Dipeptidyl Peptidase 4 Inhibitors in Type 2 Diabetes Mellitus Management: Pharmacophore Virtual Screening, Molecular Docking, Pharmacokinetic Evaluations, and Conceptual DFT Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Workflow Applicability and Future Research Direction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [PubMed]
- Skyler, J.S.; Bergenstal, R.; Bonow, R.O.; Buse, J.; Deedwania, P.; Gale, E.A.; Howard, B.V.; Kirkman, M.S.; Kosiborod, M.; Reaven, P.; et al. Intensive glycemic control and the prevention of vardiovascular events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials: A position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J. Am. Coll. Cardiol. 2009, 53, 298–304. [Google Scholar] [PubMed]
- Cui, J.; Liu, Y.; Li, Y.; Xu, F.; Liu, Y. Type 2 Diabetes and Myocardial Infarction: Recent Clinical Evidence and Perspective. Front. Cardiovasc. Med. 2021, 24, 644189. [Google Scholar] [CrossRef] [PubMed]
- Putaala, J.; Liebkind, R.; Gordin, D.; Thorn, L.M.; Haapaniemi, E.; Forsblom, C.; Groop, P.-H.; Kaste, M.; Tatlisumak, T. Diabetes mellitus and ischemic stroke in the young: Clinical features and long-term prognosis. Neurology 2011, 76, 1831–1837. [Google Scholar] [CrossRef]
- Chen, R.; Ovbiagele, B.; Feng, W. Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. Am. J. Med. Sci. 2016, 351, 380–386. [Google Scholar] [CrossRef]
- La Sala, L.; Prattichizzo, F.; Ceriello, A. The Link between Diabetes and Atherosclerosis. Eur. J. Prev. Cardiol. 2019, 26, 15–24. [Google Scholar] [CrossRef]
- Ye, J.; Li, L.; Wang, M.; Ma, Q.; Tian, Y.; Zhang, Q.; Liu, J.; Li, B.; Zhang, B.; Liu, H.; et al. Diabetes Mellitus Promotes the Development of Atherosclerosis: The Role of NLRP3. Front. Immunol. 2022, 29, 900254. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, F.; Xie, Y.; Mo, Y.; Zhang, X.; Liu, C. Diabetic endothelial microangiopathy and pulmonary dysfunction. Front. Endocrinol. 2023, 14, 1073878. [Google Scholar] [CrossRef]
- Gutman, M.; Kaplan, O.; Skornick, Y.; Klausner, J.M.; Lelcuk, S.; Rozin, R.R. Gangrene of the lower limbs in diabetic patients: A malignant complication. Am. J. Surg. 1987, 154, 305–308. [Google Scholar] [CrossRef]
- Gao, L.; Li, T.; Wang, S.; Wang, J. Comprehensive treatment of diabetic hallux gangrene with lower extremity vascular disease: A case report. J. Int. Med. Res. 2019, 47, 6374–6384. [Google Scholar] [CrossRef]
- Casanova, L.; Hughes, F.; Preshaw, P. Diabetes and periodontal disease: A two-way relationship. Br. Dent. J. 2014, 217, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.-W.; Bonventre, J.V. Acute Kidney Injury and Progression of Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Hotta, N. A new perspective on the biguanide, metformin therapy in type 2 diabetes and lactic acidosis. J. Diabetes Investig. 2019, 10, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.; Marques, D.; Barra, C.; Rosendo-Silva, D.; Costa, A.; Rodrigues, T.; Gasparini, P.; Melo, B.F.; Sacramento, J.F.; Seiça, R.; et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol. Metabol. 2021, 51, 101241. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Sharma, S.; Khan, Y. DPP-4 inhibitors for treating T2DM-hype or hope? an analysis based on the current literature. Front. Mol. Biosci. 2023, 10, 1130625. [Google Scholar] [CrossRef]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef]
- Hinnen, D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Kang, B.; Zhou, J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc. Diabetol. 2022, 21, 83. [Google Scholar] [CrossRef]
- Zhang, S.; Qi, Z.; Wang, Y.; Song, D.; Zhu, D. Effect of sodium-glucose transporter 2 inhibitors on sarcopenia in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1203666. [Google Scholar] [CrossRef]
- Chiarelli, F.; Di Marzio, D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: Current evidence and future perspectives. Vasc. Health. Risk Manag. 2008, 4, 297–304. [Google Scholar]
- Frkic, R.L.; Richter, K.; Bruning, J.B. The therapeutic potential of inhibiting PPARγ phosphorylation to treat type 2 diabetes. J. Biol. Chem. 2021, 297, 101030. [Google Scholar] [CrossRef]
- Zhou, K.; Lansang, M.C. Diabetes Mellitus and Infections. In Endotext; MDText.com: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK569326/ (accessed on 30 August 2023).
