Degassing Dissolved Oxygen through Bubbling: The Contribution and Control of Vapor Bubbles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Background Physics
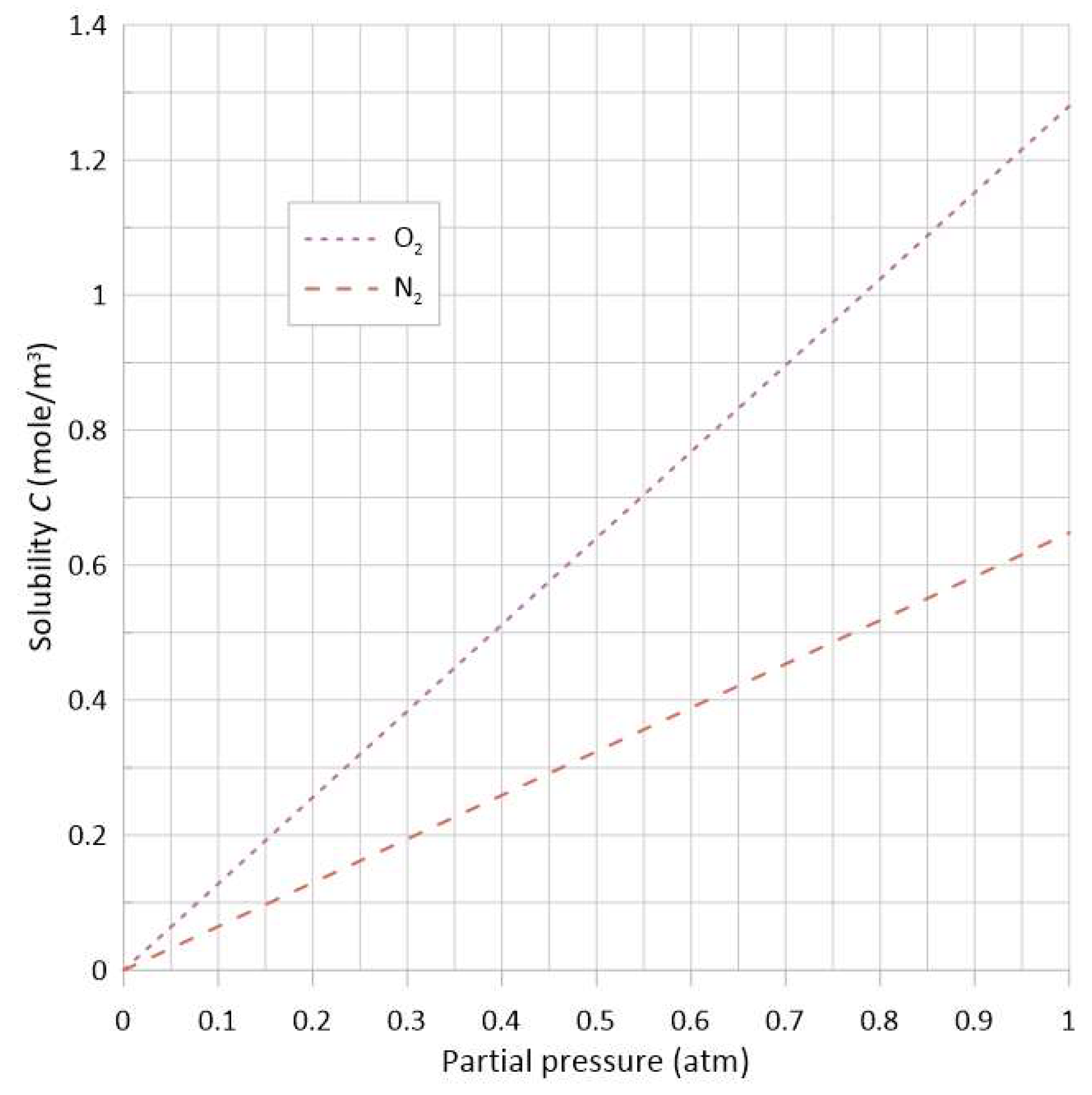
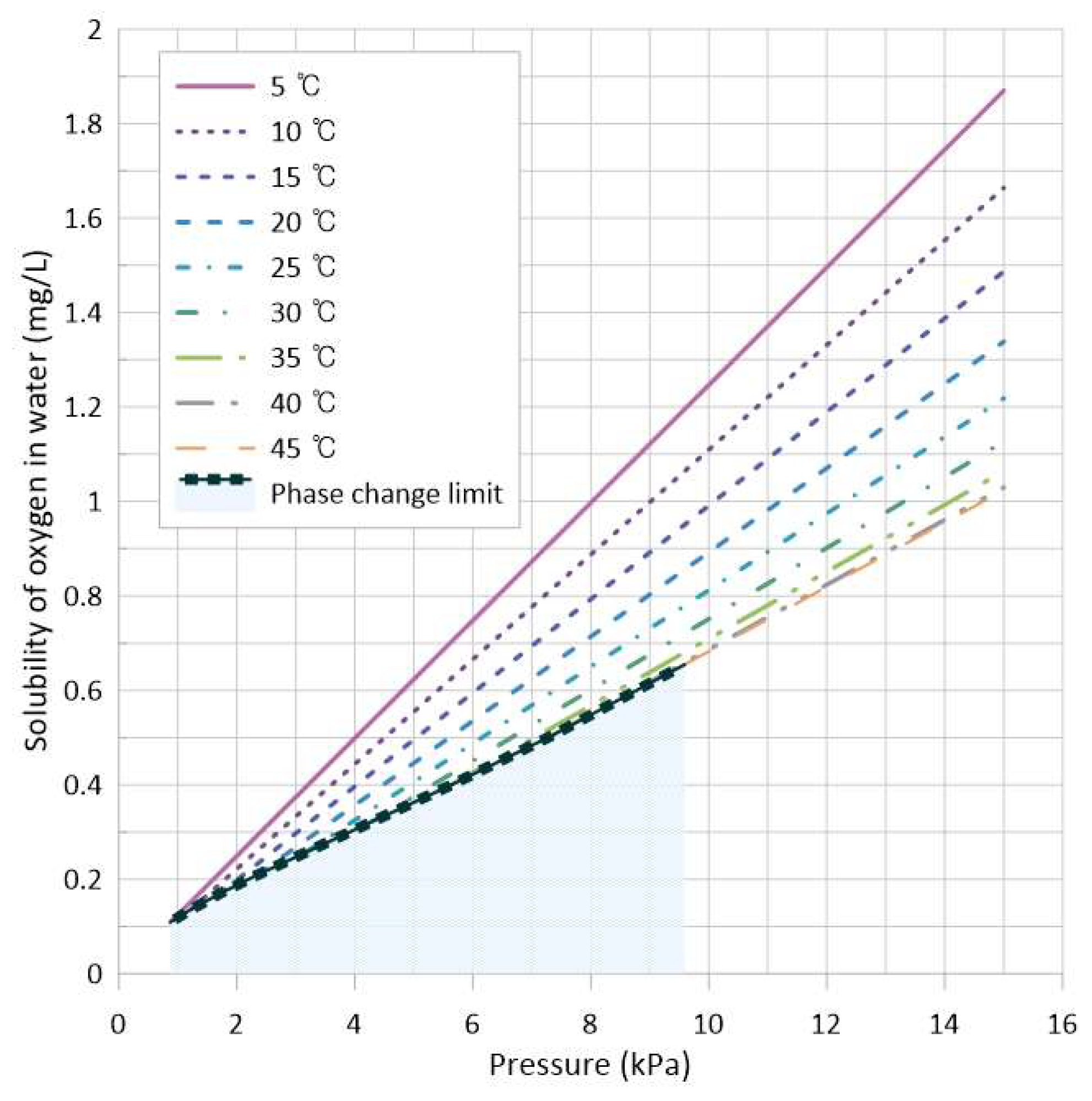
2.1.1. Solubility Behavior with Respect to Temperature and Pressure
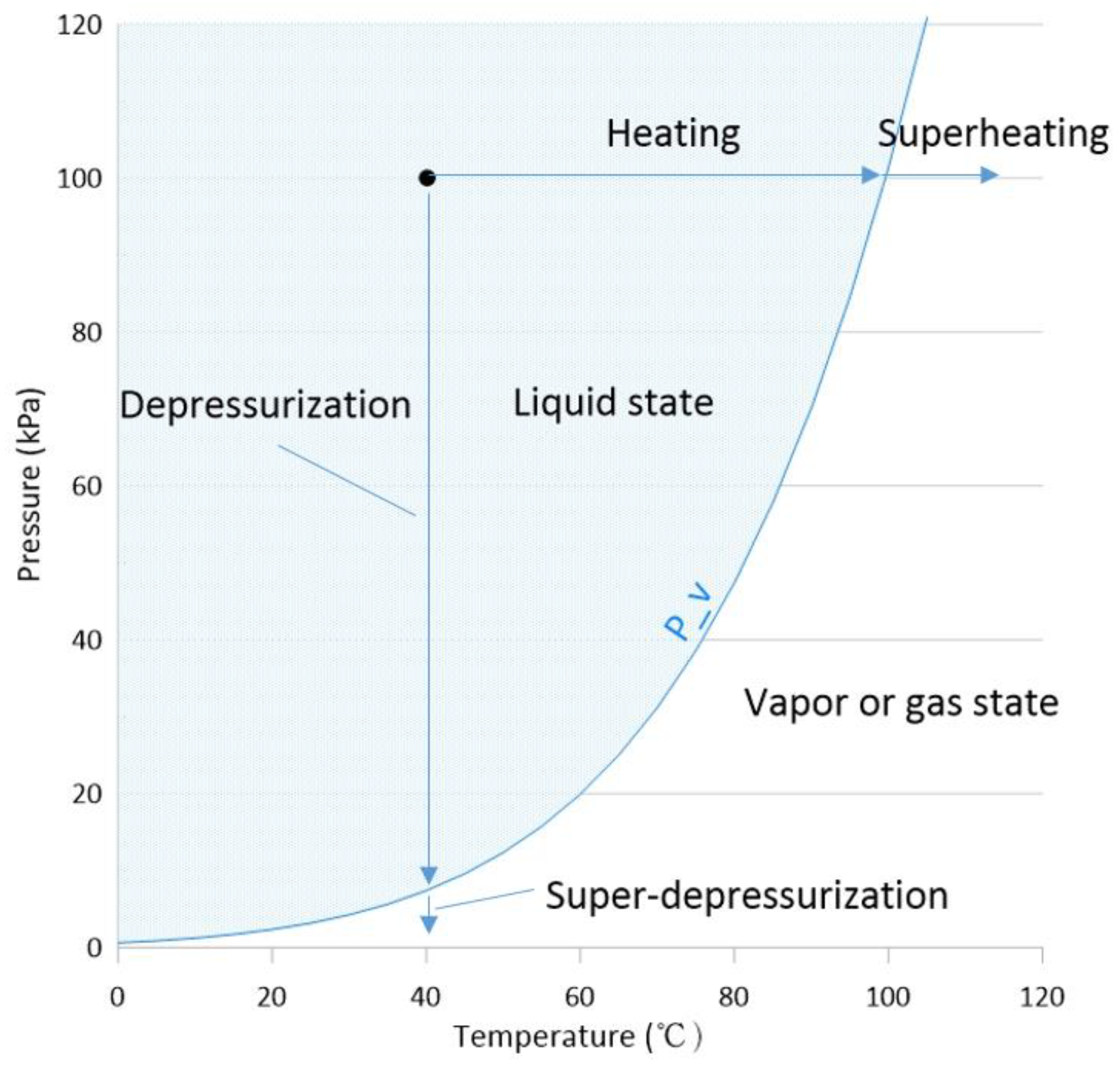
2.1.2. Bubble Generation—Tension vs. Superheating
“If a pure liquid at a subcooled state is depressurized at constant temperature, and pressure is reduced below that of the saturated vapor pressure without significant nucleation sites, the depressurization may lead to continuation of the state down the theoretical isotherm to a point where the pressure is below the saturated vapor pressure. The pressure difference between this local pressure and the saturated vapor pressure is the magnitude of the tension.” Brennen also notes that “the necessary condition for vapor bubble generation is related to the extent of tension and the duration.”
