Assessment of Cadmium and Copper Adsorption by Two Agricultural Soils from Romania and Tunisia: Risk of Water Resource Pollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cadmium and Copper Solutions
2.2. Preparation and Characterization of the Soils
2.3. Adsorption Experiment of Cadmium and Copper by the Soils
2.4. Adsorption Modeling
3. Results
3.1. Soil Properties
3.2. Adsorption Kinetics of Copper and Cadmium in Romanian and Tunisian Soils
3.3. Adsorption Isotherm of Copper and Cadmium in Romanian and Tunisian Soils
3.4. Environmental Risk of Water Resource Pollution in Romania and Tunisia by Copper and Cadmium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Preventing Disease through Healthy Environments: Exposure to Cadmium: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Preventing Disease through Healthy Environments: Exposure to Lead: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Krajnc. Integrated criteria document, Cadmium—Effects, Appendix; National Institute of Public Health and Environmental Protection: Bilthoven, The Netherlands, 1987. [Google Scholar]
- Agarwal, S.K.; Tiwari, S.C.; Dash, S.C. Spectrum of poisoning requiring hemodialysis in a tertiarycare hospital in India. Int. J. Artif. Organs 1993, 16, 20–22. [Google Scholar] [CrossRef]
- Low, B.A.; Donohue, J.M.; Bartley, C.B. Backflow Prevention Failures and Copper Poisonings Associated with Post-Mix Soft Drink Dispensers; NSF International: Ann Arbor, MI, USA, 1996. [Google Scholar]
- Stenhammar, L. Diarrhoea following contamination of drinking water with copper. Eur. J. Med. Res. 1996, 4, 217–218. [Google Scholar]
- Cordos, E.; Roman, C.; Ponta, M.; Frentiu, T.; Rautiu, R. Evaluation of soil pollution with copper, lead, zinc and cadmium in the mining area Baia Mare. Rev. De Chim.-Buchar. 2007, 58, 470–474. [Google Scholar]
- Ulmanu, M.; Anger, I.; Gament, E.; Olanescu, G.; Predescu, C.; Sonaciu, M. Effect of a Romanian zeolite on heavy metals transfer from polluted soil to corn, mustard and oat. UPB Sci. Bull. B. 2006, 68, 67–78. [Google Scholar]
