Magnesium Spinel Ferrites Development for FDM 3D-Printing Material for Microwave Absorption
Abstract
1. Introduction
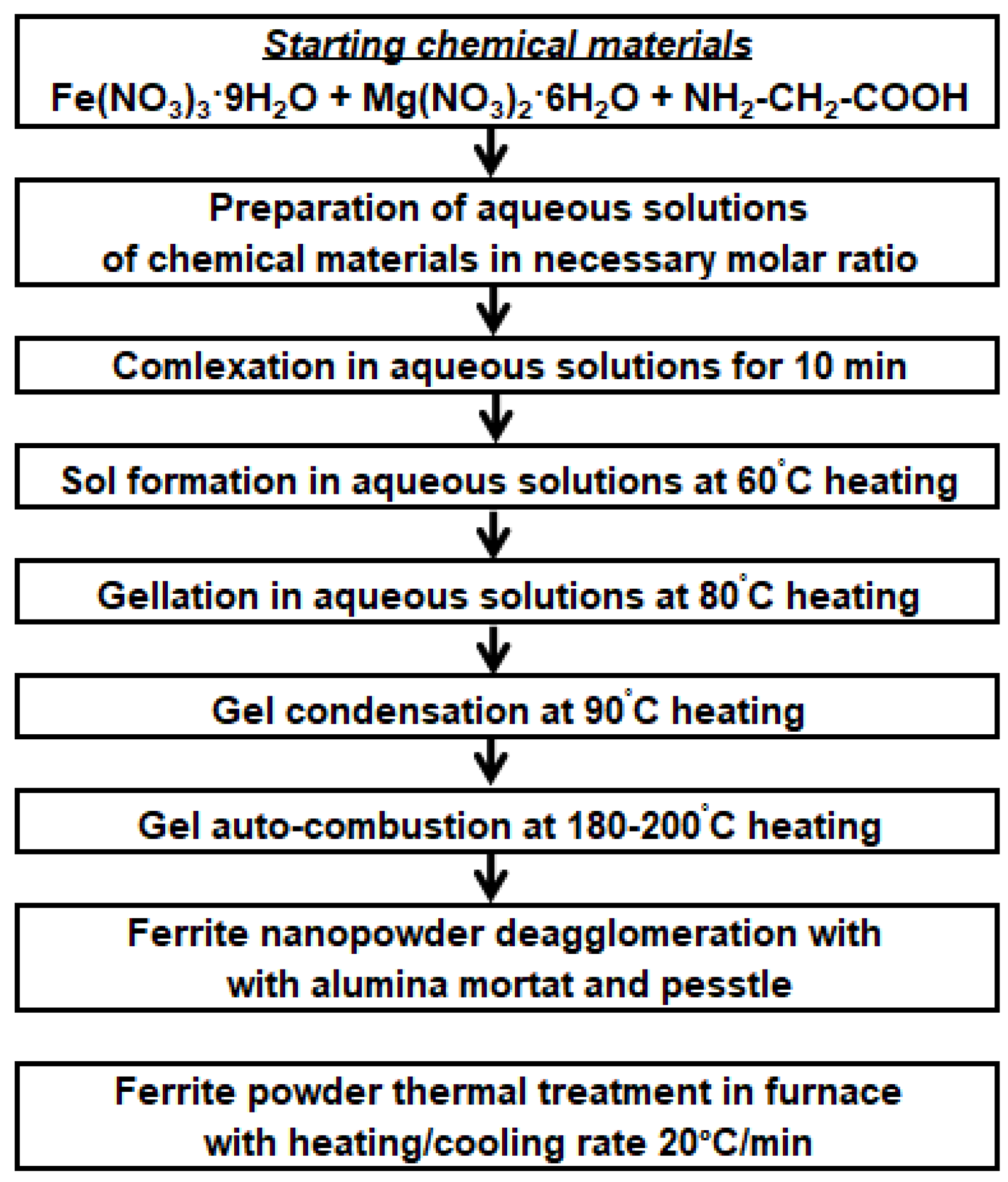
2. Materials and Methods
3. Results
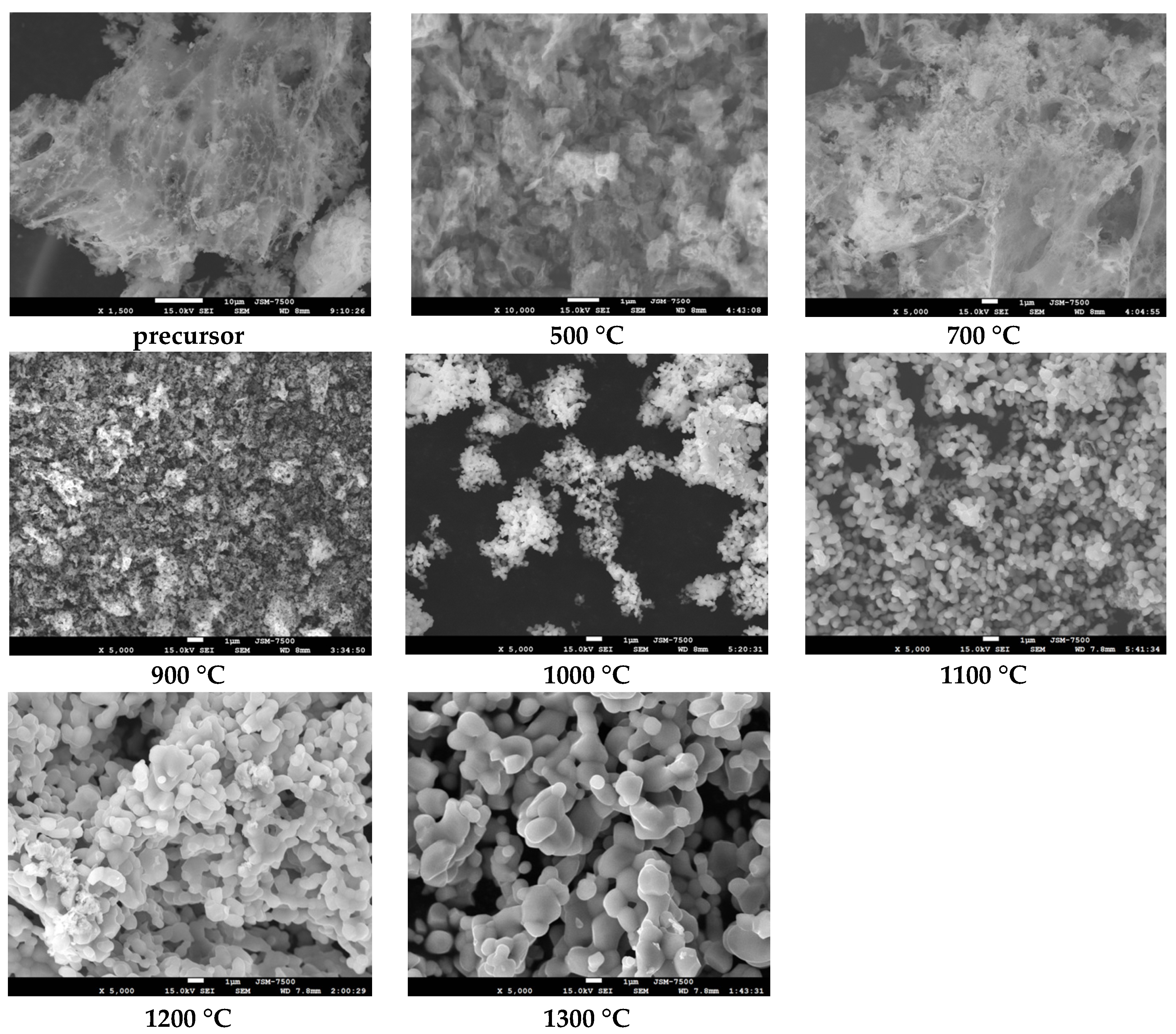
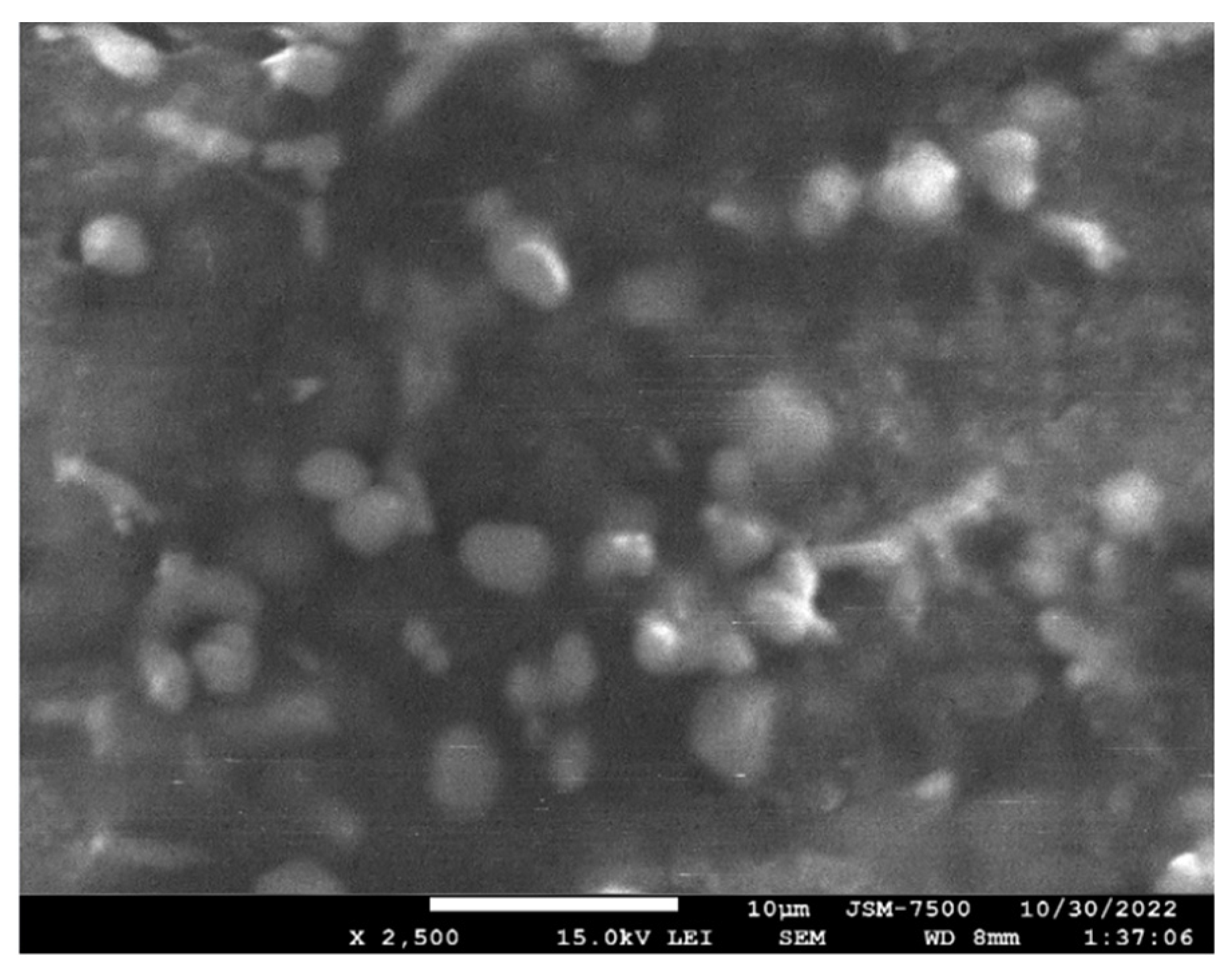
3.1. Electron Microscopy
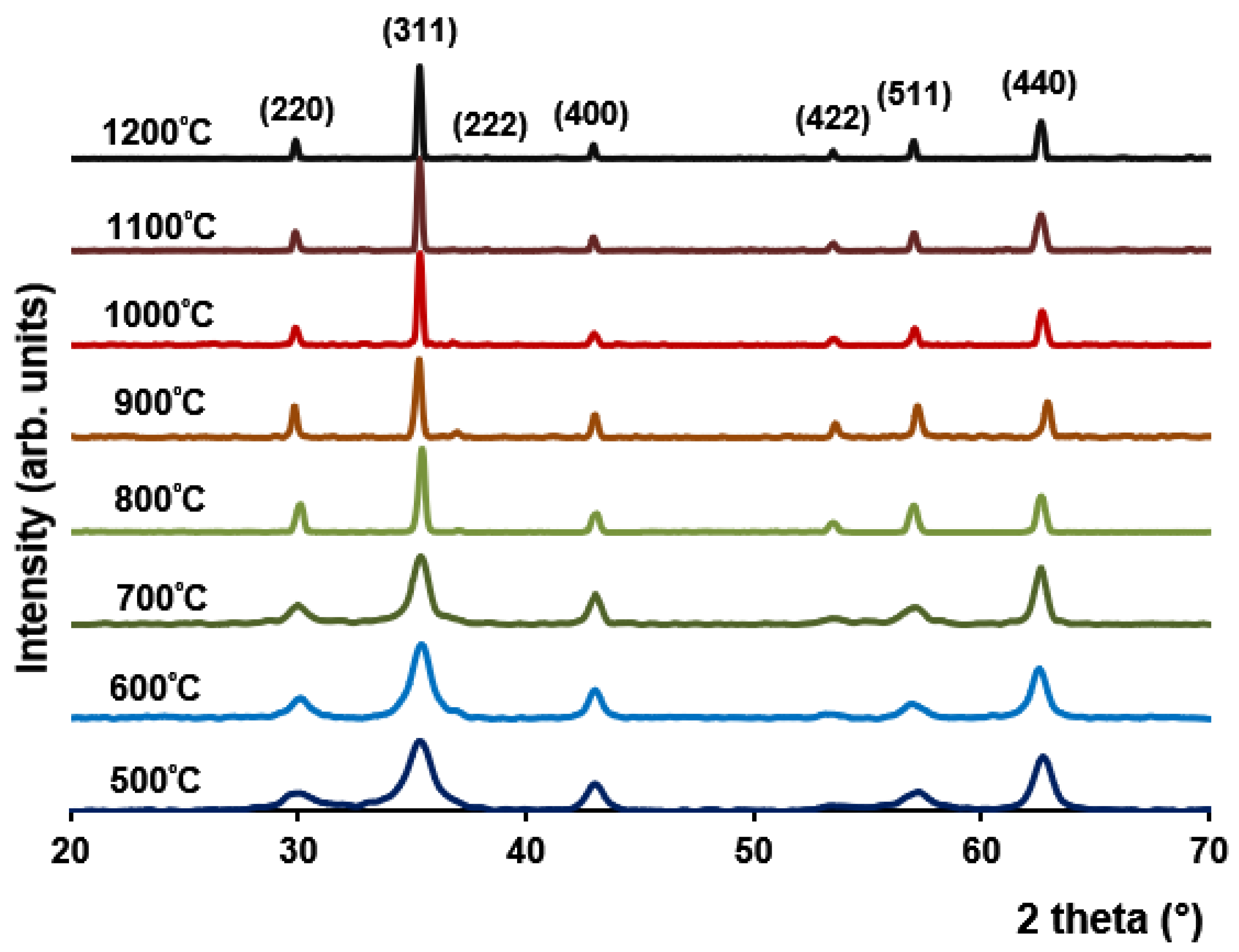
3.2. XRD Analysis
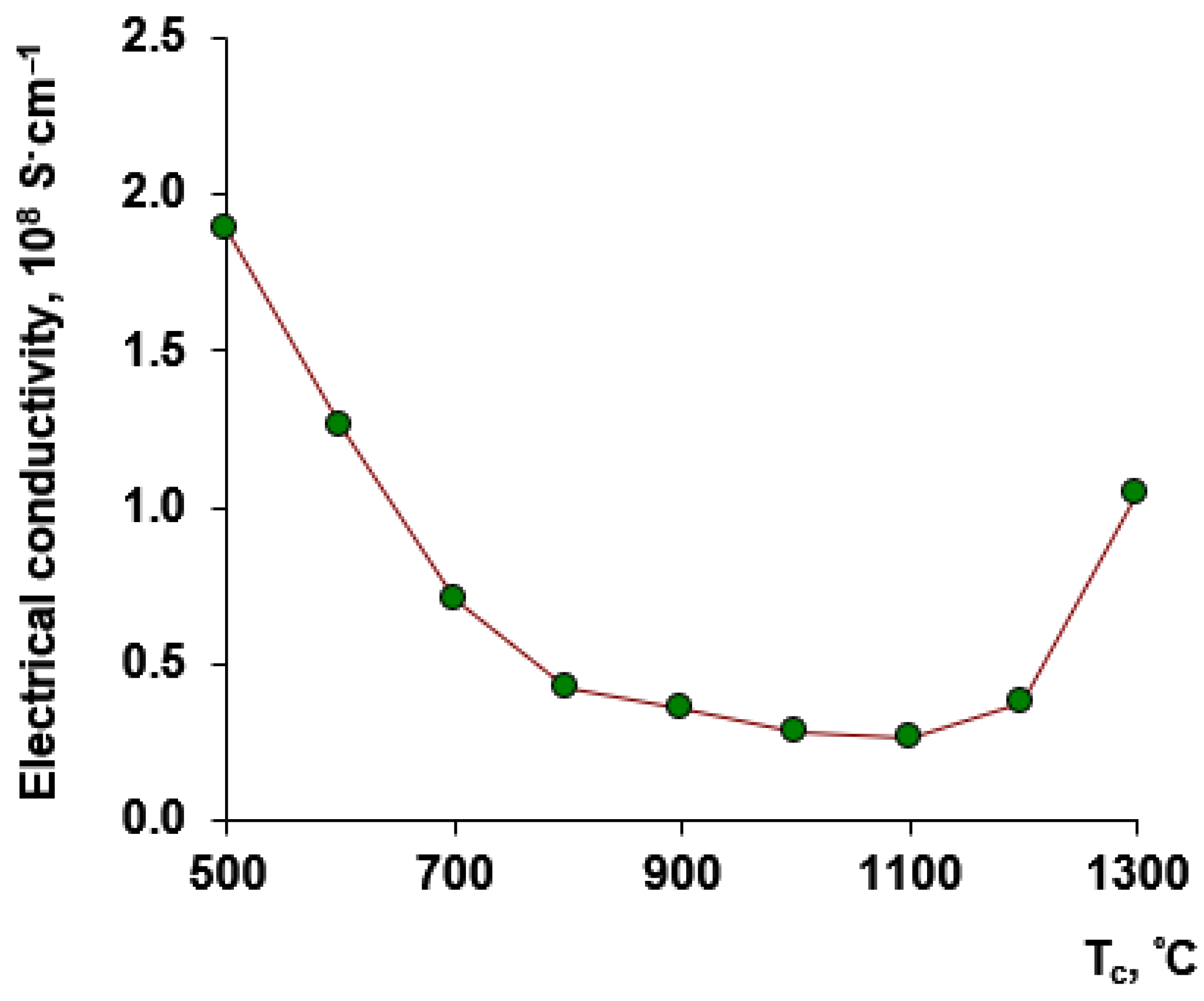
3.3. DC Electrical Conductivity
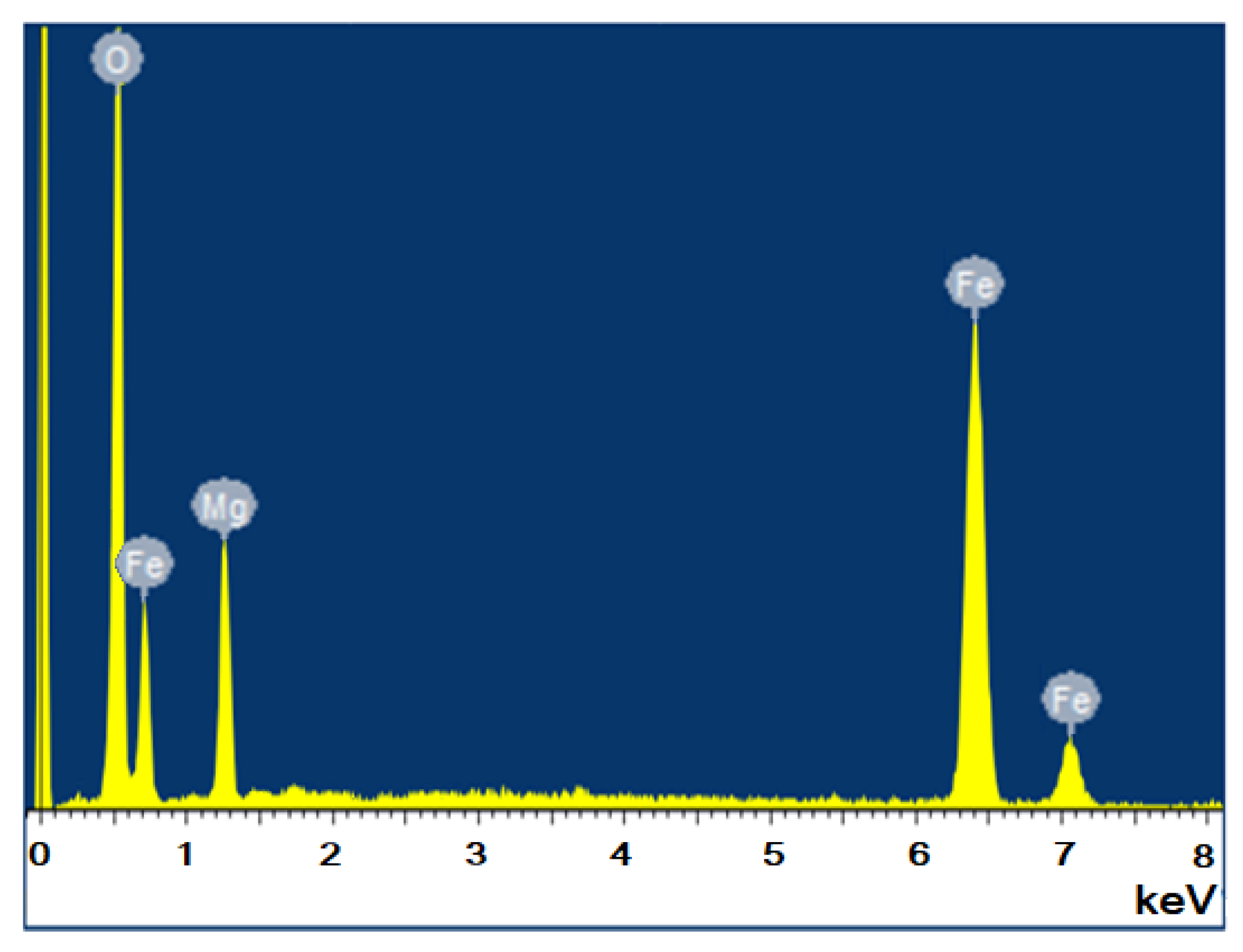
3.4. EDX Analysis
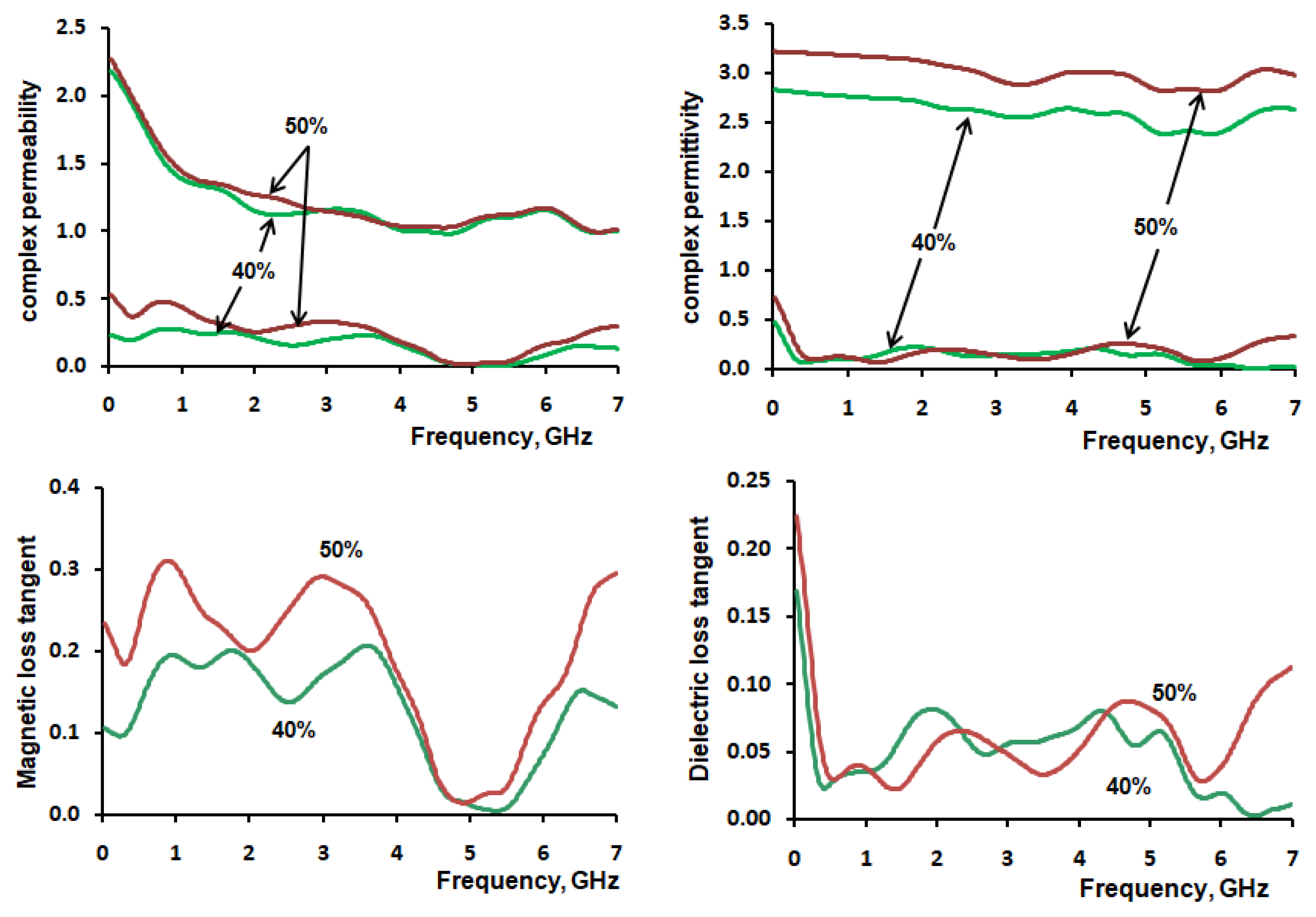
3.5. VNA Measurement MgFe2O4–Paraffin Composites
3.6. 3D FDM Printing MgFe2O4–PLA
3.7. VNA Measurement MgFe2O4–PLA Composites
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aliyan, N.; Mirkazemi, S.M.; Masoudpanah, S.M.; Akbari, S. The effect of post-calcination on cation distributions and magnetic properties of the coprecipitated MgFe2O4 nanoparticles. Appl. Phys. A 2017, 123, 446. [Google Scholar] [CrossRef]
- Mahato, D.K.; Majumder, S.; Banerjee, S. Large polaron tunneling, magnetic and impedance analysis of magnesium ferrite nanocrystallite. Appl. Surf. Sci. 2017, 413, 149–159. [Google Scholar] [CrossRef]
- Araújo, J.; Araujo-Barbosa, S.; Souza, A.; Iglesias, C.; Xavier, J.; Souza, P.; Cid, C.P.; Azevedo, S.; da Silva, R.; Correa, M.; et al. Tuning structural, magnetic, electrical, and dielectric properties of MgFe2O4 synthesized by sol-gel followed by heat treatment. J. Phys. Chem. Sol. 2021, 154, 110051. [Google Scholar] [CrossRef]
