Abstract
ABTS (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid) is a widely used compound for determining the total antioxidant capacity (TAC) of plant extracts, food, clinical fluids, etc. This photometric assay is based on the reduction by the presence of antioxidant compounds of a well-known metastable radical () which can be formed via several different approaches and be used in many different determination methodologies such as automated photometric measures in microplates, clinical robots, valuable titrations, and previous liquid chromatographic separation. Another interesting aspect is that, in some cases, the ABTS/TAC method permits sequential hydrophilic and lipophilic antioxidant activity determinations, obtaining total antioxidant activity values through the summatory data of both types of antioxidants. In this work, we present a review of several aspects of the ABTS/TAC, highlighting the major achievements that have made this method so widely used, e.g., ABTS radical formation in hydrophilic or lipophilic reaction media, measurement strategies, automatization, and adaptation to high-throughput systems, as well as the pros and cons. Moreover, some recent examples of ABTS/TAC method applications in plant, human, and animal samples are discussed.
1. Introduction
One of the most common analyses carried out on plant extracts, food, clinical fluids, and other sources is that of antioxidant capacity. The 2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid, ABTS) method (ABTS/TAC or ABTS/TEAC) is one of the most widely used in different research areas such as food science technology, agriculture, plant science, and nutrition. Its widespread use in many aspects of research has resulted in an increasing in the numbers of citation over the years (Figure 1).
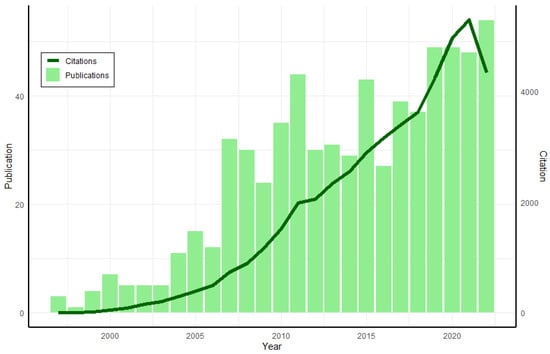
Figure 1.
ABTS/TAC method publications and citations (1997–2022). Source: Web of Science, search: ABTS AND TEAC (all fields).
ABTS was initially used in the detection of fecal occult blood [1] and as a reagent in the determination of glucose [2] in a glucose oxidase/peroxidase assay. ABTS was also used in the determination of peroxidase activity and by kinetic studies [3,4,5,6]. The reaction product of ABTS is a radical cation of the oxidized form of ABTS (), which was later used in the determination of the antioxidant capacity of biological samples.
The ABTS/TAC assay is a spectrophotometric method that uses the oxidized ABTS radical cation () to react with antioxidants to reduce the ABTS radical and lose its bluish green color. has several characteristics that make it suitable for colorimetric assays; it has several absorbance peaks at different wavelengths [3,7], it has a high extinction coefficient, its solubility in water is high, and it is also soluble in organic media [8]. The redox potential () for ABTS/ is 0.68 V [9], high enough to react with most antioxidant compounds [10,11]. There are two main mechanisms involved in the reaction between radicals and antioxidants, the hydrogen atom transfer reaction (HAT) involving a single-step movement of a hydrogen atom [12,13,14], and the electron transfer reaction (SET) where a single electron is transferred to reduce a compound [13,14,15]. The ABTS assay mainly follows the SET mechanism, although HAT can also be applied [12].
The first widely recognized use of as a reactive to measure the antioxidant capacity of a sample or pure compound was by Miller et al. [16] using the reaction of myoglobin/H2O2 to produce the bluish green , although there were some previously published procedures from other authors (see below). Subsequently, the method was modified in certain aspects, such as the form of generation and the parameter to estimate [7].
In this study, we review several aspects of the ABTS/TAC method, from ABTS radical generation to the different strategies used to determine photometric measurements. The major achievements that have led to this method being so widely applied, such as the possibilities of ABTS radical generation in hydrophilic or lipophilic reaction media, the possible automatization and adaptation to a high-throughput system, and several recent examples in different biological materials are discussed. Lastly, the pros and cons of these methods are analyzed.
