A Review of Mo-Si Intermetallic Compounds as Ultrahigh-Temperature Materials
Abstract
1. Introduction
2. Mo-Si Series Intermetallic Compounds
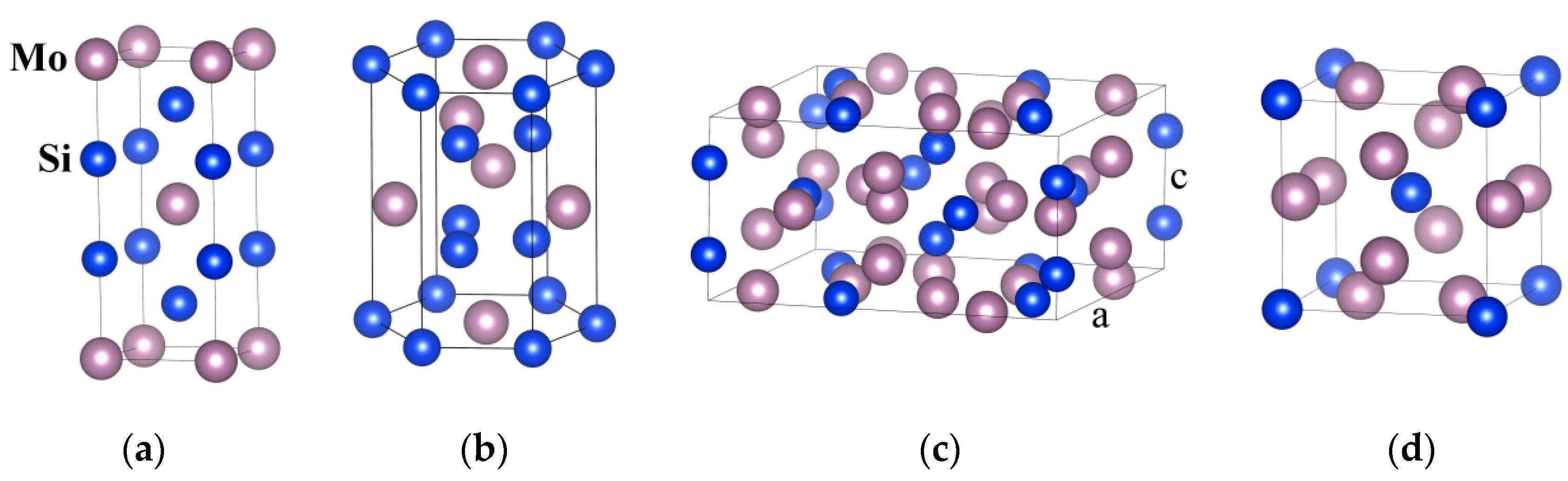
2.1. Crystal Structure and Performance Characteristics
| Alloy Type | Crystal Structure | Melting Point /°C | Density/ (g·cm−3) | Hardness/ GPa | CET/ K−1 | Fracture Toughness /(MPa·m1/2) | BDTT/ °C |
|---|---|---|---|---|---|---|---|
| MoSi2 | C11b-C40 | 2030 | 6.24 | 10.5 | 8.1 × 10−6 | 2.9 | 900–1000 |
| Mo5Si3 | D8m | 2180 | 8.19 | 12.85 | 5.2 × 10−6 | 3.2 | - |
| Mo3Si | A15 | 2025 | 8.97 | 11.48 | 9.0 × 10−6 | 3.5 | 1400 |
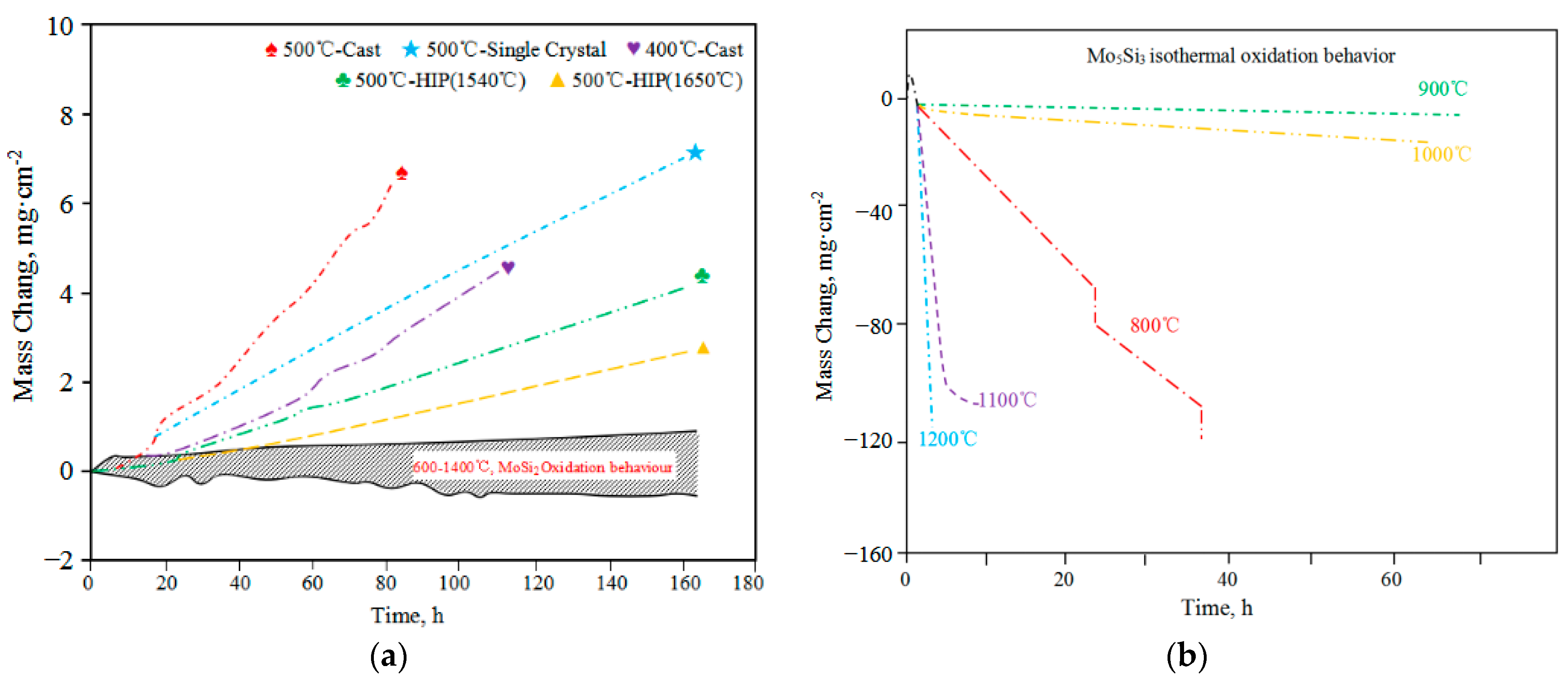
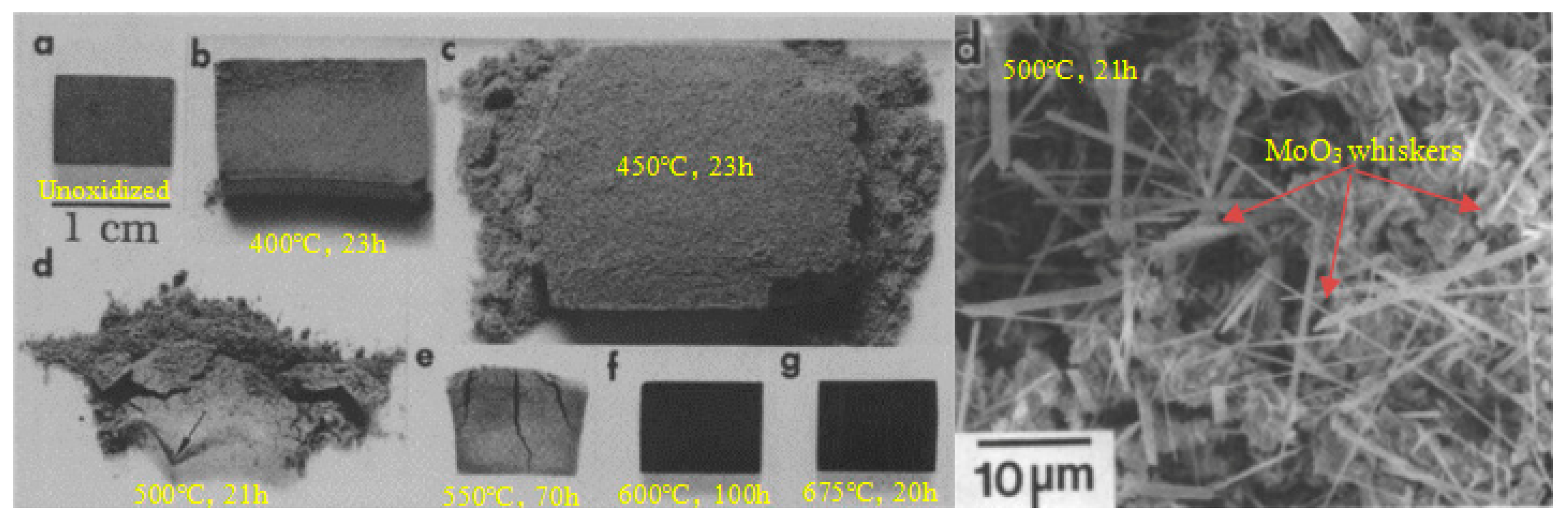
2.2. High-Temperature Performance and Issues in Application
3. Modification of Mo-Si Series Intermetallic Compounds
3.1. Alloying Research
3.2. Compounding Research
3.3. Research on the Preparation of Single Crystals and Ultrafine Crystals
4. Preparation Process of Mo-Si-Based UHTMs
5. Application of Mo-Si Series Intermetallic Compounds
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, R.; Kasama, A.; Fujikura, M.; Iwanaga, I.; Tanaka, H.; Matsumura, Y. Newly developed niobium-based superalloys for elevated temperature application. In Proceedings of the International Symposium on Niobium for High Temperature Applications, Araxa, Brazil, 1–3 December 2003. [Google Scholar]
- Zhang, Q.; Chang, Y.; Gu, L. Study of microstructure of nickel-based superalloys at high temperatures. Scr. Mater. 2017, 126, 55–57. [Google Scholar] [CrossRef]
- Lemberg, J.A.; Ritchie, R.O. Mo-Si-B Alloys for Ultrahigh-Temperature Structural Applications. Adv. Mater. 2012, 24, 3445–3480. [Google Scholar] [CrossRef] [PubMed]
- Soboyejo, W.O.; Srivatsan, T.S. Advanced Structural Materials: Properties, Design Optimization, and Applications (Materials Engineering); CRS Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Chan, K.F.; Zaid, M.H.M.; Mamat, M.S.; Liza, S.; Tanemura, M.; Yaakob, Y. Recent developments in carbon nanotubes-reinforced ceramic matrix composites: A review on dispersion and densification techniques. Crystals 2021, 11, 457. [Google Scholar] [CrossRef]
- Guo, X.J.; Si, X.Q.; Li, C.; Zhao, S.H.; Liu, Y.X.; Wang, Z.Q.; He, Z.J.; Wang, X.Y.; Cao, J. Active brazing of C/C composites and single crystal Ni-based superalloy: Interfacial microstructure and formation mechanism. J. Alloys Compd. 2021, 886, 161183. [Google Scholar] [CrossRef]
- Lu, J.H.; Li, H.J.; Li, K.Z.; Wang, L.Y.; Bai, R.C.; Wang, C. New application of intercalation on improving toughness of carbon/carbon composites. Mater. Chem. Phys. 2006, 96, 376–380. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, M. Intermetallic compound catalysts: Synthetic scheme, structure characterization and catalytic application. J. Mater. Chem. 2020, 8, 2207–2221. [Google Scholar] [CrossRef]
- Pan, Y.; Pu, D.; Liu, G. Influence of Mo concentration on the structure, mechanical and thermodynamic properties of Mo–Al compounds from first-principles calculations. Vacuum 2020, 175, 109291. [Google Scholar] [CrossRef]
- Li, B.; Li, W.H.; Wang, S.X.; Zhang, G.J.; Sun, J. Ductility of Mo-12Si-8.5B alloys doped with lanthanum oxide by the liquid-liquid doping method. J. Alloys Compd. 2015, 642, 34–39. [Google Scholar] [CrossRef]
- Kuchino, J.; Kurokawa, K.; Shibayama, T.; Takahashi, H. Effect of microstructure on oxidation resistance of MoSi2 fabricated by spark plasma sintering. Vacuum 2004, 73, 623–628. [Google Scholar] [CrossRef]
- Maxwell, W.A. Study of molybdenum disilicide for elevated temperature applications. In Proceedings of the Metallurgy and Materials Information Meet, Oak Ridge, TN, USA, 16–18 April 1951; pp. 16–18. [Google Scholar]