- Zampiva, R.Y.S.; Kaufmann, C.G., Jr.; Alve, A.K.; Bergmann, C.P. Influence of the Fuel Composition and the Fuel/Oxidizer Ratio on the Combustion Solution Synthesis of MgFe2O4 Catalyst Nanoparticles. FME Trans. 2018, 46, 157–164. [Google Scholar]
- Gerle, A.; Piotrowski, J.; Podworny, J. The Influence of Order in the Cation Sublattice of MgAl2O4, MgCr2O4 and MgFe2O4 Spinels on the Kinetics of Topochemical Reactions with Sulphur Oxides. J. Ceram. Sci. Technol. 2017, 8, 183–192. [Google Scholar]
- De Hoyos-Sifuentes, D.H.; Reséndiz-Hernández, P.J.; Díaz-Guillén, J.A.; Ochoa-Palacios, R.M.; Altamirano-Guerrero, G. Synthesis and characterization of MgFe2O4 nanoparticles and PEG-coated MgFe2O4 nanocomposite. J. Mater. Res. Technol. 2022, 18, 3130–3142. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, M. Comparative studies on impact of synthesis methods on structural and magnetic properties of magnesium ferrite nanoparticles. Process. Appl. Ceram. 2014, 8, 137–143. [Google Scholar] [CrossRef]
- Hussein, S.I.; Elkady, A.S.; Rashad, M.M.; Mostafa, A.G.; Megahid, R.M. Structural and magnetic properties of magnesium ferrite nanoparticles prepared via EDTA-based sol–gel reaction. J. Magn. Magn. Mater. 2015, 379, 9–15. [Google Scholar] [CrossRef]