2. Different Strategies for the ABTS/TAC Method
The generation of can be achieved via different strategies, but enzymatic and chemical methods are the most widely used (Figure 2). Miller et al. [16] used the myoglobin/H2O2 system to oxidize ABTS; this procedure encloses many steps and is laborious to perform. Previously, the ABTS radical () was generated using a H2O2/peroxidase (HRP) system for the determination of the flavonoid naringin [17] and indole-3-carbinol (indole-3-methanol) [18,19], but the method was not presented until 1996 by Arnao et al. [20]. Here, was generated using a faster, easier, and a more controllable method, whereby the reaction medium is controlled by the H2O2 quantities present due to the well-known stoichiometry of the reaction (2 mol per mol H2O2) [3,6]. Table 1 shows a chronological perspective of the ABTS/TAC method and its different approaches.
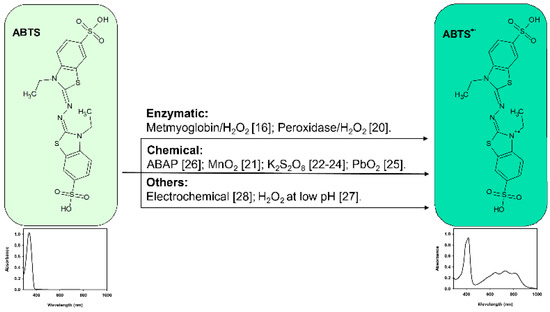
Figure 2.
Generation strategies [16,20,21,22,23,24,25,27,28].

Table 1.
Milestones in the development of the ABTS/TAC method.
Subsequently, different chemical oxidative reagents have been used for the ABTS radical generation such as MnO2 [21], potassium persulfate [22,23,24], PbO2 [25], 2,2’-azobis-(2-amidopropane) (ABAP) [26], or H2O2 at low pH [27]. Other generation methods include electrochemical oxidation [28,29] and peroxidase-like nanozyme [30,31].
generation is the first step in this antioxidant assay, which can be achieved either in the presence of the antioxidant or before antioxidant addition. These different ways of generating the lead to two different ways to quantify the antioxidant capacity: (i) kinetic and (ii) end-point approach [7]. In the ABTS method of Miller et al. [16], a kinetic approach was used, where the inhibition of the reaction due to the presence of the antioxidant is measured after a fixed time. Arnao et al. [17,20] also used this approach to determine the lag-time but recorded the delay in the appearance of the steady-state generation of . Thus, not only was the antioxidant capacity of the samples determined, but the possible inhibitory effects on the reaction by some compounds present in the samples were also evaluated [20].
In order to simplify the kinetic strategy measurements, several end-point methods were developed, in which media with preformed were used [17,18,19]. In this case, the subsequent addition of the sample containing antioxidants produced a decoloration (bleaching) of the [22,38,39]. This approach results in the method easiest to perform, eliminating the interaction of the antioxidant with the reactant enzymes, and making it suitable for high-throughput analysis. Although the end-point strategy has solved some of the problems observed in kinetic assays, new difficulties have arisen, such as the different reaction speeds of the different antioxidants present in the samples with , obtaining fast- and slow-reacting antioxidant types [40,41]. Recently, some novel and practical procedures to determine the antioxidant capacity have been proposed to improve ABTS/TAC determination through the estimation of exponential curve-fitting [24], inhibition percentage [27], or redox titration [25].
3. ABTS/TAC Methods for Hydrophilic and Lipophilic Antioxidants
Initially, the ABTS/TAC assay was developed for hydrophilic determination, estimating hydrophilic antioxidants such as organic acids, amino acids, glutathione, and phenols, among other antioxidants that can be solubilized in water. In this situation, fat-soluble antioxidants would apparently be dispersed in the aqueous medium, in a manner in which they could not be accurately measured. The development of a lipophilic method for determining these fat-soluble antioxidants was proposed by Miller [21] to measure the antioxidant capacity of carotenoids, using an ABTS radical generated in aqueous medium after dissolving carotenoid samples in hexane/acetone. Using the ability of horseradish peroxidase (HRP) to act in organic media, Cano et al. [8] proposed a modified ABTS/TAC method to generate directly in organic media, where different organic solvents were tested for peroxidase reactivity and stability.
