Amine-Based Deep Eutectic Solvents for Alizarin Extraction from Aqueous Media
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the DESs
3. Characterization and Instruments
3.1. Instruments
3.2. Liquid–Liquid Extraction Study
3.3. Single Parameter Optimization Process
3.3.1. Effect of Initial pH of Solution
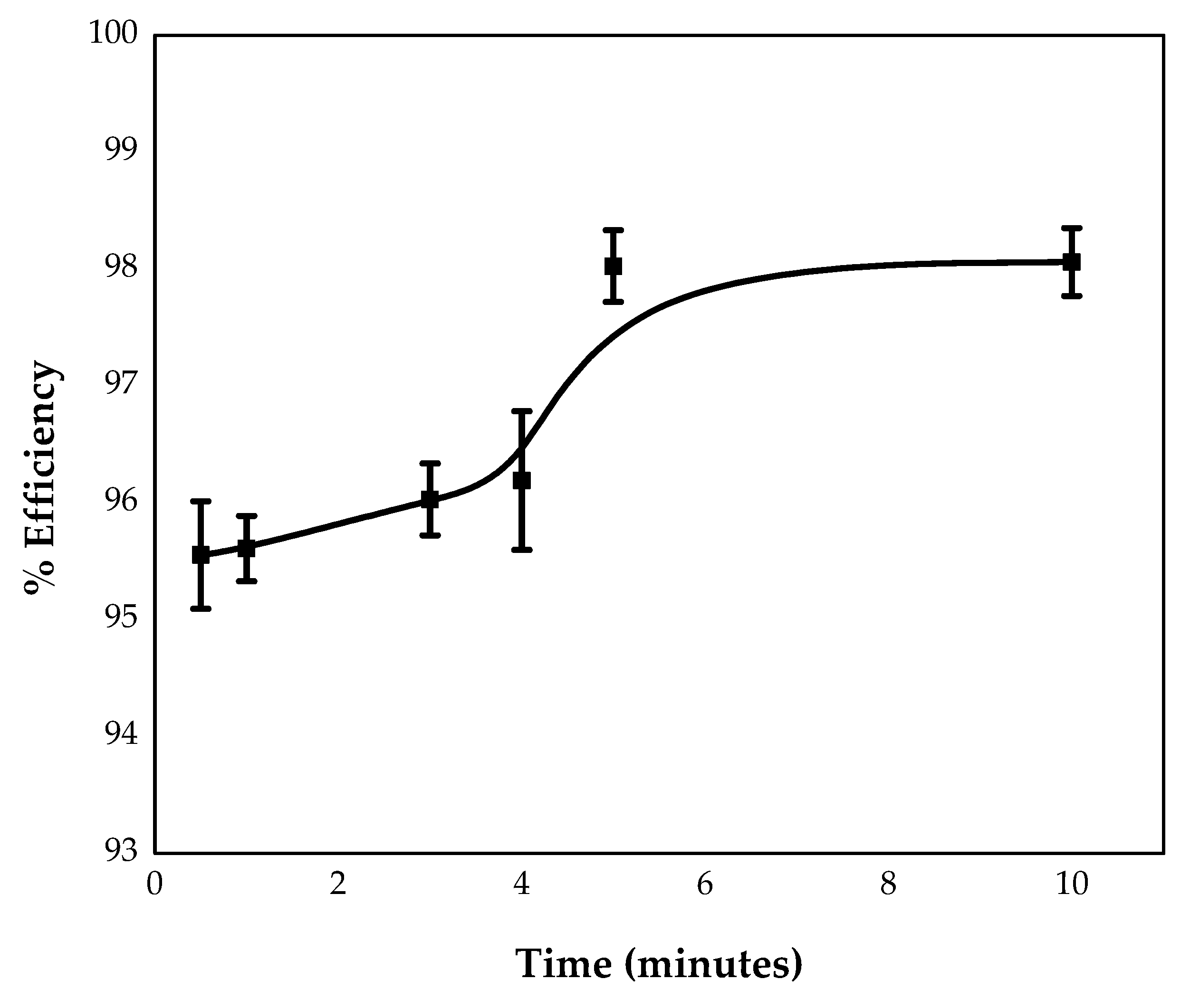
3.3.2. Effect of Time
3.3.3. Effect of Initial Concentration of Alizarin
3.3.4. Effect of Volume Ratio
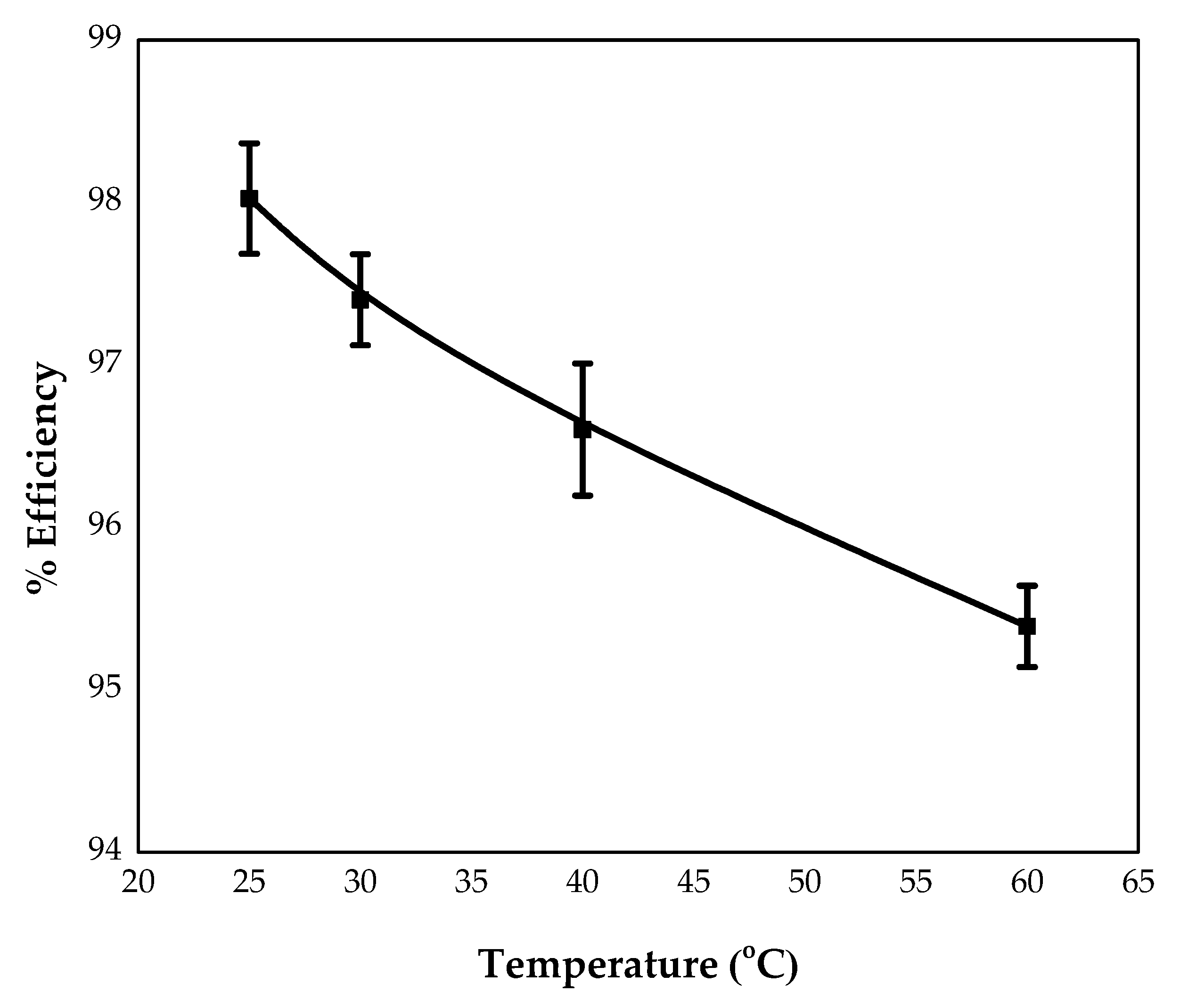
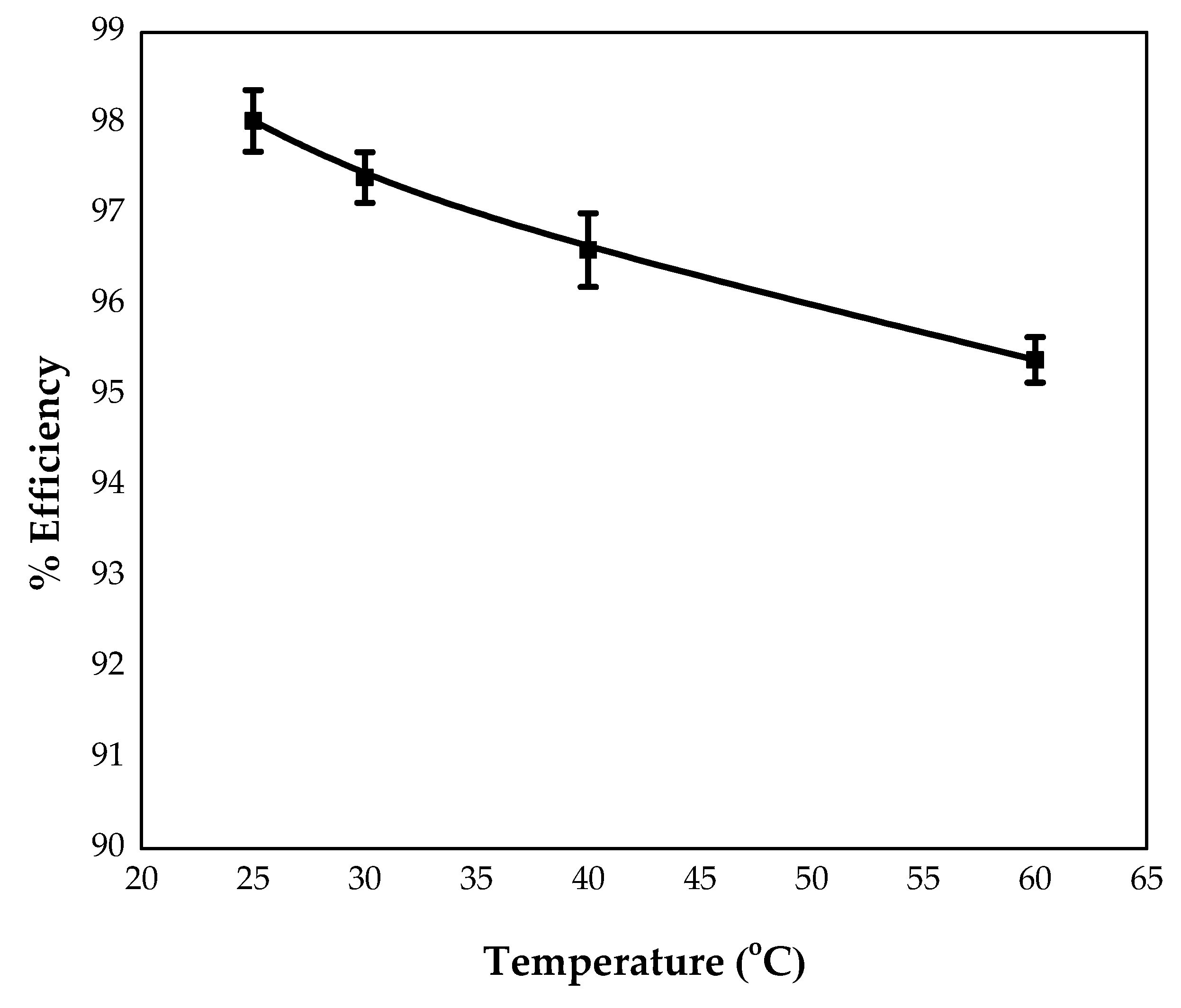
3.3.5. Effect of Temperature
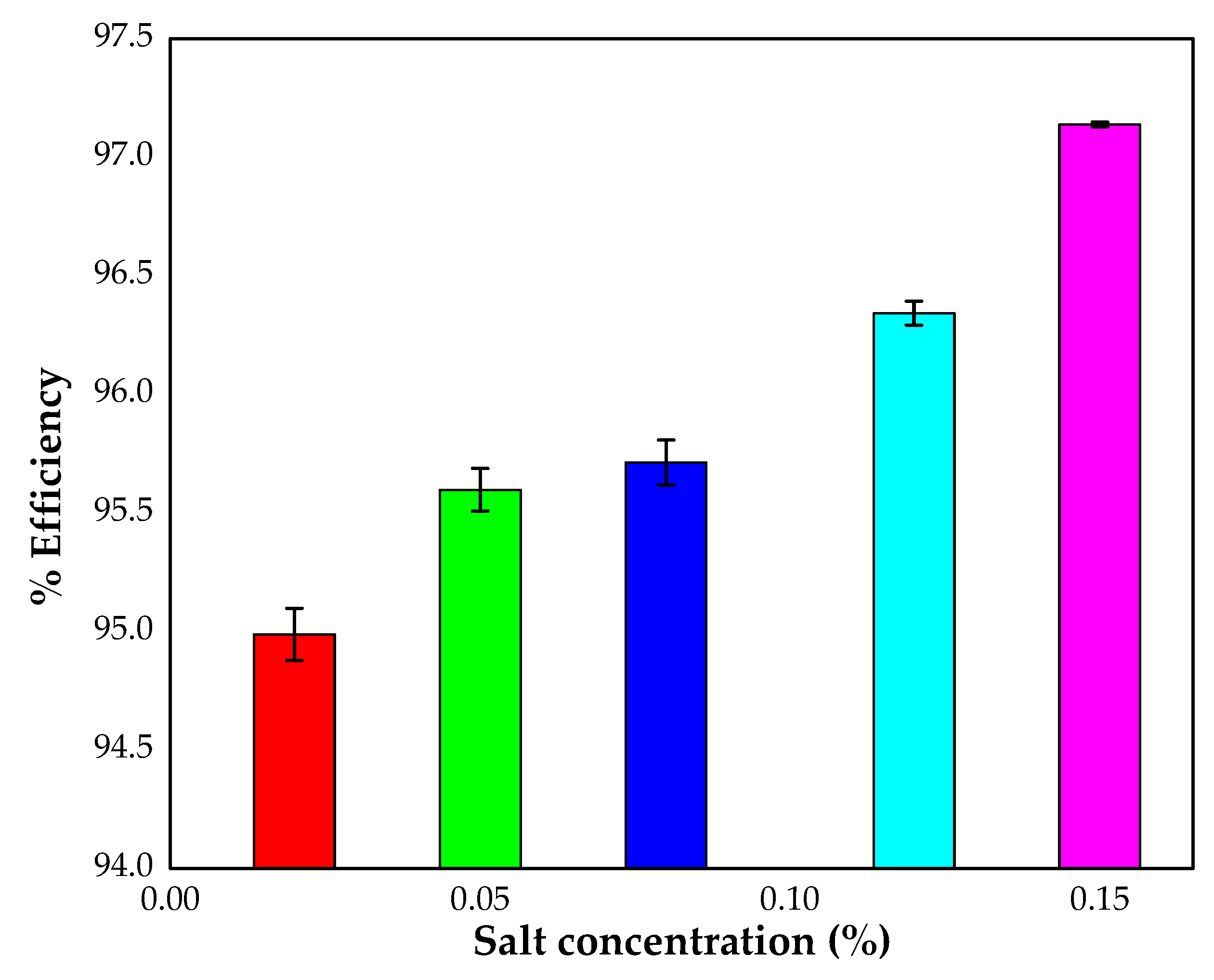
3.3.6. Effect of Salt
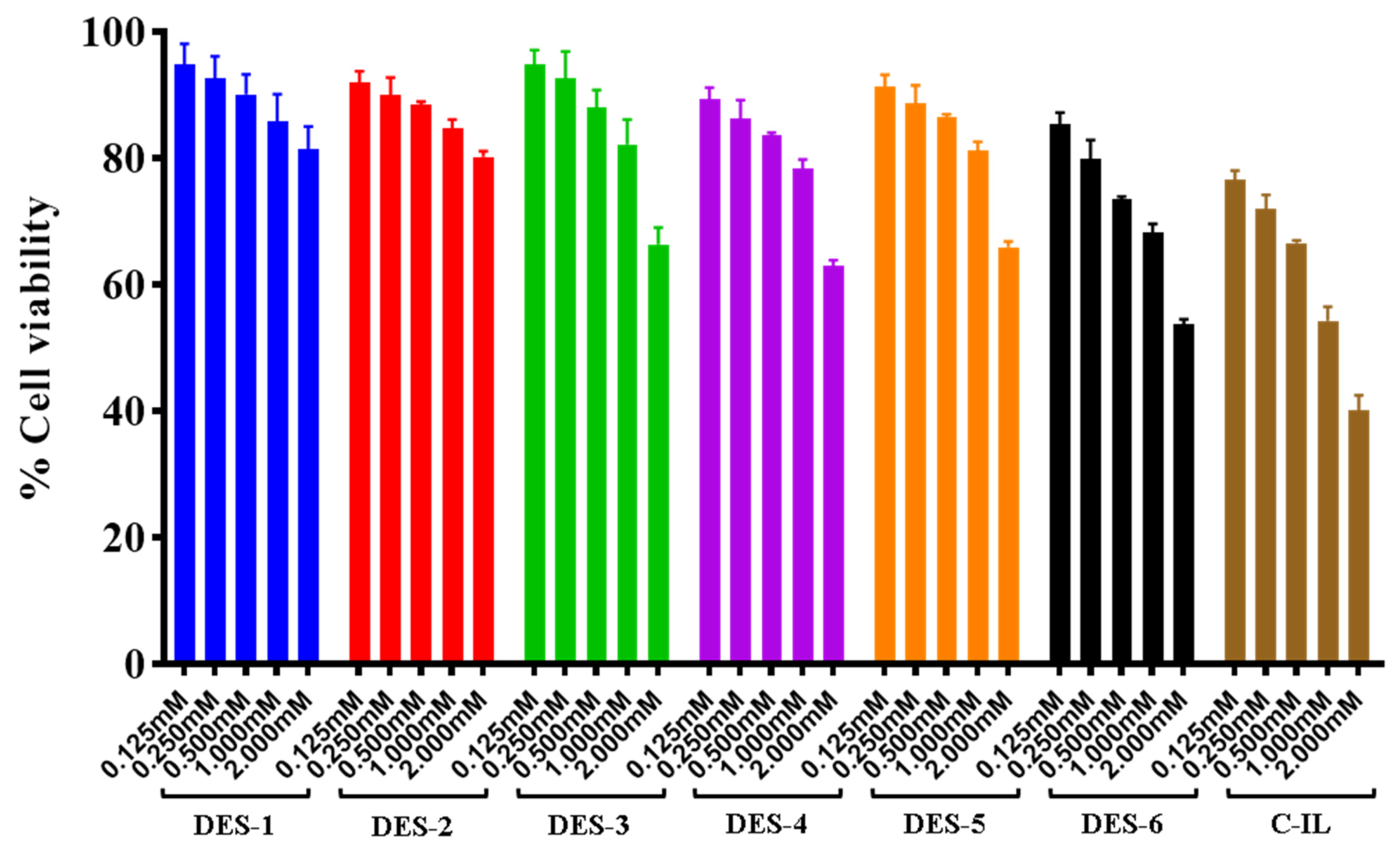
3.4. Cell Viability Assays
4. Results and Discussion
4.1. Process Optimization
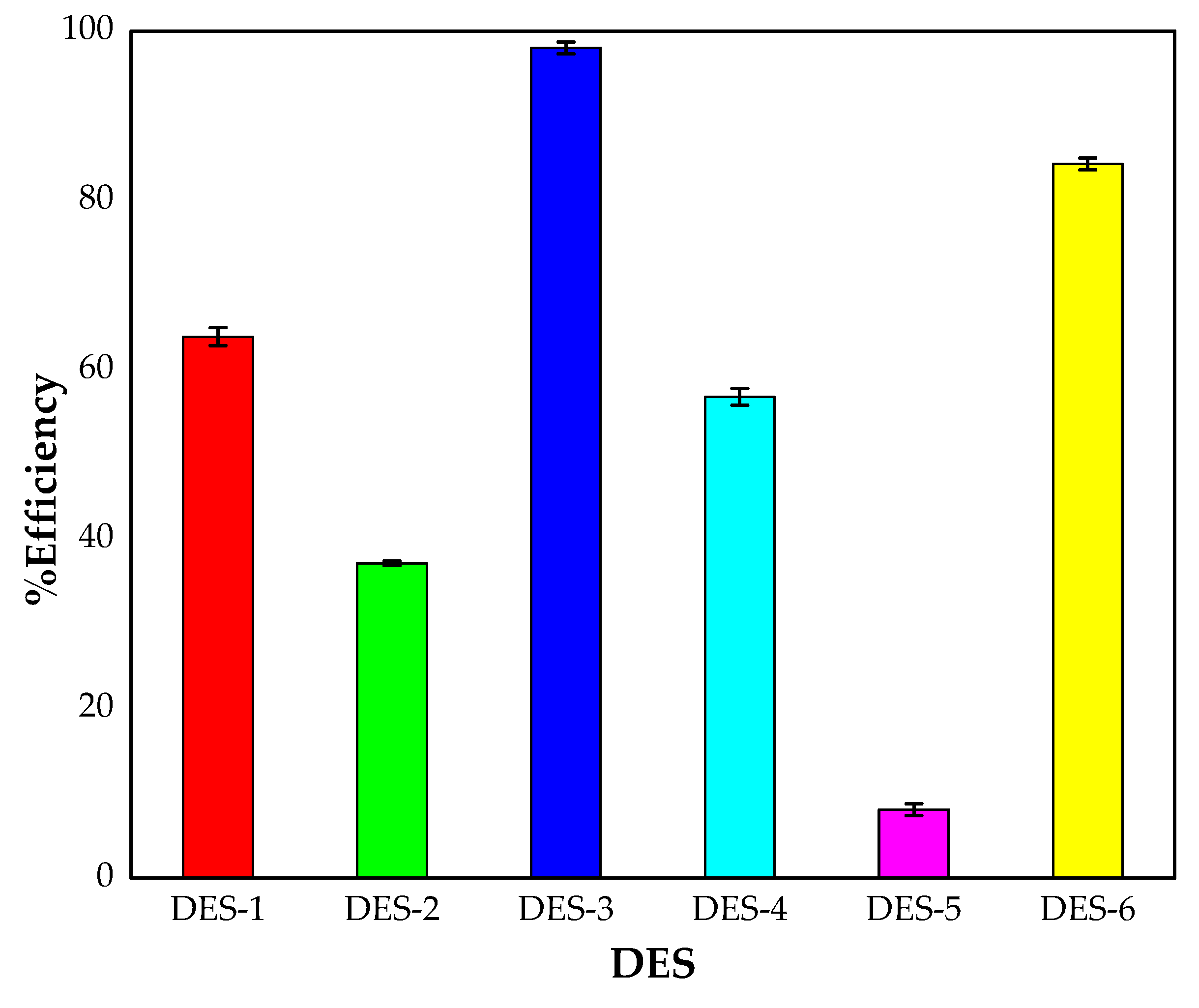
4.1.1. Screening of the DESs
4.1.2. Effect of pH
4.1.3. Effect of Time
4.1.4. Effect of Initial Concentration of Alizarin
4.1.5. Effect of Volume Ratio
4.1.6. Effect of Temperature
4.1.7. Effect of Salt (Ionic Strength of Alizarin Solution)
4.1.8. Thermodynamic Analysis
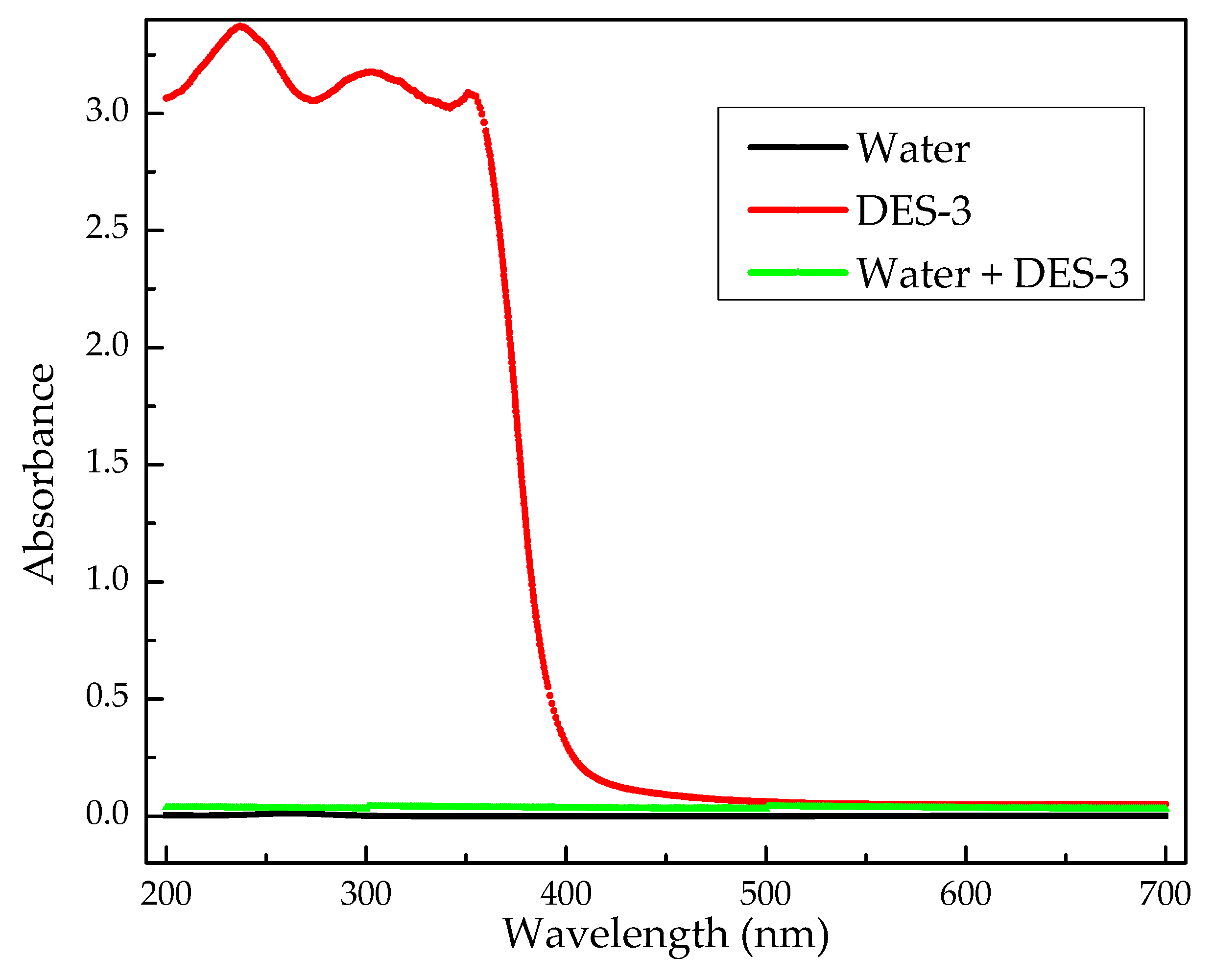
4.2. Solubility of DES-3 in Water
4.3. Thermogravimetric Analysis (TGA) of DES-3
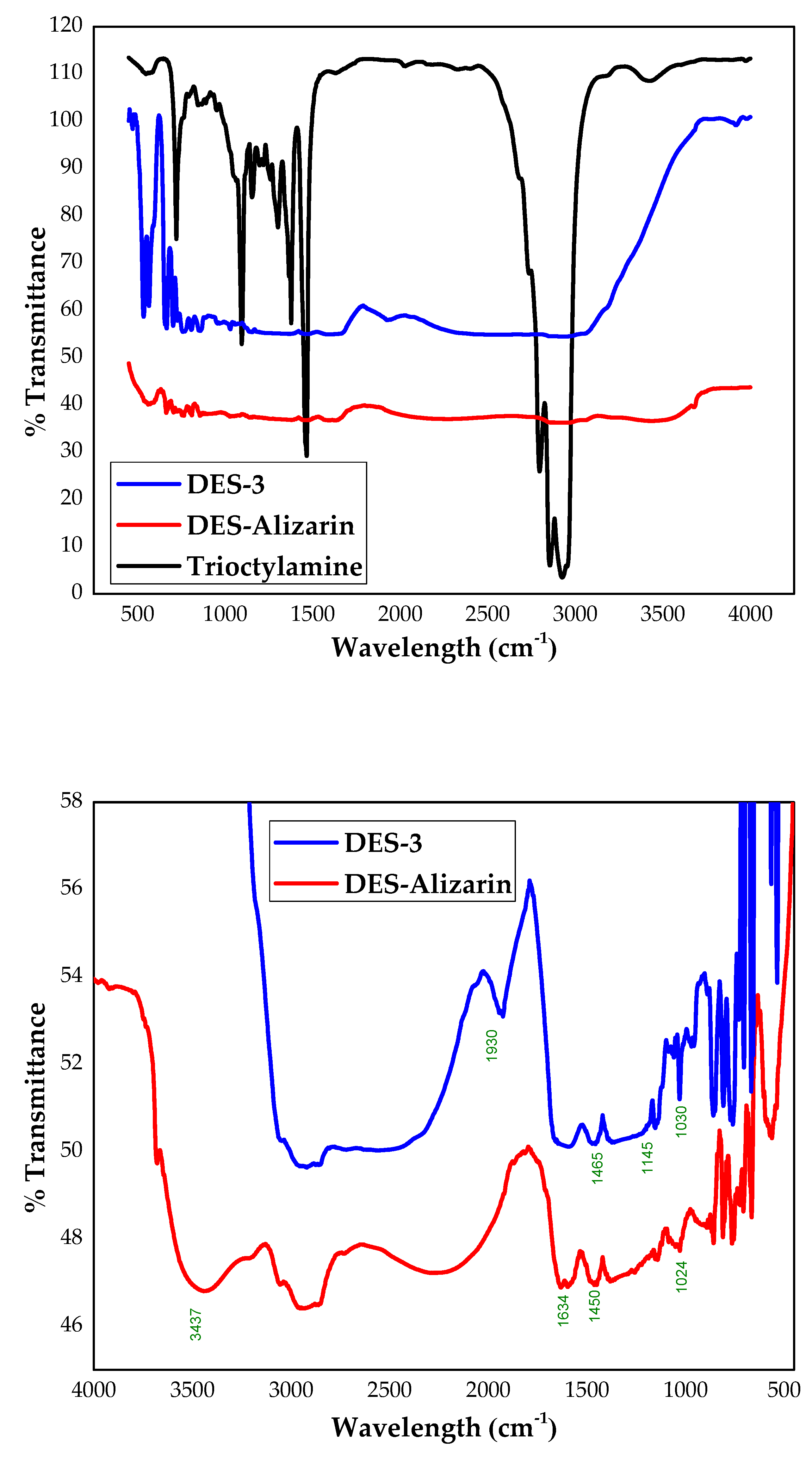
4.4. FTIR Analysis
4.5. Cell Viability Assays
5. Comparative Study with Literature
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, K.-T.; Cerniglia, C.E. Mutagenicity of azo dyes: Structure-activity relationships. Mutat. Res. Genet. Toxicol. 1992, 277, 201–220. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Li, X.; Luo, C.; Lu, F.; Qiu, H. Removal of alizarin red from water environment using magnetic chitosan with Alizarin Red as imprinted molecules. Colloids Surf. B Biointerfaces 2012, 91, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Lalhriatpuia, C.; Lee, S.-M.; Kong, S.-H. Efficient application of nano-TiO2 thin films in the photocatalytic removal of Alizarin Yellow from aqueous solutions. Appl. Surf. Sci. 2015, 353, 275–283. [Google Scholar] [CrossRef]
