Experimental Study on Enrichment of Indole in Wash Oil by a Combined Process of Extraction and Re-Extraction
Abstract
:1. Introduction
2. Experimental Method and Conditions
2.1. Equilibrium Extraction
2.2. Equilibrium Re-Extraction
3. Results and Discussion
3.1. Calculation Formula
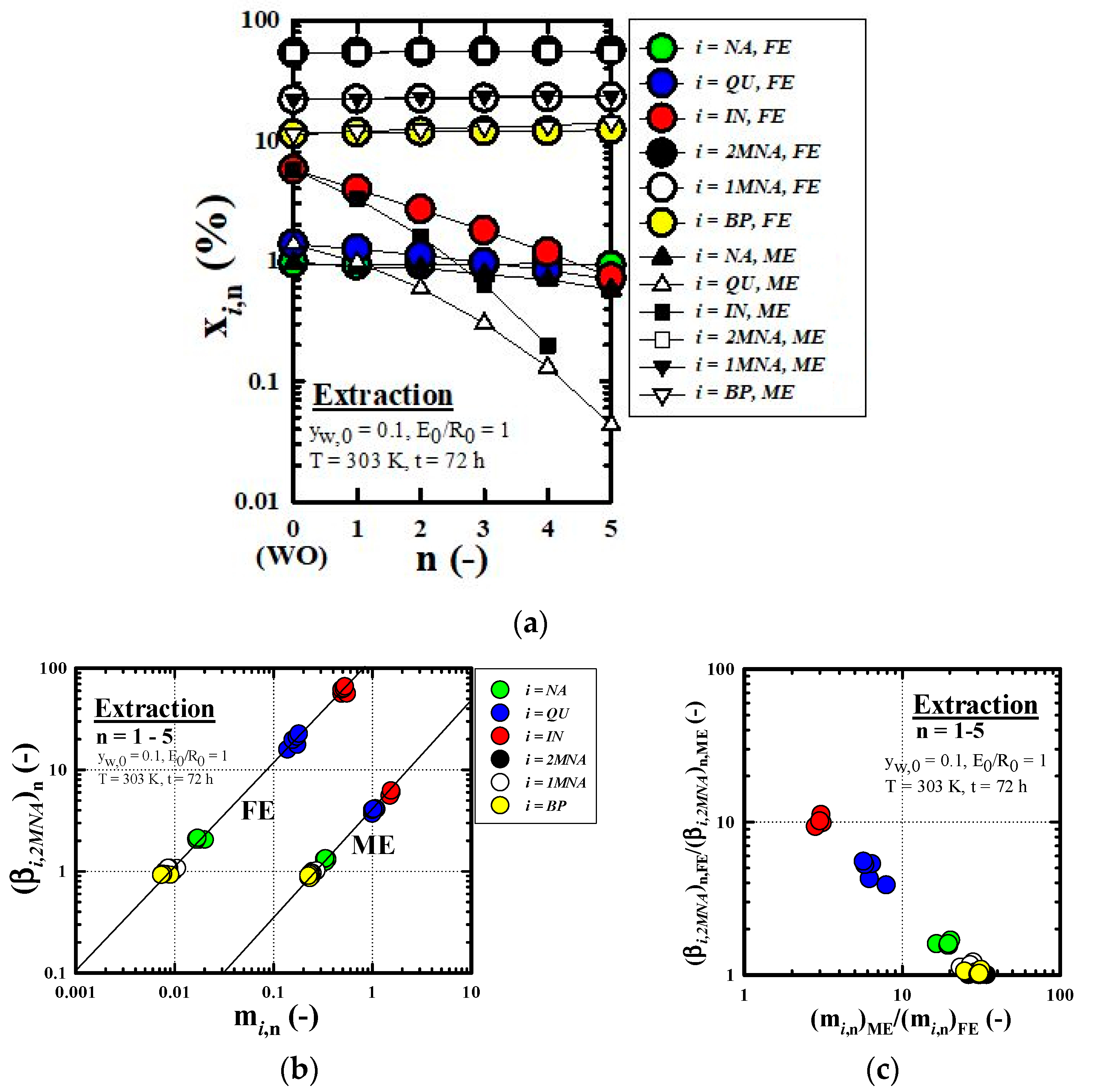
3.2. Equilibrium Extraction
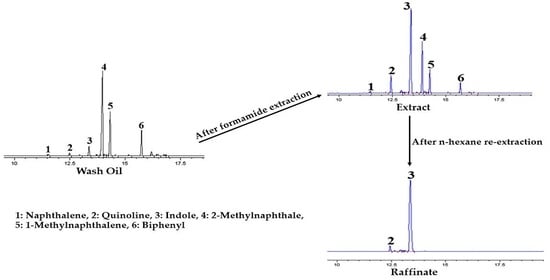
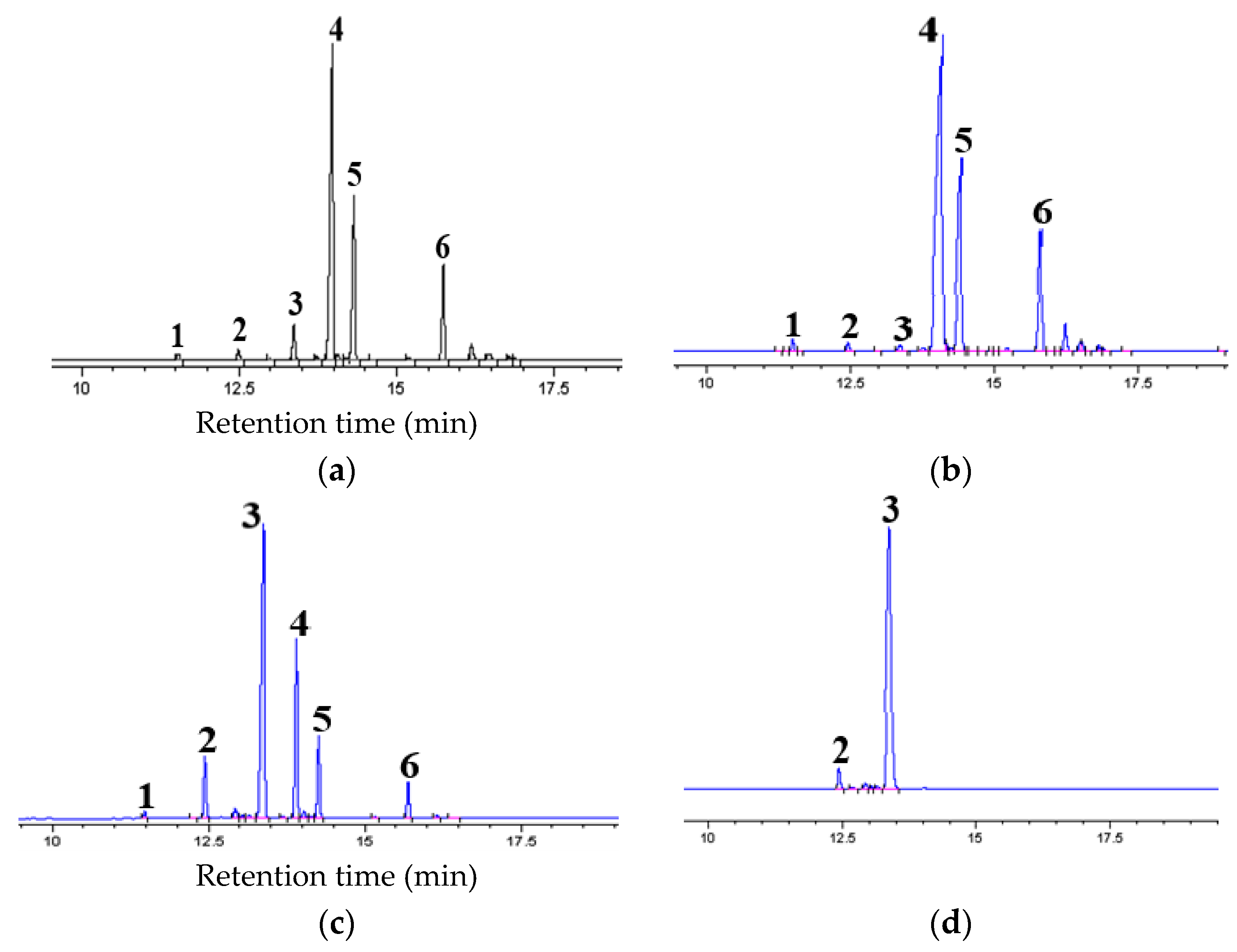
3.2.1. GC Chromatogram of Wash Oil (Extraction Feed)
3.2.2. Confirmation of Equilibration Time
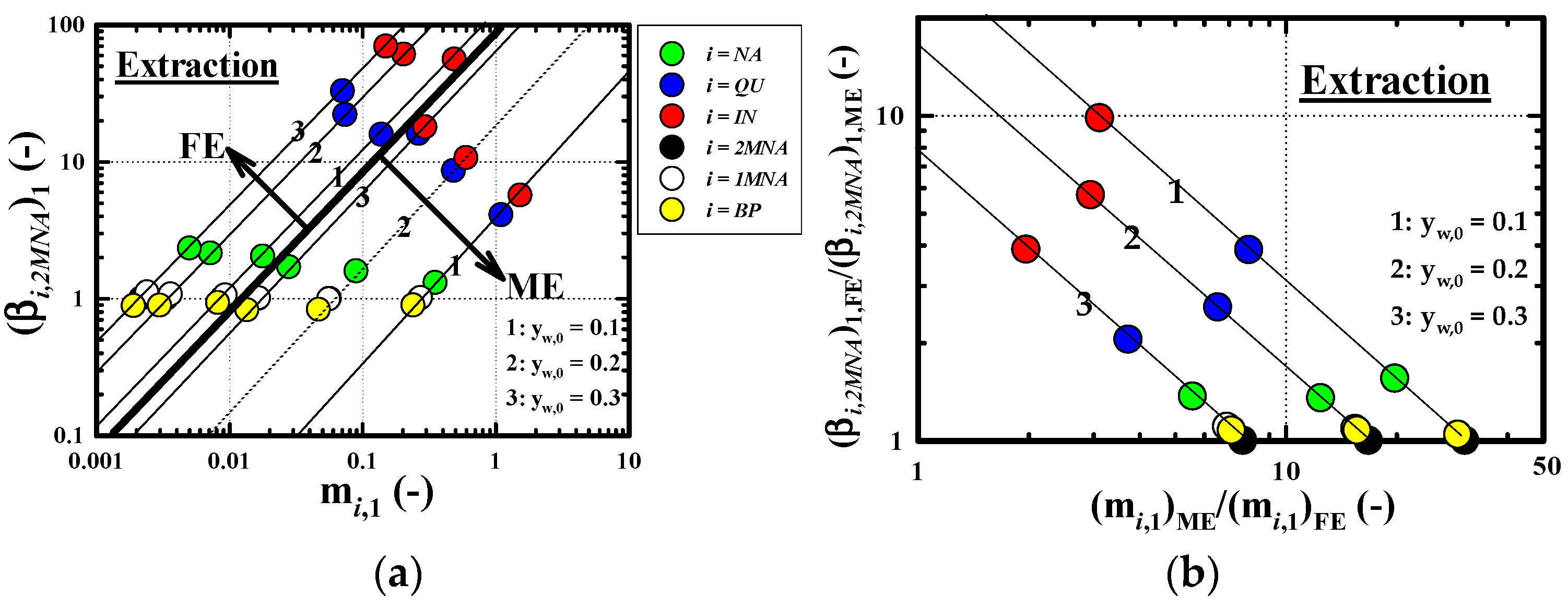
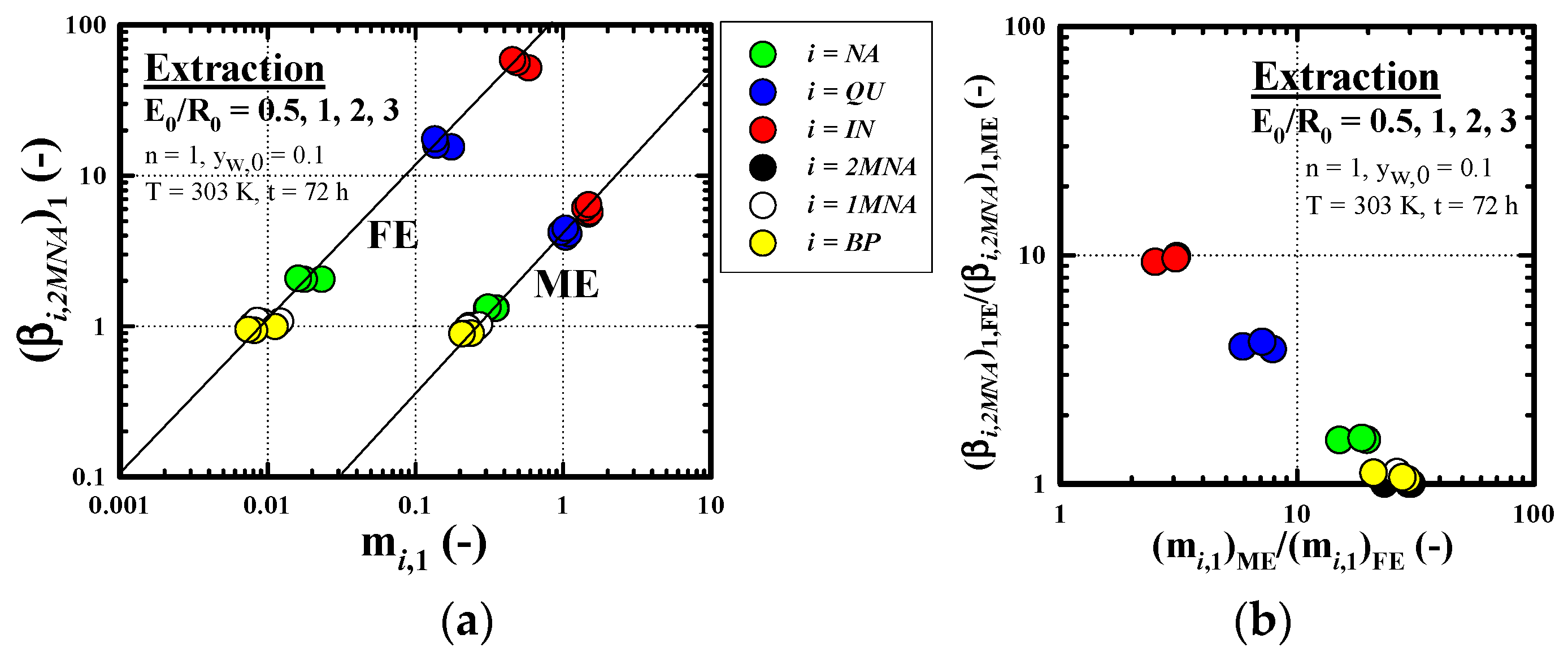
3.2.3. Recovery Performance of IN
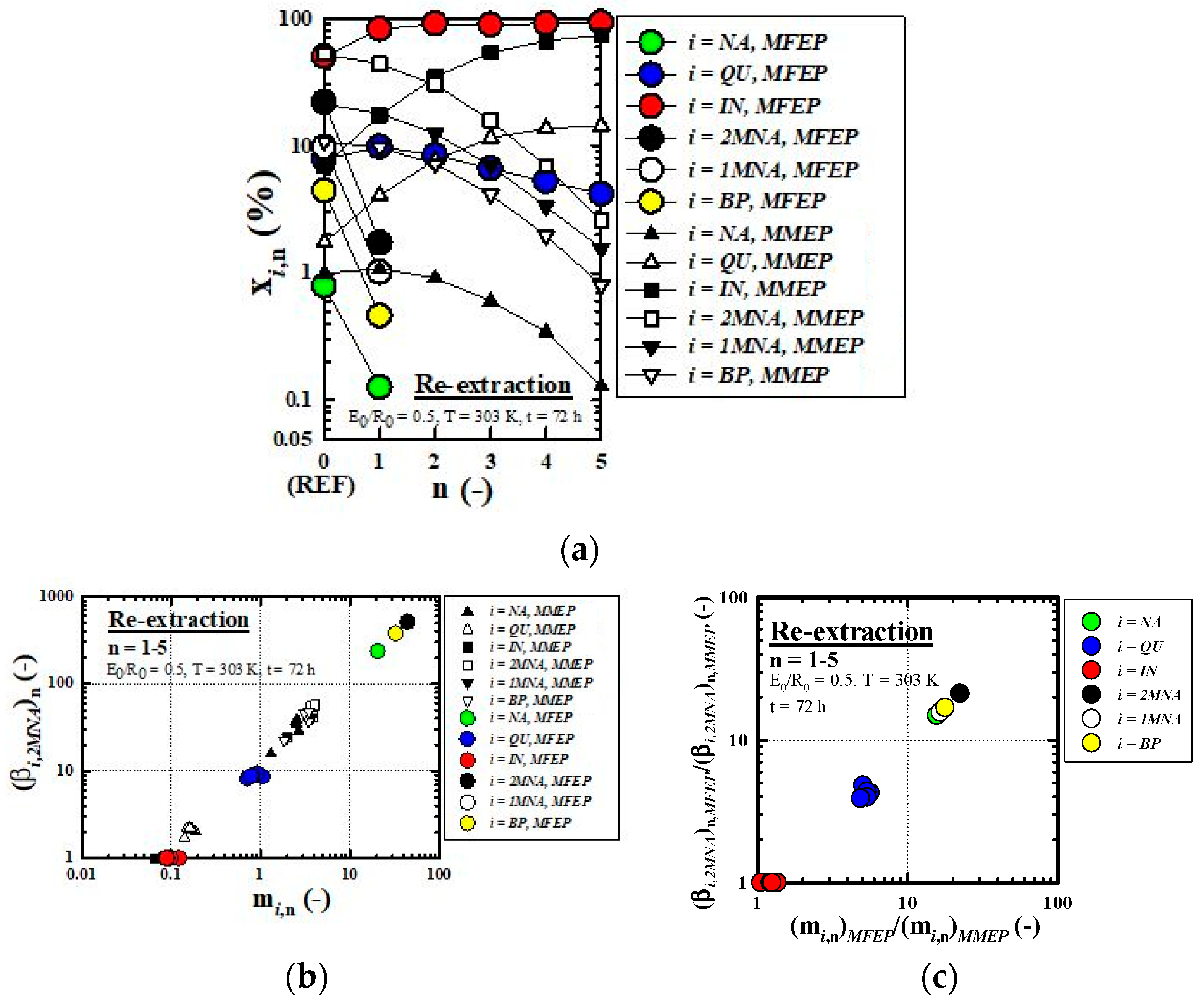
3.3. Equilibrium Re-Extraction
3.3.1. GC Chromatogram of MFEP (Re-Extraction Feed)
3.3.2. Enrichment Performance of IN
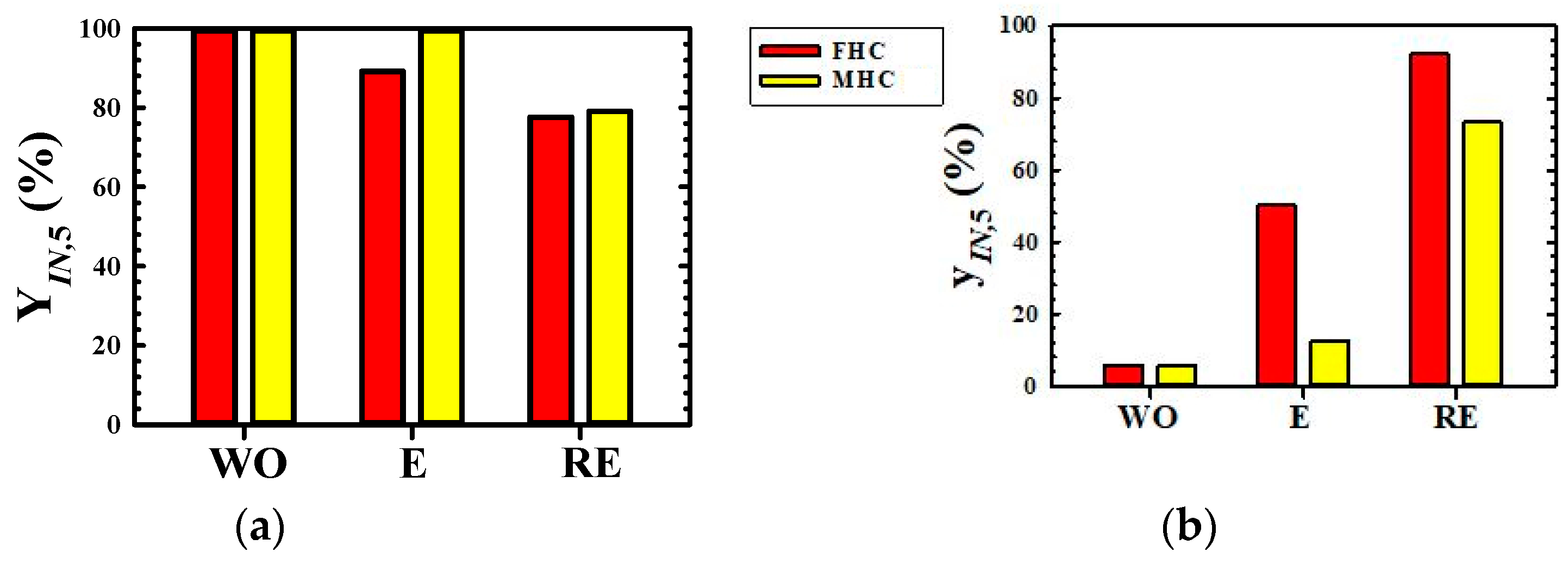
3.4. Comparison of Combined Enrichment Efficiency
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, S.J. Separation and purification of indole in model coal tar fraction of 9 compounds system. Polycycl. Aromat. Compd. 