The importance of obtaining a method which was capable of measuring both antioxidant capacities (hydrophilic and lipophilic) using the same reactive chromogen led to the possibility of the determination of the total antioxidant activity (TAA) as a combination of hydrophilic antioxidant activity (HAA) and lipophilic antioxidant activity (LAA) (TAA = HAA + LAA) (Figure 3). This was demonstrated in our studies in different plant material such as lettuce [42], tomato [43], grapes [44], citrus fruits [45,46], spinach [47], lupin [48], cereals [49], artichoke [50], Quercus tree [51], and chamomiles [52], as well as in foodstuffs such as vegetable soups [33], wine [53,54], and beers [38], and in animal material such as rat [55,56,57], canine [58], and human plasma [59], in addition to kidney, liver, and brain organs [56,60], and boar seminal samples [61]. Table 2 shows some of the obtained values using the ABTS/TAC method for different products, with a relative classification according to their antioxidant capacity.
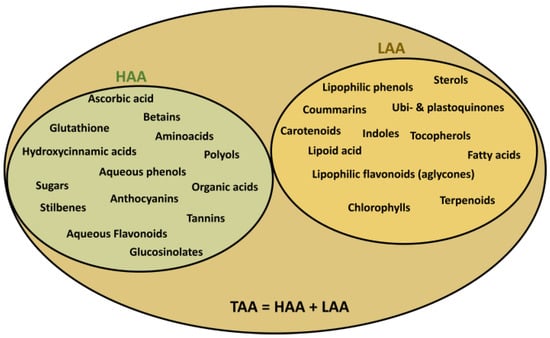
Figure 3.
Total antioxidant activity (TAA) as summation of the hydrophilic (HAA) and lipophilic (LAA) activities, as well as their components.

Table 2.
High, moderate, and low TAC ranking of different products according to published values.
4. Adaptation of the ABTS Assay to Different Techniques
The increase in the use of the ABTS/TAC assay for determining the antioxidant capacity and the rise in studies on the relationship between structure compound and antioxidant capacity led to the adaption of the different methods discussed for use in high-throughput systems. Adaptations have been made for several techniques such as microplate readers, high-performance liquid chromatography (HPLC), flow-injection assay (FIA), stopped-flow techniques, and automated analysis equipment (Figure 4).
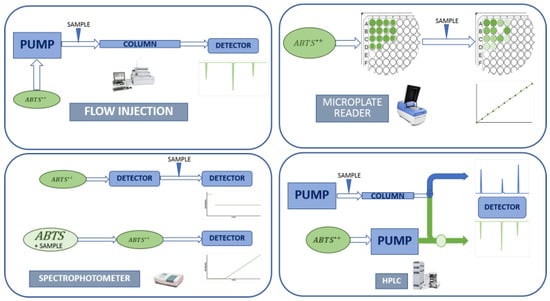
Figure 4.
Adaptation of ABTS/TAC method to different high-throughput systems.
A microplate adaptation was developed by Laigth et al. in 1999 [32] for measuring antioxidant activity in rat plasma. The microplate assay was adapted to the preformed ABTS radical strategy [8,33] or in-well microplate ABTS radical generation [69]. Cano et al. [8] adapted the lipophilic ABTS/H2O2/HRP end-point method to a microplate reader for determining the antioxidant capacity of lipophilic α-tocopherol and β-carotene.
The ABTS/H2O2/HRP generation method was also adapted to an HPLC system, in a post-column ABTS radical reaction [70,71], and applied to both hydrophilic and lipophilic antioxidants. In this case, a better understanding of the peak separate compounds with antioxidant capacity was obtained, while quantifying the compound content and its respective hydro- or lipophilic antioxidant activity.
Recently, two new interesting adaptations have been developed, one using a paper device as support for the antioxidant determination using ABTS assay together with the Folin–Ciocâlteu and CUPRA assays [37], and the other using a peroxidase-like nanozyme to generate [30], measuring the level of TAC with a portable device, while promoting a large-scale measurement of antioxidant activity using a smartphone [31].
