Tailoring Dry Microparticles for Pulmonary Drug Delivery: Ultrasonic Spray Freeze-Drying with Mannitol and Salbutamol Sulphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Solution Preparation
2.2. Spray Freeze-Drying
2.3. Morphological Characterisation
2.4. Surface Area and Pore Size Analysis
2.5. X-ray Diffraction
2.6. In Vitro Drug Deposition
2.7. Statistical Analysis
3. Results and Discussion
3.1. Particle Size and Morphology
3.2. Particle Surface Area and Porosity
3.3. Crystalline Properties of Particles
3.4. In Vitro Aerodynamic Properties of Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, S.P. Delivering drugs to the lungs: The history of repurposing in the treatment of respiratory diseases. Adv. Drug Deliv. Rev. 2018, 133, 5–18. [Google Scholar] [CrossRef]
- Newman, S.P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Jain, H.; Bairagi, A.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Recent advances in the development of microparticles for pulmonary administration. Drug Discov. Today 2020, 25, 1865–1872. [Google Scholar] [CrossRef]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef]
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present and future. Expert. Opin. Drug Deliv. 2017, 14, 499–512. [Google Scholar] [CrossRef]
- Murgia, X.; De Souza Carvalho, C.; Lehr, C.M. Overcoming the pulmonary barrier: New insights to improve the efficiency of inhaled therapeutics. Eur. J. Nanomed. 2014, 6, 157–169. [Google Scholar] [CrossRef]
