Temperature-Programmed Reduction of NiO/Al2O3 by Biochar In Situ Generated from Citric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Impregnation Experiments
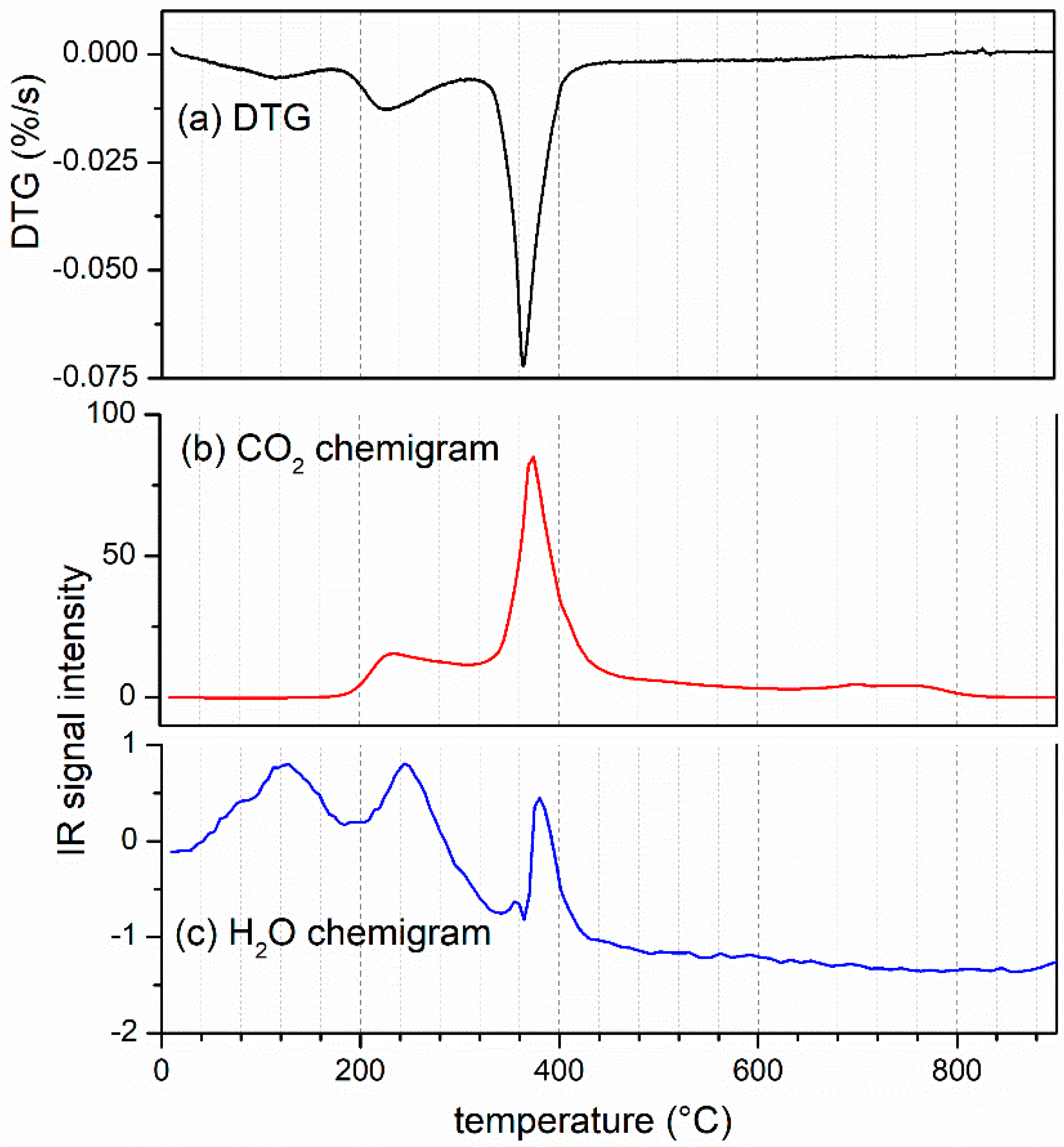
2.2. TGA–FTIR Experiments
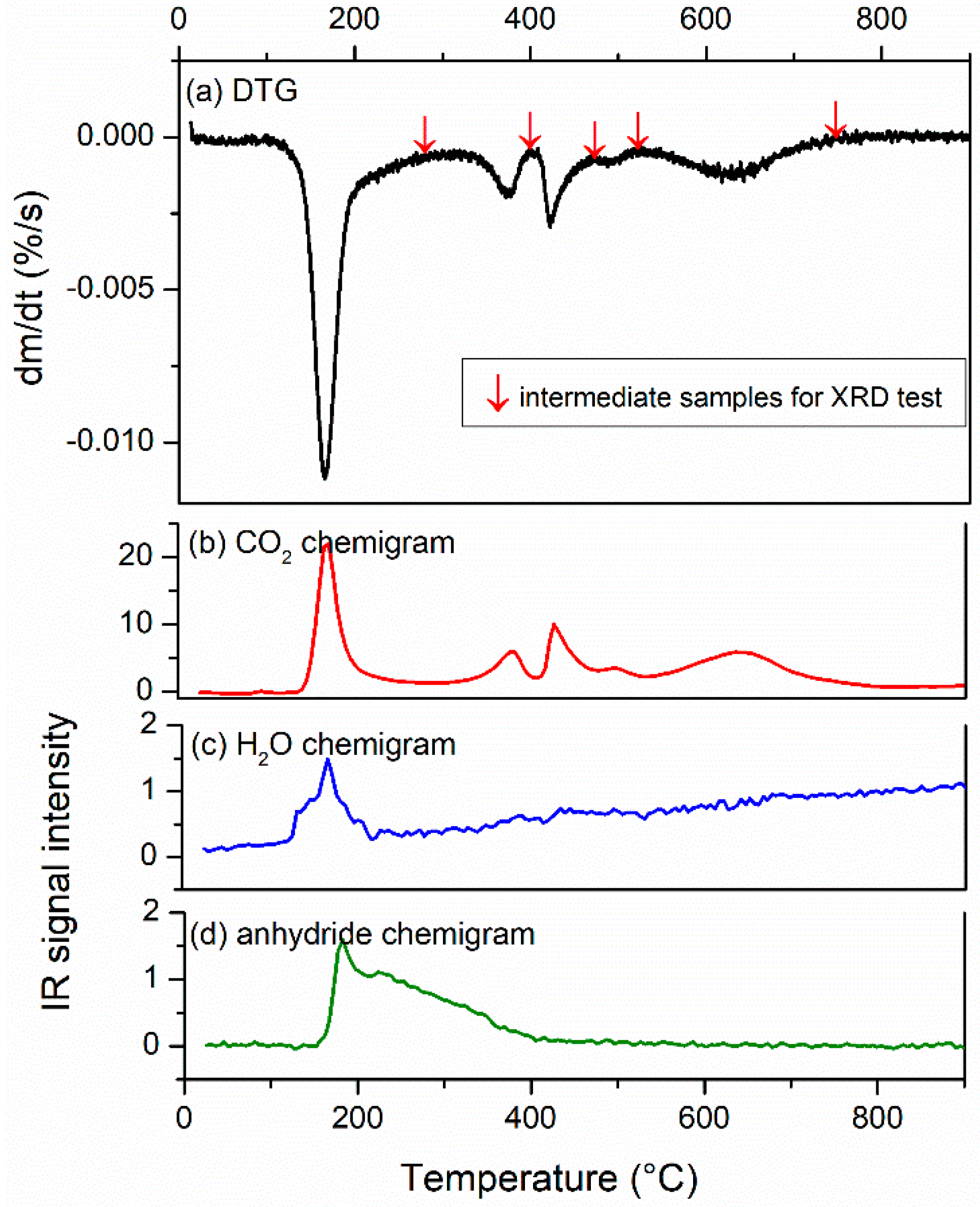
2.3. Acquisition of Intermediates
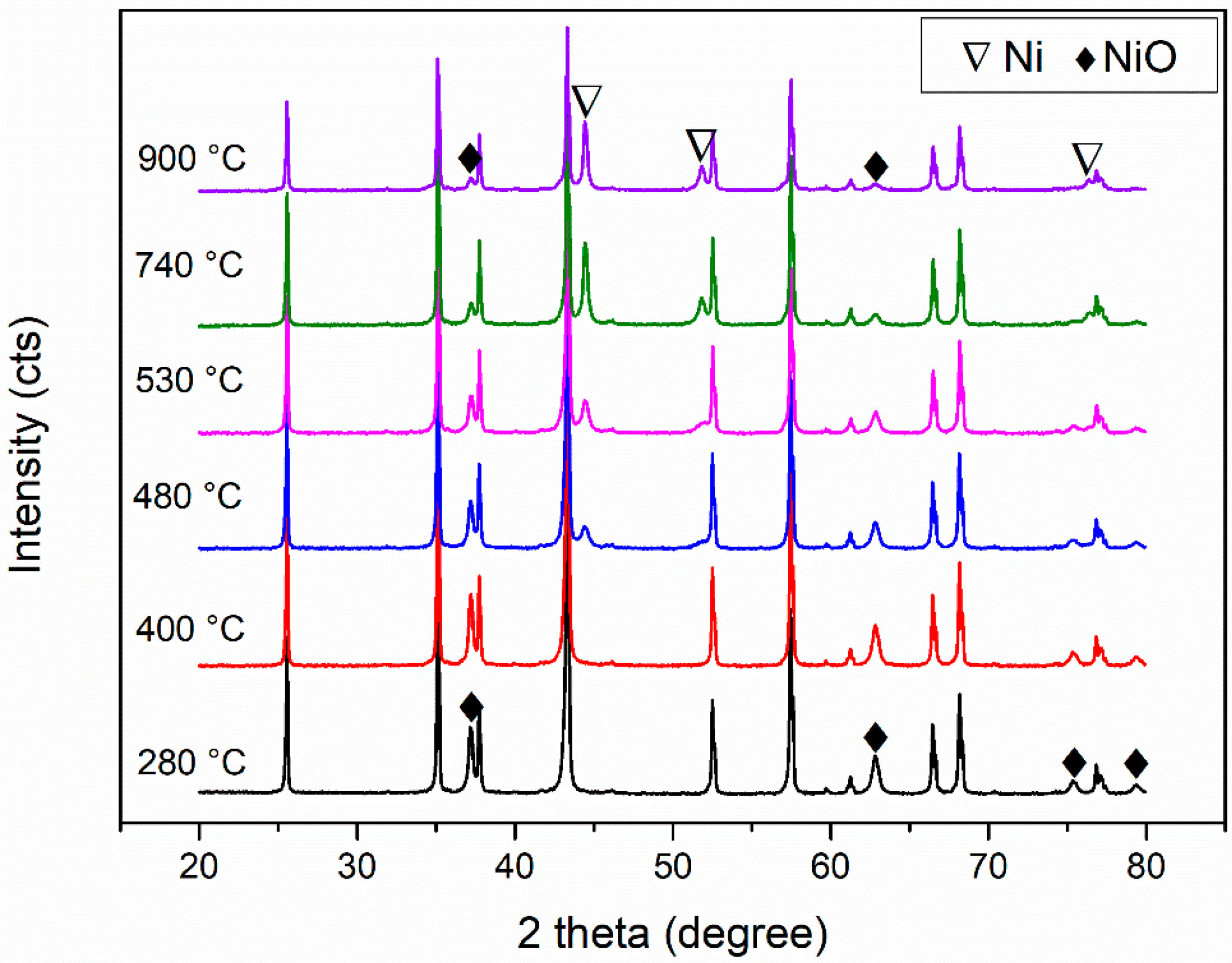
2.4. XRD Characterization and Rietveld Refinement
2.5. CHNO Elemental Analysis
2.6. Temperature Programmed Oxidation (TPO) Tests
3. Results and Discussion
3.1. Process Analysis (TGA–FTIR and XRD Results)
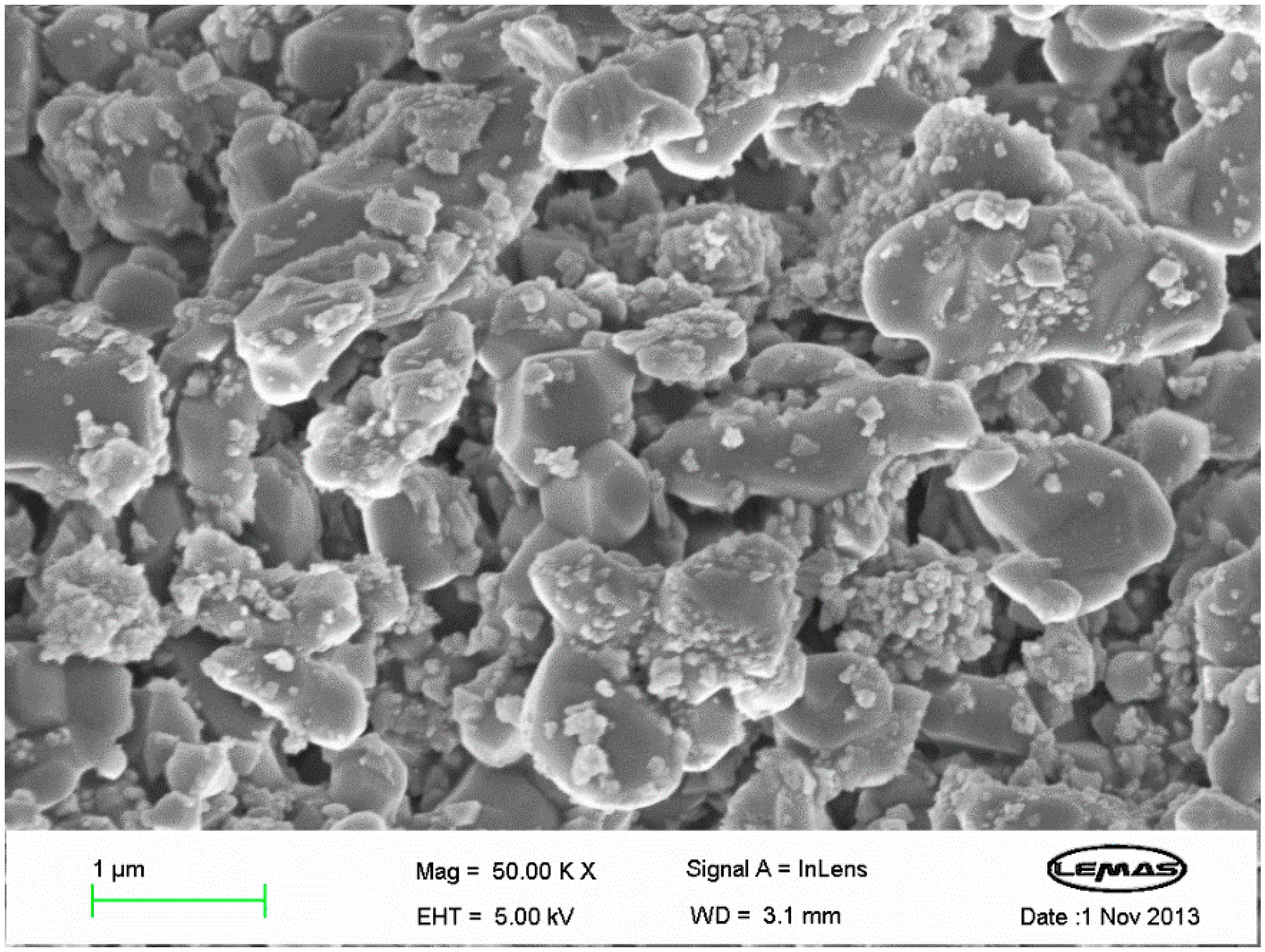
3.2. Characteristics of the In Situ Generated Biochar
3.2.1. Elementary Composition (CHNO Elemental Analysis Results)
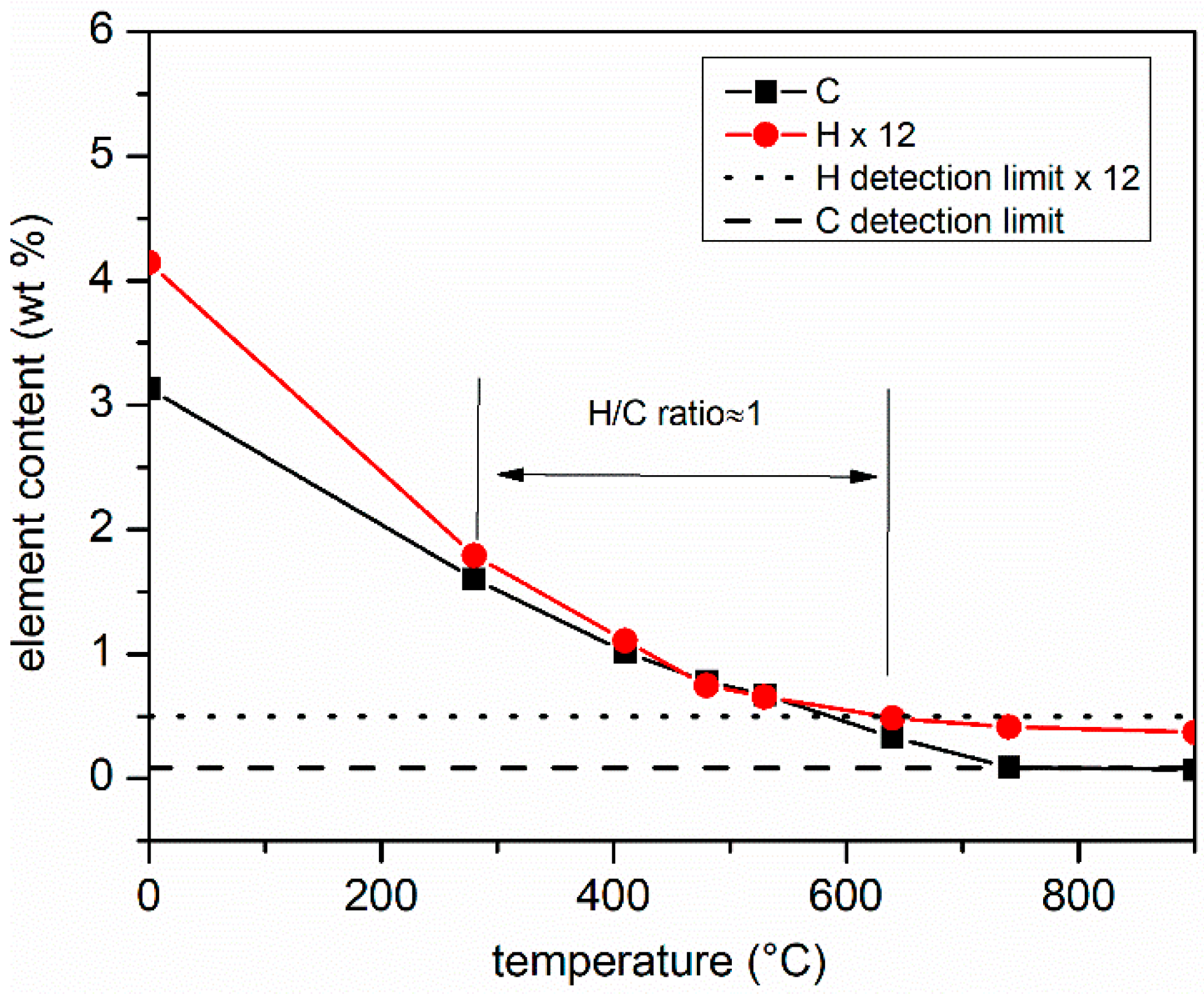
3.2.2. Reactivity and Homogeneity (TPO Results)
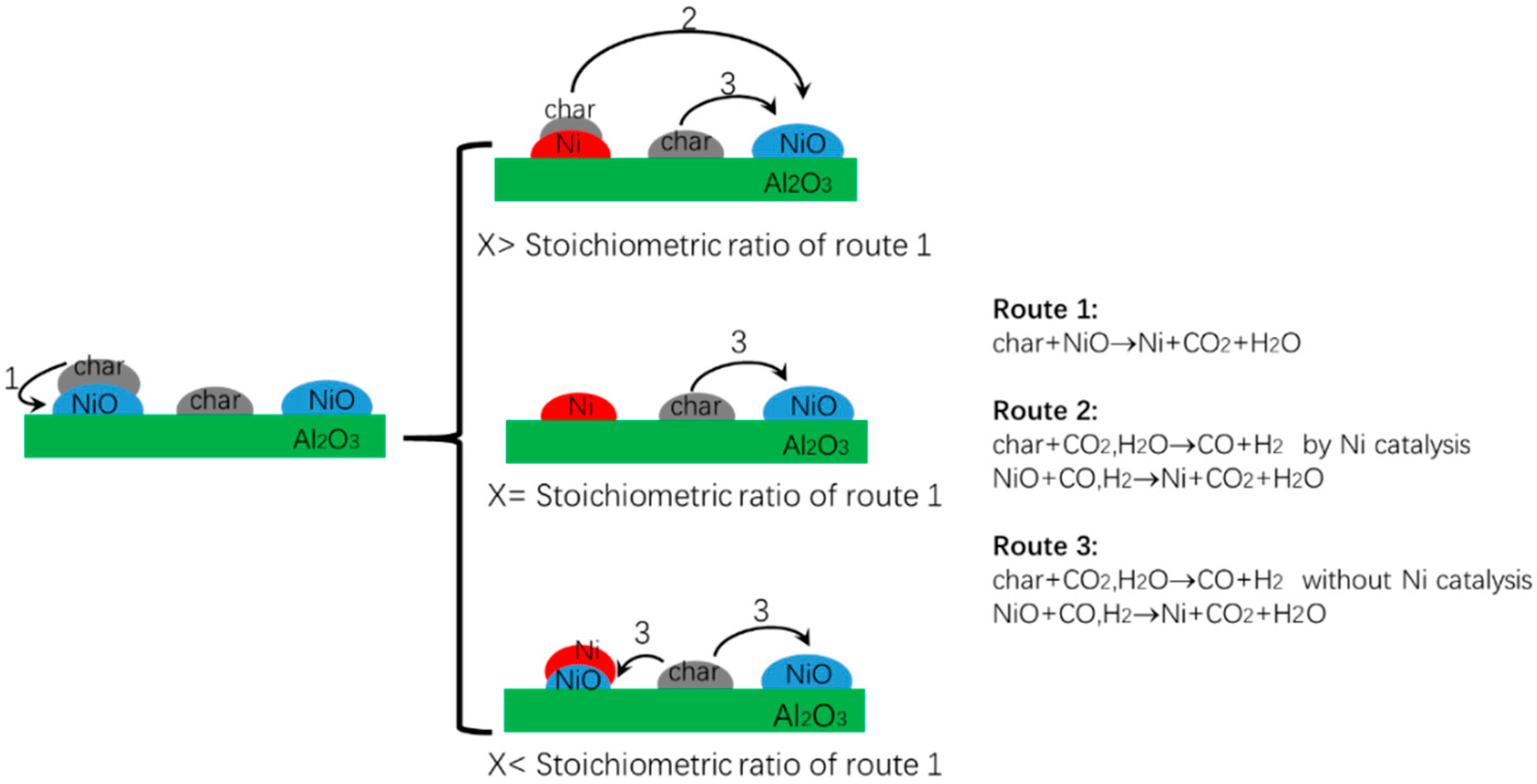
3.3. Influences of Al2O3 Support on Reduction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krzywanski, J.; Czakiert, T.; Nowak, W.; Shimizu, T.; Zylka, A.; Idziak, K.; Sosnowski, M.; Grabowska, K. Gaseous emissions from advanced CLC and oxyfuel fluidized bed combustion of coal and biomass in a complex geometry facility: A comprehensive model. Energy 2022, 251, 123896. [Google Scholar] [CrossRef]