- Durrani, S.K.; Naz, S.; Mehmood, M.; Nadeem, M.; Siddique, M. Structural, impedance and Mössbauer studies of magnesium ferrite synthesized via sol–gel auto-combustion process. J. Saudi Chem. Soc. 2017, 21, 899–910. [Google Scholar] [CrossRef]
- Easwari, M.; Jesurani, S. Synthesis & Magnetic Properties of Magnesium Ferrite (MgFe2O4) Nanoparticles Via Sol Gel Method. Int. Res. J. Eng. Technol. 2017, 4, 110–113. [Google Scholar]
- Naidu, V.; Seeni, S.M.; Maraikkayar, M.A.; Sivaranjani, S.; Kalpana, M.; Jeeva, R.K.; Pandian, S. Engineered meta magneto dielectric substrate for microstrip patch antenna miniaturization with reduced return loss. Int. J. Appl. Eng. 2015, 10, 30474–30479. [Google Scholar]
- Lee, C.-K.; McGhee, J.; Tsipogiannis, C.; Zhang, S.; Cadman, D.; Goulas, A.; Whittaker, T.; Gheisari, R.; Engstrom, D.; Vardaxoglou, J.; et al. Evaluation of Microwave Characterization Methods for Additively Manufactured Materials. Designs 2019, 3, 47. [Google Scholar] [CrossRef]
- Shamray, I.I.; Buz’Ko, V.Y.; Goryachko, A.I. Changes in the Structure and Properties of Nickel-Zinc Spinel Nanoferrites Series for 3D-Printing. IOP Conf. Ser. Mater. Sci. Eng. 2020, 969, 012101. [Google Scholar] [CrossRef]
- Wang, Y.; Castles, F.; Grant, P.S. 3D Printing of NiZn ferrite/ABS Magnetic Composites for Electromagnetic Devices. MRS Proc. 2015, 1788, 29–35. [Google Scholar] [CrossRef]