- Muralidharan, P.; Malapit, M.; Mallory, E.; Hayes, D.; Mansour, H.M. Inhalable nanoparticulate powders for respiratory delivery. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1189–1199. [Google Scholar] [CrossRef]
- Hassan, M.S.; Lau, R.W.M. Effect of particle shape on dry particle inhalation: Study of flowability, aerosolization, and deposition properties. AAPS PharmSciTech 2009, 10, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Praphawatvet, T.; Peters, J.I.; Williams, R.O. Inhaled nanoparticles—An updated review. Int. J. Pharm. 2020, 587, 119671. [Google Scholar] [CrossRef]
- Chaurasiya, B.; Zhao, Y.Y. Dry powder for pulmonary delivery: A comprehensive review. Pharmaceutics 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-Based Drug Delivery System: The Magic Bullet for the Treatment of Chronic Pulmonary Diseases. Mol. Pharm. 2021, 18, 3671–3718. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Muneer, S.; Izake, E.L.; Islam, N. Impact of Leucine and Magnesium Stearate on the Physicochemical Properties and Aerosolization Behavior of Wet Milled Inhalable Ibuprofen Microparticles for Developing Dry Powder Inhaler Formulation. Pharmaceutics 2023, 15, 674. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhang, X.; Huang, Y.; Zhao, Z.; Liao, Q.; Xu, J.; Huang, Z.; Zhang, J.; Wu, C.Y.; Pan, X.; et al. Nanoporous mannitol carrier prepared by non-organic solvent spray drying technique to enhance the aerosolization performance for dry powder inhalation. Sci. Rep. 2017, 7, 46517. [Google Scholar] [CrossRef]
