Abstract
Thallium (I) was removed from aqueous solution by using gamma-alumina nanoparticles (γANPs) materials as nano adsorbents. Varied experimental conditions such as adsorbent dose, agitation time, initial concentration, pH, and temperature effects were carried out in batch conditions in view of the optimization of thallium (I) adsorption and the identification of the adsorption mechanisms in the system γANPs-Tl. The pH effect indicated a remarkable increase in the quantity of Tl(I) removed for pH values ranging from 4 to 8, an almost constant magnitude for pH values between 8 and 10, and a decrease for pH values above 10. Considering an initial Tl(I) concentration of 20 µg/L and an adsorbent dose of 1 g/L at a pH value of 8.5, the removal was achieved at 95.12 ± 0.02% efficiency. The pseudo-second-order kinetics and the Freundlich isotherm perfectly described the adsorption mechanism. The process of thallium (I) adsorption reaction, as highlighted by thermodynamic investigations, was found to be spontaneous and exothermic with coexistence of physisorption and chemisorption with a dominance of physisorption. The diffusion model predicted multi-linearity, suggesting an involvement of surface spread and intraparticle diffusion in the sorption process. Thallium removal was effective by using γANPs as nano adsorbents.
1. Introduction
As a typical rare element in the nature, thallium (Tl) is well known for being widely spread at a very low concentration level [1,2,3]. It does not belong to the group of heavy metals which are essential to living organisms. Because of its high toxicity, it has attracted more and more attention [4,5,6,7]. The toxicity of thallium (I) is higher than those of Hg, Cd, Pb and Cu [8]. Its acute toxicity to humans brings about vomiting, diarrhea, temporary loss of hair; affects the nervous system, lungs, heart, liver, and kidneys; and can even lead to death [9]. Epidemiological data showed that exposure to Tl during pregnancy may also have negative effects on birth outcomes, such as fetal death, congenital malformations, or decreased birth weight [10,11]. An exposure to Tl concentration levels in the range of 0.1 to 100 μg/L may be considered chronic [12,13,14]. As a result, the World Health Organization describes thallium and its compounds as major hazardous wastes [15].
Many countries have established stringent criteria for Tl concentration in water/wastewater in order to mitigate the risk of diseases due to thallium exposure through aqueous medium. Examples of criteria can be found in references [6,7]. In the United States, the maximum Tl level in drinking water and wastewater discharge has been set at 2 and 140 μg/L, respectively, by the USEPA [6,16,17,18,19]. In China, the Tl concentration limit has been lowered to 0.1 μg/L and 2 μg/L for drinking water and industrial wastewater, respectively, in some provinces [6,7]. Yet, thallium is unregulated in most countries, including Burkina Faso.
Thallium found in the environment comes from two sources: natural sources and anthropogenic sources. Thallium concentrations in natural waters are highly dependent on the parent rock with which the water interacts [5,20]. The two oxidation states of thallium that are usually present in aquatic environment are Tl(I) and Tl(III). Since both forms are considered as highly toxic, their exposure to living organisms may lead to health hazard [6]. In Burkina Faso, thallium concentrations ranging from 0.0732 µg/L to 3.94 µg/L have been detected in ground waters in a village named Yamtenga [21]. Our first investigations on thallium contamination in these ground waters led to the conclusion that the thallium source is naturally linked to the source rock by water—rock interaction mechanisms [22]. These ground waters were therefore considered potential sources of environmental pollution in thallium and of exposure for humans. Since thallium is not a conventional pollutant, its presence in the ground waters paved the way for the necessity of developing appropriate methods, in our context, for the removal of trace amounts of thallium to prevent its pollution to humans and the environment. Tl(I) is very mobile and generally occurs in stable compounds in natural waters, it appears to be very difficult to remove with conventional methods. As a result, more investigations are needed for Tl(I) removal in groundwater [4,23].
Different reliable methods—such as adsorption [24,25], chemical precipitation/coagulation [26,27,28], ion exchange [29,30], and solvent extraction [31,32]—were used for the removal of Tl(I) removal from aqueous solutions. Chemical precipitation/coagulation is extensively used in industry because of its efficiency in removing Tl pollutants. However, this method is unsuitable for a use at low Tl concentrations because it may generate toxic sludge. There are advantages in using ion exchange methods, which are indeed their low maintenance cost and good performance. These advantages make them the most extensively used methods for wastewater treatment. However, large-scale applications were found to be limited by fouling problems. Solvent extraction is generally linked to high efficiency and selectivity, but it is not appropriate for any composition of the solution. In addition, the large amounts of organic waste produced during the treatment process limited the scope of application [6,24]. Because of their high cost, the sophisticated technology which is involved, and the risks of toxicity in some circumstances, all of the above techniques may appear to be difficult to implement in developing countries. In contrast, thallium removal which occurs through adsorption process onto appropriate adsorbents is strongly preferred because of its simplicity of use, its cost effectiveness, ease of operation, and high efficiency. Moreover, the process could be applied to a great quantity of water and could be implemented in rural environments. Whereas many efforts are focusing on the development of adsorption processes, there is still a need for finding effective, inexpensive, and efficient adsorbents materials.
A number of adsorbents—such as carbon materials [33,34], mineral materials [35,36], biomass materials [37], Prussian blue and analogues [38,39], manganese oxides [40,41,42,43,44], and titanium-based materials [45,46,47]—have been the subject of a lot of investigations for Tl(I) removal from aqueous solutions. In general, most of these adsorbents have insufficient porosity and surface area which limit their effectiveness in the adsorption process (Table S1 in “Supplementary Material SI”). The attributes that determine the adsorption efficiency for most of the adsorbents—porosity, pore size and specific surface area, and the removal of pollutants at trace levels—constitute a major challenge [48,49,50]. As a matter of fact, the removal of trace pollutants by adsorption requires adsorbents with large functional groups and a large specific surface area. This has therefore sparked a renewed interest in nanotechnology for this category of sorbents.
To overcome the limitations of traditional adsorbents, new generations of ad-sorbents (nano adsorbents) for water treatment system have been introduced [51,52]. As opposed to traditional adsorbents, nano adsorbents show exceptional characteristics such as a large specific surface area, good conductivity and selectivity. They are chemically reactive and optically active. Moreover, they show interesting catalytic and magnetic properties. A large surface provides a large number of active sites for the attachment of pollutants to the nano adsorbent. These properties make them more adsorbent and attractive compared to conventional materials [53,54,55,56]. Literature results reported high adsorption capacities onto nanomaterials due to their large specific surface areas, their surface reactivity and their mobility in solution [57]. Zero-valence metal nanoparticles, metal oxide nanoparticles, carbon nanotubes (CNTs), and nanocomposites belong to the most widely investigated nanomaterials for water and wastewater treatment.
Nanoscale metal oxides such as alumina nanoparticles are well-known adsorbents used for inorganic pollutants removal. They exist in several forms [58], among which gamma-alumina nanoparticles were found to be more promising materials as solid-phase adsorbents [59,60]. The effectiveness of gamma-alumina nanoparticles in the adsorption process is attributed to a combination of favorable textural properties: a proper distribution of pores size, a high specific surface area and surface chemical properties that can be either acidic or basic [61]. Gamma-alumina nanoparticles, obtained from commercial routes, have been used to investigate the removal of several metal ions, such as Ni(II), Zn(II), Cu(II) and Cd(II), and Tl(III) as well [62,63,64,65,66]. It is worth noting that nowadays, research efforts are currently focusing on the production of gamma-alumina nanoparticles with controlled porosity at the nanometer scale, the objective being to obtain materials with suitable properties for the elimination of pollutants up to the trace state. Recently, in an attempt to boost the additional value of natural local bauxites, they were used as precursors in the synthesis of low-cost gamma-alumina nanoparticles (γANPs) [67]. The γANPs were subsequently functionalized with CTAB and the physicochemical properties of the CTAB-functionalized γANPs (γANPs-CTAB) were investigated in view of bisphenol-A removal [67]. Indeed, the quest for simple, low-cost, and high-performance water purification processes in developing countries requires the use of local materials that are natural materials, good sorbents, inexpensive, and represent a viable replacement for high-cost starting materials and toxic precursors that are involved in synthesis processes. In our context, large deposits of bauxite ores are widely available in the Center-North region of Burkina Faso. Bauxite is a raw material which is well known as the best source of aluminum for the synthesis of nano gamma-alumina powder [67]. In this regard, specific attention has been drawn to the low-cost synthesis of gamma-alumina nanoparticles for pollutants removal from aqueous solutions [67]. However, to the best of our knowledge, gamma-alumina nanoparticles—synthesized from natural local bauxites—have not yet been used for the interaction with thallium (I) ions. To date, few studies have reported adsorption behavior and removal efficiency of thallium (I) at low concentration. As a matter of fact, most of the investigations were carried out at high thallium (I) concentration ranging from 1–150 mg.L−1, whereas thallium is found to be in the range of a few μg.L−1 in real polluted water sources [11,16,28]. In this paper, the removal of Tl(I) ions at low concentration, through adsorption onto synthesized gamma-alumina nanoparticles, was investigated for the first time.
The objective of the current paper was to investigate the removal of thallium (I) from aqueous solutions by adsorption onto γANPs, a special emphasis being put on the effects of several parameters—such as adsorbent dosage, contact time (kinetics), initial concentration (isotherms), pH, and temperature—on the adsorption process.
2. Materials and Methods
2.1. Materials
A local natural bauxite (BA) was used as precursor in the synthesis of γ-alumina nanoparticles (γANPs). The BA material was collected in Foulou village whose geographical localization is the following: center-north region of Burkina Faso; coordinates: 13°13′35,0′′ N and 01°32′56,7′′ W. The γ-alumina nanoparticle materials were synthesized by utilizing the following chemicals: hydrochloric acid (HCl, 37% extra pure, Loba Chemie, and Ahmedabad, India) and ethanol (99.8%, Fluka, Darmstadt, Germany). The N-cetyl-N, N, N, trimethylammonium bromide (CTAB), as surfactant, was purchased from Sigma-Aldrich (≥99% purity, Saint-Louis (Missouri)). The bromide ions were tested using silver nitrate (AgNO3 ≥ 99.8% purity, Sigma-Aldrich).
The purchase of analytical grade sodium hydroxide pellets (NaOH ≥ 99% purity) and hydrophilic PTFE was made from VWR International and Sigma Aldrich, respectively. All experiments were carried out using milli-Q water which was produced by the Milli-Q (Millipore Corporation, New York, NY, USA) water purification system.
2.2. Methods
2.2.1. Synthesis and Characterization of Gamma-Alumina Nanoparticles (γ-ANPs)
The sol–gel process, adapted from previous works by Dani Gustaman Syarif et al., 2015 and P. Manivasakan et al., 2009 [68,69], was carried out for the synthesis of the γ-ANPs using local bauxite. The procedure involved a stirring of the following mixture for 2 h: a quantity of finely ground bauxite, sodium hydroxide and water. After decantation, the red mud precipitate was separated from the filtrate by filtration. The fairly clear suspension was then cooled. A CTAB solution was added to the filtrate to minimize agglomerations and improve the textural properties of the synthesized alumina nanoparticles. By addition of HCl, the filtrate was precipitated. The precipitate was filtered off and washed extensively with milli-Q water and then with ethanol to remove impurities. Subsequently, the precipitate was heated to 200 °C to form boehmite (AlOOH) which was calcined at 900 °C for 4 h to give alumina nanoparticles. Figure 1 represents the visual appearance of the synthesized γ-ANPs.

Figure 1.
Appearance of γ-ANPs.
