Advances of Nano-Enabled ZnFe2O4 Based-Gas Sensors for VOC Detection and Their Potential Applications: A Review
Abstract
:1. Introduction
| Material | Target Gas | Response (Ra/Rg)/Concentration (ppm) | Limit of Detection (ppm) | Response/Recovery Times (s) | Operating Temperature (°C) | Ref |
|---|---|---|---|---|---|---|
| ZnFe2O4 | Acetone | 192/90 | - | 3/21 | 120 | [28] |
| ZnO/ZnFe2O4 | Acetone | 92.9/90 | 0.18 | 7.7/17.3 | 120 | [29] |
| ZnFe2O4/SnO2 | Acetone | 120/100 | 0.10 | 30/197 | 210 | [30] |
| Sn-ZnO/ZnFe2O4 | Triethylamine | 28.1/10 | <0.2 | -/7 | 270 | [31] |
| Au-ZnO | Isoprene | 1371/1 | 0.006 | - | 350 | [32] |
| ZnO-CuO | Acetone | 11.1/1 | 0.04 | - | 200 | [33] |
| Pd-SnO2 | Ethylene | 11.1/100 | 0.05 | 1/- | 250 | [34] |
| SnO2-CuO | Ethanol | 8/100 | - | 4/10 | 320 | [35] |
| SnO2 | Ethanol | 59.6/40 | - | 105/100 | 150 | [36] |
| CuO/WO3 | Xylene | 6.3/50 | - | 5.5/16 | 260 | [37] |
| TiO2 | Acetone | 12/100 | - | 3/421 | 320 | [38] |
| CoTiO3/TiO2 | Benzene | 33.4/50 | 0.1 | 49/9 | RT | [39] |
| TiO2 | Ethanol | 2.2/100 | - | - | RT | [40] |
2. Gas Sensors
2.1. Semiconductor Metal Oxides (SMO) Based Gas Sensors
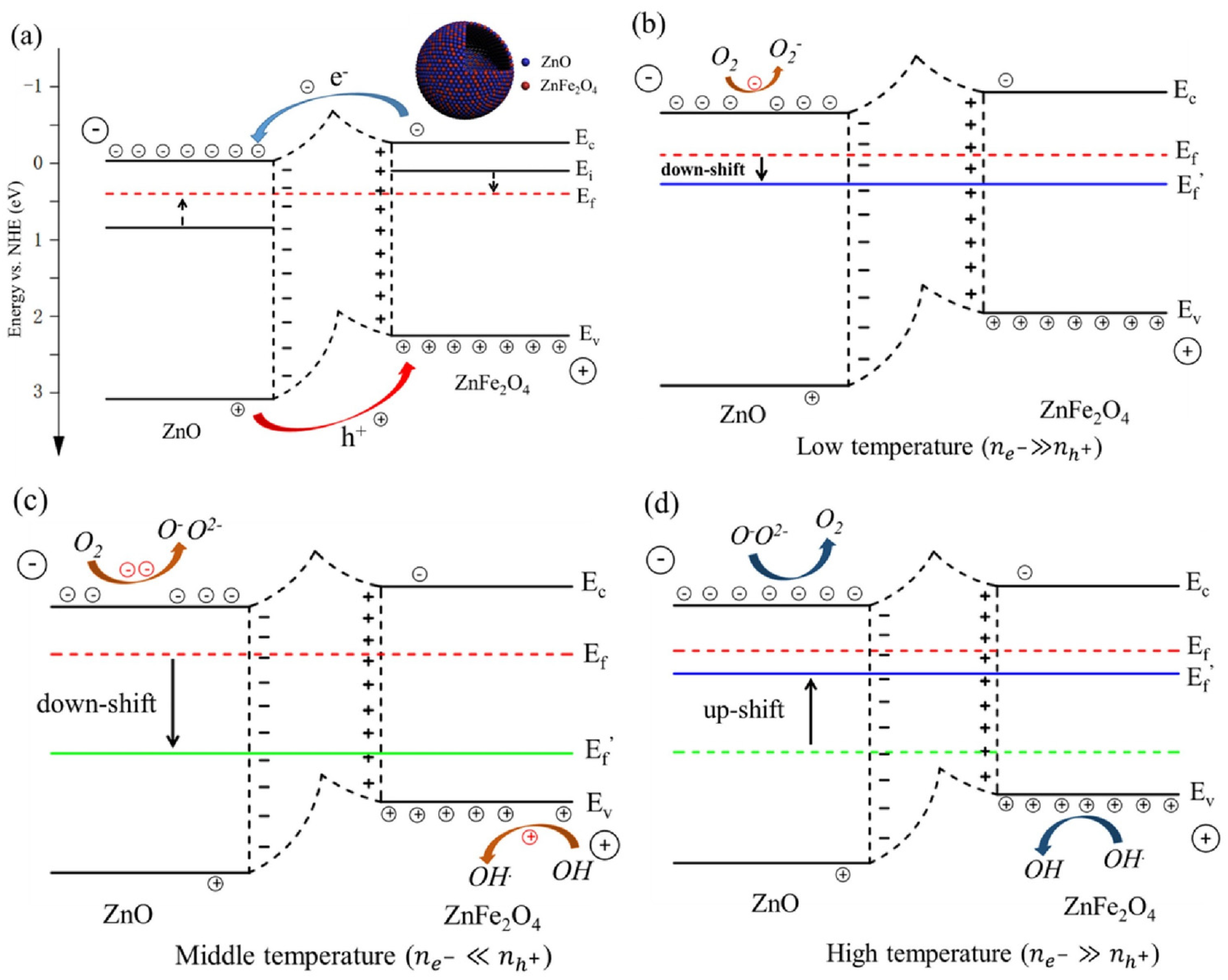
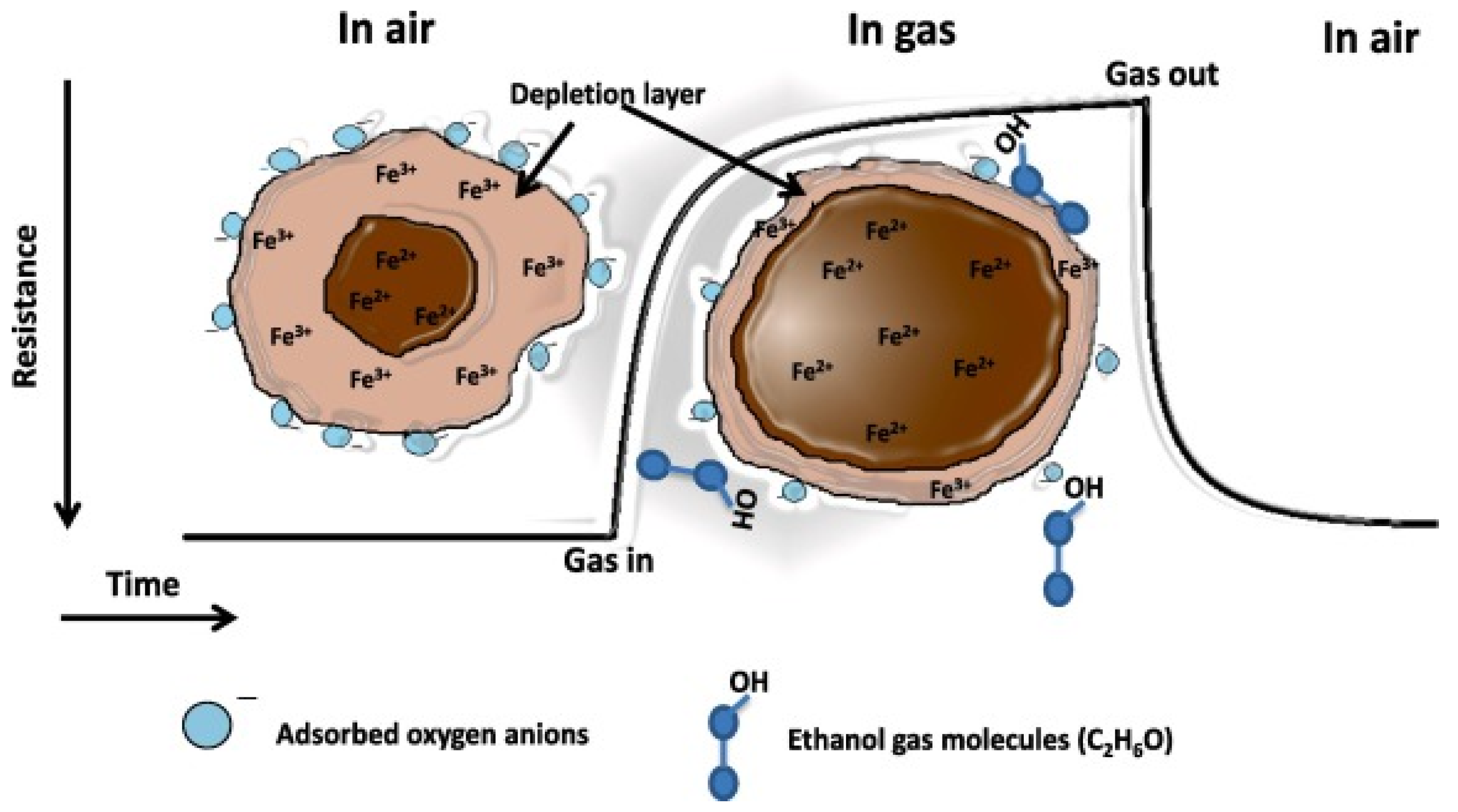
2.2. ZnFe2O4 Spinel Structure and Gas Sensing Mechanism
3. Challenges and Limitations of ZnFe2O4 Sensors
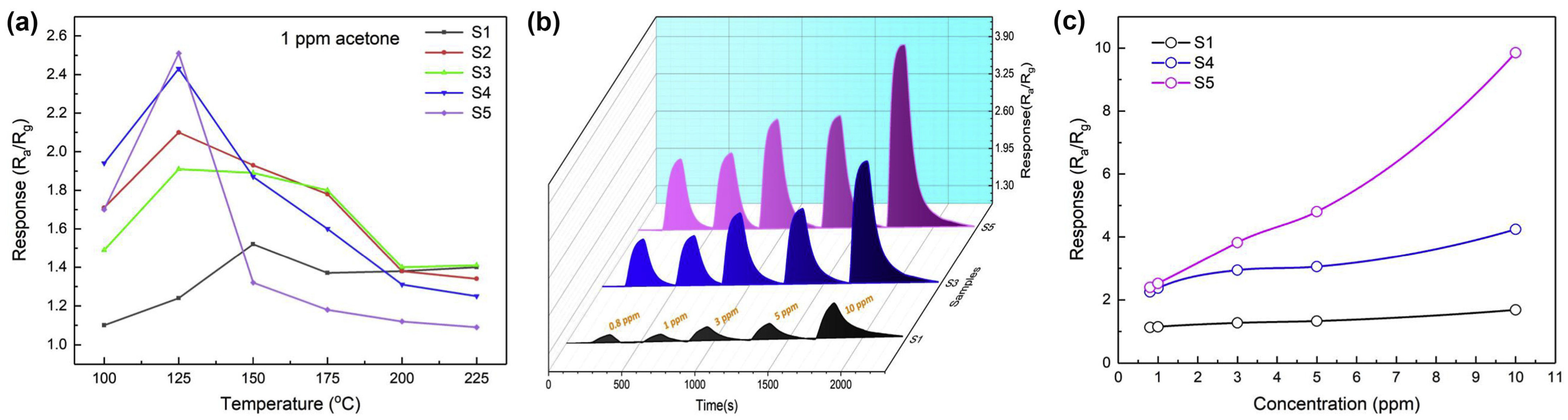
4. Strategies for the Enhancement of ZnFe2O4 Sensing Properties
4.1. Effects of Zn2+ Metal Ion Substitution
4.2. Effects of Structural Morphology
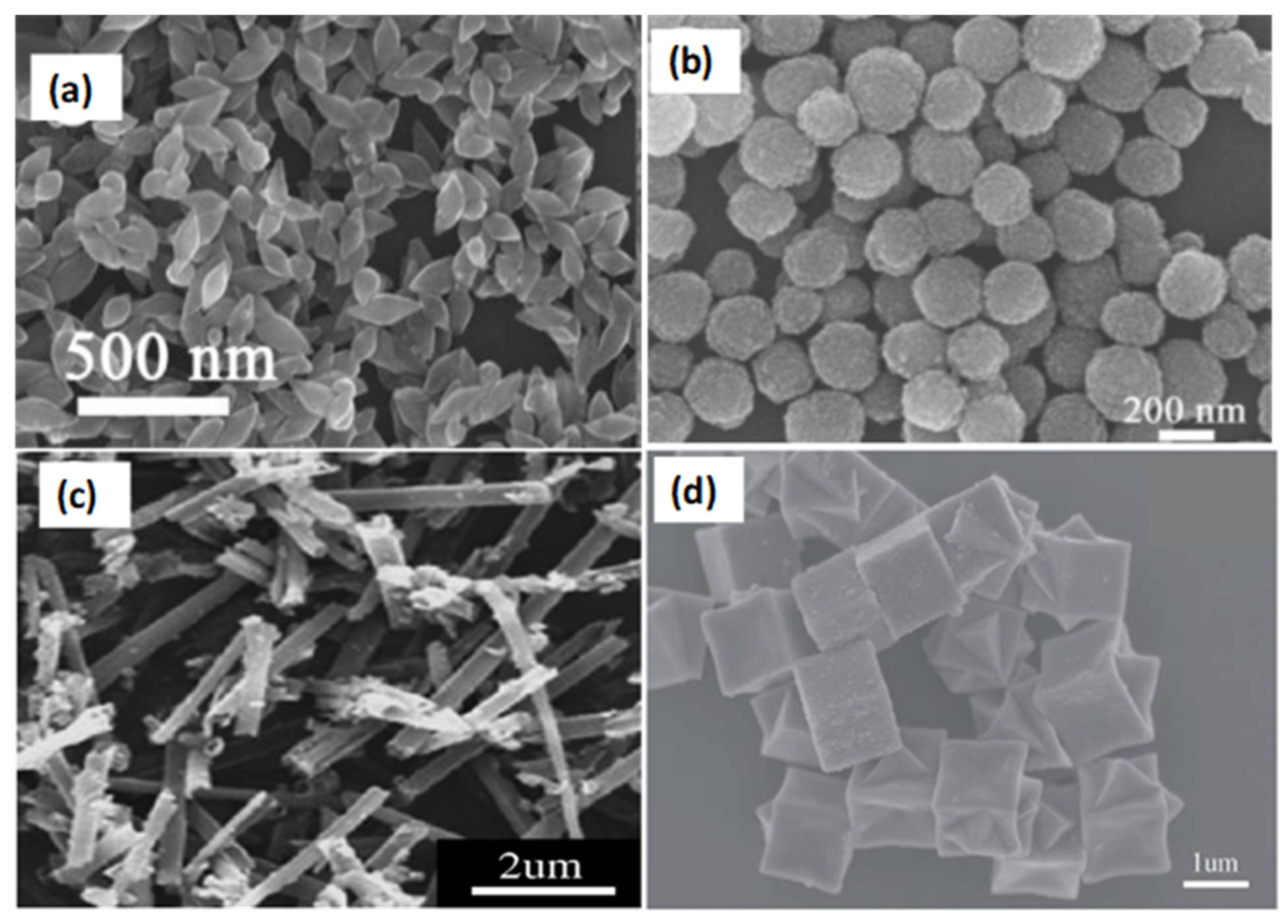