- Casqueiro, J.; Janine, C.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16, S27–S36. [Google Scholar] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Ozougwu, J.C.; Obimba, K.C.; Belonwu, C.D.; Unakalamba, C.B. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef]
- Weyer, C.; Bogardus, C.; Mott, D.M.; Pratley, R.E. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Investig. 1999, 104, 787–794. [Google Scholar] [CrossRef]
- Rahman, S.U.; Ali, H.S.; Jafari, B.; Zaib, S.; Hameed, A.; Al-Kahraman, Y.M.S.A.; Langer, P.; Iqbal, J. Structure-Based Virtual Screening of Dipeptidyl Peptidase 4 Inhibitors and their In vitro Analysis. Comput. Biol. Chem. 2021, 91, 107326–107353. [Google Scholar] [CrossRef]
- Meduru, H.; Wang, Y.-T.; Tsai, J.J.P.; Chen, Y.-C. Finding a Potential Dipeptidyl Peptidase-4 (DPP-4) Inhibitor for Type-2 Diabetes Treatment Based on Molecular Docking, Pharmacophore Generation, and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2016, 17, 920. [Google Scholar] [CrossRef]
- Crisan, L.; Avram, S.; Pacureanu, L. Pharmacophore-based screening and drug repurposing exemplified on glycogen synthase kinase-3 inhibitors. Mol. Divers. 2017, 21, 385–405. [Google Scholar] [CrossRef]
- Pacureanu, L.; Bora, A.; Crisan, L. New Insights on the Activity and Selectivity of MAO-B Inhibitors through In Silico Methods. Int. J. Mol. Sci. 2023, 24, 9583. [Google Scholar] [CrossRef]
- Hermansyah, O.; Bustamam, A.; Yanuar, A. Virtual screening of dipeptidyl peptidase-4 inhibitors using quantitative structure–activity relationship-based artificial intelligence and molecular docking of hit compounds. Comput. Biol. Chem. 2021, 95, 107597–107608. [Google Scholar] [CrossRef] [PubMed]
- Ivan, D.; Crisan, L.; Funar-Timofei, S.; Mracec, M. A quantitative structure–activity relationships study for the anti-HIV-1 activities of 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine derivatives using the multiple linear regression and partial least squares methodologies. J. Serb. Chem. Soc. 2013, 78, 495–506. [Google Scholar] [CrossRef]
- Crisan, L.; Pacureanu, L.; Avram, S.; Bora, A.; Avram, S.; Kurunczi, L. PLS and shape-based similarity analysis of maleimides–GSK-3 inhibitors. J. Enzym. Inhib. Med. Chem. 2014, 29, 599–610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Syam, Y.M.; Anwar, M.M.; Abd El-Karim, S.S.; Elseginy, S.A.; Essa, B.M.; Sakr, T.M. New quinoxaline compounds as DPP-4 inhibitors and hypoglycemics: Design, synthesis, computational and bio-distribution studies. RSC Adv. 2021, 11, 36989–37011. [Google Scholar] [CrossRef] [PubMed]