5. Recent Applications of the ABTS/TAC Assay
Since the first use of this assay for determining the antioxidant capacity (see Table 1) it has become one of the most used methods, only surpassed by the DPPH method [45]. Many research fields have used the ABTS/TAC method in their studies, such as food technology, agriculture, plant science, nutrition, and clinical [7]. Some of the latest studies in different fields of research are discussed below.
In a study carried out in dogs, different ABTS/TAC methods including the method of Miller et al. with metmyoglobin [16], the method of generated at low pH [27], and the method of Arnao et al. with peroxidase [38] were compared in terms of total antioxidant capacity determinations in the canine serum of healthy and inflammatory bowel disease-affected dogs [58]. The three ABTS/TAC methods assayed showed acceptable results, with an imprecision of less than 15%, but only the H2O2/peroxidase assay showed significant differences between the two dog samples, along with a higher correlation between observed and expected antioxidant activities in the canine sample dilution assay. After validation of the methods for their use in canine serum, the authors recommended metmyoglobin and peroxidase methods for ABTS radical generation [58].
A study on gestational diabetes mellitus in rats used the ABTS/TAC assay of Arnao et al. [38] for determining the hydrophilic antioxidant capacity in serum samples, describing a direct correlation between treatment and an increase in HAA, confirmed by a low oxidative marker [57,59].
The ABTS/TAC assay was used in the characterization and comparison of wild chamomile plants [52], using the hydrophilic and lipophilic determination of the peroxidase method [8]. In this study, differences between different studied chamomile plants in root, stem, leaf, and flower extracts were described, with relevant differences in HAA and LAA depending on the organ and the chamomile species [52]. Furthermore, an excellent correlation between the antioxidant capacities (HAA, LAA, and TAA) and the phenol and flavonoid content (aqueous, organic, and total) was found.
In another interesting study, the antioxidant activity of six byproducts from artichoke industrial processing was analyzed and compared [50]. These biowastes are very rich in phytochemicals with potential health benefits to humans and animals [72]. The content of phenols and flavonoids, and the antioxidant capacities, amongst other parameters, were analyzed, with selective thermal treatment in artichoke hearts and bracts; the most promising byproducts were found closest to the artichoke heart. In this type of study, the ABTS/TAC method was demonstrated to be a fast and reliable technique, in terms of the processing line, the antioxidant properties of food, and its byproducts.
In the last few years, some reviews on antioxidant activity assays have been published, where some controversies and limitations of different antioxidant assay approaches were discussed [7,13,73,74]. Certain problems in the use of persulfate for ABTS oxidation, and difficulties in the kinetic approach for antioxidants with multiple hydroxyl groups have been described [13]. Apak et al. provided a list of desirable characteristics for an ideal antioxidant assay [13,14], most of which are accomplished by the ABTS/TAC assay using peroxidase. These characteristics include physiological pH (the ABTS assay can be run at different pH), stable and reproducible probes (the ABTS radical is a metastable radical with a peroxidative controlled reaction), activity against aqueous and organic antioxidants (the ABTS assay is compatible with both aqueous (HAA) and lipophilic (LAA) antioxidants), absorption in the visible spectrophotometric region, preferably beyond 500–700 nm to avoid interference from chlorophylls and anthocyanins ( has various spectral maxima at 414, 734, and 800 nm in the visible region), and optimal redox potential ( is able to oxidize the most important antioxidants) [13,14].
The important role of antioxidants in the response to oxidative stress in biological material and, therefore, in the prevention of many diseases and dysfunctions has increased the search for new antioxidant compounds, as well as the evaluation of their antioxidant activity in vitro. Obviously, these assays must be contrasted with other in vivo antioxidant studies. For example, some studies comparing the in vitro and in vivo antioxidant activities between different antioxidant assays were recently presented, highlighting the priority to determine the in vivo effect of antioxidants and its correlation with the in vitro antioxidant activity in interesting nutrients [75].
