Strain Development, Substrate Utilization, and Downstream Purification of Vitamin C
Abstract
1. Introduction
2. Applications
3. Production
- -
- Reichstein process,
- -
- Two-step fermentation, and
- -
- One-step fermentation.
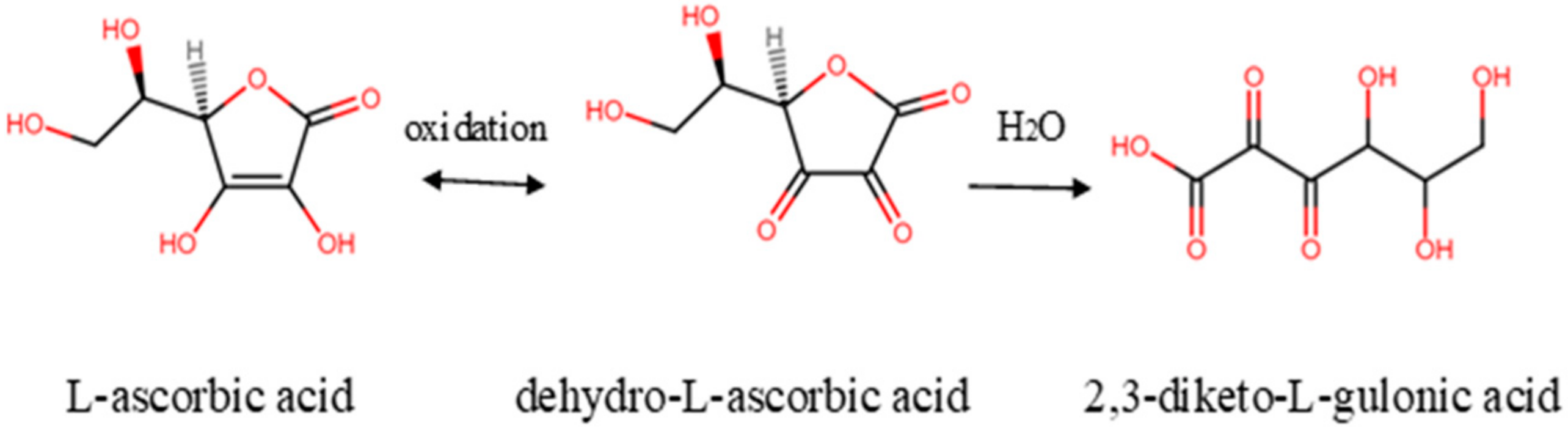
3.1. Reichstein Method
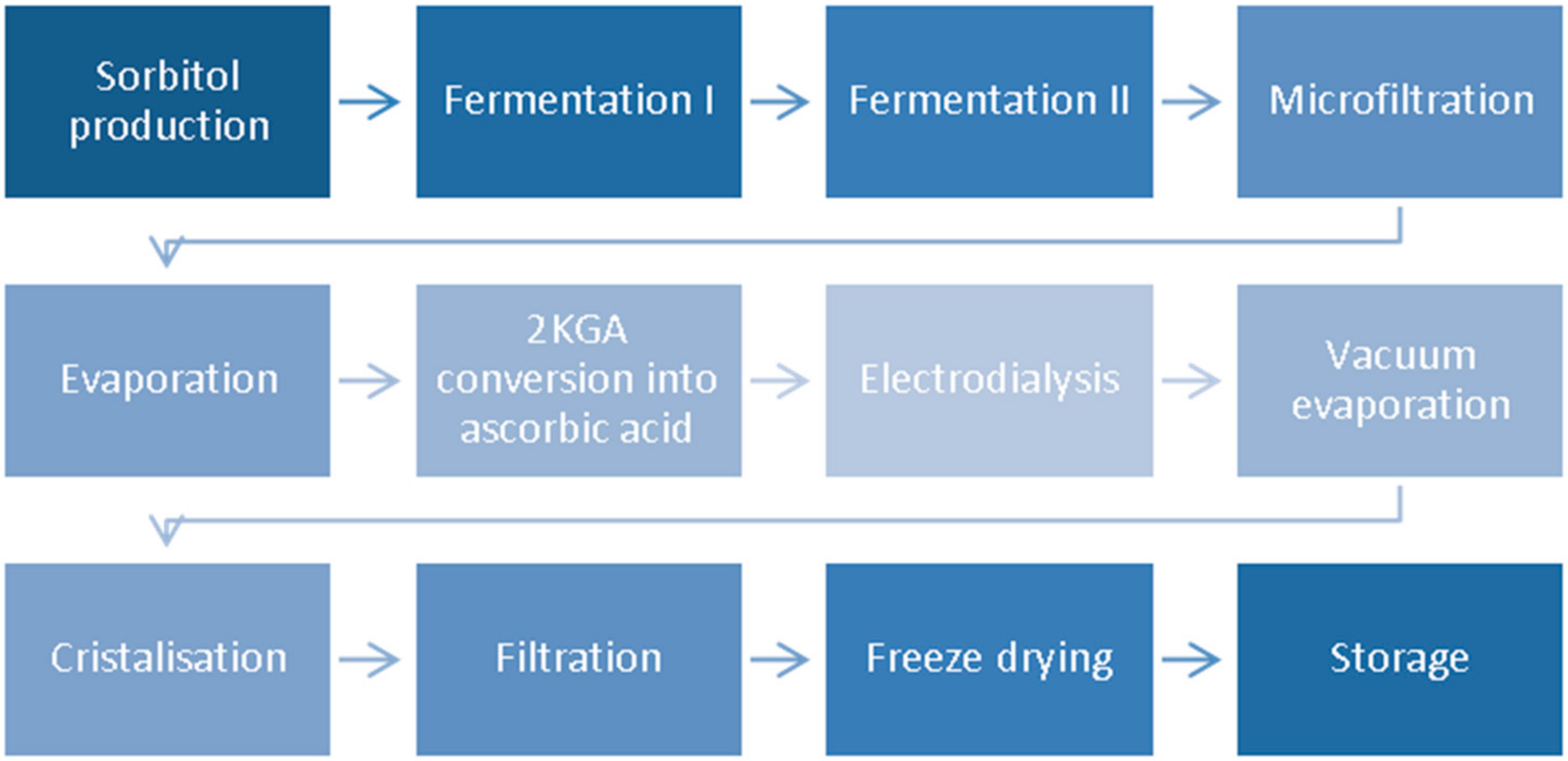
3.2. Two-Step Fermentation Process
3.3. One-Step Fermentation
4. Separation of Vitamin C
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Zeng, W.; Xu, S.; Du, G.; Zhou, J.; Chen, J. Current challenges facing one-step production of l-ascorbic acid. Biotechnol. Adv. 2018, 36, 1882–1899. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Narwal, V.; Pundir, C.S. Ascorbic acid biosensing methods: A review. Process. Biochem. 2022, 118, 11–23. [Google Scholar] [CrossRef]
- The Observatory of Economic Complexity. Available online: https://oec.world (accessed on 2 July 2022).
