Comparison of Volatile Compositions among Four Related Ligusticum chuanxiong Herbs by HS-SPME-GC-MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Materials and Chemicals
2.3. Preparation of Standard Solution
2.4. Regression Method and Correction
2.5. Preparation of n-alkanes Solution (C9–C30) and Internal Standards Solution
2.6. Preparation of Sample for GC-MS Analysis
2.7. Gas Chromatography Conditions
2.8. Mass Spectrometry Conditions
2.9. Data Analysis
3. Results
3.1. Optimization of Analytical Method
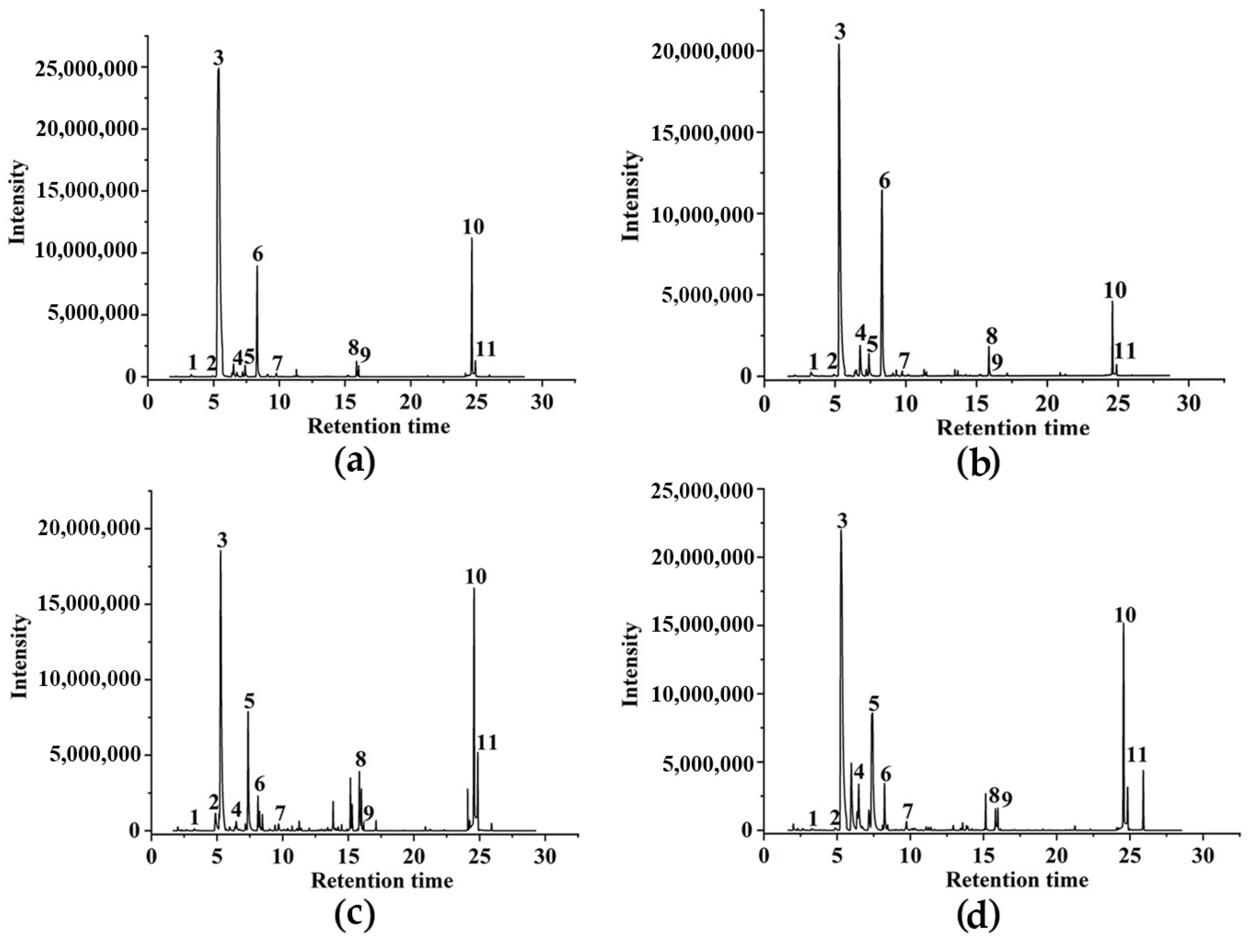
3.2. Identification of Compounds in Volatile Gas
3.3. Correct of Retention Time and Regression Equation
3.4. Method Validation
3.5. Comparing the Types of Volatile Compounds in the Four Herbs
3.6. Comparing the Contents of Volatile Compounds in the Four Herbs
3.7. Comparing the Main Volatile Compounds in the Four Herbs
3.8. Comparing the Chemical Classes of Volatile Compounds in the Four Herbs
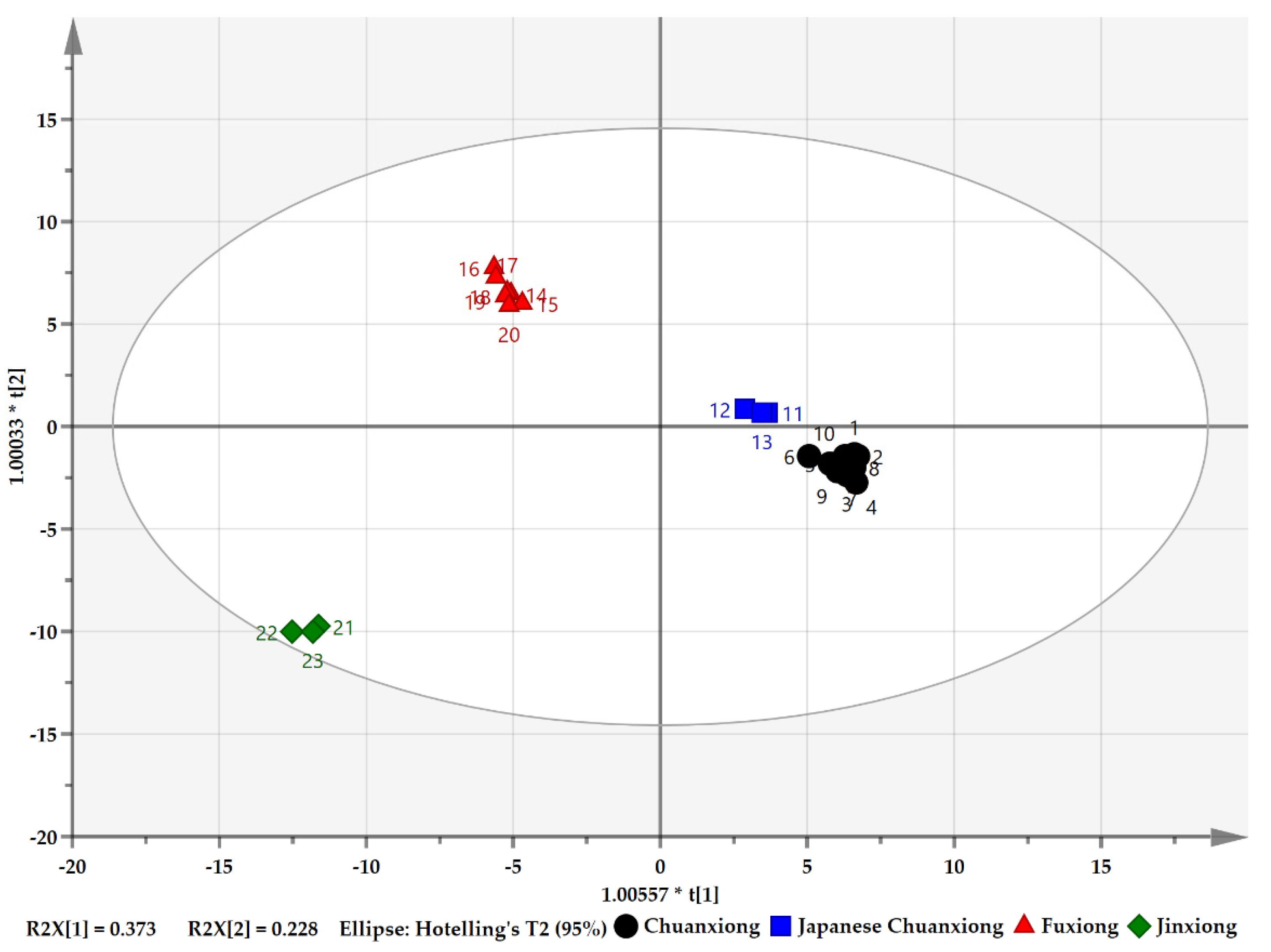
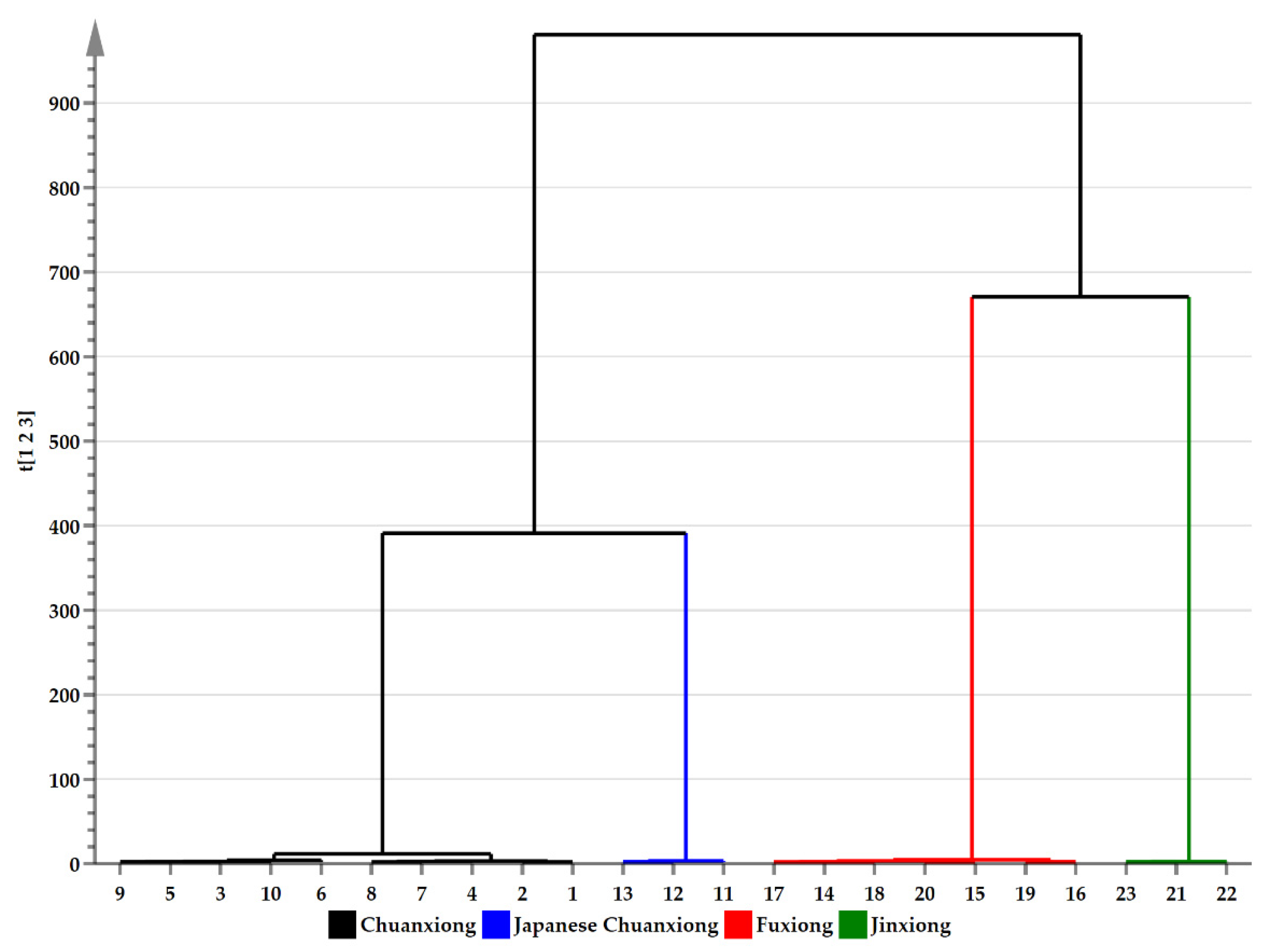
3.9. Statistical Analysis of Volatile Compounds in the Four Herbs
4. Discussion
4.1. The Developed Method Can Rapidly Detect the Natural and Real Compounds in Volatile Gas and Is a Novel Method for the Analysis of Aromatic Herbs
4.2. The Volatile Compositions Are Generally Similar, but the Amounts of Major Bioactive Compounds Are Obviously Different, Indicating That the Four Herbs Should Be Differentiated for Their Medical Uses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pharmacopoeia Committee of Japanese. Japanese Pharmacopoeia; Ministry of Health and Welfare Press: Tokyo, Japan, 2021; Volume 1, p. 1229. [Google Scholar]
- Kimura, K.; Hata, K.; Kozawa, M. Pharmacognostical studies on Umbelliferous plants XVII. Syoyakugaku Zasshi 1961, 15, 5–13. [Google Scholar]
- Suk, K.-T.; Nitta, A.Y.A.; Konoshima, M. Studies on origin of Japanese Chuanxiong. VI. Yakugaku Zasshi 1974, 94, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Choi, H.-W.; Sung, J.-S.; Bang, J.-W. Inter-genomic relationships among three medicinal herbs: Cnidium officinale, Ligusticum chuanxiong and Angelica polymorpha. Genes Genom. 2010, 32, 95–101. [Google Scholar] [CrossRef]
- Jin, W.; Cui, J.; Liu, Y. Study on the cultivation technique of Dongchuanxiong. J. Tradit. Chin. Med. 2001, 6, 26–27. [Google Scholar]
- Pu, F.D.; Watson, M. Apiaceae. Ligusticum L. Flora of China; Scientific Press: Beijing, China, 2005; Volume 14, pp. 140–150. [Google Scholar]
- Zhang, H.; Fang, S.; Wu, L. Studies on the origin of the traditional Chinese drug “Jinxiong”. Acta Pharmacol. Sin. 1990, 28, 447–482. [Google Scholar]