6. Conclusions
Despite some disadvantages observed using the ABTS/TAC method, which are shared with the commonly used DPPH method (not a natural physiological radical, large size, and slow vs. fast antioxidant reaction) [45], this method is still useful. The ABTS/TAC method is fast, with minimal processing, it is extremely versatile against different pH, different wavelengths can be selected to avoid spectrophotometric interferences, it can be used both for hydrophilic and lipophilic measurement, and it is easily adaptable to high-throughput methods (microplate, HPLC, etc.), thereby satisfying almost all requirements for an ideal antioxidant assay.
Author Contributions
A.C., J.H.-R., and A.B.M. contributed to the planning of the main ideas and visualization; A.C. was responsible first draft manuscript; A.B.M., A.C., and M.B.A. were responsible for the revision and final version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded through the project of the Ministry of Science and Innovation “R+D+I Projects”, the State Program for the Generation of Knowledge and Scientific and Technological Strengthening of the R+D+I System and R+D+I Oriented to the Challenges of Society of the State Plan for Scientific and Technical Research and Innovation 2017–2020, Grant PID2020-113029RB-I00 funded by MCIN/AEI/10.13039/501100011033. More information can be found at https://www.um.es/en/web/phytohormones/ (accessed on 30 December 2022; Phytohormones and Plant Development Lab).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deadman, N. Detection of faecal occult blood using ABTS as reagent. Clin. Chim. Acta 1973, 48, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Majkić, N.; Djordjević, S.; Berkeš, I. Eine kinetische methode zur bestimmung von “aeroben transhydrogenases”. Clin. Chim. Acta 1975, 65, 227–233. [Google Scholar] [CrossRef]
- Childs, R.E.; Bardsley, W.G. The steady-state kinetics of peroxidase with 2,2’-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem. J. 1975, 145, 93–103. [Google Scholar] [CrossRef]
- Del Río, J.A.; Acosta, M.; Bravo, J.S.; Arnao, M.B.; Sabater, F. Control del catabolismo auxínico. Inactivación de peroxidasas. In Metabolismo y Modo de Acción de Fitohormonas; Universidad de León: León, Spain; Servicio de Publicaciones: León, Spain, 1988; pp. 10–14. ISBN 84-7719-120-4. [Google Scholar]
- Acosta, M.; Arnao, M.B.; del Río, J.A.; Casas, J.L.; Sánchez-Bravo, J.; García-Cánovas, F. Inactivation of Peroxidase: Its Role in Plant Senescence. In Plant Aging: Basic and Applied Approaches; Rodríguez, R., Tamés, R.S., Durzan, D.J., Eds.; NATO ASI Series; Springer US: Boston, MA, USA, 1990; pp. 417–421. ISBN 978-1-4684-5760-5. [Google Scholar]
- Arnao, M.; Acosta, M.; del Rio, J.A.; Garcia-Canovas, F. Inactivation of peroxidase by hydrogen peroxide and its protection by a reductant agent. Biochim. Biophys. Acta (BBA) Prot. Struct. Mol. Enz. 1990, 1038, 85–89. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.B. ABTS/TEAC (2,2′-Azino-Bis-(3-Ethylbenzothiazoline-6-Sulfonic Acid)/Trolox®-Equivalent Antioxidant Capacity) Radical Scavenging Mixed-Mode Assay. In Measurement of Antioxidant Activity & Capacity; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 117–139. ISBN 978-1-119-13538-8. [Google Scholar]
- Cano, A.; Acosta, M.; Arnao, M.B. A method to measure antioxidant activity in organic media: Application to lipophilic vitamins. Redox Rep. 2000, 5, 365–370. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Walker, R.B.; Everette, J.D. Comparative Reaction Rates of Various Antioxidants with ABTS Radical Cation. J. Agric. Food Chem. 2009, 57, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2011, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Casas, J.L.; del Río, J.A.; Acosta, M.; García-Cánovas, F. An enzymatic colorimetric method for measuring naringin using 2,2’-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) in the presence of peroxidase. Anal. Biochem. 1990, 185, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.D. Study of the Decarboxylative Route of Indole-3-Acetic Acid. Stability and Reactivity of Indole-3-Carbinol. Licentiate Thesis, Faculty of Biology, University of Murcia, Murcia, Spain, 1992. [Google Scholar]