- Khelifi, F.; Melki, A.; Hamed, Y.; Adamo, P.; Caporale, A.G. Environmental and human health risk assessment of potentially toxic elements in soil, sediments, and ore-processing wastes from a mining area of southwestern Tunisia. Environ. Geochem. Health 2019, 42, 4125–4139. [Google Scholar] [CrossRef] [PubMed]
- Ghemari, C.; Waterlot, C.; Ayari, A.; Leclercq, J.; Douay, F.; Nasri-Ammar, K. Assessment of heavy metals in soil and terrestrial isopod Porcelliolaevis in Tunisian industrialized areas. Environ. Earth Sci. 2017, 76, 623. [Google Scholar] [CrossRef]
- Boussen, S.; Soubrand, M.; Bril, H.; Ouerfelli, K.; Abdeljaouad, S. Transfer of lead, zinc and cadmium from mine tailings to wheat (Triticumaestivum) in carbonated Mediterranean (Northern Tunisia) soils. Geoderma 2013, 192, 227–236. [Google Scholar] [CrossRef]
- Abdelwaheb, M.; Jebali, K.; Dhaouadi, H.; Dridi-Dhaouadi, S. Adsorption of nitrate, phosphate, nickel and lead on soils: Risk of groundwater contamination. Ecotoxicol. Environ. Saf. 2019, 179, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Mingorance, M.D.; Barahona, E.; Fernández-Gálvez, J. Guidelines for improving organic carbon recovery by the wet oxidation method. Chemosphere 2007, 68, 409–413. [Google Scholar] [CrossRef]
- Henriksen, A.; Selmer-Olsen, A.R. Automatic methods for determining nitrate and nitrite in water and soil extracts. Analyst 1970, 95, 514–518. [Google Scholar] [CrossRef]
- Ye, Z.H.; Shu, W.S.; Zhang, Z.Q.; Lan, C.Y.; Wong, M.H. Evaluation of major constraints to revegetation of lead/zinc mine tailings using bioassay techniques. Chemosphere 2002, 47, 1103–1111. [Google Scholar] [CrossRef]
- Xing, R.; Yang, Z.; Zhou, Y.; Wang, S. Rapid determination of total sulfur content in green liquors by turbidimetric method. Bio Resour. 2020, 15, 721–728. [Google Scholar]
- Hajrasuliha, S.; Cassel, D.K.; Rezainejad, Y. Estimation of chloride ion concentration in saline soils from measurement of electrical conductivity of saturated soil extracts. Geoderma 1991, 49, 117–127. [Google Scholar] [CrossRef]