- Gündeşli, M.A.; Kafkas, N.E.; Okatan, V.; Usanmaz, S. Identification and characterisation of volatile compounds determined by Hs/Gcms technıque in pulp of ‘Abbas’ Fig (Ficus carica L.) variety. Pak. J. Agri. Sci. 2020, 57, 623–629. [Google Scholar] [CrossRef]
- Wan, D.; Peng, C.; Zhang, H. Records of Genuine Medicinal Materials in Sichuan; Sichuan Science and Technology Press: Chengdu, China, 2005; pp. 4–12. [Google Scholar]
- Kafkas, N.E.; Gündeşli, M.A. Fig Flavor. Advances in Fig Research and Sustainable Production; CABI: Wallingford, UK, 2022; pp. 364–386. [Google Scholar] [CrossRef]
- Yang, C.; Wang, C.; Wang, M.; Qin, X.; Hao, G.; Kang, M.; Hu, X.; Cheng, Y.; Shen, J. A novel deodorization method of edible oil by using ethanol steam at low temperature. J. Food Sci. 2021, 86, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Dal Prá, V.; Dolwitsch, C.B.; da Silveira, G.D.; Porte, L.; Frizzo, C.; Tres, M.V.; Mossi, V.; Mazutti, M.A.; do Nascimento, P.C.; Bohrer, D.; et al. Supercritical CO2 extraction, chemical characterisation and antioxidant potential of Brassica oleracea var capitata against HO, O2− and ROO. Food Chem. 2013, 141, 3954–3959. [Google Scholar] [CrossRef] [PubMed]
- Zahiri, E.; Khandaghi, J.; Farajzadeh, M.A.; Afshar Mogaddam, M.R. Combination of dispersive solid phase extraction with solidification organic drop-dispersive liquid-liquid microextraction based on deep eutectic solvent for extraction of organophosphorous pesticides from edible oil samples. J. Chromatogr. A 2020, 1627, 461390. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y. The Study on Variations of Chemical Componets and Antioxidant Activity of Red Wine During Decanting Process. Ph.D. Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2011. [Google Scholar]
- Zhang, C.; Qi, M.; Shao, Q.; Zhou, S.; Fu, R. Analysis of the volatile compounds in Ligusticum chuanxiong Hort. using HS-SPME-GC-MS. J. Pharm. Biomed. Anal. 2007, 44, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; He, R.; Long, M.; Li, Y.; Zheng, Y.; Lin, L.; Liang, D.; Zhang, X.; Liao, M.; Lv, X.; et al. Comparison of the fruit volatile profiles of five muscadine grape cultivars (Vitis rotundifolia Michx.) using HS-SPME-GC/MS combined with multivariate statistical analysis. Front. Plant Sci. 2021, 12, 728891. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Bishop, K.S.; Zhang, J.; Chen, D.; Quek, S.Y. Characterization of phenolic compounds and aroma active compounds in feijoa juice from four New Zealand grown cultivars by LC-MS and HS-SPME-GC-O-MS. Food Res. Int. 2020, 129, 108873. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Duan, S.; Dang, Y.; Zhang, X.; Xia, K.; Liu, S.; Han, X.; Wen, J.; Li, Z.; Wang, X.; et al. Origin identification of Chinese Maca using electronic nose coupled with GC-MS. Sci. Rep. 2019, 9, 12216. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Pollen morphology and ITS relationship of Ligusticum sinense cv. Chuanxiong, L. sinense cv. Fuxiong and L. sinense cv. Acta Bot. Yunnanica 1990, 2, 173–178, 238–240. [Google Scholar]
- Zhou, J.; Gao, Y.; Wei, J.; Liu, Z.; Downie, S.R. Molecular phylogenetics of Ligusticum (Apiaceae) based on nrDNA ITS sequences: Rampant polyphyly, placement of the Chinese endemic species, and a much-reduced circumscription of the genus. Int. J. Plant Sci. 2020, 181, 306–323. [Google Scholar] [CrossRef]
- Ren, T.; Xie, D.; Peng, C.; Gui, L.; Price, M.; Zhou, S.; He, X. Molecular evolution and phylogenetic relationships of Ligusticum (Apiaceae) inferred from the whole plastome sequences. BMC Ecol. Evol. 2022, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Sha, X.; Xiong, M.; Zhong, W.; Wei, Y.; Li, M.; Tao, S.; Mou, F.; Peng, F.; Zhang, C. Uncovering dynamic evolution in the plastid genome of seven Ligusticum species provides insights into species discrimination and phylogenetic implications. Sci. Rep. 2021, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopoeia Committee of Chinese. Pharmacopoeia of the People’s Republic of China; Medical Science Press: Beijing, China, 2020; Volume 1, p. 42. [Google Scholar]
- He, Y. Literature research and clinical application of Japanese Siwu Decoction. Foreign Med. Sci. Tradit. Chin. Med. 1994, 5, 1–4. [Google Scholar]
| No. | Herbal Name | Plant Species | Collection Area | Collection Year/Month |
|---|---|---|---|---|
| 1 | Chuanxiong | Ligusticum chuanxiong Hort. | Dujiangyan, Sichuan, China | 2020.05 |
| 2 | Chuanxiong | Ligusticum chuanxiong Hort. | Dujiangyan, Sichuan, China | 2020.05 |
| 3 | Chuanxiong | Ligusticum chuanxiong Hort. | Dujiangyan, Sichuan, China | 2021.06 |
| 4 | Chuanxiong | Ligusticum chuanxiong Hort. | Dujiangyan, Sichuan, China | 2021.06 |
| 5 | Chuanxiong | Ligusticum chuanxiong Hort. | Dujiangyan, Sichuan, China | 2021.06 |
| 6 | Chuanxiong | Ligusticum chuanxiong Hort. | Dujiangyan, Sichuan, China | 2021.06 |
| 7 | Chuanxiong | Ligusticum chuanxiong Hort. | Dujiangyan, Sichuan, China | 2021.06 |
| 8 | Chuanxiong | Ligusticum chuanxiong Hort. | Pengzhou, Sichuan, China | 2020.05 |
| 9 | Chuanxiong | Ligusticum chuanxiong Hort. | Pengzhou, Sichuan, China | 2020.05 |
| 10 | Chuanxiong | Ligusticum chuanxiong Hort. | Shifang, Sichuan, China | 2020.05 |
| 11 | Japanese Chuanxiong | Cnidium officinale Makino | Pengzhou, Sichuan, China | 2014.10 |