2.1.3. Diffusion Potential of Steam and Vapor Bubbles
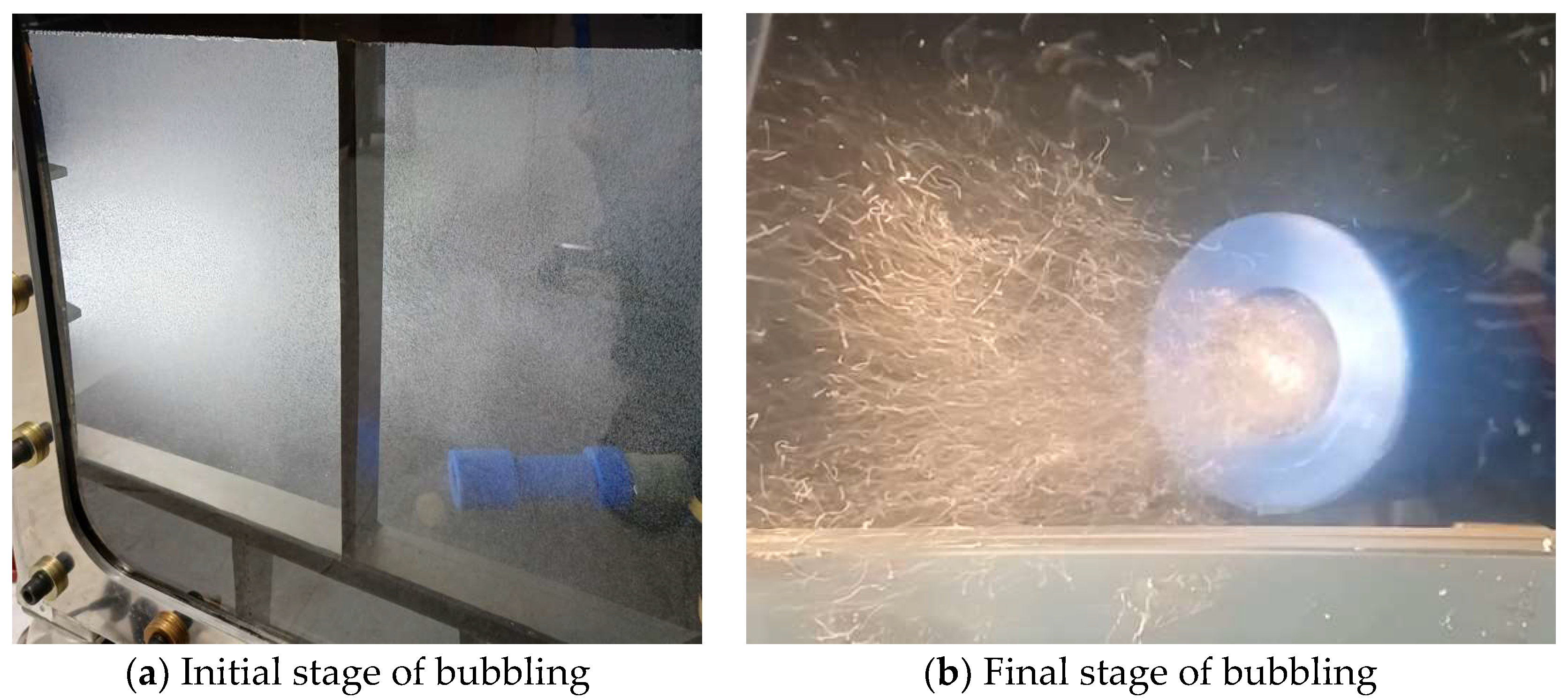
2.1.4. Bubble Diffusion
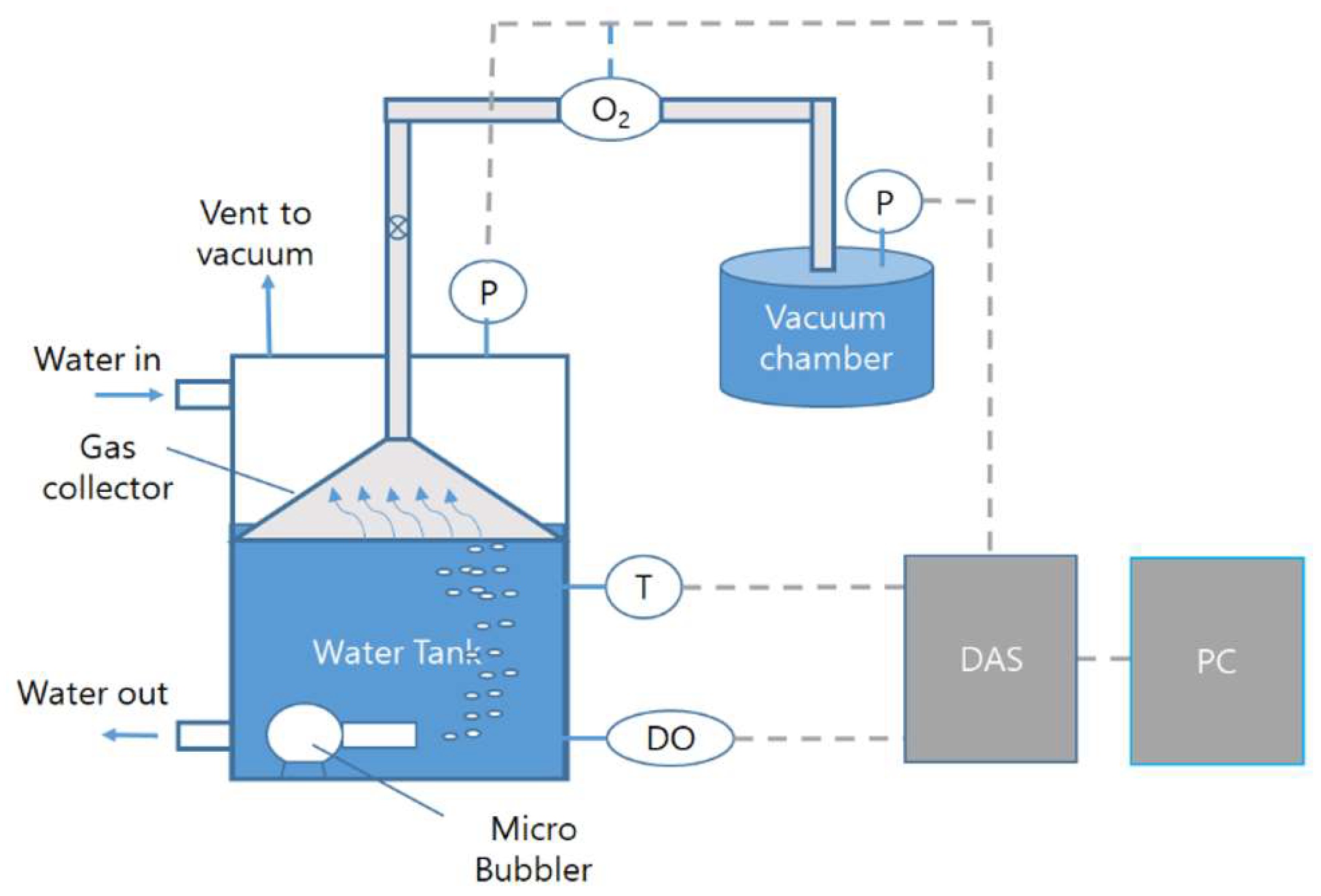
2.2. Experimental Setup and Test Methods
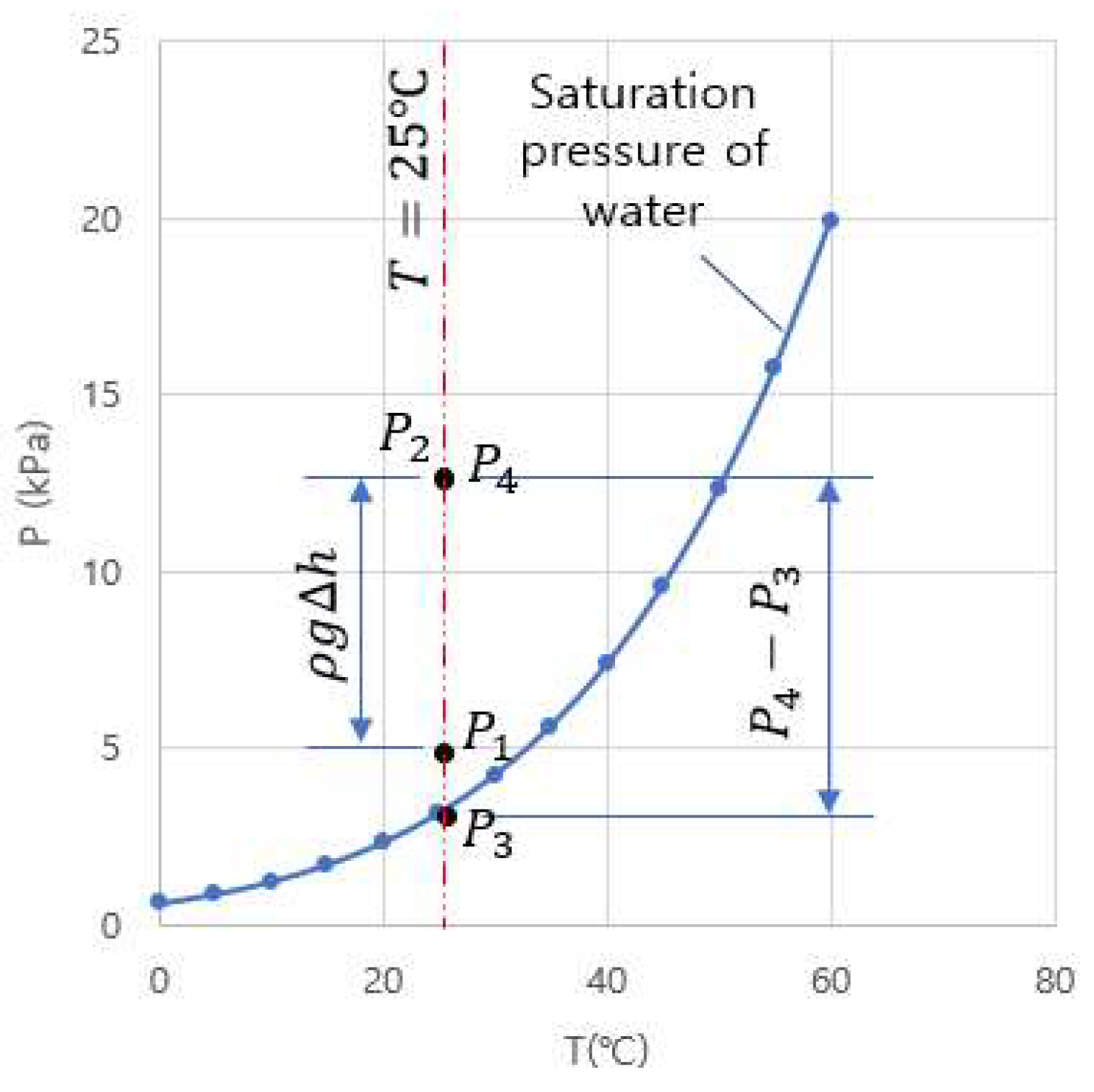
2.3. Vacuum Bubbling Model and Analysis of Test Condition
2.3.1. Vacuum Bubbling Model
- -
- water temperature ;
- -
- nozzle depth ∆h = 0.8 m;
- -
- ullage pressure ;
- -
- fixed flow rate through the nozzle (driven by a pump using DC power of 20 W).