- Hachem, M.; Sharma, B.K.; El Naggar, A.; Pilankar, I.; Anwar, N. Systematic approaches for soil analysis in forensic investigation. In Proceedings of the 2020 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 4 February–9 April 2020; pp. 1–5. [Google Scholar]
- Dhouibi, N.; Binous, H.; Dhaouadi, H.; Dridi-Dhaouadi, S. Hydrodistillation residues of Centaureanicaeensis plant for copper and zinc ions removal: Novel concepts for waste re-use. J. Clean. Prod. 2020, 261, 121106. [Google Scholar] [CrossRef]
- Abdelwaheb, M.; Ayeb, A.; Dhaouadi, H.; Dridi-Dhaouadi, S.; Peña, A. Risk of water contamination: Adsorption of dimethoate on a Mediterranean soil. Int. J. Environ. Stud. 2022, 1–20. [Google Scholar] [CrossRef]
- Tor, A.; Danaoglu, N.; Arslan, G.; Cengeloglu, Y. Removal of fluoride from water by using granular red mud: Batch and column studies. J. Hazard. Mater. 2009, 164, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hsini, N.; Abdelwaheb, M.; Dhaouadi, H.; Dridi-Dhaouadi, S. Valorization of solid wastes from Dittrichia essential oil extraction as biosorbents for cadmium removal: Biosorbent characterizations and isotherm modeling. Int. J. Environ. Sci. Technol. 2020, 17, 4611–4622. [Google Scholar] [CrossRef]
- Girish, C.R. Various isotherm models for multicomponent adsorption: A review. Int. J. Civ. Eng. Technol. 2017, 8, 80–86. [Google Scholar]
- Annabi, M.; Bahri, H.; Latiri, K. Statut Organique et Respiration Microbienne des sols du Nord de la Tunisie; Biotechnol. Agron. Soc. Environ. 2009, 13, 401–408. [Google Scholar]
- Cristian, P.; Radu, L.; Andrei, V.; Rovena, L.A. Organic carbon sequestration and nitrogen content in forest soils versus arable soils within a heavy-clay Phaeozem landscape: A Romanian case study. Arch. Agron. Soil Sci. 2020, 66, 2026–2038. [Google Scholar] [CrossRef]
