biofuels and Chemicals: A Review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Demler, M.; Weuster-Botz, D. Reaction Engineering Analysis of Hydrogenotrophic Production of Acetic Acid by Acetobacterium woodii. Biotechnol. Bioeng. 2011, 108, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Kantzow, C.; Mayer, A.; Weuster-Botz, D. Continuous Gas Fermentation by Acetobacterium woodii in a Submerged Membrane Reactor with Full Cell Retention. J. Biotechnol. 2015, 212, 11–18. [Google Scholar] [CrossRef]
- Steger, F.; Rachbauer, L.; Windhagauer, M.; Montgomery, L.F.R.; Bochmann, G. Optimisation of Continuous Gas Fermentation by Immobilisation of Acetate-Producing Acetobacterium woodii. Anaerobe 2017, 46, 96–103. [Google Scholar] [CrossRef]
- Riegler, P.; Bieringer, E.; Chrusciel, T.; Stärz, M.; Löwe, H.; Weuster-Botz, D. Continuous Conversion of CO2/H2 with Clostridium aceticum in Biofilm Reactors. Bioresour. Technol. 2019, 291, 121760. [Google Scholar] [CrossRef]
- Heijstra, B.D.; Leang, C.; Juminaga, A. Gas Fermentation: Cellular Engineering Possibilities and Scale Up. Microb. Cell Fact. 2017, 16, 60. [Google Scholar] [CrossRef]
- Fu, B.; Jin, X.; Conrad, R.; Liu, H.; Liu, H. Competition between Chemolithotrophic Acetogenesis and Hydrogenotrophic Methanogenesis for Exogenous H2/CO2 in Anaerobically Digested Sludge: Impact of Temperature. Front. Microbiol. 2019, 10, 2418. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, Ö.; Cetecioglu, Z. Volatile Fatty Acid Production from Semi-Synthetic Milk Processing Wastewater under Alkali PH: The Pearls and Pitfalls of Microbial Culture. Bioresour. Technol. 2020, 297, 122415. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Fernández-Naveira, Á.; Veiga, M.C.; Kennes, C. Impact of Cyclic PH Shifts on Carbon Monoxide Fermentation to Ethanol by Clostridium autoethanogenum. Fuel 2016, 178, 56–62. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic PH Measurement and Homeostasis in Bacteria and Archaea. In Advances in Microbial Physiology; Elsevier: Cambridge, MA, USA, 2009; Volume 55, ISBN 9780123747907. [Google Scholar]
- Fujishima, S.; Miyahara, T.; Noike, T. Effect of moisture content on anaerobic digestion of dewatered sludge: Ammonia inhibition to carbohydrate removal and methane production. Water Sci. Technol. 2000, 41, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lay, J.J.; Li, Y.Y.; Noike, T. Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res. 1997, 31, 1518–1524. [Google Scholar] [CrossRef]
- Hoffmann, S.; Justesen, T. Effect of temperature, humidity and exposure to oxygen on the survival of anaerobic bacteria. J. Med. Microbiol. 1980, 13, 609–612. [Google Scholar] [CrossRef]
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current status of biogas upgrading for direct biomethane use: A review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Wan, J.; Jing, Y.; Zhang, S.; Angelidaki, I.; Luo, G. Mesophilic and Thermophilic Alkaline Fermentation of Waste Activated Sludge for Hydrogen Production: Focusing on Homoacetogenesis. Water Res. 2016, 102, 524–532. [Google Scholar] [CrossRef]