- Kauss, O.; Obert, S.; Bogomol, I.; Wablat, T.; Krüger, M. Temperature resistance of Mo3Si: Phase stability, microhardness, and creep properties. Metals 2021, 11, 564. [Google Scholar] [CrossRef]
- Yan, X.; Grytsiv, A.; Rogl, P.; Pomjakushin, V.; Palm, M. Direct observation of incommensurate structure in Mo3Si. J. Phase Equilib. Diff. 2016, 56, 660–666. [Google Scholar]
- Liu, Y.; Shao, G.; Tsakiropoulos, P. Thermodynamic reassessment of the Mo–Si and Al–Mo–Si systems. Intermetallics 2000, 8, 953–962. [Google Scholar] [CrossRef]
- Nunes, C.A.; Sakidja, R.; Dong, Z.; Perepezko, J.H. Liquidus projection for the Mo-rich portion of the Mo–Si–B ternary system. Intermetallics 2000, 8, 327–337. [Google Scholar] [CrossRef]
- Akinc, M.; Meyer, M.K.; Kramer, M.J.; Thom, A.J.; Huebsch, J.J.; Cook, B. Boron-doped molybdenum silicides for structural applications. Mater. Sci. Eng. A 1999, 261, 16–23. [Google Scholar] [CrossRef]
- Rosales, I.; Schneibel, J.H. Stoichiometry and mechanical properties of Mo3Si. Intermetallics 2000, 8, 885–889. [Google Scholar] [CrossRef]
- Shah, D.M.; Berczik, D.; Anton, D.L.; Hecht, R. Appraisal of other silicides as structural materials. Mater. Sci. Eng. A 1992, 155, 45–57. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, J.G.; Rosales, I.; Ca Sales, M.; Serna, S.; Martinez, L. Corrosion performance of molybdenum silicides in acid solutions. Mater. Sci. Eng. A 2004, 371, 217–221. [Google Scholar] [CrossRef]
- Perepezko, J.H.; Lange, A.; Heilmaier, M.; Sossamann, T.A. Oxidation behavior of pack-cemented Si-B oxidation protection coatings for Mo-Si-B alloys at 1300 degrees C. Surf. Coat. Technol. 2015, 266, 57–63. [Google Scholar]
- Yeh, C.L.; Chen, W.H. Combustion synthesis of MoSi2 and MoSi2–Mo5Si3 composites. J. Alloys Compd. 2007, 438, 165–170. [Google Scholar] [CrossRef]
- Huang, Y.A.; Wang, Z.M.; Zhang, L.Q.; Wei, S.Z. Pore/skeleton structure and compressive strength of porous Mo3Si-Mo5Si3-Mo5SiB2 intermetallic compounds prepared by spark plasma sintering and homogenization treatment. J. Alloys Compd. 2021, 856, 158150. [Google Scholar] [CrossRef]
- Kumar, N.; Nezhdanov, A.V.; Smertin, R.M.; Polkovnikov, V.N.; Yunin, P.A.; Garakhin, S.A.; Chkhalo, N.I.; Mashin, A.I.; Kudryashov, M.A.; Usanov, D.A. Phase-microstructure of Mo/Si nanoscale multilayer and intermetallic compound formation in interfaces. Intermetallics 2020, 125, 106872. [Google Scholar] [CrossRef]
- Rosales, I.; Schneibel, J.H.; Heatherly, L.; Horton, J.A.; Campillo, B. High temperature deformation of A15 Mo3Si single crystals. Scr. Mater. 2003, 48, 185–190. [Google Scholar] [CrossRef]
- Sharif, A.A.; Misra, A.; Petrovic, J.J.; Mitchell, T.E. Solid solution hardening and softening in MoSi2 alloys. Scr. Mater. 2001, 44, 879–884. [Google Scholar] [CrossRef]
- Mashayekh, S.; Baharvandi, H.R. Effects of MoSi2 content on the microstructure and chemical evolution during the densification of HfB2-based composites. Mater. Chem. Phys. 2021, 258, 123960. [Google Scholar] [CrossRef]
- Ito, K.; Matsuda, K.; Shirai, Y.; Inui, H.; Yamaguchi, M. Brittle-ductile behavior of single crystals of MoSi2. Mater. Sci. Eng. A 1999, 261, 99–105. [Google Scholar] [CrossRef]
- Chu, F.; Thoma, D.J.; McClellan, K.J.; Peralta, P. Mo5Si3 single crystals: Physical properties and mechanical behavior. Mater. Sci. Eng. A 1999, 261, 44–52. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, X.D.; Yang, L.M.; Wang, F. The vacancy defects and oxygen atoms occupation effects on mechanical and electronic properties of Mo5Si3 silicides. Commun. Theor. Phys. 2021, 73, 121–129. [Google Scholar] [CrossRef]
- Hagihara, K.; Maeda, S.; Nakano, T.; Umakoshi, Y. Indentation fracture behavior of (Mo0.85Nb0.15)Si2 crystals with C40 single-phase and MoSi2(C11b)/NbSi2(C40) duplex-phase with oriented lamellae. Sci. Technol. Adv. Mat. 2004, 5, 11–17. [Google Scholar] [CrossRef]