- Alonge, T.A.; Ojo, O.I.; Adejumobi, M.A. Electrical conductivity based classification and mapping of salt affected soils in kampe-omi irrigation scheme. GSJ 2018, 6, 1075. [Google Scholar]
- Mechri, M.; Patil, S.B.; Saidi, W.; Hajri, R.; Jarrahi, T.; Gharbi, A.; Jedidi, N. Soil organic carbon and nitrogen status under fallow and cereal-legume species in a Tunisian semi-arid conditions. Eur. J. Earth Environ. 2016, 3, 1–13. [Google Scholar]
- Halmajan, H.V.; Nastase, D.; Vasile, G.; Paun, V.; Stoian, F.; Gidea, M. Fertilization practices in oilseed rape in Romania. Agriculture 2007, 63, 69–72. [Google Scholar]
- Rahardjo, H.; Aung, K.K.; Leong, E.C.; Rezaur, R.B. Characteristics of residual soils in Singapore as formed by weathering. Eng. Geol. 2004, 73, 157–169. [Google Scholar] [CrossRef]
- Giles, C.H.; Mac Ewan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- WHO. Copper in Drinking-Water, Background Document for Preparation of WHO Guidelines for Drinking-Water Quality: Geneva; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- WHO. Cadmium in Drinking-Water, Background Document for Preparation of WHO Guidelines for Drinking-Water Quality, Geneva; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Eighmy, T.T.; Crannell, B.S.; Butler, L.G.; Cartledge, F.K.; Emery, E.F.; Oblas, D.; Francis, C.A. Heavy metal stabilization in municipal solid waste combustion dry scrubber residue using soluble phosphate. Environ. Sci. Technol. 1997, 31, 3330–3338. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, D. In situ immobilization of Cu (II) in soils using a new class of iron phosphate nanoparticles. Chemosphere 2007, 68, 1867–1876. [Google Scholar] [CrossRef]
- Lee, H.H.; Owens, V.N.; Park, S.; Kim, J.; Hong, C.O. Adsorption and precipitation of cadmium affected by chemical form and addition rate of phosphate in soils having different levels of cadmium. Chemosphere 2018, 206, 369–375. [Google Scholar] [CrossRef]
- Yong, R.N.; Sheremata, T.W. Effect of chloride ions on adsorption of cadmium from a landfill leachate. Can. Geotech. J. 1991, 28, 378–387. [Google Scholar] [CrossRef]