- Aghdasinia, H.; Khataee, A.; Sheikhi, M.; Takhtfiroozeh, P. Pilot plant fluidized-bed reactor for degradation of basic blue 3 in heterogeneous fenton process in the presence of natural magnetite. Environ. Prog. Sustain. Energy 2017, 36, 1039–1048. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: Effect of temperature. Process Biochem. 2000, 36, 431–439. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kavitha, D. Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dye. Pigment. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Isanejad, M.; Arzani, M.; Mahdavi, H.R.; Mohammadi, T. Novel amine modification of ZIF-8 for improving simultaneous removal of cationic dyes from aqueous solutions using supported liquid membrane. J. Mol. Liq. 2017, 225, 800–809. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Alanazi, F.K.; Alsarra, I.A. Removal of alizarin red from aqueous solution by ethyl acetate green nanoemulsions. Water Sci. Technol. 2014, 70, 1569–1574. [Google Scholar] [CrossRef]
- Kansal, S.K.; Lamba, R.; Mehta, S.K.; Umar, A. Photocatalytic degradation of Alizarin Red S using simply synthesized ZnO nanoparticles. Mater. Lett. 2013, 106, 385–389. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Vedaraman, N.; Surianarayanan, M.; Macfarlane, D.R. Extraction and recovery of azo dyes into an ionic liquid. Talanta 2006, 69, 1059–1062. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination 2014, 349, 145–154. [Google Scholar]