- Ye, T.; Yu, J.; Luo, Q.; Wang, S.; Chan, H.-K. Inhalable clarithromycin liposomal dry powders using ultrasonic spray freeze drying. Powder Technol. 2017, 305, 63–70. [Google Scholar] [CrossRef]
- Jara, M.O.; Warnken, Z.N.; Sahakijpijarn, S.; Moon, C.; Maier, E.Y.; Christensen, D.J.; Koleng, J.J.; Peters, J.I.; Hackman Maier, S.D.; Williams Iii, R.O. Niclosamide inhalation powder made by thin-film freezing: Multi-dose tolerability and exposure in rats and pharmacokinetics in hamsters. Int. J. Pharm. 2021, 603, 120701. [Google Scholar] [CrossRef]
- Kim, Y.H.; Shing, K.S. Supercritical fluid-micronized ipratropium bromide for pulmonary drug delivery. Powder Technol. 2008, 182, 25–32. [Google Scholar] [CrossRef]
- Adali, M.B.; Barresi, A.A.; Boccardo, G.; Pisano, R. Spray freeze-drying as a solution to continuous manufacturing of pharmaceutical products in bulk. Processes 2020, 8, 709. [Google Scholar] [CrossRef]
- Duong, T.; López-Iglesias, C.; Szewczyk, P.K.; Stachewicz, U.; Barros, J.; Alvarez-Lorenzo, C.; Alnaief, M.; García-González, C.A. A Pathway From Porous Particle Technology Toward Tailoring Aerogels for Pulmonary Drug Administration. Front. Bioeng. Biotechnol. 2021, 9, 671381. [Google Scholar] [CrossRef]
- Vishali, D.A.; Monisha, J.; Sivakamasundari, S.K.; Moses, J.A.; Anandharamakrishnan, C. Spray freeze drying: Emerging applications in drug delivery. J. Control. Release 2019, 300, 93–101. [Google Scholar] [CrossRef]
- Yu, H.; Tran, T.-T.; Teo, J.; Hadinoto, K. Dry powder aerosols of curcumin-chitosan nanoparticle complex prepared by spray freeze drying and their antimicrobial efficacy against common respiratory bacterial pathogens. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 34–42. [Google Scholar] [CrossRef]