2.3.2. Analysis of Test Conditions
3. Results
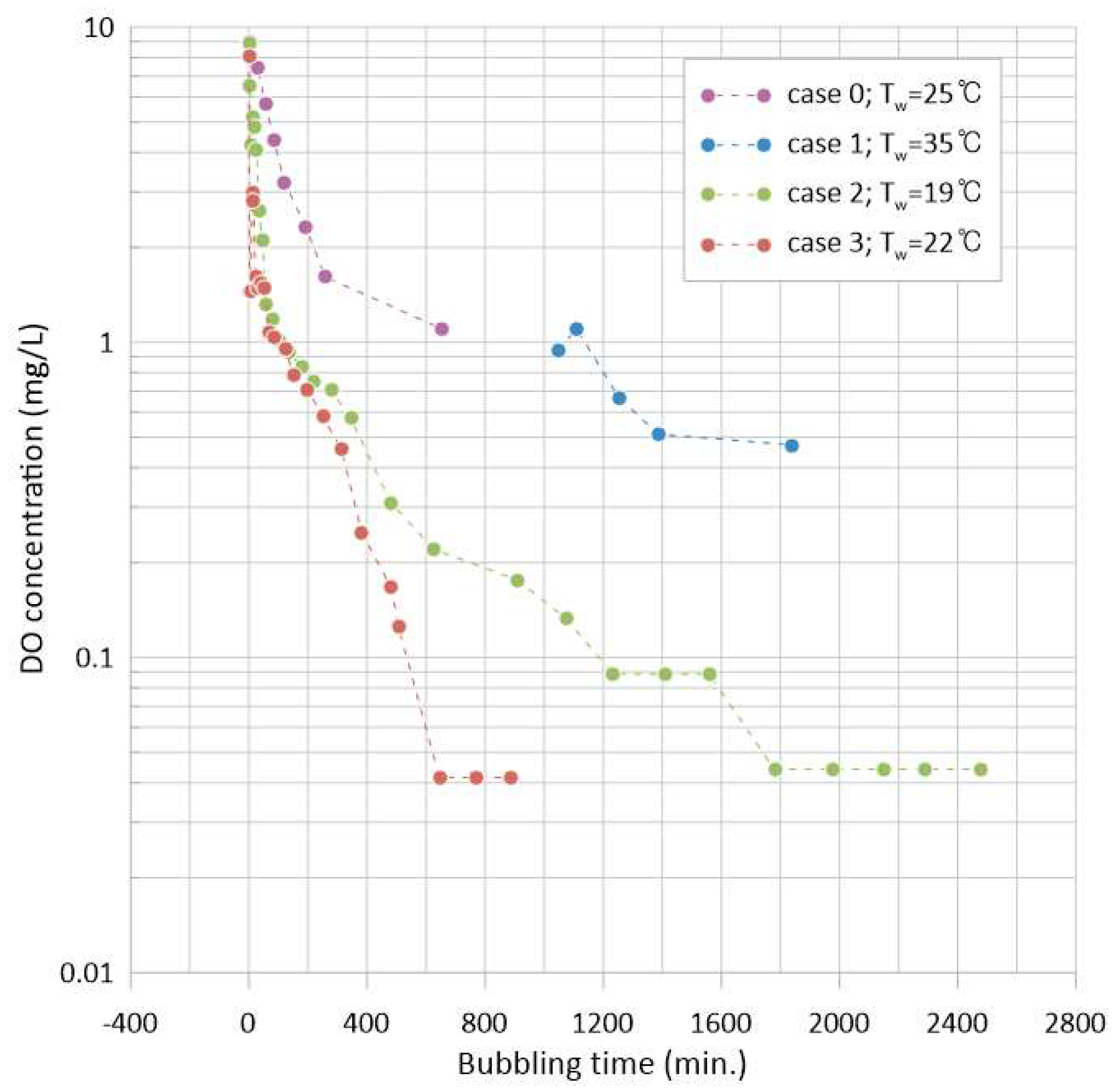
3.1. Variation of DO Concentration (Minimum DO and Time)
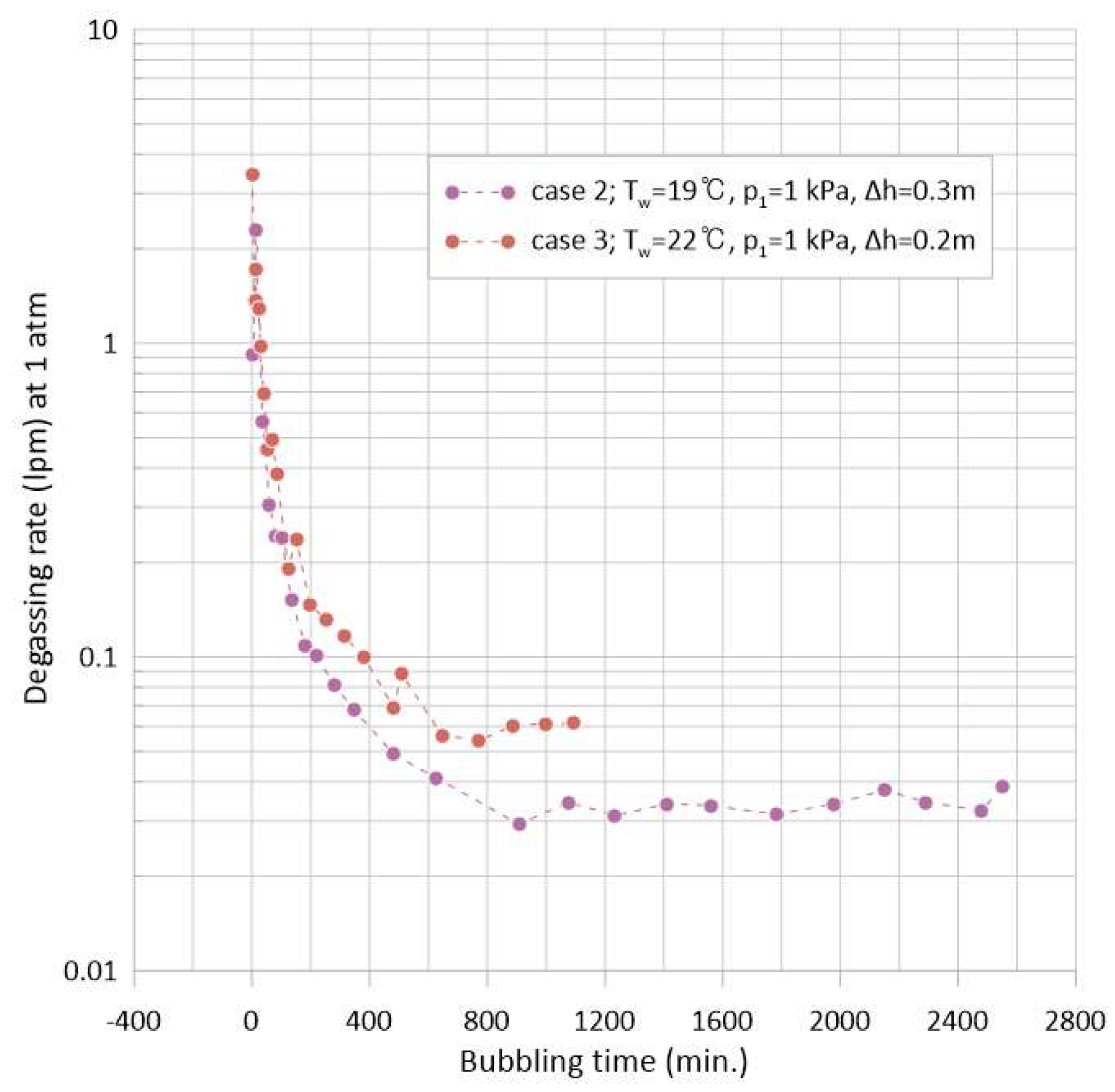
3.2. Degassing Rate and the Rate Model
3.3. Energy Consumption for Degassing
4. Discussion
- (1)
- The two gas sources, supersaturated solutes and water vapor, contributed simultaneously, with different contribution fractions.
- (2)
- The degassing rate of the vapor is assumed to be constant and determinable (from experiments).
- (3)
- The extracted gas is composed of vapor bubbles, as determined in (2), and supersaturated solutes, which are responsible for the remaining degassing rate.
- (1)
- The total rate of the volume change for the extracted gas was determined from the experiment: .
- (2)
- The total rate of the volume change for the extracted gas was interpreted as the sum of the vapor bubbles and dissolved gases (O2 and N2):
- (3)
- The initial composition of the supersaturated solutes was estimated based on the measured DO concentration or the assumed equilibrium conditions.
- (4)
- As time advances, was updated by measurements or using correlation equations, as shown in Figure 14.
5. Conclusions
- Vapor bubbles with a DO volume fraction of less than 10−6, due to the effect of volume expansion during the phase change process, are responsible for mass diffusion at the lowest DO concentration levels, close to zero.
- The conditions for the generation and retention of vapor bubbles under vacuum are explicitly delineated in this study, along with other influential variables. The tension ( at the bubbler nozzle throat defines the vapor generation condition, while the deviation from the phase-change pressure ( downstream of the bubbler nozzle defines the condition for vapor bubble retention in the present experimental system. The effect of these factors was demonstrated through experiments (cases 2 and 3), showing that even under the same vessel pressure = 1 kPa, the time required for a zero mg/L reading of DO concentration could be reduced by 60% depending on the nozzle depth change in the present study, which is one of the influential parameters herein.