- Arnao, M.B.; Sanchez-Bravo, J.; Acosta, M. Indole-3-carbinol as a scavenger of free radicals. Biochem. Mol. Biol. Int. 1996, 39, 1125–1134. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J.; García-Cánovas, F.; Acosta, M. Inhibition by L-ascorbic acid and other antioxidants of the 2,2’-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) oxidation catalyzed by peroxidase. A new approach for determining total antioxidant status of foods. Anal. Biochem. 1996, 236, 255–261. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2′-azino-bis-(3-ethylenebenzothiazoline-6-sulfonic acid radical cation decolorization assay. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 379–389. [Google Scholar]
- Arts, M.J.T.J.; Dallinga, J.S.; Voss, H.-P.; Haenen, G.R.M.M.; Bast, A. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. 2004, 88, 567–570. [Google Scholar] [CrossRef]
- Geletii, Y.V.; Balavoine, G.G.A.; Efimov, O.N.; Kulikova, V.S. The Determination of Total Concentration and Activity of Antioxidants in Foodstuffs. Russ. J. Bioorganic Chem. 2002, 28, 501–514. [Google Scholar] [CrossRef]
- Campos, A.M.; Lissi, E.A. Evaluation of the antioxidant capacity of herbal teas by a procedure based on the bleaching of ABTS radical cations. Bol. Soc. Chil. Quim. 1995, 40, 375–381. [Google Scholar]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.M.; Domínguez, C.; Guillén, D.A.; Barroso, C.G. Determination of antioxidant power of red and white wines by a new electrochemical method and tis correlation with polyphenolic content. J. Agric. Food Chem. 2002, 50, 3112–3115. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.M.; Guillén, D.A.; Barroso, C.G. Development of an electrochemical method for the determination of antioxidant activity. Application to grape-derived products. Eur. Food Res. Technol. 2003, 216, 445–448. [Google Scholar] [CrossRef]
- Liang, Y.; Li, R.; Sun, H.; Dan, J.; Su, Z.; Kang, Y.; Zhang, Q.; Shi, S.; Wang, J.; Zhang, W. Functionalized natural melanin nanoparticle mimics natural peroxidase for total antioxidant capacity determination. Sens. Actuators B Chem. 2022, 359, 131541. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Li, X.; Liu, R.; Sang, Y.; Wang, X.; Wang, S. Nanozyme-enabled sensing strategies for determining the total antioxidant capacity of food samples. Food Chem. 2022, 384, 132412. [Google Scholar] [CrossRef]
- Laight, D.W.; Gunnarsson, P.; Kaw, A.V.; Änggård, E.E.; Carrier, M.J. Physiological microassay of plasma total antioxidant status in a model of endothelial dysfunction in the rat following experimental oxidant stress in vivo. Environ. Toxicol. Pharmacol. 1999, 7, 27–31. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Koleva, I.I.; Niederländer, H.A.G.; van Beek, T.A. Application of ABTS radical cation for selective on-line detection of radical scavengers in HPLC eluates. Anal. Chem. 2001, 73, 3373–3381. [Google Scholar] [CrossRef]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-Ring Chemistry and Antioxidant Activity: Fast Reaction Kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Pellegrini, N.; Del Rio, D.; Colombi, B.; Bianchi, M.; Brighenti, F. Application of the 2,2‘-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) Radical Cation Assay to a Flow Injection System for the Evaluation of Antioxidant Activity of Some Pure Compounds and Beverages. J. Agric. Food Chem. 2002, 51, 260–264. [Google Scholar] [CrossRef]