- Alsamghan, A.S.; Alwabli, A.S.; Abadi, M.; Alsaleem, S.A.; Anbari, D.M.; Alomari, A.S.; Alzahrani, O.; Alam, Q.; Tarique, M. From sequence analysis of DPP-4 to molecular docking based searching of its inhibitors. Bioinformation 2020, 16, 444–451. [Google Scholar] [PubMed]
- Crisan, L.; Istrate, D.; Bora, A.; Pacureanu, L. Virtual screening and drug repurposing experiments to identify potential novel selective MAO-B inhibitors for Parkinson’s disease treatment. Mol. Div. 2021, 25, 1775–1794. [Google Scholar] [CrossRef]
- Crisan, L.; Bora, A. Small Molecules of Natural Origin as Potential Anti-HIV Agents: A Computational Approach. Life 2021, 11, 722. [Google Scholar] [CrossRef]
- Pantaleão, S.Q.; Maltarollo, V.G.; Araujo, S.C.; Gertrudesc, J.C.; Honorio, K.M. Molecular docking studies and 2D analyses of DPP-4 inhibitors as candidates in the treatment of diabetes. Mol. Biosyst. 2015, 11, 3188–3193. [Google Scholar] [CrossRef]
- Qi, J.-H.; Chen, P.-y.; Cai, D.-y.; Wang, Y.; Wei, Y.-l.; He, S.-p.; Zhou, W. Exploring novel targets of sitagliptin for type 2 diabetes mellitus: Network pharmacology, molecular docking, molecular dynamics simulation, and SPR approaches. Front. Endocrinol. 2023, 13, 1096655–1096668. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, M.; Pan, F.; Li, J.; Dou, R.; Wang, X.; Wang, Y.; He, Y.; Wang, S.; Cai, S. In silico analysis of novel dipeptidyl peptidase-IV inhibitory peptides released from Macadamia integrifolia antimicrobial protein 2 (MiAMP2) and the possible pathways involved in diabetes protection. Curr. Res. Nutr. Food Sci. 2021, 4, 603–611. [Google Scholar] [CrossRef]
- Singh, A.; Mishra, A. Molecular dynamics simulation and free energy calculation studies of Coagulin L as dipeptidyl peptidase-4 inhibitor. J. Biomol. Struct. Dyn. 2022, 40, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Shoombuatong, W.; Prachayasittikul, V.; Anuwongcharoen, N.; Songtawee, N.; Monnor, T.; Prachayasittikul, S.; Prachayasittikul, V.; Nantasenamat, C. Navigating the chemical space of dipeptidyl peptidase-4 inhibitors. Drug Des. Devel. Ther. 2015, 9, 4515–4549. [Google Scholar] [PubMed]
- Visa, A.; Plesu, N.; Maranescu, B.; Ilia, G.; Borota, A.; Crisan, L. Combined Experimental and Theoretical Insights into the Corrosion Inhibition Activity on Carbon Steel Iron of Phosphonic Acids. Molecules 2021, 26, 135. [Google Scholar] [CrossRef] [PubMed]