| ZnFe2O4 Morphology | Surface Area (m2g−1) | Target Gas | Concentration (ppm) | Reproducibility (Cycles)/Stabilty (Days) | Ra/Rg | Ref. |
|---|---|---|---|---|---|---|
| Mesoporous nanostructures | 103.6 | Acetone | 100 | -/30 | 11.6 | [77] |
| Porous Olives | 96.5 | Ethanol | 200 | 8/7 | 223 | [75] |
| Porous nanospheres | 59.0 | Acetone | 30 | 3/30 | 11.8 | [70] |
| Monodisperse nanospheres | 87.40 | Toluene | 1/100 | 5/20 | 1.41/9.98 | [71] |
| Hollow urchin-like core-shell spheres | 50.62 | Toluene | 100 | 6/30 | 79 | [78] |
| Porous nanospheres | 59.0 | Acetone | 100 | 3/30 | 42.1 | [70] |
| Porous nanorods | 82.01 | Acetone | 100 | -/30 | 52.8 | [73] |
| Hollow spheres | 48.1 | Ethylene glycol | 100 | -/8 weeks | 35.5 | [72] |

4.3. Effects of Generated Surface Defects
4.4. Effects of Surface Doping
4.5. Heterostructures

| ZnFe2O4 Heterostructure | Morphology | Interface/ Heterojunction | Target Gas | Concentration (ppm) | Reproducibilty (Cycles)/Stability (Days) | Ra/Rg | Ref. |
|---|---|---|---|---|---|---|---|
| ZnO/ZnFe2O4 | Lychee-like core, shell, and hollow microsphere | n-n junction | Acetone | 100 | -/20 | 36.6 | [97] |
| CuO@ZnFe2O4 | Yolk shell microspheres | p-n junction | Xylene | 100 | 5/60 | 24 | [92] |
| ZnFe2O4/ZnO | Flower-like microstructures | n-n junction | Acetone | 50 | 6/60 | 8.3 | [98] |
| ZnO/ZnFe2O4 | Microsphere | n-p-n junction | Benzene | 1 | -/- | 1.1 | [94] |
| ZnO/ZnFe2O4 | Tetrapod-like | n-n junction | Ethanol | 100 | -/30 | 13.97 | [73] |
| ZnFe2O4/ZnO | Nanosheets assembled into microspheres | n-n junction | Trimethylamine | 100 | 10/30 | 31.5 | [99] |