- Dâas, A.; Hamdaoui, O. Extraction of anionic dye from aqueous solutions by emulsion liquid membrane. J. Hazard. Mater. 2010, 178, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Chagas, N.V.; Quinaia, S.P.; Anaissi, F.J.; Santos, J.M.; Felsner, M.L.; Justi, K.C. Clay and charcoal composites: Characterisation and application of factorial design analysis for dye adsorption. Chem. Pap. 2014, 68, 553–563. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manage. 2009, 90, 1663–1679. [Google Scholar]
- Liu, C.-H.; Wu, J.-S.; Chiu, H.-C.; Suen, S.-Y.; Chu, K.H. Removal of anionic reactive dyes from water using anion exchange membranes as adsorbers. Water Res. 2007, 41, 1491–1500. [Google Scholar] [CrossRef]
- Padervand, M.; Tasviri, M.; Gholami, M. Effective photocatalytic degradation of an azo dye over nanosized Ag/AgBr-modified TiO2 loaded on zeolite. Chem. Pap. 2011, 65, 280–288. [Google Scholar] [CrossRef]
- Kaur, P.; Rajani, N.; Kumawat, P.; Singh, N.; Kushwaha, J.P. Performance and mechanism of dye extraction from aqueous solution using synthesized deep eutectic solvents. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 85–91. [Google Scholar] [CrossRef]
- Khan, A.S.; Nasrullah, A.; Ullah, Z.; Bhat, A.; Ghanem, O.B.; Muhammad, N.; Rashid, M.U.; Man, Z. Thermophysical properties and ecotoxicity of new nitrile functionalised protic ionic liquids. J. Mol. Liq. 2018, 249, 583–590. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Arvina, A.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Muhammad, N. Dicationic imidazolium based ionic liquids: Synthesis and properties. J. Mol. Liq. 2017, 227, 98–105. [Google Scholar] [CrossRef]
- Khan, A.S.; Nasrullah, A.; Ullah, F.; Muhammad, N.; Kubra, S.; Din, I.U.; Mutahir, Z. Effect of imidazolium’s ionic liquids with different anions and alkyl chain length on phytotoxicity and biochemical analysis of maize seedling. J. Mol. Liq. 2021, 321, 114491. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Hashim, M.A. Functionalization of CNTs surface with phosphonuim based deep eutectic solvents for arsenic removal from water. Appl. Surf. Sci. 2016, 389, 216–226. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Tome, L.I.N.; Baiao, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Mehrabi, N.; Haq, U.F.A.; Reza, M.T.; Aich, N. Using Deep Eutectic Solvent for Conjugation of Fe3O4 Nanoparticles onto Graphene Oxide for Water Pollutant Removal. 2020. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/60c7499c337d6c1d2de27748 (accessed on 15 January 2022).
- Fu, L.; Hu, X.; Yu, S.; Guo, Y.; Liu, H.; Zhang, W.; Lou, Y.; Li, D.; Yu, Q. Sustainable wastewater treatment by deep eutectic solvents and natural silk for radioactive iodine capture. Water Sci. Technol. 2019, 80, 1683–1691. [Google Scholar] [CrossRef]
- Almustafa, G.; Sulaiman, R.; Kumar, M.; Adeyemi, I.; Arafat, H.A.; AlNashef, I. Boron extraction from aqueous medium using novel hydrophobic deep eutectic solvents. Chem. Eng. J. 2020, 395, 125173. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, D.; Zhao, Y.; Li, T.; Zhang, K.; Fan, J. Highly efficient extraction/separation of Cr (VI) by a new family of hydrophobic deep eutectic solvents. Chemosphere 2020, 241, 125082. [Google Scholar] [CrossRef]
- Flieger, J.; Tatarczak-Michalewska, M.; Groszek, A.; Blicharska, E. Determination of Alizarin Red S by Ionic Liquid-Based Extraction and High-Performance Liquid Chromatography. Anal. Lett. 2016, 49, 1997–2005. [Google Scholar] [CrossRef]
- Akbar, N.; Khan, N.A.; Sagathevan, K.; Iqbal, M.; Tawab, A.; Siddiqui, R. Gut bacteria of Cuora amboinensis (turtle) produce broad-spectrum antibacterial molecules. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Akbar, N.; Siddiqui, R.; Iqbal, M.; Sagathevan, K.; Kim, K.S.; Habib, F.; Khan, N.A. Gut Bacteria of Rattus rattus (Rat) Produce Broad-Spectrum Antibacterial Lipopeptides. ACS Omega 2021, 6, 12261–12273. [Google Scholar] [CrossRef]