- The total energy consumption required to complete the degassing of the water body to 0 mg/L at room temperature was measured and reported in the present study. The energy used during the experiments includes the electric power for a water pump and a vacuum pump. The minimum deaeration time for 0.36 m3 of water was found to be 998 min (case 3), with a total electricity consumption of 0.829 kWh (2.31 kWh/m3).
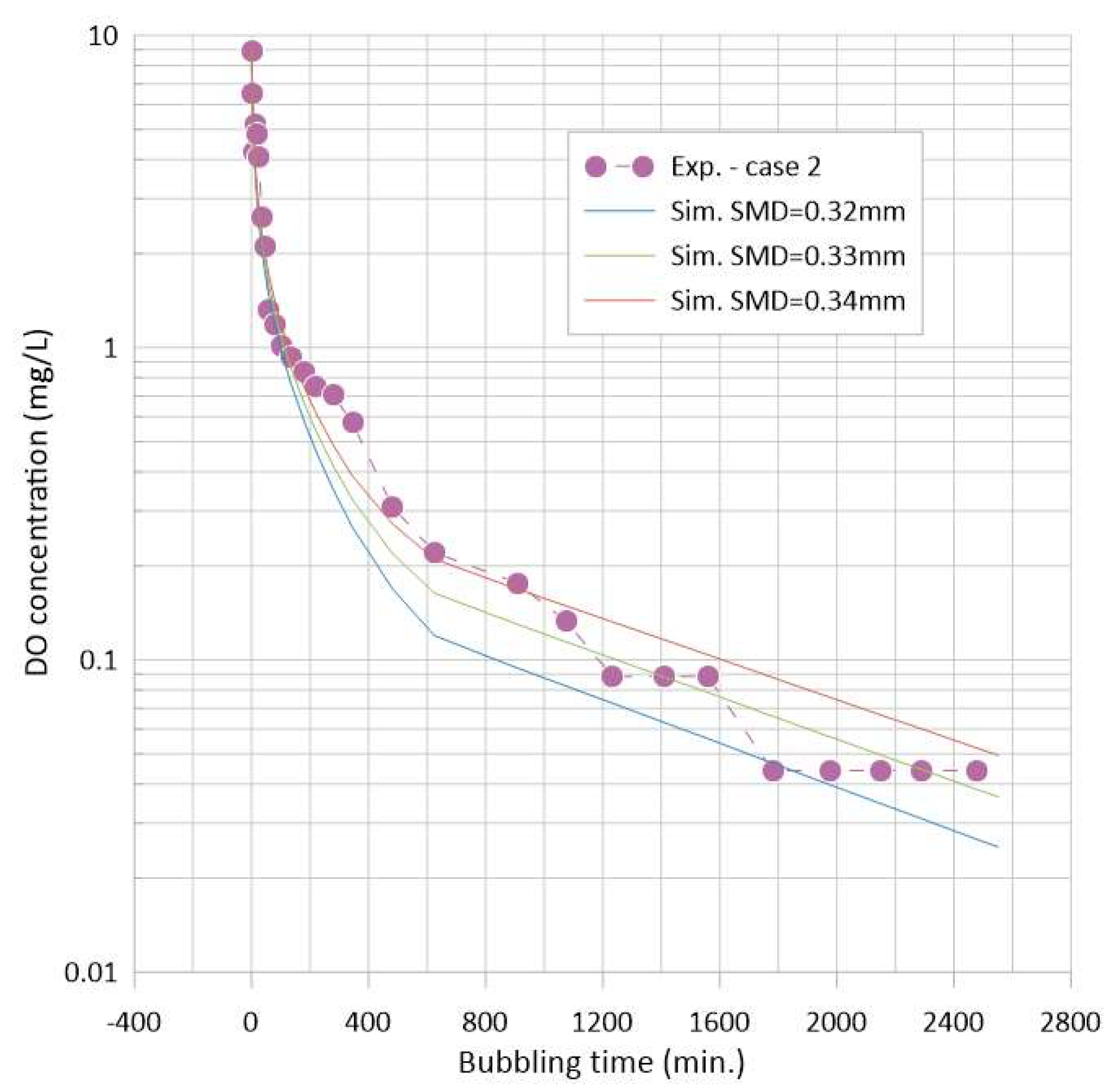
- Although case-specific, attempts were made to analyze the test results and to develop a predictive model for the present approach. Based on the estimated SMD of the initial vapor bubbles, the entire bubbling behavior could be reproduced using the discrete-bubble model with the measured degassing rate. Further efforts are encouraged for model refinement and validation.
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, J.W.; Heo, P.W.; Kim, T.S. Theoretical model and experimental validation for underwater oxygen extraction for realizing artificial gills. Sens. Actuators A Phys. 2018, 284, 103–111. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Melhuish, C.; Greenman, J. Artificial gills for robots: MFC behavior in water. Bioinsp. Biomim. 2007, 2, S83–S93. [Google Scholar] [CrossRef] [PubMed]
- Bodner, A. Available online: http://www.likeafish.biz (accessed on 3 November 2023).
- Kim, G.B.; Kim, S.J.; Kim, M.H.; Hong, C.U.; Kang, H.S. Development of a hollow fiber membrane module for using implantable artificial lung. J. Membr. Sci. 2009, 326, 130–136. [Google Scholar] [CrossRef]
- Water Treatment: Aeration and Gas Stripping—TU Delft OpenCourseWare. Available online: https://ocw.tudelft.nl/wp-content/uploads/Aeration0and-gas-stripping-1.pdf (accessed on 24 December 2021).
- Arthur, D. How Does Deaerator Work? Available online: http://watertreatmentbasics.com/how-does-deaerator-work/ (accessed on 24 December 2021).
- Greenbank, G.R.; Wright, P.A. The deaeration of raw whole milk before heat treatment as a factor in retarding the development of the tallowy flavor in its dried product. J. Dairy Sci. 1951, 34, 815–818. [Google Scholar] [CrossRef]
- Carlsson, H.; Jönsson, C. Separation of Air Bubbles from Milk in a Deaeration Process. 2012. Available online: https://www.researchgate.net/publication/267687974 (accessed on 3 November 2023).
