Cadmium-Tolerant Bacteria in Cacao Farms from Antioquia, Colombia: Isolation, Characterization and Potential Use to Mitigate Cadmium Contamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples and Physical-Chemical Characterization
2.2. Isolation and Purification of Cadmium-Tolerant Bacteria (CdtB)
2.3. CdtB Identification, DNA Extraction and PCR Amplification of the 16S rRNA Partial Gene
2.4. Metabolic Characterization Using Isothermal Microcalorimetry (IMC)
2.5. Characterization of Cd Immobilization Bacterial Capacities
2.6. Biolog Gen III Metabolic Characterization
2.7. Bioaugmentation of CdtB and Mineral Amendment in the Selected Cacao Farms
2.8. Data Analysis
2.8.1. Phylogenetic Tree
2.8.2. Kinetic Growth and Thermodynamic Parameters
2.8.3. Soil Cadmium and Physical Chemical Properties
3. Results
3.1. Soil Taxonomic Sub-Group, Cacao Varieties and CdtB Isolates
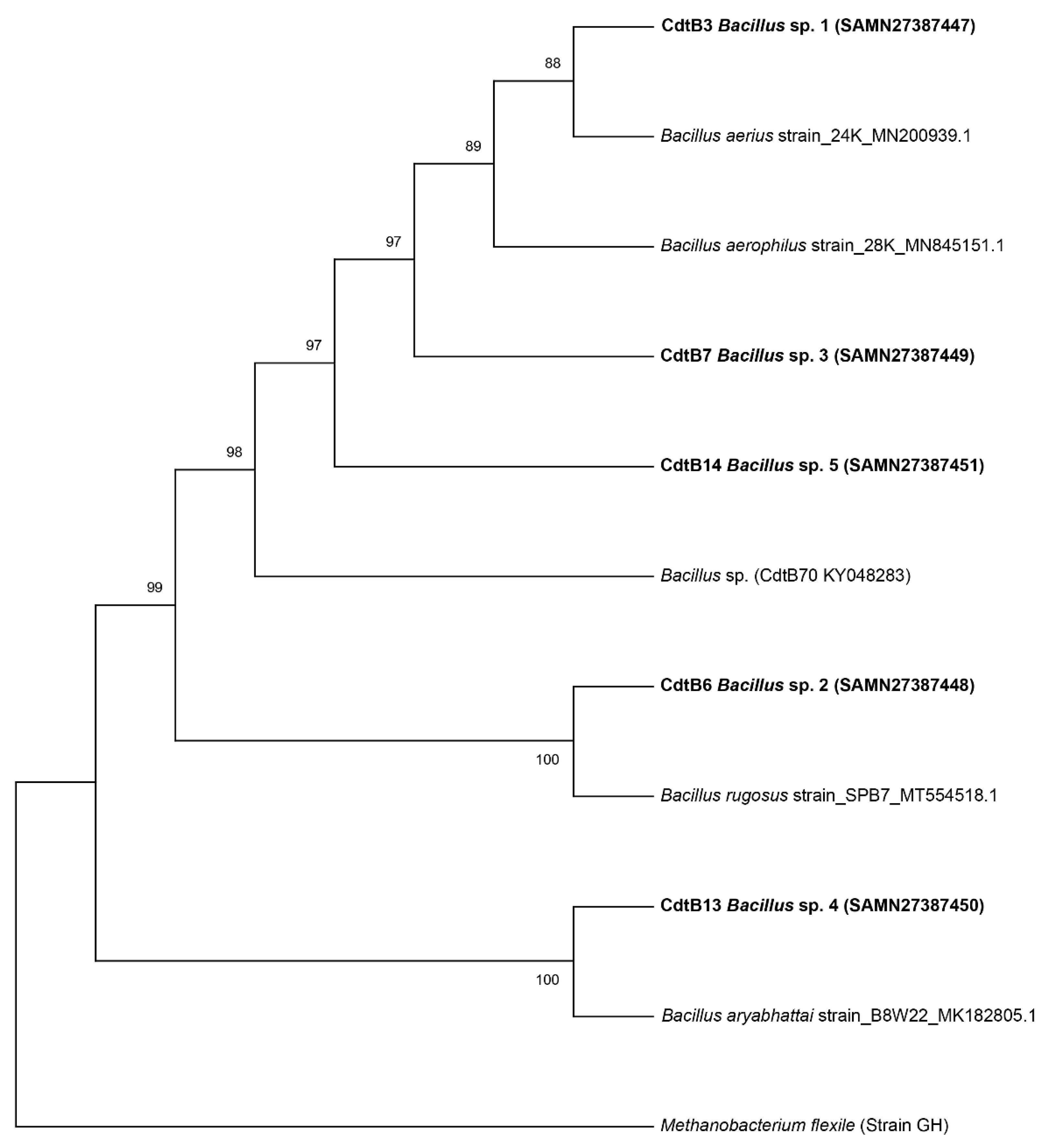
3.2. Identification of Bacterial Strains by 16S rRNA
3.3. Determining CdtB Metabolic Activity Using IMC
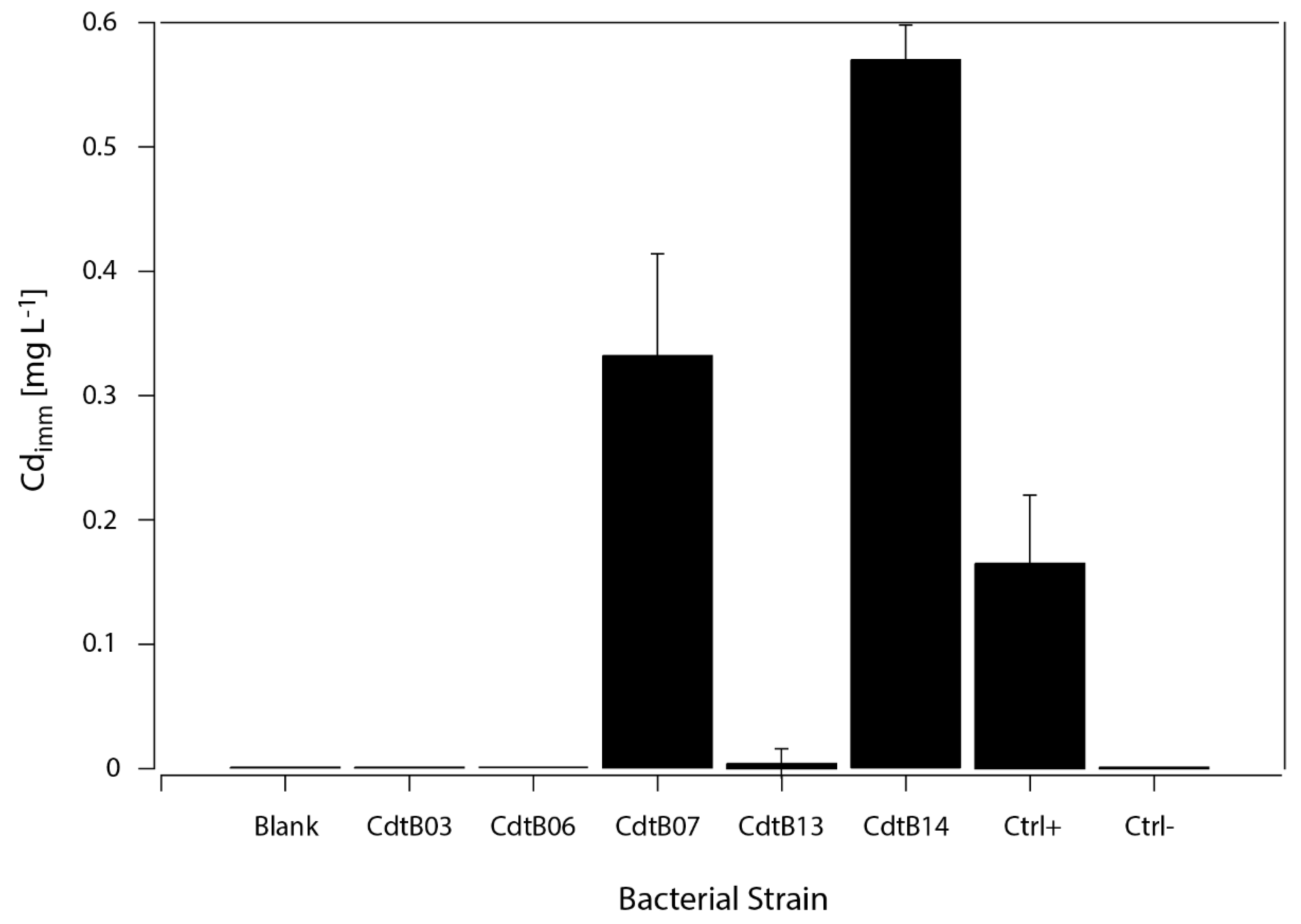
3.4. Cd Immobilization by CdtB
3.5. Application of Bioaugmented Inoculum of CdtB Strains and Zeolite in the Selected Cacao Farms
4. Discussion
4.1. Soil Properties and Cd Availability
4.2. Identification of Potentially Highly Effective CdtB Strains for Bioremediation
4.3. Metabolic Plasticity of CdtB for a Challenging Environment
4.4. Preliminary Results of the Co-Application of CdtB and Zeolite in the Selected Cacao Farms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, M.; Chen, W.; Dai, H.; Wang, X.; Yang, L.; Kang, Y.; Sun, H.; Wang, L. Cadmium-induced hormesis effect in medicinal herbs improves the efficiency of safe utilization for low cadmium-contaminated farmland soil. Ecotoxicol. Environ. Saf. 2021, 225, 112724. [Google Scholar] [CrossRef]
- Bravo, D.; Braissant, O. Cadmium Tolerant Bacteria: Current Trends and Applications in Agriculture. Lett. Appl. Microbiol. 2021. accepted. [Google Scholar] [CrossRef]