- Carpenter, K.J. The discovery of vitamin C. Ann. Nutr. Metab. 2012, 61, 259–264. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, M.J. (Ed.) The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed.; Whitehouse Station: Hunterdon, NJ, USA, 2006; p. 136. [Google Scholar]
- Jingyan, S.; Yuwen, L.; Zhiyong, W.; Cunxin, W. Investigation of thermal decomposition of ascorbic acid by TG-FTIR and thermal kinetics analysis. J. Pharm. Biomed. 2013, 77, 116–119. [Google Scholar] [CrossRef]
- Munoz-Munoz, J.L.; Garcia-Molina, F.; García-Ruiz, P.A.; Varon, R.; Tudela, J.; García-Cánovas, F.; Rodriguez-Lopez, J.N. Stereospecific inactivation of tyrosinase by l- and d-ascorbic acid. BBA Proteins Proteom. 2009, 1794, 244–253. [Google Scholar] [CrossRef]
- Herbig, A.L.; Renard, C.; Renard, M.G.C.C. Factors that impact the stability of vitamin C at intermediate temperatures in a food matrix. Food. Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef]
- Pappenberger, G.; Hohmann, H.P. Industrial production of l-Ascorbic acid (Vitamin C) and d-isoascorbic acid. Adv. Biochem. Eng. Biotechnol. 2013, 143, 143–188. [Google Scholar] [CrossRef]
- Bauernfeind, J.C. Ascorbic Acid Technology in Agricultural, Pharmaceutical, Food, and Industrial Applications. Adv. Chem. Ser. 1982, 200, 395–497. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. The Journal of Nutrition Critical Review New Developments and Novel Therapeutic Perspectives for Vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef]
- Hodges, R.E.; Hood, J.; Canham, J.E.; Sauberlich, H.E.; Baker, E.M. Clinical manifestations of ascorbic acid deficiency in man. Am. J. Clin. Nutr. 1971, 24, 432–443. [Google Scholar] [CrossRef]
- Akmal, M.; Qadri, J.Q.; Al-Waili, N.S.; Thangal, S.; Haq, A.; Saloom, K.Y. Improvement in human semen quality after oral supplementation of vitamin C. J. Med. Food 2006, 9, 440–442. [Google Scholar] [CrossRef]
- Kocot, J.; Luchowska-Kocot, D.; Kiełczykowska, M.; Musik, I.; Kurzepa, J. Does Vitamin C Influence Neurodegenerative Diseases and Psychiatric Disorders? Nutrients 2017, 9, 659. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102–117. [Google Scholar] [CrossRef]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic acid in skin health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Lin, S.H.; Agalloco, J. Degradation kinetics of ascorbic acid. Process. Biochem. 1979, 14, 22–24. [Google Scholar]
- Torregrosa, F.; Esteve, M.J.; Frígola, A.; Cortés, C. Ascorbic acid stability during refrigerated storage of orange–carrot juice treated by high pulsed electric field and comparison with pasteurized juice. J. Food Eng. 2006, 73, 339–345. [Google Scholar] [CrossRef]
- Berlinet, C.; Brat, P.; Brillouet, J.-M.; Ducruet, V. Ascorbic acid, aroma compounds, and browning of orange juices related to PET packaging materials and pH. J. Sci. Food Agric. 2006, 86, 2206–2212. [Google Scholar] [CrossRef]