- Qian, Y.; Yao, Z.; Lin, H.; Zhou, J. Mechanical and microwave absorption properties of 3D-printed Li0.44Zn0.2Fe2.36O4/polylactic acid composites using fused deposition modeling. J. Mater. Sci. Mater. Electron. 2018, 29, 19296–19307. [Google Scholar] [CrossRef]
- Xicong, Y.; Qi, G.; Enyi, H.; Chao, Y.; Bin, O.; Peng, Y.; Haihua, W. Mechanical and microwave absorbing properties of Mn-Zn ferrite/polylactic acid composites formed by fused deposition modeling. Acta Mater. Compos. Sin. 2022, 10, 1–13. [Google Scholar]
- Haihua, W.; Zhenglang, H.; Yutian, L.; Peng, Q.; Li, L.; Jianxin, Z. Electromagnetic absorption properties and mechanical properties of Fe-Ni alloy/polylactic acid composites fabricated by fused deposition modeling. Acta Mater. Compos. Sin. 2022, 39, 172–182. [Google Scholar]
- Khot, V.M.; Salunkhe, A.B.; Phadatare, M.R.; Pawar, S.H. Formation, microstructure and magnetic properties of nanocrystalline MgFe2O4. Mater. Chem. Phys. 2012, 132, 782–787. [Google Scholar] [CrossRef]
- Rothwell, E.J.; Frasch, J.L.; Ellison, S.M.; Chahal, P.; Ouedraogo, R.O. Analysis of the Nicolson-Ross-Weir Method for Characterizing the Electromagnetic Properties of Engineered Materials. Prog. Electromagn. Res. C 2016, 157, 31–47. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I.; Parise, J.B. Cation ordering in magnesioferrite, MgFe2O4, to 982 °C using in situ synchrotron X-ray powder diffraction. Am. Mineral. 2005, 90, 219–228. [Google Scholar] [CrossRef]