| 12 | Japanese Chuanxiong | Cnidium officinale Makino | Pengzhou, Sichuan, China | 2014.10 |
| 13 | Japanese Chuanxiong | Cnidium officinale Makino | Pengzhou, Sichuan, China | 2014.10 |
| 14 | Fuxiong | Ligusticum sinense ‘Fuxiong’ | Jiujiang, Jiangxi, China | 2021.07 |
| 15 | Fuxiong | Ligusticum sinense ‘Fuxiong’ | Jiujiang, Jiangxi, China | 2021.07 |
| 16 | Fuxiong | Ligusticum sinense ‘Fuxiong’ | Jiujiang, Jiangxi, China | 2021.07 |
| 17 | Fuxiong | Ligusticum sinense ‘Fuxiong’ | Jiujiang, Jiangxi, China | 2021.07 |
| 18 | Fuxiong | Ligusticum sinense ‘Fuxiong’ | Yushan, Hubei, China | 2021.10 |
| 19 | Fuxiong | Ligusticum sinense ‘Fuxiong’ | Yushan, Hubei, China | 2021.10 |
| 20 | Fuxiong | Ligusticum sinense ‘Fuxiong’ | Yushan, Hubei, China | 2021.10 |
| 21 | Jinxiong | Ligusticum sinense ‘Jinxiong’ | Tengchong, Yunnan, China | 2021.11 |
| 22 | Jinxiong | Ligusticum sinense ‘Jinxiong’ | Tengchong, Yunnan, China | 2021.11 |
| 23 | Jinxiong | Ligusticum sinense ‘Jinxiong’ | Tengchong, Yunnan, China | 2021.11 |
| No. | Component | Chuanxiong (ng/g, n = 10) | Japanese Chuanxiong (ng/g, n = 3) | Fuxiong (ng/g, n = 7) | Jinxiong (ng/g, n = 3) |
|---|---|---|---|---|---|
| Phthalides | |||||
| 1 | Ligustilide | 1047 ± 276.2 | 800.7 ± 1.332 | 1715 ± 386.7 | 1533 ± 25.30 |
| 2 | Senkyunolide A | 850.8 ± 416.8 | 529.5 ± 39.86 | 1069 ± 149.6 | 1007 ± 7.554 |
| 3 | Butylphthalide | 56.70 ± 14.00 | 21.72 ± 1.612 | 71.70 ± 32.29 | 77.90 ± 0.550 |
| 4 | 3-Butylidenephthalide | 25.41 ± 17.31 | 37.98 ± 6.219 | 312.2 ± 105.5 | 982.7 ± 6.463 |
| Terpenoids | |||||
| 5 | alpha-Pinene | 797.7 ± 333.7 | 1447 ± 68.84 | 762.6 ± 166.1 | 936.1 ± 10.88 |
| 6 | Limonene | 188.9 ± 107.8 | 193.8 ± 65.49 | 293.5 ± 85.97 | 1107 ± 28.13 |
| 7 | Linalool | 117.5 ± 56.84 | 277.9 ± 5.390 | 245.1 ± 41.35 | 246.1± 9.239 |
| 8 | beta-Pinene | 75.29 ± 30.93 | 252.4 ± 119.4 | 1008 ± 159.4 | 274.6 ± 2.615 |
| 9 | Verbenone | 9.919 ± 4.070 | + | 2.038 ± 1.698 | 26.13 ± 0.689 |
| 10 | Borneol | 4.472 ± 1.625 | 43.91 ± 2.016 | 66.94 ± 20.76 | 63.26 ± 1.479 |
| 11 | 2-Methylisoborneol | 4.405 ± 1.385 | 5.820 ± 0.734 | 23.11 ± 7.818 | 3.636 ± 0.287 |
| 12 | alpha-Terpineol | 3.200 ± 0.555 | 25.58 ± 3.900 | 28.03 ± 8.355 | 2.284 ± 0.085 |
| 13 | Verbenol | + | 21.78 ± 1.596 | 13.95 ± 4.250 | 22.63 ± 0.285 |
| 14 | Geraniol | + | 2.113 ± 0.257 | 2.592 ± 3.199 | 1.432 ± 0.123 |
| 15 | Camphor | + | 1.531 ± 0.027 | 1.763 ± 0.879 | 2.278 ± 0.207 |
| 16 | L-Menthol | + | + | 4.876 ± 2.253 | + |
| 17 | alpha-Ionone | + | + | + | + |
| 18 | Eucalyptol | + | - | - | - |
| 19 | beta-Ionone | - | - | + | + |
| Organic acids | |||||