- González-Orozco, C.E.; Porcel, M. Two centuries of changes in Andean crop distribution. J. Biogeogr. 2021, 48, 1972–1980. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Joint FAO/WHO Food Standards Program Codex Committee on Food Import and Export Inspection and Certification Systems; Discussion paper on the need for further guidance on traceability/product tracing CX/FICS 07/16/7; Codex Alimentarius Commission: Surfers Paradise, Australia, 2007. [Google Scholar]
- EU, E. Commission Regulation (EU) No 488/2014 of 12 May 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs. Off. J. Eur. Union 2014, 488, 2014. [Google Scholar]
- Argüello, D.; Chavez, E.; Lauryssen, F.; Vanderschueren, R.; Smolders, E.; Montalvo, D. Soil properties and agronomic factors affecting cadmium concentrations in cacao beans: A nationwide survey in Ecuador. Sci. Total Environ. 2019, 649, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Bravo, D.; Leon-Moreno, C.; Martínez, C.A.; Varón-Ramírez, V.M.; Araujo-Carrillo, G.A.; Vargas, R.; Quiroga-Mateus, R.; Zamora, A.; Rodríguez, E.A.G. The first national survey of cadmium in cacao farm soil in Colombia. Agronomy 2021, 11, 761. [Google Scholar] [CrossRef]
- Bravo, D.; Pardo-Díaz, S.; Benavides-Erazo, J.; Rengifo-Estrada, G.; Braissant, O.; Leon-Moreno, C. Cadmium and cadmium-tolerant soil bacteria in cacao crops from northeastern Colombia. J. Appl. Microbiol. 2018, 124, 1175–1194. [Google Scholar] [CrossRef] [PubMed]
- Minari, G.D.; Saran, L.M.; Constancio, M.T.L.; da Silva, R.C.; Rosalen, D.L.; de Melo, W.J.; Alves, L.M.C. Bioremediation potential of new cadmium, chromium, and nickel-resistant bacteria isolated from tropical agricultural soil. Ecotoxicol. Environ. Saf. 2020, 204, 111038. [Google Scholar] [CrossRef]
- Shahid, M.; Javed, M.T.; Mushtaq, A.; Akram, M.S.; Mahmood, F.; Ahmed, T.; Noman, M.; Azeem, M. Microbe-mediated mitigation of cadmium toxicity in plants. In Cadmium Toxicity and Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 427–449. [Google Scholar]
- Tian, W.; Li, L.; Xiao, X.; Wu, H.; Wang, Y.; Hu, Z.; Begum, N.; Zou, Y.; Lou, L.; Chang, M. Identification of a plant endophytic growth-promoting bacteria capable of inhibiting cadmium uptake in rice. J. Appl. Microbiol. 2022, 132, 520–531. [Google Scholar] [CrossRef]
- Njoku, K.; Akinyede, O.; Obidi, O. Microbial remediation of heavy metals contaminated media by Bacillus megaterium and Rhizopus stolonifer. Sci. Afr. 2020, 10, e00545. [Google Scholar] [CrossRef]