- Puangbanlang, C.; Sirivibulkovit, K.; Nacapricha, D.; Sameenoi, Y. A paper-based device for simultaneous determination of antioxidant activity and total phenolic content in food samples. Talanta 2019, 198, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. Methods to Measure the Antioxidant Activity in Plant Material. A Comparative Discussion. Free. Radic. Res. 1999, 31, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.; Haenen, G.R.M.M.; van den Berg, H.; Bast, A.A.L.T. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Tian, X.; Schaich, K.M. Effects of Molecular Structure on Kinetics and Dynamics of the Trolox Equivalent Antioxidant Capacity Assay with ABTS+•. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A. Three ABTS•+ radical cation-based approaches for the evaluation of antioxidant activity: Fast- and slow-reacting antioxidant behavior. Chem. Pap. 2018, 72, 1917–1925. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.B. Hydrophilic and Lipophilic Antioxidant Activity in Different Leaves of Three Lettuce Varieties. Int. J. Food Prop. 2005, 8, 521–528. [Google Scholar] [CrossRef]
- Cano, A.; Acosta, M.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activity changes during on-vine ripening of tomatoes (Lycopersicon esculentum Mill.). Postharvest Biol. Technol. 2003, 28, 59–65. [Google Scholar] [CrossRef]
- Alcolea, J.F.; Cano, A.; Acosta, M.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activities of grapes. Food/Nahrung 2002, 46, 353–356. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Cano, A.; Arnao, M.B. Actividad Antioxidante Hidrofílica y Lipofílica y Contenido En Vitamina C De Zumos De Naranja Comerciales: Relación Con Sus Características Organolépticas Lipophilic and Hydrophilic Antioxidant Activity and Vitamin C Content of Commercial Orange Juices: Correlation with Organoleptic Parameters Actividade Antioxidante Hidrofílica E Lipofílica E Contido En Vitamina C De Zumos De Laranxa Comerciais: Relación Coas Características Organolépticas. Cienc. Tecnol. Aliment. 2004, 4, 185–189. [Google Scholar]
- Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Hydrophilic and Lipophilic Antioxidant Activity in Spinach Leaves (Spinacia oleracea L.); UMH: Orihuela, Spain, 2003; Volume 1, pp. 233–236. [Google Scholar]
- Cano, A.; Artés, F.; Arnao, M.B.; Sánchez-Bravo, J.; Acosta, M. Inhibition of Etiolated Lupin Hypocotyl Growth and Rooting by Peroxides, Ascorbate and Glutathione. J. Plant Physiol. 1996, 147, 721–728. [Google Scholar] [CrossRef]
- Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Changes in hydrophilic antioxidant activity in Avena sativa and Triticum aestivum leaves of different age during de-etiolation and high-light treatment. J. Plant Res. 2006, 119, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.Á.; Castillo, J.; Zamora, S. Chemical and functional properties of the different by-products of artichoke (Cynara scolymus L.) from industrial canning processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef]
- El Omari, B.; Fleck, I.; Aranda, X.; Abadía, A.; Cano, A.; Arnao, M.B. Total antioxidant activity in Quercus ilex resprouts after fire. Plant Physiol. Biochem. 2003, 41, 41–47. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Castillo, M.E.C.; Cano, A.; Hernández-Ruiz, J.; Lamarti, A.; Arnao, M.B. Comparative study of wild chamomile plants from the north-west of Morocco: Bioactive components and total antioxidant activity. J. Med. Plants Res. 2021, 5, 431–441. [Google Scholar]
- Alcolea, J.F.; Cano, A.; Acosta, M.; Arnao, M.B. Antioxidant Qualities of the Fruit and the Wine of the Variety of a Cabernet-Sauvignon; Instituto Canario de Investigaciones Agrarias: Tenerife, Spain, 2000; pp. 229–232. [Google Scholar]
- Alcolea, J.; Cano, A.; Acosta, M.; Arnao, M.B. Determination of the hydrophilic and lipophilic antioxidant activity of white- and red wines during the wine-making process. Ital. J. Food Sci. 2003, 15, 207–214. [Google Scholar]
- Plaza, F.; Arnao, M.B.; Zamora, S.; Madrid, J.; Rol de Lama, M. Validación de un microensayo con ABTS+ para cuantificar la contribución de la melatonina al estatus antioxidante total del plasma de rata. Nutr. Hosp. 2001, 16, 202. [Google Scholar]