| 20 | Ethylic acid | 250.7 ± 82.80 | 239.6 ± 23.76 | - | - |
| 21 | Butyric acid | 151.5 ± 66.49 | 285.0 ± 11.39 | 183.9 ± 74.33 | 79.83 ± 0.570 |
| 22 | Caproic acid | 42.53 ± 17.83 | 217.4 ± 5.023 | 25.12 ± 12.55 | 9.235 ± 0.206 |
| 23 | n-Valeric acid | 30.69 ± 19.30 | 130.2 ± 23.11 | 49.25 ± 18.41 | 12.97 ± 0.693 |
| 24 | Isovaleric acid | 21.78 ± 9.769 | 36.77 ± 2.373 | 68.37 ± 9.079 | 41.17 ± 0.948 |
| 25 | Phenylacetic acid | 6.612 ± 0.235 | 6.398 ± 0.010 | 6.613 ± 0.300 | 6.570 ± 0.103 |
| 26 | 2-Methyl butyric acid | 6.333 ± 2.966 | 16.15 ± 4.662 | 21.29 ± 5.477 | 7.130 ± 0.398 |
| 27 | Propionic acid | 6.281 ± 2.764 | 39.12 ± 1.442 | 36.31 ± 9.761 | 8.317 ± 0.217 |
| 28 | Enanthic acid | 5.437 ± 2.162 | + | + | + |
| 29 | Isobutyric acid | 4.645 ± 1.367 | 5.141 ± 0.627 | 14.37 ± 6.171 | 12.43 ± 1.100 |
| 30 | Pelargonic acid | 1.457 ± 0.521 | 3.758 ± 0.445 | 7.366 ± 3.543 | 11.52 ± 1.396 |
| 31 | Isocaproic acid | + | 2.235 ± 0.514 | 103.3 ± 53.46 | 63.95 ± 1.193 |
| 32 | Capric acid | - | + | 4.233 ± 2.612 | 1.918 ± 0.081 |
| 33 | Lauric acid | - | + | 1.354 ± 1.062 | 1.617 ± 0.002 |
| 34 | Caprylic acid | - | - | 4.347 ± 2.673 | 1.851 ± 0.036 |
| Ketones | |||||
| 35 | 2-Undecanone | 406.2 ± 289.9 | 1568 ± 8.220 | 529.0 ± 61.98 | 168.5 ± 1.170 |
| 36 | 2-Heptanone | 19.27 ± 10.21 | 12.70 ± 3.520 | 6.445 ± 4.461 | 16.56 ± 2.181 |
| 37 | 2-Nonanone | 6.463 ± 3.472 | 14.39 ± 2.878 | 10.68 ± 3.185 | 20.50 ± 1.459 |
| 38 | 5-Nonanone | 1.846 ± 0.827 | + | 14.45 ± 7.659 | 23.43 ± 2.776 |
| 39 | Benzophenone | + | 48.14 ± 6.421 | + | 45.11 ± 0.650 |
| 40 | Diacetyl | + | + | 30.14 ± 7.288 | 66.72 ± 0.650 |
| 41 | 5-Hexene-2-one | + | + | 2.686 ± 0.904 | + |
| 42 | Acetophenone | + | + | 2.032 ± 0.554 | 2.545 ± 0.061 |
| 43 | Isophorone | + | + | + | 1.237 ± 0.068 |
| 44 | Benzyl acetone | + | + | + | + |
| 45 | 3-Heptanone | + | + | + | + |
| 46 | Acetoin | - | - | - | 846.4 ± 20.81 |
| 47 | 2-Hexanone | - | - | - | + |
| Aromatics | |||||
| 48 | Toluene | 246.0 ± 80.09 | 248.6 ± 114.4 | 42.95 ± 11.01 | 72.04 ± 2.430 |
| 49 | alpha-Methylstyrene | 34.16 ± 17.42 | 21.51 ± 5.808 | 83.37 ± 18.19 | 178.2 ± 1.415 |
| 50 | Ethylbenzene | 14.08 ± 7.531 | + | + | 7.696 ± 0.489 |
| 51 | 1,2,4,5-Tetramethylbenzene | 5.916 ± 2.600 | 24.19 ± 4.405 | 8.317 ± 2.403 | 4.439 ± 0.293 |
| 52 | o-Xylene | 3.009 ± 1.458 | 14.98 ± 0.969 | 1.150 ± 0.210 | 2.757 ± 0.083 |
| 53 | Styrene | + | 23.90 ± 2.978 | 3.232 ± 1.075 | 2.273 ± 0.238 |
| 54 | p-Xylene | + | + | + | 1.350 ± 0.193 |
| 55 | 2-Methylnaphthalene | + | + | + | + |
| 56 | 1-Methylnaphthalene | + | + | + | + |
| 57 | m-Xylene | + | - | + | + |
| 58 | Naphthalene | - | + | + | + |