- Xu, D.; Tong, A.; Fan, L.-S. State of Scale-Up Development in Chemical Looping Technology for Biomass Conversions: A Review and Perspectives. Waste Biomass Valorization 2022, 13, 1363–1383. [Google Scholar] [CrossRef]
- Coppola, A.; Scala, F. Chemical Looping for Combustion of Solid Biomass: A Review. Energy Fuels 2021, 35, 19248–19265. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Ghiat, I.; McKay, G.; Mackey, H.; Elkhalifa, S.; Al-Ansari, T. A comprehensive review of biomass based thermochemical conversion technologies integrated with CO2 capture and utilisation within BECCS networks. Resour. Conserv. Recycl. 2021, 173, 105734. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Hu, M.; Tian, Y.; Jin, X.; Ma, S.; Xu, T.; Hu, Z.; Liu, S.; Guo, D.; et al. Chemical looping combustion of biomass using metal ferrites as oxygen carriers. Chem. Eng. J. 2017, 312, 252–262. [Google Scholar] [CrossRef]
- Mendiara, T.; Adanez-Rubio, I.; Gayan, P.; Abad, A.; de Diego, L.F.; Garcia-Labiano, F.; Adanez, J. Process Comparison for Biomass Combustion: InSitu Gasification-Chemical Looping Combustion (iG-CLC) versus Chemical Looping with Oxygen Uncoupling (CLOU). Energy Technol. 2016, 4, 1130–1136. [Google Scholar] [CrossRef]