2019, 39, 60–72. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, J.; Guo, Z.; Gao, J.; Xu, D.; Ma, Y.; Zhang, L.; Wang, Y. Experimental and quantum chemical calculations investigations of morpholine-based ionic liquids as extractants for efficient extraction of nitrogen heterocyclic neutral com-pounds. Fuel 2023, 333, 126446. [Google Scholar] [CrossRef]
- Kim, S.J. Purification of indole contained in wash oil by combination of extraction and crystallization (Part 1: Recovery and concentration of indole contained in wash oil by solvent extraction). Molecules 2022, 27, 5331. [Google Scholar] [CrossRef] [PubMed]
- Ukegawa, K.; Matsumura, A.; Kodera, Y.; Kondo, T.; Nakayama, T.; Tanabe, H.; Yoshida, S.; Mito, Y. Solvent extraction of nitrogen compounds from a coal tar fraction. (Part 1). Effect of extraction conditions on the extraction rate and the selectivities of nitrogen compounds. J. Jpn. Pet. Inst. 1990, 33, 250–254. [Google Scholar] [CrossRef]
- Kim, S.J.; Chun, Y.J. Separation of nitrogen heterocyclic compounds from model coal tar fraction by solvent extraction. Sep. Sci. Technol. 2005, 40, 2095–2109. [Google Scholar]
- Salim, C.; Egashira, R. Separation of coal tar distillate by solvent extraction—Separation of extract phase using distillation. J. Jpn. Pet. Inst. 2006, 49, 326–334. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Chen, A.; Ji, Y. Extraction behavior of indole from simulated wash oil using halogen-free ionic liquids. ACS Omega 2021, 6, 16623–16630. [Google Scholar] [CrossRef]
- Xiao, R.H.; Gao, W.H. Study on the recovery of indole from coal tar wash oil. Coal Convers. 1998, 21, 59–61. [Google Scholar]
- Mamoru, Y.; Tomonori, K. Separation and purification of indole from coal tar by supercritical fluid extraction. J. Chem. Eng. Jpn. 1993, 26, 153–158. [Google Scholar]
- Feng, Y.; Yang, E.; Dang, L.; Wei, H. Liquid–liquid phase equilibrium for ternary mixtures of formamide (or ethylene glycol, or monoethanolamine) + indole + 2-methylnaphthalene at 308.15 K. Fluid Phase Equilibria 2015, 398, 10–14. [Google Scholar] [CrossRef]