- Isleroglu, H.; Turker, I. Evaluation of Process Conditions for Ultrasonic Spray-Freeze Drying of Transglutaminase. Food Technol. Biotechnol. 2020, 58, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chan, A.Y.L.; Chow, M.Y.T.; Lo, F.F.K.; Qiu, Y.; Kwok, P.C.L.; Lam, J.K.W. Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery. Asian J. Pharm. Sci. 2018, 13, 163–172. [Google Scholar] [CrossRef] [PubMed]
- D’Addio, S.M.; Chan, J.G.Y.; Kwok, P.C.L.; Prud’Homme, R.K.; Chan, H.K. Constant size, variable density aerosol particles by ultrasonic spray freeze drying. Int. J. Pharm. 2012, 427, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Wanning, S.; Süverkrüp, R.; Lamprecht, A. Pharmaceutical spray freeze drying. Int. J. Pharm. 2015, 488, 136–153. [Google Scholar] [CrossRef]
- Yu, H.; Teo, J.; Chew, J.W.; Hadinoto, K. Dry powder inhaler formulation of high-payload antibiotic nanoparticle complex intended for bronchiectasis therapy: Spray drying versus spray freeze drying preparation. Int. J. Pharm. 2016, 499, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Ng, M.L.L.; Kho, K.; Hadinoto, K. Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: Effect of freeze-drying adjuvants. Int. J. Pharm. 2011, 404, 289–300. [Google Scholar] [CrossRef]
- Keyhan Shokouh, M.; Faghihi, H.; Darabi, M.; Mirmoeini, M.; Vatanara, A. Formulation and evaluation of inhalable microparticles of Rizatriptan Benzoate processed by spray freeze-drying. J. Drug Deliv. Sci. Technol. 2021, 62, 102356. [Google Scholar] [CrossRef]