- Crisan, L.; Borota, A.; Bora, A.; Pacureanu, P. Diarylthiazole and Diarylimidazole Selective COX-1 Inhibitors Analysis through Pharmacophore Modeling, Virtual Screening, and DFT-Based Approaches. Struct. Chem. 2019, 30, 2311–2326. [Google Scholar] [CrossRef]
- Mohammad, B.D.; Baig, M.S.; Bhandari, N.; Siddiqui, F.A.; Khan, S.L.; Ahmad, Z.; Khan, F.S.; Tagde, P.; Jeandet, P. Heterocyclic Compounds as Dipeptidyl Peptidase-IV Inhibitors with Special Emphasis on Oxadiazoles as Potent Anti-Diabetic Agents. Molecules 2022, 27, 6001. [Google Scholar] [CrossRef]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Makrilakis, K. The Role of DPP-4 Inhibitors in the Treatment Algorithm of Type 2 Diabetes Mellitus: When to Select, What to Expect. Int. J. Environ. Res. Public Health 2019, 16, 2720. [Google Scholar] [CrossRef]
- Tarapués, M.; Cereza, G.; Figueras, A. Association of musculoskeletal complaints and gliptin use: Review of spontaneous reports. Pharmacoepidemiol. Drug Saf. 2013, 22, 1115–1118. [Google Scholar] [CrossRef]
- Nader, M.A. Inhibition of anaphylaxis like reaction and mast cell activation by Sitagliptin. Int. Immunopharmacol. 2011, 11, 1052–1056. [Google Scholar] [CrossRef]
- Rendell, M.; Drincic, A.; Andukuri, R. Alogliptin benzoate for the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2012, 13, 553–563. [Google Scholar] [CrossRef]
- Nabeno, M.; Akahoshi, F.; Kishida, H.; Miyaguchi, I.; Tanaka, Y.; Ishii, S.; Kadowaki, T. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem. Biophys. Res. Comm. 2013, 434, 191–196. [Google Scholar] [CrossRef]
- Arulmozhiraja, S.; Matsuo, N.; Ishitsubo, E.; Okazaki, S.; Shimano, H.; Tokiwa, H. Comparative Binding Analysis of Dipeptidyl Peptidase IV (DPP-4) with Antidiabetic Drugs-An Ab Initio Fragment Molecular Orbital Study. PLoS ONE 2016, 11, e0166275. [Google Scholar] [CrossRef] [PubMed]
- Lambeir, A.M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.; Alam, O.; Siddiqui, N.; Jha, M.; Manaithiya, A.; Bawa, S.; Sharma, N.; Alshehri, S.; Alam, P.; Shakeel, F. Insight into Structure Activity Relationship of DPP-4 Inhibitors for Development of Antidiabetic Agents. Molecules 2023, 28, 5860. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Q.; Zhang, C.; Yang, W.; Liu, H.; Lv, Z.; Liu, J.; Jiao, Z. Inhibition of Dipeptidyl Peptidase-4 by Flavonoids: Structure–Activity Relationship, Kinetics and Interaction Mechanism. Front. Nutr. 2022, 9, 892426. [Google Scholar] [CrossRef]