- Gateshki, M.; Petkov, V.; Pradhan, S.K.; Vogt, T. Structure of nanocrystalline MgFe2O4 from X-ray diffraction, Rietveld and atomic pair distribution function analysis. J. Appl. Cryst. 2005, 38, 772–779. [Google Scholar] [CrossRef]
- O‘Neil, H.; Annersten, H.; Virgo, D. The temperature dependence of the cation distribution in magnesioferrite (MgFe2O4) from powder XRD structural refinements and Mössbauer spectroscopy. Am. Mineral. 1992, 77, 725–740. [Google Scholar]
- Harrison, R.J.; Putnis, A. Determination of the mechanism of cation ordering in magnesioferrite (MgFe2O4) from the time and temperature-dependence of magnetic susceptibility. Phys. Chem. Miner. 1999, 26, 322–332. [Google Scholar] [CrossRef]
- Tian, N.; Wang, J.W.; Li, F.; Mei, Z.; Lu, Z.X.; Ge, L.L.; You, C.Y. Enhanced microwave absorption of Fe flakes with magnesium ferrite cladding. J. Magn. Magn. Mater. 2012, 324, 4175–4178. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Zhuang, J. Synthesis for powder of nano-MgFe2O4/Fe2O3 by citrate sol-gel and absorbs characteristic of electromagnetic wave. J. Func. Mater. 2005, 36, 1839–1841. [Google Scholar]