- Ogienko, A.G.; Bogdanova, E.G.; Trofimov, N.A.; Myz, S.A.; Ogienko, A.A.; Kolesov, B.A.; Yunoshev, A.S.; Zubikov, N.V.; Manakov, A.Y.; Boldyrev, V.V.; et al. Large porous particles for respiratory drug delivery. Glycine-based formulations. Eur. J. Pharm. Sci. 2017, 110, 148–156. [Google Scholar] [CrossRef]
- Wanning, S.; Süverkrüp, R.; Lamprecht, A. Impact of excipient choice on the aerodynamic performance of inhalable spray-freeze-dried powders. Int. J. Pharm. 2020, 586, 119564. [Google Scholar] [CrossRef]
- Leung, S.S.Y.; Wong, J.; Guerra, H.V.; Samnick, K.; Prud’homme, R.K.; Chan, H.-K. Porous mannitol carrier for pulmonary delivery of cyclosporine A nanoparticles. AAPS J. 2017, 19, 578–586. [Google Scholar] [CrossRef]
- Liao, Q.; Lam, I.C.H.; Lin, H.H.S.; Wan, L.T.L.; Lo, J.C.K.; Tai, W.; Kwok, P.C.L.; Lam, J.K.W. Effect of formulation and inhaler parameters on the dispersion of spray freeze dried voriconazole particles. Int. J. Pharm. 2020, 584, 119444. [Google Scholar] [CrossRef]
- Parsian, A.R.; Vatanara, A.; Rahmati, M.R.; Gilani, K.; Khosravi, K.M.; Najafabadi, A.R. Inhalable budesonide porous microparticles tailored by spray freeze drying technique. Powder Technol. 2014, 260, 36–41. [Google Scholar] [CrossRef]
- Emami, F.; Vatanara, A.; Vakhshiteh, F.; Kim, Y.; Kim, T.W.; Na, D.H. Amino acid-based stable adalimumab formulation in spray freeze-dried microparticles for pulmonary delivery. J. Drug Deliv. Sci. Technol. 2019, 54, 101249. [Google Scholar] [CrossRef]