| ZnO/ZnFe2O4 | Hierarchical kiwifruit-like | n-n junction | Triethylamine | 100 | 6/30 | 40.15 | [98] |
| ZnFe2O4/α-Fe2O3 | Porous microrods | n-n junction | Triethylamine | 100 | 4/30 | 42.4 | [96] |
| Fe2O3/ZnFe2O4 | Porous spindles | n-n junction | Triethylamine | 20 | 3/- | 60.24 | [100] |
| CaFe2O4/ZnFe2O4 | Walnut | p-n junction | Isoprene | 30 | 9/30 | 19.5 | [101] |
| ZnFe2O4/(Fe-ZnO) | Nanocomposite | n-n junction | Acetone | 100 | 3/- | 30.8 | [102] |
5. Applications of ZnFe2O4 Gas Sensor
6. Future Perspective
7. Conclusions
- The development of novel synthesis procedures with excellent control of morphology can create access to more active sites.
- Different noble metals (i.e., Ag, Au, and Pd) can improve the sensing properties by chemically or electronically sensitizing ZnFe2O4.
- Introduction of structural defects by controlling the concertation of Fe2+ in the octahedral site and narrowing the band-gap of ZnFe2O4 to enhance sensing properties.
- Heterostructure design to form hybrid nanocomposites with either p-type or n-type materials, which will result in a new depletion layer that enhances the sensing response.
- Integration of several ZnFe2O4 nanostructures with different morphologies to produce a sensor array with enhanced sensing capabilities for specific applications in the food industry.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Volatile Organic Compound Gas Sensor Market Size Report, 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/volatile-organic-compound-gas-sensor-market (accessed on 11 November 2020).