- Sunseri, J.; Koes, D.R. Pharmit: Interactive exploration of chemical space. Nucleic Acids Res. 2016, 44, W442–W448. [Google Scholar] [CrossRef]
- Zhang, Z.; Wallace, M.B.; Feng, J.; Stafford, J.A.; Skene, R.J.; Shi, L.; Lee, B.; Aertgeerts, K.; Jennings, A.; Xu, R.; et al. Design and Synthesis of Pyrimidinone and Pyrimidinedione Inhibitors of Dipeptidyl Peptidase IV. J. Med. Chem. 2011, 54, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Wang, L.; Beconi, M.; Eiermann, G.J.; Fisher, M.H.; He, H.; Hickey, G.J.; Kowalchick, J.E.; Leiting, B.; Lyons, K.; et al. (2R)-4-Oxo-4-[3-(Trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin- 7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: A Potent, Orally Active Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2005, 48, 141–151. [Google Scholar] [CrossRef]
- Eckhardt, M.; Langkopf, E.; Mark, M.; Tadayyon, M.; Thomas, L.; Nar, H.; Pfrengle, W.; Guth, B.; Lotz, R.; Sieger, P.; et al. 8-(3-(R)-Aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a Highly Potent, Selective, Long-Acting, and Orally Bioavailable DPP-4 Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2007, 50, 6450–6453. [Google Scholar] [CrossRef]
- MakeReceptor, v. 3.5.0.4; OpenEye Scientific Software Inc.: Santa Fe, NM, USA, 2020.
- Istrate, D.; Crisan, L. Natural Compounds as DPP-4 Inhibitors: 3D-Similarity Search, ADME Toxicity, and Molecular Docking Approaches. Symmetry 2022, 14, 1842. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-4: LigPrep; Schrödinger LLC: New York, NY, USA, 2022.
- OMEGA, 4.0.0.4; OpenEye Scientific Software: Santa Fe, NM, USA, 2019. Available online: http://www.eyesopen.com (accessed on 30 August 2023).
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef] [PubMed]
- FRED, 3.5.0.4; OpenEye Scientific Software: Santa Fe, NM, USA. Available online: http://www.eyesopen.com (accessed on 30 August 2023).
- Kelley, B.P.; Brown, S.P.; Warren, G.L.; Muchmore, S.W. POSIT: Flexible Shape-Guided Docking for Pose Prediction. J. Chem. Inf. Model. 2015, 55, 1771–1780. [Google Scholar] [CrossRef]
- McGann, M. FRED Pose Prediction and Virtual Screening Accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [Google Scholar] [CrossRef] [PubMed]
- McGann, M. FRED and HYBRID docking performance on standardized datasets. J. Comput. Aided Mol. Des. 2012, 26, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2022-4: Maestro, v. 13.4.134; Schrödinger LLC: New York, NY, USA, 2022.