| Aldehydes | |||||
| 59 | trans-2-Heptenal | 146.3 ± 52.27 | 182.2 ± 37.88 | 63.32 ± 27.77 | 512.3 ± 9.237 |
| 60 | n-Dodecanal | 10.85 ± 3.367 | 27.02 ± 2.698 | 34.00 ± 13.17 | 306.6 ± 1.381 |
| 61 | 2-Nonenal | 7.093 ± 4.352 | 7.968 ± 2.153 | - | - |
| 62 | trans-2-Decenal | 6.883 ± 3.384 | 6.579 ± 0.213 | 20.63 ± 3.676 | 51.85 ± 0.582 |
| 63 | trans,trans-2,4-Nonadienal | 5.423 ± 1.750 | 5.095 ± 0.717 | 46.79 ± 9.876 | 43.97 ± 0.818 |
| 64 | Benzaldehyde | 4.888 ± 2.584 | 15.18 ± 4.482 | 28.63 ± 3.785 | + |
| 65 | Octanal | 2.893 ± 1.778 | 18.79 ± 2.964 | 20.86 ± 4.684 | - |
| 66 | n-Decanal | 2.059 ± 1.380 | 1.012 ± 0.200 | 2.310 ± 0.690 | 1.528 ± 0.207 |
| 67 | Phenylacetaldehyde | 1.288 ± 0.276 | + | 3.230 ± 1.539 | + |
| 68 | 4,5-Epoxy-(E)-2-decenal | 1.023 ± 0.494 | + | 2.454 ± 0.802 | 13.88 ± 0.520 |
| 69 | Salicylaldehyde | + | + | 6.367 ± 1.603 | 1.585 ± 0.178 |
| 70 | trans,trans-2,4-Heptadienal | + | + | + | + |
| 71 | Hexanal | - | 50.53 ± 27.65 | 37.20 ± 6.896 | 3.221 ± 0.116 |
| 72 | trans,trans-2,4-Decadienal | - | + | 1.007 ± 0.351 | + |
| Pyrazines | |||||
| 73 | 2-Ethylpyrazine | 101.6 ± 51.58 | 25.37 ± 2.615 | 232.6 ± 47.45 | 542.1 ± 15.60 |
| 74 | 2,3-Dimethylpyrazine | 1.902 ± 0.993 | 1.522 ± 0.097 | 7.433 ± 0.932 | 12.10 ± 0.216 |
| 75 | 2-Isopropyl-3-methoxypyrazine | + | + | + | + |
| 76 | 2-Isobutyl-3-methoxy pyrazine | + | + | + | + |
| 77 | 2-Methylpyrazine | + | + | 1.746 ± 0.336 | + |
| Alcohols | |||||
| 78 | 1-Octanol | 11.31 ± 6.357 | 76.55 ± 4.184 | 11.03 ± 2.921 | 183.3 ± 1.744 |
| 79 | Benzyl alcohol | 7.046 ± 3.049 | 17.83 ± 0.979 | 8.074 ± 4.790 | 1.643 ± 0.042 |
| 80 | 2-Nonanol | 2.438 ± 1.666 | 7.968 ± 0.604 | 15.61 ± 11.01 | 1.732 ± 0.213 |
| 81 | 1-Tetradecanol | 1.046 ± 0.357 | - | - | 14.77 ± 0.542 |
| 82 | 1-Dodecanol | + | + | 1.767 ± 0.770 | 32.34 ± 0.231 |
| 83 | 1-Undecanol | - | + | 2.050 ± 0.936 | + |
| 84 | 2-Phenylethanol | - | - | - | 3.252 ± 0.013 |
| 85 | 1-Pentanol | - | 3.119 ± 0.225 | - | - |
| 86 | 2-Ethyl-1-hexanol | - | - | 8.34 ± 8.039 | 3.244 ± 0.269 |
| Esters | |||||
| 87 | Ethyl acetate | 10.23 ± 6.942 | 3.195 ± 1.161 | 173.3 ± 34.61 | 173.2 ± 8.945 |
| 88 | n-Hexyl acetate | 1.997 ± 0.674 | 3.980 ± 0.342 | 1.129 ± 0.457 | 6.266 ± 0.156 |
| 89 | n-Butyl acetate | 1.795 ± 0.804 | + | 2.662 ± 1.808 | 9.478 ± 0.150 |
| 90 | 1-Methoxy-2-propyl acetate | + | 2.431 ± 0.699 | + | + |
| 91 | Methyl methacrylate | + | 1.669 ± 0.530 | + | 1.046 ± 0.087 |
| 92 | Ethyl sorbate | + | 1.012 ± 0.200 | 6.218 ± 1.155 | 37.61 ± 0.119 |
| 93 | Methyl salicylate | + | + | 3.396 ± 0.669 | 4.452 ± 0.257 |
| 94 | gamma-Octalactone | + | + | + | + |