- Mandoj, F.; Nardis, S.; Di Natale, C.; Paolesse, R. Porphyrinoid Thin Films for Chemical Sensing. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 422–443. [Google Scholar]
- Kassal, P.; Steinberg, M.D.; Steinberg, I.M. Wireless chemical sensors and biosensors: A review. Sens. Actuators B Chem. 2018, 266, 228–245. [Google Scholar] [CrossRef]
- Promphet, N.; Ummartyotin, S.; Ngeontae, W.; Puthongkham, P.; Rodthongkum, N. Non-invasive wearable chemical sensors in real-life applications. Anal. Chim. Acta 2021, 1179, 338643. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Korditis, I.; Swart, H.C.; Motaung, D.E. Facile control of room temperature nitrogen dioxide gas selectivity induced by copper oxide nanoplatelets. J. Colloid Interface Sci. 2020, 560, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Liu, T.; Wen, B.; Gong, C.; Wei, G.; Su, Z. Recent Advances in the Construction of Flexible Sensors for Biomedical Applications. Biotechnol. J. 2020, 15, e2000094. [Google Scholar] [CrossRef]
- Lu, Q.; Xie, X.; Parlikad, A.K.; Schooling, J.M.; Konstantinou, E. Moving from Building Information Models to Digital Twins for Operation and Maintenance. Proc. Inst. Civ. Eng. Smart Infrastruct. Constr. 2020, 174, 46–56. [Google Scholar] [CrossRef]
- Tonezzer, M.; Le, D.T.T.; Iannotta, S.; Van Hieu, N. Selective discrimination of hazardous gases using one single metal oxide resistive sensor. Sens. Actuators B Chem. 2018, 277, 121–128. [Google Scholar] [CrossRef]
- Yang, B.; Myung, N.V.; Tran, T. 1D Metal Oxide Semiconductor Materials for Chemiresistive Gas Sensors: A Review. Adv. Electron. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Singh, R.C.; Singh, M.P.; Virk, H.S. Applications of Nanostructured Materials as Gas Sensors. Solid State Phenom. 2013, 201, 131–158. [Google Scholar] [CrossRef]
- Duarte, J.; Rodrigues, F.; Castelo Branco, J. Sensing Technology Applications in the Mining Industry—A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2334. [Google Scholar] [CrossRef]
- Sarnoski, P.J.; O’Keefe, S.F.; Jahncke, M.L.; Mallikarjunan, P.; Flick, G.J. Analysis of crab meat volatiles as possible spoilage indicators for blue crab (Callinectes sapidus) meat by gas chromatography–mass spectrometry. Food Chem. 2010, 122, 930–935. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Ropartz, D.; García-Campaña, A.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion Mobility Spectrometry in Food Analysis: Principles, Current Applications and Future Trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, G.; Martinsdottir, E.; Jonsson, E.H. Rapid Gas Sensor Measurements to Determine Spoilage of Capelin (Mallotus villosus). J. Agric. Food Chem. 1997, 45, 2654–2659. [Google Scholar] [CrossRef]
- Camara, M.; Gharbi, N.; Lenouvel, A.; Behr, M.; Guignard, C.; Orlewski, P.; Evers, D. Detection and Quantification of Natural Contaminants of Wine by Gas Chromatography–Differential Ion Mobility Spectrometry (GC-DMS). J. Agric. Food Chem. 2013, 61, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D. Recent Trends on the Use of Infrared Spectroscopy to Trace and Authenticate Natural and Agricultural Food Products. Appl. Spectrosc. Rev. 2012, 47, 518–530. [Google Scholar] [CrossRef]
- Mustafa, F.; Othman, A.; Andreescu, S. Cerium oxide-based hypoxanthine biosensor for Fish spoilage monitoring. Sens. Actuators B Chem. 2021, 332, 129435. [Google Scholar] [CrossRef]
- Carvalho, M.; Ribeiro, P.; Romão, V.; Cardoso, S. Smart fingertip sensor for food quality control: Fruit maturity assessment with a magnetic device. J. Magn. Magn. Mater. 2021, 536, 168116. [Google Scholar] [CrossRef]
- Šutka, A.; Gross, K.A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuators B Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Allen, G.C.; Harris, S.J.; Jutson, J.A.; Dyke, J.M. A study of a number of mixed transition metal oxide spinels using X-ray photoelectron spectroscopy. Appl. Surf. Sci. 1989, 37, 111–134. [Google Scholar] [CrossRef]
- Gao, D.; Shi, Z.; Xu, Y.; Zhang, J.; Yang, G.; Zhang, J.; Wang, X.; Xue, D. Synthesis, Magnetic Anisotropy and Optical Properties of Preferred Oriented Zinc Ferrite Nanowire Arrays. Nanoscale Res. Lett. 2010, 5, 1289–1294. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, L.; Zhai, Q.; Lu, C. Synthesis, Properties, and Formation Mechanism of Zinc Ferrite Hollow Spheres. J. Am. Ceram. Soc. 2007, 90, 1959–1962. [Google Scholar] [CrossRef]
- Lemine, O.M.; Bououdina, M.; Sajieddine, M.; Al-Saie, A.M.; Shafi, M.; Khatab, A.; Al-hilali, M.; Henini, M. Synthesis, structural, magnetic and optical properties of nanocrystalline ZnFe2O4. Phys. B Condens. Matter 2011, 406, 1989–1994. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Niu, H.; Mao, C.; Zhang, S.; Shen, Y. ZnFe2O4 nanoparticles: Synthesis, characterization, and enhanced gas sensing property for acetone. Sens. Actuators B Chem. 2015, 221, 55–62. [Google Scholar] [CrossRef]
- Li, K.; Luo, Y.; Gao, L.; Li, T.; Duan, G. Au-Decorated ZnFe2O4 Yolk-Shell Spheres for Trace Sensing of Chlorobenzene. ACS Appl. Mater. Interfaces 2020, 12, 16792–16804. [Google Scholar] [CrossRef]
- Li, X.; Han, C.; Lu, D.; Shao, C.; Li, X.; Liu, Y. Highly electron-depleted ZnO/ZnFe2O4/Au hollow meshes as an advanced material for gas sensing application. Sens. Actuators B Chem. 2019, 297, 126769. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Mhlongo, G.H. Highly selective acetone detection displayed by a surface engineered fiber-like ZnFe2O4 based-sensor following heat-treatment ramping rate variation. Mater. Lett. 2023, 330, 133214. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Shingange, K.; Mhlongo, G.H. ZnO/ZnFe2O4 heterostructure for conductometric acetone gas sensors. Sens. Actuators B Chem. 2023, 377, 133027. [Google Scholar] [CrossRef]