- Chen, W.; Liu, Q.; Zhu, X.; Fu, M. One-step in situ growth of magnesium ferrite nanorods on graphene and their microwave-absorbing properties. Appl. Organomet. Chem. 2018, 32, e4017. [Google Scholar] [CrossRef]
- Tang, H.; Xu, B.; Wang, S.; Zhang, Y.G. MgFe2O4 nanoparticles preparation and its electromagnetic properties. J. Func. Mater. 2013, 44, 776–779. [Google Scholar]
- Vyzulin, S.A.; Buz’ko, V.Y.; Kalikintseva, D.A.; Goryachko, A.I.; Sarin, L.I.; Kolantsov, O.A.; Syr’ev, N.E. Magnetic and dielectric properties of composites based on magnetic microspheres. J. Phys. Conf. Ser. 2019, 1389, 012161. [Google Scholar] [CrossRef]
- Goryachko, A.I.; Ivanin, S.N.; Buz’ko, V.Y. Electromagnetic properties of composite materials based on Nd-compound modified Fe-Si-Al alloy. IOP Conf. Ser. Mater. Sci. Eng. 2020, 969, 012019. [Google Scholar] [CrossRef]
- Vyzulin, S.A.; Miroshnichenko, E.L.; Kalikintseva, D.A.; Buz’ko, V.Y. Investigation of microwave absorption properties of nanosized nickel-zinc ferrites powders. In Proceedings of the 2017 Radiation and Scattering of Electromagnetic Waves (RSEMW), Divnomorskoe, Russia, 26–30 June 2017; pp. 152–154. [Google Scholar]
- Vyzulin, S.A.; Kalikintseva, D.A.; Miroshnichenko, E.L.; Buz’Ko, V.Y.; Goryachko, A.I. Microwave Absorption Properties of Nickel–Zinc Ferrites Synthesized by Different Means. Bull. Russ. Acad. Sci. Phys. 2018, 82, 943–945. [Google Scholar] [CrossRef]