- Wu, H.; Fan, S.; Chen, H.; Shen, J.; Geng, Y.; Peng, L.; Du, H. Effects of Cu2+ and pH on the binding of alizarin red S to bovine serum albumin based on the analysis of protein conformation. Anal. Methods 2014, 6, 4729–4733. [Google Scholar] [CrossRef]
- Kakavandi, F.H.; Rahimi, M.; Baniamer, M.; Mahdavi, H.R. Performance evaluation of Alizarin extraction from aqueous solutions in a microfluidic system. Chem. Pap. 2017, 71, 2521–2532. [Google Scholar] [CrossRef]
- Thasneema, K.; Dipin, T.; Thayyil, M.S.; Sahu, P.K.; Messali, M.; Rosalin, T.; Elyas, K.; Saharuba, P.; Anjitha, T.; Hadda, T.B. Removal of toxic heavy metals, phenolic compounds and textile dyes from industrial waste water using phosphonium based ionic liquids. J. Mol. Liq. 2021, 323, 114645. [Google Scholar] [CrossRef]
- Xu, K.; Xu, P.; Wang, Y. Aqueous biphasic systems formed by hydrophilic and hydrophobic deep eutectic solvents for the partitioning of dyes. Talanta 2020, 213, 120839. [Google Scholar] [CrossRef]
- Thasneema, K.; Thayyil, M.S.; Rosalin, T.; Elyas, K.; Dipin, T.; Sahu, P.K.; Kumar, N.K.; Saheer, V.; Messali, M.; Hadda, T.B. Thermal and spectroscopic investigations on three phosphonium based ionic liquids for industrial and biological applications. J. Mol. Liq. 2020, 307, 112960. [Google Scholar]
- Kottummal, T.K.; Pilathottathil, S.; Thayyil, M.S.; Perumal, P.M.; Sreekala, K.K.N.; Guruswamy, G.; Chaluvalappil, S.V.; Poovingal, N.N.M. Dielectric relaxation and electrochemical studies on trihexyl tetradecyl phosphonium chloride ionic liquid. J. Mol. Liq. 2018, 252, 488–494. [Google Scholar] [CrossRef]
- Saw, P.K.; Prajapati, A.K.; Mondal, M.K. The extraction of Cr (VI) from aqueous solution with a mixture of TEA and TOA as synergic extractant by using different diluents. J. Mol. Liq. 2018, 269, 101–109. [Google Scholar] [CrossRef]