- U.S. Department of Energy. Deaerators in Industrial Steam Systems. (Brochure).; 2012. Available online: https://www.energy.gov/sites/prod/files/2014/05/f16/steam18_steam_systems.pdf (accessed on 3 November 2023).
- 3MTM Liqui-CelTM Membrane Contactors. A High Level of Consistency and Control. Available online: https://multimedia.3m.com/mws/media/2091084O/3m-spsd-liqui-cel-general-brochure.pdf (accessed on 21 August 2023).
- Vacuum Deaerator. Eurowater, Vacuum Deaerator|Remove Oxygen and Carbon Dioxide from Make-Up Water. Available online: https://www.eurowater.com/en/water-treatment-plants/deaeration/vacuum-deaerator (accessed on 3 November 2023).
- Vacuum Deaeration, EWT Water Technology, Vacuum Deaeration. EWT Water Technology. Available online: https://www.ewt-wasser.de/en/product/vacuum-deaerator.html (accessed on 3 November 2023).
- WhittierTM Vacuum Deaerators. Industrial Grade Degasification. Available online: https://www.veoliawatertechnologies.com/en/technologies/whittier-vacuum-deaerators (accessed on 21 August 2023).
- American Water Chemicals®. Deaeration Systems. Available online: https://www.membranechemicals.com/water-treatment/deaeration-systems/ (accessed on 21 August 2023).
- Zero Gas Vacuum Deaerator. Available online: https://www.cannonartes.com/systems-equipment/deaeration/zerogas-vacuum-deaerators/ (accessed on 21 August 2023).
- Vacuum Deaerator (DEB). Available online: https://bachiller.com/en/vacuum-deaerator/ (accessed on 21 August 2023).
- PerMix. PerMix Vacuum Deaerator. Available online: https://www.permixmixers.com/liquid-mixers/permix-vacuum-deaerator/ (accessed on 21 August 2023).
- TechniBlend. Beverage Deaeration. Available online: https://www.techniblend.com/products/beverage-deaeration/ (accessed on 21 August 2023).
- High Vacuum Water Deaeration V2WD. Available online: https://bbt.corosys.com/en/solutions/degassing/high-vacuum-water-deaeration-v2wd/ (accessed on 21 August 2023).
- Ulfa Technology Co. Ltd. Operations Manual for Developer Degassing System (UDO-3006B-1). (In Korean). Available online: https://www.ulfatech.com/home/wp-content/uploads/pdf/UDO-3006B.pdf (accessed on 2 December 2022).