- Rosales, I. Synthesis and characterization of Mo3Si single crystal. J. Cryst. Growth 2008, 310, 3833–3836. [Google Scholar] [CrossRef]
- Harada, Y.; Morinaga, M.; Saso, D.; Takata, M.; Sakata, M. Refinement in crystal structure of MoSi2. Intermetallics 1998, 6, 523–527. [Google Scholar] [CrossRef]
- Tanaka, K.; Inui, H.; Yamaguchi, M.; Koiwa, M. Directional atomic bonds in MoSi2 and other transition-metal disilicides with the C11b, C40 and C54 structures. Mater. Sci. Eng. A 1999, 261, 158–164. [Google Scholar] [CrossRef]
- Aronsson, B.; Tjomsland, O.; Lundén, R.; Prydz, H. The Crystal Structure of Mo5Si3 and W5Si3. Acta Chem. Scand. 1955, 9, 1107–1110. [Google Scholar] [CrossRef]
- Lehmann, M.; Saemann-Ischenko, G.; Adrian, H.; Nölscher, C. Disordered A15 compounds from the Matthias-Valley: Mo3Ge and Mo3Si. Phys. B + C 1981, 107, 473–474. [Google Scholar] [CrossRef]
- Vasudévan, A.K.; Petrovic, J.J. A comparative overview of molybdenum disilicide composites. Mater. Sci. Eng. A 1992, 155, 1–17. [Google Scholar] [CrossRef]
- Cottom, B.A.; Mayo, M.J. Fracture toughness of nanocrystalline ZrO2-3mol% Y2O3 determined by vickers indentation. Scr. Mater. 1996, 34, 809–814. [Google Scholar] [CrossRef]
- Meschter, P.J. Low-temperature oxidation of molybdenum disilicide. Metall. Trans. A 1992, 23, 1763–1772. [Google Scholar] [CrossRef]
- Berztiss, D.A.; Cerchiara, R.R.; Gulbransen, E.A.; Pettit, F.S.; Meier, G.H. Oxidation of MoSi2 and comparison with other silicide materials. Mater. Sci. Eng. A 1992, 155, 165–181. [Google Scholar] [CrossRef]
- Chou, T.C.; Nieh, T.G. Mechanism of MoSi2 pest during low temperature oxidation. J. Mater. Res. 1993, 8, 214–226. [Google Scholar] [CrossRef]
- Vilasi, S. Oxidation behaviour of arc-melted and uniaxial hot pressed MoSi2 at 500 °C. Intermetallics 2010, 18, 2267–2274. [Google Scholar]
- Kurokawa, K.; Houzumi, H.; Saeki, I.; Takahashi, H. Low temperature oxidation of fully dense and porous MoSi2. Mater. Sci. Eng. A 1999, 261, 292–299. [Google Scholar] [CrossRef]
- Wang, D.Z.; Zuo, T.Y.; Liu, X.Y. Present status and development trend of MoSi2-based high temperature structural materials. Mater. Rep. 1997, 4, 53–56. (In Chinese) [Google Scholar]
- Zhang, F.; Zhang, L.T.; Shan, A.D.; Wu, J.S. Oxidation of stoichiometric poly-and single-crystalline MoSi2 at 773 K. Intermetallics 2006, 14, 406–411. [Google Scholar] [CrossRef]
- Kumar, N.K.; Mitra, R.; Das, J. Effect of moist environment on the oxidation behavior of Mo76-xSi14B10Fex (x = 0, 0.5, 1 at.%) ultrafine composites in the range of 700–800 °C. Corros. Sci. 2019, 155, 86–96. [Google Scholar] [CrossRef]
- Tortorelli, P.F.; Schneibel, J.H.; More, K.; Pint, B.A. Oxidation-sulfidation behavior of multi-phase Mo-Si-B alloys. Mater. Sci. Forum 2004, 461–464, 1063–1072. [Google Scholar] [CrossRef]
- Obert, S.; Kauffmann, A.; Seils, S.; Schellert, S.; Weber, M.; Gorr, B.; Christ, H.J.; Heilmaier, M. On the chemical and microstructural requirements for the pesting-resistance of Mo-Si-Ti alloys. J. Mater. Res. Technol. 2020, 9, 8556–8567. [Google Scholar] [CrossRef]
- Ochiai, S. Influence of the addition of Cr and Al elements to the Mo3Si intermetallic alloy on the phase construction and the oxidation behavior. Mater. Sci. Forum 2003, 426, 1771–1776. [Google Scholar] [CrossRef]
- Klissurski, D.; Mancheva, M.; Iordanova, R.; Kunev, B. Synthesis of Cr2 (MoO4)3 from mechanically activated precursors. Chem. Sustain. Dev. 2005, 13, 229–232. [Google Scholar]
- Hou, H.D.; Ning, X.J.; Wang, Q.S.; Gao, B.; Liu, Y.B.; Liu, Y. Preparation of Mo(Si, Al)2 feedstock used for air plasma spraying. Chin. J. Nonferrous Met. 2016, 26, 2939–2946. [Google Scholar] [CrossRef]
- Mitra, R.; Rao, V.; Rao, A.V. Effect of small aluminum additions on microstructure and mechanical properties of molybdenum di-silicide. Intermetallics 1999, 7, 213–232. [Google Scholar] [CrossRef]