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the chemical beauty of drugs. Nat. Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Delivery Rev. 1997, 23, 4–25. [Google Scholar] [CrossRef]
- Schrödinger Release 2022-4: Jaguar; Schrödinger LLC: New York, NY, USA, 2022.
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
- Gill, P.M.W.; Johnson, B.G.; Pople, J.A.; Frisch, M.J. The performance of the Becke—Lee—Yang—Parr (B—LYP) density functional theory with various basis sets. Chem. Phys. Lett. 1992, 197, 499–505. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. 1988, B37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. Role of Frontier Orbitals in Chemical Reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. Why is the dual descriptor a more accurate local reactivity descriptor than Fukui functions? J. Math. Chem. 2015, 53, 451–465. [Google Scholar] [CrossRef]
- Alves, M.J.; Froufe, H.J.C.; Costa, A.F.T.; Santos, A.F.; Oliveira, L.G.; Osório, S.R.M.; Abreu, R.M.V.; Pintado, M.; Ferreira, I.C.F. Docking studies in target proteins involved in antibacterial action mechanisms: Extending the knowledge on standard antibiotics to antimicrobial mushroom compounds. Molecules 2014, 19, 1672–1684. [Google Scholar] [CrossRef]
- Houston, D.R.; Walkinshaw, M.D. Consensus docking: Improving the reliability of docking in a virtual screening context. J. Chem. Inf. Model. 2013, 53, 384–390. [Google Scholar] [CrossRef]
- Barnes, T.M.; Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. Vehicles for Drug Delivery and Cosmetic Moisturizers: Review and Comparison. Pharmaceutics 2021, 12, 2012. [Google Scholar] [CrossRef]
- Ng, K.W.; Lau, W.M. Skin Deep: The basics of Human Skin Structure and Drug Penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement, 1st ed.; Dragicevic, N., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar]
- Faver, J.; Merz, K.M., Jr. The Utility of the HSAB Principle via the Fukui Function in Biological Systems. J. Chem. Theory Comput. 2010, 9, 548–559. [Google Scholar] [CrossRef]
- Zamora, P.P.; Bieger, K.; Cuchillo, A.; Tello, A.; Muena, J.P. Theoretical determination of a reaction intermediate: Fukui function analysis, dual reactivity descriptor and activation energy. J. Mol. Struct. 2021, 1227, 129369. [Google Scholar] [CrossRef]