ICP (ICP-AES-IRIS Intrepid II XSP model) was carried out to achieve elementary chemical analysis. The BET analysis, using the BELSORP MAX instrument running with the Bel Japan Inc. software, made it possible to determine the specific surface area and pore volume of the γANPs samples. The morphological characterization of the γANPs samples was achieved by running scanning electron microscopy (SEM HITACHI SU8020 computer-controlled with the software EDX SDD, Thermo Scientific, Mons, Belgium). To characterize the adsorbent materials γANPs, X-ray powder diffraction (XRPD) was carried out on a D8 Advance Davinci Bruker X-ray generator diffractometer (working at 40 mA generator current and 40 kV generator voltage with Cu-Kα radiation (λ = 1.54060 Å)). The XRPD data were recorded at a scan speed of 0.02° s−1 and in the 2θ angles values ranging from 5 to 70°. Functional groups on the γANPs samples were identified by using Fourier-transform infrared (FTIR) spectroscopy on a Shimadzu FTIR-8400S spectrometer.
2.2.2. Determination of pHZPC of the Adsorbent (γANPs)
The zero-point charge of the adsorbent (pHZPC) was determined by the solid addition method [60,70]. We used a mixture of 50 mL of 0.01 M NaCl and 200 mg of alumina nanoparticles in different flasks. We adjusted the pH of the different flasks, respectively, in the range 2.0–12.0 by adding 0.1 mol. L−1 of HCl or NaOH solutions. The flasks were then agitated at 500 rpm for 24 h and the pH of the solutions was measured by a pH meter.
2.2.3. Determination of Thallium (I) Ions Concentration
Thallium (I) ions determination was carried out by differential pulse anodic stripping voltammetry (DPASV) analysis. The experimental set-up comprises a potentiostat 910 PSTAT (Metrohm), a stand containing a measuring cell in which are placed: a working electrode with a rotating disc electrode (EDI 101), the electrode being a wax impregnated carbon paste electrode (diameter 3 mm) modified with a mercury film; a silver chloride Ag/AgCl reference electrode, saturated KCl (potential 208 mV at 25 °C compared to the normal hydrogen electrode ENH); a platinum auxiliary electrode; and an argon supply for solution bubbling. The potentiostat is controlled by a computer with the software PSTAT for the execution of the commands as well as the data acquisition.
All solutions were prepared with milli-Q water. Standard solutions were prepared from mercury nitrate (purity > 99.99%, Merck, Darmstadt), thallium nitrate (purity > 99.995%, Sigma Aldrich, Saint-Louis (Missouri)) dissolved in 0.1 mol.L−1 Hydrochloric acid HCl (trace analysis, Certipur, Merck, Darmstadt) which was also used as the supporting electrolyte. A certified standard of thallium 1 g.L−1 (Certipur, Merck, Darmstadt) was also used for the preparation of standard solutions. The solution was bubbled through a stream of pure argon.
The working electrode used for the determination of thallium is a thin film of mercury deposited on carbon paste electrode (CPE) impregnated with wax. This deposition is achieved using a mercuric salt solution prepared from Hg(NO3)2. Differential pulse anodic redissolution voltammetry (DPASV) was used for the quantitative determination of Tl(I) ions. The supporting electrolyte used was a 0.1 mol.L−1 hydrochloric acid HCl solution. For optimization studies, 19.9 mL of standard solution prepared in the supporting electrolyte and 100 µL of 10−3 mol.L−1 Hg2+ ions were introduced into the electrochemical cell. For the analyses of Tl(I) concentration before and after adsorption, we used 19.9 mL of supporting electrolyte, 20 mL of water sample and 100 µL of 10−3 mol.L−1 Hg2+ ions. Then, bubbling with argon is carried out for approximately 10 min and the voltammogram is recorded by varying the potential from the most negative to the most positive. This procedure is repeated after adding a small quantity of the desired ion. The standard addition method is used to measure the concentration of thallium.
The thallium concentrations determined by DPASV at the mercury film modified wax impregnated CPE modified were statistically confirmed with ICP measurements of the same solutions, and we found that the results were validated at 98.7%.
2.3. Batch Adsorption Investigations of Thallium (I)
At different pH values and various initial Tl(I) concentrations, the adsorption experiments of thallium (I) onto γANPs were conducted by agitating the Tl(I) solution with γANPs (adsorbent dosage varying from 0.05 g to 0.4 g). The experiments were carried out at different contact times varying from 0 to 120 min, under a defined constant stirring speed. All batch experiences were carried out in triplicate at room temperature.
The concentration of thallium (I) before and after adsorption was determined using the DPASV at a mercury film modified wax impregnated carbon paste electrode. After agitation in all cases, the solution was centrifuged at 4000 rpm and the suspension was filtered through 0.45 µm hydrophilic PTFE membrane filters. A volume of 20 mL was employed for the analyses.
The amount of the adsorbed thallium (µg/g) in all the experiments was calculated using the Equation (1):
Ci and Ce are initial and equilibrium concentrations of thallium (µg/L), respectively, V is the volume (mL), and M is the adsorbent mass (g).
The percentage of thallium (% Tl) removed is calculated using the Equation (2):
Ci and Ce are initial and equilibrium concentrations of Tl (µg/L), respectively.
The pH value was adjusted to a value of around 8.5 for all solutions used for Tl(I) adsorption experimentation.
To investigate the pH effect on the adsorption of Tl(I) on γANPs, the pH of a solution of 20 µg/L was adjusted at values ranging from 2 to 12 and further 100 mL of each prepared solution and 100 mg of γANPs were placed in a bottle of 150 mL and the prepared solutions were shaken for 2 h with a horizontal shaker.
To investigate the adsorbent dose effect, different amounts of γANPs (from 0.05 to 0.4 g) were added in 100 mL of 20 µg/L Tl(I) aqueous solution for 2 h.
The effect of the agitation time on adsorption was carried out by placing 100 mg of the adsorbent (γANPs) and 100 mL of a Tl(I) solution at concentration of 20 µg/L in a 150 mL bottle. The bottle was then stirred at a speed of 600 rpm for various contact times varying from 0 to 120 min at the ambient temperature.
Pseudo-first-order and pseudo-second-order models were used to investigate the adsorption kinetics of Tl(I) by γANPs (Equations (3) and (4) respectively) [71].
The graphical representation of ln () as a function of time is a straight line. Therefore, the pseudo-first-order model is confirmed only if the graphical representation of the experimental data lead to a straight line as expected by the theoretical expression, with a good coefficient of determination.
The graphical representation of t/Qe as a function of time is a straight line. Therefore, the pseudo-second-order model is confirmed only if the graphical representation of the experimental data lead to a straight line as expected by the theoretical expression, with a good coefficient of determination.
The Langmuir and Freundlich models were applied to fit the equilibrium data (Equations (5) and (6)). These isotherms are listed as follows [71]:
Langmuir isotherm model
where Qm (mg⁄g) is the Langmuir monolayer sorption capacity and KL (L⁄mg) is the Langmuir coefficient.
Freundlich isotherm model
where KF (mg/g) and n are Freundlich constants corresponding to the adsorption capacity and the adsorption intensity, respectively.
Intraparticle diffusion model
Pseudo-first-order and pseudo-second-order models are limited to furnish the diffusion mechanism which occur in certain circumstances. The intraparticle diffusion model was then introduced by Weber and Morris (1963) [72] to describe the rate determining step of the adsorption. In this model, the adsorbate removal is proportional to t1/2 rather than t. Equation 7 is employed to evaluate the intraparticle diffusion model.
Kdiff and C are the constant of intraparticle diffusion (mg/g.min1/2) and a constant for any experiment (mg/g), respectively. They are determined by plotting Qt versus t1/2. When the obtained curve is a straight line, the intraparticle diffusion intervenes in the process. The intraparticle diffusion is only considered to be the controlling step of the adsorption process when this line passes through the origin.
A desorption test with ultrapure water was carried out using the spent nano-alumina materials, which were obtained after adsorption of Tl(I) under the optimal conditions. This involved mixing 0.1 g of spent nanoalumina (or Tl(I)-loaded nanoalumina) with 0.1 L of ultrapure water which was then stirred for 24 h. The Tl(I)-loaded nanoalumina was dried before use. After 24 h, the solid phase was separated from the solution by filtration and the concentrations of Tl(I) in the test solution were measured. Then the quantities of Tl(I) adsorbed were calculated. The quantities of Tl(I) remaining on the surface of the gamma alumina nanoparticles were also calculated by taking the difference between the initial quantity of Tl(I) in the exhausted nanoalumina (19.024 µg/g of nanoalumina approximately) and the quantity of Tl(I) desorbed from the Tl(I)-loaded nanoalumina.
2.4. Temperature Effect
In general, the adsorption phenomenon is always accompanied by a thermal process [73,74] which can be either exothermic (ΔH < 0) or endothermic (ΔH > 0). The measurement of the isosteric heat of adsorption ΔHx is the main criterion for differentiating chemisorption from physisorption [75]. To evaluate the effect of temperature and thermodynamic parameters, the amounts of Tl(I) removed by γAl2O3 were evaluated at 303, 313, 323 and 333 K. The thermodynamic data obtained provide information on the mechanisms associated with the thallium adsorption by gamma alumina nanoparticles. The standard free energy ∆G0 of an adsorption process is related to the equilibrium constant by the classical Van’t Hoff equation:
where ΔG0 is the Gibb free energy change (kJ.mol−1), R is the ideal gas constant (8.314 J.mol−1.K−1), and T is the absolute temperature (K). The distribution coefficient Kd of adsorption is calculated as:
where C0 and Ce represent the initial concentration and the equilibrium concentration, respectively, of the solute (µg/L); V is the volume of the suspension (mg/L); m is the mass of the adsorbent (g).
ΔG0 = −RT.lnKd
The standard enthalpy (ΔH in kJ.mol−1) and standard entropy (ΔS in kJ·mol−1.K−1) variations can be calculated from the Equation (10):
The lnKd curve as a function of 1/T gives a portion of straight line with slope −ΔH/RT and ordinate at the origin ΔS/R.
The most relevant thermodynamic property describing the effects of heat during the adsorption process is the isosteric heat of sorption [76]. It is a useful parameter providing indications for the characterization and optimization of the adsorption process as well as for the surface heterogeneity of the adsorbent. The expression for isosteric heat of sorption is given below.
where K is a constant while Ce is the equilibrium concentration of Tl(I) in solution and the other parameters retain their usual meanings as previously defined. The isosteric heat of sorption ∆Hx was obtained from the slope of the plot of LnCe versus 1/T.
The procedure consisted of introducing 100 mL of the Tl(I) solution (20 µg/L) into a 200 mL Erlenmeyer flask with 100 mg of γAl2O3. The mixtures are subjected to sonication for 2 h using a BRANSONIC brand ultrasonic sonicator at temperatures of 303, 313, 323 and 333 K controlled by a HANNA Checktemp probe. Each supernatant is centrifuged and filtered with a 0.45 µm pore PTFE filter. The quantities of Tl(I) adsorbed are calculated using relation (3). The amounts of Tl(I), adsorbed by γAl2O3 at these temperatures, made it possible to evaluate thermodynamic parameters such as the free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) variations associated with the adsorption of Tl(I).
3. Results and Discussion
3.1. Characterization of Synthesized γANPs
The chemical composition of the synthesized gamma alumina nanoparticles is summarized in Table 1. The results show that the predominant oxides of the γANP samples include aluminum oxide, silicon oxide, and other oxides present in trace amounts. The ICP output values are summed to obtain the total chemical composition. Our results are in agreement with others data published in the literature [77,78].

Table 1.
Chemical composition of γANPs.
The specific surface area of γANPs was about 187 m2/g, while the pore volume was about 0.58 cm3/g and pore diameter about 9.8 nm. In general, specific surface area values greater than 125 m2/g indicate a good stability of the material [79,80]. This suggests that the synthesized γANPs are very stable.
Figure 2a shows the SEM image of the synthesized γANPs. The sample crystals reached a porous and spongy shape, and the crystal morphology showed regular pores and sufficient volume for surface reactions. SEM images of the synthesized nano-alumina show low bulk density and large pore size of the nanoparticles which can be explained by the evaporation of ethanol in the synthesis process [81]. The EDX spectrum in Figure 2b clearly shows the peak of Al and O as major constituents, which confirms the formation of alumina nanoparticles.