- Tortorici, P.C. Diffusion and Phase Formation in Selected Re-Fractory Metal Silicides. Ph.D. Thesis, Department of Materials Science and Engineering. Purdue University, West Lafayette, IN, USA, 1997. [Google Scholar]
- Pan, K.; Yang, Y.; Wei, S.; Wu, H.H.; Mao, X. Oxidation behavior of Mo-Si-B alloys at medium-to-high temperatures. J. Mater. Sci. Technol. 2020, 60, 113–127. [Google Scholar] [CrossRef]
- Choi, W.J.; Park, C.W.; Park, J.H.; Kim, Y.D.; Byun, J.M. Research trends of the Mo-Si-B alloys as next generation ultra-high-temperature alloys. J. Korean Powder Metall. Inst. 2019, 26, 156–165. [Google Scholar] [CrossRef]
- Li, R.; Li, B.; Chen, X.; Wang, J.; Wang, T.; Gong, Y.; Ren, S.; Zhang, G. Variation of phase composition of Mo-Si-B alloys induced by boron and their mechanical properties and oxidation resistance. Mater. Sci. Eng. A 2019, 749, 196–209. [Google Scholar] [CrossRef]
- Sharif, A.A.; Misra, A.; Mitchell, T.E. Deformation mechanisms of polycrystalline MoSi2 alloyed with 1 at.% Nb. Mater. Sci. Eng. A 2003, 358, 279–287. [Google Scholar] [CrossRef]
- Yanagihara, K.; Maruyama, T.; Nagata, K. Effect of third elements on the pesting suppression of Mo-Si-X intermetallics (X = Al, Ta, Ti, Zr and Y). Intermetallics 1996, 4, S133–S139. [Google Scholar] [CrossRef]
- Dasgupta, T.; Umarji, A.M. Improved ductility and oxidation resistance in Nb and Al co-substituted MoSi2. Intermetallics 2008, 16, 739–744. [Google Scholar] [CrossRef]
- Obert, S.; Kauffmann, A.; Heilmaier, M. Characterisation of the oxidation and creep behaviour of novel Mo-Si-Ti alloys. Acta Mater. 2020, 184, 132–142. [Google Scholar] [CrossRef]
- Mitchell, T.E.; Misra, A. Structure and mechanical properties of (Mo, Re)Si2 alloys. Mater. Sci. Eng. A 1999, 261, 106–112. [Google Scholar] [CrossRef]
- Misra, A.; Sharif, A.A.; Petrovic, J.J.; Mitchell, T.E. Rapid solution hardening at elevated temperatures by substitutional Re alloying in MoSi2. Acta Mater. 2000, 48, 925–932. [Google Scholar] [CrossRef]
- Huicochea, C.; Rosales, I.; Castañeda, I.E.; Uruchurtu, J. Corrosion resistance of Mo3Si with niobium additions in hydrochloric acid. Port. Electrochim. Acta 2007, 25, 195–204. [Google Scholar] [CrossRef]
- Zhang, G.J.; Yue, X.M.; Tadahiko, W. Addition effects of aluminum and in-situ formation of alumina in MoSi2. J. Mater. Sci. 1999, 34, 997–1001. [Google Scholar] [CrossRef]
- Kowalik, R.W.; Hebsur, M.G. Cyclic oxidation study of MoSi2–Si3N4 base composites. Mater. Sci. Eng. A 1999, 261, 300–303. [Google Scholar] [CrossRef]
- Soboyejo, W.O.; Ye, F.; Chen, L.C.; Bahtishi, N.; Schwartz, D.S.; Lederich, R.J. Effects of reinforcement morphology on the fatigue and fracture behavior of MoSi2/Nb composites. Acta Mater. 1996, 44, 2027–2041. [Google Scholar] [CrossRef]
- Stoloff, N.S. An overview of powder processing of silicides and their composites. Mater. Sci. Eng. A 1999, 261, 169–180. [Google Scholar] [CrossRef]
- Ghadami, S.; Taheri-Nassaj, E.; Baharvandi, H.R. Novel HfB2-SiC-MoSi2 composites by reactive spark plasma sintering. J. Alloys Compd. 2019, 809, 151705. [Google Scholar] [CrossRef]
- Ghadami, S.; Taheri-Nassaj, E.; Baharvandi, H.R.; Ghadami, F. Effect of in situ SiC and MoSi2 phases on the oxidation behavior of HfB2-based composites. Ceram. Int. 2020, 46, 20299–20305. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Z.; Liu, L.; Xu, H.; Mao, Z. Effect of Nano-ZrO2 on the Microstructure and High Temperature Tribological Properties of MoSi2 Coating. J. Therm. Spray Technol. 2013, 22, 873–881. [Google Scholar] [CrossRef]
- Petrovic, J.J. Mechanical behavior of MoSi2 and MoSi2 composites. Mater. Sci. Eng. A 1995, 192–193, 31–37. [Google Scholar] [CrossRef]