- Singla, A.; Verma, D.; Lal, B.; Sarma, P.M. Enrichment and Optimization of Anaerobic Bacterial Mixed Culture for Conversion of Syngas to Ethanol. Bioresour. Technol. 2014, 172, 41–49. [Google Scholar] [CrossRef]
- Omar, B.; Abou-Shanab, R.; El-Gammal, M.; Fotidis, I.A.; Kougias, P.G.; Zhang, Y.; Angelidaki, I. Simultaneous Biogas Upgrading and Biochemicals Production Using Anaerobic Bacterial Mixed Cultures. Water Res. 2018, 142, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Chaikitkaew, S.; Seengenyoung, J.; Mamimin, C.; Birkeland, N.K.; Reungsang, A.; O-Thong, S. Simultaneous Biogas Upgrading and Acetic Acid Production by Homoacetogens Consortium Enriched from Peatland Soil. Bioresour. Technol. Rep. 2021, 15, 100701. [Google Scholar] [CrossRef]
- Omar, B.; El-Gammal, M.; Abou-Shanab, R.; Fotidis, I.A.; Angelidaki, I.; Zhang, Y. Biogas Upgrading and Biochemical Production from Gas Fermentation: Impact of Microbial Community and Gas Composition. Bioresour. Technol. 2019, 286, 121413. [Google Scholar] [CrossRef] [PubMed]




| Solution A (g/L) | Solution B (g/L) | Solution C (g/L) | Solution D (g/L) | Solution E (mg/L) |
|---|---|---|---|---|
| NH4Cl-100 | K2HPO4·3H2O-200 | Resazurin-0.5 | FeCl2·4H2O-2 | Biotin-2 |
| NaCl-10 | H3BO3-0.05 | Folic acid-2 | ||
| MgCl2·6H2O-10 | ZnCl2-0.05 | Pyridoxine acid-10 | ||
| CaCl2·2H2O-5 | CuCl2·2H2O-0.038 | Riboflavin-5 | ||
| MnCl2·4H2O-0.05 | Thiamine hydrochloride-5 | |||
| (NH4)6MO7O24·4H2O-0.05 | Cynocobalamine-0.1 | |||
| AlCl3-0.05 | Nicotinic acid-5 | |||
| CoCl2·6H2O-0.05 | P-aminobenzoic acid-5 | |||
| NiCl2·6H2O-0.092 | Lipoic acid-5 | |||
| EDTA-0.5 | DL-pantothenic acid-5 | |||
| Conc. HCl-1 mL | ||||
| Na2SeO3·5H2O-0.1 |
| Experiments | Parameter | Operating Condition | ||
|---|---|---|---|---|
| 1 | Effect of different pH values | |||
| pH | 6 | 7 | 8 | |
| Biogas ratio | 3:2 (CH4:CO2) | |||
| 2 | Effect of different temperatures (°C) | |||
| Temperature (°C) | 30 | 35 | 40 | |
| Biogas ratio | 3:2 (CH4:CO2) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, A.; Chawade, A.; Ikram, M.M.; Saharan, V.K.; Pareek, N.; Vivekanand, V. Biogas Upgradation by CO2 Sequestration and Simultaneous Production of Acetic Acid by Novel Isolated Bacteria. Processes 2023, 11, 3163. https://doi.org/10.3390/pr11113163
Upadhyay A, Chawade A, Ikram MM, Saharan VK, Pareek N, Vivekanand V. Biogas Upgradation by CO2 Sequestration and Simultaneous Production of Acetic Acid by Novel Isolated Bacteria. Processes. 2023; 11(11):3163. https://doi.org/10.3390/pr11113163
Chicago/Turabian StyleUpadhyay, Apoorva, Aakash Chawade, Mohd Mohsin Ikram, Virendra Kumar Saharan, Nidhi Pareek, and Vivekanand Vivekanand. 2023. "Biogas Upgradation by CO2 Sequestration and Simultaneous Production of Acetic Acid by Novel Isolated Bacteria" Processes 11, no. 11: 3163. https://doi.org/10.3390/pr11113163
APA StyleUpadhyay, A., Chawade, A., Ikram, M. M., Saharan, V. K., Pareek, N., & Vivekanand, V. (2023). Biogas Upgradation by CO2 Sequestration and Simultaneous Production of Acetic Acid by Novel Isolated Bacteria. Processes, 11(11), 3163. https://doi.org/10.3390/pr11113163