- Mercali, G.D.; Jaeschke, D.P.; Tessaro, I.C.; Marczak, L.D.F. Study of vitamin C degradation in acerola pulp during ohmic and conventional heat treatment. Food Sci. Biotechnol. 2012, 47, 91–95. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Tsai, Y.C.; Fu, C.C.; Wu, J.S.B. Degradation of ascorbic acid in ethanolic solutions. J. Agric. Food Chem. 2012, 60, 10696–10701. [Google Scholar] [CrossRef]
- Mercali, G.D.; Schwartz, S.; Marczak, L.D.F.; Tessaro, I.C.; Sastry, S. Effect of the electric field frequency on ascorbic acid degradation during thermal treatment by ohmic heating. J. Agric. Food Chem. 2014, 62, 5865–5870. [Google Scholar] [CrossRef]
- Soares, N.F.F.; Hotchkiss, J.H. Comparative effects of De-aeration and package permeability on ascorbic acid loss in refrigerated orange juice. Packag. Technol. Sci. 1999, 12, 111–118. [Google Scholar] [CrossRef]
- Bosch, V.; Cilla, A.; García-Llatas, G.; Gilabert, V.; Boix, R.; Alegría, A. Kinetics of ascorbic acid degradation in fruit-based infant foods during storage. J. Food Eng. 2013, 116, 298–303. [Google Scholar] [CrossRef]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef]
- Hiedra, R.; Lo, K.B.; Elbashabsheh, M.; Gul, F.; Wright, R.M.; Albano, J.; Azmaiparashvili, Z.; Apontec, G.P. The use of IV vitamin C for patients with COVID-19: A case series. Expert Rev. Anti Infect. Ther. 2020, 18, 1259–1261. [Google Scholar] [CrossRef]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study. Aging 2021, 13, 7020–7034. [Google Scholar] [CrossRef]
- Sugisawa, T.; Miyazaki, T.; Hoshino, T. Microbial production of l-ascorbic acid from d-sorbitol, l-sorbose, l-gulose, and l-sorbosone by Ketogulonicigenium vulgare DSM 4025. Biosci. Biotechnol. Biochem. 2005, 69, 659–662. [Google Scholar] [CrossRef][Green Version]
- Kuivanen, J.; Penttilä, M.; Richard, P. Metabolic engineering of the fungal d-galacturonate pathway for l-ascorbic acid production. Microb. Cell Fact. 2015, 14, 2. [Google Scholar] [CrossRef]
- Gao, L.; Hu, Y.; Liu, J.; Du, G.; Zhou, J.; Chen, J. Stepwise metabolic engineering of Gluconobacter oxydans WSH-003 for the direct production of 2-keto-l-gulonic acid from d-sorbitol. Metab. Eng. 2014, 24, 30–37. [Google Scholar] [CrossRef]
- Chen, S.; Jia, N.; Ding, M.Z.; Yuan, Y.J. Comparative analysis of l-sorbose dehydrogenase by docking strategy for 2-keto-l-gulonic acid production in Ketogulonicigenium vulgare and Bacillus endophyticus consortium. J. Ind. Microbiol. Biotechnol. 2016, 43, 1507–1516. [Google Scholar] [CrossRef]
- Nassif, L.A. The Production of 2-Keto-l-Gulonic Acid by Different Gluconobacter Strains. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 1997. [Google Scholar]
- Yang, Y.; Gao, M.; Yu, X.; Zhang, Y.; Lyu, S. Optimization of medium composition for two-step fermentation of vitamin C based on artificial neural network–genetic algorithm techniques. Biotechnol. Biotec. Equip. 2015, 29, 1128–1134. [Google Scholar] [CrossRef]
- Lyu, S.; Guo, Z.; Pan, J.; Yang, Y.; Yang, W.; Chen, H.; Zhang, Z. Effect of rare earth elements on vitamin C fermentation by mixed cultures. Int. J. Agric. Biol. 2014, 16, 1135–1140. [Google Scholar]