- Lambert, M.; Leven, B.; Green, R. New methods of cleaning up heavy metal in soils and water. Environ. Sci. Technol. Lett. 2000, 7, 133–163. [Google Scholar]
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapu, R.; Li, Y.; Baligar, V.C. Evaluation of soil amendments as a remediation alternative for cadmium-contaminated soils under cacao plantations. Environ. Sci. Pollut. Res. 2016, 23, 17571–17580. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Y.; Chen, Q.; Li, Y.; Guo, D.; Nie, X.; Peng, X. Assessment of heavy metal pollution and the effect on bacterial community in acidic and neutral soils. Ecol. Indic. 2020, 117, 106626. [Google Scholar] [CrossRef]
- Gil, J.P.; López-Zuleta, S.; Quiroga-Mateus, R.Y.; Benavides-Erazo, J.; Chaali, N.; Bravo, D. Cadmium distribution in soils, soil litter and cacao beans: A case study from Colombia. Int. J. Environ. Sci. Technol. 2021, 19, 2455–2476. [Google Scholar] [CrossRef]
- IGAC. Suelos y Tierras de Colombia—Subdirección de Agrología, Tomo I; Imprenta Nacional de Colombia: Bogotá, Colombia, 2015; pp. 1–72. [Google Scholar]
- Bravo, D.; Braissant, O.; Cailleau, G.; Verrecchia, E.; Junier, P. Isolation and characterization of oxalotrophic bacteria from tropical soils. Arch. Microbiol. 2015, 197, 65–77. [Google Scholar] [CrossRef]
- Bravo, D.; Benavides-Erazo, J. The use of a two-dimensional electrical resistivity tomography (2D-ERT) as a technique for cadmium determination in cacao crop soils. Appl. Sci. 2020, 10, 4149. [Google Scholar] [CrossRef]
- EPA. Method 3051. Microwave assisted acid digestion of sediments, sludges, soils, and oils. Revision 0. In Test Methods for Evaluating Solid Wastes. Physical/Chemical Methods. SW-846; U.S. Government Printing Office: Washington, DC, USA, 1994. [Google Scholar]
- McBride, M.B. A comparison of reliability of soil cadmium determination by standard spectrometric methods. J. Environ. Qual. 2011, 40, 1863–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peech, M. Hydrogen-ion activity. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1965, 9, 914–926. [Google Scholar]