| 95 | gamma-Decalactone | + | + | + | + |
| 96 | gamma-Dodecalactone | + | + | + | + |
| 97 | 2-Ethoxyethyl acetate | - | + | - | 1.173 ± 0.319 |
| 98 | Ethyl-2-methylbutyrate | - | + | - | + |
| 99 | sec-Butyl acetate | - | - | + | + |
| Phenols | |||||
| 100 | Methyleugenol | 3.275 ± 0.763 | 3.541 ± 1.465 | 6.622 ± 2.278 | 238.3 ± 0.411 |
| 101 | Vanillin | 2.017 ± 1.097 | 11.34 ± 4.201 | 3.995 ± 1.234 | 3.310 ± 0.239 |
| 102 | Eugenol | + | 4.435 ± 0.347 | + | 22.38 ± 0.365 |
| 103 | 6-Chloro-o-cresol | + | + | 3.312 ± 1.158 | 8.473 ± 0.384 |
| 104 | m-Cresol | + | + | + | 1199 ± 78.04 |
| 105 | p-Cresol | + | + | + | 432.2 ± 9.016 |
| 106 | p-Ethylphenol | + | + | + | 5.326 ± 0.217 |
| 107 | o-Cresol | + | + | + | 7.189 ± 0.176 |
| 108 | 2,3-Xylenol | + | + | + | + |
| 109 | p-Ethylguaiacol | + | + | + | + |
| 110 | p-Propylphenol | + | + | + | + |
| 111 | Phenol | - | + | + | 3.692 ± 0.017 |
| 112 | Geosmin | - | + | + | + |
| 113 | Guaiacol | - | - | - | + |
| Pyridines | |||||
| 114 | 5-Ethyl-2-methylpyridine | 2.487 ± 1.609 | 4.651 ± 1.421 | 5.920 ± 1.676 | 7.609 ± 0.236 |
| 115 | 3-Ethyl-4-methylpyridine | + | + | 6.877 ± 0.885 | + |
| 116 | 2-n-Propylpyridine | + | + | 1.075 ± 0.289 | 1.112 ± 0.013 |
| Ethers | |||||
| 117 | Diethyl disulfide | 1.207 ± 0.789 | 1.271 ± 0.203 | 10.63 ± 3.640 | 56.00 ± 2.100 |
| 118 | Dimethyl disulfide | + | 1.432 ± 0.299 | + | 1.150 ± 0.463 |
| 119 | Butyl cellosolve | + | + | + | 3.208 ± 0.011 |
| 120 | Dimethyl trisulfide | - | + | + | + |
| 121 | 2,4,6-Trichloroanisole | - | - | + | + |
| 122 | 2-Phenoxyethanol | - | - | - | 4.546 ± 0.323 |
| Others | |||||
| 123 | 5-Methyl furfural | + | + | 3.012 ± 0.813 | + |
| 124 | p-Dichlorobenzene | + | + | + | + |
| 125 | Caprolactam | + | + | + | + |
| 126 | Coumarin | + | + | + | + |
| 127 | Skatole | + | + | + | + |
| 128 | Benzothiazole | + | + | + | - |
| No. | Chuanxiong | Japanese Chuanxiong | Fuxiong | Jinxiong | ||||
|---|---|---|---|---|---|---|---|---|
| Compound | Ratio (%) | Compound | Ratio (%) | Compound | Ratio (%) | Compound | Ratio (%) | |
| 1 | Ligustilide | 22.5 | 2-Undecanone | 22.0 | Ligustilide | 22.4 | Ligustilide | 12.8 |
| 2 | Senkyunolide A | 17.9 | alpha-Pinene | 20.3 | Senkyunolide A | 13.3 | m-Cresol | 10.0 |
| 3 | alpha-Pinene | 16.6 | Ligustilide | 11.2 | beta-Pinene | 13.2 | Limonene | 9.2 |
| 4 | 2-Undecanone | 8.1 | Senkyunolide A | 7.4 | alpha-Pinene | 10.0 | Senkyunolide A | 8.4 |
| 5 | Ethylic acid | 5.2 | Butyric acid | 4.0 | 2-Undecanone | 7.0 | 3-Butylidenephthalide | 8.2 |
| 6 | Toluene | 5.1 | Linalool | 3.9 | 3-Butylidenephthalide | 4.1 | alpha-Pinene | 7.8 |