- Takagi, Y.; Sugisawa, T.; Hoshino, T. Continuous 2-Keto-l-gulonic acid fermentation by mixed culture of Ketogulonicigenium vulgare DSM 4025 and Bacillus megaterium or Xanthomonas maltophilia. Appl. Microbiol. Biotechnol. 2010, 86, 469–480. [Google Scholar] [CrossRef]
- Yang, W.; Han, L.; Mandlaa, M.; Zhang, H.; Zhang, Z.; Xu, H. A plate method for rapid screening of Ketogulonicigenium vulgare mutants for enhanced 2-keto-l-gulonic acid production. Braz. J. Microbiol. 2017, 48, 397–402. [Google Scholar] [CrossRef]
- Hancock, R.D.; Viola, R. The use of micro-organisms for l-ascorbic acid production: Current status and future perspectives. Appl. Microbiol. Biotechnol. 2001, 56, 567–576. [Google Scholar] [CrossRef]
- Zou, W.; Liu, L.; Chen, J. Structure, mechanism and regulation of an artificial microbial ecosystem for vitamin C production. Crit. Rev. Microbiol. 2013, 39, 247–255. [Google Scholar] [CrossRef]
- Lim, S.M.; Lau, M.S.L.; Tiong, E.I.J.; Goon, M.M.; Lau, R.J.C.; Yeo, W.S.; Lau, S.Y.; Mubarak, N.M. Process design and economic studies of two-step fermentation for production of ascorbic acid. SN Appl. Sci. 2020, 2, 816. [Google Scholar] [CrossRef]
- Boudrant, J. Microbial processes for ascorbic acid biosynthesis: A review. Enzym. Microb. Technol. 1990, 12, 322–329. [Google Scholar] [CrossRef]
- Survase, S.A.; Bajaj, I.B.; Singhal, R.S. Biotechnological Production of Vitamins. Food Technol. Biotechnol. 2006, 44, 381–396. [Google Scholar]
- Yang, W.; Xu, H. Industrial Fermentation of Vitamin C. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Vandamme, E.J., Revuelta, J.L., Eds.; Wiley: Weinheim, Germany, 2016; pp. 161–192. [Google Scholar] [CrossRef]
- Sugisawa, T.; Ojima, S.; Hoshino, T.; Matzinger, P.K. Isolation and Characterization of a New Vitamin C Producing Enzyme (l-Gulono-γ-lactone Dehydrogenase) of Bacterial Origin. Biosci. Biotechnol. Biochem. 1995, 59, 190–196. [Google Scholar] [CrossRef][Green Version]
- Kaswurm, V.; van Hecke, W.; Kulbe, K.D.; Ludwig, R. Engineering of a bi-enzymatic reaction for efficient production of the ascorbic acid precursor 2-keto-l-gulonic acid. Biochem. Eng. J. 2013, 79, 104–111. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Shi, Z.; Liu, L.; Chen, J. Manipulation of B. megaterium growth for efficient 2-KLG production by K. vulgare. Process Biochem. 2010, 45, 602–606. [Google Scholar] [CrossRef]
- Sauer, M.; Branduardi, P.; Valli, M.; Porro, D. Production of l-ascorbic acid by metabolically engineered Saccharomyces cerevisiae and Zygosaccharomyces bailii. Appl. Environ. Microbiol. 2004, 70, 6086–6091. [Google Scholar] [CrossRef]
- Zhou, M.; Bi, Y.; Ding, M.; Yuan, Y. One-Step Biosynthesis of Vitamin C in Saccharomyces cerevisiae. Front. Microbiol. 2021, 12, 643472. [Google Scholar] [CrossRef]
- Running, J.A.; Huss, R.J.; Olson, P.T. Heterotrophic production of ascorbic acid by microalgae. J. Appl. Phycol. 1994, 6, 99–104. [Google Scholar] [CrossRef]
- Running, J.A.; Severson, D.K.; Schneider, K.J. Extracellular production of l-ascorbic acid by Chlorella protothecoides, Prototheca species, and mutants of P. moriformis during aerobic culturing at low pH. J. Ind. Microbiol. Biotechnol. 2002, 29, 93–98. [Google Scholar] [CrossRef]