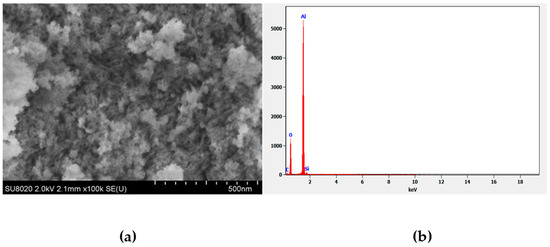
Figure 2.
(a) Scanning electron microscope (SEM) image; (b) EDX spectrum.
The XRPD patterns of the samples before and after calcination at 900 °C are shown in Figure 3. It can be seen that the main phase found in the non-calcined samples was boehmite (JCPDS 21-1307), which is transformed into γ-alumina (JCPDS Map 29-0063) after calcination. Similar profiles of γ-alumina were obtained by other authors. We can ob-serve three sharp peaks with high intensity at 2θ values of 37.8°, 45.7°, and 66.9°, respectively. These peaks indicate that the γANPs were synthesized with high crystallinity [82,83,84]. The crystallite sizes were also calculated using the Scherrer equation (Equation (10)):
where K is a constant generally taken as ~0.9, λ is the wavelength of the incident radiation, β is the full width of diffraction peak at half maximum intensity (FWHM), and θ is the diffraction angle. The calculated crystallite sizes were found to be in the range of 4.1 nm for nano size synthesized γ-alumina [81].
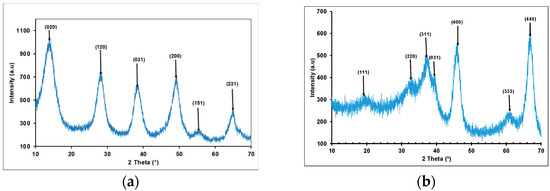
Figure 3.
(a) X-ray diffraction of boehmite; (b) X-ray of γANPs.
The assessment of the surface functional groups of the adsorbent material was car-ried out by FT-IR spectrum analysis (Figure 4). The FT-IR spectrum of the synthesized γANPs shows a broad band at 3457 cm−1 attributed to the stretch band of hydroxyl groups on the surface of alumina. Moreover, a weak band at 1641 cm−1 is associated with the stretching vibration band of the Al-OH bond, and a symmetrical Al-O-Al stretching vibration band seen at 520 cm−1 corresponds to the characteristic vibration of Al2O3. The same observations were obtained for γ-alumina in other studies [60,85,86,87].
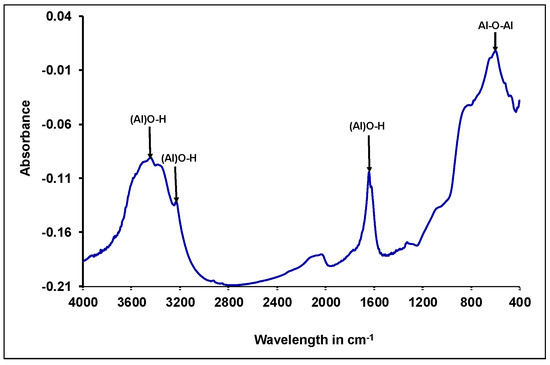
Figure 4.
FT-IR spectrum of γANPs.
Figure 5 shows the zero point charge (pHZPC) of the synthesized alumina nanoparticles. This figure indicates that the pHZPC value of synthesized γANPs is 8. This value is similar to that reported on other alumina nanoparticles used as adsorbents [60,88,89]. The γANPs synthesized in this study are positively charged for pH values below 8 and negatively charged for values above 8.
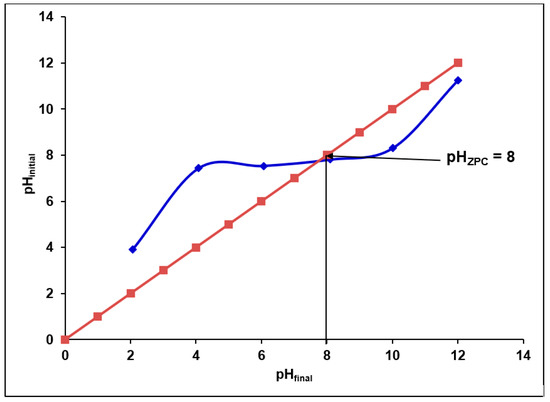
Figure 5.
Determination of the zero point charge (pHZPC) of synthesized alumina nanoparticles.
3.2. Batch Investigations of Thallium Adsorption onto γANPs
3.2.1. Effect of Initial pH
pH values ranging from 2 to 12 were selected to investigate the influence of the pH suspension on the optimum conditions for the adsorption process. According to the Pourbaix diagram of thallium in aqueous solution, Tl(I) is soluble as the species of Tl+ or Tl(OH)aq at a wide pH range (0–14) [34,42,90,91]. However, Tl+ is the species which is likely to be more easily adsorbed, through electrostatic attraction on a negatively charged surface. Figure 6 shows that the quantity of Tl(I) adsorbed by the alumina nanoparticles increases with the pH increase up to 8 and stays at a constant level when the pH value varies between 8 and 10. When the pH value is greater than 10, a decrease in the quantity of Tl(I) adsorbed is observed.
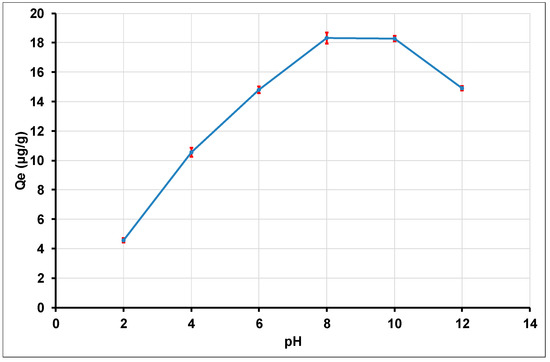
Figure 6.
Effect of initial pH on the adsorption of Tl(I) by γANPs.
Physical and chemical properties of alumina play an important role in understanding the adsorption behavior in terms of active sites involved in adsorption process. It is known that the active sites on alumina surface are reactive hydroxyl groups. The properties of the surface of alumina strongly depend on pH. Indeed, in acidic medium when the pH values are smaller than the pHzpc value of the investigated alumina nanoparticles, which is 8, the surface is positively charged (Figure 7). At a basic medium (pH > pHzpc), the surface is charged negatively (Figure 7).
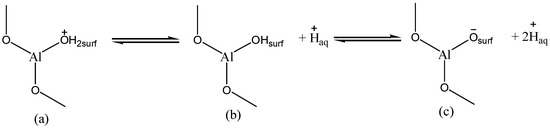
Figure 7.
Properties of γANPs.surface: (a) acidic pH, positive charge; (b) point of zero charge; (c) basic pH, negative charge.
From the literature, it is also known that the spatial distribution of the reactive hydroxyl groups is rather complex [92]. Characterizing alumina by fluorescence spectroscopy, Metivier, R. et al. (2003) demonstrated that the reactive hydroxyl groups are not homogenously distributed on alumina surface, but they are rather clustered into regions of high density [92]. In addition in our context, ICP results show that although the predominant oxide of the γANP samples is aluminum oxide, they also contain other alkali oxides present in trace amounts. As a result, the surface properties of the synthesized nano alumina will depend on reactive hydroxyl groups not homogeneously distributed as well as the impurities which are also present at the surface. The surface charge formation and the strong dependence of the properties on the pH are considered in the following discussion.
For pH values below the pHzpc value, the surface of the nano alumina carries a positive charge due to the surface protonation (Figure 7). In this context, attraction of Tl+ ions is not favored on the reactive sites. However, due to the complexity of the surface charge formation, Tl+ ions are likely to be adsorbed at a small extent on other sites present at the surface. Hence, by increasing the pH from 4 up to 8, the adsorption rate gradually increases with the increasing pH up to 8, due to these phenomena which are not exactly defined. When the pH values are greater than the pHzpc, the negative charge of alumina nanoparticles favor the sorption of Tl+, as a result of the electrostatic attraction which involves Tl+ ions and the negative surface sites of alumina nanoparticles. Thus, in the pH range from 8 to 10, a constant and significant adsorption of Tl+ on the surface of γ-alumina was noted, suggesting the predominance of Tl+ in this range of pH. The optimum pH for the removal of Tl(I) on γ-alumina is therefore in the pH range from 8 to 10. Similar results were reported by Pu, Y. et al. (2013) who investigated the removal of thallium (I) by multiwall carbon nanotubes [34]. In addition, for pH values smaller than 4, it is noted that the elimination of Tl+ ions from the aqueous medium gradually decreases, indicating that the removal of Tl+ by γAl2O3 is highly influenced in an acidic medium. As a matter of fact, for pH values smaller than 4 (pH < 4), the gamma-alumina nanoparticles dissolve and the consequence is a modification of the surface properties of the materials [93], which thus brings about a drastic reduction in the adsorption of γAl2O3. Xiaoliu, H. et al. (2015) reported similar results on the removal of thallium (I) by manganese dioxide nanoparticles, with aggregation of MnO2 and leaching of Mn under acidic conditions at pH 4.0 [42]. When the pH values are above 10, the adsorption capacity decreases. Indeed, at pH values higher than 10 (pH > 10), the aqueous solution is enriched with hydroxyl ions (OH−). This enrichment of the solution in hydroxyl leads to the conversion of part of the Tl+ ions in solution into TlOH(aq) [94]. According to Lin, T.S. et al. (1998) Tl+ is the dominant species of Tl(I) for pH < 11.7, and at pH > 11.7, Tl+ is converted into TlOH(aq) [94]. In our context, we found an adsorption decrease from the pH value of 10, with a Tl(I) concentration taken as 20 μg.L−1 (or 9.78.10−8 mol.L−1). In principle, the same observation could have been made here if the pH step variation was set smaller. However, the pH step variation was set at a value of 2 in the present experiment, which results in a lower pH value than 11.7. Further experiments are needed to assess the process in the pH range from 10 to 12. Indeed, when describing Tl species, the real difficulty is the diverse values which are used as for the hydrolysis constants of Tl(I) [6]. An accurate calculation which takes into account the concentration of Tl+ ions and the real part of Tl+ which is converted into TlOH(aq) will certainly lead to an exact pH value of conversion depending on the ratio log [Tl(OH)aq]/[Tl+] [SI]. The decrease in the adsorption of Tl+ on the gamma alumina is therefore justified by the coexistence of the Tl+ and Tl(OH)(aq) species in the solution where only the Tl+ species are likely to interact with the negatively charged gamma alumina surface.
3.2.2. Effect of the Agitation Time on Tl(I) Adsorption and Adsorption Kinetics
Effect of the Agitation Time on Tl(I) Adsorption
The effect of the agitation time on the adsorption process was studied to evaluate the quantity of Tl(I) which is adsorbed at different times (Figure 8). Assessing the equilibrium time is a key element in batch adsorption, since it represents an important economically factor for the treatment of polluted water systems [95]. The results obtained are plotted as Qe = f(t), Figure 8. The analysis of the curve indicates a rapid change in the adsorption rate of thallium by gamma alumina nanoparticles, which occurs during the first 30 min, and then a stagnation is observed over the rest of the process until saturation is reached. The fast adsorption kinetics which is observed during the early minutes can be interpreted as an easy access to a large number of active sites which are available on the material surface at the beginning of the adsorption process. For high contact times, Tl(I) needs more time to diffuse into pores of the adsorbent; the remainder of the non-adsorbed amount can be interpreted by saturation of the adsorbent. The constant level, observed after the first 30 min, represents the surface reaction stage, which is characterized by the binding of adsorbate particles due to interactions between adsorbate particles and adsorbent active sites. Thus, the Tl(I) removal by gamma alumina nanoparticles constitutes a rapid process along with an adsorption equilibrium which is reached during the first 30 min of the process. Figure 8 also indicates that the maximum amount of Tl(I) adsorbed by γAl2O3 was 19.05 ± 0.23 µg/g (about 95.26% removal rate). This rapid adsorption on the surface of alumina nanoparticles has also been described for previous investigations on various inorganic species, such as Cu (II), Zn (II) and Ni (II) [62,63]. Previous similar data, describing the adsorption kinetics of Tl(I) on sawdust, indicated that the adsorption equilibrium occurred at a maximum removal time of 7 min [96]. In addition, investigations carried out on multi-walled carbon nanotubes (MWCNT) indicated that the adsorption rate of Tl(I) was also rapid [34].