- Gibala, R.; Ghosh, A.K.; Van Aken, D.C.; Srolovitz, D.J.; Basu, A.; Chang, H.; Mason, D.P.; Yang, W. Mechanical behavior and interface design of MoSi2-based alloys and composites. Mater. Sci. Eng. A 1992, 155, 147–158. [Google Scholar] [CrossRef]
- Cook, J.; Khan, A.; Lee, E.; Mahapatra, R. Oxidation of MoSi2-based composites. Mater. Sci. Eng. A 1992, 155, 183–198. [Google Scholar] [CrossRef]
- Sun, L.; Pan, J. Fabrication and characterization of TiC-particle-reinforced MoSi2 composites. J. Eur. Ceram. Soc. 2002, 22, 791–796. [Google Scholar] [CrossRef]
- Schliephake, D.; Kauffmann, A.; Cong, X.; Gombola, C.; Azim, M.; Gorr, B.; Christ, H.J.; Heilmaier, M. Constitution, oxidation and creep of eutectic and eutectoid Mo-Si-Ti alloys. Intermetallics 2019, 104, 133–142. [Google Scholar] [CrossRef]
- Narciso-Romero, F.J.; Arpón-Carballo, R.; Rodríguez-Reinoso, F.; Komatsu, M. Synthesis of a (MoSi2, Mo5Si3)/SiC composite using an in situ solid-state displacement reaction between Mo2C and Si. J. Ceram. Soc. Jpn. 2000, 108, 957–959. [Google Scholar] [CrossRef][Green Version]
- Joachim, H.S.; Kramer, M.J.; Richard, N.W. Processing and mechanical properties of a molybdenum silicide with the composition Mo–12Si–8.5B (at. %). Intermetallics 2001, 9, 25–31. [Google Scholar]
- Krakhmalev, P.V.; Ström, E.; Sundberg, M.; Li, C. Processing, microstructure and properties of C40Mo(Si, Al)2/Al2O3 composites. Mater. Sci. Eng. A 2003, 360, 207–213. [Google Scholar] [CrossRef]
- Dong, Y.O.; Kim, H.C.; Jin, K.Y.; Shon, I.J. One step synthesis of dense MOSi2-SiC composite by high-frequency induction heated combustion and its mechanical properties. J. Alloys Compd. 2005, 395, 174–180. [Google Scholar]
- Fang, C.; Xu, J.; Hou, Z. In situ pressureless sintering of SiC/MoSi2 composites. Ceram. Int. 2012, 38, 2767–2772. [Google Scholar]
- Xu, J.; Chen, F.; Tan, F. In-situ preparation of SiC–MoSi2 composite by microwave reaction sintering. Ceram. Int. 2012, 38, 6895–6898. [Google Scholar] [CrossRef]
- Wang, J.; Feng, P.; Niu, J.; Guo, R.; Akhtar, F. Synthesis, microstructure and properties of MoSi2-5vol%Al2O3 Composites. Ceram. Int. 2014, 40, 16381–16387. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, G.; Wang, X.; Wang, Z.; Feng, P. Effects of raw materials on synthesis, microstructure and properties of MoSi2-10 Vol% SiC composites. Trans.-Indian Ceram. Soc. 2016, 75, 33–39. [Google Scholar] [CrossRef]
- Zhu, G.M.; Wang, X.H.; Feng, P.Z.; Liu, Z.S.; Niu, J.A. Synthesis and properties of MoSi2–MoB–SiC ceramics. J. Am. Ceram. Soc. 2016, 99, 1147–1150. [Google Scholar] [CrossRef]
- Vorotilo, S.; Potanin, A.Y.; Pogozhev, Y.S.; Levashov, E.A.; Kochetov, N.A.; Kovalev, D.Y. Self-propagating high-temperature synthesis of advanced ceramics MoSi2–HfB2–MoB. Ceram. Int. 2019, 45, 96–107. [Google Scholar] [CrossRef]
- Benavente, R.; Salvador, M.D.; Bloem, C.; Gutierrez, C.F.; Alcazar, C.; Moreno, R.; Borrell, A. Tribological behavior of TZ4YS-MoSi2 composites obtained by spark plasma sintering. J. Eur. Ceram. Soc. 2021, 41, 7155–7163. [Google Scholar] [CrossRef]
- Nie, X.W.; Lu, Q. Fracture toughness of ZrO2-SiC/MoSi2 composite ceramics prepared by powder metallurgy. Ceram. Int. 2021, 47, 19700–19708. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Q.; Shao, X.; Ma, J.; Wang, C.; Huang, B. Microstructure, mechanical properties and oxidation resistance of Mo5Si3–Al2O3 composite. Mater. Sci. Eng. A 2014, 592, 12–18. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ishikawa, K.; Inui, H. Creep deformation of single crystals of binary and some ternary MoSi2 with the C11b structure. Intermetallics 2000, 8, 1159–1168. [Google Scholar]
- Fu, X.W.; Yang, W.Y.; Sun, Z.Q.; Zhang, L.Q.; Zhu, J. High temperature creep behavior of in situ synthesized MoSi2-30%SiC composite. Trans. Nonferrous Met. Soc. China 2002, 12, 686–690. [Google Scholar]