- Wu, H.-C.; Ku, Y.; Tsai, H.-H.; Kuo, Y.-L.; Tseng, Y.-H. Rice husk as solid fuel for chemical looping combustion in an annular dual-tube moving bed reactor. Chem. Eng. J. 2015, 280, 82–89. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, H.; Liu, Y.; Dang, H.; Ye, L.; Conejio, A.N.; Xu, R. Review on biomass metallurgy: Pretreatment technology, metallurgical mechanism and process design. Int. J. Miner. Metall. Mater. 2022, 29, 1133–1149. [Google Scholar] [CrossRef]
- Murakami, T.; Higashi, R.; Maruoka, D.; Kasai, E. Reduction of Iron Ore by Uncarbonized Biomass in a Rotary Kiln Type Furnace. Isij Int. 2021, 61, 2971–2978. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, L.; Cang, D.; Li, J.; Li, X.; Xu, C.C. Current status and potential of biomass utilization in ferrous metallurgical industry. Renew. Sustain. Energy Rev. 2017, 68, 511–524. [Google Scholar] [CrossRef]
- Ye, Q.; Peng, Z.; Li, G.; Liu, Y.; Liu, M.; Ye, L.; Wang, L.; Rao, M.; Jiang, T. Rapid microwave-assisted reduction of ferromanganese spinel with biochar: Correlation between phase transformation and heating mechanism. J. Clean. Prod. 2021, 286, 124919. [Google Scholar] [CrossRef]
- Abd Rashid, R.Z.; Salleh, H.M.; Ani, M.H.; Yunus, N.A.; Akiyama, T.; Purwanto, H. Reduction of low grade iron ore pellet using palm kernel shell. Renew. Energy 2014, 63, 617–623. [Google Scholar] [CrossRef]