- Liang, W.; Chow, M.Y.T.; Chow, S.F.; Chan, H.-K.; Kwok, P.C.L.; Lam, J.K.W. Using two-fluid nozzle for spray freeze drying to produce porous powder formulation of naked siRNA for inhalation. Int. J. Pharm. 2018, 552, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef]
- Thakral, S.; Sonje, J.; Munjal, B.; Bhatnagar, B.; Suryanarayanan, R. Mannitol as an Excipient for Lyophilized Injectable Formulations. J. Pharm. Sci. 2023, 112, 19–35. [Google Scholar] [CrossRef]
- Adali, M.B.; Barresi, A.; Boccardo, G.; Montalbano, G.; Pisano, R. Ultrasonic spray freeze-drying of sucrose and mannitol-based formulations: Impact of the atomization conditions on the particle morphology and drying performance. Dry. Technol. 2023, 41, 251–261. [Google Scholar] [CrossRef]
- Oddone, I.; Barresi, A.A.; Pisano, R. Influence of controlled ice nucleation on the freeze-drying of pharmaceutical products: The secondary drying step. Int. J. Pharm. 2017, 524, 134–140. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 503–619. [Google Scholar] [CrossRef]
- Niwa, T.; Mizutani, D.; Danjo, K. Spray Freeze-Dried Porous Microparticles of a Poorly Water-Soluble Drug for Respiratory Delivery. Chem. Pharm. Bull. 2012, 60, 870–876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehta, M.; Bhardwaj, S.P.; Suryanarayanan, R. Controlling the physical form of mannitol in freeze-dried systems. Eur. J. Pharm. Biopharm. 2013, 85, 207–213. [Google Scholar] [CrossRef] [PubMed]
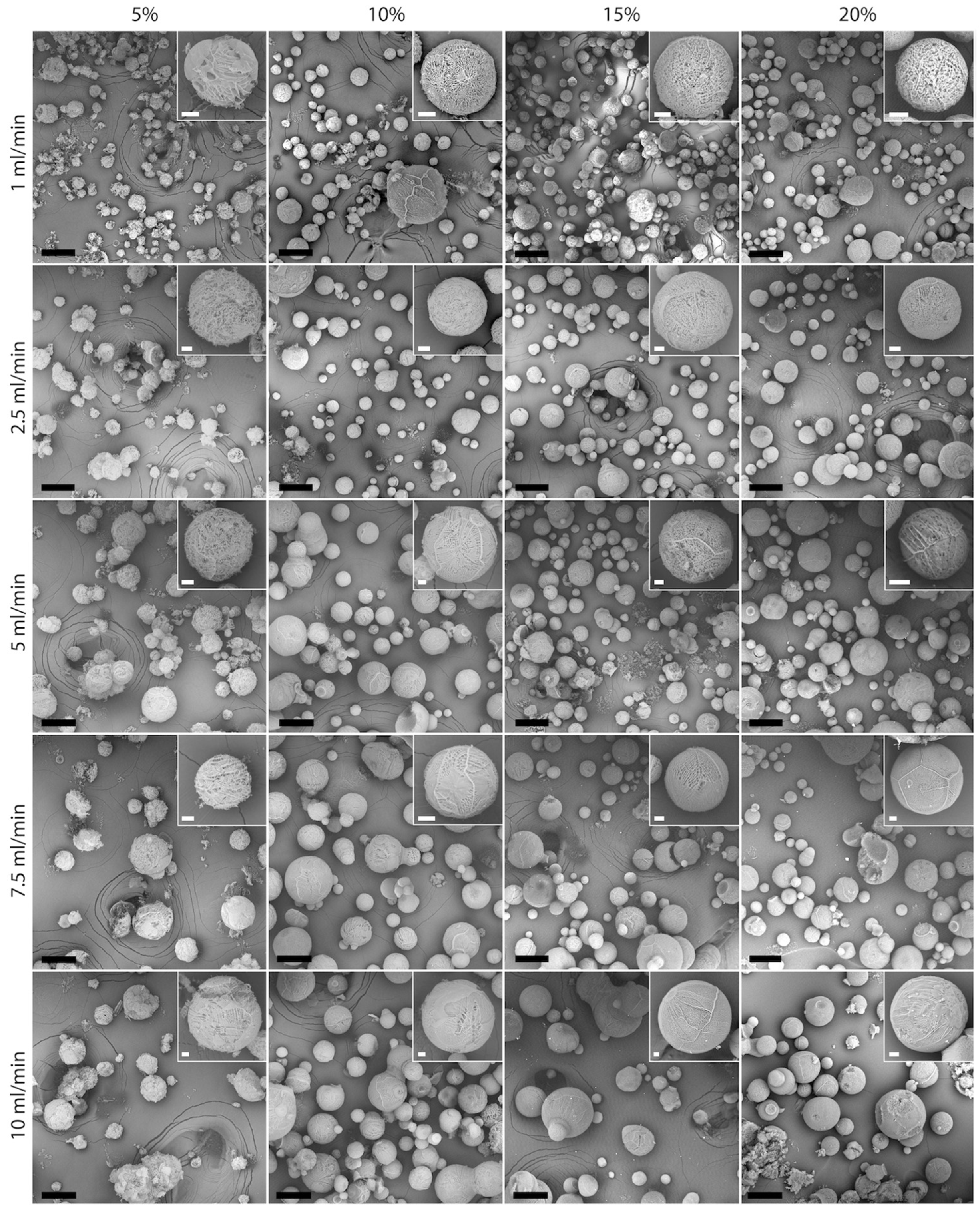