- Allison, L.; Richards, L. Diagnosis and Improvement of Saline and Alkali Soils; Soil and Water Conservative Research Branch, Agricultural Research Service: Riverdale, MD, USA, 1954. [Google Scholar]
- Shuman, L.; Duncan, R. Soil exchangeable cations and aluminum measured by ammonium chloride, potassium chloride, and ammonium acetate. Commun. Soil Sci. Plant Anal. 1990, 21, 1217–1228. [Google Scholar] [CrossRef]
- Coleman, M.V.; Thomas, D.J.D. The Structure of silicon oxide films. Phys. Status Solidi B 1967, 22, 593–602. [Google Scholar] [CrossRef]
- Pratt, P.; Bair, F. A comparison of three reagents for the extraction of aluminum from soils. Soil Sci. 1961, 91, 357–359. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Mergeay, M. Heavy metal resistances in microbial ecosystems. In Molecular Microbial Ecology Manual; Akkermans, A.D.L., Van Elsas, J.D., De Bruijn, F.J., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 439–455. [Google Scholar]
- Braissant, O.; Wirz, D.; Göpfert, B.; Daniels, A.U. Use of isothermal microcalorimetry to monitor microbial activities. FEMS Microbiol. Lett. 2010, 303, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bravo, D.; Braissant, O.; Solokhina, A.; Clerc, M.; Daniels, A.U.; Verrecchia, E.; Junier, P. Use of an isothermal microcalorimetry assay to characterize microbial oxalotrophic activity. FEMS Microbiol. Ecol. 2011, 78, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Braissant, O.; Wirz, D.; Göpfert, B.; Daniels, A.U. “The heat is on”: Rapid microcalorimetric detection of mycobacteria in culture. Tuberculosis 2010, 90, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Latimer, P. Light scattering and absorption as methods of studying cell population parameters. Annu. Rev. Biophys. 1982, 11, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Gałązka, A.; Tyśkiewicz, R.; Jaroszuk-Ściseł, J. Endophytic bacteria potentially promote plant growth by synthesizing different metabolites and their phenotypic/physiological profiles in the biolog gen iii microplateTM test. Int. J. Mol. Sci. 2019, 20, 5283. [Google Scholar] [CrossRef] [Green Version]
- Braissant, O.; Bachmann, A.; Bonkat, G. Microcalorimetric assays for measuring cell growth and metabolic activity: Methodology and applications. Methods 2015, 76, 27–34. [Google Scholar] [CrossRef]