- Bucher Unipektin. Deaerator for the Food Industry. Available online: https://www.directindustry.com/prod/bucher-unipektin-ag/product-89865-2522723.html (accessed on 2 December 2022).
- Eurowater. Vacuum Deaerator. Available online: https://www.eurowater.com/en/water-treatment-plants/deaeration/vacuum-deaerator (accessed on 2 December 2022).
- OMVE. Vacuum Deaerator DEA 310. Available online: https://www.directindustry.com/prod/omve-lab-pilot-equipment/product-50514-349872.html (accessed on 2 December 2022).
- Chiggiato, P. Vacuum Technology for Superconducting Devices. In Proceedings of the CAS-CERN Accelerator School: Superconductivity for Accelerators, Erice, Italy, 24 April–4 May 2013; Bailey, R., Ed.; CERN-2014-005 (CERN, Geneva, 2014). [Google Scholar]
- Hong, J.A.; Lee, J.S.; Jun, Y.-D. Degassing Dissolved Oxygen through Bubbles under a Vacuum Condition. In Proceedings of the 7th Thermal and Fluids Engineering Conference (TFEC), Las Vegas, NV, USA, 15–18 May 2022. [Google Scholar]
- Jun, Y.-D. Degassing behavior of water through bubbling near a vapor pressure condition. In Proceedings of the 16th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics (HEFAT2022-ATE), Amsterdam, The Netherlands, 8–10 August 2022; pp. 277–282. [Google Scholar]
- Engineering ToolBox. Solubility of Air in Water. 2004. Available online: https://www.engineeringtoolbox.com/air-solubility-water-d_639.html (accessed on 17 March 2022).
- Yoo, S.H. (Ed.) Encyclopedia of Soil Science; Seoul National University Press: Seoul, Republic of Korea, 2000; pp. 443–444. ISBN 89-521-0204-5. (In Korean) [Google Scholar]
- Klein, S. Engineering Equation Solver (EES) by F-Chart Software, Accompanying Thermodynamics an Engineering Approach, 7th Edition in SI Units; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Brennen, C.E. Cavitation and Bubble Dynamics; Oxford University Press: New York, NY, USA, 1995; pp. 16–17. [Google Scholar]
- Incropera, F.P.; Dewitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, Sixth Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 882–884. [Google Scholar]
- Wüest, A.; Brooks, N.H.; Imboden, D. Bubble Plume Modeling for Lake Restoration. Water Resour. Res. 1992, 28, 3235–3250. [Google Scholar] [CrossRef]
- McGinnis, D.F.; Little, J.C. Predicting diffused-bubble oxygen transfer rate using the discrete-bubble model. Water Res. 2002, 36, 4627–4635. [Google Scholar] [CrossRef] [PubMed]








| Application | Required DO Level (mg/L) | Method | Source (s) |
|---|---|---|---|
| Boiler feed water | ≤0.005 | Pressure deaerator | U.S. DOE * [9] |
| Food industry | ≤0.01 | Packing w/gas | Bucher Unipektin [21] |
| Beverage | ≤0.01 | Vac. Packing w/o gas | Corosys [19] |
| District heating | ≤0.2 | Vac. Evap. w/fillers | Eurowater [22] |
| Lab and pilot equipment | ≤0.5 | Vac. Disc. and spray | OMVE ** [23] |
| Pressure Boundaries (mbar) | Pressure Boundaries (Pa) | |
|---|---|---|
| Low vacuum (LV) | ||
| Medium vacuum (MV) | 1– | |
| High vacuum (HV) | ||
| Ultra-high vacuum (UHV) | ||
| Extreme vacuum (EV) | < | < |
| Species | Unit | Henry’s Constant H |
|---|---|---|
| O2 | mol/(L·atm) | |
| N2 |
| Property or Variable Name | Correlation Equation | Range |
|---|---|---|
| H () | (T in °C) | Not available |
(T in °C) | ||
( | m | |
| m | ||
( | m | |
| m | ||
| m |
| Case | (kPa) | (m) | (kPa) | (kPa) | (kPa) | (°C) | (kPa) | (kPa) | (kPa) |
|---|---|---|---|---|---|---|---|---|---|
| 0 (Baseline) | 0.8 | 12.82 | 9.65 1 | 3.17 | 25 | 3.17 | 0 | 9.65 | |
| 1 | 5 | 0.8 | 12.82 | 9.65 1 | 3.17 | 35 | 5.63 | 2.46 | 7.19 |
| 2 | 1 | 0.3 | 3.93 | 9.65 1 | (−5.72) | 18.6 | 2.16 | (7.88) | 1.77 |
| 3 | 1 | 0.2 | 2.96 | 9.65 1 | (−6.69) | 22 | 2.67 | (9.36) | 0.29 |
| Test Case | Water Vol. (m3) | Total Bubbling Time (min.) | Epump (kWh) | Evac·p (kWh) | Etotal (kWh) |
|---|---|---|---|---|---|
| Case 2 | 0.40 | 2551 | 0.847 | 0.822 | 1.669 |
| Case 3 | 0.36 | 998 | 0.331 | 0.467 | 0.798 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, Y.-D. Degassing Dissolved Oxygen through Bubbling: The Contribution and Control of Vapor Bubbles. Processes 2023, 11, 3158. https://doi.org/10.3390/pr11113158
Jun Y-D. Degassing Dissolved Oxygen through Bubbling: The Contribution and Control of Vapor Bubbles. Processes. 2023; 11(11):3158. https://doi.org/10.3390/pr11113158
Chicago/Turabian StyleJun, Yong-Du. 2023. "Degassing Dissolved Oxygen through Bubbling: The Contribution and Control of Vapor Bubbles" Processes 11, no. 11: 3158. https://doi.org/10.3390/pr11113158
APA StyleJun, Y.-D. (2023). Degassing Dissolved Oxygen through Bubbling: The Contribution and Control of Vapor Bubbles. Processes, 11(11), 3158. https://doi.org/10.3390/pr11113158