- Sakanishi, K.; Obata, H.; Mochida, I.; Sakaki, T. Capture and recovery of indole from methylnaphthalene oil in a continuous supercritical CO2 extraction apparatus over a fixed bed of anion-exchange resin. Ind. Eng. Chem. Res. 1996, 35, 335–337. [Google Scholar] [CrossRef]
- Sakanishi, K.; Obata, H.; Mochida, I.; Sakaki, T. Removal and recovery of quinoline bases from methylnaphthalene oil in a semicontinuous supercritical CO2 separation apparatus with a fixed bed of supported aluminum sulfate. Ind. Eng. Chem. Res. 1995, 34, 4118–4124. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, T.; Gao, P.; Gao, J.; Xu, D.; Zhao, P.; Zhang, L.; Wang, Y. Separation of indole by designed ionic liquids with dual functional chemical sites: Mechanism exploration and experimental validation. J. Environ. Chem. Eng. 2021, 9, 105971. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, Y.; Ren, S.; Wu, W. Highly efficient separation of indole from model wash oil using tetraethyl ammonium amino acid ionic liquids. Sep. Purif. Technol. 2021, 258, 117997. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, D.; Gao, J.; Zhou, S.; Zhao, L.; Zhang, Z. Extraction and mechanism for the separation of neutral N-compounds from coal tar by ionic liquids. Fuel 2017, 194, 27–35. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, M.; Gao, J.; Zhang, L.; Zhou, S.; Wang, Y. Separation of heterocyclic nitrogen compounds from coal tar fractions via ionic liquids: COSMO-SAC screening and experimental study. Chem. Eng. Commun. 2019, 206, 1199–1217. [Google Scholar] [CrossRef]
- Jiao, T.; Zhuang, X.; He, H.; Zhao, L.; Li, C.; Chen, H.; Zhang, S. An ionic liquid extraction process for the separation of indole from wash oil. Green Chem. 2015, 17, 3783–3790. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, Y.; Ren, S.; Niu, M.; Yao, C.; Wu, W. Efficient extraction of indole from wash oil by quaternary ammonium salts via forming deep eutectic solvents. Fuel 2018, 215, 330–338. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sato, Y.; Ebina, T.; Yokoyama, C.; Takahasi, S.; Mito, Y.; Tanabe, H.; Nishiguchi, N.; Nagaoka, K. Separation of high purity indole from coal tar by high pressure crystallization. Fuel 1991, 70, 565–566. [Google Scholar] [CrossRef]