- Martin, Y.C.; Kofron, J.L.; Traphagen, L.M. Do Structurally Similar Molecules Have Similar Biological Activity? J. Med. Chem. 2002, 45, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT online: Collection of Open Natural Products database. J. Cheminform. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Jiang, Q.; Liu, J.; Shen, Z. Establishment of a dipeptidyl peptidases (DPP) 8/9 expressing cell model for evaluating the selectivity of DPP4 inhibitors. J. Pharmacol. Toxicol. Methods 2015, 71, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Z.; Wallace, M.B.; Stafford, J.A.; Kaldor, S.W.; Kassel, D.B.; Navre, M.; Shi, L.; Skene, R.J.; Asakawa, T.; et al. Discovery of alogliptin: A potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. J. Med. Chem. 2007, 50, 2297–2300. [Google Scholar] [CrossRef]

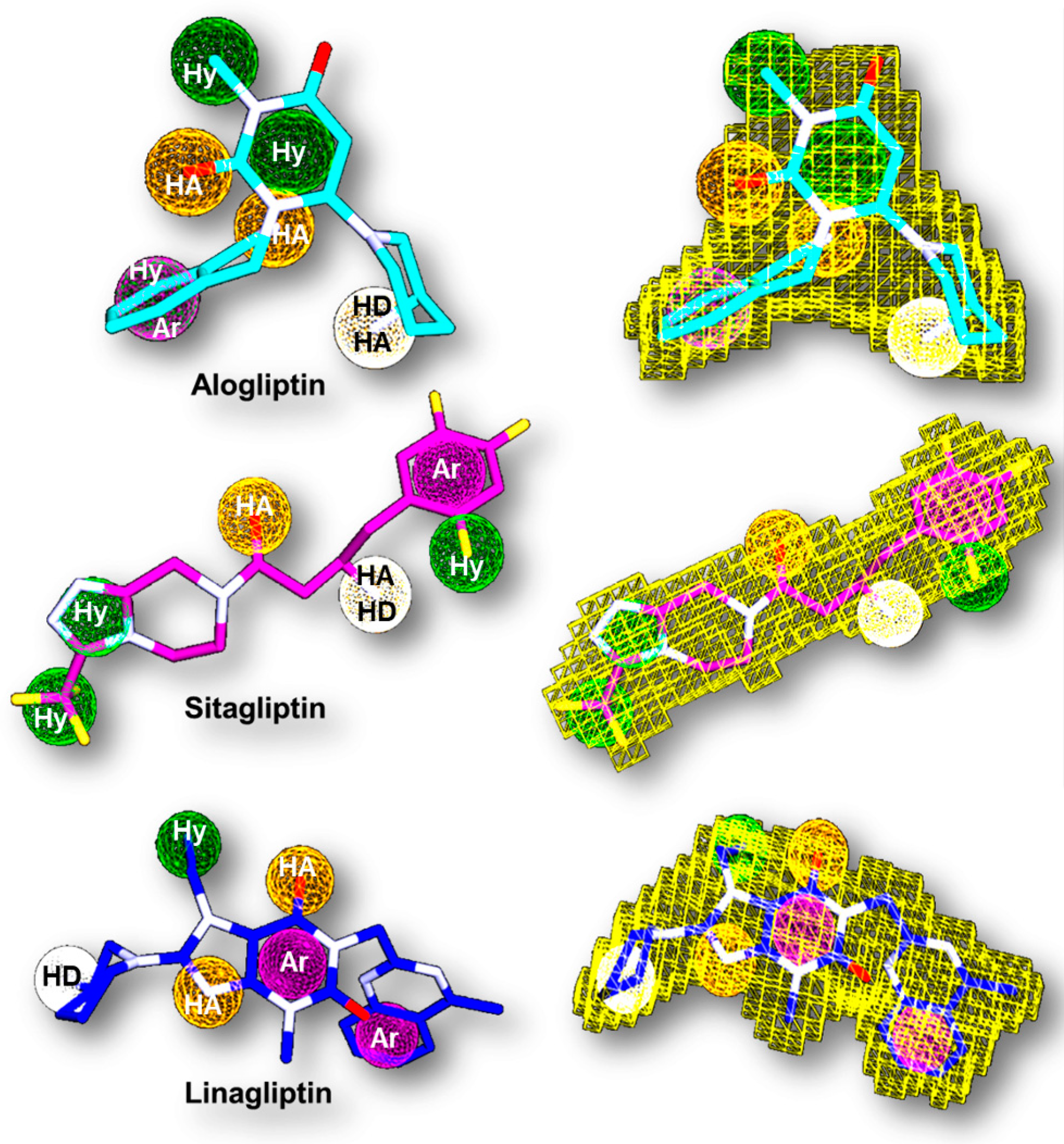

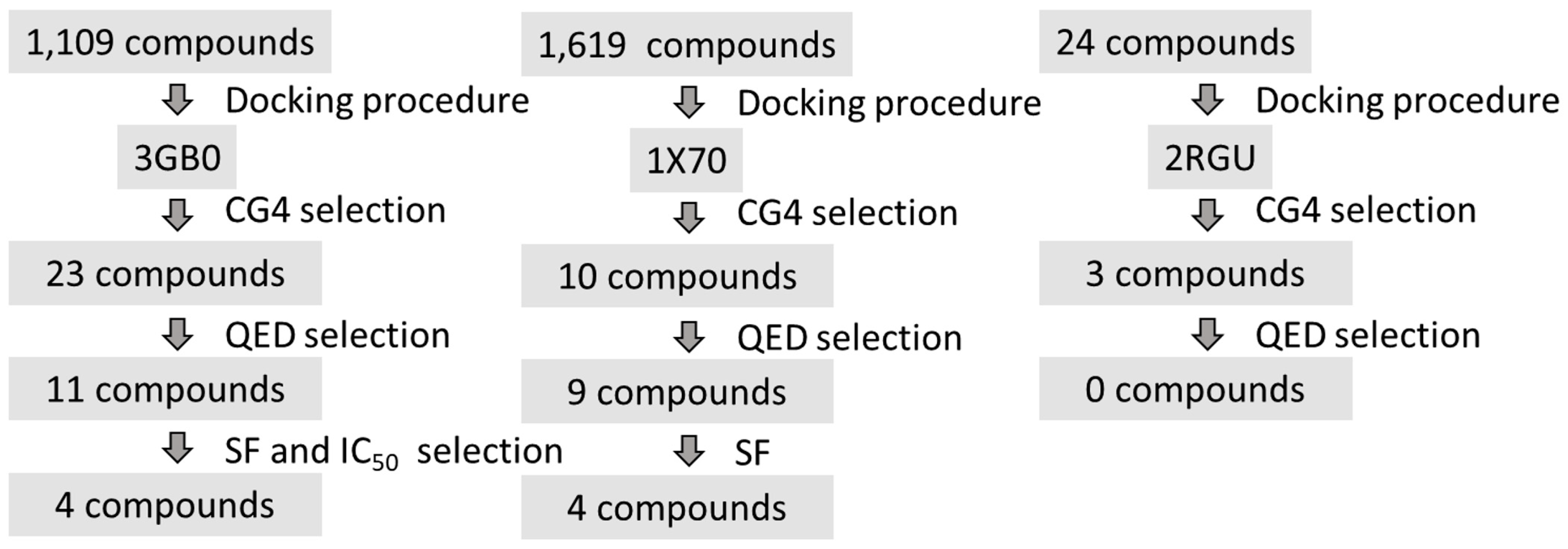
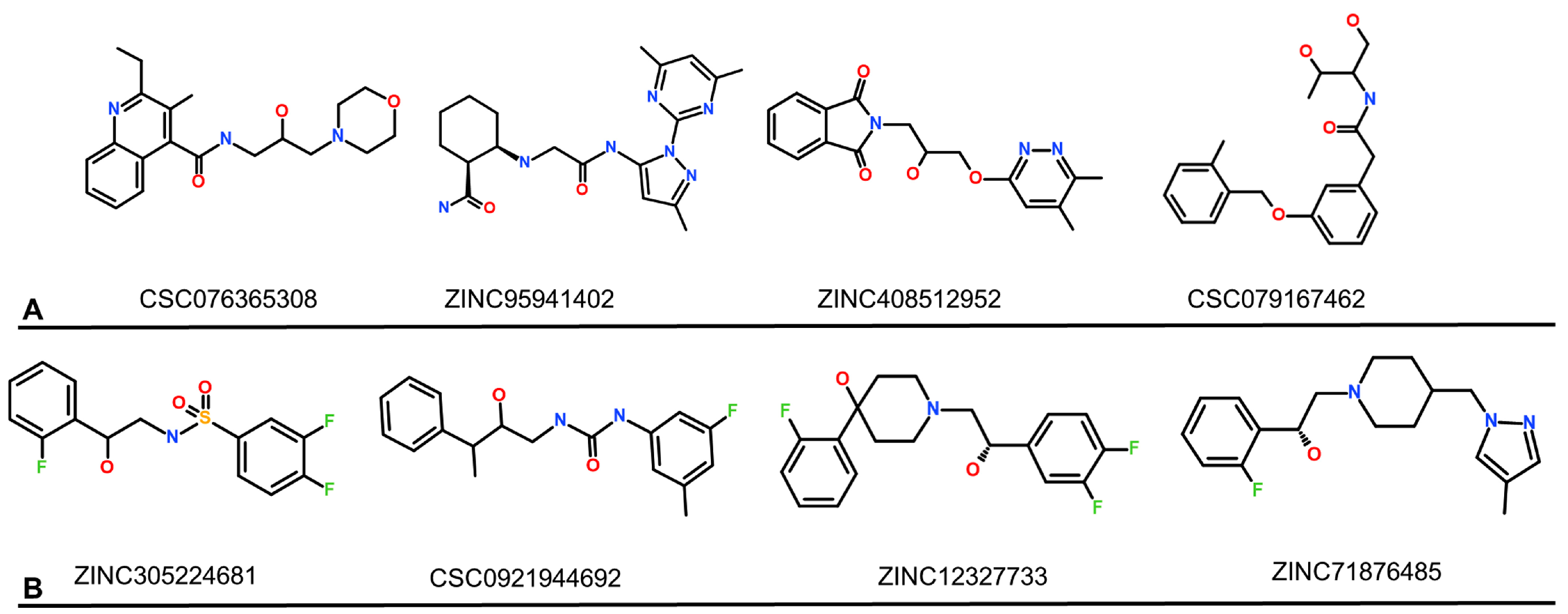
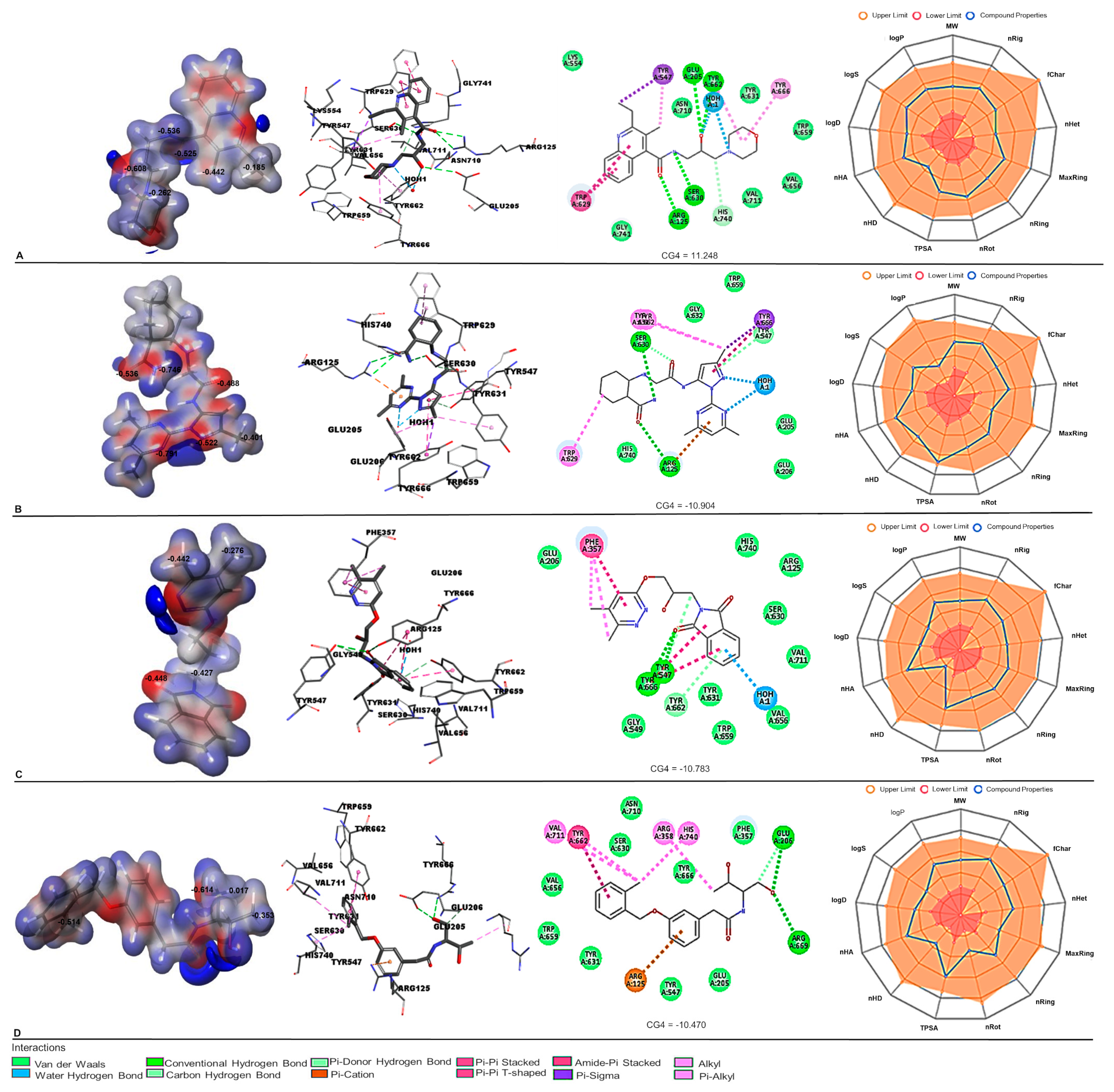

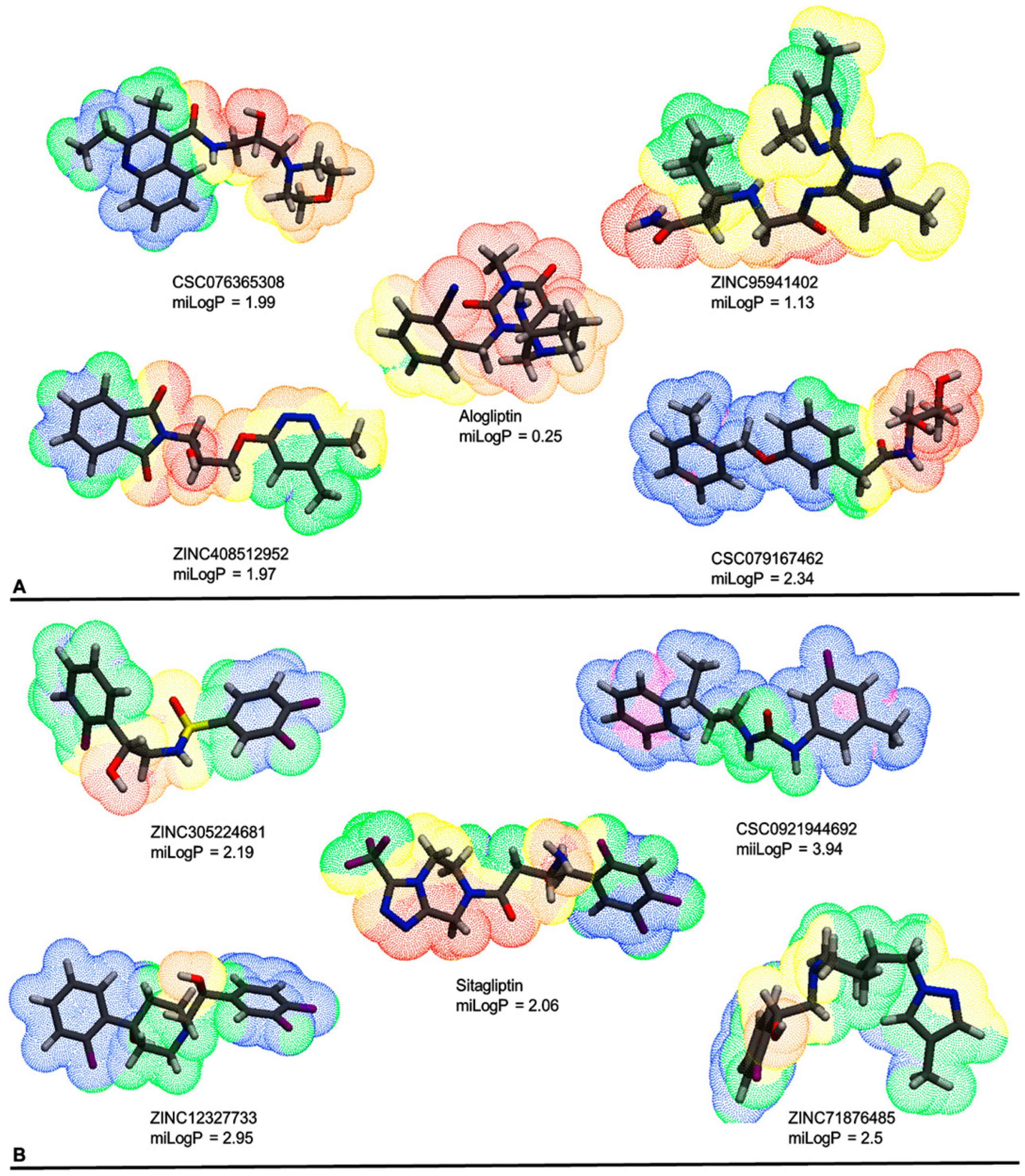
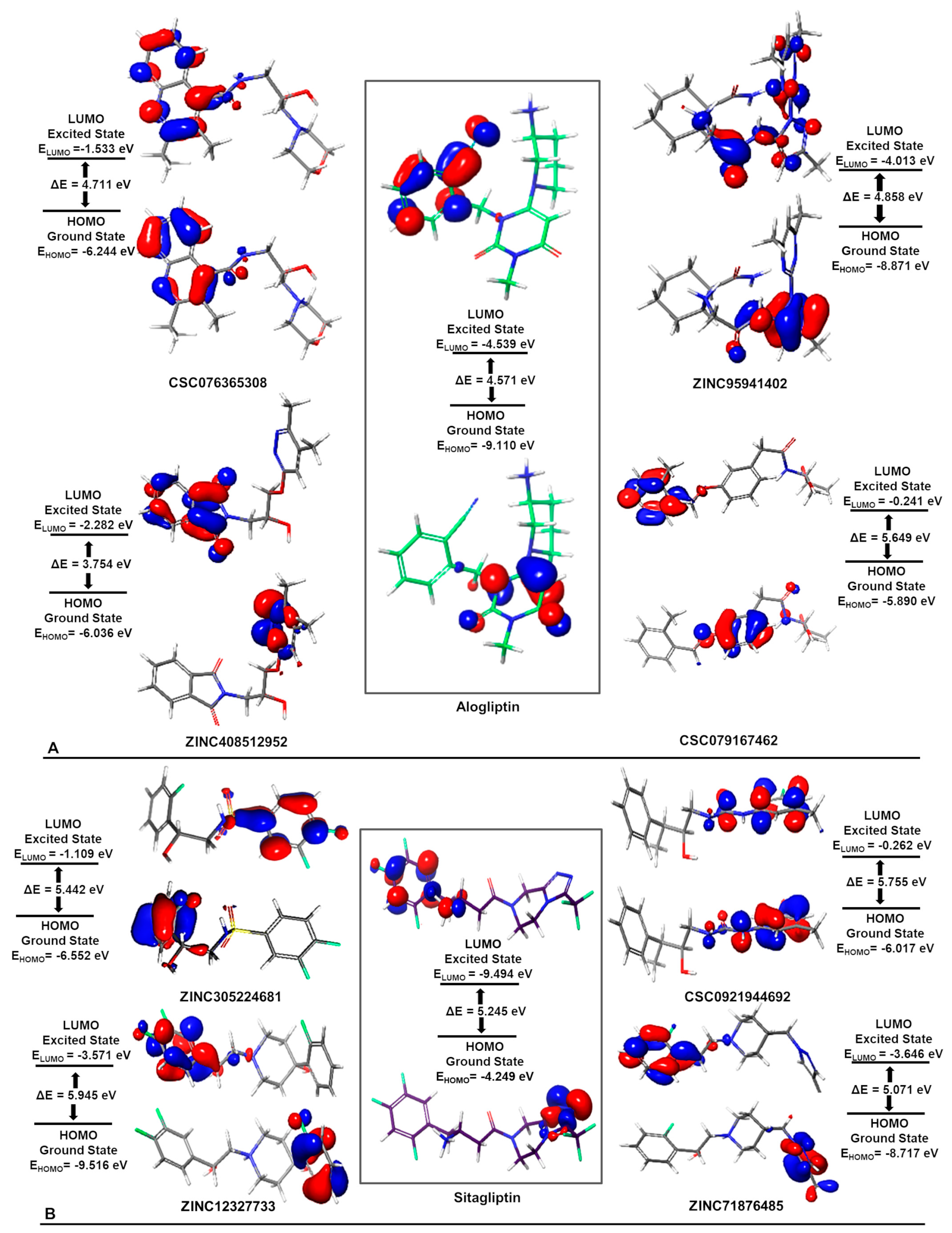
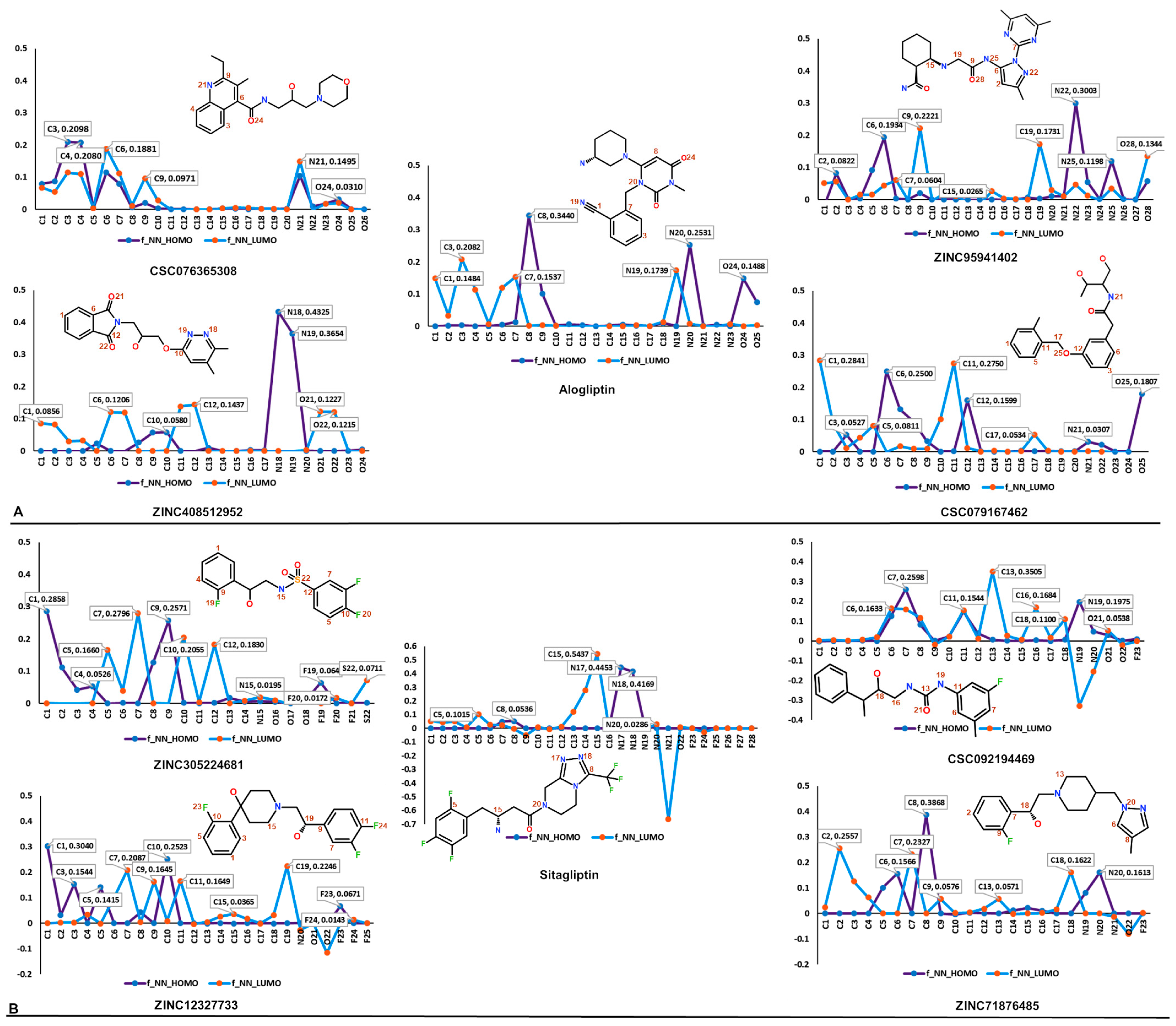
| Database | No of Molecules | Alogliptin (Molecules “Hits”) | Sitagliptin (Molecules “Hits”) | Linagliptin (Molecules “Hits”) |
|---|---|---|---|---|
| MolPot | 4,807,813 | 107 | 83 | 4 |
| CHEMBL30 | 1,998,181 | 138 | 55 | 8 |
| ChemDiv(2015) | 1,456,120 | 16 | 8 | 1 |
| ChemSpace | 50,181,678 | 326 | 413 | 1 |
| MCULE | 45,257,086 | 190 | 718 | 1 |
| MCULE-ULTIMATE | 126,471,502 | 178 | 14 | 2 |
| LabNetwork | 1,794,286 | 58 | 11 | 2 |
| ZINC | 12,921,916 | 154 | 328 | 5 |
| TOTAL | 244,888,582 | 1109 | 1619 | 24 |
| (A) | |||||
| CSC076365308 | ZINC95941402 | ZINC408512952 | CSC079167462 | Alogliptin | |
| MW | 357.210 | 385.220 | 327.120 | 343.180 | 339.170 |
| Volume | 372.349 | 387.613 | 323.978 | 365.066 | 345.687 |
| Density | 0.959 | 0.994 | 1.010 | 0.940 | 0.981 |
| nHA | 6 | 9 | 7 | 5 | 7 |
| nHD | 2 | 4 | 1 | 3 | 2 |
| nRot | 7 | 6 | 5 | 9 | 3 |
| nRing | 3 | 3 | 3 | 2 | 3 |
| MaxRing | 10 | 6 | 9 | 6 | 6 |
| nHet | 6 | 9 | 7 | 5 | 7 |
| fChar | 0 | 0 | 0 | 0 | 0 |
| nRig | 18 | 20 | 18 | 13 | 21 |
| Flexibility | 0.389 | 0.300 | 0.278 | 0.692 | 0.143 |
| Stereo Centers | 1 | 2 | 1 | 2 | 1 |
| TPSA | 74.690 | 131.050 | 92.620 | 78.790 | 97.050 |
| logS | −1.511 | −1.832 | −2.903 | −2.535 | −2.103 |
| logP | 1.740 | 0.78 | 1.63 | 2.128 | 1.185 |
| logD | 1.714 | 1.619 | 1.497 | 2.508 | 1.452 |
| PAINS | 0 alerts | 0 alerts | 0 alerts | 0 alerts | 0 alerts |
| Lipinski Rule | Accepted | Accepted | Accepted | Accepted | Accepted |
| Pfizer Rule | Accepted | Accepted | Accepted | Accepted | Accepted |
| Npscore | −1.407 | −0.929 | −1.042 | −0.482 | −1.318 |