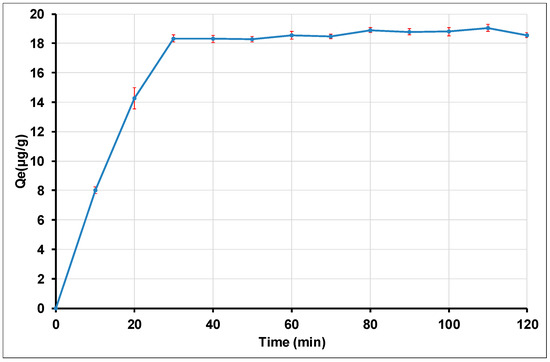
Figure 8.
Effect of agitation time of Tl(I) adsorption on γANPs.
Investigation of Adsorption Kinetics Using Linear and Quadratic Models
Adsorption kinetics was investigated through pseudo-first order and pseudo-second order models. The kinetic data of the adsorption of Tl+ by γAl2O3 were fitted using linear and quadratic models (Figure 9 and Figure 10).
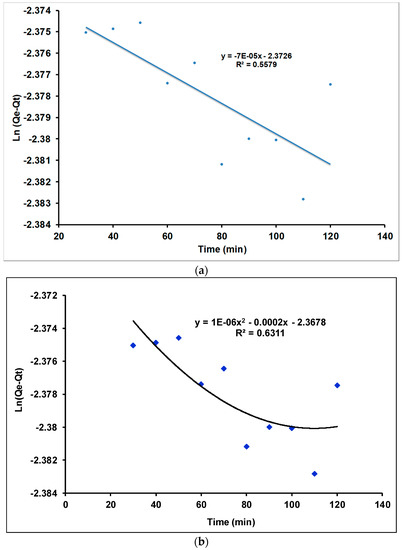
Figure 9.
Pseudo-first-order model of Tl(I) adsorption on the γANPs: (a) linear model and (b) quadratic model.
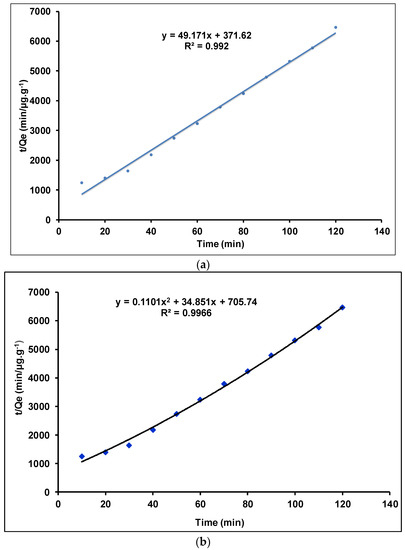
Figure 10.
Pseudo-second-order model of Tl(I) adsorption on the γANPs: (a) linear model and (b) quadratic model.
Figure 9 indicates that the coefficients of determination of both linear and quadratic models are very low, indicating a poor correlation between the ordinate (Ln (Qe − Qt) and the abscissa (time). As a result, the linear and quadratic models are not appropriate to describe the pseudo-first order model. Furthermore, with an R2 value equals to 0.5579 the linear pseudo-first-order model (R2 equals to 0.5579) cannot be considered for the fitting of the kinetic data of the present work.
Figure 10 shows that the coefficients of determination (R2) obtained with both linear and quadratic models are almost similar and very high, 0.992 and 0.9966, respectively, indicating a strong correlation between the ordinate (t/Qe) and the abscissa (time). Although both linear and quadratic models lead to high values of the coefficients of determination (R2), only the linear adjustment provides a straight line as expected by the theoretical expression (Equation (4)). Since the accuracy of the fit of an adsorption model to experimental data is usually assessed as a function of the magnitude of the coefficient of determination, the linear pseudo-second-order model with an R2 value being close to unity is retained in the present work for the description of the kinetic data.
Table 2 summarizes the experimental kinetic constants and determination coefficients as calculated using the linear adjustment for both pseudo-first- and pseudo-second- order models, for the elimination of Tl+ onto 0.1 g of γAl2O3 as adsorbent dose. As already stated, the experimental results are better fitted by the linear pseudo-second-order kinetic model (Figure 10a) than the linear pseudo-first-order model. This observation is assessed by the coefficient of determination of the pseudo-second-order kinetic model which is greater than 0.99 [97,98]. It is therefore suggested that chemisorption is involved in the adsorption process [97,98]. This result is similar to those reported for the adsorption of thallium (I) on the following adsorbents: multiwall carbon nanotubes [34], polyacriamide-aluminosilicate composites [97], and FeOOH-MnO2 nanocomposites [98]. Generally, the experimental data of the adsorption of Tl(I) on several adsorbents are better interpreted by the pseudo-second-order kinetic model [34,97,98]. Taking into account the work by Vithanage, M. et al. (2016) describing the fact that kinetic order is dependent on initial concentrations, it is suggested that the pseudo-second-order kinetic model is more adaptable to experimental data when polluting species are less abundant (low initial concentration) than the available adsorption sites [99]. In other words, adsorption kinetics of Tl+ at the γAl2O3/(Tl+ solution) interface could be expressed in terms of Tl(I) concentration.

Table 2.
Kinetic constants of Tl(I) removal on γANPs.
Intraparticle Diffusion of Thallium Adsorption by Gamma Nano Alumina
Generally, sorption processes have a complex nature, in which both surface sorption and intraparticle diffusion can occur. In order to determine the limiting step of the Tl(I) sorption process on nano aluminas, the experimental data were analyzed by applying the Weber–Morris method. This model can be used to determine whether external transport or intraparticle transport governs the rate of sorption processes.
Figure 11 represents the intraparticle diffusion modeling curve for gamma alumina nanoparticles for 100 mg as adsorbent dose. Analysis of the curve (Figure 11) shows that two linear parts are involved. The first part ranges from 10 min to 30 min and the second part from 30 min to 120 min. While the steeply rising first part defines initial rapid absorption by boundary layer effects, the plateau-like second part, which is associated with intraparticle diffusion, takes place after completion of outer surface coverage by the first process. This multilinearity of the curve suggests that external mass transfer and intraparticle diffusion are involved in different phases of the Tl sorption process on gamma alumina nanoparticles [100,101,102,103]. Senol, Z.M et al. (2010) [97] obtained similar results on the elimination of Tl(I) onto polyacryamide-aluminosilicate composites. The fact that the straight line is not passing through the origin indicates that the intraparticle diffusion could not be considered the limiting step, and that other processes are involved in the sorption of Tl by gamma-alumina nanoparticles [102,103]. As a matter of fact, the correlation coefficient (R2) of the intraparticle diffusion model is equal to 0.5662. This value suggests that the adsorption process of Tl(I) is more complex and that mechanisms other than intraparticle diffusion are involved in the sorption of Tl(I) on the studied gamma-alumina nanoparticles [102,104].
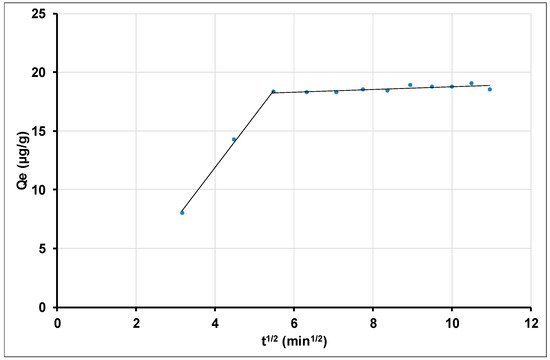
Figure 11.
Intra-particle diffusion modeling for the adsorption of Tl(I) on the gamma-alumina nanoparticles.
3.2.3. Effect of Adsorbent Dose
The adsorbent dose effect is illustrated in Figure 12. It is observed that the removal efficiency increases from 0% to 95.12 ± 0.02% with the increase in the adsorbent dose from 0 to 0.05 g. This increase in the percentage of adsorption observed as a function of the mass of the alumina nanoparticles is a consequence of the availability of the active adsorption sites for Tl+ ions. These active sites increase with the amount of adsorbent up to the mass of 0.05 g. At low adsorbent doses, Tl+ ions easily access the adsorption sites of alumina nanoparticles, resulting in a rapid increase in the amount adsorbed with the adsorbent mass [105]. Beyond 0.05 g as mass of adsorbent, the number of accessible free sites becomes stable. The percentage of adsorption thus remains constant up to 0.4 g of the adsorbent. Consequently, the adsorbent dose was set at a fixed value of 0.1 g, which was considered appropriate for the remainder of the investigations.
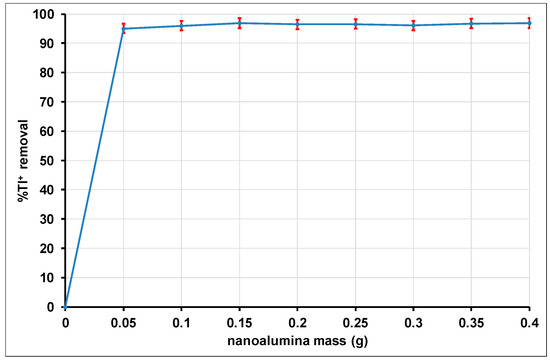
Figure 12.
Effect of the adsorbent dose on the adsorption of Tl(I).
3.2.4. Effect of Initial Concentration of Tl Solution and Isotherms
Effect of Initial Concentration of Tl(I) Solution
To study the effect of the initial concentration, a mass of 0.1 g of alumina nanoparticles is mixed with 100 mL of Tl+ solution with an initial concentration varying from 5 to 120 µg/L, and the residual concentration or equilibrium concentration (Ce) is determined in each case. The adsorption is carried out at pH 8.5 and at room temperature.
Figure 13 indicates the quantities of Tl(I) removed per gram of adsorbent (adsorption capacity Qe, µg/g) at different initial concentrations and for 120 min as the agitation time at 500 rpm. As illustrated in Figure 13, the adsorption capacity of alumina nanoparticles increases with the increase of the initial concentration of Tl(I) until the available active sites become saturated. These results indicate that Qe increases rapidly to reach the value of 30 µg/g, which corresponds to an initial concentration of 30 µg/L of Tl+. The curve Qe = f(Ce) follows two regimes: the first one is relatively considered a straight line up to 30 µg/g; the second one turns to a parabolic behavior afterward. Thus, the initial thallium concentration affects the adsorption of thallium on alumina nanoparticles. Indeed, below 30 µg/L as thallium (I) initial concentration, the alumina nanoparticles’ active sites are more available and this leads to a better adsorption. However, the gradual saturation of the active sites induces a less effective adsorption after 30 µg/L. In these investigations, when the initial thallium concentration increases from 5 to 120 µg/L, the adsorbed thallium (Qe) values increase from 3.94 ± 0.02 to 111.34 ± 0.05 µg/g. Similar results were reported by Pu, Y. et al. (2013) on the removal of thallium (I) on carbon nanotubes [34]. Given the toxicity of thallium and its low concentration in drinking water, a residual concentration above 2 µg/L still remains a hazard to consumers. An adsorbent capable of reducing the residual concentration below the USEPA recommended standard concentration (2 µg/L) is essential. Thus, the adsorbents required for this purpose must have a high affinity and a high charge capacity for Tl(I). In general, thallium is removed from aqueous media, but the residual concentration remains above the recommended standard concentration. Luo, Z. et al. (2022) removed 95.85% of thallium (I) from an aqueous solution using 800 µg.L−1 as the test solution and a montmorillonite biochar composite as adsorbent. The resulting residual concentration of Tl(I) was 33.2 µg.L−1 [106]. Zhang, G. et al. (2022) starting from a solution of 1.6 mg.L−1 of thallium (I), eliminated 93.44% and 98.25% of Tl(I) by using pyrolysis residue and low-grade pyrolusite, respectively. The residual concentrations obtained were 104.96 µg.L−1 and 17.5 µg.L−1 for pyrolysis residue and low-grade pyrolusite, respectively [18]. All of these adsorbent materials, which were reported in the literature, showed a high capacity to remove thallium species in an aqueous medium. However, the residual concentration which was above 2 µg/L remained an obstacle to the consumption of the purified water as drinking water. In our case, the exceptional performance of the gamma-alumina nanoparticles, synthesized from local bauxite, led to a deep purification of synthetic water contaminated with Tl(I), with a residual concentration of less than 2 µg/L at a pH of 8.5. Therefore, the investigated gamma-alumina nanoparticles synthesized from local bauxite could present a high potential as an adsorbent for the treatment of thallium-contaminated groundwater. A comparative table (Table 3) is given to take into account the comparison of the results in the present work with those obtained from similar previously conducted studies.