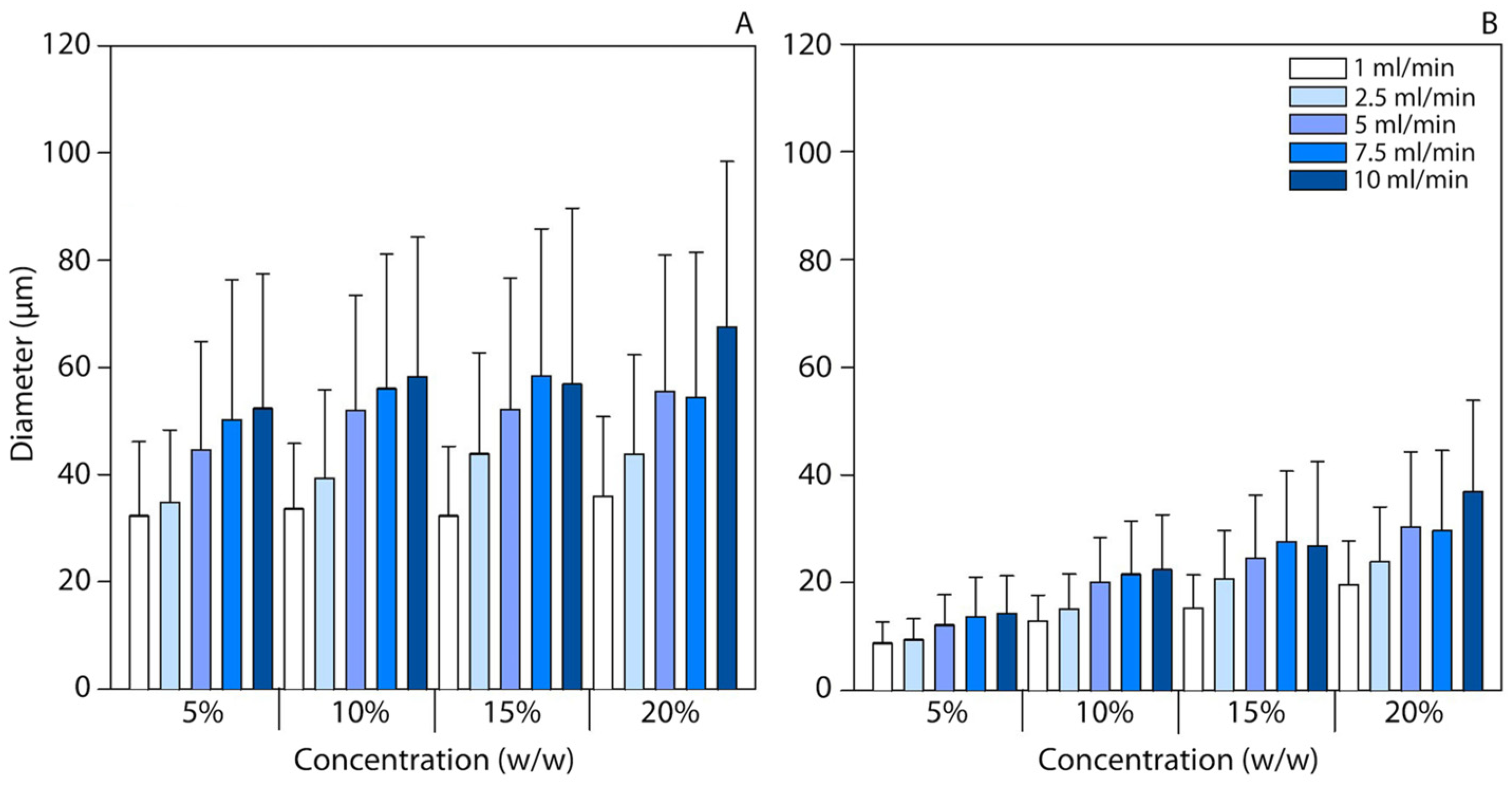
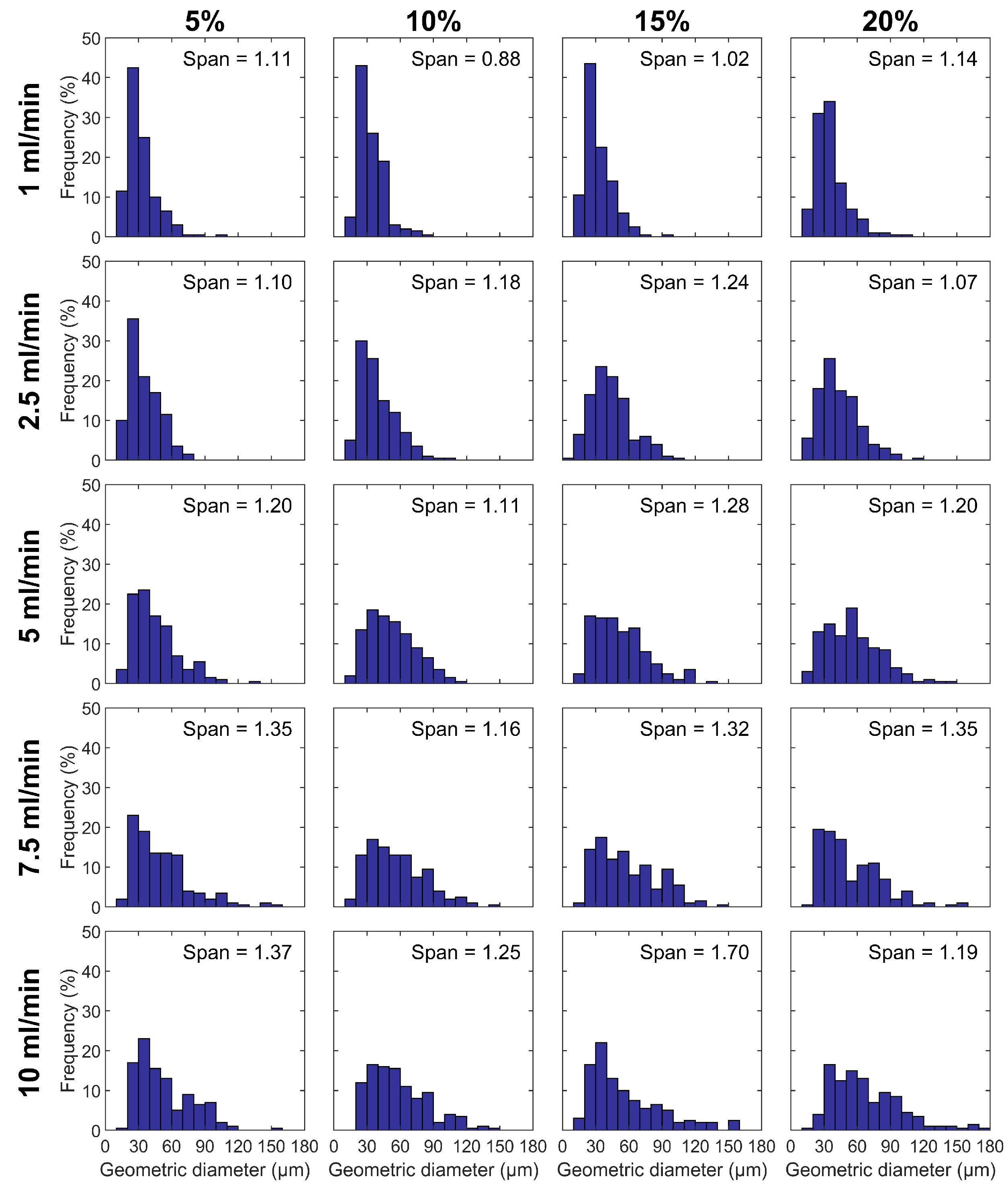
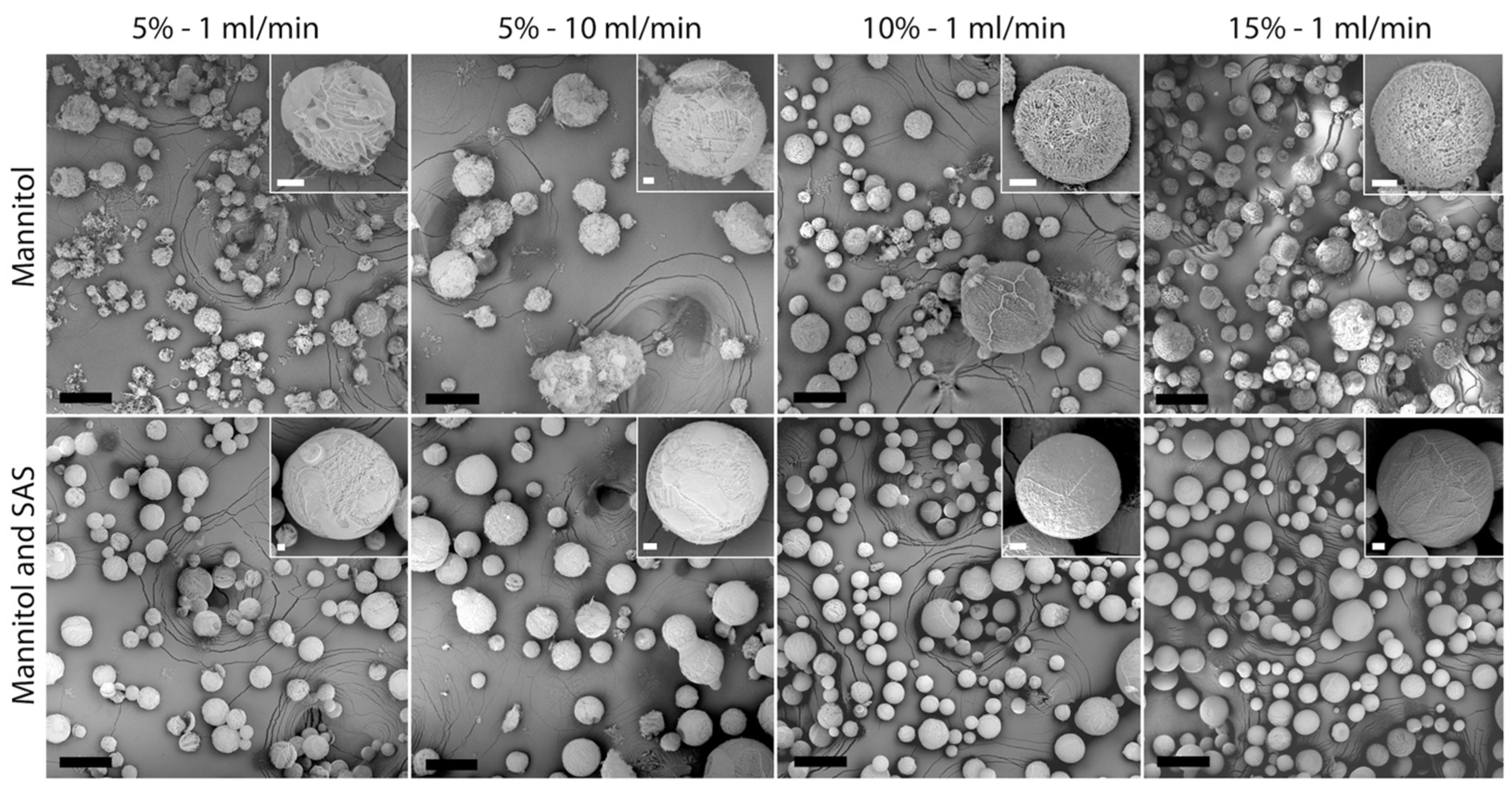
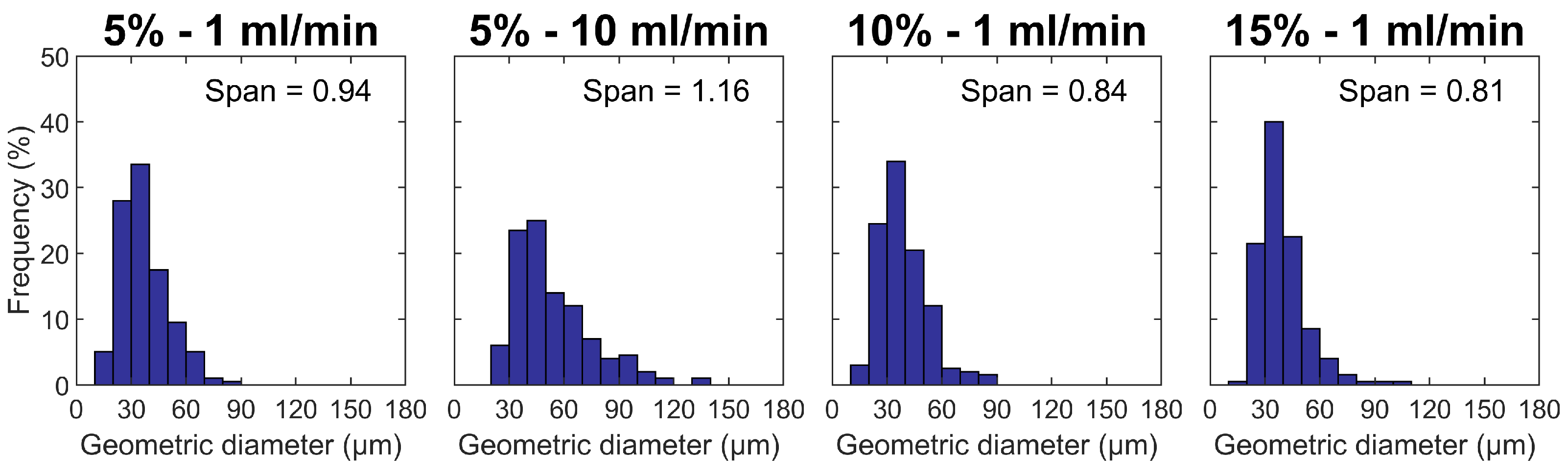

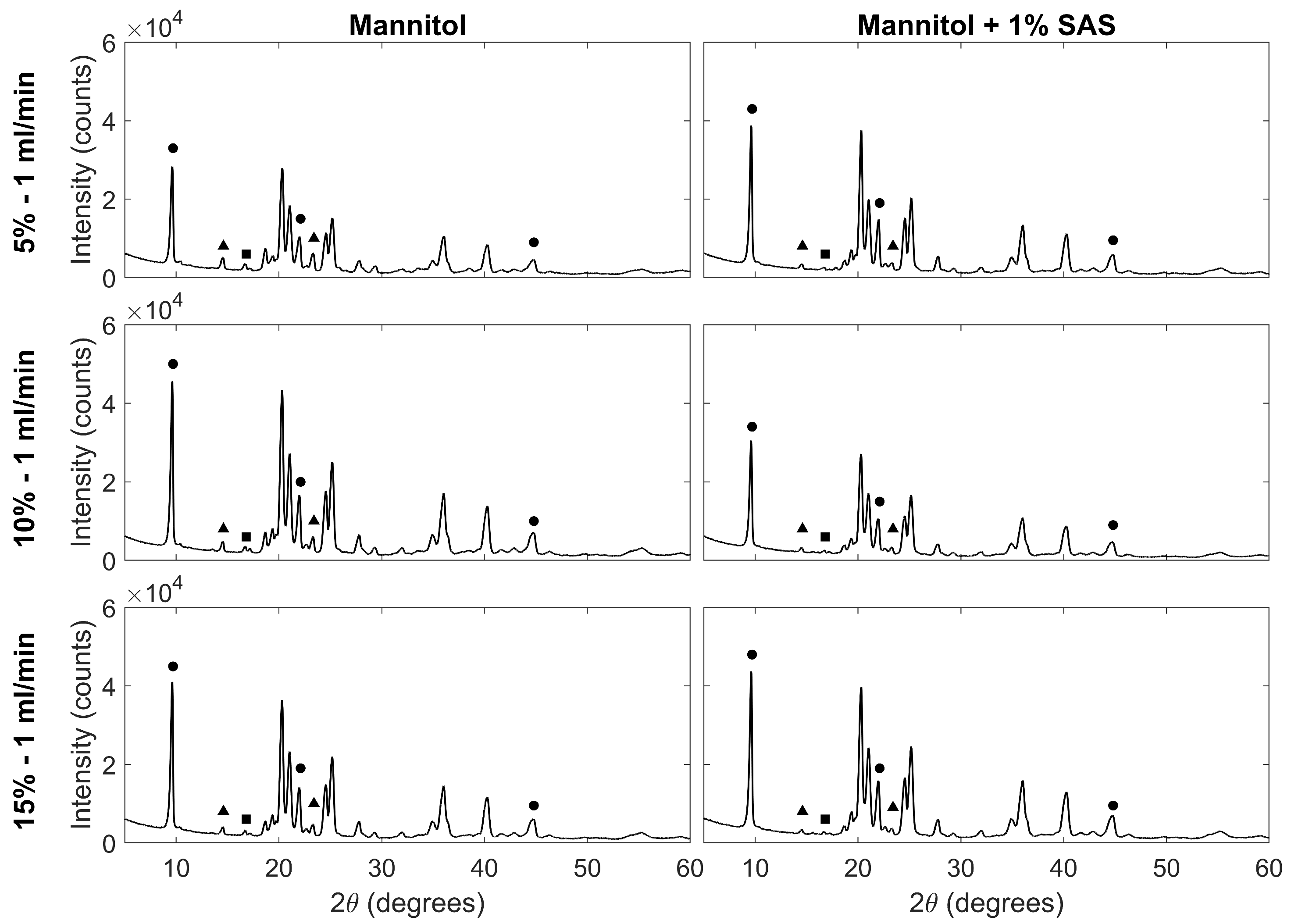
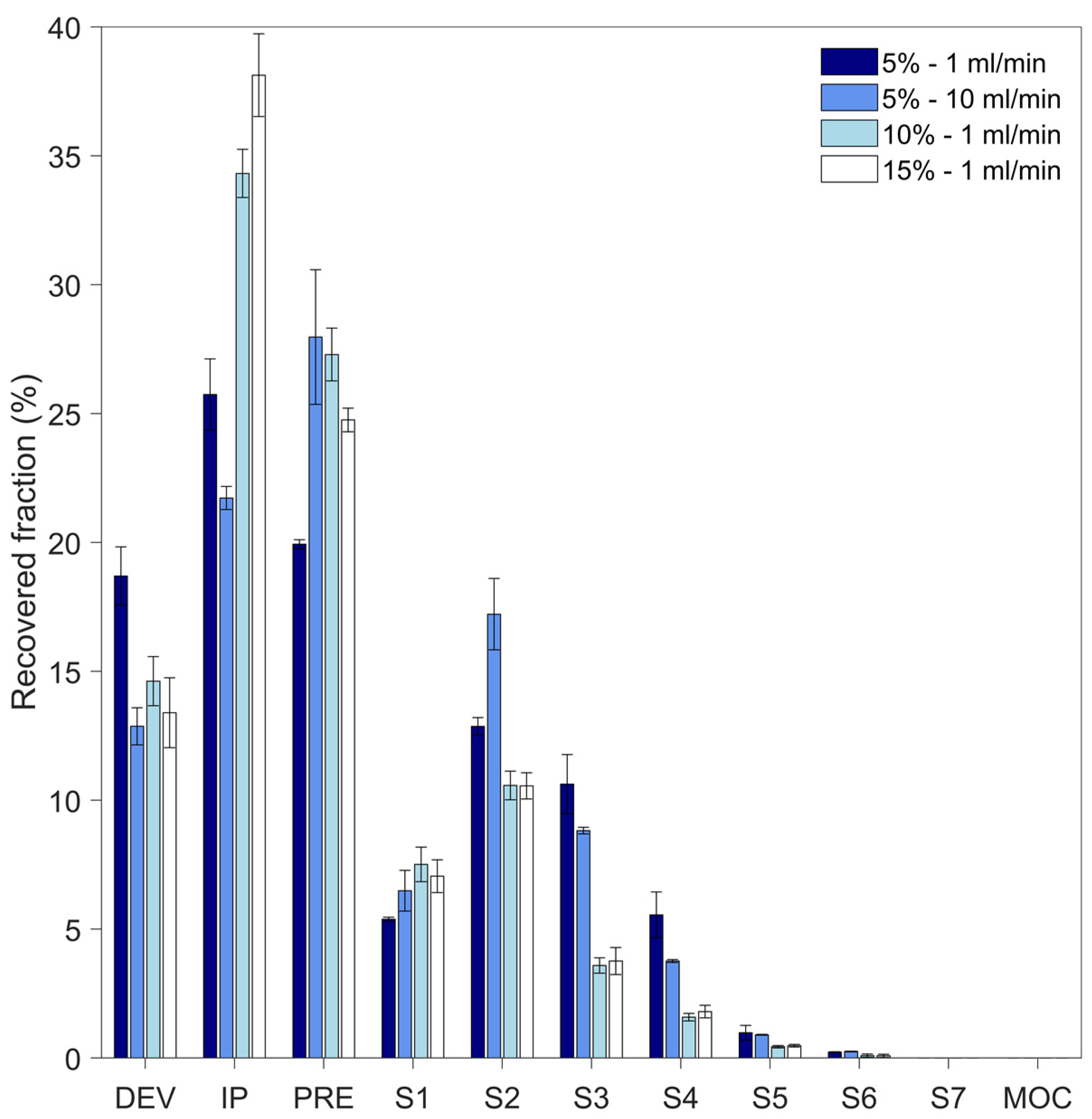
| Composition | % (w/w) | Feed Flow Rate (mL/min) | BET Surface Area (m2 g−1) | Pore Size (nm) | Peak Ratio | |
|---|---|---|---|---|---|---|
| BJH Adsorption | BJH Desorption | |||||
| Mannitol | 5 | 1 | 8.14 ± 0.09 | 11.1 | 10.4 | 1.9 |
| 2.5 | 8.03 ± 0.08 | 11.0 | 10.1 | 2.2 | ||
| 5 | 5.78 ± 0.05 | 14.5 | 12.5 | 1.7 | ||
| 7.5 | 9.19 ± 0.05 | 9.8 | 9.0 | 2.3 | ||
| 10 | 9.92 ± 0.10 | 10.3 | 9.8 | 1.8 | ||
| 10 | 1 | 7.60 ± 0.03 | 10.3 | 9.3 | 3.0 | |
| 2.5 | 7.14 ± 0.05 | 10.0 | 9.2 | 3.8 | ||
| 5 | 8.10 ± 0.04 | 10.1 | 9.3 | 3.5 | ||
| 7.5 | 9.81 ± 0.05 | 9.9 | 9.0 | 3.1 | ||
| 10 | 6.66 ± 0.06 | 11.0 | 10.1 | 3.4 | ||
| 15 | 1 | 5.97 ± 0.06 | 11.6 | 10.8 | 4.2 | |
| 2.5 | 5.92 ± 0.06 | 11.9 | 10.6 | 6.0 | ||
| 5 | 5.52 ± 0.05 | 12.0 | 10.9 | 5.0 | ||
| 7.5 | 8.30 ± 0.10 | 11.7 | 11.2 | 4.9 | ||
| 10 | 8.68 ± 0.08 | 10.2 | 9.4 | 5.5 | ||
| 20 | 1 | 6.73 ± 0.06 | 11.1 | 10.5 | 3.1 | |
| 2.5 | 6.04 ± 0.05 | 11.9 | 11.3 | 4.4 | ||
| 5 | 7.68 ± 0.06 | 10.7 | 9.6 | 4.9 | ||
| 7.5 | 10.10 ± 0.11 | 10.7 | 11.5 | 4.5 | ||
| 10 | 6.63 ± 0.05 | 10.9 | 9.8 | 4.2 | ||
| Mannitol and SAS | 5 | 1 | 21.62 ± 0.10 | 10.9 | 10.2 | 5.8 |
| 10 | 21.66 ± 0.09 | 10.0 | 9.3 | 3.1 | ||
| 10 | 1 | 23.86 ± 0.09 | 10.4 | 9.8 | 5.4 | |
| 15 | 1 | 22.90 ± 0.11 | 10.3 | 9.6 | 7.2 | |