- Espinosa, C.; López-Jiménez, J.A.; Pérez-Llamas, F.; Guardiola, F.A.; Esteban, M.A.; Arnao, M.B.; Zamora, S. Long-term intake of white tea prevents oxidative damage caused by adriamycin in kidney of rats. J. Sci. Food Agric. 2015, 96, 3079–3087. [Google Scholar] [CrossRef]
- Gázquez, A.; Rodríguez, F.; Sánchez-Campillo, M.; Martínez-Gascón, L.E.; Arnao, M.B.; Saura-Garre, P.; Albaladejo-Otón, M.D.; Larqué, E. Adiponectin agonist treatment in diabetic pregnant rats. J. Endocrinol. 2021, 251, 1–13. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Arnao, M.B.; Cerón, J.J. Validation of three automated assays for total antioxidant capacity determination in canine serum samples. J. Veter. Diagn. Investig. 2016, 28, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Gázquez, A.; Sánchez-Campillo, M.; Rodriguez, F.; Arnao, M.B.; Larqué, E. Effect of Adiponectin Agonist Administration during Pregnancy on Oxidative and Inflammatory Status in the Adult Offspring of Diabetic Rats; Karger: Basel, Switzerland, 2020; Volume 76, p. 69. [Google Scholar]
- Espinosa, C.; López-Jiménez, J.A.; Cabrera, L.; Larqué, E.; Almajano, M.P.; Arnao, M.B.; Zamora, S.; Pérez-Llamas, F. Protective effect of white tea extract against acute oxidative injury caused by adriamycin in different tissues. Food Chem. 2012, 134, 1780–1785. [Google Scholar] [CrossRef]
- Hernández, M.; Cano, A.; Arnao, M.B.; Lucas, X.; Vázquez, J.; Martinez, E.; Roca, J. Antioxidant Capacity of Boar Seminal Plasma. Reprod. Fertil. Dev. 2005, 17, 283. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Barreiros, L.; Reis, S.; Segundo, M.A. Kinetic matching approach applied to ABTS assay for high-throughput determination of total antioxidant capacity of food products. J. Food Compos. Anal. 2014, 33, 187–194. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Campos, A.M.; Lissi, E.A. Total antioxidant potential of Chilean wines. Nutr. Res. 1996, 16, 385–389. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M.M. Total antioxidant activity in plant material and its interest in food technology. Recent Research Development Series. Agric. Food Chem. 1998, 2, 893–905. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.A. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink. Food Chem. 1997, 60, 331–337. [Google Scholar] [CrossRef]
- Queirós, R.B.; Tafulo, P.A.R.; Sales, M.G.F. Assessing and Comparing the Total Antioxidant Capacity of Commercial Beverages: Application to Beers, Wines, Waters and Soft Drinks Using TRAP, TEAC and FRAP Methods. Comb. Chem. High Throughput Screen. 2013, 16, 22–31. [Google Scholar] [CrossRef]
- Kambayashi, Y.; Binh, N.T.; Asakura, H.W.; Hibino, Y.; Hitomi, Y.; Nakamura, H.; Ogino, K. Efficient Assay for Total Antioxidant Capacity in Human Plasma Using a 96-Well Microplate. J. Clin. Biochem. Nutr. 2009, 44, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Alcaraz, O.; Acosta, M.; Arnao, M.B. On-line antioxidant activity determination: Comparison of hydrophilic and lipophilic antioxidant activity using the ABTS•+ assay. Redox Rep. 2002, 7, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Acosta, M.; Cano, A. Antioxidant Activity: An Adaptation for Measurement by HPLC. In Encyclopedia of Chromatography; CRC Press: New York, NY, USA, 2005; pp. 105–110. [Google Scholar] [CrossRef]
- Zayed, A.; Farag, M.A. Valorization, extraction optimization and technology advancements of artichoke biowastes: Food and non-food applications. LWT 2020, 132, 109883. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Prenzler, P.D.; Ryan, D.; Robards, K. (Eds.) Handbook of Antioxidant Methodology: Approaches to activity Determination; Food Chemistry, Function and Analysis No28; Royal Society of Chemistry: Cambridge, UK, 2021; ISBN 978-1-83916-155-1. [Google Scholar]
- Mota, J.C.; Almeida, P.P.; Freitas, M.Q.; Stockler-Pinto, M.B.; Guimarães, J.T. Far from being a simple question: The complexity between in vitro and in vivo responses from nutrients and bioactive compounds with antioxidant potential. Food Chem. 2023, 402, 134351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).