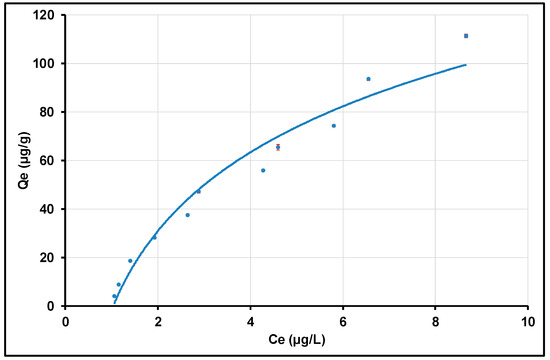
Figure 13.
Initial concentration effect on the Tl(I) adsorption by γANPs.

Table 3.
Comparison of the present results with those reported in literature.
From Table 3, it can be noted that the residual concentration of Tl(I) in the present study remains below the USEPA recommended standard concentration in comparison with those reported for other adsorbents.
Investigations of Adsorption Isotherms Using Linear and Quadratic Models
The relationship between Qe (amount of adsorbate adsorbed by the adsorbent) and Ce (concentration of adsorbate remaining in the solution after the system has reached equilibrium) is described in terms of adsorption isotherms. The equilibrium data were adjusted to the Langmuir and Freundlich models using linear and quadratic models. The Langmuir and Freundlich models are shown in Figure 14 and Figure 15, respectively.
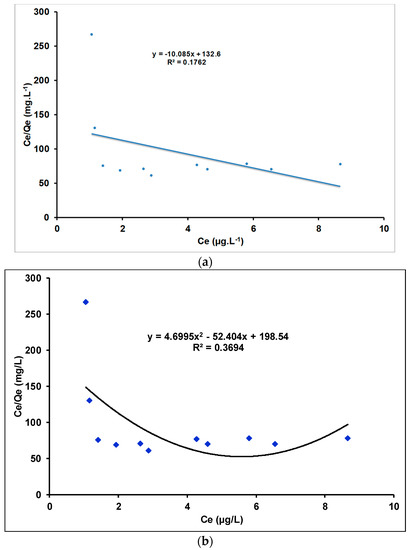
Figure 14.
Langmuir isotherm of Tl(I) adsorption on γANPs: (a) linear model and (b) quadratic model.
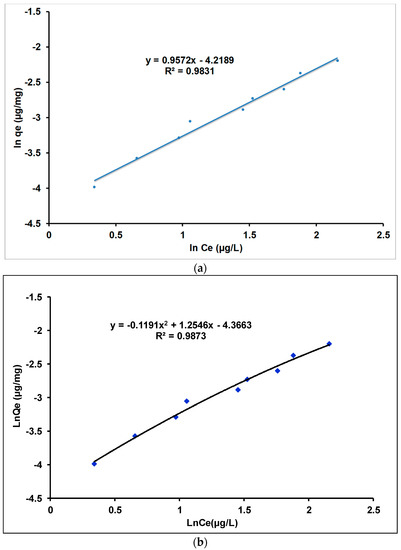
Figure 15.
Freundlich isotherm of Tl(I) adsorption on γANPs: (a) linear model and (b) quadratic model.
Figure 14 indicates that the coefficients of determination of both linear and quadratic models are very low, indicating a poor correlation between the ordinate (Ce/Qe) and the abscissa (Ce). As a result, the linear and quadratic models are not appropriate to describe the Langmuir isotherm model. Furthermore, the linear adjustment of the Langmuir isotherm model (R2 equals 0.1762) cannot be considered for the fitting of the isotherm data of the present work.
Figure 15 shows that the coefficients of determination (R2) obtained with both linear and quadratic models are almost similar and very high, 0.9831 and 0.9873, respectively, indicating a strong correlation between the ordinate (LnQe) and the abscissa (LnCe). Although both linear and quadratic models lead to high values of the coefficients of determination (R2), only the linear adjustment of the Freundlich isotherm provides a straight line as expected by the theoretical expression (Equation 6). The linear fitting of the Freundlich isotherm model is therefore retained for the description of the isotherm data in the present work.
The parameters of thallium (I) adsorption isotherms were evaluated at room temperature and are presented in Table 4. The values obtained for the coefficient of determination (R2) indicate that the thallium (I) adsorption data correspond better to the Freundlich isotherm (Figure 15a) than to the Langmuir isotherm (Figure 14a) at low initial concentrations. The Freundlich isotherm is suitable for data obtained at low Ce values (contaminant concentration in equilibrium solution). These results are in agreement with other similar investigations dealing with the removal of thallium (I) by zeolites and manganese dioxide nanoparticles [90,98]. The value of n determined in the Freundlich isotherm is greater than one, which implies 1/n < 1. This result supports the fact that the adsorption process is better described by the Freundlich isotherm and would indicate a chemisorption process [113,114]. In addition, isotherms with n > 1 are classified as L-type isotherms reflecting a high affinity between adsorbate and adsorbent and indicating a process of chemisorption in the Tl(I) removal [115,116].

Table 4.
Isotherm parameters of Tl(I) adsorption on γANPs.
The values of the various parameters of the Freundlich model (Table 4) indicate an affinity between the alumina nanoparticles and thallium (I), suggesting that the synthesized alumina nanoparticles have a high potential for Tl(I) removal. The Freundlich isotherm applies to both monolayers (chemisorption) and multilayer adsorption (physisorption). The Freundlich isotherm model is constructed on the hypothesis that adsorbate is likely to adsorb onto the heterogeneous adsorbent surface [117,118]. Table 4 presents the isotherm adsorption parameters of thallium (I) on alumina nanoparticles.
3.3. Temperature Effect and Thermodynamic Parameters of Tl(I) Adsorption on γANPs
The amounts of Tl(I) adsorbed at equilibrium as a function of temperature are represented in Figure 16. This Figure shows a decrease in the amount of Tl(I) adsorbed at equilibrium as the temperature increases. This result suggests that the nano-alumina adsorption reaction of Tl(I) is favorable at low temperatures. Indeed, all of the Qe values for temperatures ranging from 303 to 333 K are located between 18.65 and 15.96 µg of Tl(I)/g of nano alumina particles. Therefore, the temperature affects the process of adsorption of Tl(I) by nano alumina particles. This reduction in Qe values when the temperature increases may be attributed to the exothermic nature of the adsorption reaction [119,120].
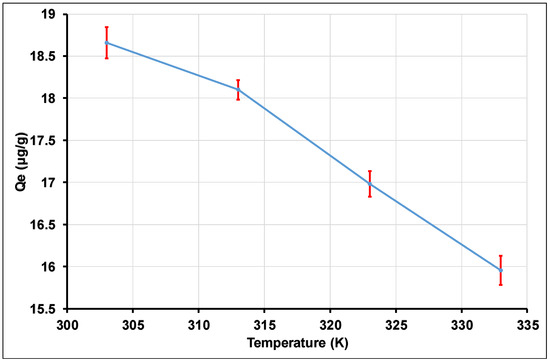
Figure 16.
Effect of temperature.
The thermodynamic parameters are presented in Table 5. The free enthalpy variation values are as follows: −22.923 ≤ ∆G ≤ −24.031 kJ/mol. These values provide a measure of the spontaneity of the adsorption process. The ∆G values of Tl(I) adsorption are negative at all temperatures studied and become less negative with the increase in temperature. These results suggests that the adsorption process is spontaneous, feasible, and that the degree of spontaneity decreases with the increase in temperature, mainly due to the dominance of physisorption over chemisorption [121,122,123]. In general, an increase in the above-mentioned values with an increase in temperature indicates that the adsorption process is more favorable at lower temperatures. This situation suggests that the mobility of Tl(I) in the solution increases with the decrease in temperature, leading to a higher affinity of Tl (I) on the adsorbent at low temperature [124]. The resulting values of ∆H are −36.09 kJ/mol. This negative value of ∆H is indicative of an exothermic adsorption process for the gamma alumina nanoparticles materials [87,125]. This result is confirmed by the decrease in the adsorption capacity of Tl(I) with the increase in temperature.

Table 5.
Thermodynamic parameters of Tl(I) adsorption by nano alumina.
From ∆H and ∆G values, we conclude that the process of adsorption of Tl(I) by gamma alumina nanoparticles materials is spontaneous and exothermic. Wen Liu et al. also reported that adsorption of Tl(I) by titanate nanotubes was exothermic and spontaneous [45]. However, R. Soltani et al. reported that sorption of Tl(I) by mesoporous silica functionalized by thiol groups was rather endothermic and spontaneous [126]. According to Chidozie, C.N. et al. (2021) in an exothermic reaction, less energy is required for bond breakage than is required for bond formation [127]. The exothermicity of the nano-alumina binding reaction of Tl(I) may be explained by the fact that the Tl(I) ions are well solvated in water and are therefore enveloped in a hydration shell. The total energy absorbed to dehydrate Tl(I) is less than the total energy released during the formation of the bond between thallium (I) and nano-alumina, which results in the release of additional energy in the form of heat [34,45]. Negative values of ΔS suggest a decrease in randomness or disorder at the solid–liquid interface during the adsorption of Tl(I) on gamma alumina [128,129]. Negative values of ΔS and ΔH indicate a spontaneous adsorption process.
3.4. Mechanism of Thallium Adsorption onto γANPs
The adsorption mechanisms of Tl(I) on alumina nanoparticles are discussed compiling the previous results obtained from the pH effect, kinetic analysis, adsorption isotherm, thermodynamic parameters, and model of diffusion. Generally, the pollutant sorption process is complex in nature and can be governed by one or more processes.
From the effect of pH, it can be concluded that thallium adsorption on the surface of γ-alumina is mainly governed by the pH values, with a selective adsorption of Tl+ species on the negatively charged reactive hydroxyl sites for pH values located between 8 and 10. This type of adsorption could occur through bond formation (inner-sphere complexes). Although at low pH (4 < pH < 8) the reactive hydroxyls are positively charged, there is an adsorption of Tl(I), suggesting that the mechanism of adsorption is not based on the deposition of Tl hydroxide species [91]. In this later adsorption, the metal cations are weakly bound to the surface as outer-sphere complexes, and are more mobile than those present in inner-sphere complexes [130]. It seems highly probable that both mechanisms intervene in the sorption process. This was confirmed by the desorption experiment which was carried out at spent γ-alumina samples (Tl(I)-loaded alumina), which were obtained after the adsorption of Tl+ under optimum conditions. We found that the residual concentration of thallium in the filtrate was around 11.98 µg/L, i.e., around 63% of Tl physisorbed and therefore 32% of Tl(I) adsorbed. This is confirmed by the results obtained from the thermodynamic parameters which show a dominance of physisorption over chemisorption.
The pseudo-second-order model and the Freundlich isotherm obtained from the kinetic study and the adsorption isotherms, respectively, support the involvement of chemisorption in the process of adsorption of Tl by the γ-alumina nanoparticles. Analysis of intra-particle diffusion of thallium (I) sorption shows that the curve is non-linear and exhibits two distinct steps. This multi-linearity underlines that two mechanisms—namely external mass transfer and intra-particle diffusion—are involved in the different stages of Tl(I) sorption.