- Abt, E.; Robin, L.P. Perspective on Cadmium and Lead in Cocoa and Chocolate. J. Agric. Food Chem. 2020, 68, 13008–13015. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [Green Version]
- Wade, J.; Ac-Pangan, M.; Favoretto, V.R.; Taylor, A.J.; Engeseth, N.; Margenot, A.J. Drivers of cadmium accumulation in Theobroma cacao L. beans: A quantitative synthesis of soil-plant relationships across the cacao belt. PLoS ONE 2022, 17, e0261989. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Xu, C.; Wang, F.; Tian, L.; Wang, Y.; Chen, H.; Yong, Z.; Choi, M.M.F.; Bramanti, E.; Maskow, T. An in vitro microcalorimetric method for studying the toxic effect of cadmium on microbial activity of an agricultural soil. Ecotoxicology 2007, 16, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Bravo, D. Bacterial cadmium-immobilization activity measured by isothermal microcalorimetry in cacao-growing soils from Colombia. Front. Environ. Sci. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Martin, G.; Guggiari, M.; Bravo, D.; Zopfi, J.; Cailleau, G.; Aragno, M.; Job, D.; Verrecchia, E.; Junier, P. Fungi, bacteria and soil pH: The oxalate–carbonate pathway as a model for metabolic interaction. Environ. Microbiol. 2012, 14, 2960–2970. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent. Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Prasad, V.; Chauhan, P.S.; Lata, C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front. Plant Sci. 2017, 8, 1510. [Google Scholar] [CrossRef] [Green Version]
- Bravo, D.; Santander, M.; Rodriguez, J.; Escobar, S.; Gideon, R.; Atkinson, R. ‘From Soil to Chocolate Bar’: Identifying Critical Steps in the Journey of Cadmium in a Colombian Cacao Plantation. Food Addit. Contam. Part A 2022. accepted. [Google Scholar] [CrossRef]
- Luo, L.Y.; Xie, L.L.; Jin, D.C.; Mi, B.B.; Wang, D.H.; Li, X.F.; Dai, X.Z.; Zou, X.X.; Zhang, Z.; Ma, Y.Q.; et al. Bacterial community response to cadmium contamination of agricultural paddy soil. Appl. Soil Ecol. 2019, 139, 100–106. [Google Scholar] [CrossRef]