- Mitra, R.; Sadananda, K.; Feng, C.R. Effect of microstructural parameters and Al alloying on creep behavior, threshold stress and activation volumes of molybdenum disilicides. Intermetallics 2004, 12, 827–836. [Google Scholar] [CrossRef]
- Ballokova, B.; Hvizdos, P.; Besterci, M.; Zumdick, M.; Boehm, A. Creep testing of MoSi2—Bases composites. High Temp. Mater. Process. 2006, 25, 139–142. [Google Scholar] [CrossRef]
- Hochmuth, C.; Schliephake, D.; Völkl, R.; Heilmaier, M.; Glatzel, U. Influence of zirconium content on microstructure and creep properties of Mo–9Si–8B alloys. Intermetallics 2014, 48, 3–9. [Google Scholar] [CrossRef]
- Hasemann, G.; Bogomol, I.; Schliephake, D.; Loboda, P.I.; Krüger, M. Microstructure and creep properties of a near-eutectic directionally solidified multiphase Mo–Si–B alloy. Intermetallics 2014, 48, 28–33. [Google Scholar] [CrossRef]
- Ballokova, B.; Besterci, M.; Hvizdos, P. Creep behaviour and fracture analysis of MoSi2 based composites. High Temp. Mater. Process. 2015, 34, 317–323. [Google Scholar] [CrossRef]
- Petrovic, J.J.; Vasudevan, A.K. Key developments in high temperature structural silicides. Mater. Sci. Eng. A 1999, 261, 1–5. [Google Scholar] [CrossRef]
- Liang, J.; Liu, X.B.; Ke, J.; Luo, Y.S.; Liang, L. Preparation and high temperature oxidation resistance of laser deposited Ti5Si3/MoSi2/Mo5Si3 reinforced α-Ti/NiTi composite coatings. Surf. Coat. Technol. 2019, 372, 56–64. [Google Scholar] [CrossRef]
- Mason, D.P.; Van Aken, D.C. On the creep of directionally solidified MoSi2-Mo5Si3 eutectics. Acta Metall. Mater. 1995, 43, 1201–1210. [Google Scholar] [CrossRef]
- Arreguin, Z.J.; Turenne, S.; Martel, A.; Benaissa, A. Microwave sintering of MoSi2-Mo5Si3 to promote a final nanometer-scale microstructure and suppressing of pesting phenomenon. Mater. Charact. 2012, 68, 117–122. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, P.; Wang, X.; Zhang, H.; Liu, Y. Combustion synthesis and mechanical properties of MoSi2-ZrB2-SiC ceramics. J. Ceram. Soc. 2018, 126, 504–509. [Google Scholar] [CrossRef]
- Guo, S.Q.; Nishimura, T.; Mizuguchi, T.; Kagawa, Y. Mechanical properties of hot-pressed ZrB2–MoSi2–SiC composites. J. Eur. Ceram. Soc. 2008, 28, 1891–1898. [Google Scholar] [CrossRef]
- Maloy, S.A.; Mitchell, T.E.; Heuer, A.H. High temperature plastic anisotropy in MoSi2 single crystals. Acta Metall. Mater. 1995, 43, 657–668. [Google Scholar] [CrossRef]
- Zou, Z.X.; Xiang, J.Z.; Xu, S.Y. Theoretical derivation of hall-petch relationship and discussion of its applicable range. Phys. Exam. Test. 2012, 30, 13–17. [Google Scholar]
- Karch, J.; Birringer, R.; Gleiter, H. Ceramics ductile at low temperature. Nature 1987, 330, 556–558. [Google Scholar] [CrossRef]
- Bohn, R.; Haubold, T.; Birringer, R.; Gleiter, H. Nanocrystalline intermetallic compounds-An approach to ductility? Scr. Metall. Mater. 1991, 25, 811–816. [Google Scholar] [CrossRef]
- Cao, G.; Geng, L.; Zheng, Z.; Naka, M. The oxidation of nanocrystalline Ni3Al fabricated by mechanical alloying and spark plasma sintering. Intermetallics 2007, 15, 1672–1677. [Google Scholar] [CrossRef]
- Yoshimi, K. Advanced design and casting process development of MoSiB-based ultra-high temperature materials. Impact 2018, 2018, 53–55. [Google Scholar] [CrossRef]
- Hebsur, M.G. Development and characterization of SiC(f)/MoSi2–Si3N4(p) hybrid composites. Mater. Sci. Eng. A 1999, 261, 24–37. [Google Scholar] [CrossRef]
- Krüger, M.; Schmelzer, J.; Helmecke, M. Similarities and differences in mechanical alloying processes of V-Si-B and Mo-Si-B powders. Metals 2016, 6, 241. [Google Scholar] [CrossRef]
- Lu, S.S.; Zhou, J.S.; Wang, L.Q.; Liang, J. Influence of MoSi2 on the microstructure and elevated-temperature wear properties of Inconel 718 coating fabricated by laser cladding. Surf. Coat. Technol. 2021, 424, 127665. [Google Scholar] [CrossRef]