The data obtained in this work support the hypothesis that the sorption of Tl(I) onto gamma alumina is a complex process. Further investigations are needed on spent γ-alumina nanoparticles samples to assess the exact mechanism.
4. Conclusions
This study presented the removal of Tl(I) onto synthesized γ-alumina nanoparticles (γANPs) with a crystallite size of 4.1 nm. The experimental investigations showed that we achieved a high adsorption rate for Tl(I) removal from aqueous solution. The experimental results were better fitted by the pseudo-second order kinetic model, and the Freundlich isotherm described the isotherm model. We found that electrostatic interactions, at pH values ranging from 8 to 10, governed the adsorption process. At pH greater than 10, Tl(OH) influences the adsorption process, and as a result, we observed a decrease in the interaction between the adsorbent surface and the thallium ions (Tl+) ions. The thallium (I) adsorption process was spontaneous and the reaction is exothermic. The coexistence of physisorption, and chemisorption with a dominance of physisorption was demonstrated. In addition, it was found that intraparticle diffusion is involved in the process. The potential of γANPs, synthesized from local bauxite raw materials, as adsorbents for thallium (I) removal from aqueous solutions was clearly established, considering the residual concentration which was below the recommended USEPA standard concentration.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10091826/s1, Table S1: Comparison of adsorbents surfaces used for thallium (I) removal.
Author Contributions
Conceptualization, O.R.K., C.B., I.Z. and B.G.; Methodology, O.R.K., C.B., I.Z. and B.G.; Investigation, O.R.K.; Writing—original draft preparation, O.R.K.; Writing—review and editing, O.R.K., C.B., I.Z. and B.G.; Supervision, C.B., I.Z. and B.G.; Funding acquisition, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the International Science Programme (ISP), Uppsala, Sweden, BUF: 02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
International Science Programme (ISP), Uppsala, Sweden, is gratefully acknowledged for its financial support. The authors thank the National Funds for Development, Innovation and Research of Burkina Faso (FONRID) for the support of the publication cost. Ollé Rodrigue Kam (O.R.K) would like to thank Pr. Anne-Lise Hantson (Université de Mons, UMons, Belgique) for his stay at the Umons (Faculté Polytechnique, Département Génie des Procédés Chimiques et Biochimiques).
Conflicts of Interest
The authors have declared that no competing interest exist.
References
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Belzile, N.; Chen, Y.-W. Thallium in the environment : A critical review focused on natural waters, soils, sediments and airborne particles. Appl. Geochem. 2017, 84, 218–243. [Google Scholar]
- Juan, L.; Xuwen, L.; Yuqing, S.; Daniel, C.W.; Jianying, Q.; Weilong, Z.; Nuo, L.; Meiling, Y.; Jin, W.; Holger, L.; et al. Thallium pollution in China and removal technologies for waters: A review. Environ. Int. 2019, 126, 771–790. [Google Scholar]
- Peter, A.L.J.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef]
- Qi, J.; Lai, Y.; Liang, C.; Yan, S.; Huang, K.; Pan, W.; Feng, L.; Jiang, L.; Zhu, P.; Hao, J.; et al. Prenatal thallium exposure and poor growth in early childhood: A prospective birth cohort study. Environ. Int. 2019, 123, 224–230. [Google Scholar] [CrossRef]
- Xu, H.; Luo, Y.; Wang, P.; Zhu, J.; Yang, Z.; Liu, Z. Removal of thallium in water/wastewater: A review. Water Res. 2019, 165, 1–26. [Google Scholar]
- Xiao, T.F.; Yang, F.; Li, S.H.; Zheng, B.S.; Ning, Z.P. Thallium pollution in China: A geo-environmental perspective. Sci. Total Environ. 2012, 421–422, 51–58. [Google Scholar] [CrossRef]
- Saeid, A.; Mahmood, C.; Mohammad, H.; Entezari, M.J.H.; Narjes, G. On-line preconcentration of ultra-trace thallium (I) in water samples with titanium dioxide nanoparticles and determination by graphite furnace atomic absorption spectrometry. Arab. J. Chem. 2012, 9, 1833–1839. [Google Scholar]
- Viraraghavan, T.; Asha, S. Thallium: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 39–44. [Google Scholar]
- Robert, S.H.; Robert, H. Thallium Poisoning During Pregnancy: A Case Report and Comprehensive Literature Review. J. Toxicol. Clin. Toxicol. 2000, 38, 767–775. [Google Scholar]
- Zhang, G.; Luo, J.; Cao, H.; Hu, S.; Li, H.; Wu, Z.; Xie, Y.; Li, X. Highly efficient removal of thallium (I) by facilely fabricated amorphous titanium dioxide from water and wastewater. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Beatrice, C.; Massimo, O.; Alessandro, U.; Roberto, G.; Massimo, O.; Riccardo, P.; Emilia, B. Human exposure to thallium through tap water: A study from Valdicastello Carducci and Pietrasanta (northern Tuscany, Italy). Sci. Total Environ. 2016, 548–549, 33–42. [Google Scholar]
- Cristian, B.; Massimo, O.; Giovanni, O.; Lepore, F.A.; Simone, V. Thallium-rich rust scales in drinkable water distribution systems: A case study from northern Tuscany, Italy. Sci. Total Environ. 2017, 587–588, 491–501. [Google Scholar]
- Campanella, B.; Casiot, C.; Onor, M.; Perotti, M.; Petrini, R.; Bramanti, E. Thallium release from acid mine drainages: Speciation in river and tap water from Valdicastello mining district (northwest Tuscany). Talanta 2017, 171, 255–261. [Google Scholar] [CrossRef]
- Liu, J.; Lippold, H.; Lippmann-Pipke, J.; Chen, Y. Sorption of thallium (I) onto geological materials: Influence of pH and humic matter. Chemosphere 2011, 82, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Nuvolone, D.; Petri, D.; Aprea, M.C.; Bertelloni, S.; Voller, F.; Aragona, I. Thallium Contamination of Drinking Water: Health Implications in a Residential Cohort Study in Tuscany (Italy). Int. J. Environ. Res. Public Health 2021, 18, 1–5. [Google Scholar]
- United States Environmental Protection Agency (US EPA). Edition of the Drinking Water Standards and Health Advisories Office of Water U.S. Environmental Protection Agency; United States Environmental Protection Agency (US EPA): Washington, DC, USA, 2018; pp. 3–8.
- Zhang, G.; Yang, H.; Li, Z.; Jiang, M.; Zhang, Q. Comparative investigation on removal of thallium (Ⅰ) from wastewater using low-grade pyrolusite and pyrolysis residue derived from oily sludge: Performance, mechanism and application. Groundw. Sustain. Dev. 2022, 16, 1–5. [Google Scholar] [CrossRef]
- Twidwell, L.G.; Williams-Beam, C. Potential Technologies for Removing Thallium from Mine and Process Wastewater: An Abbreviated Annotation of the Literature. Eur. J. Miner. Processing Environ. Prot. 2002, 2, 1–10. [Google Scholar]
- Zhou, T.; Fan, Y.; Yuan, F.; Cooke, D.; Zhang, X.; Li, L. A preliminary investigation and evaluation of the thallium environmental impacts of the unmined Xiangquan thallium-only deposit in Hexian. China Environ. Geol. 2008, 54, 131–145. [Google Scholar] [CrossRef]
- Mahamane, A.A.; Guel, B. Caractérisations physico-chimiques des eaux souterraines de la localité de Yamtenga (Burkina Faso). Int. J. Biol. Chem. Sci. 2015, 9, 517–533. [Google Scholar] [CrossRef][Green Version]
- Kam, O.R.; Bakouan, C.; Zongo, I.; Guel, B. Assessing the Source of Thallium Contamination in Ground and Surface Waters in the Locality of Yamtenga (Burkina Faso): Correlation with Some Heavy Metal Ions. Int. Res. J. Pure Appl. Chem. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Li, H.; Lin, M.; Xiao, T.; Long, J.; Liu, F.; Li, Y.; Liu, Y.; Liao, D.; Chen, Z.; Zhang, P.; et al. Highly efficient removal of thallium (I) from wastewater via hypochlorite catalytic oxidation coupled with adsorption by hydrochar coated nickel ferrite composite. J. Hazard. Mater. 2020, 388, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiong, Y.; Cheng, X.; Hou, X.; Yang, Y.; Tian, Y.; You, J.; Xu, L. Adsorptive removal of trace thallium (I) from wastewater: A review and new perspectives. J. Hazard. Mater. 2020, 393, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, S.; Cao, J.; Tsang, D.C.W.; Beiyuan, J.; Peng, Y.; Fang, F.; She, J.; Yin, M.; Shen, N.; et al. Highly efficient removal of thallium in wastewater by MnFe2O4-biochar composite. J. Hazard. Mater. 2021, 401, 1–34. [Google Scholar] [CrossRef]
- Davies, M.; Figueroa, L.; Wildeman, T.; Bucknam, C. The oxidative precipitation of thallium at alkaline pH for treatment of mining influenced water. Mine Water Environ. 2016, 35, 77–85. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wang, X.; Huang, Z.; Xu, C.; Yang, T.; Zhao, X.; Qi, J.; Ma, J. Highly efficient removal of trace thallium from contaminated source waters with ferrate: Role of in situ formed ferric nanoparticle. Water Res. 2017, 124, 149–157. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Huang, H.; Huang, Z.; Xu, C.; Guo, G.; He, H.; Ma, J. Treatment of trace thallium in contaminated source waters by ferrate peroxidation and poly aluminium chloride coagulation. Sep. Purif. Technol. 2019, 227, 1–47. [Google Scholar]
- Sinyakova, M.A.; Semenova, E.A.; Gamuletskaya, O.A. Ion exchange of copper (II), lanthanum (III), thallium (I), and mercury (II) on the “polysurmin” substance. Russ. J. Gen. Chem. 2014, 84, 2516–2520. [Google Scholar] [CrossRef]