- Shan, S.; Guo, Z.; Lei, P.; Wang, Y.; Li, Y.; Cheng, W.; Zhang, M.; Wu, S.; Yi, H. Simultaneous mitigation of tissue cadmium and lead accumulation in rice via sulfate-reducing bacterium. Ecotox. Environ. Safe 2019, 169, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Siripornadulsil, S.; Siripornadulsil, W. Cadmium-tolerant bacteria reduce the uptake of cadmium in rice: Potential for microbial bioremediation. Ecotox. Environ. Safe 2013, 94, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Poonguzhali, S.; Sa, T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 2007, 69, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, A.P.; Milani, R.F.; Efraim, P.; Morgano, M.A.; Tfouni, S.A.V. Cd and Pb in cocoa beans: Occurrence and effects of chocolate processing. Food Contr. 2021, 119, 107455. [Google Scholar] [CrossRef]


| Farm | Treatment | Planting Year | Cacao Cultivars | Soil Sub-Group | ID CdtB Isolates |
|---|---|---|---|---|---|
| A | Control | 2016 | TCS01, TCS06, TCS13, TCS19, CCN51 | Oxic Dystrudepts | CdtB3, CdtB13 |
| B | Strain A + zeolite | 2010 | FSV41, FSV155, FCH8, FTA2, CCN51 | Typic Kandiudox | None |
| C | Strain B + zeolite | 2015 | FSV41, Hybrids | Typic Kandiudox | CdtB6, CdtB7. |
| D | Zeolite | 2011 | CCN51, ICS95 | Typic Kandiudox | CdtB14. |
| Strain | µ [h−1] | µmax [h−1] | λ [h−1] | Qmax [J] | p-Value |
|---|---|---|---|---|---|
| CdtB3 | 0.100 ± 0.005 | 0.100 ± 0.006 | 0.191 ± 0.009 | 0.067 ± 0.005 | 0.997 |
| CdtB6 | 0.200 ± 0.007 | 0.300 ± 0.009 | 2.137 ± 0.006 | 0.063 ± 0.004 | 0.997 |
| CdtB7 | 0.100 ± 0.002 | 0.200 ± 0.003 | 0.572 ± 0.007 | 0.071 ± 0.006 | 0.997 |
| CdtB13 | 0.100 ± 0.004 | 0.100 ± 0.005 | 2.382 ± 0.004 | 0.071 ± 0.007 | 0.997 |
| CdtB14 | 0.300 ± 0.006 | 0.400 ± 0.007 | 3.757 ± 0.005 | 0.090 ± 0.006 | 0.997 |
| CdtB41 E. cloacae | 0.000 ± 0.000 | 0.001 ± 0.001 | 3.304 ± 0.003 | 0.069 ± 0.007 | 0.997 |
| E. coli K12 DSM498 | 0.000 ± 0.000 | 0.000 ± 0.000 | 3.005 ± 0.005 | 0.052 ± 0.005 | 0.997 |
| Mergeay + Cd | 0.000 ± 0.000 | 0.001 ± 0.001 | 0.110 ± 0.003 | 0.005 ± 0.002 | 0.997 |
| Farm | Time | pH | EC | SOM | CEC | Ca | Mg | K | AlH | Al | P | Cd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (dS·m−1) | (%) | (cmol+·kg−1) | (mg·kg−1) | |||||||||