- He, L.; Hu, J.; Yuan, Q.; Xia, Z.; Jin, L.; Gao, H.; Fan, L.; Chu, X.; Meng, F. Synthesis of porous ZnFe2O4/SnO2 core-shell spheres for high-performance acetone gas sensing. Sens. Actuators B Chem. 2023, 378, 133123. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Yang, M.; Tang, L.; Wang, Q.; Sun, Y.; Xu, Y. Novel strategy to construct porous Sn-doped ZnO/ZnFe2O4 heterostructures for superior triethylamine detection. Mater. Sci. Semicond. Process. 2021, 125, 105643. [Google Scholar] [CrossRef]
- Saito, N.; Haneda, H.; Watanabe, K.; Shimanoe, K.; Sakaguchi, I. Highly sensitive isoprene gas sensor using Au-loaded pyramid-shaped ZnO particles. Sens. Actuators B Chem. 2021, 326, 128999. [Google Scholar] [CrossRef]
- Lee, J.E.; Lim, C.K.; Park, H.J.; Song, H.; Choi, S.; Lee, D. ZnO–CuO Core-Hollow Cube Nanostructures for Highly Sensitive Acetone Gas Sensors at the ppb Level. ACS Appl. Mater. Interfaces 2020, 12, 35688–35697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Duan, Z.; Yuan, Z.; Li, X.; Si, W.; Liu, B.; Zhang, Y.; Jiang, Y.; Tai, H. High performance ethylene sensor based on palladium-loaded tin oxide: Application in fruit quality detection. Chin. Chem. Lett. 2020, 31, 2045–2049. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Wang, B.; Pei, S. Hydrothermal synthesis of SnO2-CuO composite nanoparticles as a fast-response ethanol gas sensor. J. Alloys Compd. 2021, 886, 161299. [Google Scholar] [CrossRef]
- Motsoeneng, R.G.; Kortidis, I.; Ray, S.S.; Motaung, D.E. Designing SnO2 Nanostructure-Based Sensors with Tailored Selectivity toward Propanol and Ethanol Vapors. ACS Omega 2019, 4, 13696. [Google Scholar] [CrossRef]
- Guo, M.; Luo, N.; Chen, Y.; Fan, Y.; Wang, X.; Xu, J. Fast-response MEMS xylene gas sensor based on CuO/WO3 hierarchical structure. J. Hazard. Mater. 2022, 429, 127471. [Google Scholar] [CrossRef]
- Cao, S.; Sui, N.; Zhang, P.; Zhou, T.; Tu, J.; Zhang, T. TiO2 nanostructures with different crystal phases for sensitive acetone gas sensors. J. Colloid Interface Sci. 2022, 607, 357–366. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Mi, Q. A high-performance room temperature benzene gas sensor based on CoTiO3 covered TiO2 nanospheres decorated with Pd nanoparticles. Sens. Actuators B Chem. 2022, 350, 130830. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Y.; Meng, D.; Wang, K.; Wang, C. A novel room temperature ethanol gas sensor based on 3D hierarchical flower-like TiO2 microstructures. Mater. Lett. 2020, 277, 128372. [Google Scholar] [CrossRef]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A New Detector for Gaseous Components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Yadav, R.S.; KuritKa, I.; Vilcakova, J.; Urbanek, P.; Machovsky, M.; Masar, M.; Holek, M. Structural, magnetic, optical, dielectric, electrical and modulus spectroscopic characteristics of ZnFe2O4 spinel ferrite nanoparticles synthesized via honey mediated sol gel combustion method. J. Phys. Chem. Solids 2017, 110, 87–99. [Google Scholar] [CrossRef]
- Devan, R.S.; Kolekar, Y.D.; Chougule, B.K. Effect of cobalt substitution on the properties of nickel copper ferrite. J. Physics Condens. Matter 2006, 18, 9809–9821. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Sadeghi, H.; Morisako, A. Simulation of DC-hopping conduction in spinel ferrites using free electron gas model. J. Magn. Magn. Mater. 2007, 316, e283–e286. [Google Scholar] [CrossRef]
- Verwey, E.J.; Haayman, P.W.; Romeijn, F.C. Physical Properties and Cation Arrangement of Oxides with Spinel Structures II. Electronic Conductivity. J. Chem. Phys. 1947, 15, 181–187. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Mdlalose, W.B.; Mhlongo, G.H. Size-tunable ferromagnetic ZnFe2O4 nanoparticles and their ethanol detection capabilities. Appl. Surf. Sci. 2020, 508, 144863. [Google Scholar] [CrossRef]
- Neethirajan, S.; Jayas, D.S.; Sadistap, S. Carbon Dioxide (CO2) Sensors for the Agri-food Industry—A Review. Food Bioprocess Technol. 2008, 2, 115–121. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators B Chem. 2011, 156, 527–538. [Google Scholar] [CrossRef]
- Darshare, S.L.; Deshmulch, R.G.; Surgavanshi, S.S.; Mulla, I.S. Gas sensing properties of Zinc Ferrite Nanoparticles Synthesized by the Molten-Salt Route. Am. Ceram. Soc. 2008, 91, 2724–2726. [Google Scholar] [CrossRef]
- Sutka, A.; Zavickis, J.; Mezinskis, G.; Jakovlevs, D.; Barloti, J. Ethanol monitoring by ZnFe2O4 thin film obtained by spray pyrolysis. Sens. Actuators B Chem. 2013, 176, 330–334. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Faisal, M.; Asiri, A.M.; Alamry, K.A. Highly sensitive formaldehyde chemical sensor based on hydrothermally prepared spinel ZnFe2O4 nanorods. Sens. Actuators B Chem. 2012, 171–172, 932–937. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, W.; Xie, C.; Wang, A.; Zeng, D.; Hu, M. Electrical conductivity and gas sensitivity to VOCs of V-doped ZnFe2O4 nanoparticles. Mater. Lett. 2006, 60, 1374–1378. [Google Scholar] [CrossRef]
- Park, M.; Kim, J.; Kim, K.J.; Lee, J.; Kim, J.H.; Yamauchi, Y. Porous nanoarchitectures of spinel-type transition metal oxides for electrochemical energy storage systems. Phys. Chem. Chem. Phys. PCCP 2015, 17, 3963–3977. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels; crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Mineral. 1983, 68, 181. [Google Scholar]