| QED | 0.820 | 0.693 | 0.828 | 0.685 | 0.873 |
| CG4 | −11.248 | −10.904 | −10.783 | −10.470 | −10.404 |
| (B) | |||||
| ZINC305224681 | CSC092194469 | ZINC12327733 | ZINC71876485 | Sitagliptin | |
| MW | 331.050 | 316.160 | 351.140 | 317.190 | 407.120 |
| Volume | 291.848 | 329.958 | 342.472 | 328.881 | 343.983 |
| Density | 1.134 | 0.958 | 1.025 | 0.964 | 1.184 |
| nHA | 4 | 4 | 3 | 4 | 6 |
| nHD | 2 | 3 | 2 | 1 | 2 |
| nRot | 5 | 7 | 4 | 5 | 6 |
| nRing | 2 | 2 | 3 | 3 | 3 |
| MaxRing | 6 | 6 | 6 | 6 | 9 |
| nHet | 8 | 5 | 6 | 5 | 12 |
| fChar | 0 | 0 | 0 | 0 | 0 |
| nRig | 14 | 13 | 18 | 17 | 17 |
| Flexibility | 0.357 | 0.538 | 0.222 | 0.294 | 0.353 |
| Stereo Centers | 1 | 2 | 1 | 1 | 1 |
| TPSA | 66.400 | 61.360 | 43.700 | 41.290 | 77.040 |
| logS | −3.063 | −4.277 | −3.219 | −1.797 | −0.783 |
| logP | 2.518 | 3.453 | 2.664 | 2.314 | 0.694 |
| logD | 2.406 | 3.831 | 2.872 | 2.223 | 1.932 |
| PAINS | 0 alerts | 0 alerts | 0 alerts | 0 alerts | 0 alerts |
| Lipinski Rule | Accepted | Accepted | Accepted | Accepted | Accepted |
| Pfizer Rule | Accepted | Rejected | Accepted | Accepted | Accepted |
| Npscore | −1.854 | −1.260 | −0.950 | −1.884 | −1.404 |
| QED | 0.882 | 0.791 | 0.890 | 0.922 | 0.672 |
| CG4 | −11.107 | −10.968 | −10.712 | −10.540 | −10.500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istrate, D.; Crisan, L. Dipeptidyl Peptidase 4 Inhibitors in Type 2 Diabetes Mellitus Management: Pharmacophore Virtual Screening, Molecular Docking, Pharmacokinetic Evaluations, and Conceptual DFT Analysis. Processes 2023, 11, 3100. https://doi.org/10.3390/pr11113100
Istrate D, Crisan L. Dipeptidyl Peptidase 4 Inhibitors in Type 2 Diabetes Mellitus Management: Pharmacophore Virtual Screening, Molecular Docking, Pharmacokinetic Evaluations, and Conceptual DFT Analysis. Processes. 2023; 11(11):3100. https://doi.org/10.3390/pr11113100
Chicago/Turabian StyleIstrate, Daniela, and Luminita Crisan. 2023. "Dipeptidyl Peptidase 4 Inhibitors in Type 2 Diabetes Mellitus Management: Pharmacophore Virtual Screening, Molecular Docking, Pharmacokinetic Evaluations, and Conceptual DFT Analysis" Processes 11, no. 11: 3100. https://doi.org/10.3390/pr11113100
APA StyleIstrate, D., & Crisan, L. (2023). Dipeptidyl Peptidase 4 Inhibitors in Type 2 Diabetes Mellitus Management: Pharmacophore Virtual Screening, Molecular Docking, Pharmacokinetic Evaluations, and Conceptual DFT Analysis. Processes, 11(11), 3100. https://doi.org/10.3390/pr11113100