- Li, H.; Chena, Y.; Long, J.; Jiang, D.; Liu, J.; Li, S.; Qi, J.; Zhang, P.; Wang, J.; Gong, J.; et al. Simultaneous removal of thallium and chloride from a highly saline industrial wastewater using modified anion exchange resins. J. Hazard. Mater. 2017, 333, 179–185. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yin, G.Y.; Hu, Z.G. Extraction and separation of gallium, indium and thallium with several carboxylic acids from chloride media. Talanta 2003, 59, 905–912. [Google Scholar] [CrossRef]
- Hassanien, M.M.; Kenawy, I.M.; Mostafa, M.R.; El-Dellay, H. Extraction of gallium, indium and thallium from aquatic media using amino silica gel modifed by gallic acid. Microchim. Acta 2011, 172, 137–145. [Google Scholar] [CrossRef]
- Hanaf, A. Adsorption of cesium, thallium, strontium and cobalt radionuclides using activated carbon. Asian J. Chem. 2010, 1, 292–300. [Google Scholar] [CrossRef]
- Pu, Y.; Yang, X.; Zheng, H.; Wang, D.; Su, Y.; He, J. Adsorption and desorption of thallium (I) on multiwalled carbon nanotubes. Chem. Eng. J. 2013, 219, 403–410. [Google Scholar] [CrossRef]
- Deng, H.M.; Chen, Y.H.; Wu, H.H.; Liu, T.; Wang, Y.L.; Wu, G.Y.; Ye, H.P. Adsorption of Tl (I) on Na-montmorillonite and kaolinite from aqueous solutions. Environ. Earth Sci. 2016, 75, 1–10. [Google Scholar] [CrossRef]
- Wick, S.; Baeyens, B.; Fernandes, M.M.; Voegelin, A. Thallium adsorption onto illite. Environ. Sci. Technol. 2018, 52, 571–580. [Google Scholar] [CrossRef]
- Birungi, Z.S.; Chirwa, E.M.N. The adsorption potential and recovery of thallium using green micro-algae from eutrophic water sources. J. Hazard. Mater. 2015, 299, 67–77. [Google Scholar] [CrossRef]
- Sangvanich, T.; Sukwarotwat, V.; Wiacek, R.J.; Grudzien, R.M.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C.; Yantasee, W. Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica. J. Hazard. Mater. 2010, 182, 225–231. [Google Scholar] [CrossRef]
- Vincent, T.; Taulemesse, J.-M.; Dauvergne, A.; Chanut, T.; Testa, F.; Guibal, E. Thallium (I) sorption using Prussian blue immobilized in alginate capsules. Carbohyd. Polym. 2014, 99, 517–526. [Google Scholar] [CrossRef]
- Wan, S.; Ma, M.; Lu, L.; Qian, L.; Xu, S.; Xue, Y.; Ma, Z. Selective capture of thallium (I) ion from aqueous solutions by amorphous hydrous manganese dioxide. Chem. Eng. J. 2014, 239, 200–206. [Google Scholar] [CrossRef]
- Pan, B.C.; Wan, S.L.; Zhang, S.J.; Guo, Q.W.; Xu, Z.C.; Lv, L.; Zhang, W.M. Recyclable polymer-based nano-hydrous manganese dioxide for highly efficient Tl (I) removal from water. Sci. China Chem. 2014, 57, 763–771. [Google Scholar] [CrossRef]
- Huangfu, X.; Jiang, J.; Lu, X.; Wang, Y.; Liu, Y.; Pang, S.-Y.; Cheng, H.; Zhang, X.; Ma, J. Adsorption and oxidation of thallium (I) by a nanosized manganese dioxide. Water Air Soil Pollut. 2015, 226, 1–9. [Google Scholar] [CrossRef]
- Li, K.; Li, H.; Xiao, T.; Long, J.; Zhang, G.; Li, Y.; Liu, X.; Liang, Z.; Zheng, F.; Zhang, P. Synthesis of manganese dioxide with different morphologies for thallium removal from wastewater. J. Environ. Manag. 2019, 251, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wick, S.; Pena, J.; Voegelin, A. Thallium sorption onto manganese oxides. Environ. Sci. Technol. 2019, 53, 13168–13178. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, P.; Borthwick, A.G.L.; Chen, H.; Ni, J.R. Adsorption mechanisms of thallium (I) and thallium (III) by titanate nanotubes: Ion-exchange and co-precipitation. J. Colloid Interface Sci. 2014, 423, 67–75. [Google Scholar] [CrossRef]
- Zhang, G.S.; Fan, F.; Li, X.P.; Qi, J.Y.; Chen, Y.H. Superior adsorption of thallium (I) on titanium peroxide: Performance and mechanism. Chem. Eng. J. 2018, 331, 471–479. [Google Scholar] [CrossRef]
- Wang, N.; Su, Z.; Deng, N.; Qiu, Y.; Ma, L.; Wang, J.; Chen, Y.; Hu, K.; Huang, C.; Xiao, T. Removal of thallium (I) from aqueous solutions using titanate nanomaterials: The performance and the influence of morphology. Sci. Total Environ. 2020, 717, 1–13. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Z.; Liu, J.; Jiang, G. Effective heavy metal removal from aqueous systems by thiol functionalized magnetic mesoporous silica. J. Hazard. Mater. 2011, 192, 277–283. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.J. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Singh, U.; Kaushal, R. Treatment of wastewater with low cost adsorbent—A review. Int. J. Tech. Non-Tech. Res. 2013, 4, 33–42. [Google Scholar]
- Förstner, U.; Wittmann, G.T.W. Metals Pollution in the Aquatic Environment, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Kalfa, O.M.; Yalcinkaya, O.; Turker, A.R. Synthesis of nano B2O3/TiO2 composite material as a new solid phase extractor and its application to preconcentration and separation of cadmium. J. Hazard. Mater. 2009, 166, 455–461. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, P.; Guo, L. Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta 2005, 68, 25–30. [Google Scholar] [CrossRef]
- Hurt, R.H.; Monthioux, M.; Kane, A. Toxicology of carbon nanomaterials: Status, trends and perspectives on the special issues. Carbon 2006, 44, 1028–1033. [Google Scholar] [CrossRef]
- Ilisz, I.; Dombi, A.; Mogyorósi, K.; Dékány, I. Photocatalytic water treatment with different TiO2 nanoparticles and hydrophilic/hydrophobic layer silicate adsorbents. Coll. Surf. A Physicochem. Eng. Asp. 2004, 230, 89–97. [Google Scholar] [CrossRef]
- Bruno, B.; Sarah, M.L.; Guillaume, N.; Muriel, P.; Jean, L.; Daniel, R.T.; Charles, T. Metal Phosphonates Applied to Biotechnologies: A Novel Approach to Oligonucleotide Microarrays. Chemistry 2005, 11, 1980–1988. [Google Scholar]
- Taiba, N.; Tayyiba, D. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar]
- Li, J.; Shi, Y.; Cai, Y.; Mou, S.; Jiang, G. Adsorption of di-ethylphthalate from aqueous solutions with surfactant-coated nano/microsized alumina. Chem. Eng. J. 2008, 140, 214–220. [Google Scholar] [CrossRef]
- Wasan, T.A.-R.; Omar, F. A-R.; Noor, M.A. Preparation of a Modified Nanoalumina Sorbent for the Removal of Alizarin Yellow R and Methylene Blue Dyes from Aqueous Solutions. J. Chem. 2016, 9, 1–12. [Google Scholar]
- RIBEIRO, A.P. Alumines Macro- Mésoporeuses Produites par Procédé Sol-Gel Pour une Application en Catalyse Hétérogène. Doctoral Thesis, PARIS-SUD University, Bures-sur-Yvette, France, 2015. [Google Scholar]
- Siahpoosh, S.M.; Salahi, E.; Hessari, F.A.; Mobasherpour, I. Synthesis of γ-Alumina Nanoparticles with High-Surface-Area via Sol-Gel Method and Their Performance for the Removal of Nickel from Aqueous Solution. Bull. Société R. Sci. Liège 2016, 85, 912–934. [Google Scholar] [CrossRef]
- Wang, R.Y.; Zhang, W.; Zhang, L.Y.; Hua, T.; Tang, G.; Peng, X.Q.; Hao, M.H.; Zuo, Q.T. Adsorption characteristics of Cu (II) and Zn (II) by nano-alumina material synthesized by the sol-gel method in batch mode. Environ Sci. Pollut. Res. Int. 2019, 26, 1595–1605. [Google Scholar] [CrossRef]
- Alizadeh, P.; Asghari, A.; Hemmati, M. Efficient determination of some potentially toxic metal ions from real samples via modified nano-γ-alumina-based solid-phase extraction followed by flame atomic absorption spectrometric analysis. Int. J. Environ. Anal. Chem. 2017, 97, 230–246. [Google Scholar] [CrossRef]
- Koju, N.K.; Xin, S.; Qing, W.; Zhihao, H.; Claudio, C. Cadmium removal from simulated groundwater using alumina nanoparticules: Behaviors and mechanisms. Environ. Pollut. 2018, 240, 255–266. [Google Scholar] [CrossRef]
- Lei, Z.; Ting, H.; Min, Z.; Xingjia, G.; Zhu, Y. Studies on the capability and behavior of adsorption of thallium on nano-Al2O3. J. Hazard Mater. 2008, 157, 352–357. [Google Scholar]
- Kam, O.R.; Garikoé, I.; Bakouan, C.; Guel, B. Low-Cost Synthesis of Alumina Nanoparticles and Their Usage for Bisphenol-A Removal from Aqueous Solutions. Processes 2021, 9, 1709. [Google Scholar] [CrossRef]
- Syarif, D.G.; Prajitno, D.H.; Umar, E. Synthesis of A12O3 Nanoparticles from Local Bauxite for Water-A12O3 Nanofluids egy. J. Phys. Conf. Ser. 2017, 799, 1–8. [Google Scholar]
- Manivasakan, P.; Rajendran, V.; Rauta, P.R.; Sahu, B.B.; Panda, B.K. Direct synthesis of nano alumina from natural bauxite. Adv.Mater. Res. 2009, 67, 143–148. [Google Scholar]
- Vijayakumar, G.; Tamilarasan, R.; Dharmendirakumar, M. Adsorption, Kinetic, Equilibrium and Thermodynamic studies on the removal of basic dye Rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J. Mater. Environ. Sci. 2012, 3, 157–170. [Google Scholar]
- Musah, M.; Azeh, Y.; Mathew, J.T.; Umar, M.T.; Abdulhamid, Z.; Muhammad, A.I. Adsorption Kinetics and Isotherm Models: A Review. CaJoST 2022, 1, 20–26. [Google Scholar] [CrossRef]
- Weber, W.J.; and Morris, J.C. Kinetics of adsorption of carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Ramesh, A.; Lee, D.J.; Wong, C.J.W. Thermodynamic parameters for adsorption equilibrium of heavy metals and dyes from wastewater with low-cost adsorbents. J. Colloid Interface Sci. 2005, 291, 588–592. [Google Scholar] [CrossRef]
- El-Rahman, K.M.A.; El-Kamash, A.M.; El-Sourougy, M.R.; Abdel-Moniem, N.M. Thermodynamic modeling for the removal of Cs, Sr, Ca and Mg ions from aqueous waste solutions using zeolite. A. J. Radioanal. Nucl. Chem. 2006, 268, 221–230. [Google Scholar] [CrossRef]
- Freitas, A.F.; Mendes, M.F.; Coelho, G.L.V. Thermodynamic study of fatty acids adsorption on different adsorbents. J. Chem. Thermodyn. 2007, 39, 1027–1037. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S. Insight into adsorption thermodynamics. In Thermodynamics; Tadashi, M., Ed.; InTechOpen: Vienna, Austria, 2011; pp. 350–364. Available online: http://www.intechopen.com/books/thermodynamics/insight-into-adsorption-thermodynamics (accessed on 14 January 2011).