- Sutka, A.; Mezinskis, G.; Lusis, A.; Stingaciu, M. Gas sensing properties of Zn-doped p-type nickel ferrite. Sens. Actuators B Chem. 2012, 171–172, 354–360. [Google Scholar] [CrossRef]
- Patil, R.P.; Delekar, S.D.; Mane, D.R.; Hankare, P.P. Synthesis, structural and magnetic properties of different metal ion substituted nanocrystalline zinc ferrite. Results Phys. 2013, 3, 129–133. [Google Scholar] [CrossRef]
- Ebrahimi, H.R.; Parish, M.; Amiri, G.R.; Bahraminejad, B.; Fatahian, S. Synthesis, characterization and gas sensitivity investigation of Ni0.5Zn0.5Fe2O4 nanoparticles. J. Magn. Magn. Mater. 2016, 414, 55–58. [Google Scholar] [CrossRef]
- Mukherjee, C.; Mondal, D.; Sarkar, M.; Das, J. Nanocrystalline nickel zinc ferrite thick film as an efficient alcohol sensor at room temperature. Int. J. F Environ. Agric. Biotechnol. 2017, 2, 799–804. [Google Scholar]
- El-Sayed, A.M.; Hamzawy, E.M.A. Structure and Magnetic Properties of Nickel–Zinc Ferrite Nanoparticles Prepared by Glass Crystallization Method. Monatsh. Chem. 2006, 137, 1119–1125. [Google Scholar] [CrossRef]
- Shen, X.; Xiang, J.; Song, F.; Liu, M. Characterization and magnetic properties of electrospun Co1−xZnxFe2O4 nanofibers. Appl. Phys. A 2010, 99, 189–195. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Pal, M.; Dutta, A. Structural, optical and electrical properties of chemically derived nickel substituted zinc ferrite nanocrystals. Mater. Chem. Phys. 2011, 127, v–viii. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Rathore, D.; Kurchania, R.; Pandey, R.K. Fabrication of Ni1−xZnxFe2O4 (x=0, 0.5 and 1) nanoparticles gas sensor for some reducing gases. Sens. Actuators A Phys. 2013, 199, 236–240. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Mhlongo, G.H. Enhanced Propanol Response Behavior of ZnFe2O4 NP-Based Active Sensing Layer Induced by Film Thickness Optimization. Processes 2021, 9, 1791. [Google Scholar] [CrossRef]
- Wu, K.; Lu, Y.; Liu, Y.; Liu, Y.; Shen, M.; Debliquy, M.; Zhang, C. Synthesis and acetone sensing properties of copper (Cu2+) substituted zinc ferrite hollow micro-nanospheres. Ceram. Int. 2020, 46, 28835–28843. [Google Scholar] [CrossRef]
- Pal, J.; Pal, T. Faceted Metal and Metal Oxide Nanoparticles: Design, Fabrication and Catalysis. Proc. Natl. Acad. Sci. USA 2020, 117, 19168–19177. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Ji, W.; Shen, Z.; Tang, Q.; Fan, M. NH3 molecule adsorption on spinel-type ZnFe2O4 surface: A DFT and experimental comparison study. Appl. Surf. Sci. 2018, 442, 778–786. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Cheng, F.; Chen, J. ZnFe2O4 tubes: Synthesis and application to gas sensors with high sensitivity and low-energy consumption. Sens. Actuators B Chem. 2007, 120, 403–410. [Google Scholar] [CrossRef]
- Tiemann, M. Porous Metal Oxides as Gas Sensors. Chem. A Eur. J. 2007, 13, 8376–8388. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Wang, C.; Sun, P.; Hu, X.; Li, X.; Shimanoe, K.; Yamazoe, N.; Lu, G. Highly sensitive acetone gas sensor based on porous ZnFe2O4 nanospheres. Sens. Actuators B Chem. 2015, 206, 577–583. [Google Scholar] [CrossRef]
- Dong, C.; Liu, X.; Xiao, X.; Du, S.; Wang, Y. Monodisperse ZnFe2O4 nanospheres synthesized by a nonaqueous route for a highly slective low-ppm-level toluene gas sensor. Sens. Actuators B Chem. 2017, 239, 1231–1236. [Google Scholar] [CrossRef]
- Yang, H.; Bai, X.; Hao, P.; Tian, J.; Bo, Y.; Wang, X.; Liu, H. A simple gas sensor based on zinc ferrite hollow spheres: Highly sensitivity, excellent selectivity and long-term stability. Sens. Actuators B Chem. 2019, 280, 34–40. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Dun, M.; Huang, X. Porous ZnFe2O4 nanorods with net-worked nanostructure for highly sensor response and fast response acetone gas sensor. Sens. Actuators B Chem. 2017, 248, 85–91. [Google Scholar] [CrossRef]
- Qu, F.; Shang, W.; Thomas, T.; Ruan, S.; Yang, M. Self-template derived ZnFe2O4 double-shell microspheres for chemresistive gas sensing. Sens. Actuators B Chem. 2018, 265, 625–631. [Google Scholar] [CrossRef]
- Sun, K.; Song, X.; Wang, X.; Li, X.; Tan, Z. Annealing temperature-dependent porous ZnFe2O4 olives derived from bimetallic organic frameworks for high-performance ethanol gas sensing. Mater. Chem. Phys. 2020, 241, 122379. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, X.; Gong, Y.; Han, N.; Liu, H.; Chen, Y. MOF-derived hierarchical ZnO/ZnFe2O4 hollow cubes for enhanced acetone gas-sensing performance. RSC Adv. 2017, 7, 34609–34617. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Yang, Q.; Gao, Y.; Sun, P.; Zhang, T.; Lu, G. Mesoporous ZnFe2O4 prepared through hard template and its acetone sensing properties. Mater. Lett. 2016, 183, 378–381. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Li, M.; Li, Z.; Yuan, Y.; Yang, X.; Guo, L. Highly efficient toluene gas sensor based on spinel structured hollow urchin-like core-shell ZnFe2O4 spheres. Sens. Actuators B Chem. 2021, 349, 130734. [Google Scholar] [CrossRef]
- Gritsyna, V.T.; Afanasyev-Charkin, I.V.; Kobyakov, V.A.; Sickafus, K.E. Structure and electronic states of defects in spinel of different compositions MgO.nAI2O3:Me. J. Am. Ceram. Soc. 1999, 82, 3365. [Google Scholar] [CrossRef]
- El-Sayed, A.M. Electrical conductivity of nickel–zinc and Cr substituted nickel–zinc ferrites. Mater. Chem. Phys. 2003, 82, 583–587. [Google Scholar] [CrossRef]