- Ma, Q.; Bi, Y.H.; Wang, E.X.; Zhai, B.B.; Dong, X.T.; Qiao, B. Integrated proteomic and metabolomic analysis of a reconstructed three species microbial consortium for one-step fermentation of 2-keto-l-gulonicacid, the precursor of vitamin C. J. Ind. Microbiol. Biotechnol. 2019, 46, 21–31. [Google Scholar] [CrossRef]
- Rosa, J.C.C.; Colombo, L.T.; Alvim, M.C.T.; Avonce, N.; van Dijck, P.; Passos, F.M.L. Metabolic engineering of Kluyveromyces lactis for l-ascorbic acid (vitamin C) biosynthesis. Microb. Cell Fact. 2013, 12, 59. [Google Scholar] [CrossRef]
- Banjo, T.; Kareem, S.; Popoola, T.; Akinloye, O. Microbial Production of Ascorbic Acid from Brewery Spent Grain (BSG) by Aspergillus flavus and Aspergillus tamarii. Food Appl. Biosci. J. 2018, 6, 93–105. [Google Scholar] [CrossRef]
- Banjo, T.; Kareem, S.; Akinduti, P.; Popoola, T.; Akinloye, O. Optimization and production of ascorbic acid by fusant cell of Aspergillus flavus and Aspergillus tamarii. J. King Saud Univ. Sci. 2019, 31, 931–936. [Google Scholar] [CrossRef]
- Blaga, A.C.; Malutan, T. Selective separation of vitamin C by reactive extraction. J. Chem. Eng. Data 2012, 57, 431–435. [Google Scholar] [CrossRef]
- Poletto, P.; Álvarez-Rivera, G.; López, G.D.; Borges, O.M.A.; Mendiola, J.A.; Ibáñez, E.; Cifuentes, A. Recovery of ascorbic acid, phenolic compounds and carotenoids from acerola by-products: An opportunity for their valorization. LWT 2021, 146, 111654. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Joshiba, G.J. Separation and Purification of Vitamins: Vitamins B1, B2, B6, C and K1. In Applications of Ion Exchange Materials in Biomedical Industries; Springer International Publishing: Cham, Switzerland, 2019; pp. 177–187. [Google Scholar] [CrossRef]
- Ghislain De Troostembergh, J.-C.M.-P.; Debonne, A.I.; Obyn, W.R.; Gwenaëlle, C.; Peuzet, M. Process for the Manufacture of 2-Keto-l-Gulonic Acid. WIPO Patent WO 2003 016508 A2, 23 February 2003. [Google Scholar]
- Kumar, S.; Pandey, S.; Wasewar, K.L.; Ak, N.; Uslu, H. Reactive Extraction as an Intensifying Approach for the Recovery of Organic Acids from Aqueous Solution: A Comprehensive Review on Experimental and Theoretical Studies. J. Chem. Eng. Data 2021, 66, 1557–1573. [Google Scholar] [CrossRef]






| Microorganism | Culture Medium | Fermentation Method | Concentration | Bibliography |
|---|---|---|---|---|
| K.vulgarae DSM 4025 | 8%d-sorbitol, 0.25% MgSO4·7H2O, 3.0% CSL, 5.0% baker’s yeast, 0.5% urea, 0.05% glycerol, 1.5% CaCO3, and 0.15% antifoam at 30 °C and 180 rpm | Batch | 80 g/L vitamin C | [28] |
| AspergiliusnigerATCC 1015 (CBS 113.46) | 10 g/L glucose, 6 g/L NaNO3, 0.52 g/L KCl, 0.52 g/L MgCl2, and 1.52 g/L KH2PO4, with pH = 3 | Batch | 20 g/L vitamin C | [29] |
| G. oxidans (pGUC-k0203-GS-k0095-pqqABCDE) | 50 g/L sorbitol and 10 g/L yeast extract (for industrial fermentation it may be replaced by CSL) | Batch | 150 g/L 2-KGA | [30] |