- Fulda, B.; Voegelin, A.; Ehlert, K.; Kretzschmar, R. Redox transformation, solid phase speciation and solution dynamics of copper during soil reduction and reoxidation as affected by sulfate availability. Geochim. Cosmochim. 2013, 123, 385–402. [Google Scholar] [CrossRef]
- Ruangcharus, C.; Kim, S.U.; Hong, C.O. Mechanism of cadmium immobilization in phosphate-amended arable soils. Appl. Biol. Chem. 2020, 63, 36. [Google Scholar] [CrossRef]
- Christensen, J.B.; Jensen, D.L.; Christensen, T.H. Effect of dissolved organic carbon on the mobility of cadmium, nickel and zinc in leachate polluted groundwater. Water Res. 1996, 30, 3037–3049. [Google Scholar] [CrossRef]
- Pommery, J.; Ebenga, J.P.; Imbenotte, M.; Palavit, G.; Erb, F. Etude de la complexation du cadmium par un acide humique de reference. WaterResearch 1988, 22, 185–189. [Google Scholar] [CrossRef]
- Zaring, D. Agriculture, nonpoint source pollution, and regulatory control: The clean water act’s bleak present and future. Harv. Envtl. L. Rev. 1996, 20, 515. [Google Scholar]
- Brady, B.; Pagenkopf, G.K. Cadmium complexation by soil fulvic acid. Can. J. Chem. 1978, 56, 2331–2336. [Google Scholar] [CrossRef]
- Parat, C.; Chaussod, R.; Lévêque, J.; Dousset, S.; Andreux, F. The relationship between copper accumulated in vineyard calcareous soils and soil organic matter and iron. Eur. J. Soil Sci. 2002, 53, 663–670. [Google Scholar] [CrossRef]
- Ruellan, A.; Delétang, J. Les Phénomènes D’échange de Cations et D’anions dans les Sols; Office de la recherche scientifique et technique outre-mer: Paris, France, 1967. [Google Scholar]


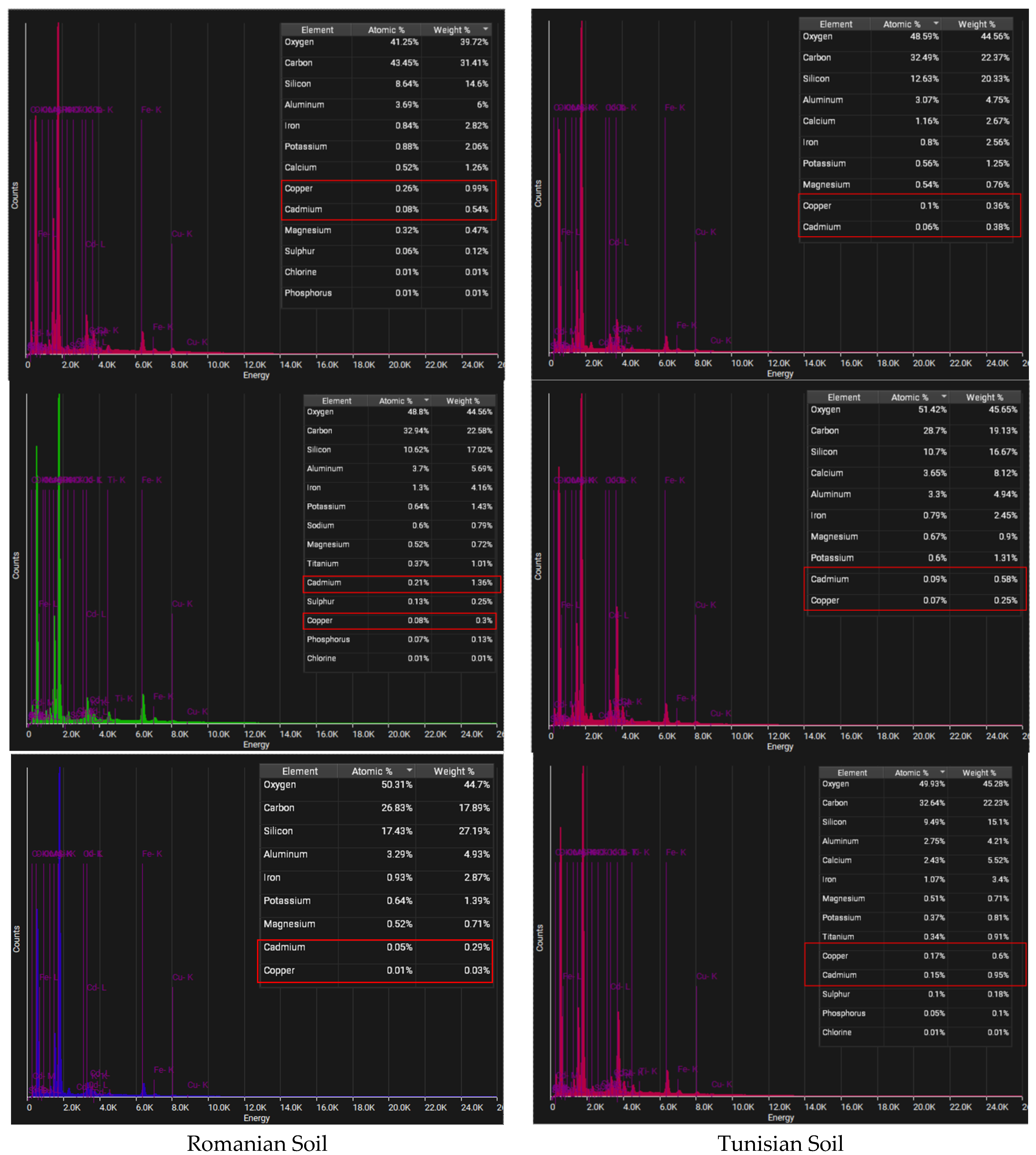

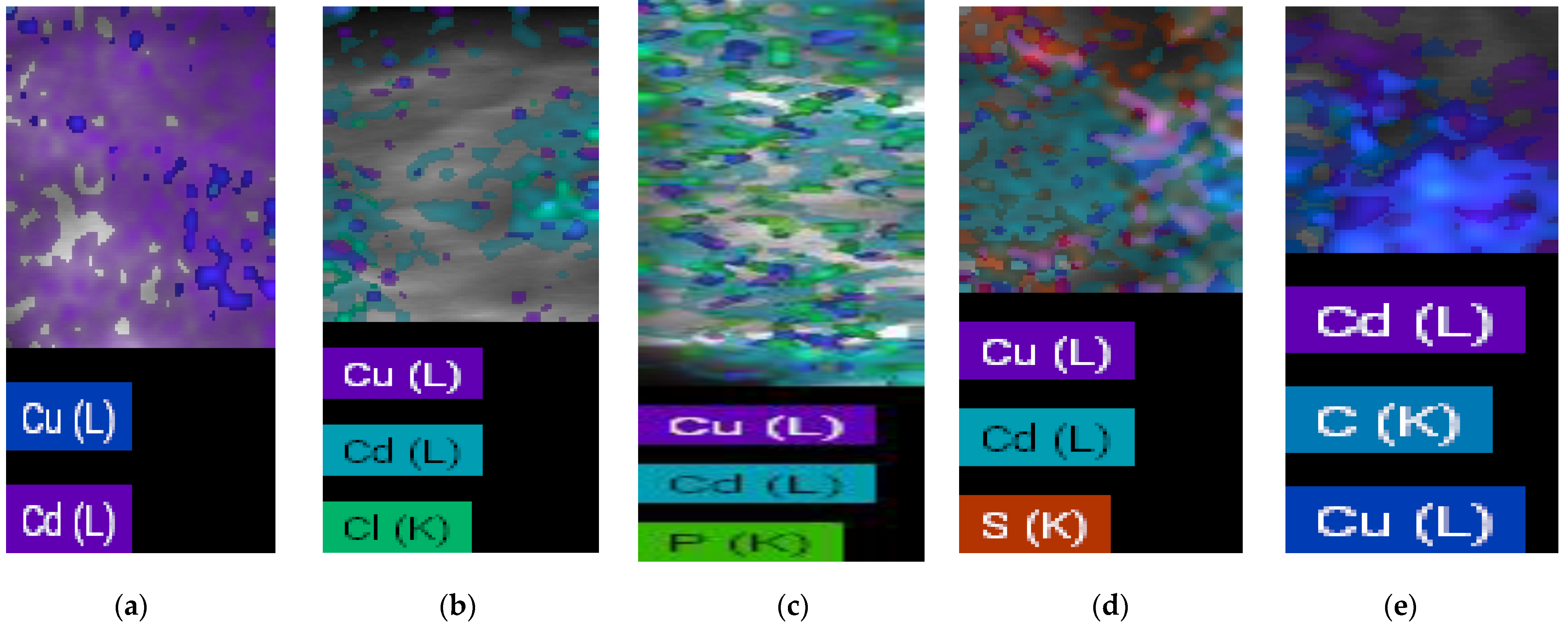
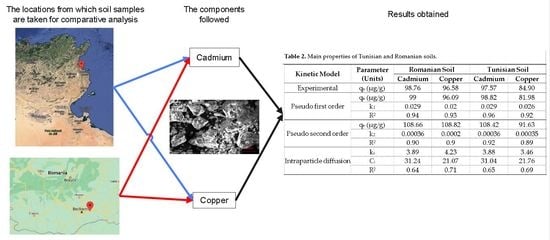
| Parameter | Tunisian Soil | Romanian Soil |
|---|---|---|
| Sand (%) | 46.67 | 56 |
| Silt (%) | 3.33 | 8 |
| Clay (%) | 50 | 36 |
| Organic carbon (%) | 2.72 | 2.67 |
| Cationic exchange capacity (CEC) (mmol/100 g) | 36.89 | 13.33 |
| pH | 8.15 | 6.6 |
| pHpzc | 7.95 | 6.84 |