- Sutka, A.; Mezinskis, G.; Lusis, A.; Jakovlevs, D. Influence of iron non-stoichiometry on spinel zinc ferrite gas sensing properties. Sens. Actuators B Chem. 2012, 171–172, 204–209. [Google Scholar] [CrossRef]
- Peng, S.; Wang, Z.; Liu, R.; Bi, J.; Wu, J. Controlled oxygen vacancies of ZnFe2O4 with superior gas sensing properties prepared via a facile one-step self-catalyzed treatment. Sens. Actuators B Chem. 2019, 288, 649–655. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and Metal Oxide Nanoparticles in Chemiresistors: Does the Nanoscale Matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Suman, P.H.; Kim, J.J.; Tuller, H.L.; Orlandi, M.O. Investigation of electronic and chemical sensitization effects promoted by Pt and Pd nanoparticles on single-crystalline SnO nanobelt-based gas sensors. Sens. Actuators B Chem. 2019, 301, 127055. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Mhlongo, G.H. Evaluation of the effects of Au addition into ZnFe2O4 nanostructures on acetone detection capabilities. Mater. Res. Bull. 2021, 142, 111395. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, P.; Wang, Y.; Xu, L.; Zhang, B.; Lv, C.; Ma, J.; Zhang, Y. Sb-doped three-dimensional ZnFe2O4 macroporous spheres for N-butanol chemiresistive gas sensors. Sens. Actuators B Chem. 2020, 320, 128384. [Google Scholar] [CrossRef]
- Kwon, H.; Yoon, J.; Lee, Y.; Kim, D.Y.; Baek, C.; Kim, J.K. An array of metal oxides nanoscale hetero p-n junctions toward designable and highly-selective gas sensors. Sens. Actuators B Chem. 2018, 255, 1663–1670. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sens. Actuators B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Liu, F.; Chu, X.; Dong, Y.; Zhang, W.; Sun, W.; Shen, L. Acetone gas sensors based on graphene-ZnFe2O4 composite prepared by solvothermal method. Sens. Actuators B Chem. 2013, 188, 469–474. [Google Scholar] [CrossRef]
- Zhang, N.; Ruan, S.; Han, J.; Yin, Y.; Li, X.; Liu, C.; Adimi, S.; Wen, S.; Xu, Y. Oxygen vacancies dominated CuO@ZnFe2O4 yolk-shell microspheres for robust and selective detection of xylene. Sens. Actuators B Chem. 2019, 295, 117–126. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Chen, Y.; Chen, J.; Gong, Y.; Han, N. Abnormal n-p-n type conductivity transition of hollow ZnO/ZnFe2O4 nanostructures during gas sensing process: The role of ZnO-ZnFe2O4 hetero-interface. Sens. Actuators B Chem. 2017, 253, 144–155. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Shao, M.; Huang, J.; Deng, X.; Hou, P.; Xu, X. Fabrication of ZnO/ZnFe2O4 hollow nanocages through metal organic frameworks route with enhanced gas sensing properties. Sens. Actuators B Chem. 2017, 251, 27–33. [Google Scholar] [CrossRef]
- Li, Y.; Luo, N.; Sun, G.; Zhang, B.; Ma, G.; Jin, H.; Wang, Y.; Cao, J.; Zhang, Z. Facile synthesis of ZnFe2O4/α-Fe2O3 porous microrods with enhanced TEA-sensing performance. J. Alloys Compd. 2018, 737, 255–262. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.; Liu, D.; Lin, G.; Wan, J.; Jiang, H.; Lai, X.; Hao, S.; Liu, X. Lychee-like ZnO/ZnFe2O4 core-shell hollow microsphere for improving acetone gas sensing performance. Ceram. Int. 2020, 46, 5960–5967. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Wang, T.; Liu, J.; Sun, P.; Chuai, X.; Lu, G. Enhanced gas sensing characteristics of the flower-like ZnFe2O4/ZnO microstructures. Sens. Actuators B Chem. 2017, 248, 902–909. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, C.; He, L.; Zhang, K.; Zhang, J.; Jin, L.; Asiri, A.M.; Alamry, K.A.; Chu, X. ZnFe2O4/ZnO nanosheets assembled microspheres for high performance trimethylamine gas sensing. J. Alloys Compd. 2020, 849, 156461. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, J.; Song, P.; Li, J.; Yang, Z.; Wang, Q. Spindle-like Fe2O3/ZnFe2O4 porous nanocomposites derived from metal-organic frameworks with excellent sensing performance towards triethylamine. Sens. Actuators B Chem. 2020, 317, 128205. [Google Scholar] [CrossRef]
- Guo, W.; Huang, L.; Liu, X.; Wang, J.; Zhang, J. Enhanced isoprene gas sensing performance based on p-CaFe2O4/n-ZnFe2O4 heterojunction composites. Sens. Actuators B Chem. 2022, 354, 131243. [Google Scholar] [CrossRef]
- Cao, E.; Guo, Z.; Song, G.; Zhang, Y.; Hao, W.; Sun, L.; Nie, Z. MOF-derived ZnFe2O4/(Fe-ZnO) nanocomposites with enhanced acetone sensing performance. Sens. Actuators B Chem. 2020, 325, 128783. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Sun, H.; Sun, P.; Liang, X.; Liu, F.; Hu, X.; Lu, G. Nanosheet-assembled ZnFe2O4 hollow microspheres for high-sensitive acetone sensor. ACS Appl. Mater. Interfaces 2015, 7, 15414. [Google Scholar] [CrossRef]
- Morgott, D.A. Acetone. In Patty’s Toxicology; Wiley Online Library: New York, NY, USA, 2001; pp. 735–752. [Google Scholar]
- Fleming-Jones, M.E.; Smith, R.E. Volatile Organic Compounds in Foods: A Five Year Study. J. Agric. Food Chem. 2003, 51, 8120–8127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, R.; Fang, W.; Li, G.; Guo, Y.; Qin, Z. Triethylamine as an initiator for cracking of heptane. Energy 2006, 31, 2773–2790. [Google Scholar] [CrossRef]
- Brahmachari, G.; Nayek, N.; Nurjamal, K.; Karmakar, I.; Begam, S. Triethylamine—A Versatile Organocatalyst in Organic Transformations: A Decade Update. Synthesis 2018, 50, 4145–4164. [Google Scholar] [CrossRef]
- Karmakar, M.; Das, P.P.; Pal, M.M.; Mondal, B.B.; Majumder, S.B.; Mukherjee, K.K. Acetone and ethanol sensing characteristics of magnesium zinc ferrite nano-particulate chemi-resistive sensor. J. Mater. Sci. 2014, 49, 5766. [Google Scholar] [CrossRef]