- Mohanty, A.; Devi, N.; Sukla, L.B.; Swain, N. Liquid-liquid extraction of Co (II) from nitrate solution using TOA. Mater. Today Proc. 2020, 30, 262–266. [Google Scholar] [CrossRef]
- al Kaisy, G.M.J.; Mutalib, M.I.A.; Leveque, J.M.; Rao, T. Novel low viscosity ammonium-based ionic liquids with carboxylate anions: Synthesis, characterization, and thermophysical properties. J. Mol. Liq. 2017, 230, 565–573. [Google Scholar] [CrossRef]
- Khan, A.S.; Ibrahim, T.; Akbar, N.; Khamis, M.I.; Siddiqui, R.; Nancarrow, P.; Mjalli, F.S.; Khan, N.A.; Jabbar, N.A. Application of protic ammonium-based ionic liquids with carboxylate anions for phenol extraction from aqueous solution and their cytotoxicity on human cells. J. Mol. Liq. 2021, 342, 117447. [Google Scholar] [CrossRef]
- Macário, I.; Oliveira, H.; Menezes, A.; Ventura, S.; Pereira, J.; Gonçalves, A.; Coutinho, J.; Gonçalves, F. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayyan, M.; Looi, C.Y.; Hayyan, A.; Wong, W.F.; Hashim, M.A. In vitro and in vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS ONE 2015, 10, e0117934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, F.; Asumana, C.; Yu, G. Extraction of soluble dyes from aqueous solutions with quaternary ammonium-based ionic liquids. Sep. Purif. Technol. 2013, 106, 105–109. [Google Scholar] [CrossRef]
- Muthuraman, G.; Palanivelu, K. Selective extraction and separation of textile anionic dyes from aqueous solution by tetrabutyl ammonium bromide. Dye. Pigment. 2005, 64, 251–257. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Coutinho, J.A.P.; Fernandes, A.M.; Freire, M.G. Complete removal of textile dyes from aqueous media using ionic-liquid-based aqueous two-phase systems. Sep. Purif. Technol. 2014, 128, 58–66. [Google Scholar] [CrossRef]