| EC (µS · cm−1) | 384.1 | 220.12 |
| [P] (ppm) | 375 | 100.89 |
| [S] (ppm) | 25 | 6.76 |
| [NO3−] (ppm) | 20 | 3.42 |
| [NO2−] (ppm) | 0.5 | 0.5 |
| [Cl−] (ppm) | 32 | 16 |
| Kinetic Model | Parameter (Units) | Romanian Soil | Tunisian Soil | ||
|---|---|---|---|---|---|
| Cadmium | Copper | Cadmium | Copper | ||
| Experimental | qe (µg/g) | 98.76 | 96.58 | 97.57 | 84.90 |
| Pseudo first order | qe (µg/g) | 99 | 96.09 | 98.82 | 81.98 |
| k1 | 0.029 | 0.02 | 0.029 | 0.026 | |
| R2 | 0.94 | 0.93 | 0.96 | 0.92 | |
| Pseudo second order | qe (µg/g) | 108.66 | 108.82 | 108.42 | 91.63 |
| k2 | 0.00036 | 0.0002 | 0.00036 | 0.00035 | |
| R2 | 0.90 | 0.9 | 0.92 | 0.89 | |
| Intraparticle diffusion | ki | 3.89 | 4.23 | 3.88 | 3.46 |
| Ci | 31.24 | 21.07 | 31.04 | 21.76 | |
| R2 | 0.64 | 0.71 | 0.65 | 0.69 | |
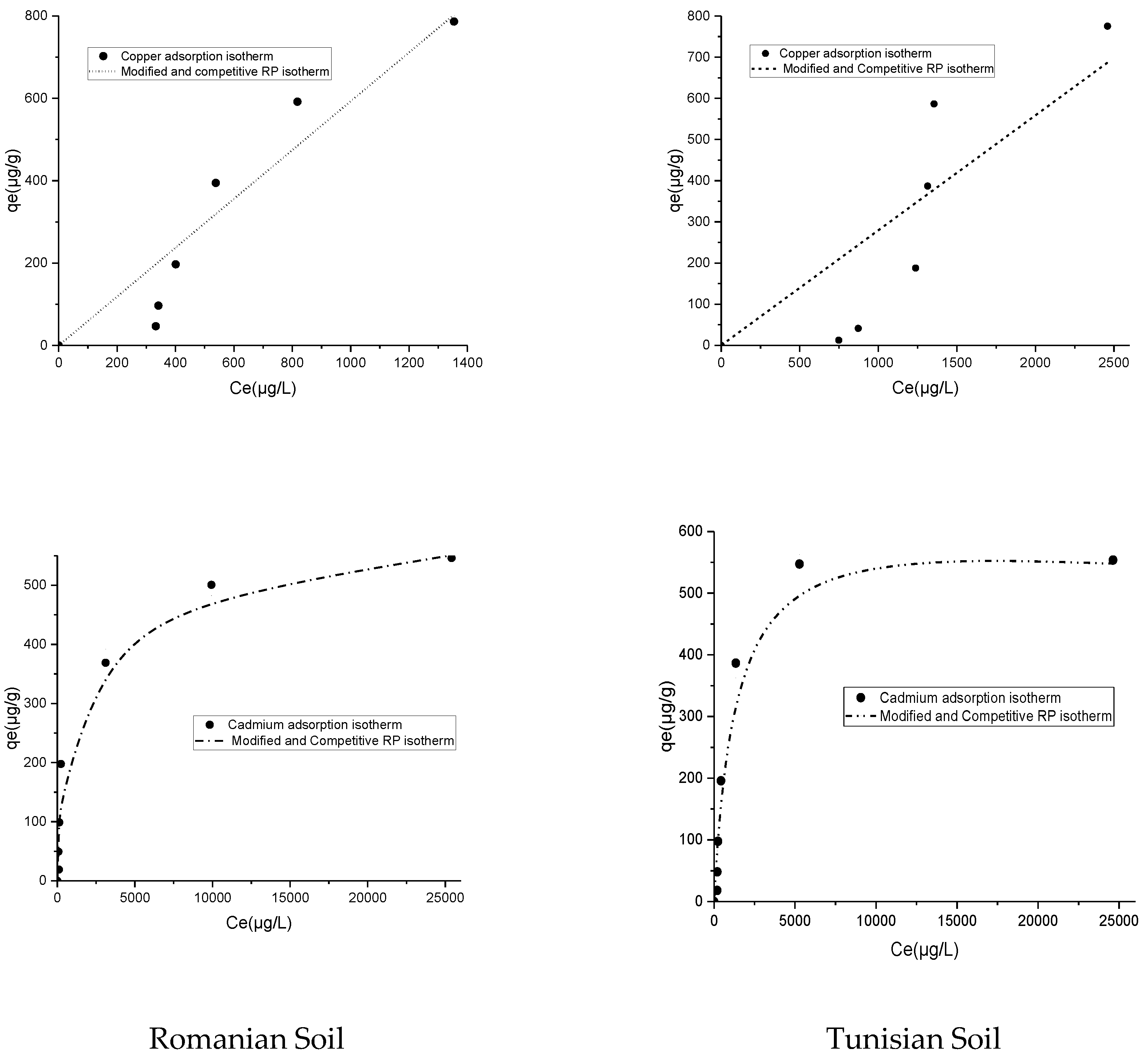
| Isotherm Model | Parameter (Units) | Romanian Soil | Tunisian Soil | ||
|---|---|---|---|---|---|
| Cadmium | Copper | Cadmium | Copper | ||
| Experimental | qeexp (µg/g) | 545.9 | 786.5 | 553.7 | 775.4 |
| Langmuir | qmax (µg/g) | 524.6 | 111,082 | 618.65 | 23,793 |
| KL (L·μg-1) | 0.0015 | 5.4 × 10−6 | 0.00091 | 1.13 × 10−5 | |
| R2 | 0.96 | 0.88 | 0.96 | 0.58 | |
| Freundlich | 1/nF | 0.31 | 1.12 | 0.31 | 1.5 |
| KF (m·L1/nF·µg1−1/nF·g−1) | 26.76 | 0.25 | 28.43 | 0.0062 | |
| R2 | 0.93 | 0.9 | 0.78 | 0.78 | |
| Redlich–Peterson | KRP | 1.08 | 0.59 | 0.44 | 0.28 |
| aRP | 0.006 | 0.001 | 0.0001 | 0.0016 | |
| bRP | 0.88 | 0.031 | 1.16 | 0.001 | |
| R2 | 0.98 | 0.88 | 0.98 | 0.63 | |
| Isotherm Model | Parameter (Units) | Romanian Soil | Tunisian Soil | ||
|---|---|---|---|---|---|
| Cadmium | Copper | Cadmium | Copper | ||
| Experimental | qeexp (µg/g) | 545.9 | 786.5 | 553.7 | 775.4 |
| Modified competitive Langmuir | qmax (µg/g) | 524.62 | 590 | 553.7 | 775 |
| KL1 (L·μg−1) | 0.0018 | 0.0015 | 0.002 | 41.41 | |
| KL2 (L·μg−1) | 5.4 × 10−6 | 1.14 × 10−5 | 1.1 × 10−7 | 1.2 × 10−5 | |
| n1 | 32.51 | 0.00015 | 1.1 × 10−5 | 112,042 | |