- Nemufulwi, M.I.; Swart, H.C.; Mhlongo, G.H. A comprehensive comparison study on magnetic behaviour, defects-related emission and Ni substitution to clarify the origin of enhanced acetone detection capabilities. Sens. Actuators B Chem. 2021, 339, 129860. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Q.; Zheng, B.; You, J.; Luo, Y. Synthesis and acetone gas sensing properties of Ag activated hollow sphere structured ZnFe2O4. Ceram. Int. 2018, 44, 20700–20707. [Google Scholar] [CrossRef]
- Zhai, C.; Zhao, Q.; Gu, K.; Xing, D.; Zhang, M. Ultra-fast response and recovery of triethylamine gas sensors using a MOF-based ZnO/ZnFe2O4 structures. J. Alloys Compd. 2019, 784, 660–667. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Han, L.; Li, X.; Xu, Y. Hierarchical kiwifruit-like ZnO/ZnFe2O4 heterostructure for high-sensitive triethylamine gaseous sensor. Sens. Actuators B Chem. 2021, 344, 130251. [Google Scholar] [CrossRef]
- Liu, S.; Guan, M.; Li, X.; Guo, Y. Light irradiation enhanced triethylamine gas sensing materials based on ZnO/ZnFe2O4 composites. Sens. Actuators B Chem. 2016, 236, 350–357. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Cunniffe, C.; Nicholson, M.; Arshak, A. Preparation and characterisation of ZnFe2O4/ZnO polymer nanocomposite sensors for the detection of alcohol vapours. Superlattices Microstruct. 2007, 42, 479–488. [Google Scholar] [CrossRef]
- Hayasaka, T.; Lin, A.; Copa, V.C.; Lopez, L.P.; Loberternos, R.A.; Ballesteros, L.I.M.; Kubota, Y.; Liu, Y.; Salvador, A.A.; Lin, L. An electronic nose using a single graphene FET and machine learning for water, methanol, and ethanol. Microsyst. Nanoeng. 2020, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cho, M.; Li, Y.; He, T.; Ahn, J.; Park, J.; Ren, T.; Lee, C.; Park, I. Machine learning-enabled textile-based graphene gas sensing with energy harvesting-assisted IoT application. Nano Energy 2021, 86, 106035. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Song, Z.; Ye, W.; Fan, Z. Smart gas sensor arrays powered by artificial intelligence. J. Semicond. 2019, 40, 111601. [Google Scholar] [CrossRef]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on Smart Gas Sensing Technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef]



| Gas | Source of Emission/Product | Detection Test/Application | SMO Used | Detection Range (ppm)/Environmental Setting | Ref. |
|---|---|---|---|---|---|
| Acetone and Ethanol | Household and industrial products, laboratories, and chemical industries | Safety purposes | Mg0.5Zn0.5 Fe2O4 | 20–200/dry air RH, RT | [108] |
| Propanol | Alcoholic beverages | Quality and classification of wines | ZnFe2O4 NPs | 0.5–40/10–80%, 120 °C | [64] |
| Acetone | Human breath, fish products | Diabetes diagnostics and spoilage detection | ZnFe2O4 nanoparticles | 0.5–40/0–60%, 120 °C | [109] |
| n-butanol | petroleum refineries and insect repellents, | Human health and safety | Sb-doped 3D ZnFe2O4 MPs | 0.049–200/25–90 RH, 250 °C | [88] |
| Toluene | lacquers, medicine, pesticides, leather manufacturing, and explosives | Environmental and human safety, Human health | urchin-like hollow core-shell ZnO/ZnFe2O4 ZnFe2O4 | 0.2–100/10–98% RH, 275 °C 0.2–100/10–98% RH, 250 °C | [78] |
| Toluene | Chemical Industry | Environmental safety and human safety | Monodisperse ZnFe2O4 nanospheres | 1–100/Dry air. 300 °C | [71] |
| Acetone | Human breath | Acetone-threat or diabetes-breathalyzer tests | Double-shelled ZnFe2O4 microsphere | 0.13–200/Dry air, 206 °C | [74] |
| Acetone | Human breath, industry | Diagnosis of diabetes, industrial processes, and health control | ZnO/ZnFe2O4/Au 0.125%Graphene-ZnFe2O4 ZnFe2O4 hollow sphere and Ag-ZnFe2O4 | 0.3–200/33–95% RH, 225 °C 1–1000/Dry air, 275 °C 0.8–500/25–100% Rh, 175 °C | [27,91,110] |
| Ethanol | Human breath; alcohol beverages | Medical or clinical applications; brewing process control. | ZnFe2O4 thin film | 1–50/Dry air, 390 °C | [50] |
| Triethylamine (TEA) | Meat, wastewater, dead fish, and marine products | Meat spoilage, environmental monitoring, and human health protection | MOF-ZnO/ZnFe2O4 | 2–100/Dry air, 170 °C | [111] |
| TEA | Industrial production | Industrial monitoring | kiwifruit-like ZnO/ZnFe2O4 ZnO/ZnFe2O4 | 1–200/dry air, 200 °C 5–1000/dry air, 80 and 240 °C | [112,113] |
| Propanol | Industrial plants | Leak detection | ZnFe2O4/ZnO polymer nanocomposite | 500–5000/Dry air, N/A | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemufulwi, M.I.; Swart, H.C.; Mhlongo, G.H. Advances of Nano-Enabled ZnFe2O4 Based-Gas Sensors for VOC Detection and Their Potential Applications: A Review. Processes 2023, 11, 3122. https://doi.org/10.3390/pr11113122
Nemufulwi MI, Swart HC, Mhlongo GH. Advances of Nano-Enabled ZnFe2O4 Based-Gas Sensors for VOC Detection and Their Potential Applications: A Review. Processes. 2023; 11(11):3122. https://doi.org/10.3390/pr11113122
Chicago/Turabian StyleNemufulwi, Murendeni I., Hendrik C. Swart, and Gugu H. Mhlongo. 2023. "Advances of Nano-Enabled ZnFe2O4 Based-Gas Sensors for VOC Detection and Their Potential Applications: A Review" Processes 11, no. 11: 3122. https://doi.org/10.3390/pr11113122
APA StyleNemufulwi, M. I., Swart, H. C., & Mhlongo, G. H. (2023). Advances of Nano-Enabled ZnFe2O4 Based-Gas Sensors for VOC Detection and Their Potential Applications: A Review. Processes, 11(11), 3122. https://doi.org/10.3390/pr11113122