- Razmara, R.S.; Daneshfar, A.; Sahrai, R. Determination of methylene blue and sunset yellow in wastewater and food samples using salting-out assisted liquid–liquid extraction. J. Ind. Eng. Chem. 2011, 17, 533–536. [Google Scholar] [CrossRef]
- Muthuraman, G.; Teng, T.T. Solvent extraction of methyl violet with salicylic acid from aqueous acidic solutions. Desalination 2010, 263, 113–117. [Google Scholar] [CrossRef]

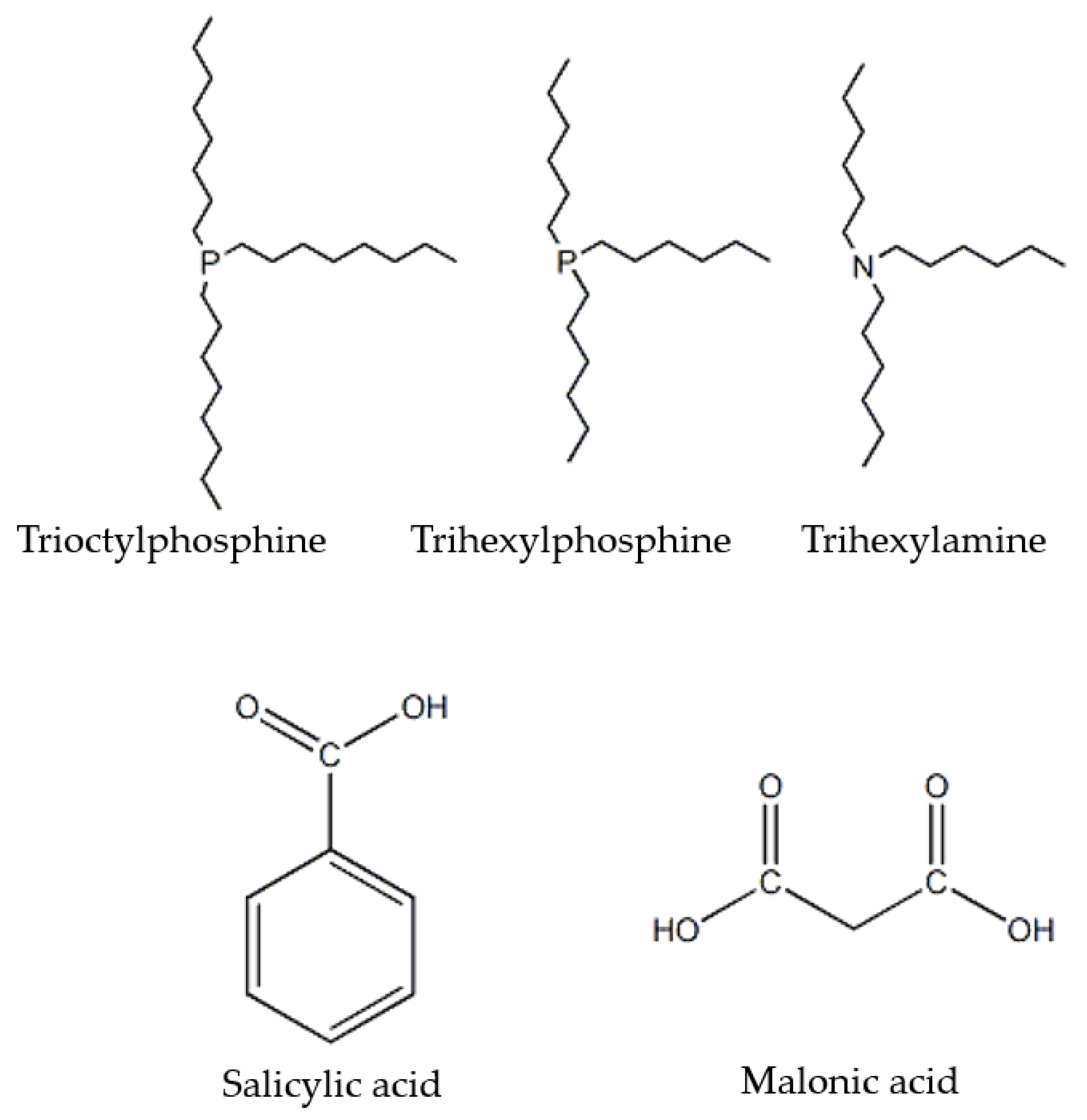



| Name | Materials | Amounts (g) | Molar Ratio |
|---|---|---|---|
| DES-1 | Salicylic acid | 0.69 | 1:1 |
| Trioctylphosphine | 1.85 | ||
| DES-2 | Salicylic acid | 0.69 | 1:1 |
| Trihexylamine | 1.01 | ||
| DES-3 | Salicylic acid | 0.69 | 1:1 |
| Trioctylamine | 1.77 | ||
| DES-4 | Malonic acid | 0.52 | 1:1 |
| Trioctylphosphine | 1.85 | ||
| DES-5 | Malonic acid | 1.04 | 1:1 |
| Trihexylamine | 1.01 | ||
| DES-6 | Malonic acid | 0.52 | 1:1 |
| Trioctylamine | 1.77 |
| Studied Parameter | Value | Fixed Parameters | ||||
|---|---|---|---|---|---|---|
| pH | Time (mins) | Ci (mg/L) | DES Dosage (mL) | Temperature (°C) | ||
| pH | 2–12 | - | 5 | 200 | 0.15 | 25 |
| Time | 0.5–10 min | 6.37 | - | 1000 | 0.15 | 25 |
| Initial concentration | 200–2000 mg/L | 6.37 | 5 | - | 0.15 | 25 |
| Volumetric ratio | 1:10 to 1:60 | 6.37 | 5 | 1000 | - | 25 |
| Temperature | 25–60 °C | 6.37 | 5 | 1000 | 0.15 | - |
| Temperature (K) | |||
|---|---|---|---|
| 298.15 | −9.67 | −0.32 | 0.031 |
| 303.15 | −9.13 | −0.89 | 0.027 |
| 313.15 | −8.17 | −14.66 | −0.02 |
| 333.15 | −8.39 | −82.31 | −0.22 |
| Extraction Solvent | Target Analyte | %E | Reference |
|---|---|---|---|
| Tricaprylmethylammonium thiocyanate | Methyl orange | 89.09 | [44] |
| Methylene blue | 64.14 | ||
| Tetrabutyl ammonium bromide | Anionic dyes | 98 | [45] |