| n2 | 2,844,253 | 2.2 | 2,844,253 | 2,844,253 | |
| R2 | 0.7 | 0.45 | 0.6 | 0.16 | |
| Modified competitive Freundlich | x1 | 3.82 | 2.72 | 3.77 | 1.15 |
| x2 | 3.22 | 3.44 | 3.17 | 0.0001 | |
| z1 | 10−7 | 10−7 | 10−7 | 10−7 | |
| Kf1 | 26.77 | 0.25 | 28.43 | 0.006 | |
| n1 | 1.10 | 0.54 | 1.1 | 2.73 | |
| y2 | 10–8 | 10–8 | 10–8 | 10–8 | |
| R2 | 0.92 | 0.90 | 0.78 | 0.78 | |
| Modified competitive Redlich–Peterson | KRP1 | 153.4 | 67.4 | 0.0017 | 71.57 |
| n1 | 0.15 | 0.15 | 0.004 | 224.9 | |
| n2 | 0.01 | 0.01 | 1.1 × 10−5 | 9.8 × 10−6 | |
| aRP1 | 1.077 | 7.39 | 2 × 10−6 | 9.3 × 10−7 | |
| aRP2 | 932.77 | 772.11 | 0.0016 | 0.13 | |
| bRP1 | 0.89 | 0.001 | 1.16 | 10,883 | |
| bRP2 | 0.0001 | 9.9 × 10−5 | 0.001 | 0.0001 | |
| R2 | 0.98 | 0.88 | 0.98 | 0.86 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelwaheb, M.; Nedeff, V.; Dridi-Dhaouadi, S.; Moșneguțu, E.; Barsan, N.; Chițimus, A.-D. Assessment of Cadmium and Copper Adsorption by Two Agricultural Soils from Romania and Tunisia: Risk of Water Resource Pollution. Processes 2022, 10, 1802. https://doi.org/10.3390/pr10091802
Abdelwaheb M, Nedeff V, Dridi-Dhaouadi S, Moșneguțu E, Barsan N, Chițimus A-D. Assessment of Cadmium and Copper Adsorption by Two Agricultural Soils from Romania and Tunisia: Risk of Water Resource Pollution. Processes. 2022; 10(9):1802. https://doi.org/10.3390/pr10091802
Chicago/Turabian StyleAbdelwaheb, Mohamed, Valentin Nedeff, Sonia Dridi-Dhaouadi, Emilian Moșneguțu, Narcis Barsan, and Alexandra-Dana Chițimus. 2022. "Assessment of Cadmium and Copper Adsorption by Two Agricultural Soils from Romania and Tunisia: Risk of Water Resource Pollution" Processes 10, no. 9: 1802. https://doi.org/10.3390/pr10091802
APA StyleAbdelwaheb, M., Nedeff, V., Dridi-Dhaouadi, S., Moșneguțu, E., Barsan, N., & Chițimus, A.-D. (2022). Assessment of Cadmium and Copper Adsorption by Two Agricultural Soils from Romania and Tunisia: Risk of Water Resource Pollution. Processes, 10(9), 1802. https://doi.org/10.3390/pr10091802