- Strezov, V. Iron ore reduction using sawdust: Experimental analysis and kinetic modelling. Renew. Energy 2006, 31, 1892–1905. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Nguyen, H.K.D.; Pham, V.V.; Do, H.T. Preparation of Ni/biochar Catalyst for Hydrotreating of Bio-Oil from Microalgae Biomass. Catal. Lett. 2016, 146, 2381–2391. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Wan, C.; Liu, J.; Gao, J.; Yu, F.; Zhang, J.; Cai, Z. Iron nanoparticles in situ encapsulated in biochar-based carbon as an effective catalyst for the conversion of biomass-derived syngas to liquid hydrocarbons. Green Chem. 2013, 15, 1631–1640. [Google Scholar] [CrossRef]
- Yao, D.; Hu, Q.; Wang, D.; Yang, H.; Wu, C.; Wang, X.; Chen, H. Hydrogen production from biomass gasification using biochar as a catalyst/support. Bioresour. Technol. 2016, 216, 159–164. [Google Scholar] [CrossRef]
- Lyon, R.K.; Cole, J.A. Unmixed combustion: An alternative to fire. Combust. Flame 2000, 121, 249–261. [Google Scholar] [CrossRef]
- Ishida, M.; Jin, H. A new advanced power-generation system using chemical-looping combustion. Energy 1994, 19, 415–422. [Google Scholar] [CrossRef]
- Hossain, M.M.; de Lasa, H.I. Chemical-looping combustion (CLC) for inherent separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Mendiara, T.; Abad, A.; de Diego, L.F.; Garcia-Labiano, F.; Gayan, P.; Adanez, J. Biomass combustion in a CLC system using an iron ore as an oxygen carrier. Int. J. Greenh. Gas Control 2013, 19, 322–330. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L.; Gu, H.; Song, T.; Xiao, J. Sewage sludge combustion in a CLC process using nickel-based oxygen carrier. Chem. Eng. J. 2015, 260, 631–641. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Cao, Y.; Pan, W.-P. Investigation of chemical looping combustion by solid fuels. 1. Process analysis. Energy Fuels 2006, 20, 1836–1844. [Google Scholar] [CrossRef]
- Cao, Y.; Casenas, B.; Pan, W.-P. Investigation of chemical looping combustion by solid fuels. 2. Redox reaction kinetics and product characterization with coal, biomass, and solid waste as solid fuels and CuO as an oxygen carrier. Energy Fuels 2006, 20, 1845–1854. [Google Scholar] [CrossRef]
- Siriwardane, R.; Tian, H.; Miller, D.; Richards, G.; Simonyi, T.; Poston, J. Evaluation of reaction mechanism of coal–metal oxide interactions in chemical-looping combustion. Combust. Flame 2010, 157, 2198–2208. [Google Scholar] [CrossRef]
- Chen, L.; Bao, J.; Kong, L.; Combs, M.; Nikolic, H.S.; Fan, Z.; Liu, K. The direct solid-solid reaction between coal char and iron-based oxygen carrier and its contribution to solid-fueled chemical looping combustion. Appl. Energy 2016, 184, 9–18. [Google Scholar] [CrossRef]
- Lyngfelt, A. Chemical-looping combustion of solid fuels—Status of development. Appl. Energy 2014, 113, 1869–1873. [Google Scholar] [CrossRef]