- Cook, B.A.; Bonino, C.A.; Trainham, J.A. Solid-state processing of oxidation-resistant molybdenum borosilicide composites for ultra-high-temperature applications. J. Mater. Sci. 2014, 49, 7750–7759. [Google Scholar] [CrossRef]
- Murakami, T.; Sasaki, S.; Ito, K.; Inui, H.; Yamaguchi, M. Microstructure of Nb substrates coated with Mo (Si, Al)2–Al2O3 composite and B-doped Mo5Si3 layers by spark plasma sintering. Intermetallics 2004, 12, 749–754. [Google Scholar] [CrossRef]
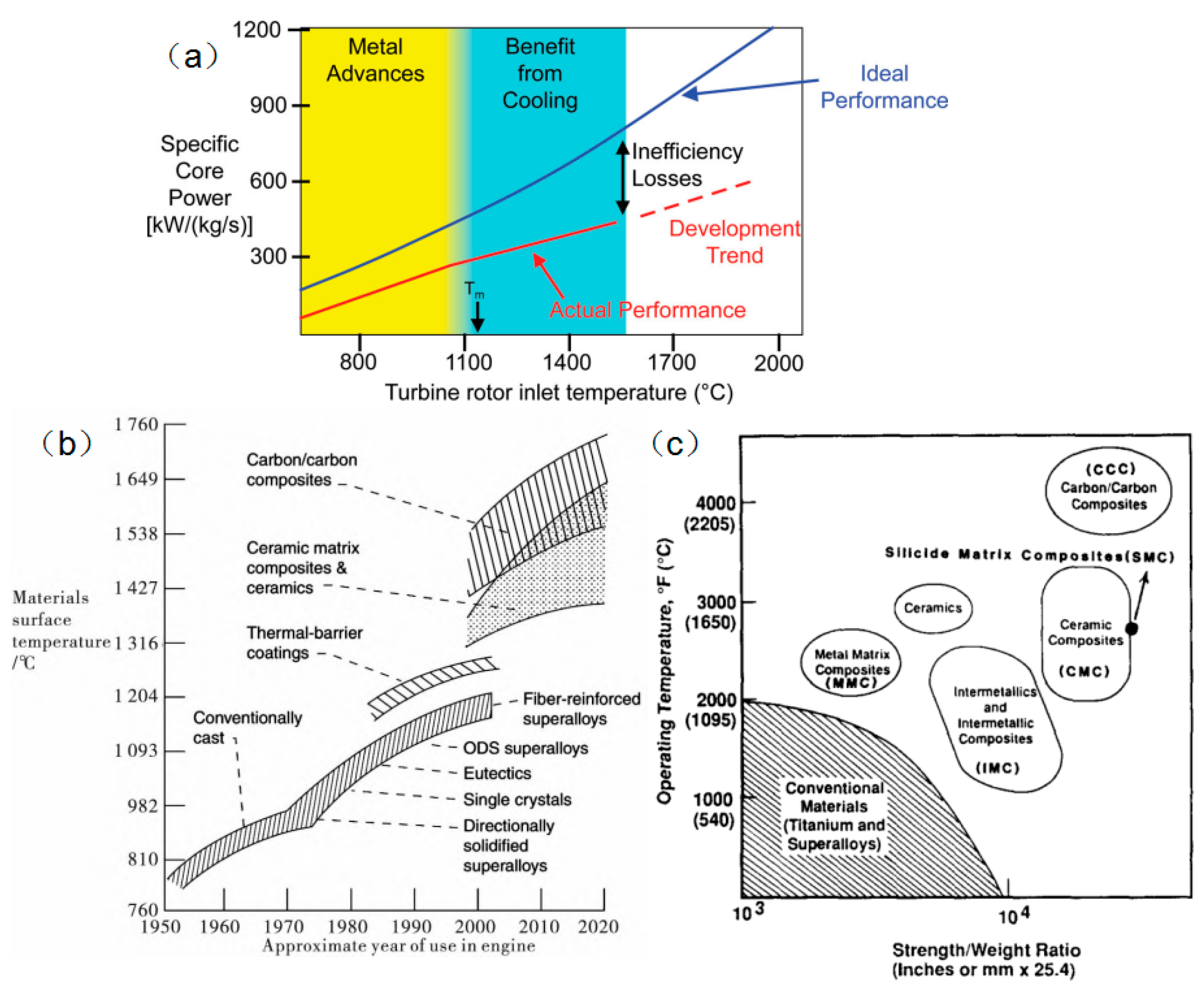

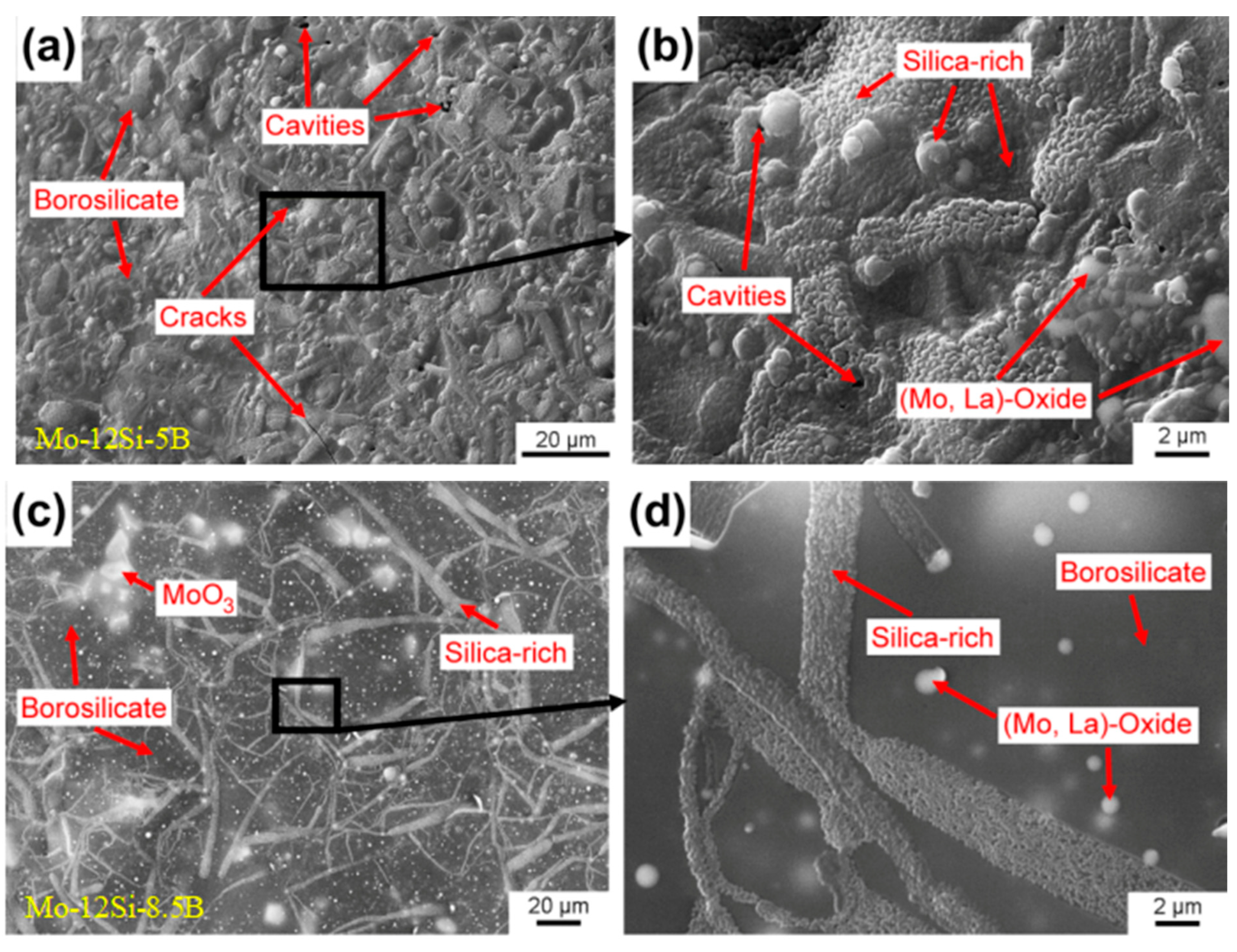
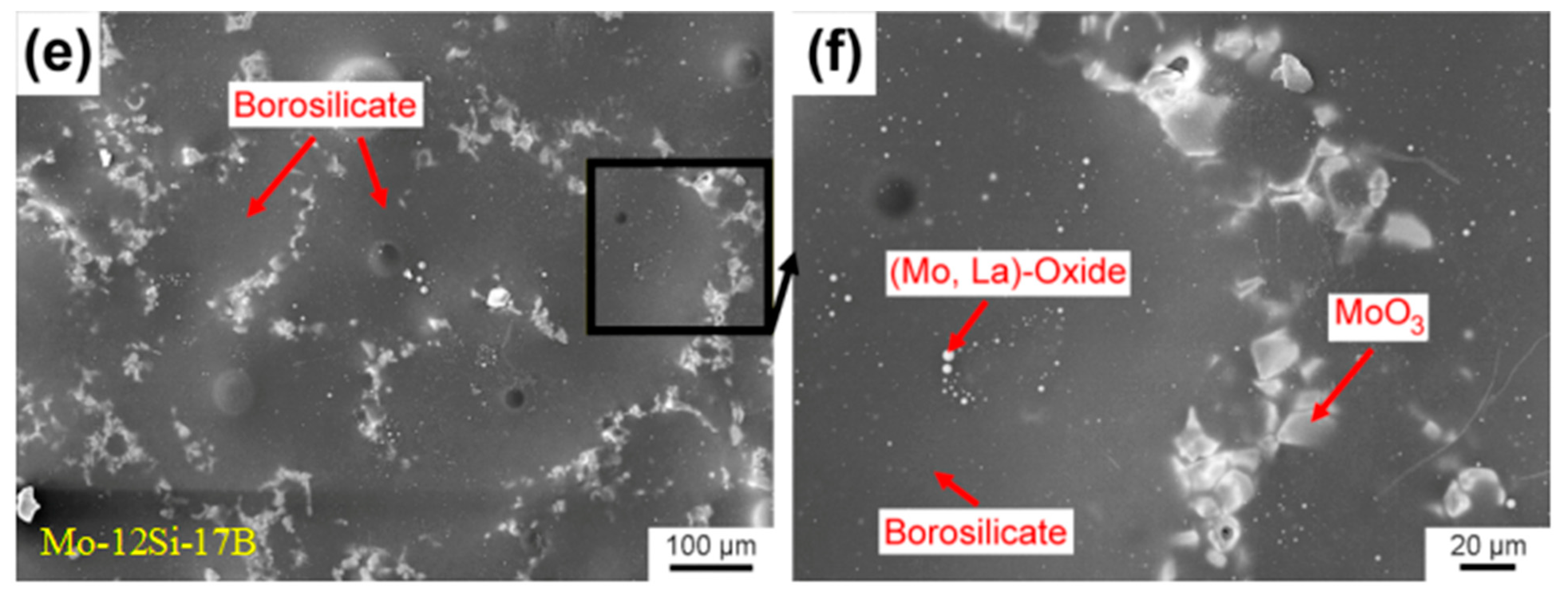



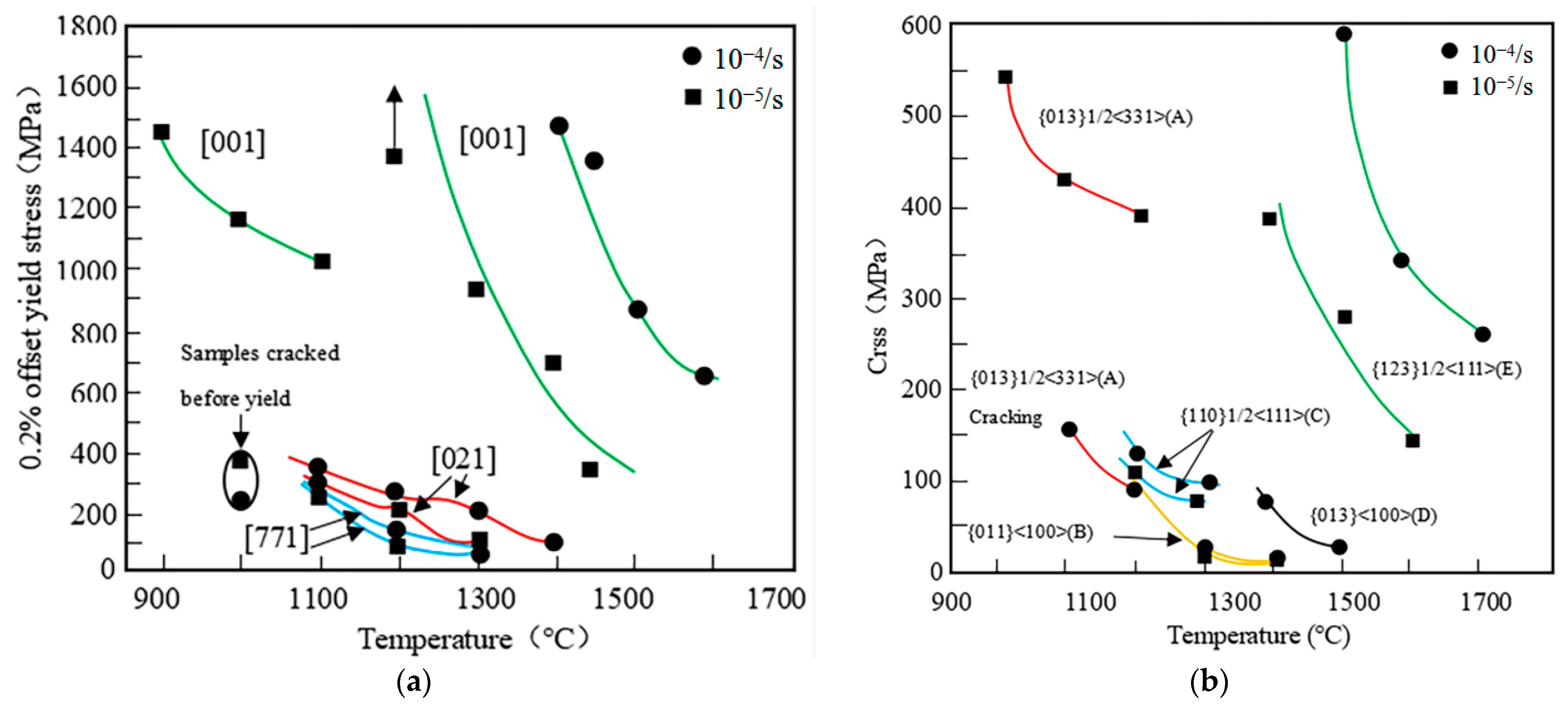
| Temperature Range (°C) | Materials System | Main Limitation (s) |
|---|---|---|
| 650–1200 | Nickel aluminides Molybdenum disilicides | Damage tolerance and creep resistance Damage tolerance |
| 1200–1400 | Molybdenum disilicides Niobium silicides | Damage tolerance and creep resistance Damage tolerance |
| 1400–1800 | Ceramic matrix composites | Damage tolerance |
| 1800–2000 | Carbon–carbon composites | Oxidation resistance |
| Alloy Type | Crystal Structure | Melting Point/°C | Density/(g·cm−3) |
|---|---|---|---|
| Ta5Si3 | D81 | 2505 | 13.40 |
| Nb5Si3 | D81 | 2484 | 7.16 |
| W5Si3 | D8m | 1370 | 14.5 |
| Zr5S i3 | D88 | 2327 | 5.99 |
| Mo5Si3 | D8m | 2180 | 8.24 |
| V5Si3 | D8m | 2150 | 5.27 |
| Ti5Si3 | D88 | 2130 | 4.32 |
| TaSi2 | C40 | 2220 | 9.10 |
| WSi2 | C11b | 2160 | 9.86 |
| MoSi2 | C11b | 2030 | 6.24 |
| MoSi3 | A15 | 2025 | 8.97 |
| ZrSi | - | 2150 | 5.94 |
| Alloy Type | Theoretical Si Concentration (at.%) | Actual Si Concentration (at.%) | Consistent |
|---|---|---|---|
| Mo3Si | 25 | 24 [18] | slightly off-stoichiometric |
| Mo5Si3 | 37.5 | 37.5–40 | off-stoichiometric |
| MoSi2 | 66.7 | 66.7 | stoichiometric |
| Silicide | Oxidation Temperature (°C) | Oxidizing Time (h) | Mass Change (mg cm−2) |
|---|---|---|---|
| MoSi2 | 1200 | 4 | +0.3 |
| 1500 | 4 | +1.3 | |
| WSi2 | 1200 | 4 | −17 |
| 1500 | 4 | −23 | |
| NbSi2 | 1200 | 6 | +40 |
| TaSi2 | 1200 | 4 | +60 |
| TiSi2 | 1200 | 4 | +22 |
| Mo5Si3 | 1500 | 4 | −67 |
| W5Si3 | 1500 | 4 | −205 |
| Ta5Si3 | 1500 | 1 | +125 |
| Ti5Si3 | 1500 | 2 | +32 |
| Type of Reinforcement | Highest Fracture Toughness (MPa·m−1/2) |
|---|---|
| Refractory metal (Nb, W, MO) wires | >15 |
| 20 vol.% Ta particles | 10 |
| 20 vol.% ZrO, particles | 7.8 |
| 20 vol.% Sic whiskers | 4.4 |
| 20 vol.% SIC particles | 4.0 |
| Polycrystalline MoSi2 | 3 |
| Material | CTE (°C−1) @ 1000 °C | Tm (°C) |
|---|---|---|
| MoSi2 | 8.5 × 10−6 | 2030 |
| TiC | 7.7 × 10−6 | 3250 |
| Mo5Si3 | 6.7 × 10−6 (@ 500 °C) | 2180 |
| HfC | 6.25 × 10−6 | 3890 |
| HfB2 | 5.5 × 10−6 | 3250 |
| SiO2 (vitreous) | 0.55 × 10−6 | 1710 |
| CaO | 13.1 × 10−6 | 2570 |
| MgO | 15.7 × 10−6 | 2800 |
| Ce20 | 14 × 10−6 | 2600 |
| Phase | CTE/K−1 | Compatibility | Performance Variation after Compounding | Mechanism |
|---|---|---|---|---|
| SiC [41,69,70] | 4.7–5.0 × 10−6 | C | Limited toughening effect at low temperatures, improved strength and oxidation resistance of MoSi2 at high temperature, a great difference in thermal expansion coefficient causing cracking. | Crack deflection; Dispersed phase strengthening |
| ZrO2 [70,71] | 8.8 × 10−6 | R | Three times the improvement for fracture toughness of MoSi2 at room temperature, improved strength of MoSi2 at high temperatures, poor thermodynamic compatibility with MoSi2. | Phase transformation toughening |
| Si3N4 [70,72] | 3.0 × 10−6 | C | Fracture toughness reaching 15 MPa·m1/2 at room temperature, creep rate of 10–8 s at 1200 °C, inhibiting pesting oxidation of MoSi2. | Microbridge toughening |