- Goryachko, A.I.; Ivanin, S.N.; Buzko, V.Y. Synthesis, Microstructural and Electromagnetic Characteristics of Cobalt-Zinc Ferrite. Condens. Matter Interphas. 2020, 22, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Buz’Ko, V.Y.; Shamrai, I.I.; Ryabova, M.Y.; Kireeva, G.V.; Goryachko, A.I. Properties of Nickel Zinc Ferrite Nanopowders Prepared by Different Methods. Inorg. Mater. 2021, 57, 38–43. [Google Scholar] [CrossRef]



| Sample | Tm, °C | Tc, °C | Tg, °C |
|---|---|---|---|
| 40% (wt.) MgFe2O4-PLA | 112.1 | 97.8 | 81.5 |
| 50% (wt.) MgFe2O4-PLA | 111.7 | 98.7 | 90.2 |
| Calcination Temperature, °C | 500 | 600 | 700 | 800 | 900 | 1000 | 1100 | 1200 |
|---|---|---|---|---|---|---|---|---|
| Dm, nm | 10.2 | 11.8 | 15.1 | 29.7 | 31.6 | 48.0 | 57.3 | 74.1 |
| Material | Dielectric Constant | Magnetic Loss Tangent | Dielectric Loss Tangent |
|---|---|---|---|
| Paraffin | 2.211 ± 0.029 | 0.004 ± 0.002 | 0.0023 ± 0.012 |
| PLA-printed | 2.220 ± 0.027 | 0.003 ± 0.002 | 0.0043 ± 0.015 |
| Material | RLmax, dB | Frequency at RLmax, GHz | Reference |
|---|---|---|---|
| 40% (wt.) MgFe2O4-PLA, 10 mm | −4.28 | 3.00 | This work |
| 35% (wt.) Ni0.5Zn0.5Fe2O4-PLA, 10 mm | −4.49 | 4.22 | [13] |
| 40% (wt.) Mn0.6Zn0.4Fe2O4-PLA, 7.4 mm | −7.2 | 5.7 | [16] |