- Kung, H.H. Transition Metal Oxides: Surface Chemistry and Catalysis; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Sharma, S.; Vastola, F.; Walker, P., Jr. Reduction of nickel oxide by carbon: II. Interaction between nickel oxide and natural graphite. Carbon 1997, 35, 529–533. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Richardson, J.; Lei, M.; Turk, B.; Forster, K.; Twigg, M.V. Reduction of model steam reforming catalysts: NiO/α-Al2O3. Appl. Catal. A Gen. 1994, 110, 217–237. [Google Scholar] [CrossRef]
- Ostrovski, O.; Zhang, G.Q. Reduction and carburization of metal oxides by methane-containing gas. Aiche J. 2006, 52, 300–310. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Li, C.-Z. NiO reduction with hydrogen and light hydrocarbons: Contrast between SiO2-supported and unsupported NiO nanoparticles. Appl. Catal. A Gen. 2011, 398, 187–194. [Google Scholar] [CrossRef]
- Quah, E.B.H.; Li, C.-Z. Roles of desorbed radicals and reaction products during the oxidation of methane using a nickel mesh catalyst. Appl. Catal. A Gen. 2004, 258, 63–71. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.-Z. Coke formation and reaction pathways of catalyst-surface-generated radicals during the pyrolysis of ethane using Ni mesh catalyst. Appl. Catal. A Gen. 2007, 316, 90–99. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Lee, W.J.; Li, C.-Z. Positive and negative catalytic effects of a nickel mesh catalyst for the partial oxidation of ethane. Chem. Eng. J. 2009, 147, 307–315. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Feng, Y.; Liu, R.; Zhao, K.; Zheng, A.; Chang, S.; Zhao, Z.; Li, H. Characteristics of biomass gasification using chemical looping with iron ore as an oxygen carrier. Int. J. Hydrogen Energy 2013, 38, 14568–14575. [Google Scholar] [CrossRef]
- El-Guindy, M.I.; Davenport, W.G. Kinetics and mechanism of llmenite reduction with graphite. Metall. Trans. 1970, 1, 1729–1734. [Google Scholar] [CrossRef]
- Sharma, S.; Vastola, F.; Walker, P., Jr. Reduction of nickel oxide by carbon: III. Kinetic studies of the interaction between nickel oxide and natural graphite. Carbon 1997, 35, 535–541. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Alsammerrai, D.A. Thermal-Decomposition of Citric-Acid. Thermochim. Acta 1986, 98, 119–126. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzynski, L. Thermal behaviour of citric acid and isomeric aconitic acids. J. Therm. Anal. Calorim. 2011, 104, 731–735. [Google Scholar] [CrossRef]
- Cheng, F.; Dupont, V.; Twigg, M.V. Temperature-programmed reduction of nickel steam reforming catalyst with glucose. Appl. Catal. A Gen. 2016, 527, 1–8. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Z.; Chen, B. H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci. Rep. 2016, 6, 22644. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; de Lasa, H.I. Reactivity and stability of Co-Ni/Al2O3 oxygen carrier in multicycle CLC. AIChE J. 2007, 53, 1817–1829. [Google Scholar] [CrossRef]
- Hu, X.; Lu, G. Inhibition of methane formation in steam reforming reactions through modification of Ni catalyst and the reactants. Green Chem. 2009, 11, 724–732. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.; Li, X. Temperature-Programmed Reduction of NiO/Al2O3 by Biochar In Situ Generated from Citric Acid. Processes 2022, 10, 1542. https://doi.org/10.3390/pr10081542
Cheng F, Li X. Temperature-Programmed Reduction of NiO/Al2O3 by Biochar In Situ Generated from Citric Acid. Processes. 2022; 10(8):1542. https://doi.org/10.3390/pr10081542
Chicago/Turabian StyleCheng, Feng, and Xiuwei Li. 2022. "Temperature-Programmed Reduction of NiO/Al2O3 by Biochar In Situ Generated from Citric Acid" Processes 10, no. 8: 1542. https://doi.org/10.3390/pr10081542
APA StyleCheng, F., & Li, X. (2022). Temperature-Programmed Reduction of NiO/Al2O3 by Biochar In Situ Generated from Citric Acid. Processes, 10(8), 1542. https://doi.org/10.3390/pr10081542