| A | Before ‡ | 5.91 ± 0.34 | 0.34 ± 0.03 | 3.40 ± 0.16 | 7.92 ± 1.80 | 6.04 ± 1.57 | 1.43 ± 0.30 | 0.23 ± 0.04 | 0.14 ± 0.08 | 0.06 ± 0.05 | 3.87 ± 0 | 1.68 ± 0.03 |
| After | 4.67 | 0.22- | 4.07 | 3.49 | 1.34 | 0.39 | 0.13 | 0.83 | 0.46 | 2.66 | 1.68 ± 0.04 * | |
| B | Before ‡ | 5.47 ± 0.47 | 0.24 ± 0.03 | 3.55 ± 0.07 | 7.07 ± 2.27 | 5.21 ± 2.14 | 1.05 ± 0.65 | 0.15 ± 0.06 | 0.59 ± 0.59 | 0.42 ± 0.42 | 12.41 ± 2.09 | 1.93 ± 0.06 |
| After | 4.78 | 0.21 | 2.79 | 3.76 | 1.40 | 0.66 | 0.09 | 1.56 | 1.18 | 3.87 | 1.62 ± 0.01 * | |
| C | Before ‡ | 4.73 ± 0.03 | 0.32 ± 0.05 | 3.57 ± 0.34 | 6.76 ± 0.34 | 2.77 ± 0.33 | 0.91 ± 0.04 | 0.14 ± 0.01 | 2.87 ± 0.03 | 2.31 ± 0.01 | 3.87 ± 0 | 1.49 ± 0.04 |
| After | 6.06 | 0.34 | 5.52 | 11.41 | 8.64 | 2.57 | 0.15 | ND | ND | 6.05 | 1.19 ± 0 * | |
| D | Before ‡ | 5.06 ± 0.04 | 0.17 ± 0.01 | 2.68 ± 0.17 | 3.74 ± 0.17 | 1.84 ± 0.15 | 0.80 ± 0.07 | 0.32 ± 0.08 | 0.69 ± 0.12 | 0.39 ± 0.12 | 5.30 ± 1.43 | 1.31 ± 0.02 |
| After | 5.77 | 0.16 | 2.64 | 4.25 | 2.83 | 1.13 | 0.24 | ND | ND | 3.87 | 1.24 ± 0.01 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroga-Mateus, R.; López-Zuleta, S.; Chávez, E.; Bravo, D. Cadmium-Tolerant Bacteria in Cacao Farms from Antioquia, Colombia: Isolation, Characterization and Potential Use to Mitigate Cadmium Contamination. Processes 2022, 10, 1457. https://doi.org/10.3390/pr10081457
Quiroga-Mateus R, López-Zuleta S, Chávez E, Bravo D. Cadmium-Tolerant Bacteria in Cacao Farms from Antioquia, Colombia: Isolation, Characterization and Potential Use to Mitigate Cadmium Contamination. Processes. 2022; 10(8):1457. https://doi.org/10.3390/pr10081457
Chicago/Turabian StyleQuiroga-Mateus, Ruth, Santiago López-Zuleta, Eduardo Chávez, and Daniel Bravo. 2022. "Cadmium-Tolerant Bacteria in Cacao Farms from Antioquia, Colombia: Isolation, Characterization and Potential Use to Mitigate Cadmium Contamination" Processes 10, no. 8: 1457. https://doi.org/10.3390/pr10081457
APA StyleQuiroga-Mateus, R., López-Zuleta, S., Chávez, E., & Bravo, D. (2022). Cadmium-Tolerant Bacteria in Cacao Farms from Antioquia, Colombia: Isolation, Characterization and Potential Use to Mitigate Cadmium Contamination. Processes, 10(8), 1457. https://doi.org/10.3390/pr10081457