| 7 | Limonene | 3.7 | beta-Pinene | 3.5 | Limonene | 3.8 | Acetoin | 7.1 |
| 8 | Butyric acid | 3.1 | Ethylic acid | 3.5 | Linalool | 3.3 | 2-Ethylpyrazine | 4.5 |
| 9 | trans-2-Heptenal | 3.0 | Toluene | 3.4 | 2-Ethylpyrazine | 3.1 | trans-2-Heptenal | 4.3 |
| 10 | Linalool | 2.4 | Caproic acid | 3.1 | Butyric acid | 2.5 | p-Cresol | 3.6 |
| No. | Chemical Classes | Chuanxiong | Japanese Chuanxiong | Fuxiong | Jinxiong | ||||
|---|---|---|---|---|---|---|---|---|---|
| Compound Number | Content Percentage | Compound Number | Content Percentage | Compound Number | Content Percentage | Compound Number | Content Percentage | ||
| 1 | Phthalides | 4 | 42.17 | 4 | 16.51 | 4 | 40.58 | 4 | 30.07 |
| 2 | Terpenoids | 15 | 24.84 | 13 | 33.14 | 14 | 32.24 | 14 | 22.44 |
| 3 | Organic acids | 12 | 10.96 | 14 | 14.33 | 14 | 7.03 | 14 | 2.16 |
| 4 | Ketones | 11 | 8.71 | 11 | 23.98 | 11 | 7.89 | 13 | 9.96 |
| 5 | Aromatics | 10 | 6.27 | 10 | 4.86 | 11 | 1.86 | 11 | 2.26 |
| 6 | Aldehydes | 12 | 3.90 | 14 | 4.73 | 14 | 3.62 | 13 | 7.84 |
| 7 | Pyrazines | 5 | 2.09 | 5 | 0.40 | 5 | 3.18 | 4 | 4.64 |
| 8 | Alcohols | 5 | 0.46 | 6 | 1.40 | 5 | 0.50 | 7 | 2.00 |
| 9 | Esters | 10 | 0.32 | 12 | 0.22 | 11 | 2.50 | 13 | 1.96 |
| 10 | Phenols | 11 | 0.14 | 13 | 0.30 | 13 | 0.21 | 14 | 16.04 |
| 11 | Pyridines | 3 | 0.06 | 3 | 0.08 | 3 | 0.19 | 3 | 0.08 |
| 12 | Ethers | 3 | 0.04 | 4 | 0.04 | 5 | 0.15 | 6 | 0.54 |
| 13 | Others | 6 | 0.03 | 6 | 0.01 | 6 | 0.05 | 4 | 0.003 |
| Total | 106 | 100 | 115 | 100 | 116 | 100 | 120 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Chen, J.; Li, W.; Song, S.; Li, X.; Yu, H.; Long, F.; Chen, R.; Bao, X.; Chan, K.; et al. Comparison of Volatile Compositions among Four Related Ligusticum chuanxiong Herbs by HS-SPME-GC-MS. Processes 2023, 11, 196. https://doi.org/10.3390/pr11010196
Huang S, Chen J, Li W, Song S, Li X, Yu H, Long F, Chen R, Bao X, Chan K, et al. Comparison of Volatile Compositions among Four Related Ligusticum chuanxiong Herbs by HS-SPME-GC-MS. Processes. 2023; 11(1):196. https://doi.org/10.3390/pr11010196
Chicago/Turabian StyleHuang, Shiwei, Jiamei Chen, Wan Li, Shanghong Song, Xiaoxue Li, Han Yu, Fei Long, Rong Chen, Xiaoming Bao, Kelvin Chan, and et al. 2023. "Comparison of Volatile Compositions among Four Related Ligusticum chuanxiong Herbs by HS-SPME-GC-MS" Processes 11, no. 1: 196. https://doi.org/10.3390/pr11010196
APA StyleHuang, S., Chen, J., Li, W., Song, S., Li, X., Yu, H., Long, F., Chen, R., Bao, X., Chan, K., & Lu, G. (2023). Comparison of Volatile Compositions among Four Related Ligusticum chuanxiong Herbs by HS-SPME-GC-MS. Processes, 11(1), 196. https://doi.org/10.3390/pr11010196