| Al2O3 [64,70] | 8.3 × 10−6 | R | Fracture toughness improvement of MoSi2 at room temperature; poor thermodynamic compatibility with MoSi2, strength and fracture toughness improvement of Mo5Si3. | Crack pinning; Fine grain strengthening |
| TiB2 [70,73] | 8.1 × 10−6 | C | Improved strength of MoSi2 at high temperatures and improved toughness of MoSi2 at room temperature. | Crack deflection |
| ZrB2 [70,73] | 8.3 × 10−6 | R | Improved MoSi2 fracture toughness. | Crack deflection; Microbridge toughening |
| TiC [66,74] | 7.4 × 10−6 | C | Improved brittleness and strength of MoSi2 at room temperature. | Enhanced dispersion |
| Nbf/Nbp [66,70] | 7.2 × 10−6 | R | Fibers can help to enhance MoSi2 fracture toughness 1–3 times, promote crack production, decrease strength at high temperature; particles help to increase fracture toughness by 20–30%. | Plastic deformation; Refining and toughening |
| Tap/Wp [71] | 6.6/4.5 × 10−6 | - | Limited improvement of MoSi2 fracture toughness, enhanced strength at high temperatures. | Fine grain strengthening |
| Preparation Methods | Mechanisms | Disadvantages |
|---|---|---|
| Casting [107,108] | Vacuum arc melting and high-temperature annealing | Produces drawbacks such as cracks, dispersed shrinkage, shrinkage cavity, and coarse grains; the microstructure is hard to be controlled |
| Directional solidification [107] | Establishes a specific temperature gradient to allow solidification along a specific orientation | Produces thermal cracking in the transition part due to large temperature difference; produces stress and deformation after solidification |
| Mechanical alloying [107,109] | Repeated ball milling, crushing, and cold welding to achieve complete alloying of the mixture. | Powder pollution; the difficulty informing metastable alloy powder; the lack of flexibility in operation |
| Self-propagating high-temperature synthesis [107,109] | The chemical reaction of the powder releases a lot of heat to make the reaction continue | The reaction is hard to be controlled; the density of the obtained material is low |
| Hot pressing; hot isostatic pressing [107,109] | Simultaneous powder pressing and pressure sintering | Requires long forming and holding time; is prone to oxidation; produces thermal stress during the cooling process |
| Laser cladding [107,110] | The high-energy density laser beam causes the material to melt rapidly and solidify | Difficulty in preparing large bulk material; high equipment requirement |
| Solid-state displacement reaction [111] | In situ reaction for 2, 3 elements or compounds to form new compounds. | Slow and expensive solid-state replacement reaction |
| Low vacuum plasma deposition [112] | In the low vacuum environment, the melting powder deposits with the high-speed plasma flow hitting the substrate | Limitations on the surface of the deposited film; complicated reaction; degraded quality of the film |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Zheng, B.; Wu, C.; Li, P.; Xue, T.; Wu, J.; Han, F.; Chen, Y. A Review of Mo-Si Intermetallic Compounds as Ultrahigh-Temperature Materials. Processes 2022, 10, 1772. https://doi.org/10.3390/pr10091772
Jiang L, Zheng B, Wu C, Li P, Xue T, Wu J, Han F, Chen Y. A Review of Mo-Si Intermetallic Compounds as Ultrahigh-Temperature Materials. Processes. 2022; 10(9):1772. https://doi.org/10.3390/pr10091772
Chicago/Turabian StyleJiang, Liang, Bin Zheng, Changsong Wu, Pengxiang Li, Tong Xue, Jiandong Wu, Fenglan Han, and Yuhong Chen. 2022. "A Review of Mo-Si Intermetallic Compounds as Ultrahigh-Temperature Materials" Processes 10, no. 9: 1772. https://doi.org/10.3390/pr10091772
APA StyleJiang, L., Zheng, B., Wu, C., Li, P., Xue, T., Wu, J., Han, F., & Chen, Y. (2022). A Review of Mo-Si Intermetallic Compounds as Ultrahigh-Temperature Materials. Processes, 10(9), 1772. https://doi.org/10.3390/pr10091772