- Bawa, S.G.; Ahmed, A.S.; Okonkwo, P.C. The study of Thermal effect on the surface properties of Gamma-Alumina synthesized from Kankara Kaolin. Niger. J. Technol. 2016, 35, 66–70. [Google Scholar]
- Paranjpe, K.Y. Alpha, Beta and Gamma Alumina as a catalyst—A Review. Pharma Innov. J. 2017, 6, 236–238. [Google Scholar]
- Meteab, H.S.; Karem, H.A.; Salih, W.K. Synthesis and characterization of nano gamma aluminium oxide from iraqi bauxite using extraction method. ARPN J. Eng. Appl. Sci. 2018, 13, 814–818. [Google Scholar]
- Rodríguez, J.A.; García, M.F. Synthesis, Properties, and Applications of Oxide Nanomaterials; John Wiley & Sons: Hobeken, NJ, USA, 2007. [Google Scholar]
- Rahmanpour, O.; Shariati, A.; Nikou, M.R.K. New Method for Synthesis Nano Size γ-Al2O3 Catalyst for Dehydration of Methanol to Dimethyl Ether. Int. J. Chem. Eng. Appl. 2012, 3, 125–128. [Google Scholar] [CrossRef]
- Yimin, L.; Dianqing, L.; Pinggui, T.; Yongjun, F. A simple and promoter free way to synthesize spherical γ-alumina with high hydrothermal stability. Mater. Lett. 2015, 155, 75–77. [Google Scholar]
- Bazyari, A.; Mortazavi, Y.; Khodadadi, A.A.; Thompson, L.T.; Tafreshi, R.; Zaker, A.; Ajenifujah, O.T. Effects of alumina phases as nickel supports on deep reactive adsorption of (4,6-dimethyl) dibenzothiophene: Comparison between γ, δ, and θ-alumina. Appl. Catal. B Environ. 2016, 180, 312–323. [Google Scholar] [CrossRef]
- Ysla, F.A.; Martins, A.R.; Rodrigo Estevam, C.; Cesário, F.d.V.; Adriana, D.B.; Carvalho, L.S. A Simple Way to Produce γ-Alumina from Aluminum Cans by Precipitation Reactions. Mater. Res. 2016, 19, 977–982. [Google Scholar]
- Khosla, E.; Kaur, S.; Dave, P.N. Mechanistic study of adsorption of acid orange-7 over aluminum oxide nanoparticles. J. Eng. 2013, 1–9. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Srivastava, V.; Upadhyay, S.N.; Weng, C.H. Alumina nanoparticles for the removal of Ni (II) from aqueous solutions. Ind. Eng. Chem. Res. 2008, 47, 8095–8100. [Google Scholar] [CrossRef]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Simultaneous removal of heavy-metal ions in wastewater samples using nanoalumina modified with 2,4-dinitrophenylhydrazine. J. Hazard. Mater. 2010, 181, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, C.Y.; Wu, L.Y.; Ge, M.F.; Song, W.G. Superb fluoride and arsenic removal performance of highly order mesoporous aluminas. J. Hazard. Mater. 2011, 198, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Weng, C.H.; Singh, V.K.; Sharma, Y.C. Adsorption of nickel ions from aqueous solutions by nano alumina: Kinetic, mass transfer, and equilibrium studies. J. Chem. Eng. Data. 2011, 56, 1414–1422. [Google Scholar] [CrossRef]
- Williams-Beam, C.; Twidwell, L.G. Removal of thallium from wastewater. Hydrometallurgy. 2003, 2, 1717–1727. [Google Scholar]
- Zhang, G.; Liu, R.; Qu, J. Removal of phosphate from water by a Fe-Mn binary oxide adsorbent. J. Colloid Interface Sci. 2009, 335, 168–174. [Google Scholar] [CrossRef]
- Metivier, R.; Leray, I.; Lefevre, J.P.; Roy-Auberger, M.; Zanier-Szydlowskic, N.; Valeur, B. Characterization of alumina surfaces by fluorescence spectroscopy Part 2. Photophysics of a bound pyrene derivative as a probe of the spatial distribution of reactive hydroxyl groups. Phys. Chem. 2003, 5, 758–766. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Interfacial characterization of α-alumina with small surface area by streaming potential and chromatography. Colloids Surf. A. 2013, 436, 148–157. [Google Scholar] [CrossRef]
- Lin, T.S.; Nriagu, J. Revised hydrolysis constants for thallium (I) and thallium (III) and the environmental Implications. J. Air Waste Manag. Assoc. 1998, 48, 151–156. [Google Scholar] [CrossRef]
- Tavlieva, M.P.; Genieva, S.D.; Georgieva, V.G.; Vlaev, L.T. Kinetic study of brilliant green adsorption from aqueous solution onto white rice husk ash. J. Colloid Interface Sci. 2013, 409, 112–122. [Google Scholar] [CrossRef]
- Memon, S.Q.; Memon, N.; Solangi, A.R.; Memon, J.U.R. Sawdust: A green and economical sorbent for thallium removal. Chem. Eng. J. 2008, 140, 235–240. [Google Scholar] [CrossRef]
- Senol, Z.M.; Ulusoy, U. Thallium adsorption onto polyacryamide-aluminosilicate composites: A Tl isotope tracer study. Chem. Eng. J. 2010, 162, 97–105. [Google Scholar] [CrossRef]
- Meiking, C.; Pingxiao, W.; Langfen, Y.; Shuai, L.; Bo, R.; Haihui, H.; Nengwu, Z.; Zhang, L. FeOOH-loaded MnO2 nano-composite: An efficient emergency material for thallium pollution incident. J. Environ. Manag. 2017, 192, 31–38. [Google Scholar]
- Vithanage, M.; Mayakaduwa, S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef]
- Khan, M.N.; Wasim, A.A.; Fazal-Ur-Rehmangu, S. Statistically Optimized Adsorption of Pb (II) Ion on Corn Husk Activated Carbon—An Application of Response Surface Method. Gazi Univ. J. Sci. 2022, 35, 421–432. [Google Scholar]
- Co¸skun, Y.I.; Aksuner, N.; Yanik, J. Sandpaper wastes as adsorbent for the removal of brilliant green and malachite green dye. Acta Chim. Slov. 2019, 66, 402–413. [Google Scholar] [CrossRef]
- Betianu, C.S.; Cozma, P.; Rosca, M.; Ungureanu, C.E.-D.; Mămăligă, I.; Gavrilescu, M. Sorption of Organic Pollutants onto Soils: Surface Diffusion Mechanism of Congo Red Azo Dye. Processes 2020, 8, 1–19. [Google Scholar]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K.; Mishra, I.M. Removal of Congo red from aqueous solution by bagasse fly ash and activated carbon: Kinetic study and equilibrium isotherm analyses. Chemosphere 2005, 61, 492–501. [Google Scholar] [CrossRef]
- Periyaraman, P.M.; Karan, S.; Ponnusamy, S.K.; Vaidyanathan, V.; Vasanthakumar, S.; Dhanasekaran, A.; Subramanian, S. Adsorption of an anionic dye onto native and chemically modified agricultural waste. Environ. Eng. Manag. J. 2019, 18, 257–270. [Google Scholar]
- Makhlouf, M.; Hamacha, R.; Villièras, F.; Bengueddach, A. Kinetics and thermodynamics adsorption of phenolic compounds on organic-inorganic hybrid mesoporous material. Int. J. Innov. Appl. Stud. 2013, 3, 1116–1124. [Google Scholar]
- Habumugisha, T.; Eric, C.; Luo, Z.; Kayiranga, A.; Nkinahamira, F.; Ndayishimiye, J.C.; Yan, C. Thallium removal by the montmorillonite biochar composite: Insights and environmental implications. Desalination Water Treat. 2022, 1–5. [Google Scholar]
- Li, H.S.; Li, X.W.; Chen, Y.H.; Long, J.Y.; Zhang, G.S.; Xiao, T.F.; Zhang, P.; Li, C.L.; Zhuang, L.Z.; Huang, W.Y. Removal and recovery of thallium from aqueous solutions via a magnetite-mediated reversible adsorption-desorption process. J. Clean. Prod. 2018, 199, 705–715. [Google Scholar] [CrossRef]
- Li, H.S.; Li, X.W.; Xiao, T.F.; Chen, Y.H.; Long, J.Y.; Zhang, G.S.; Zhang, P.; Li, C.L.; Zhuang, L.Z.; Li, K.K. Efficient removal of thallium (I) from wastewater using flower-like manganese dioxide coated magnetic pyrite cinder. Chem. Eng. J. 2018, 353, 867–877. [Google Scholar] [CrossRef]
- Li, H.S.; Chen, Y.H.; Long, J.Y.; Li, X.W.; Jiang, D.Q.; Zhang, P.; Qi, J.Y.; Huang, X.X.; Liu, J.; Xu, R.B.; et al. Removal of thallium from aqueous solutions using Fe-Mn binary oxides. J. Hazard Mater. 2017, 338, 296–305. [Google Scholar] [CrossRef]
- Khavidaki, H.D.; Fekri, M.H. Removing thallium (I) ion from aqueous solutions using modified ZnO nanopowder. J. Adv. Chem. 2015, 11, 3777–3788. [Google Scholar] [CrossRef]
- Sabermahani, F.; Mahani, N.M.; Noraldiny, M. Removal of thallium (I) by activated carbon prepared from apricot nucleus shell and modified with rhodamine B. Toxin Rev. 2017, 36, 154–160. [Google Scholar] [CrossRef]
- Li, H.S.; Xiong, J.F.; Xiao, T.F.; Long, J.Y.; Wang, Q.M.; Li, K.K.; Liu, X.M.; Zhang, G.S.; Zhang, H.G. Biochar derived from watermelon rinds as regenerable adsorbent for efficient removal of thallium (I) from wastewater. Process Saf. Environ. Prot. 2019, 127, 257–266. [Google Scholar] [CrossRef]
- Nimibofa, A.; Augustus, N.E.; Donbebe, W. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 1–11. [Google Scholar]
- Jiang, J.Q.; Coopern, C.; Ouki, S. Comparison of modified montmorillonite adsorbents-part I: Preparation, characterization and phenol adsorption. Chemosphere 2002, 47, 711–716. [Google Scholar] [CrossRef]
- Taha, M.R.; Ahmad, K.; Aziz, A.A.; Chik, Z. Geoenvironmental Aspects of Tropical Residual Soils. In Tropical Residual Soils Engineering, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Hardiljeet, K.B.; Meera, J.; Denis, M.O. kinetics and thermodynamics of cadmium ion removal by adsorption onto nanozerovalent iron particles. J. Hasardous Mater. 2011, 186, 458–465. [Google Scholar]
- Yang, C.H. Statiscal mechanical study on the Freunlich isotherm equation. J. Colloid Interface Sci. 1998, 208, 379–387. [Google Scholar] [CrossRef]
- Mohammad, G.B.H.I.; Chan, N.W.; Haliza, A.R.; Nor, A.Z. Freundlich Isotherm Equilibrium Equations in Determining Effectiveness a Low Cost Absorbent to Heavy Metal Removal In Wastewater (Leachate) At Teluk Kitang Landfill, Pengkalan Chepa, Kelantan, Malaysia. J. Geogr. Earth Sci. 2013, 1, 1–8. [Google Scholar]
- Sarkar, B.; Megharaj, M.; Xi, Y.; Naidu, R. Surface charge characteristics of organopalygorskites and adsorption of p-nitrophenol in flow-through reactor system. Chem. Eng. J. 2012, 185–186, 35–43. [Google Scholar] [CrossRef]
- Ozcan, A.S.; Ozcan, A. Adsorption of acid dyes from aqueous solutions onto acid activated bentonite. J. Colloid Interface Sci. 2004, 276, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Shreadah, M.A.; Ahmed, A.; Heiba, H.F. Multi-component adsorption of Pb (II), Cd (II), and Ni (II) onto Egyptian Na activated bentonite; equilibrium, kinetics, thermodynamics, and application for seawater desalination. J. Environ Chem. Eng. 2016, 4, 1166–1180. [Google Scholar] [CrossRef]
- Rahmani, A.; Mousavi, H.Z.; Fazli, M. Effect of nanostructure alumina on adsorption of heavy metals. Desalination. 2010, 253, 94–100. [Google Scholar] [CrossRef]
- Karatas, M. Removal of Pb (II) from water by natural zeolitic tuff: Kinetics and thermodynamics. J. Hazard. Mater. 2012, 199–200, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Sheela, T.; Nayaka, Y.A.; Viswanatha, R.; Basavanna, S.; Venkatesha, T. Kinetics and thermodynamics studies on the adsorption of Zn (II), Cd (II) and Hg (II) from aqueous solution using zinc oxide nanoparticles. Powder Technol. 2012, 217, 163–170. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, C.; Wang, J.; Liu, Q.; Li, R.; Yang, P.; Zhang, M. Removal of uranium (VI) from aqueous solutions by magnetic Schiff base: Kinetic and thermodynamic investigation. Chem Eng J. 2012, 198, 412–419. [Google Scholar] [CrossRef]
- Roozbeh, S.; Azam, M.; Saeed, S. Facile one-pot synthesis of thiol-functionalized mesoporous silica submicrospheres for Tl (I) adsorption: Isotherm, kinetic and thermodynamic studies. J. Hazard Mater. 2019, 371, 146–155. [Google Scholar]
- Chidozie, C.N.; Akambende, E.A.; Cordelia, N.M.; Praise, G.C.E.; Nkpa, M.O. Equilibrium and thermodynamic investigation of biosorption of nickel from water by activated carbon made from palm kernel chaff. Sci. Rep. 2021, 11, 1–20. [Google Scholar]
- El-Eswed, R.I.Y.; Al-Muhtaseb, A.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar]
- Liu, Q.-S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Rahnemaie, R.; Hiemstra, T.; van Riemsdijk, W.H. Inner- and outer-sphere complexation of ions at the goethite–solution interface. J. Colloid Interface Sci. 2006, 297, 379–388. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).