- Mochida, I.; Fei, Y.Q.; Sakanishi, K. Capture and recovery of basic nitrogen species in coal tar pitch, using nickel sulfate as adsorbent. Chem. Lett. 1990, 19, 515–518. [Google Scholar] [CrossRef]
- Uemasu, I.; Nakayama, T. Concentration of indole in coal tar using α-cyclodextrin as the host for inclusion complexation. J. Inclus. Phenom. Molec. Recogn. Chem. 1989, 7, 327–331. [Google Scholar] [CrossRef]
- Uemasu, I. Effect of methanol-water mixture solvent on concentration of indole in coal tar using β-cyclodextrin as complexing agent. J. Jpn. Pet. Inst. 1991, 34, 371–374. [Google Scholar] [CrossRef]

| Compound | Physical Property | Composition (wt%) | ||
|---|---|---|---|---|
| b.p. * [K] | m.p. ** [K] | Extraction | Re-Extraction | |
| Naphthalene (NA, C10H8) | 491 | 351–353 | 0.960 | 0.013 |
| Quinoline (QU, C9H7N) | 511 | 257 | 1.379 | 0.157 |
| Indole (IN, C8H7N) | 526 | 325 | 5.753 | 0.993 |
| 2-Methylnaphthalene (2MNA, C11H10) | 514–515 | 307–309 | 53.251 | 0.391 |
| 1-Methylnaphthalene (1MNA, C11H10) | 513–516 | 251 | 21.854 | 0.165 |
| Biphenyl (BP, C12H10) | 528 | 342 | 11.339 | 0.074 |
| Others | 5.464 | 98.207 (with solvent) | ||
| Gas Chromatograph | HP 6890 (Hewlett Packard Co., Palo Alto, CA, USA) |
|---|---|
| Column | Capillary column HP-1 |
| length [m] inner diameter [mm] | 60 0.32 |
| film [µm] | 0.25 |
| Carrier gas | N2 |
| split ratio [-] | 0.025 |
| flow rate in column [mL/min] | 2 |
| Sample volume [µL] | 1 |
| Injection temperature [K] | 523 |
| Column temperature [K] | 383–593 |
| temperature program [K/min] | 0 (for 0–3 min) 5 (383–523 K) 14 (523–593 K) |
| Detector (FID) temperature [K] | 593 |
| Equilibrium Extraction | Equilibrium Re-Extraction | |
|---|---|---|
| Feed: wash oil or raffinate phase * | MFEP ** or raffinate phase *** | |
| Solvent: aqueous solution of formamide | n-hexane | |
| Shaking time, t (h) | 72 | 72 |
| Stage number of batch co-current Equilibrium extraction, n (-) | 1–5 | 1–5 |
| Temperature, T (K) | 303 | 303 |
| Volume of fresh solvent (E0–E4), (mL) | 320 | 320 |
| Volume fraction of water in solvent in initial, yw,0 (-) | 0.1–0.3 | − |
| Volume ratio of solvent to feed in initial, E0/R0 (-) | 0.5–3 | 0.5–3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J. Experimental Study on Enrichment of Indole in Wash Oil by a Combined Process of Extraction and Re-Extraction. Processes 2023, 11, 3097. https://doi.org/10.3390/pr11113097
Kim SJ. Experimental Study on Enrichment of Indole in Wash Oil by a Combined Process of Extraction and Re-Extraction. Processes. 2023; 11(11):3097. https://doi.org/10.3390/pr11113097
Chicago/Turabian StyleKim, Su Jin. 2023. "Experimental Study on Enrichment of Indole in Wash Oil by a Combined Process of Extraction and Re-Extraction" Processes 11, no. 11: 3097. https://doi.org/10.3390/pr11113097
APA StyleKim, S. J. (2023). Experimental Study on Enrichment of Indole in Wash Oil by a Combined Process of Extraction and Re-Extraction. Processes, 11(11), 3097. https://doi.org/10.3390/pr11113097