| N-butyl, N-methyl pyrrolidinium bis(trifluoromethanesulfonyl) amide | Navy 5RE (acid blue) | 98 | [10] |
| Acid red | 98 | ||
| Phosphonium based ILs | Chloranilic acid | ~100 | [46] |
| Indigo blue | |||
| Sudan III | |||
| Acetonitrile | Methylene blue | 60 | [47] |
| Sunset yellow | 70 | ||
| Hexafluoroisopropanol (HFIP)-based DESs | Methylene blue | [35] | |
| Tartrazine | |||
| Sudan Ⅲ | |||
| Salicylic acid | Methyl violet | 96 | [48] |
| Lycolic acid and choline chloride based DESs | Eriochrome black T | 90 | [17] |
| Butyl-methyl imidazolium chloride and potassium dihydrogen phosphate | Alizarin Red S | 95 | [29] |
| DES-3 | Alizarin | 98.02 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasir, N.; Khan, A.S.; Akbar, N.; Hassan, M.F.; Ibrahim, T.H.; Khamis, M.; Siddiqui, R.; Khan, N.A.; Nancarrow, P. Amine-Based Deep Eutectic Solvents for Alizarin Extraction from Aqueous Media. Processes 2022, 10, 794. https://doi.org/10.3390/pr10040794
Yasir N, Khan AS, Akbar N, Hassan MF, Ibrahim TH, Khamis M, Siddiqui R, Khan NA, Nancarrow P. Amine-Based Deep Eutectic Solvents for Alizarin Extraction from Aqueous Media. Processes. 2022; 10(4):794. https://doi.org/10.3390/pr10040794
Chicago/Turabian StyleYasir, Nihal, Amir Sada Khan, Noor Akbar, Muhammad Faheem Hassan, Taleb H. Ibrahim, Mustafa Khamis, Ruqaiyyah Siddiqui, Naveed Ahmed Khan, and Paul Nancarrow. 2022. "Amine-Based Deep Eutectic Solvents for Alizarin Extraction from Aqueous Media" Processes 10, no. 4: 794. https://doi.org/10.3390/pr10040794
APA StyleYasir, N., Khan, A. S., Akbar, N., Hassan, M. F., Ibrahim, T. H., Khamis, M., Siddiqui, R., Khan, N. A., & Nancarrow, P. (2022). Amine-Based Deep Eutectic Solvents for Alizarin Extraction from Aqueous Media. Processes, 10(4), 794. https://doi.org/10.3390/pr10040794