| 40% (wt.) FeNi50-PLA, 3.0 mm | −6.2 | 12.7 | [17] |
| 50% (wt.) magnetic microspheres-paraffin 10 mm | −6.55 | 3.38 | [28] |
| 40% (wt.) Fe-Si-Al alloy micropowder-paraffin, 5 mm | −16.76 | 4.57 | [29] |
| 50% (wt.) Co0.5Zn0.5Fe2O4-paraffin, 10 mm | −9.09 | 4.26 | [32] |
| 50% (wt.) Ni0.5Zn0.5Fe2O4-paraffin, 10 mm | −7.58 | 4.42 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzko, V.; Ivanin, S.; Goryachko, A.; Shutkin, I.; Pushankina, P.; Petriev, I. Magnesium Spinel Ferrites Development for FDM 3D-Printing Material for Microwave Absorption. Processes 2023, 11, 60. https://doi.org/10.3390/pr11010060
Buzko V, Ivanin S, Goryachko A, Shutkin I, Pushankina P, Petriev I. Magnesium Spinel Ferrites Development for FDM 3D-Printing Material for Microwave Absorption. Processes. 2023; 11(1):60. https://doi.org/10.3390/pr11010060
Chicago/Turabian StyleBuzko, Vladimir, Sergey Ivanin, Alexander Goryachko, Ivan Shutkin, Polina Pushankina, and Iliya Petriev. 2023. "Magnesium Spinel Ferrites Development for FDM 3D-Printing Material for Microwave Absorption" Processes 11, no. 1: 60. https://doi.org/10.3390/pr11010060
APA StyleBuzko, V., Ivanin, S., Goryachko, A., Shutkin, I., Pushankina, P., & Petriev, I. (2023). Magnesium Spinel Ferrites Development for FDM 3D-Printing Material for Microwave Absorption. Processes, 11(1), 60. https://doi.org/10.3390/pr11010060