| % (w/w) Solid | Flow Rate (mL min−1) | EF (%) | FPF (%) | MMAD (µm) | GSD (-) |
|---|---|---|---|---|---|
| 5 | 1 | 81.3 ± 1.1 | 26 ± 3 | 4.4 ± 0.2 | 1.72 ± 0.04 |
| 5 | 10 | 87.1 ± 0.7 | 21.2 ± 0.5 | 5.0 ± 0.1 | 1.68 ± 0.06 |
| 10 | 1 | 85 ± 1 | 9.5 ± 0.8 | 6.1 ± 0.2 | 1.75 ± 0.02 |
| 15 | 1 | 86.6 ± 1.3 | 9.9 ± 1.3 | 6.0 ± 0.3 | 1.77 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasero, L.; Susa, F.; Chiavarino, R.; Limongi, T.; Sulpizi, A.; Guidi, T.; Pisano, R. Tailoring Dry Microparticles for Pulmonary Drug Delivery: Ultrasonic Spray Freeze-Drying with Mannitol and Salbutamol Sulphate. Processes 2023, 11, 3096. https://doi.org/10.3390/pr11113096
Pasero L, Susa F, Chiavarino R, Limongi T, Sulpizi A, Guidi T, Pisano R. Tailoring Dry Microparticles for Pulmonary Drug Delivery: Ultrasonic Spray Freeze-Drying with Mannitol and Salbutamol Sulphate. Processes. 2023; 11(11):3096. https://doi.org/10.3390/pr11113096
Chicago/Turabian StylePasero, Lorena, Francesca Susa, Riccardo Chiavarino, Tania Limongi, Adamo Sulpizi, Tomaso Guidi, and Roberto Pisano. 2023. "Tailoring Dry Microparticles for Pulmonary Drug Delivery: Ultrasonic Spray Freeze-Drying with Mannitol and Salbutamol Sulphate" Processes 11, no. 11: 3096. https://doi.org/10.3390/pr11113096
APA StylePasero, L., Susa, F., Chiavarino, R., Limongi, T., Sulpizi, A., Guidi, T., & Pisano, R. (2023). Tailoring Dry Microparticles for Pulmonary Drug Delivery: Ultrasonic Spray Freeze-Drying with Mannitol and Salbutamol Sulphate. Processes, 11(11), 3096. https://doi.org/10.3390/pr11113096