| G. oxydans-ss-pqqABCDE | 2% l-sorbose, 0.3% CSL, 1% peptone, 0.3% beef extract, 0.3% yeast extract, 0.1% urea, 0.1% KH2PO4, 0.02% MgSO4, and 0.2% CaCO3 at 30 °C | Batch | 150 g/L 2-KGA | [31] |
| G. oxydans IFO 3293 | (w/v) 10.5% l-sorbose, 0.05% glycerol, 1.5% yeast extract, 0.25% MgSO4∙H2O, and 2.5% CaCO3, with pH = 7.2 | Batch | 105 g/L 2-KGA | [32] |
| G. oxydans ATCC 621 | (w/v) 10.5% sorbose, 0.05% glycerol, 1.5% yeast extract, 0.25% MgSO4∙H2O, and 2.5% CaCO3, with pH = 7.2 | Batch | 105 g/L 2-KGA | [32] |
| K. vulgare, Bacillus subtilis A9 | 92.5 g/L l-sorbose, 10.2 g/L urea, 16 g/L CSL, 3,96 g/L CaCO3, and 0.28 g/L MgSO4 at 29 °C | Batch | 92.5 g/L 2-KGA | [33] |
| K. vulgare, B. megaterium 25-B | 90 g/L l-sorbose, 10 g/L CSL, 12 g/L urea, 1 g/L KH2PO4, 0.2 g/L MgSO4, and1 g/L CaCO3at 29 °C | Batch | 90 g/L 2-KGA | [34] |
| K. vulgare DSM 4025, B. megaterium DSM 4026 and Xanthomonas maltophilia IFO 12692 | 5/L g d-glucose, 5 g/L beef extract, 5 g/L polypeptone, 3 g/L NaCl (pH 7.0 before sterilization), and 120 g/L l-sorbose | Single-stage continuous fermentation | 90 g/L 2-KGA | [35] |
| K. vulgare 65, B. megaterium 2980 | 80.0 g/L l-sorbose, 12.0 g/L carbamide (sterilized separately), 15.0 g/L CSL, 1.0 g/L KH2PO4, 0.2 g/L MgSO4·7H2O, and 1.0 g/L of CaCO3, with pH = 6.7–7.0 and at 29 °C | Batch | 80 g/L 2-KGA | [36] |
| First Step Fermentation | Second Step Fermentation |
|---|---|
| Microorganism: G. oxydans | Microorganisms: Ketogulonigenium vulgare and Bacillus megaterium |
| Fermentation time: 14–24 h | Duration: 40–70 h |
| Optimum temperature: 30–32 °C | Optimal temperature: 29 °C |
| pH: 6 | Optimal pH: 7 (corrected with NaOH) |
| Oxygen transfer rate: 300–500 mmol/L·h | Rate of oxygen transmission: 100 mmol/L·h |
| Type of fermenter: air lift fermenter | Initial concentration of substrate: (l-sorbose) 10 g/L with constant addition between 10–30 h |
| Productivity: 13 g/L·h | Final product concentration: 90–110 g/L |
| Culture Medium 1 | Culture Medium 2 |
|---|---|
| Glucose 20 g/L | Glucose 20 g/L |
| Yeast nitrogen base without amino acids, 6.7 g/L | Yeast extract 10 g/L |
| Uracil 0.02 g/L, Histidine 0.02 g/L Tryptophan 0.02 g/L Leucine 0.1 g/L | Peptone 20 g/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucaliuc, A.; Cîșlaru, A.; Kloetzer, L.; Blaga, A.C. Strain Development, Substrate Utilization, and Downstream Purification of Vitamin C. Processes 2022, 10, 1595. https://doi.org/10.3390/pr10081595
Tucaliuc A, Cîșlaru A, Kloetzer L, Blaga AC. Strain Development, Substrate Utilization, and Downstream Purification of Vitamin C. Processes. 2022; 10(8):1595. https://doi.org/10.3390/pr10081595
Chicago/Turabian StyleTucaliuc, Alexandra, Ana Cîșlaru, Lenuţa Kloetzer, and Alexandra Cristina Blaga. 2022. "Strain Development, Substrate Utilization, and Downstream Purification of Vitamin C" Processes 10, no. 8: 1595. https://doi.org/10.3390/pr10081595
APA StyleTucaliuc, A., Cîșlaru, A., Kloetzer, L., & Blaga, A. C. (2022). Strain Development, Substrate Utilization, and Downstream Purification of Vitamin C. Processes, 10(8), 1595. https://doi.org/10.3390/pr10081595